Abstract
Total bilateral limbal stem cell deficiency leading to loss of corneal clarity, potential vision loss, pain, photophobia, and keratoplasty failure cannot be treated by autologous limbal transplantation, and allogeneic limbal transplantation requires subsequent immunosuppressive treatment. Cultured autologous oral mucosal epithelial cells have been shown to be safe and effective alternatives. These cells can be transplanted on supports or without support after detachment from the culture dishes. Dispase, known for epidermal sheet detachment, is reported as not usable for oral mucosa. The objective was to find an optimized detachment method providing a sufficiently resistant and adhesive cultured oral mucosal epithelium (COME), which can be grafted without sutures. Enzymatic treatments (dispase or collagenase at different concentrations) were compared to enzyme-free mechanical detachment. Histological immunofluorescence (IF) and Western blotting (WB) were used to examine the impact on adhesion markers (laminin-332, β1-integrin, and type VII collagen) and junctional markers (E-cadherin, P-cadherin). Finally, the COME ability to adhere to the cornea and produce a differentiated epithelium 15 d after grafting onto an ex vivo porcine stroma model were investigated by histology, IF, and transmission electron microscopy. Collagenase at 0.5 mg/mL and dispase at 5 mg/mL were selected for comparative study on adhesive expression marker by IF and WB showed that levels of basement membrane proteins and cell–cell and cell–matrix junction proteins were not significantly different between the 3 detachment methods. Collagenase 0.5 mg/mL was selected for the next step validation because of the better reproducibility, 100% success (vs. 33% with dispase 5 mg/mL). Grafted onto porcine de-epithelialized corneal stroma, collagenase 0.5 mg/mL detached COME were found to adhere, stratify, and continue to ensure renewal of the epithelium. For COME, collagenase 0.5 mg/mL enzymatic detachment was selected and validated on its resistance and adhesive marker expression as well as their anchorage onto our new ex vivo de-epithelialized stroma model.
Keywords: limbal stem cell deficiency, cultivated oral mucosa epithelium, enzymatic detachment, ex vivo cornea model, stem cell therapy
Introduction
By definition, transplant surgery in patients with total bilateral limbal stem cell deficiency (LSCD) cannot use autologous limbus or cultured autologous limbal epithelium. Allogeneic limbal epithelium in the form of a keratolimbal allograft from cadaveric tissue or conjunctival limbal allografts from living related donors can be performed1–4, but they require posttransplant immunesuppression which has a high risk of side effects. Moreover, the long-term success rate (1 y) for this type of graft is low and gradually decreases over time5.
Cultured autologous oral mucosal epithelial cells (CAOMECS) were demonstrated to be an alternative solution to treating this problem in animals6,7 and subsequently in humans8. Initially, CAOMECS were cultured on carriers such as amniotic membrane9–16 which made them easy to handle for transplantation but required sutures causing secondary inflammation. Later, a thermosensitive polymer was developed by Okano (UpCell Insert, CellSeed Inc, Tokyo, Japan)17. Sheets grown on this polymer could be detached without enzymatic processing, thus basement membrane remained intact and allowed rapid attachment to the stroma without requiring any sutures.
We tested this system in a clinical trial involving 25 patients (26 eyes) with bilateral corneal LSCD caused by burns, Stevens-Johnson syndrome, and ocular pemphigoid. These patients are typically refractory to transplantation of donor cornea, but results showed CAOMECS to be safe and to effectively reconstruct the ocular surface18. Like the corneal epithelium, the epithelium of the oral mucosa (OM) can act as a barrier protecting the cornea; it reduces pain and allows healing of corneal ulcers. In addition, transplanted oral mucosal sheets delay conjunctival invasion and the neovascularization responsible for rejection of corneal grafts. Finally, its smooth and transparent surface contributes to improving the quality of vision and visual acuity (VA) by reducing dispersion and distortion of the light. For patients with healthy stroma, an increase in VA was possible with no need for other treatment. For those with a severely deteriorated stroma, penetrating keratoplasty was performed 1-y postgraft and improved VA was achieved. The long-term results of this trial demonstrated that CAOMECS contain the stem cells necessary for long-lived renewal of the epithelium and that they restored the epithelial function of the cornea by delaying neovascularization and conjunctivalisation8.
The manufacturer’s claims in relation to the performance of UpCell Insert focused on the fact that it avoided the need for enzymatic detachment, producing a support-free easy-to-graft CAOMECS with preserved basement membrane. Unfortunately, UpCell Insert is no longer available in Europe. We therefore decided to test different detachment methods to obtain a culture oral mucosa epithelium (COME). Our aim was to identify a method producing a COME that is sufficiently resistant to be grafted junctions to give rise to a sheet and not to a cell suspension as trypsin does. Moreover, it must be of Good Manufacturing Practice (GMP) grade because of the clinical applications. So, only dispase and collagenase were found to meet the criteria. Thermolysin used for epithelium separation or sheet detachment was rejected because no GMP grade was available. On the other hand, the neutral protease dispase has been recommended for several decades and was successfully used in our lab in 1988 to detach cultured epidermal sheets for burn treatment19. We also selected collagenase used for isolation of different cell types which are already intended for transplantation into humans. This enzyme degrading the collagen and preserving cell–cell junctions has been recommended for separation of limbal epithelium from the stroma while preserving highly adhesive stem cells17.
In this study, we compared the resistance and capacity to adhere to the stroma of the samples obtained after detachment by enzymatic (dispase or collagenase) or mechanical methods. Our assessment was based on the expression of adhesion markers such as laminin-332, measured by immunofluorescence (IF) and Western blotting (WB), and their ability to adhere and to produce a living, differentiated epithelium on a new ex vivo porcine stroma model.
