Abstract
Study Design:
Retrospective case study.
Objective:
To evaluate the trends and demographics of recombinant human bone morphogenetic protein 2 (rhBMP2) utilization in single-level anterior lumbar interbody fusion (ALIF) in the United States.
Methods:
Patients who underwent single-level ALIF from 2005 to 2011 were identified by searching ICD-9 diagnosis and procedure codes in the PearlDiver Patient Records Database (PearlDiver Technologies, Fort Wayne, IN), a national database of orthopedic insurance records. The year of procedure, age, gender, and region of the United States were analyzed for each patient.
Results:
A total of 921 patients were identified who underwent a single-level ALIF in this study. The average rate of single-level ALIF with rhBMP2 utilization increased (35%-48%) from 2005 to 2009, but sharply decreased to 16.7% in 2010 and 15.0% in 2011. The overall incidence of single-level ALIF without rhBMP2 (0.20 cases per 100 000 patients) was more than twice of the incidence of single-level ALIF with rhBMP2 (0.09 cases per 100 000 patients). The average rate of single-level ALIF with rhBMP2 utilization is highest in West (41.4%), followed by Midwest (33.3%), South (26.5%) and Northeast (22.2%). The highest incidence of single-level ALIF with rhBMP2 was observed in the group aged less than 65 years (compared with any other age groups, P < .001), with an incidence of 0.21 per 100 000 patients.
Conclusions:
The incidence of rhBMP2 utilization in single-level ALIF increased from 2006 to 2009, but decreased in 2010 and 2011. The Northeast region had the lowest incidence of rhBMP2 utilization. The group aged less than 65 years trended to have the higher incidence of single-level ALIF with rhBMP2 utilization.
Keywords: recombinant human bone morphogenetic protein 2 (rhBMP2), anterior lumbar interbody fusion (ALIF), demographics, single-level
Introduction
The procedure of anterior lumbar interbody fusion (ALIF) is commonly performed on patients suffering from pain and neurological symptoms associated with disorders of the lumbar spine caused by disc degeneration or trauma. An important objective of the ALIF procedure is solid arthrodesis of the spinal segment.1 Recombinant human bone morphogenetic protein (rhBMP) belongs to a family of differentiation factors that promote bone creation and remodeling.2 Clinical use of rhBMP was approved by the US Food and Drug Administration (FDA) in 2002 for surgery of the anterior lumbar spine to promote bone fusion.3 The use of rhBMP2 may decrease pseudarthrosis and eliminate the bone graft harvest morbidity associated with procurement of iliac crest bone graft material. However, questions about complications associated with the use of rhBMP2 in humans have been reported.4-9 The FDA issued Public Health Notification in 2008 regarding the utilization of rhBMP2 in the anterior cervical spine fusion.10 The trend of rhBMP2 utilization in single-level ALIF has not yet been reported.
The primary objective of this study is to further define the epidemiology, patient demographics of rhBMP2 utilization in single-level ALIF from a national private insurance database from 2005-2011.
Materials and Methods
Patients undergoing single-level ALIF with rhBMP2 utilization were searched by use of the PearlDiver Patient Records Database (PearlDiver Technologies, Fort Wayne, IN). This database is a national insurance database with the largest contribution being from UnitedHealth Group (Decatur, IL). For the years 2005 to 2011, more than 49 million patient records exist in the database with an International Classification of Diseases, Ninth Revision (ICD-9) code or Current Procedural Terminology (CPT) code. Access to the database was granted by PearlDiver Technologies for the purpose of academic orthopedic research. The database was stored on a password-protected computer in our laboratory. By use of the database, CPT and ICD-9 codes can be searched in isolation or in combination with one another. The search results yield the number of patients with the searched code or combination of codes in each year, 5-year age group, and region of the United States.
We conducted a search of the database to identify all patients undergoing single-level ALIF surgery with/without rhBMP2 utilization between 2005 and 2011. Patients were identified by entry of ICD-9 code 84.51 (“Insertion of interbody spinal fusion device”) and CPT code 22558 (“Anterior approach for lumbar fusion [anterior retroperitoneal exposure]”), and were further identified by combination or exclusion of ICD-9 code 84.52 (“Insertion of recombinant bone morphogenetic protein [rhBMP]”), as listed in Table 1. Patients with ICD-9 code 81.30- 81.39 for “correction of pseudarthrosis of spine, refusion of spine,” CPT code 22585 for “each additional interspace” were excluded. The codes were searched so as to exclude the same code being counted more than once for the same patient. Patients were stratified by gender (female, male), geographic region (Midwest, Northeast, South, West) (Table 2) and age group (<65, 65-69, 70-74, 75-79, 80-84, >84 years). The database stratifies patients by age in 5-year increments.
