Abstract
In this study, we explored the effect of the water extract of Cinnamomum osmophloeum Kanehira (COK) leaves on hair growth by in vitro and in vivo assays. Using an in vitro 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, it was found that the proliferation of rat vibrissae and human hair dermal papilla cells (hDPCs) was significantly enhanced by the COK leaf extract treatment. As determined by quantitative real-time polymerase chain reaction (RT-PCR), the messenger RNA (mRNA) levels of some hair growth–related factors including vascular endothelial growth factor, keratinocyte growth factor (KGF), and transforming growth factor-β2 were found to be higher in the cultured hDPCs exposed to COK leaf extract than those in the untreated control group. In the hair-depilated C57BL/6 mouse model, the stimulation of hair growth was demonstrated in the group of COK leaf extract treatment. Both photographical and histological observations revealed the promotion of the anagen phase in the hair growth cycle by the COK leaf extract in the C57BL/6 mice. Finally, the ultra performance liquid chromatography (UPLC) showed that the COK extract contained mostly cinnamic aldehyde and a small amount of cinnamic acid. The results suggest that the COK leaf extract may find use for the treatment of hair loss.
Keywords: dermal papilla cells, Cinnamomum osmophloeum Kanehira, hair growth
Introduction
Hair loss, hair thinning, or alopecia are caused by a variety of factors such as aging or disease states1. More specifically, it can be caused by health conditions and medical treatment such as chronic inflammatory disease2, nutritional deficiency, hormone imbalance, stress, or chemotherapy1. An increasing number of people suffer from hair loss or alopecia3, and their mental health is consequently affected2. Two medicines, minoxidil and finasteride, have been approved by the U.S. Food and Drug Administration for treatment of alopecia. Unfortunately, the efficacies of these 2 drugs are limited, variable, transient, and accompanied by unpredictable side effects1. Therefore, alternative and efficient treatments are still needed2.
In hair structure, the part above the epidermis is the hair shaft, and the part under the epidermis is the hair follicle. Hair follicles, which are the hair roots, are stem cells composed of dermal sheath cells and dermal papilla cells (DPCs). DPCs are surrounded by capillaries that aid in the transport of nutrients for DPC growth and, by doing so, help regulate the hair growth cycle. Generally, the hair growth cycle is divided into 4 main stages: the growth phase, regeneration phase (anagen), degeneration phase (catagen), and rest phase (telogen)4,5. Among these, the anagen phase is the most active phase for DPC and hair follicle growth2,6. DPCs control the number of matrix cells in the anagen phase and enlarge hair follicle size that might directly adjust the hair growth cycle6,7. In the anagen phase, the growing hair follicle is richly supplied with nutrition via blood vessels8. Growth factors derived from DPCs also have chemotactic effects on the surrounding cells that help stimulate hair growth9,10. Among them, vascular endothelial growth factor (VEGF) is an important mediator of angiogenesis in hair follicle growth and cycling as it improves perifollicular vascularization, which increases hair growth, and hair follicle size8. Keratinocyte growth factor (KGF) is an endogenous mediator of hair follicle growth, development, and differentiation, and it does so by stimulating sebaceous glands and epidermal and hair follicle keratinocytes11. Insulin-like growth factor-1 (IGF-1) is essential for hair growth, and it does so by regulating cellular proliferation and migration during hair follicle development12. Transforming growth factor-β2 (TGF-β2) signaling at the ligand or receptor level significantly impaired hair folliculogenesis and maturation13. Hepatocyte growth factor (HGF), a paracrine factor secreted by follicular papilla cells, promotes follicular growth via acting on neighboring follicular epithelial cells14.
The Cinnamomum cassia bark is a traditional folk medicine herb and flavoring agent with interesting and varied biological activities15. Cinnamomum osmophloeum Kanehira (COK) is an indigenous plant of Taiwan belonging to the genus Cinnamon, and its leaf is commonly used in food, beverages, and pharmaceuticals. The chemical constituents of the essential oil of COK leaves are similar to those of C. cassia bark16. For the study reported herein, we investigated the effects of the COK leaf extract on the proliferation of cultured human DPCs observed the turning on VEGF, KGF, and TGF-β2 messenger RNA (mRNA) expression in the cells and found 2 main compounds in the COK leaf extract. Finally, the COK extracts showed the same capacity on the dorsal hair growth of C57BL/6 mice.
Materials and Methods
Plant Material
Fresh COK leaves were collected from the Wen Jia-bao mountain plantation in Fenglin (Hualien, Taiwan). The authenticity of the leaves was confirmed by the Forestry Bureau Council of Agriculture, Taiwan. The collected leaves were washed, air-dried in the shade, and stored in an airtight container at 25 °C.