Materials and Methods
Origin, Isolation, and Culture of Cultured Oral Mucosal Epithelium
Epithelial cells were isolated from biopsies of normal human OM taken from the nonkeratinized cheek region of the mouth (Declaration to Research Ministry, n° AC-2013-1846). All samples were obtained from patients undergoing oral surgery after gathering informed consent. The epithelium was separated from the lamina propria using dispase II (Thermo Fisher Scientific, Waltham, MA, USA), 10 mg/mL for 3 h at 4 °C. After separation, the epithelium was treated with trypsin 0.5 g/l–ethylenediaminetetraacetic acid (EDTA) 0.2 g/l (Gibco®, Grand Island, NY, USA) for 20 min to extract cells, which were collected every 10 min. Epithelial cells were grown at 20,000 cells/cm2 on an irradiated feeder layer of primary human fibroblasts from dermis in 6-well plates (35 mm diameter, Becton Dickinson, Le Pont-de-Claix, France) in epithelial cell culture medium (Dulbecco’s modified Eagle’s medium (DMEM)-Ham-F12 2.78/1; Sigma-Aldrich, Darmstadt, Germany), 10% fetal calf serum (FCS) (Hyclone Laboratories, South Logan, UT, USA), 0.4 µg/mL hydrocortisone (Upjohn, Kalamazoo, MI, USA), 0.12 UI/mL insulin (Umuline, Lilly, Neuilly-sur-Seine, France), 0.033 µg/mL selenium (Aguettant Laboratories, Lyon, France), 0.4 µg/mL isoprenaline hydrochloride (Isuprel, Sterling Winthrop, Longvic, France), 2 × 10− 9 M, tri-iodothyronine (Sigma-Aldrich 10 ng/mL epidermal growth factor (EGF) (R&D Systems, Minneapolis, MN, USA), and antibiotics. Cultures were maintained for 14 d, changing medium 3 times a week until a confluent cell layer had formed.
COME Detachment by Mechanical or Enzymatic Methods
Four days after reaching confluence, COME were detached. For mechanical detachment, COME were detached using a scraper. For enzymatic detachment, after rinsing 3 times with phosphate-buffered saline 1× (PBS) (Gibco®), COME were detached from the culture support by treatment with dispase or collagenase. After adding enzymatic solutions, plates were incubated (37 °C, 5% CO2) until sheet detachment. Detached sheets were immediately transferred with a polyvinylidene fluoride (PVDF) ring to wells containing DMEM.
Determining Optimal Concentrations of Dispase and Collagenase
COMEs were detached from the bottom of the wells by a gentle enzymatic treatment at different enzyme concentrations. As dispase II at 5 mg/mL is validated in our lab for epidermal cell sheet detachment, we chose to use this concentration and test 2 others obtained by 2-fold serial dilutions 2.5 and 1.25 mg/mL to minimize the potential enzymatic degradation. For collagenase, the tested concentrations were 1 mg/mL according to Chen20 and 2-fold serial dilutions corresponding to 0.5 and 0.25 mg/mL. From 6 OM donors, 3 were used for dispase detachment and 3 for collagenase detachment. For each enzymatic concentration, the detachment was performed on 3 COME for each donor to validate the concentration to use for the detachment. Incubation time, macroscopic integrity, and handling time were measured to compare the quality of detachment for each COME from the 6 donors.
Comparing Detachment Methods Based on Sheet Integrity and Levels of Adhesive and Junctional Markers
Enzymatic detachment of COME using dispase or collagenase treatment was compared to mechanical detachment by measuring levels of basement membrane markers, laminin-332, β1-integrin, and type VII collagen, as well as cadherins as junctional markers. Expression level of the adhesive and junctional markers was measured on COME following detachment using the optimal enzyme concentrations determined in the previous step (5 mg/mL dispase and 0.5 mg/mL collagenase).
Six COME were prepared from 3 donor OM, thus 2 COME could be tested per detachment method. One cell sheet was used for WB, while the other was submitted to histology, immunohistology.
Colony Forming Efficiency (CFE) on COME after Detachment
COME detached with collagenase from 3 donors were treated with trypsin 0.5 g/l–EDTA 0.2 g/l at 37 °C to obtain a single-cell suspension. For clonogenic potential determination, cells were seeded on 3 flasks 25 cm2 for each strain at the densities of 10 cells per cm2. After cultivation for 12 to 14 d, the cells were fixed and stained with rhodamine B. Holoclones and meroclones were counted under a dissecting microscope to calculate colony forming unit. The clonogenic potential was estimated by the CFE (%) that represents the percentage of cells giving colonies:
Grafting Cultured OM Epithelium onto Ex Vivo Corneal Stroma Model
Porcine excised corneas were chosen as ex vivo model corneas because of their high bioavailability and high degree of anatomic similarity to human corneas21. Porcine corneas were harvested with authorization of ethical committee n° DR2013-30.
OM cell sheets have already been predicted to be effective in vivo in animals7,22–25 and humans10,11,12,13,14,15,16,18,26–33. The aim of this model is to demonstrate that COME detached with the selected enzyme is rapidly adherent to the stroma.
After limbal ablation with Vannas 30° scissors followed by a corneal de-epithelialization with heptanol treatment for 5 min to mimic a LSCD, the tissue was rinsed in PBS 1× and placed in Cornea Prep II medium (Eurobio, Courtaboeuf, France).
Just after detachment of the COME by the selected method, 3 COME from each of the 3 donors were immediately transferred with a PVDF ring onto de-epithelialized porcine stroma. Samples were then raised up so that they were at the air–liquid interface and cultured for 15 d in DMEM-Ham-F12 2.2/1, 8 mg/mL bovine serum albumin (BSA) Sigma-Aldrich, 0.4 µg/mL hydrocortisone, 0.12 UI/mL insulin, 50 µg/mL ascorbic acid (Bayer, Leverkusen, Germany), and antibiotics. Medium was changed 3 times a week.