Table 1.
ICD-9 Diagnosis Codes and CPT Procedure Codes Searched.
| Code | Diagnosis/Procedure |
|---|---|
| ICD-9-84.51 | Insertion of interbody spinal fusion device |
| ICD-9-84.52 | Insertion of recombinant bone morphogenetic protein (rhBMP) |
| CPT-22558 | Anterior approach for lumbar fusion (anterior retroperitoneal exposure) |
Abbreviations: ICD-9, International Classification of Diseases, Ninth Revision; CPT, Current Procedural Terminology.
Table 2.
Regional Breakdown of States.
| Region | States |
|---|---|
| Midwest | IA, KS, MN, MO, NE, IL, IN, MI, WI, OH, NO, SD |
| Northeast | CT, MA, ME, NH, NJ, PA, RI, NY, VT |
| South | AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV, PR |
| West | AK, AZ, CA, CO, ID, MT, NM, NV, OR, UT, WA, WY, HI |
When reporting results, we use the term “incidence,” calculated as the number of single-level ALIF cases with/without rhBMP2 identified per every 100 000 patients searched in a particular year, gender, age group, or region. This was done to account for differences in the number of patients in the database for a given variable. The “average rate” of ALIF with and without rhBMP2 utilization in this study was defined as the rate: incidence of ALIF with rhBMP2/(incidence of ALIF with rhBMP2+ incidence of ALIF without rhBMP2). We then used χ2 analysis to determine statistical significance with regard to gender, age, and region. Linear regression was performed to test the significance of trends over time. The level of significance was P < .05.
Results
Nine hundred twenty-one patients undergoing single-level ALIF were identified by ICD code 84.51 and CPT code 22558. There were 554 women and 367 men. Further in combination or exclusion of ICD-9 code 84.52, there were 648 patients undergoing single-level ALIF without rhBMP2, and 274 patients undergoing single-level ALIF with rhBMP2. The overall incidence of single-level ALIF without rhBMP2 (0.20 cases per 100 000 patients) was more than twice of the incidence of single-level ALIF with rhBMP2 (0.09 cases per 100 000 patients) (Figure 1). Interestingly, the rate of single-level ALIF with rhBMP2 utilization increased (35%-48%) from 2005 to 2009, but sharply decreased to 16.7% in 2010 and 15.0% in 2011 (Figure 2). Accordingly, the incidence of single-level ALIF with rhBMP2 utilization declined in 2010, and the incidence of single-level ALIF without rhBMP2 utilization increased steadily (Figure 2).
Figure 1.
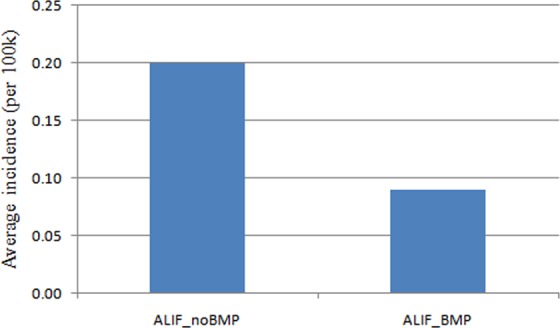
Average incidence of patients undergoing single-level ALIF with/without rhBMP from 2005 to 2011 (per 100 000 patients). ALIF, anterior lumbar interbody fusion; rhBMP, recombinant human bone morphogenetic protein.
Figure 2.
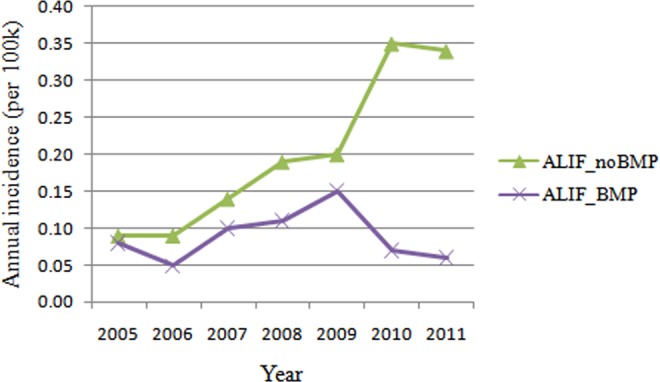
Annual incidence of patients undergoing single-level ALIF with/without rhBMP from 2005 to 2011 (per 100 000 patients). ALIF, anterior lumbar interbody fusion; rhBMP, recombinant human bone morphogenetic protein.