Extract Preparation
The COK leaf extract was prepared by processing the COK leaves (10 kg) in 15 kg hot water (96–100 °C) in a gas-heating cryogenic distillation extraction pot for 40 min. The extract was collected by cooling condensation and then weighed after removing the water under reduced pressure. The extract prepared was used for subsequent studies to evaluate its effects on hair growth in vitro and in vivo.
Culture of Follicular Papilla Cells from Rat Vibrissae
Rat DPCs were isolated from rat vibrissa follicles. The tentacle dermal tissues were dissected from Wistar rat’s upper lips, and the vibrissa hairs were trimmed away and soaked in 75% ethanol10. The tentacle dermal tissues were then put into Dulbecco’s modified eagle’s medium (DMEM, Invitrogen, Paisley, UK) containing 2% penicillin/streptomycin and were treated with 2 mg/mL collagenase type IV for 2 h. Furthermore, DPCs were isolated from hair bulbs, the cultures of DPCs were initiated by outgrowth from hair bulbs. The hair bulbs were maintained in DMEM (Invitrogen) plus 10% fetal bovine serum (FBS, Invitrogen) in the incubator environment at 37 °C, 5% CO2, and passaged every 3 d17,18.
Culture of Human DPCs
Primary human hair DPCs (hDPCs) were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) as primary cells and maintained in mesenchymal stem cell (MSC) medium (ScienCell Research Laboratories), 5% (w/v) FBS, 1% (w/v) penicillin, 1% (w/v) mesangial cell growth supplement (ScienCell Research Laboratories) under a 5% CO2, humidified environment at 37 °C. For optimal results, we followed the cell culture recommendations of the supplier. Media, FBS, and other chemicals were obtained from ScienCell2.
Cell Proliferation Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to determine the effects of the COK extract on the proliferation of the human DPCs in this study. The cells (5 × 103 cells/well) were seeded into 96-well culture plates (BD Falcon, Corning, NY, USA). After the overnight incubation, cells were treated with various concentrations of COK and minoxidil (positive control), and deionized water served as the negative control for 48 h. After drug treatment for 48 h, the culture medium was removed and replaced with 500 μg/mL of MTT solution (Sigma-Aldrich, Steinheim, Germany) for 4 h. The medium was then removed, and 100 μL of dimethyl sulfoxide (DMSO, Sigma-Aldrich) was added to lyse the cells and until the crystal violet was dissolved. Absorbance at 595 nm was detected using an enzyme-linked immunosorbent assay (ELISA) reader (PerkinElmer, Waltham, MA, USA). Cell proliferation (%) was performed as described previously2.
Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from the hDPC with RNeasy Mini Kit reagents (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized according to the manufacturer’s protocol using maxim RT Pre Mix reagents (iNtRON Biotechnology, Kyungki-Do, Korea) and the following respective forward and reverse primers: 5′-CAGCAGTCTTCCAACCCAAT-3′ and 5′-CCTGCACTCCCTCTACTTGC-3′) for IGF-1, 5′-GCCTGAAAGATATCCCGACA-3′ and 5′-TTCCATGTTCTTGTCCCACA-3′ for HGF, 5′-GACATGGATCCTGCCAACTT-3′ and 5′-GGAAGAAAGTGGGCTGTTTTT-3′ for KGF, 5′-CTACCTCCACCATGCCAAGT-3′ and 5′-GCGAGTCTGTGTTTTTGCAG-3′ for VEGF, and 5′-CGCCAACCGCGAGAAGAT-3′ and 5′-CGCCAACCGCGAGAAGAT-3′ for human β-actin, which served as the internal standard. qRT-PCR analysis was performed using FastStart Essential DNA Green Master Mix (Roche, Indianapolis, IN, USA) in a C1000 Touch Thermal Cycler instrument (Bio-Rad, Hercules, CA, USA). Quantification of marker genes was performed according to the ΔΔCt method19.
Assessment of Hair Growth in Vivo
Male C57BL/6 mice were purchased from Lasco Co., Ltd. When the mice were 7 to 8 weeks old, they were used for hair growth studies. The dorsal hairs of the mice were partially shaved with an animal clipper and then completely depilated clearly with a calcium thioglycolate solution (Sigma-Aldrich). The mice were treated with deionized water (negative control), a 1% or 20% (v/v) COK extract, or 0.5 mM minoxidil (positive control) by topical spraying 0.2 mL with observation once every day for 30 d. The treated skin was observed, photographed, and then biopsied to examine histological features at days 1, 9, 14, 21, and 30 after depilation.