All ex vivo grafted corneal stroma were cut into 3 sections for histology, immunohistochemistry (IH), and transmission electron microscopy (TEM) analysis.
Histology and Immunostaining
The COME and the grafted corneal stroma samples were fixed in 4% formaldehyde solution (Alphapath, Mudaison, France) for histological analysis. Samples were then embedded in paraffin and cut into 5 µm thick sections before staining with hematoxylin–phloxine–saffron.
IH was performed on COME after detachment and 14 d after grafting onto porcine corneal stroma. The primary antibodies used for this study were anticytokeratin 13 (dilution 1:75; Santa Cruz, Dallas, TX, USA), antilaminin-332 (dilution 1:100; Chemicon, Billerica, MA, USA), anti-β1-integrin (dilution 1:500; Santa Cruz), anti-p63 (4A4; Ventana, Tucson, AZ, USA), and anticollagen VII (dilution 1:50; Santa Cruz). To view anticytokeratin 13, antilaminin-332, anti-β1-integrin, and anticollagen VII staining, COME were embedded in optimum cutting temperature compound (Sakura Finetek France SAS, Villeneuve-d’Ascq, France) and frozen at −20 °C. Then, 5 µm sections were cut and fixed in acetone for 10 min at −20 °C prior to blocking. For all staining except cytokeratin 13 (K13), blocking buffer was PBS 1× containing 4% BSA and 5% normal goat serum. K13, blocking buffer was PBS 1× containing 4% BSA and 5% FCS. All primary antibodies were applied overnight at 4 °C. The secondary antibody was AlexaFluor 488 anti-IgG (Invitrogen, Carlsbad, CA, USA) for all primary antibodies. To detect p63 in COME, tissue constructs were first fixed in 4% formaldehyde solution and embedded in paraffin. An automated immunostaining procedure using a Ventana kit was performed on 5 µm sections. Distribution of the target protein was revealed using diaminobenzidine (DAB) enzyme substrate (Dako, Les Ulis, France), which produced a brown precipitate. Counterstaining was performed with Harris hematoxylin and bluing reagent for p63; for the other staining, Hoechst 33258 (Sigma-Aldrich) counterstaining was used to reveal cell nuclei. For all immunohistology experiments, native nonkeratinized human OM was used as a positive control. Samples were analyzed with a Zeiss LSM 510 confocal laser scanning microscope and a Nikon eclipse fluorescence microscope.
TEM
Tissue constructs were fixed with 2% glutaraldehyde, 0.1 M NaCacodylate/HCl, pH 7.4 for 2 h and postfixed with 1% osmium tetroxide, and 0.15 M NaCacodylate/HCl, pH 7.4 for 1 h. After dehydration in graded ethanol solutions, samples were embedded in Epon which was polymerized at 60 °C for 72 h. The blocks were cut using an ultramicrotome and sections measuring 60 to 80 nm thick were contrasted with uranyl acetate and lead citrate. Samples were viewed using a Philips CM 120 transmission electron microscope.
WB
Whole-cell lysates were prepared from samples obtained through 3 different experiments using cold radio immunoprecipitation assay (RIPA) lysis buffer (20 mM tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 250 µM phenylmethylsulfonyl fluoride, 1 mM N-ethylmaleimide, 1% Nonidet P-40, 1% Triton X-100, 0.1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]). All procedures were performed at 4 °C. After centrifugation, the protein concentrations of the lysates were determined, and equivalent amounts (50 µg) of proteins were resolved on 8% SDS polyacrylamide gels. Separated proteins were transferred to nitrocellulose for immunodetection of laminin-332 subunits (pAb 4101), P-cadherin (56/P-cadherin clone, DB Transduction Laboratories, Le Pont-de-Claix, France), E-cadherin (M106, Takara, Saint-Germain-en-Laye, France), or β1-integrin (P5D2, Santa Cruz). Antibody binding was revealed by enhanced chemiluminescence. β-Actin was used as a loading control. Purified laminin-332 was used as a positive control; purification was performed as previously described34. Bands were quantified using Image J software (Version 1.50.i National Institutes of Health (NIH), Bethesda, MD, USA), and intensities were normalized relative to the actin signal. Each sample was analyzed in triplicate.
Statistical Analysis
WB data are presented as mean ± standard deviation (SD) and the Student’s t test (unpaired, 2-tailed) was used to compare groups. The data presented combine results from 3 distinct donors as specified in the legends of graphs and figures. The threshold for statistical significance was taken as P < 0.05.
Results
Determining Optimal Dispase and Collagenase Concentrations for COME Detachment
Detachment led to 3 different macroscopic aspects (Fig. 1): COME were complete (Fig. 1A) or with hole (Fig. 1B) or impossible to detach (Fig. 1C).
Fig. 1.
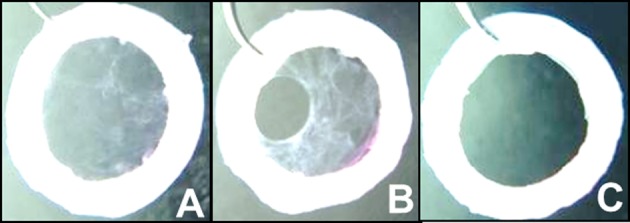
Cultured oral mucosal epithelium (COME) macroscopic aspect after detachment. (A) Complete, (B) with hole, and (C) impossible to detach. Only complete COME can be validated for graft.
For dispase, Table 1 shows the results for the handling time to harvest, which is synonymous with ease of transfer. Sheet integrity is indicated by the number of tear-free COME obtained, the number of holes, or the number of COME that were impossible to detach after dispase treatment at the concentrations tested (5, 2.5, and 1.25 mg/mL). Only the COME from 3 donors produced 3 sheets which detached without holes at the 5 and 2.5 mg/mL concentrations. The cells from the 2 other donors produced no useable COME either because holes were present or because sheets were impossible to detach. Prolonging the incubation time did not improve results; indeed, the sheet was destroyed when incubation exceeded 15 min.
