Abstract
Immune checkpoint blockade has recently emerged as an important therapeutic approach to the management of malignancies across multiple disease settings. Concomitantly, there has been an increasing appreciation for the role of radiotherapy in eliciting and promoting tumor-directed immune responses. In this review, we discuss the clinical evidence to date on combinations of radiotherapy with immune checkpoint inhibitors, both from the standpoint of safety and efficacy. We highlight important but yet-unanswered questions for this combination approach, as well as their implications for future prospective studies.
Keywords: Radiotherapy, immunotherapy, immune checkpoint blockade, checkpoint inhibitors, CTLA-4, PD-1, PD-L1, metastatic disease, abscopal effect, stereotactic body radiation therapy
Background and rationale
Within the last few years, immune checkpoint blockade (ICB) has proven to be a groundbreaking advance in the treatment of multiple types of advanced malignancies. Several seminal clinical trials have shown impressive response rates utilizing ICB, leading to a paradigm shift in clinical practice, particularly among patients with advanced or metastatic disease.1–4 However, despite remarkable outcomes in some patients, a significant proportion of patients still do not attain a clinically meaningful response, and those that do frequently demonstrate a partial response at best. In addition, although some patients achieve a durable response to ICB, many progress after an initial response, reflecting the emergence of secondary resistance.
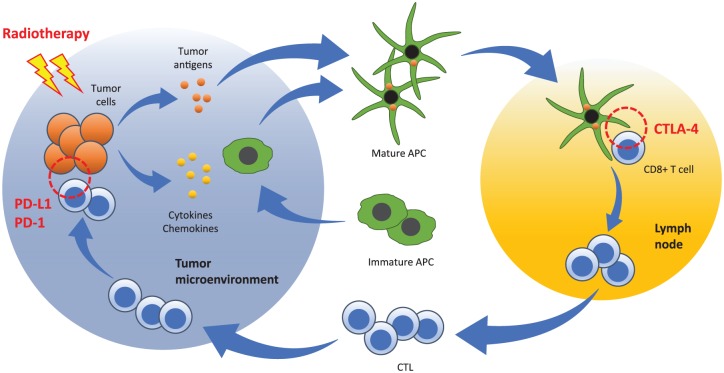
In this context, emerging preclinical and clinical data suggest an important role for radiotherapy in the potentiation and modulation of tumor immunity.5 Radiotherapy has the potential to convert immunologically ‘cold’ tumors into ‘hot’ tumors by a combination of distinct mechanisms including: (a) increasing tumor immunogenicity via the upregulation of antigenic expression, antigen processing, major histocompatibility molecules, and costimulatory signals; (b) overcoming an immunosuppressive tumor microenvironment by shifting the cytokine balance in favor of immunostimulation (e.g. by increasing the production of immunostimulatory cytokines); (c) recruiting antigen-presenting and immune effector cells to the tumor microenvironment (Figure 1).
Figure 1.
Radiation priming of a tumor-specific immune response and opportunities for combination approaches with immune checkpoint blockade immunotherapy. Radiotherapy triggers antigen release from tumor cells, and the release of cytokines and chemokines from the tumor and its microenvironment. Immature antigen-presenting cells (APCs) are recruited to the tumor microenvironment, where they uptake tumor antigens and mature. These mature APCs then traffic to tumor-draining lymph nodes, where they prime CD8+ T lymphocytes that recognize the presented tumor antigens. Activated CD8+ T cells expand into effector cytotoxic T lymphocytes (CTLs), which home to the tumor site where they recognize and kill the tumor cells. The current immune checkpoint blocking agents utilized in the clinical setting focus on the blockade of cytotoxic T lymphocyte antigen-4 (CTLA-4) at the CD8+ T-cell priming phase, and blockade of the programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) interaction at the CTL effector phase.