Histopathological Analysis
Biopsied samples of the mouse skins were obtained from punching the skin of the mice on days 1 and 14 and fixing the samples in in 3.7% formaldehyde. Tissues were then dehydrated, cleared, and infiltrated with a histoprocessor (Tissue-Tek Sakura, Tokyo, Japan) for 16 h. Then, tissues were embedded in paraffin wax, sliced into 5-µm sections, and stained with Mayer’s hematoxylin solution (Sigma-Aldrich) and eosin Y (J.T. Baker, Deventer, Holland) for histopathological analysis. Sections were examined with a microscope (IX70, Olympus, Tokyo, Japan). From the histological characterization of the sections, the number of hair follicles per square millimeter of the skin and the percentage ratio of hair follicle were determined using ImageJ software (1.49v) (National Institutes of Health (NIH), Bethesda MD, USA) fitted with an ocular micrometer. Follicles were counted manually in the dermis and subcutis layers by a blinded observer at a fixed size. Skin thickness from the epidermis to subcutis layer was also measured20.
Animal Ethics Statement
All procedures described in the study were reviewed and approved by the institutional animal care and use committee (IACUC) of National Dong Hwa University. The approval number is 101-007.
UPLC of the COK Extract
The ACQUITY UPLC system (Waters Corp., Milford, MA, USA), controlled by Waters Empower 2 software, included a binary solvent manager, sampler manager, heated column compartment, photodiode array detector, single quadrupole detector, and a C18 column (1.7 μm, 2.1 × 100 mm). The column and sample temperatures were maintained at 40 °C and 25 °C, respectively. The elution gradient was as described16, that is, the isocratic mobile phase was 10% (v/v) aqueous acetonitrile, and the flow rate was 0.8 mL/min. The detection wavelength was 280 nm. Cinnamic aldehyde (CA) and cinnamic acid (CAC) used as standards were purchased from Sigma-Aldrich and stored at −20 °C.
Statistical Analysis
All data are shown as the mean ± standard deviation (SD). For comparison of different treatments between 2 groups, the Student’s t test was used. P < 0.05 was considered significant, P < 0.01 would normally be considered highly significant, and P < 0.001 extremely significant.
Results
Effects of the COK Extract on Rat Vibrissae DPC Proliferation
To assess the benefit of COK extract in animal hair growth, we examined the impact of COK extract at different concentrations on rat DPC proliferation. Following the data reported by other groups in the past, we used 0.1 µM, 1 µM, and 10 µM minoxidil as positive controls21,22. Rat vibrissae DPCs were cultivated in 1% (v/v) FBS containing either COK extract or minoxidil for 48 h, and then an MTT assay was performed. The rat DPCs exposed to the COK extracts (0.5% and 1%) grew significantly more than the negative control group (Fig. 1). In addition, COK extract had a notably stronger effect on the proliferation of DPCs, as compared to minoxidil (Fig. 1).
Fig. 1.
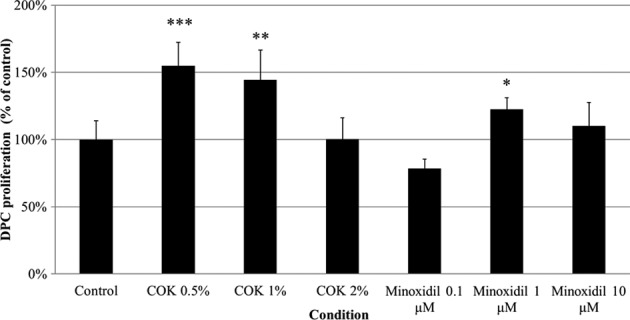
The Cinnamomum osmophloeum Kanehira extract enhances the proliferation of cultured rat vibrissae dermal papilla cells (DPCs). Cell proliferation was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Mino: minoxidil. Values are the mean ± SD. *P < 0.05 and **P < 0.01, compared with untreated cells (control).
Effects of the COK Extract on Human DPC Proliferation
To determine the benefit of COK extract in human hair growth, we assessed the impact of the COK extract on hDPC proliferation. hDPCs were cultured in 1% (v/v) FBS containing either COK extract or minoxidil for 48 h, and then an MTT assay was performed. Human DPCs exposed to the COK extract showed significantly higher proliferation than the negative control group (Fig. 2). However, COK extract had a significantly stronger effect on the proliferation of DPCs, as compared to normal (Fig. 2). Similar to minoxidil, COK extract can induce the proliferation of hDPCs.
Fig. 2.