Table 1.
COME integrity from three donors harvested with dispase at 5 mg/mL, 2.5 mg/mL and 1.25 mg/mL for two donors, and at 5 mg/mL and 2.5 mg/mL for the third donor.
| Dispase Concentration | Donor Number | Number of Sheets Obtained without Hole | Number of Sheets Obtained with Hole | Number of Sheets Impossible to Obtain | Incubation Time | Handling Time |
|---|---|---|---|---|---|---|
| 5 mg/mL | 1 | 0 | 3 | 0 | 10 min | 8 min 30 s |
| 2 | 0 | 2 | 1 | |||
| 3 | 3 | 0 | 0 | |||
| 2.5 mg/mL | 1 | 0 | 2 | 1 | 12 min | 9 min 45 s |
| 2 | 0 | 3 | 0 | |||
| 3 | 3 | 0 | 0 | |||
| 1.25 mg/mL | 1 | 0 | 0 | 3 | 16 min 30 s | 10 min 30 s |
| 2 | 0 | 1 | 2 |
The shortest times to transfer were obtained with 5 mg/mL dispase (8.5 min), at 2.5 mg/mL, the time to transfer was 9.75 min. For this reason, 5 mg/mL dispase was used in subsequent experiments. This concentration also corresponds to the concentration validated for the detachment of epidermal cell sheets for burn treatment.
For collagenase, Table 2 shows the numbers of COME obtained without holes, with holes, or that were impossible to detach. The concentrations tested were 1, 0.5, and 0.25 mg/mL. For all donors and concentrations, the main success criteria (2/3 intact detached sheets) was achieved with a similar ease of detachment as indicated by the short time to transfer (approximately 5 min) after enzymatic digestion. The digestion time was not significantly different between 1 mg/mL (48 min) and 0.5 mg/mL (56 min), but at 0.25 mg/mL, it was significantly longer (75 min). In subsequent experiments, we therefore used 0.5 mg/mL collagenase as it was the lowest enzyme concentration requiring a reasonable contact time and allowing COME harvest.
Table 2.
COME integrity from three donors harvested with collagenase at 1 mg/mL; 0.5 mg/mL and 0.25 mg/mL.
| Collagenase Concentration | Donor Number | Number of Sheets Obtained without Hole | Number of Sheets Obtained with One Hole | Number of Sheets Impossible to Obtain | Incubation Time | Handling Time |
|---|---|---|---|---|---|---|
| 1 mg/mL | 4 | 3 | 0 | 0 | 48 min | 5 min |
| 5 | 3 | 0 | 0 | |||
| 6 | 3 | 0 | 0 | |||
| 0.5 mg/mL | 4 | 3 | 0 | 0 | 56 min | 4 min 30 s |
| 5 | 3 | 0 | 0 | |||
| 6 | 3 | 0 | 0 | |||
| 0.25 mg/mL | 4 | 2 | 1 | 0 | 75 min | 5 min |
| 5 | 3 | 0 | 0 | |||
| 6 | 3 | 0 | 0 |
Comparing Methods for Epithelial Sheet Detachment
The optimal enzyme concentrations were in comparative experiments. Mechanical detachment was also tested. Cell integrity, proliferation, and expression of differentiation markers or adhesive markers were analyzed by histology, immunohistology, and WB to determine the most appropriate method.
COME Integrity
Only the enzymatic methods—5 mg/mL dispase and 0.5 mg/mL collagenase—allowed intact COME to be harvested on a PVDF ring.
Characterization of COME
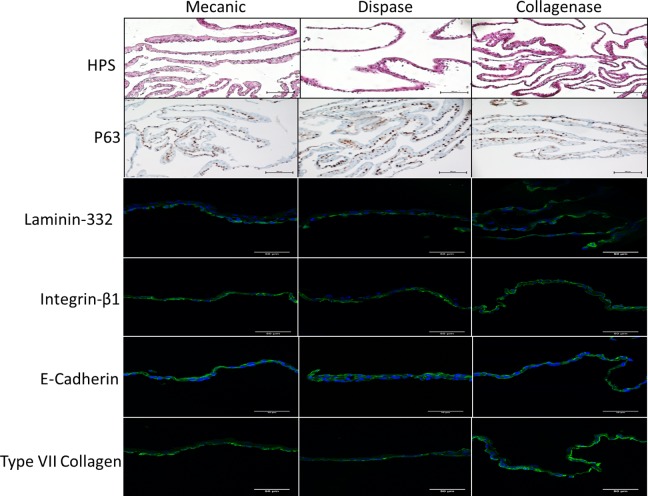
Histological analysis indicated that cells extracted (P0) from OM form a 2- to 4-layer cellular sheet. Moreover, p63, a basal stem cell marker, was expressed in all COME (Fig. 2), whatever the detachment method, indicating that it should be able to proliferate to ensure renewal of the epithelium postgraft.
Fig. 2.
Oral mucosal cell sheets from 3 donors harvested by mechanical or enzymatic methods (dispase or collagenase) were analyzed by hematoxylin–phloxine–saffron (HPS) staining (scale bars: 100 μm); expression of p63 was analyzed by immunohistochemistry (scale bar = 100 μm); and laminin-332, β1-integrin, E-cadherin, and type VII collagen levels were viewed by immunofluorescence in green color, cell nuclei are shown in blue color (scale bar = 50 μm).