The incidence of patients undergoing single-level ALIF with/without rhBMP2 declined dramatically in 2010 and 2011. In 2010, the incidence of patients undergoing single-level ALIF with rhBMP2 decreased 64.3% in male and decreased 46.7% in female (Figure 3). The male-to-female ratio of patients undergoing single-level ALIF with rhBMP2 utilization was 0.70, with 41.2% of patients undergoing single-level ALIF with rhBMP2 utilization being male patients and 58.8% female patients (Figure 4). This ratio did not differ significantly from the ratio of patients searched in the database (0.80) (χ2 = 1.303, P = .254).
Figure 3.
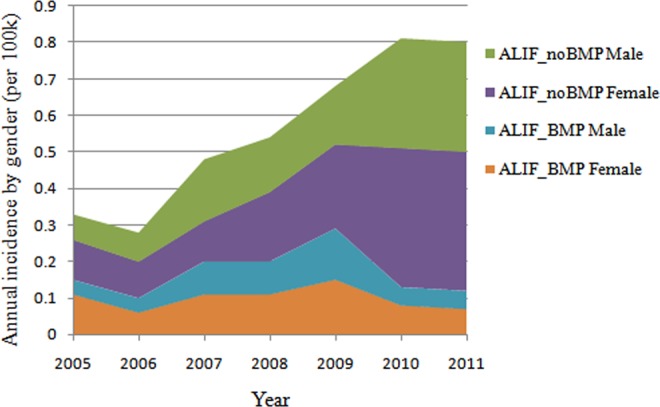
Annual incidence of patients undergoing single-level ALIF with/without rhBMP by gender from 2005 to 2011 (per 100 000 patients). ALIF, anterior lumbar interbody fusion; rhBMP, recombinant human bone morphogenetic protein.
Figure 4.
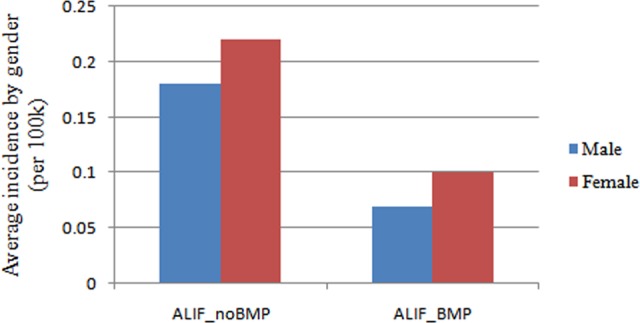
Average incidence of patients undergoing single-level ALIF with/without rhBMP by gender from 2005 to 2011 (per 100 000 patients). ALIF, anterior lumbar interbody fusion; rhBMP, recombinant human bone morphogenetic protein.
The average rate of single-level ALIF with rhBMP2 utilization is highest in West (41.4%), followed by Midwest (33.3%), South (26.5%), and Northeast (22.2%). The incidence of patients undergoing single-level ALIF with rhBMP2 declined dramatically in 2010 in all regions, especially in South (down by 70.6%) and West (down by 68.4%). In 2011, the incidence of patients undergoing single-level ALIF with rhBMP2 increased in South and West, but still decreased in Midwest and Northeast (Figure 5). Overall, the incidence of patients undergoing single-level ALIF with rhBMP2 is lowest in Northeast (0.04 cases per 100 000 patients), compared with West (0.12 cases per 100 000 patients, P = .001), Midwest (0.11 cases per 100 000 patients, P = .006), South (0.09 cases per 100 000 patients, P = .036) (Figure 6). There were no significant differences among different regions in the incidence of patients undergoing single-level ALIF without rhBMP2.
Figure 5.