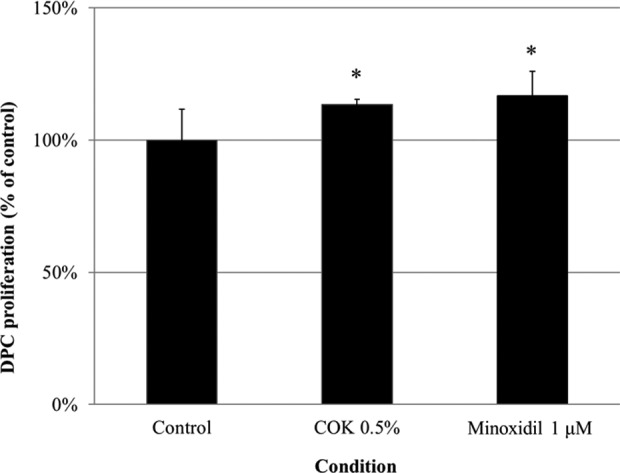
The Cinnamomum osmophloeum Kanehira extract enhances the proliferation of cultured human hair dermal papilla cells (hDPCs). Cell proliferation was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Mino: minoxidil. Values are the mean ± SD. *P < 0.05 and **P < 0.01, compared with untreated cells (control).
Effect of the COK Extract on Gene Expression
In order to elucidate the mechanism of how COK extract acts on hair growth, the mRNA expression levels of several factors related to hair growth were assessed using qPCR. The hDPCs (cell density 1 × 104 cells/cm2) were treated with either 0.5% (v/v) COK extract or minoxidil (1 µM) for 48 h, and the mRNA expression levels of HGF, IGF-1, VEGF, KGF, and TGF-β2 were measured. The treatments, both COK extract and Minoxidil, resulted in higher mRNA expression levels for all the factors tested. However, the expression profiles of the 2 treatments were not exactly the same. The COK extract treatment group achieved higher VEGF, KGF, and TGF-β2 expression levels whereas the minoxidil treatment group exhibited higher HGF, VEGF, KGF, and TGF-β2 expression in hDPCs (Fig. 3).
Fig. 3.
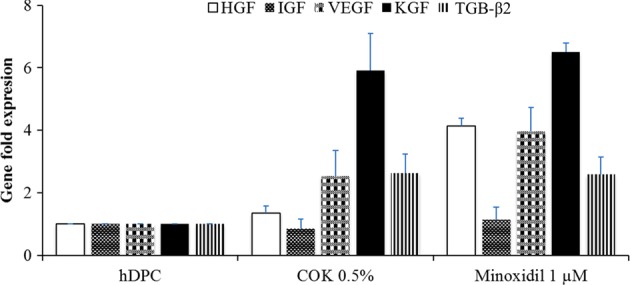
Quantitative polymerase chain reaction of growth factor genes in human hair dermal papilla cells (hDPCs). Changes in growth factor messenger RNA (mRNA) expression levels induced after different treatments for 48 h. The growth factor mRNA expression levels were normalized to that of β-actin mRNA expression with the results expressed as fold changes.
The COK Extract Promotes Hair Growth in Vivo
We then examined whether COK extract would improve the incidence of anagen phase hair growth by topically treating dorsal skin of depilated mice with COK extract. Dorsal hair of C57BL/6 mice has a time-synchronized hair growth cycle23. Depilated mouse skin in the telogen phase is pink. It becomes dark at anagen initiation, and then the skin turns gray. The COK- and minoxidil-treated mice exhibited pink skin on day 1, light gray skin on day 9, and their hair shafts were visible on day 14. More hair shafts were observed for the mice treated with 1% COK (v/v) extract than those treated with minoxidil 0.5 mM. However, the dorsal skin of the untreated group was still pink, light gray, with a little darkening on day 14 (Fig. 4). At day 14, the treatment group with 20% (v/v) COK extract had thinner hair than those treated with l% (v/v) COK extract or 0.5 mM minoxidil (Fig. 4). On day 21, the dorsal skins of all groups were in the mature anagen phase. These results demonstrate that COK extract promotes the growth of hair in C57BL/6 mice from the early telogen phase to the anagen phase. In the 1% (v/v) COK extract treatment group, the growth of hair shafts of all mice was completed by day 30, showing significantly better hair growth than the other groups.
Fig. 4.