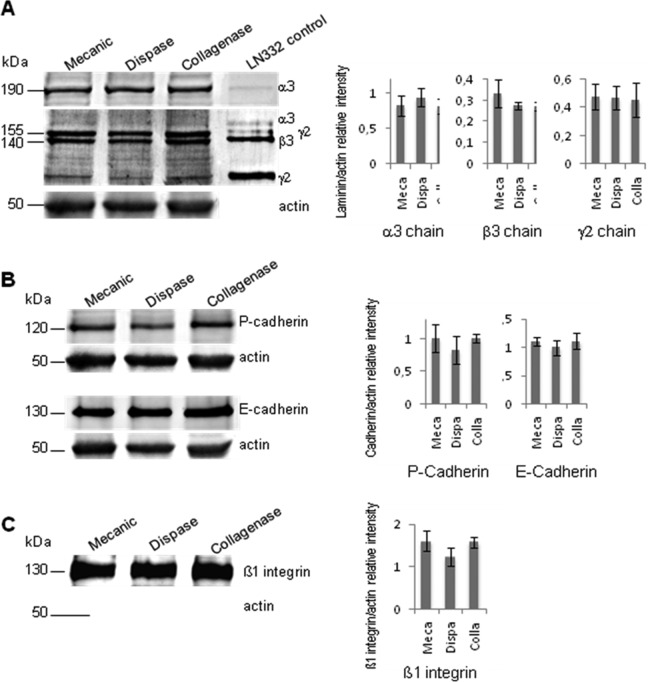
Figures 2 and 3 show representative results for levels of basement membrane and junction-specific markers: the 3 chains of laminin-332, β1-integrin, type VII collagen, and cadherins based on IF (IH) and WB analysis. Thus, WB analysis of COME lysates indicated that precursor laminin-332 in dispase- or collagenase-detached sheets was present at equivalent levels to those detected in mechanically detached sheets (Fig. 3A). These data suggest that laminin-332 retainment is unaltered by these enzymatic treatments and that subsequent adhesion of the COMEs should not be impaired. Analysis of cell–cell adhesion markers demonstrated that neither P-cadherin nor E-cadherin expression was affected by dispase or collagenase treatment (Fig. 3B). Thus, the cohesiveness and strength of the sheet were preserved. Similar results were obtained for the β1-integrin subunit (Fig. 3C), suggesting that this protein is available to induce cell–extracellular matrix interactions. Although the differences were not significant, treatment with dispase induced a slight decrease in P-cadherin, E-cadherin, and β1-integrin levels (P values 0.17, 0.27, and 0.05, respectively). For all the tested detachment methods, enzymatic or mechanic, and all donors, IF analysis indicated that basement membrane-specific markers, such as laminin-332, β1-integrin, and type VII collagen, were detectable. Single cells from COME detached by collagenase are able to form clones: indeed, CFE was 2 ± 1.0%, 11 ± 2.4%, and 20 ± 4.5% for the 3 donors, demonstrating the presence of stem cells in COME. CFE was superior or equal to 2% which is the specification for epithelial sheets for burnt patients.
Fig. 3.
Western blot analysis of levels of laminin-332 and adhesion proteins in cultured oral mucosal epitheliums (COMEs). Whole-cell lysates were prepared from COMEs and run on an 8% SDS-PAGE gel. Proteins were transferred to nitrocellulose and processed for Western blotting using antibodies against laminin-332 (A), P- and E-cadherin (B), and s1-integrin (C). Purified laminin-332 (2 μg) was used as a control (A). (A) Western blot analysis of α3 (190 kDa), β3 (140 kDa), and γ2 (155 kDa) chains of laminin-332 expressed in COMEs. Migration positions of the molecular mass markers are indicated on the left. Quantification data combine results from COMEs from 3 distinct donors.
Adhesion of Cultured OM Epithelium to Ex Vivo Porcine Cornea
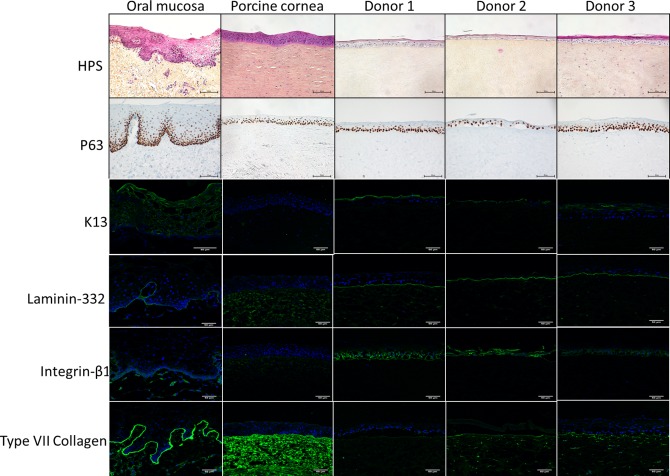
Figure 4 shows a representative result obtained with the COME from the 3 donors tested. Fifteen days postgraft, all COME formed a differentiated nonkeratinized multilayered epithelium on the porcine corneal stroma. This epithelium contained between 3 and 6 layers and was strongly attached to corneal stroma. IF analysis of K13 expression—a marker specific for human oral mucosal epithelium—confirmed that this structure was of human origin. Ungrafted porcine cornea used as a negative control did not express this marker.
Fig. 4.
Histological analysis of cultured oral mucosa epithelium grafted on ex vivo porcine cornea. Cell nuclei were stained blue with hematoxylin, the cytoplasm was stained pink with phloxine, and the extracellular matrix of connective tissue was stained orange/yellow with saffron (scale bar: 100 μm). p63 levels were analyzed by immunohistochemistry (scale bar: 100 μm); laminin-332, β1-integrin, E-cadherin, and type VII collagen levels were determined by immunofluorescence (scale bar: 50 μm).
A proliferation marker, p63, was detected in the basal layer of human OM and in all the epithelia present on porcine corneal stroma after grafting with COME.