What is the evidence for the interplay between radiotherapy and immunotherapy? In multiple preclinical studies, radiotherapy has been shown to generate tumor-specific immune responses,6–8 an effect that was lost in T cell-deficient mice6,8 or following selective depletion of CD8+ cells.8 Additional preclinical studies have shown that a combination of radiotherapy and immunotherapy with ICB demonstrate an augmented antitumor response than either therapy alone.9,10 From the clinical standpoint, there has been increasing evidence that a combination of targeted radiotherapy and immunotherapy appears to be safe and may lead to improved tumor responses. Most of the clinical evidence to date has been in the form of case reports11–15 and small nonrandomized studies.16–19 We have summarized key clinical findings below.
Toxicities associated with radiotherapy and immune checkpoint inhibitors
Three recent trials of radiotherapy and ICB (Table 1) have reported that the combination of radiotherapy and immune checkpoint inhibitors is safe and well tolerated, without obvious additive toxicities. In the University of Pennsylvania’s phase 1 trial of 22 patients with metastatic melanoma treated with radiotherapy (6 Gy × 2–3 or 8 Gy × 2–3 to one site) and ipilimumab (3 mg/kg every 3 weeks for 4 doses), no grade 4 toxicities or dose-limiting toxicities were observed.17 Among grade 3 toxicities, anemia (4/22 patients) was the most common and colitis was noted in only 1 patient.17 In Stanford’s trial of 22 patients with metastatic melanoma treated with radiotherapy (multiple dose-fractionation regimens with biologically effective dose [BED]10 range of 28.0–112.5 Gy, given to 1–2 sites) and ipilimumab (3 mg/kg every 3 weeks for 4 doses), there was 1 case of grade 4 colitis, 1 case of grade 3 colitis, and 1 case of grade 3 hypophysitis; all other adverse events were no higher than grade 2, with rash (3/22 patients) and radiation dermatitis (4/22 patients) being the most frequent.18 Lastly, the MD Anderson Cancer Center (MDACC) at the University of Texas, USA, recently conducted a phase I trial in patients with metastatic solid tumor refractory to standard therapy, utilizing stereotactic body radiotherapy (SBRT) (50 Gy in 4 fractions or 60 Gy in 10 fractions to 1 lesion) in combination with ipilimumab (3 mg/kg every 3 weeks for 4 doses) given either concurrently or sequentially.19 In this study, there were no grade 4–5 toxicities, and grade 3 toxicities were observed in 12/35 (34%) patients with only 2/35 (6%) patients experiencing dose-limiting toxicities.
Table 1.
Toxicity and efficacy outcomes in recent phase I trials of radiotherapy and immune checkpoint inhibitors.
| Institution (reference) | Primary site | n | Radiotherapy | Immunotherapy | Schedule | Nonirradiated lesions | Grade 3+ toxicities | ||
|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | |||||||
| University of Pennsylvania (17) | Melanoma | 22 | • 6 Gy × 2–3 or 8 Gy × 2–3 • 1 site |
Ipilimumab 3 mg/kg every 3 weeks × 4 | First ipilimumab 3–5 days after RT | 0/22 (0%) | 4/22 (18%) | 4/22 (18%) | • Number of patients with any grade 3 toxicity not reported • Grade 3 anemia (4/22; 18%) most common • No grade 4–5 • No DLT |
| Stanford (18) | Melanoma | 22 | • Multiple dose-fx regimens (BED10 range 28.0–112.5 Gy) • 1–2 sites |
Ipilimumab 3 mg/kg every 3 weeks × 4 | RT within 5 days of first ipilimumab | 3/22 (14%) | 3/22 (14%) | 5/22 (23%) | • 2/22 (9%) grade 3 • 1/22 (5%) grade 4 • No grade 5 |
| MD Anderson Cancer Center (19) | NSCLC, CRC, sarcoma, RCC, and others | 35 | • 50 Gy/4 fx or 60 Gy/10 fx • 1 site |
Ipilimumab 3 mg/kg every 3 weeks × 4 | RT 1 day after first ipilimumab or 1 week after second ipilimumab | 0/31 (0%) | 3/31 (10%) | 4/31 (13%) | • 12/35 (34%) grade 3 • No grade 4–5 • 2/35 (6%) with DLT |
BED, biologically effective dose; CR, complete response; CRC, colorectal carcinoma; DLT, dose-limiting toxicity; fx, fraction; NSCLC, non-small cell lung carcinoma; PR, partial response; RCC, renal cell carcinoma; RT, radiotherapy; SD, stable disease.