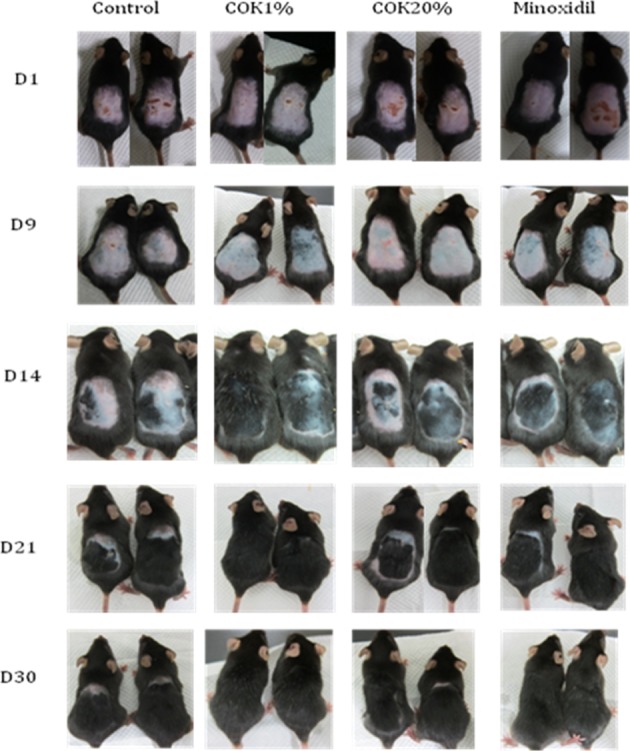
Hair growth–promoting effects of the Cinnamomum osmophloeum Kanehira (COK) extract and minoxidil. Histological comparisons of dermal papilla cell growth and proliferation and skin coloration for the untreated controls, and the minoxidil-COK extract–treated mice on days 1, 9, 14, 21, and 30.
Histological analysis of the biopsied mouse skins showed that 1% (v/v) COK extract markedly increased the number and size of the hair follicles as compared to the untreated group and the minoxidil treatment group (Fig. 5 a). This indicates that the COK extract positively affected mouse hair DPC proliferation and hair growth. The 1% and 20% (v/v) COK extracts promoted hair follicle elongation from the subcutaneous to the epidermal skin, revealing that the extract induced early onset of the anagen phase and stimulated hair growth. The histological profiles of mouse skins suggested that COK extract enhanced hair growth, increased skin thickness, and formed follicles in the subcutis layer. Animals treated with 1% COK extract, 20% COK extract, and 0.5 mM minoxidil as compared to the untreated group, all had higher percentage ratios of anagen phase to telogen phase from day 0 to day 14 (Fig. 5 b).
Fig. 5.
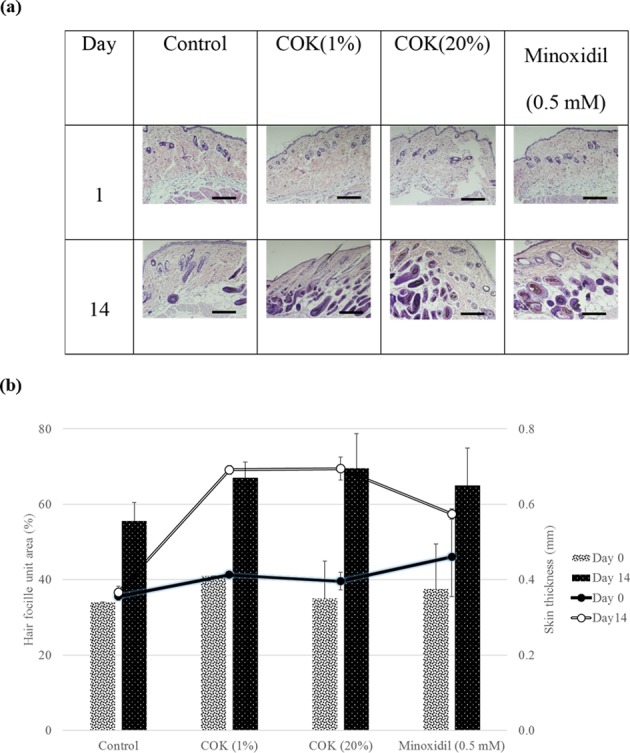
Effect of Cinnamomum osmophloeum Kanehira (COK) extract and minoxidil on the hair follicle unit area and skin thickness of mice. (a) Biopsied dorsal skin of C57BL/6 mice at days 1 and 14 was fixed and then stained with hematoxylin and eosin. Effects of topical COK extracts on C57BL/6 mouse dermal papilla cells (DPCs) during the anagen phase induction shown by representative skin samples from 4 animals. (b) Quantified slides from these samples. Scale bars = 200 µm.