Laminin-332 and type VII collagen staining decorated the epidermal–dermal junction of native human OM but not the junctions in porcine corneal stroma. This expression pattern confirmed that laminin-332 and type VII collagen deposited on porcine stroma were effectively derived from COME. For laminin-332, the staining was strong and continuous for all basal layers formed between oral mucosal sheets and porcine stroma. Staining was less intense for type VII collagen. β1-integrin was detected around all the basal cells of native human OM as well as in the epithelium of porcine stroma grafted with COME.
TEM
TEM revealed the presence of numerous hemidesmosomes between COME and porcine stroma, with anchoring fibrils and filaments (Fig. 5). Thus, the epithelium is well anchored.
Fig. 5.
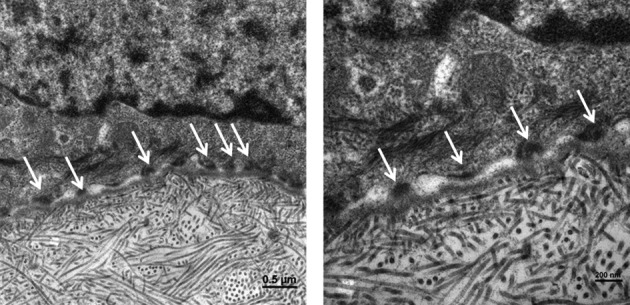
Transmission electron micrograph of a postgraft ex vivo porcine cornea. Arrows indicate the presence of hemidesmosome between epithelial cells and porcine corneal basement membrane (scale bar = 0.5 μm and 200 nm for left and right picture, respectively).
Discussion
Our results show that collagenase 0.5 mg/mL can be used for COME detachment to obtain a robust sheet. In the tests presented here, whereas COME detachment was found to be very easy and stress-free with collagenase with 100% success, with dispase the sheets were more difficult to detach, and holes formed in 67% of cases (n 9 sheets) which makes dispase-mediated detachment incompatible with clinical use. Indeed, due to the strong retraction power of the epithelia, holes increase leading to a retracted cell sheet limited at the periphery, impossible to graft on a patient’s eye. We selected collagenase at the concentration of 0.5 mg/mL, because it is the lowest concentration that provides the best reproducible results. To complete its effectiveness, we also showed that these sheets can be transferred and adhere strongly to a de-epithelialized porcine stroma.
For the transfer, we used the same PVDF ring as that used for LSCD treatment CAOMECS18. Use of this ring avoids shrinkage of the sheet, while also eliminating the need for a support such as amniotic membranes or fibrin, which is always of allogeneic origin. Because of the small surface area of the eye, this PVDF ring surrounds the area to be grafted without actually touching it, thus it should not cause lesions when it is removed. The ring can easily be removed when the graft is placed as COME adheres strongly to the corneal stroma. This strong adhesion is a major advantage, as it should allow suture-free grafting. Sutures are known to induce pain, discomfort, and inflammation and to have an impact on the patient’s quality of life. In contrast, epithelial sheets on a support do require suturing. The PVDF ring presents a major advantage compared to the large sections of Vaseline gauze commonly used to transfer epidermal sheets (ES) in that it is easy to remove. Indeed, the ES adheres to the Vaseline gauze over its whole surface area, and due to the tissue granulation any movement of the ES or the Vaseline gauze after the graft causes tearing and retraction. Vaseline gauze can therefore only be eliminated after at least 1 wk, once the epidermis is sufficiently stratified35.
Concerning the adhesiveness of the sheet, IF as well as WB showed no significant differences between the methods used to detach sheets in this study (dispase, collagenase, or mechanical detachment). In contrast, dispase was previously shown to produce a fragile sheet17. This effect was linked to dispase-induced shortening of the γ2 chain of laminin-332 and shortening of E-cadherin, which is involved in stabilizing cell–cell junctions. These results were confirmed by the observed degradation of desmosomes17. These apparent discrepancies can be explained by the fact that ES were used in the previous study, rather than OM sheets, and by the different experimental conditions. Indeed, a 4-fold lower concentration of dispase compared to the dispase concentration selected in our study (5 mg/mL) was used, requiring a longer incubation time to induce detachment: 30 min versus a mean time of 10 min in our study. Our data clearly show that sheets detached at low concentrations are already fragile when incubation exceeds 16 min at the lowest concentration of 1.25 mg/mL, this fragility made it impossible to detach intact sheets. The fragility of these sheets could be linked to the trend observed for reduced E-cadherin levels after detachment with dispase, as shown by WB, even though this difference is not significant. Given the deleterious action of overlong exposure to dispase, a maximum incubation time must be determined for each new batch. The time in contact with dispase relative to the surface area of the sheet is also very important. Indeed, we used dispase for 10 min with epidermal sheets measuring 10 cm2 or for a maximum of 20 min for ES measuring 150 cm2 18.
Although collagenase is known to degrade collagens, in particular types I, III, IV, V30, and VII36, it does not degrade type VI collagen 37 or any other adhesion and junctional proteins. Its lack of effect on cell–cell junctions in the epithelial sheet explains why the detached sheets remain resistant.
Collagenase-mediated detachment of COME was therefore used to perform ex vivo grafts on the porcine cornea model. This model of total LSCD was developed by completely destroying the limbus and the epithelium in porcine cornea. Blinking cannot be reproduced in this model, but this should not represent a problem as during clinical trials with sheets detached with UpCell Insert, the graft was protected by a contact lens for at least 1 mo and was thus not affected by blinking18. The model of COME grafted onto ex vivo porcine stroma showed that enzymatically detached epithelial sheets of OM could adhere to and anchor on the stroma and that these sheets could renew and stratify 15 d postgraft. In the conditions of this study, the epithelia became stratified and differentiated into between 2 and 4 layers before the graft and 4 to 6 layers postgraft. Moreover, the CFE showed that stem cells were preserved, so COME detached by collagenase brings epithelial stem cells able to renew epithelium. Anchoring of the epidermis to the dermis was measured based on the expression levels of markers specific for the stromal–epithelial junction: laminin-332, type VII collagen, and β1-integrin, and by its ultrastructural organization, as observed by TEM. In TEM images, hemidesmosomes were identified by their dense plaques and the presence of anchoring filaments could be seen. Adhesion markers were detected on the sheet before the graft on porcine stroma. Intracytoplasmic levels of these markers were no different than the levels measured with UpCell Insert in the previous clinical trial during all the stages of validation, and on the sheets grafted onto the 26 eyes for the 25 patients in the clinical trial18. Proteins, not degraded by enzymatic treatment, promote adhesion in synergy with the intercellular junction marker E-cadherin after grafting of the COME.