It should also be noted that a recent phase I trial of ipilimumab (3–10 mg/kg) with stereotactic radiosurgery (SRS) or whole brain radiotherapy for patients with melanoma with brain metastases showed that this combination was well tolerated, with no dose-limiting toxicity noted, and 10/16 (63%) grade 3 toxicities following radiotherapy and ipilimumab.20 In addition, there is single-institutional retrospective data supporting the safety of combining SRS with ipilimumab (3–10 mg/kg) for melanoma brain metastases, in which grade 3–4 toxicities were reported in 20% of patients.21
With the expanding interest in utilizing ICB agents that target the programmed cell death protein 1 (PD-1):programmed cell death ligand 1 (PD-L1) axis, it is also reassuring that there is emerging evidence for the safety of combining radiotherapy with PD-1 inhibitor. A recent multi-institutional retrospective analysis focused on patients with metastatic non-small cell lung carcinoma (NSCLC), melanoma, or renal cell carcinoma who were treated with palliative radiotherapy and cytotoxic T lymphocyte antigen 4 (CTLA-4) and/or PD-1 inhibitor, and showed that these combinations were generally well tolerated.22 In this analysis, the overall rate of grade 3+ immune-related adverse events (ir-AEs) was 4/105 (4%) with radiotherapy + PD-1 inhibitor and 9/45 (20%) with radiotherapy + CTLA-4 inhibitor. A total of 17 patients received both CTLA-4 and PD-1 blockade, but the rate of grade 3+ ir-AEs did not appear to be significantly higher in this group. Among patients who were treated with the ICBs sequentially, 2/13 (15%) had grade 3+ ir-AEs and among those receiving the ICBs concurrently, 1/4 (25%) had grade 3+ ir-AEs. The rates of grade 3+ ir-AEs were low regardless of radiotherapy sequencing relative to ICB as well as the temporal proximity to ICB administration, although any-grade ir-AEs trended higher when radiotherapy was given within 14 days of ICB. It was noted that there were no associations between the radiotherapy site and the specific ir-AEs noted. Altogether, these data support the safety profile of radiotherapy with ICB, with little evidence of significant additive toxicities when combination therapy is used.
Efficacy data for radiotherapy and immune checkpoint inhibitors
Among the three recent trials of radiotherapy and ICB reporting distant control outcomes (Table 1), abscopal response rates in unirradiated lesions were 10–27%, with an additional 13–23% having stable disease for overall progression-free rates of 23–50%. In the University of Pennsylvania trial, 4/22 (18%) patients had a partial response and an additional 4/22 (18%) had stable disease at distant unirradiated sites.17 In the Stanford trial, 3/22 (14%) patients achieved a systemic complete response, an additional 3/22 (14%) patients had a partial response, and 5/22 (23%) patients had stable disease at a median follow up of 55 weeks.18 In the MDACC trial, among patients who had responses assessable outside the radiotherapy field, 3/31 (10%) experienced a partial response and an additional 4/31 (13%) had stable disease lasting at least 6 months.19
In the trials limited to patients with metastatic melanoma,17–18 the abscopal response rates were 18–27% and an additional 18–23% had stable disease for overall progression-free rates of 36–50%. Although these trials did not have comparison arms without radiotherapy, the clinical outcomes compare favorably with those of patients with metastatic melanoma from large randomized phase III trials.23,24 In previously treated patients with metastatic melanoma receiving ipilimumab,23 the combined response rate in the two ipilimumab arms was 38/540 (7%) and 15/137 (11%) in the ipilimumab-only arm, and stable disease in the two ipilimumab arms was 82/540 (15%) and 24/137 (18%) in the ipilimumab-only arm. In untreated patients with melanoma receiving ipilimumab alone,24 the combined response rate was 60/315 (19%) and stable disease was noted in an additional 69/315 (22%). In the latter trial of untreated patients with melanoma,24 the best outcomes were achieved in the combined ipilimumab and nivolumab cohort, where 181/314 (58%) achieved a response, including an impressive 36/314 (11%) with a complete response, and stable disease in an additional 41/314 (13%). Along with the nivolumab-only arm, it appears that nivolumab is more active than ipilimumab in this setting. One could speculate that utilizing radiotherapy with both ipilimumab and nivolumab (or nivolumab alone) could potentially increase response rates and stable disease even more than ICB alone in this setting.