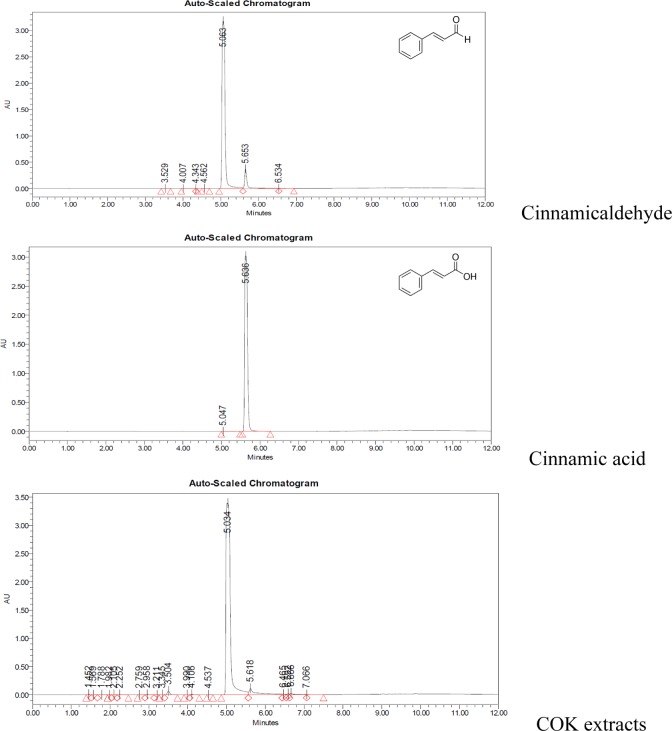
UPLC of the COK Extract
UPLCs were used to determine the components in COK extract. As compared with the data in the previous report16, the UPLC of the COK extract (Fig. 6) contained mostly CA and a small amount of CAC.
Fig. 6.
UPLC of the Cinnamomum osmophloeum Kanehira extract, and cinnamic acid and cinnamic aldehyde standards.
Discussion
COK leaves are commonly used as a traditional Chinese herbal medicine for treating dyspepsia, gastritis, and blood circulation disturbances24. The focus of this study is to explore its potential for hair growth promotion.
In the hair-depilated C57BL/6 mouse model, we found that the COK extracts can promote hair growth by increasing the skin thickness and the area of hair follicles in the skin (Fig. 5a). Based on our in vitro study, the effect may be explained by the findings that COK extract can increase the DPC number (Figs. 1 and 2) as well as increase the mRNA expression of hair growth–related factors including VEGF, KGF, and TGF-β2 (Fig. 3).
DPCs produce the epithelial follicle cells and a number of growth factors10. Such growth factors would essentially control the proliferation of epithelium follicle cells and act as a cytokine network regulating follicle growth during the anagen phase25.
VEGF acts as a major mediator of hair follicle growth and cycling and increases the size of hair follicles during the anagen phase6,10. VEGF mRNA is primarily expressed in the DPC during the anagen phase, which was also correlated with neovascularization10,26. Our results revealed that VEGF mRNA expression level of COK extract treated hDPCs was upregulated compared to the untreated group (Fig. 3). Since VEGF is a known growth modulator of the follicular papilla in the anagen stage, it stands to reason that the induction of these growth factors by COK treatment would promote the anagen stage or active hair growth. Additionally, we found that COK induced VEGF expression similar to the action of minoxidil, which was observed in hDPCs as shown in Fig. 3 27.
It was previously reported that Cinnamon and CAC can also stimulate angiogenesis by upregulating VEGF expression27 and could control the hair growth and follicle size8. As aforementioned, our UPLC shows that the COK extract contains primarily CA and a small amount of CAC (Fig. 6). Based on our study, further investigation will be required to verify whether the effect of COK extract on VEGF expression should be attributed mainly to CAC and/or to other unidentified components.
It has been previously shown that KGF promotes hair fiber elongation, protects hair follicles from apoptosis, and directly affects the growth of hair follicles28. Our data revealed that the COK leaf extract promotes KGF mRNA expression (Fig. 3). This promotion improves follicle proliferation to a greater extent than the normal control (untreated)10. The COK extract may positively affect KGF expression, thereby promoting cross talk between VEGF and KGF and resulting in auto- and cross-induction mechanisms that support keratinocyte proliferation and hair growth29,30.