We already validated by flow cytometry using CD90 marker that fibroblasts represent less than 2.5% (0.9 ± 0.7) of cells in COME after transfer. Residual fibroblasts may be autologous (from the patient’s biopsy) or allogenic (coming from the feeder layer). Even if there are remnant fibroblasts irradiated in the final product, human fibroblasts were irradiated from nonimmortalized primary cells, they were in G1 phase and they were unable to proliferate. The latter, which is irradiated, is authorized and used clinically for epidermal sheet production for burn patients (authorization no. MTI-PP08). If there are, in fact, defined media for the culture of OM epithelial cells, their comparison with medium with serum showed that the proliferative potential of the cells is greatly decreased with the defined medium EpiLife and Oral Keratinocyte Medium (OKM) compared to the DMEM + 10% calf serum medium38. Pharmaceutical validations of COME comprising in particular identity and purity tests are in progress.
In conclusion, the enzymatic detachment method using 0.5 mg/mL collagenase is a viable alternative to thermosensitive detachment. Collagenase-mediated detachment reproducibly provides an adhesive sheet that readily develops on our new ex vivo stromal model of LSCD to produce a living and differentiated epithelium. This method therefore offers hope for the success of future clinical trials.
Acknowledgment
We thank CellSeed Inc. for its closed collaboration in the clinical trial CAOMECS.
Footnotes
Ethical Approval: This study did not contain human subjects, but only human tissues obtained with written consent, and which would be disposed of as waste after a surgical operation.
Statement of Human and Animal Rights: Use of Porcine corneas (n°DR2013-30) and human corneas and oral mucosa were authorized (n°AC-2013-1846).
Statement of Informed Consent: Written informed consent was obtained from donors of oral mucosa, from a representative for corneas from deceased donors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Patricia Rousselle contribution was funded by ANR grant No. ANR-13-RPIB-0003-01.
References
- 1. Rao SK, Rajagopal R, Sitalakshmi G, Padmanabhan P. Limbal allografting from related live donors for corneal surface reconstruction. Ophthalmology. 1999;106(4):822–828. [DOI] [PubMed] [Google Scholar]
- 2. Samson CM, Nduaguba C, Baltatzis S, Foster CS. Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology. 2002;109(5):862–868. [DOI] [PubMed] [Google Scholar]
- 3. Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen-Miyajima H, Shimazaki J. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340(22):1697–1703. [DOI] [PubMed] [Google Scholar]
- 4. Shimazaki J, Shimmura S, Fujishima H, Tsubota K. Association of preoperative tear function with surgical outcome in severe Stevens-Johnson syndrome. Ophthalmology. 2000;107(8):1518–1523. [DOI] [PubMed] [Google Scholar]
- 5. Santos MS, Gomes JAP, Hofling-Lima AL, Rizzo LV, Romano AC, Belfort R. Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol. 2005;140(2):223–230. [DOI] [PubMed] [Google Scholar]
- 6. Nishida K, Yamato M, Hayashida Y, Watanabe K, Maeda N, Watanabe H, Yamamoto K, Nagai S, Kikuchi A, Tano Y, et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77(3):379–385. [DOI] [PubMed] [Google Scholar]
- 7. Hayashida Y, Nishida K, Yamato M, Watanabe K, Maeda N, Watanabe H, Kikuchi A, Okano T, Tano Y. Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface. Invest Ophthalmol Vis Sci. 2005;46(5):1632–1639. [DOI] [PubMed] [Google Scholar]
- 8. Kocaba V, Thépot A, Yamato M, Daisuke M, Kellal M, Mojallal A, Damour O, Burillon C. Long-term results of cultured autologous oral mucosa epithelial cell-sheet (CAOMECS) graft for the treatment of blindness due to bilateral limbal stem cell deficiency. J Stem Cell Res Ther. 2014;4:3. [Google Scholar]
- 9. Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88(10):1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Satake Y, Higa K, Tsubota K, Shimazaki J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118(8):1524–1530. [DOI] [PubMed] [Google Scholar]
- 11. Inatomi T, Nakamura T, Koizumi N, Sotozono C, Yokoi N, Kinoshita S. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006;141(2):267–275. [DOI] [PubMed] [Google Scholar]
- 12. Ang LPK, Nakamura T, Inatomi T, Sotozono C, Koizumi N, Yokoi N, Kinoshita S. Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch Ophthalmol. 2006;124(11):1543–1551. [DOI] [PubMed] [Google Scholar]
- 13. Inatomi T, Nakamura T, Kojyo M, Koizumi N, Sotozono C, Kinoshita S. Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. Am J Ophthalmol. 2006;142(5):757–764. [DOI] [PubMed] [Google Scholar]
- 14. Ma DHK, Kuo MT, Tsai YJ, Chen HCJ, Chen XL, Wang SF, Li L, Hsiao CH, Lin KK. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye Lond Engl. 2009;23(6):1442–1450. [DOI] [PubMed] [Google Scholar]
- 15. Chen HCJ, Yeh LK, Tsai YJ, Lai CH, Chen CC, Lai JY, Sun CC, Chang G, Hwang TL, Chen JK, et al. Expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Invest Ophthalmol Vis Sci. 2012;53(9):5615–5623. [DOI] [PubMed] [Google Scholar]