Across all three early phase trials of radiotherapy and ICB, clinical benefit was associated with immunological changes, primarily in terms of changes to the peripheral CD8+ T-cell compartment. In the University of Pennsylvania trial,17 T-cell activation changes were found to correlate with treatment response. In both the Stanford18 and MDACC19 trials, there was a correlation between changes in CD8+ T cells and clinical response. Among the case reports of abscopal effects utilizing radiotherapy and ICB (to date, all of which have utilized ipilimumab), several reported correlative outcomes demonstrating that changes in peripheral blood immune cells,12 tumor-infiltrating cytotoxic T lymphocytes (CTLs),13 and/or antibody responses12,14 were associated with the observed clinical response.
It is worth mentioning how these nonrandomized early phase studies were bookended by randomized trials utilizing radiotherapy in different contexts, with or without ICB, and showing discordant outcomes. In CA184-043, men with docetaxel-refractory metastatic castration-resistant prostate cancer were treated with palliative radiotherapy (8 Gy × 1 to 1–5 bone metastases) followed by ipilimumab (10 mg/kg every 3 weeks for 4 doses) or placebo.25 Across the entire trial cohort, the combination of radiotherapy and ipilimumab did not significantly improve overall survival. However, favorable subsets, such as men without visceral metastases and those without significant laboratory abnormalities, could be identified, thus stressing the importance of patient selection. One could argue that patients with more advanced disease are relatively more immunosuppressed, and their disease status may also be too advanced to benefit from a systemic immune response even if such a response could be successfully generated. Furthermore, 8 Gy × 1 is probably not sufficiently immunogenic when targeting metastatic lesions, given preclinical evidence that multiple fractions may be beneficial for the abscopal effect.10 In the PACIFIC trial,26 patients who received concurrent chemoradiation for unresectable stage III NSCLC showed significantly improved progression-free survival (PFS) when given the anti-PD-L1 monoclonal antibody (mAb) durvalumab after chemoradiation. Although this trial did not utilize hypofractionated radiation and was in a nonmetastatic population, it has been interpreted as indicating that the chemoradiation served as an immune priming event. If this is true, the addition of durvalumab was able to potentiate a systemic immune response, translating into a significant prolongation in PFS.
Approaches to combination therapy: key unanswered questions
While there have been efforts to pilot small clinical trials combining radiotherapy and immunotherapy to establish safety and efficacy, there remains a paucity of literature to guide rational approaches with combinations of these modalities. The challenges can be summarized as follows: (a) who are the best patients for this approach?; (b) what radiation parameters should be utilized?; (c) which immune checkpoint strategies are optimal combination partners?; (d) how do we integrate radiotherapy and immunotherapy based on the currently available evidence, including considerations for sequencing of therapies?
Disease setting
With few exceptions, the initial published trials of ICB for solid malignancies have been almost exclusively limited to the treatment-refractory metastatic setting. This should not be surprising given that most ICB strategies were initially tested in cohorts of patients who had progressed past standard-of-care therapy. More recently, there have been efforts and success in utilizing ICB and other immunotherapeutic strategies in earlier disease stages, including the first-line metastatic setting and adjuvant setting for locally advanced disease. This is a logical step for two key reasons. First, the tumor burden is lower in the earlier stages of disease, which should allow a window of opportunity for the generation of effective antitumor immunity. Second, the patient is likely to be less immunosuppressed both because of their lower disease burden and also because they are likely to have received fewer lines of myelosuppressive systemic therapy.