The potential role of TGF-β2 in hair induction by hDPCs was previously investigated, and TGF-β2 signaling at the ligand or receptor level was concluded to significantly impair hair folliculogenesis and maturation13. In Fig. 3, our result showed that the TGF-β2 mRNA levels in the COK extract treatment group or minoxidil treatment group are higher in comparison with the negative control. Accordingly, our results were consistent with the conclusion drawn by Inoue et al.13
Hair follicle cycling in young C57BL/6 mice follows the precise timescale2. The morphology of DPCs also changes during the hair growth cycle, with the cells being the largest in volume during the anagen phase and retaining their size in the resting, telogen phase2. The anagen phase, which is the active phase of the hair follicle cycle, is accompanied by an increase in the number and size of hair follicles, resulting in an increased thickness of the subcutaneous layer between the dermis and panniculus carnosus20. We observed the depilation-induced hair growth cycle of the mice. The application of 1% (v/v) COK extract induced an earlier anagen phase and prolonged the mature anagen phase when compared with the 2 control groups. As a result, histologically, we found a distinct difference in the number of hair follicles and skin thickness in the anagen phase between mice treated with the COK extract and the control groups by the hair-depilated C57BL/6 mouse model (Fig. 5 a).
However, how minoxidil acts pharmacologically and mechanistically has not been fully elucidated2. Conversely, according to our data, the effect of the COK extract may be to promote the expression of VEGF, KGF, HGF, TGB-β2, and IGF-1. However, not only growth factors but also androgens are involved in the hair cycle. The testosterone and dihydrotestosterone, the major androgens, can indirectly affect hair growth. It was reported that 5α-reductase reduces testosterone to dihydrotestosterone and in turn induces alopecia10. Future studies in optimizing the level of CA and CAC in COK extract may provide an improved treatment for hair loss or alopecia. It will be an area of interest to understand the mechanism of COK extract in treating alopecia.
The COK leaf water extract efficiently enhances hair growth, and therefore, identifying its active component(s) may lead to a new treatment for hair loss.
Acknowledgment
The authors gratefully acknowledge Mei-Hsuan Wu for her technical contributions and professor Ching-Feng Weng for his kind recommendation.
Authors’ Note: Shinn-Zong Lin and Tzyy-Wen Chiou have contributed equally to this study.
Ethical Approval: All procedures in the animal experiment described in the study were reviewed and approved by the institutional animal care and use committee (IACUC) of National Dong-Hwa University. The approval of number is 101-007.
Statement of Human and Animal Rights: Statement of Human Rights is not applicable, and all animal experiments should comply with the ARRIVE guidelines and should be carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, or the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). We followed such guidelines in the manuscript.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: Shinn-Zong Lin is the Co-Editor-in-Chief of Cell Transplantation. Neither Shinn-Zong Lin nor any of his colleagues were involved in the peer review process or decision for this manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Zhang NN, Park DK, Park HJ. Hair growth-promoting activity of hot water extract of Thuja orientalis . BMC Complement Altern Med. 2013;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park S, Shin WS, Ho J. Fructus panax ginseng extract promotes hair regeneration in C57bl/6 mice. J Ethnopharmacol. 2011;138(2):340–344. [DOI] [PubMed] [Google Scholar]
- 3. Roh SS, Kim CD, Lee MH, Hwang SL, Rang MJ, Yoon YK. The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. J Dermatol Sci. 2002;30(1):43–49. [DOI] [PubMed] [Google Scholar]
- 4. Jankovic SM, Jankovic SV. The control of hair growth. Dermatol Online J. 1998;4(1):2. [PubMed] [Google Scholar]
- 5. Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449–494. [DOI] [PubMed] [Google Scholar]
- 6. Elliott K, Stephenson TJ, Messenger AG. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: Implications for the control of hair follicle size and androgen responses. J Invest Dermatol. 1999;113(6):873–877. [DOI] [PubMed] [Google Scholar]
- 7. Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341(7):491–497. [DOI] [PubMed] [Google Scholar]