- 16. Takeda K, Nakamura T, Inatomi T, Sotozono C, Watanabe A, Kinoshita S. Ocular surface reconstruction using the combination of autologous cultivated oral mucosal epithelial transplantation and eyelid surgery for severe ocular surface disease. Am J Ophthalmol. 2011;152(2):195–201.e1. [DOI] [PubMed] [Google Scholar]
- 17. Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7(4):473–480. [DOI] [PubMed] [Google Scholar]
- 18. Burillon C, Huot L, Justin V, Nataf S, Chapuis F, Decullier E, Damour O. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. 2012;53(3):1325–1331. [DOI] [PubMed] [Google Scholar]
- 19. Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76(11):5665–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SCG. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17(5):537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanchez I, Martin R, Ussa F, Fernandez-Bueno I. The parameters of the porcine eyeball. Graefes Arch Clin Exp Ophthalmol. 2011;249(4):475–82. [DOI] [PubMed] [Google Scholar]
- 22. Bardag-Gorce F, Oliva J, Wood A, Hoft R, Pan D, Thropay J, Makalinao A, French SW, Niihara Y. Carrier-free cultured autologous oral mucosa epithelial cell sheet (CAOMECS) for corneal epithelium reconstruction: a histological study. Ocul Surf. 2015;13(2):150–163. [DOI] [PubMed] [Google Scholar]
- 23. Promprasit D, Bumroongkit K, Tocharus C, Mevatee U, Tananuvat N. Cultivation and phenotypic characterization of rabbit epithelial cells expanded ex vivo from fresh and cryopreserved limbal and oral mucosal explants. Curr Eye Res. 2015;40(3):274–281. [DOI] [PubMed] [Google Scholar]
- 24. Li W, Li Q, Wang W, Li K, Ling S, Yang Y, Liang L. A rat model of autologous oral mucosal epithelial transplantation for corneal limbal stem cell failure. Eye Sci. 2014;29(1):1–5. [PubMed] [Google Scholar]
- 25. Soma T, Hayashi R, Sugiyama H, Tsujikawa M, Kanayama S, Oie Y, Nishida K. Maintenance and distribution of epithelial stem/progenitor cells after corneal reconstruction using oral mucosal epithelial cell sheets. PLoS One. 2014;9(10):e110987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kolli S, Ahmad S, Mudhar HS, Meeny A, Lako M, Figueiredo FC. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells Dayt Ohio. 2014;32(8):2135–2146. [DOI] [PubMed] [Google Scholar]
- 27. Gaddipati S, Muralidhar R, Sangwan VS, Mariappan I, Vemuganti GK, Balasubramanian D. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J Ophthalmol. 2014;62(5):644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Priya CG, Arpitha P, Vaishali S, Prajna NV, Usha K, Sheetal K, Muthukkaruppan V. Adult human buccal epithelial stem cells: identification, ex-vivo expansion, and transplantation for corneal surface reconstruction. Eye Lond Engl. 2011;25(12):1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sotozono C, Inatomi T, Nakamura T, Koizumi N, Yokoi N, Ueta M, Matsuyama K, Kaneda H, Fukushima M, Kinoshita S. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol (Copenh.). 2014;92(6):e447–e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sotozono C, Inatomi T, Nakamura T, Koizumi N, Yokoi N, Ueta M, Matsuyama K, Miyakoda K, Kaneda H, Fukushima M, et al. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology. 2013;120(1):193–200. [DOI] [PubMed] [Google Scholar]
- 31. Hirayama M, Satake Y, Higa K, Yamaguchi T, Shimazaki J. Transplantation of cultivated oral mucosal epithelium prepared in fibrin-coated culture dishes. Invest Ophthalmol Vis Sci. 2012;53(3):1602–1609. [DOI] [PubMed] [Google Scholar]
- 32. Nakamura T, Inatomi T, Cooper LJ, Rigby H, Fullwood NJ, Kinoshita S. Phenotypic investigation of human eyes with transplanted autologous cultivated oral mucosal epithelial sheets for severe ocular surface diseases. Ophthalmology. 2007;114(6):1080–1088. [DOI] [PubMed] [Google Scholar]
- 33. Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351(12):1187–1196. [DOI] [PubMed] [Google Scholar]
- 34. Decline F, Rousselle P. Keratinocyte migration requires alpha2beta1 integrin-mediated interaction with the laminin 5 gamma2 chain. J Cell Sci. 2001;114(Pt 4):811–823. [DOI] [PubMed] [Google Scholar]
- 35. Auxenfans C, Menet V, Catherine Z, Shipkov H, Lacroix P, Bertin-Maghit M, Damour O, Braye F. Cultured autologous keratinocytes in the treatment of large and deep burns: a retrospective study over 15 years. Burns J Int Soc Burn Inj. 2015;41(1):71–79. [DOI] [PubMed] [Google Scholar]
- 36. Shi L, Ermis R, Garcia A, Telgenhoff D, Aust D. Degradation of human collagen isoforms by Clostridium collagenase and the effects of degradation products on cell migration. Int Wound J. 2010;7(2):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seltzer JL, Eisen AZ, Bauer EA, Morris NP, Glanville RW, Burgeson RE. Cleavage of type VII collagen by interstitial collagenase and type IV collagenase (gelatinase) derived from human skin. J Biol Chem. 1989;264(7):3822–3826. [PubMed] [Google Scholar]
- 38. Islam R, Eidet JR, Badian RA, Lippestad M, Messelt E, Griffith M, Dartt DA, Utheim TP. Tissue harvesting site and culture medium affect attachment, growth, and phenotype of ex vivo expanded oral mucosal epithelial cells. Sci Rep. 2017;7(1):674. [DOI] [PMC free article] [PubMed] [Google Scholar]