Conceptually, it is reasonable to consider a combination strategy of radiotherapy and ICB in early stage as well as advanced or metastatic settings. In early stage disease, ICB could be utilized after definitive therapy to prevent recurrences, including metastases. This is a useful clinical endpoint even when there are already highly effective local therapies. For example, in the setting of early stage NSCLC, it is known that either SBRT or lobectomy is highly effective for local control. However, in follow up, these patients often fail distantly, thus arguing for the importance of systemic control (perhaps following definitive local therapy). In the advanced and metastatic settings, the addition of radiotherapy to ICB may potentiate the generation of antitumor immune responses, which could treat existing metastases as well as prevent future metastases. In this scenario, it is hypothesized that the inclusion of radiation could augment both local and distant tumor control. In stage III NSCLC, there is now evidence that this strategy can be highly effective: durvalumab given after definitive chemoradiation significantly prolonged PFS compared with placebo.26 Recently, a secondary analysis of patients with metastatic NSCLC from the phase I pembrolizumab trial, KEYNOTE-001, showed that patients who had previously received radiotherapy had significantly longer PFS and overall survival than those who did not.27 Although the mechanisms underlying these observations are unknown, one potential explanation is that prior radiotherapy may have augmented tumor immunity in combination with ICB.
Patient selection
Regardless of the disease setting, there continues to be lack of a validated strategy for patient selection. How do we identify which patients will benefit most from a strategy combining radiotherapy and ICB? It should be noted that biomarker development for immunotherapy is likely to be quite complex, since therapeutic outcomes are likely to involve a combination of tumor-intrinsic factors (tumor cells and/or microenvironment) and patient-specific factors. Thus, the search for a suitable biomarker is likely to be quite broad and could potentially be undertaken at multiple levels (e.g. genome, transcriptome, proteome, immunome, and/or microbiome).
It is known that patients with higher tumor PD-L1 expression levels28,29 and those with a higher mutational burden30,31 tend to have a higher response rate to PD-1-based immunotherapy. However, it is not clear which tumor and/or patient parameters are most important in predicting response rates to radiotherapy. Conceptually, patients with higher mutational burdens, particularly those with mismatch repair deficiency or those exposed to DNA-damaging agents (e.g. platinum chemotherapy) as part of their systemic regimen, may be particularly sensitive to radiotherapy-induced cellular damage. Not only would these patient cohorts show increased sensitivity to radiation cytotoxicity, but radiotherapy may be uniquely able to utilize their tumors as in situ vaccines to generate effective antitumor immune responses. It should be noted that there are currently no validated biomarkers for responses to the combination of radiotherapy with ICB.
Radiotherapy
Although several mechanisms have been elucidated to account for the ability of radiotherapy to influence tumor immunity,5,17 the optimal radiation parameters remain unknown, particularly when combined with ICB. For example, what is the optimal radiation dose and fractionation? Should an ablative SBRT strategy be utilized, as is being tested in multiple ongoing clinical trials, or would such a strategy be counterproductive and potentially immunosuppressive?32 Preclinical evidence showed that the DNA exonuclease Trex1 is induced by high radiation doses above 12–18 Gy, resulting in an attenuation of tumor immunogenicity at this dose range.33 This finding suggests that single, ablative radiation doses may actually hinder the generation of effective antitumor immunity, potentially accounting for negative results from some prior studies.
In addition, when metastatic sites are targeted, does the site of metastasis matter? From the MDACC experience in a phase I trial of patients with metastatic solid malignancies receiving SBRT with ipilimumab, targeting liver metastases as opposed to lung metastases resulted in greater activation of T cells.19 This observation implies that in metastatic settings when multiple lesions could serve as targets for radiotherapy, the location of the targeted lesions could influence whether immunological and clinical effects are observed. An important lesson is that a negative result seen in one setting should not necessarily be interpreted broadly to indicate that a similar strategy cannot work in other settings, even those that may appear to be closely related.