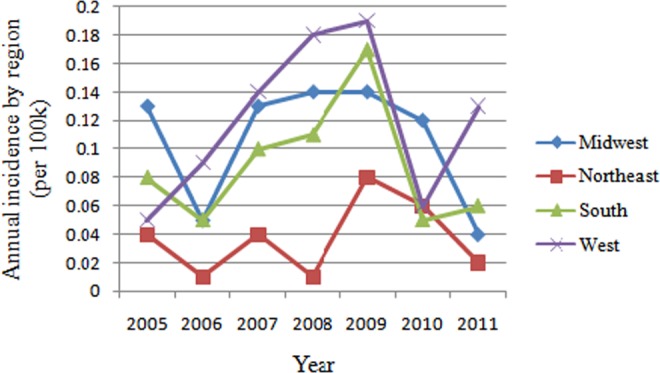
Annual i ncidence of patients undergoing single-level ALIF with/without rhBMP by region from 2005 to 2011 (per 100 000 patients). ALIF, anterior lumbar interbody fusion; rhBMP, recombinant human bone morphogenetic protein.
Figure 6.
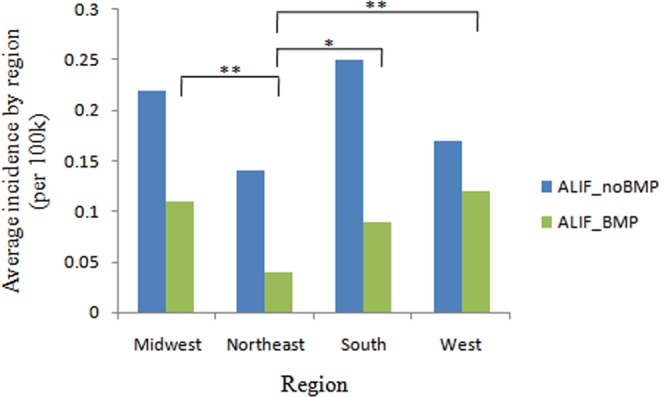
Average incidence of patients undergoing single-level ALIF with/without rhBMP by region from 2005 to 2011 (per 100 000 patients). ALIF, anterior lumbar interbody fusion; rhBMP, recombinant human bone morphogenetic protein. *P < .05, **P < .01.
Patients were categorized into 5-year age groups. The highest incidence of single-level ALIF with rhBMP2 was observed in the group aged less than 65 years (compared with any other age groups, P < .001), with an incidence of 0.21 per 100 000 patients (Figure 7). The group aged greater than 84 years had the lowest incidence of single-level ALIF with rhBMP2 (compared with other groups aged 65-79 years, P < .05), with an incidence of 0.01 per 100 000 patients. The highest incidence of single-level ALIF without rhBMP2 was observed in the group aged less than 65 years (compared with any other age groups, P < .001), with an incidence of 0.54 per 100 000 patients. The group aged greater than 84 years had the lowest incidence of single-level ALIF without rhBMP2 (compared with group aged 65-69 years, P < .05), with an incidence of 0.04 per 100 000 patients.
Figure 7.
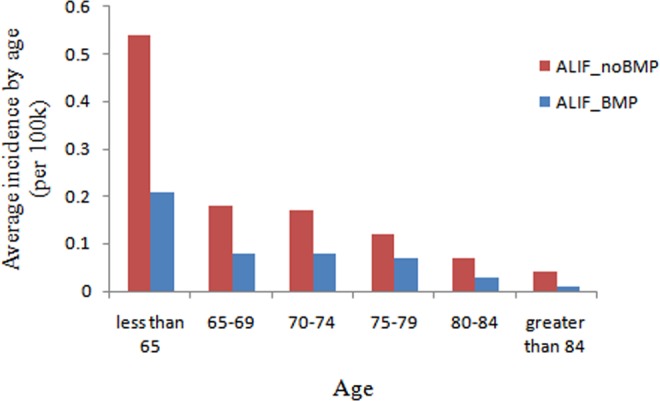
Average incidence of patients undergoing single-level ALIF with/without rhBMP by age from 2005 to 2011 (per 100 000 patients). ALIF, anterior lumbar interbody fusion; rhBMP, recombinant human bone morphogenetic protein.
Discussion
The overall rate of rhBMP2 utilization in single-level ALIF increased between 2005 and 2009 (35%-48%), but sharply dropped to 16.7% in 2010 and 15.0% in 2011. While the reasons for this drop in rhBMP-2 usage are unclear, it may be related to the concerns about potential adverse events that may occur with rhBMP2 utilization, and costs.