- 8. Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107(4):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujie T, Katoh S, Oura H, Urano Y, Arase S. The chemotactic effect of a dermal papilla cell-derived factor on outer root sheath cells. J Dermatol Sci. 2001;25(3):206–212. [DOI] [PubMed] [Google Scholar]
- 10. Rho SS, Park SJ, Hwang SL, Lee MH, Kim CD, Lee IH, Chang SY, Rang MJ. The hair growth promoting effect of Asiasari radix extract and its molecular regulation. J Dermatol Sci. 2005;38(2):89–97. [DOI] [PubMed] [Google Scholar]
- 11. Danilenko DM, Ring BD, Yanagihara D, Benson W, Wiemann B, Starnes CO, Pierce GF. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 1995;147(1):145–154. [PMC free article] [PubMed] [Google Scholar]
- 12. Su HY, Hickford JG, Bickerstaffe R, Palmer BR. Insulin-like growth factor 1 and hair growth. Dermatol Online J. 1999;5(2):1. [PubMed] [Google Scholar]
- 13. Inoue K, Aoi N, Yamauchi Y, Sato T, Suga H, Eto H, Kato H, Tabata Y, Yoshimura K. TGF-beta is specifically expressed in human dermal papilla cells and modulates hair folliculogenesis. J Cell Mol Med. 2009;13(11-12):4643–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee YR, Yamazaki M, Mitsui S, Tsuboi R, Ogawa H. Hepatocyte growth factor (HGF) activator expressed in hair follicles is involved in in vitro hgf-dependent hair follicle elongation. J Dermatol Sci. 2001;25(2):156–163. [DOI] [PubMed] [Google Scholar]
- 15. Lin TY, Liao JW, Chang ST, Wang SY. Antidyslipidemic activity of hot-water extracts from leaves of Cinnamomum osmophloeum Kaneh. Phytother Res. 2011;25(9):1317–1322. [DOI] [PubMed] [Google Scholar]
- 16. Wang YH, Avula B, Nanayakkara NP, Zhao J, Khan IA. Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food supplements in the United States. J Agric Food Chem. 2013;61(18):4470–4476. [DOI] [PubMed] [Google Scholar]
- 17. Drewa T, Joachimiak R, Kaznica A, Sarafian V, Sir J. Primary cultures from rat vibrissae as a potential cell source for in vitro construction of urinary bladder wall grafts. Transplant Proc. 2009;41(5):1932–1935. [DOI] [PubMed] [Google Scholar]
- 18. Sun XJ, Hu ZQ. isolation and in vitro culture of follicular papilla cells from rat vibrissae[in Chinese]. Nan fang yi ke da xue xue bao. 2006;26(11):1619–1620. [PubMed] [Google Scholar]
- 19. Yoshimoto K, Ma X, Guan Y, Mizoguchi M, Nakamizo A, Amano T, Hata N, Kuga D, Sasaki T. Expression of stem cell marker and receptor kinase genes in glioblastoma tissue quantified by real-time RT-PCR. Brain Tumor Pathol. 2011;28(4):291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Junlatat J, Sripanidkulchai B. Hair growth-promoting effect of Carthamus tinctorius floret extract. Phytother Res. 2014;28(7):1030–1036. [DOI] [PubMed] [Google Scholar]
- 21. Han JH, Kwon OS, Chung JH, Cho KH, Eun HC, Kim KH. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J Dermatol Sci. 2004;34(2):91–98. [DOI] [PubMed] [Google Scholar]
- 22. Kwon TR, Oh CT, Park HM, Han HJ, Ji HJ, Kim BJ. Potential synergistic effects of human placental extract and minoxidil on hair growth-promoting activity in c57bl/6j mice. Clin Exp Dermatol. 2015;40(6):672–681. [DOI] [PubMed] [Google Scholar]
- 23. Paus R, Stenn KS, Link RE. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990;122(6):777–784. [DOI] [PubMed] [Google Scholar]
- 24. Liao JC, Deng JS, Chiu CS, Hou WC, Huang SS, Shie PH, Huang GJ. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid Based Complement Alternat Med. 2012;2012:429320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rushan X, Fei H, Zhirong M, Yu-Zhang W. Identification of proteins involved in aggregation of human dermal papilla cells by proteomics. J Dermatol Sci. 2007;48(3):189–197. [DOI] [PubMed] [Google Scholar]
- 26. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 27. Choi DY, Baek YH, Huh JE, Ko JM, Woo H, Lee JD, Park DS. Stimulatory effect of Cinnamomum cassia and cinnamic acid on angiogenesis through up-regulation of VEGF and Flk-1/KDR expression. Int Immunopharmacol. 2009;9(7-8):959–967. [DOI] [PubMed] [Google Scholar]
- 28. Braun S, Krampert M, Bodo E, Kumin A, Born-Berclaz C, Paus R, Werner S. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci. 2006;119(pt 23):4841–4849. [DOI] [PubMed] [Google Scholar]
- 29. Hashimoto K. Regulation of keratinocyte function by growth factors. J Dermatol Sci. 2000;24(suppl 1):S46–S50. [DOI] [PubMed] [Google Scholar]
- 30. Lee GS, Hong EJ, Gwak KS, Park MJ, Choi KC, Choi IG, Jang JW, Jeung EB. The essential oils of Chamaecyparis obtusa promote hair growth through the induction of vascular endothelial growth factor gene. Fitoterapia. 2010;81(1):17–24. [DOI] [PubMed] [Google Scholar]