There is now increasing recognition that radiotherapy could be a double-edged sword in its effect on tumor immunity: while it may help to prime an immune response, it also has the potential to produce marked immunosuppressive effects.34 One important but often overlooked consideration is the exquisite radiosensitivity of circulating lymphocytes, and the proportion of these cells at risk based on the amount of normal vasculature exposed to any given course of radiotherapy.35 The variables considered most likely to influence the amount of normal vasculature exposed to radiotherapy are the field size and number of treatment fractions. Not surprisingly, the larger the fields, the greater the proportion of normal vasculature (and lymphocytes) exposed to radiotherapy at a given time. With respect to treatment fractions, given that lymphocytes are sensitive to radiation-induced apoptosis even at low radiation doses, a conventionally fractionated radiotherapy course has the disadvantage of exposing a greater proportion of circulating lymphocytes to additional rounds of cell killing compared with hypofractionated radiotherapy; the radiation fraction size is probably of secondary importance in this scenario. Thus, a more protracted radiation course is likely to contribute to a greater degree of lymphopenia. Significantly, multiple studies have suggested a clinically deleterious effect of radiotherapy-associated immunosuppression, demonstrating associations with inferior survival outcomes in cancer patients.36,37 In summary, the issue of hypofractionation becomes critical from two distinct standpoints: not only is it important during consideration of ablative versus nonablative stereotactic radiotherapy, but it also plays an important role in determining the degree of radiation sparing of circulating lymphocytes. Both considerations are expected to have important ramifications on the generation of effective antitumor immune responses.
ICB
To date, the ICBs utilized in the clinical setting have focused on two key immunological events: (a) the interaction of CTLA-4 on activated CD8+ cytotoxic T lymphocytes with CD80 and CD86 on antigen-presenting cells; (b) the interaction of PD-1 on effector CTLs with PD-L1 on tumor cells and tumor-associated inflammatory cells (Figure 1). The anti-CTLA-4 mAb ipilimumab is now approved by the US Food and Drug Administration (FDA) for melanoma, and another anti-CTLA-4 mAb, tremelimumab, is currently being tested in clinical trials. The PD-1:PD-L1 axis has generated significant excitement recently, and there are currently five agents utilized in the clinical setting: nivolumab and pembrolizumab, each targeting PD-1, and atezolizumab, durvalumab, and avelumab, which target PD-L1. The current FDA-approved indications for these ICB agents are shown in Table 2.
Table 2.
Food and Drug Administration-approved indications for programmed cell death protein 1 and programmed cell death ligand 1 immune checkpoint inhibitors (as of February 2018).
| Tumor type | Nivolumab
(PD-1) |
Pembrolizumab
(PD-1) |
Atezolizumab
(PD-L1) |
Durvalumab
(PD-L1) |
Avelumab
(PD-L1) |
|---|---|---|---|---|---|
| Melanoma/other skin | • Adjuvant treatment after complete resection of melanoma with lymph node involvement or metastatic disease • Unresectable or metastatic melanoma (single agent or in combination with ipilimumab) |
• Unresectable or metastatic melanoma | Metastatic Merkel cell carcinoma | ||
| NSCLC | • Metastatic NSCLC: 2L after platinum-based chemotherapy or EGFR or ALK targeted therapy | • Metastatic NSCLC: 1L (PD-L1 ⩾ 50%) • Metastatic NSCLC: 2L (PD-L1 ⩾ 1%) after platinum-containing chemotherapy or EGFR or ALK targeted therapy • Metastatic nonsquamous NSCLC: 1L in combination with carboplatin and pemetrexed |
• Metastatic NSCLC: 2L after platinum-containing chemotherapy or EGFR or ALK targeted therapy | ||
| Head and neck | • Recurrent or metastatic SCC of head and neck after platinum-based chemotherapy | • Recurrent or metastatic SCC of head and neck after platinum-containing chemotherapy | |||
| Lymphoma | • Relapsed or progressing classical Hodgkin’s lymphoma after autologous HSCT and brentuximab vedotin, or after ⩾ 3 lines of prior systemic therapy including auto-HSCT | • Refractory classical Hodgkin’s lymphoma after ⩾ 3 lines of prior systemic therapy | |||
| Genitourinary | • Advanced renal cell carcinoma after anti-angiogenic therapy • Locally advanced or metastatic urothelial carcinoma after platinum-based chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy |
• Locally advanced or metastatic urothelial carcinoma not eligible for cisplatin-containing chemotherapy • Locally advanced or metastatic urothelial carcinoma progressing on platinum-containing chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy |
• Locally advanced or metastatic urothelial carcinoma not eligible for cisplatin-containing chemotherapy • Locally advanced or metastatic urothelial carcinoma progressing on platinum-containing chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy |
• Locally advanced or metastatic urothelial carcinoma progressing during or after platinum-containing chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy | • Locally advanced or metastatic urothelial carcinoma progressing during or after prior platinum-containing chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy |
| Gastrointestinal | • MSI-H/dMMR metastatic colorectal cancer after standard fluoropyrimidine, oxaliplatin, and irinotecan • Hepatocellular carcinoma after sorafenib |
• MSI-H/dMMR metastatic colorectal cancer after fluoropyrimidine, oxaliplatin, and irinotecan • Recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma (PD-L1 CPS ⩾ 1) |
|||
| All solid tumors | • Unresectable or metastatic MSI-H/dMMR solid malignancies after standard therapy |
1L, first line; 2L, second line; ALK, anaplastic lymphoma kinase; CPS, combined positive score; dMMR; mismatch repair deficient; EGFR, epidermal growth factor receptor; HSCT, hematopoietic stem cell transplantation; MSI-H, microsatellite instability-high; NSCLC, non-small cell lung carcinoma; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; SCC, squamous cell carcinoma.
Despite the rapidly increasing indications for ICB immunotherapy, only a few studies to date have examined the combination of ICB with radiotherapy. At this point, it is not clear what should be the optimal ICB(s) and dose regimen to use in any particular setting. Should one or multiple ICBs be utilized along with radiotherapy?17,22 Should ICB dosing be adjusted when given with radiotherapy? Should ICB and radiotherapy be given concurrently12–14,18–19 or sequentially,17,19,26,27 and could this consideration be influenced by the immune checkpoint pathway being targeted? One could propose rational strategies to incorporate CTLA-4 blockade concurrently with radiotherapy (since CTLA-4 signaling occurs during the T cell-activation phase), whereas PD-1 or PD-L1 blockade could conceivably be given after radiotherapy (since this immune checkpoint step occurs during the T cell- effector phase). Whether a concurrent or sequential strategy is utilized could also be influenced by potential concerns about immune-mediated and radiotherapy-associated side effects, as well as whether any side effects could potentially be additive or synergistic in the setting of combination therapy. It is reassuring that the clinical evidence to date has suggested that most of these combination strategies appear to be safe and well tolerated in small patient cohorts. However, additional studies with longer follow up will be important to establish safety and efficacy.
An important question for future studies is whether nonresponders to ICB therapy could be converted to responders with the utilization of radiotherapy. There are some early clinical indications that radiotherapy could influence tumor response to PD-1-targeting ICB therapy,38 although it is not clear how common this phenomenon may be.
An additional consideration is whether maintenance ICB is required to sustain an immune response. One of the theoretical benefits of immunotherapy is the development of immunological memory, which could help to generate durable antitumor responses and potentially maintain patients in a progression-free state for a significant time interval. This has been supported by clinical evidence of prolonged treatment responses without evidence of progression or relapse in small subsets of treated patients. However, at this point, there is no validated tool to predict whether any given patient receiving ICB will develop a clinically significant memory response. If a memory response is detected, should that patient receive maintenance ICB to sustain their response, or could their response persist without additional ICB therapy? Conversely, in patients who do not have evidence of a durable response, does this suggest a limited memory response that could be overcome by prolonged (maintenance) ICB therapy? These many questions will inform future investigations.