We found the mean rate of rhBMP2 utilization in single-level ALIF to be highest in the Western region (41.4%), followed by Midwest (33.3%), South (26.5%), and Northeast (22.2%). Overall incidence of single-level ALIF with rhBMP2 is significantly lowest in the Northeast. In terms of rhBMP2 utilization, it seemed more conservative in the Northeast region. Interestingly, we found that the incidence of rhBMP2 utilization declined dramatically in 2010 in all regions, especially in South (down by 70.6%) and West (down by 68.4%). However in 2011, the incidence of rhBMP2 utilization increased in South and West, but still decreased in Midwest and Northeast. Therefore, South and West regions were more active in rhBMP2 utilization. In terms of various spinal fusions, Singh et al11 reported that rhBMP2 utilization rate was highest in South, which was 3 times that of Northeast, which got the lowest rate.
The greatest incidence of rhBMP2 utilization in single-level ALIF was observed in patients aged less than 65 years in this study. The trend was obvious that the lower rhBMP2 utilization in the older group. The group aged greater than 84 years had the lowest incidence of rhBMP2 utilization in single-level ALIF. From 2005 to 2011, the overall incidence of single-level ALIF increased significantly (P < .001) in patients older than 65 years. These results may reflect the aging population of the United States as people older than 65 years are expected to represent nearly 20% of the population by 2030 when compared with 12.4% in 2000.12 Cahill et al13 found that female and white patients were more likely to receive rhBMP2 in spinal fusion procedures and greater hospital charges occurred for all categories of fusions. An explanation may be that these patients may have been more likely to be osteoporotic, a known risk factor for pseudarthrosis.14 Carreon et al15 reported that in patients over 60 years old, the use of rhBMP2 was more cost-effective than ICBG for posterolateral fusion. Because there are more complications, increased need for additional treatment and revision surgery in patients older than 60 years receiving ICBG. More rigorous cost analysis studies coupled with patient outcomes may eventually allow for more specific guidance for rhBMP2 use, which may improve both treatment efficacy and cost efficiency for the patient.
There are several limitations in our study, including its retrospective nature and potential errors in ICD-9 and CPT code assignments. Additionally, we only focused on the trend of single-level ALIF within the Medicare population. Finally, the regional distribution of patients was uneven, with the South region having the largest number of patients. However, despite these limitations, we believe that the results of our study provide important information on rates of ALIF with rhBMP2 in the United States.
Conclusions
The incidence of rhBMP2 utilization in single-level ALIF increased from 2006 to 2009, but decreased in 2010 and 2011. The Northeast region had the lowest incidence of rhBMP2 utilization. The group aged less than 65 years trended to have the higher incidence of single-level ALIF with rhBMP2 utilization.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ZB—Xenco Medical (consultancy), AO Spine (consultancy, past). HJM—Dr Meisel is consultant (money paid to institution) – Regenerate Life Sciences GmbH for Zyga, DiFusion (ongoing), Co.don (paid to Dr. Meisel past); royalties from: Medtronic, Fehling Aesculap (past); owns stocks (money paid to institution) - Regenerate Life Sciences GmbH in DiFusion. STY—Dr Yoon owns stock in Phygen, Alphatec; Meditech, royalties Meditech Advisors, Stryker Spine (Paid directly to institution/employer), grant from AOSpine (Paid directly to institution/employer), research support from Biomet (Research support given to AREF), non financial research support from Nuvasive and Medtronic. JAY—Royalties: NuVasive, Osprey Medical, Amedica, Integra; Stock Ownership: Benvenue Medical, Paradigm Spine, Promethean Surgical Devices, Spinal Ventures, VertiFlex, Spinicity, ISD, Providence Medical; Private Investments: Amedica, VertiFlex, Benvenue, NuVasive; Consulting: Integra, NuVasive, Amedica, HealthTrust; Board of Directors: Durango Orthopedic Associates (None); Research Support (Staff and/or Materials): Globus Medical (Paid directly to institution/employer), NuVasive (Paid directly to institution/employer), VertiFlex (Paid directly to institution/employer), Integra (Paid directly to institution/employer). DB—Consultant – Vallum, Royalties – America, DePuy Synthes, Medtronic, Fellowship Support – AOSpine (paid directly to institution). JCW—Royalties: Aesculap, Biomet, Amedica, Seaspine , Synthes ; Stock Ownership: Fziomed; Private Investments: Promethean Spine, Paradigm spine, Benevenue, NexGen, Vertiflex, electrocore, surgitech, expanding orthopaedics, osprey, bone biologics, curative biosciences, pearldiver; Board of Directors: North American Spine Society (non-financial, reimbursement for travel for board meetings, courses, etc.), North American Spine Foundation (non-financial), Cervical Spine Research Society (non-financial, reimbursement for travel for board meetings), AO Spine/AO Foundation (honorariums for board position); Fellowship Support: AO Foundation (spine fellowship funding paid to institution).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by AOSpine and departmental funds. AOSpine is a clinical division of the AO Foundation—an independent medically guided nonprofit organization. The AOSpine Knowledge Forums are pathology focused working groups acting on behalf of AOSpine in their domain of scientific expertise. Each forum consists of a steering committee of up to 10 international spine experts who meet on a regular basis to discuss research, assess the best evidence for current practices, and formulate clinical trials to advance spine care worldwide. Study support is provided directly through AOSpine’s Research Department.