Future directions
It is an exciting time for the field of cancer immunotherapy. The positive ICB results to date, as well as recent interest in incorporating radiotherapy with immunotherapy, hold much promise for expanding therapeutic options for oncology patients in the upcoming years. Significantly, the use of ICB has now moved from the metastatic refractory setting to first-line metastatic indications, and even to potentially curative settings.26 With the increasing recognition of the ability of radiotherapy to prime and modulate tumor immune responses, the combination of radiotherapy with ICB could further expand the utility of immunotherapy across a broad range of malignancies and indications in the upcoming years.
Going forward, it will be important to design and conduct randomized clinical trials of ICB with and without radiotherapy, which will help to establish unambiguously whether radiotherapy is clinically beneficial in the setting of ICB therapy. There is an urgent need to optimize patient selection in order to determine which individuals would benefit from immunotherapy. Is limited metastatic (oligometastatic) disease the ideal scenario for a strategy combining ICB and radiotherapy? Could this also represent a potentially curative disease state, particularly when the therapeutic strategy is supported by an antitumor memory response?
In addition, there remains the need to develop effective immunomonitoring strategies, particularly those that correlate strongly with clinical endpoints. Significant associations may help to identify useful immunological biomarkers, which can then be validated in additional patient cohorts. From the translational standpoint, further elucidation of the mechanisms underlying ICB and radiotherapy combinations could also help to identify other potential therapeutic targets. Lastly, the question remains as to how the utilization of ICB and radiotherapy may influence future treatment decisions. Much is yet to be learned, and we must rely on thoughtful and rationally designed clinical trials to provide useful information to guide therapeutic decisions in the upcoming years.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Eric C. Ko  https://orcid.org/0000-0003-2308-115X
https://orcid.org/0000-0003-2308-115X
Contributor Information
Eric C. Ko, Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, USA
Silvia C. Formenti, Department of Radiation Oncology, Weill Cornell Medicine, 525 East 68th Street, N-046, Box 169, New York, NY 10065, USA.
References
- 1. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 4. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 5. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 2015; 1: 1325–1332. [DOI] [PubMed] [Google Scholar]
- 6. Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58: 862–870. [DOI] [PubMed] [Google Scholar]
- 7. Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174: 7516–7523. [DOI] [PubMed] [Google Scholar]
- 8. Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009; 114: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005; 11: 728–734. [PubMed] [Google Scholar]
- 10. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009; 15: 5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiniker SM, Chen DS, Reddy S, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol 2012; 5: 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013; 1: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stamell EF, Wolchok JD, Gnjatic S, et al. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013; 85: 293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016; 40: 25–37. [DOI] [PubMed] [Google Scholar]
- 16. Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015; 16: 795–803. [DOI] [PubMed] [Google Scholar]
- 17. Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiniker SM, Reddy SA, Maecker HT, et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys 2016; 96: 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang C, Welsh JW, de Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res 2017; 23: 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams NL, Wuthrick EJ, Kim H, et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys 2017; 99: 22–30. [DOI] [PubMed] [Google Scholar]
- 21. Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys 2015; 92: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bang A, Wilhite TJ, Pike LRG, et al. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys 2017; 98: 344–351. [DOI] [PubMed] [Google Scholar]
- 23. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 27. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017; 18: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 29. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017; 18: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Popp I, Grosu AL, Niedermann G, et al. Immune modulation by hypofractionated stereotactic radiation therapy: therapeutic implications. Radiother Oncol 2016; 120: 185–194. [DOI] [PubMed] [Google Scholar]
- 33. Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017; 8: 15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Talebian Yazdi M, Schinkelshoek MS, Loof NM, et al. Standard radiotherapy but not chemotherapy impairs systemic immunity in non-small cell lung cancer. Oncoimmunology 2016; 5: e1255393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yovino S, Kleinberg L, Grossman SA, et al. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 2013; 31: 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 2011; 17: 5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Campian JL, Ye X, Brock M, et al. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest 2013; 31: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yuan Z, Fromm A, Ahmed KA, et al. Radiotherapy rescue of a nivolumab-refractory immune response in a patient with PD-L1-negative metastatic squamous cell carcinoma of the Lung. J Thorac Oncol 2017; 12: e135–e136. [DOI] [PubMed] [Google Scholar]