References
- 1. Mobbs RJ, Chung M, Rao PJ. Bone graft substitutes for anterior lumbar interbody fusion. Orthop Surg. 2013;5:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Urist MR, Huo YK, Brownell AG, et al. Purification of bovine bone morphogenetic protein by hydroxyapatite chromatography. Proc Natl Acad Sci U S A. 1984;81:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US Food & Drug Administration. InFUSE Bone Graft/LT-CAGE Lumbar Tapered Fusion Device-P000058. https://www.fda.gov/ohrms/dockets/ac/02/slides/3828s1_03_bailey_draft/. Updated September 5, 2013. Accessed May 13, 2014.
- 4. Chrastil J, Low JB, Whang PG, Patel AA. Complications associated with the use of the recombinant human bone morphogenetic proteins for posterior interbody fusions of the lumbar spine. Spine (Phila Pa 1976). 2013;38: E1020–E1027. [DOI] [PubMed] [Google Scholar]
- 5. Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;62(5 suppl 2):ONS423–ONS431. [DOI] [PubMed] [Google Scholar]
- 6. Hoffmann MF, Jones CB, Sietsema DL. Recombinant human bone morphogenetic protein-2 (rhBMP-2) in posterolateral lumbar spine fusion: complications in the elderly. J Orthop Surg Res. 2013;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choudhry OJ, Christiano LD, Singh R, Golden BM, Liu JK. Bone morphogenetic protein-induced inflammatory cyst formation after lumbar fusion causing nerve root compression. J Neurosurg Spine. 2012;16:296–301. [DOI] [PubMed] [Google Scholar]
- 8. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine (Phila Pa 1976). 2011;36:1849–1854. [DOI] [PubMed] [Google Scholar]
- 9. Simmonds MC, Brown JV, Heirs MK, et al. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013;158:877–889. [DOI] [PubMed] [Google Scholar]
- 10. US Food & Drug Administration. FDA Public Health Notification: Life-Threatening Complications Associated with Recombinant Human Bone Morphogenetic Protein in Cervical Spine Fusion. http://www.fda.gov/medicaldevices/safety/alertsandnotices/publichealthnotifications/ucm062000.htm. Updated March 21, 2013. Accessed May 13, 2014.
- 11. Singh K, Nandyala SV, Marquez-Lara A, Fineberg SJ. Epidemiological trends in the utilization of bone morphogenetic protein in spinal fusions from 2002 to 2011. Spine (Phila Pa 1976). 2014;39:491–496. [DOI] [PubMed] [Google Scholar]
- 12. Administration on Aging. Aging statistics. http://www.aoa.gov/Aging_Statistics/. Accessed May 13, 2014.
- 13. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302:58–66. [DOI] [PubMed] [Google Scholar]
- 14. Stephen AB, Wallace WA. The management of osteoporosis. J Bone Joint Surg Br. 2001;83:316–323. [DOI] [PubMed] [Google Scholar]
- 15. Carreon LY, Glassman SD, Djurasovic M, et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion in patients over 60 years of age: a cost-utility study. Spine (Phila Pa 1976). 2009;34:238–243. [DOI] [PubMed] [Google Scholar]


