Abstract
Cell therapy has been shown to be a key clinical therapeutic option for central nervous system diseases or damage. Standardization of clinical cell therapy procedures is an important task for professional associations devoted to cell therapy. The Chinese Branch of the International Association of Neurorestoratology (IANR) completed the first set of guidelines governing the clinical application of neurorestoration in 2011. The IANR and the Chinese Association of Neurorestoratology (CANR) collaborated to propose the current version “Clinical Cell Therapy Guidelines for Neurorestoration (IANR/CANR 2017)”. The IANR council board members and CANR committee members approved this proposal on September 1, 2016, and recommend it to clinical practitioners of cellular therapy. These guidelines include items of cell type nomenclature, cell quality control, minimal suggested cell doses, patient-informed consent, indications for undergoing cell therapy, contraindications for undergoing cell therapy, documentation of procedure and therapy, safety evaluation, efficacy evaluation, policy of repeated treatments, do not charge patients for unproven therapies, basic principles of cell therapy, and publishing responsibility.
Keywords: cell therapy, neurorestoration, clinical application guideline neurorestoratology
Introduction
The Chinese Branch of the International Association of Neurorestoratology (IANR) established the first guidelines governing the clinical application of neurorestoration in 2011 (“Chinese Clinical Standard of Neurorestorative Cell Therapy”)1. These guidelines were revised in 2012 (“Standard Recommendation for the Application of Chinese Clinical Cell Therapy For Neurorestoration”)2, in 2015 (“Chinese Clinical Application Guideline of Neurorestorative Cell Therapy)3, and in 2016 (“Clinical Cell Therapy Guidelines for Neurorestoration, China Version 2016”)4. The guideline and its revisions have played a significant role in standardizing cell therapy practice in China.
Clinical cell therapies have become increasingly popular around the world. IANR and the Neurorestoratology Professional Committee of the Chinese Medical Doctor Association (Chinese Association of Neurorestoratology [CANR]) collaborated to propose “Clinical Cell Therapy Guidelines for Neurorestoration (IANR/CANR 2017)” based on the Chinese version of the guidelines and approved in principle by the IANR council board members and CANR committee members on September 1, 2016. The document was subsequently edited and literature citations were complemented. The finalized guidelines were then approved by all IANR/CANR members by email communication.
IANR/CANR hopes that these guidelines will be accepted as the applied reference standard for cell therapy of neurological diseases and damage worldwide. In particular, these guidelines may be useful to guide researchers who transplant cells into the brain and spinal cord for therapeutic research purposes. For the detailed protocol and rules of general cell therapies, researchers should first follow the regulations and policies of local governments in their respective countries. Given the rapidly advancing state of the field, the IANR/CANR will amend and update the existing guidelines to reflect the newest results demonstrated in preclinical research, translational studies, and evidence-based clinical studies.
Neurorestoratology is an emerging discipline at the intersection of clinical medicine and neuroscience. Its goal is to restore, promote, and maintain the integrity of impaired or lost neuronal functions and/or structures5.
The “Beijing Declaration of IANR” (agreed upon at the IANR 2015 Conference in Tehran) declared as its fundamental tenet that “functional recovery is possible after central nervous system (CNS) injury and neurodegeneration” and noted that “cell therapies may become a key clinical therapeutic option for acute, subacute and/or chronic CNS diseases or damage”5. More than 30 types of cells have been identified through preclinical studies as having the capacity for neurorestoration6–66.
The US Food and Drug Administration (Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products) divided cell therapy products into stem cell–derived cell therapy products and mature/functionally differentiated cell-derived cell therapy products (http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm). Stem cell–derived cell therapy products include embryonic stem cells (ESCs), induced pluripotent stem cells, and adult (multipotent) stem cells. The last one contains neural stem cells (NSCs) and mesenchymal stem cells of different types. Mature/functionally differentiated cell-derived cell therapy products include (1) specialized functional cells such as neural progenitor or precursor cells, olfactory ensheathing cells (OECs), Schwann precursor cells, oligodendroglia precursors, neural-restricted precursors, glial-restricted precursors, neutrophils, neurons, astrocytes, myoblasts, and so on; and (2) nonspecialized functional cells such as bone marrow or umbilical cord blood mononuclear cells, umbilical cord or adipose stromal cells, and fibroblasts and lymphocytes63,64,67–71. Even though there is some disagreement or controversy concerning the nomenclature of MSCs, so far the majority has accepted the MSC standard criteria made by the International Society for Cellular Therapy to identify MSCs72,73. While MSCs containing mesenchymal stem cells are able to differentiate into other (adipocytes, chondrocytes, osteocytes, etc.) kinds of cells when cultured in special media for differentiation, this kind of study can be referred to as mesenchymal stem cell research. In those cases, MSCs are cultured for expansion without differentiation; we may refer to this type of study as MSC research. Currently, there remains some misuse of these MSC standard criteria to identify their culturing and expanding MSCs and call them mesenchymal stem cells.
Due to concerns over tumorigenicity and difficulties in controlling differentiation of pluripotent or multipotent stem cells, stem cell–derived cell therapy products require more extensive preclinical and clinical testing. The clinical guidelines presented in this document apply more to mature/functionally differentiated cell therapy. To date, clinical trials of treatments based on those categories of cells have been carried out in over 40 countries with documented safety and functional neurological improvement for patients with CNS diseases and damage74–114.
Recommended Standards for Personnel and Institutions Conducting Cell Therapies
Equipment
Institutions applying cell therapies to patients must have certified laboratory facilities and equipment that comply with the relevant national standards for ensuring cell quality control (Fig. 1).
Fig. 1.

Recommended standards for personnel and institutions. The standards mainly contain 3 parts: equipment, personal and institutional review board, and ethics committee approval.
Personnel
Clinical personnel
Physicians performing cell transplantation procedures should have documented professional training and certification required to ensure high-level competency in this field. They should have passed all certification examinations recommended by the relevant professional societies or associations.
Laboratory personnel
Directors of cell preparation laboratories should have achieved a high professional rank. Technicians involved in cell preparation should have undergone all relevant professional training and passed all certification examinations in cell preparation recommended by the relevant professional societies or associations.
Oversight personnel
Inspectors assessing cell quality should have undergone all relevant professional training and passed all certification examinations in cell preparation recommended by the relevant professional societies or associations (Fig. 1).
Institutional Review Board and Ethics Committee Approval
All clinical studies or treatment involving cell therapies and human participants must be reviewed and approved by the appropriate institutional review board or ethics committee (Fig. 1).
Provisions
These guidelines include the following provisions: cell type nomenclature, cell quality control, minimal suggested cell doses, patient informed consent, indications for undergoing cell therapy, contraindications for undergoing cell therapy, documentation of procedure and therapy, safety evaluation, efficacy evaluation, policy of repeated treatments, do not charge patients for unproven therapies, basic principles of cell therapy, and publishing responsibility (Fig. 2).
Fig. 2.
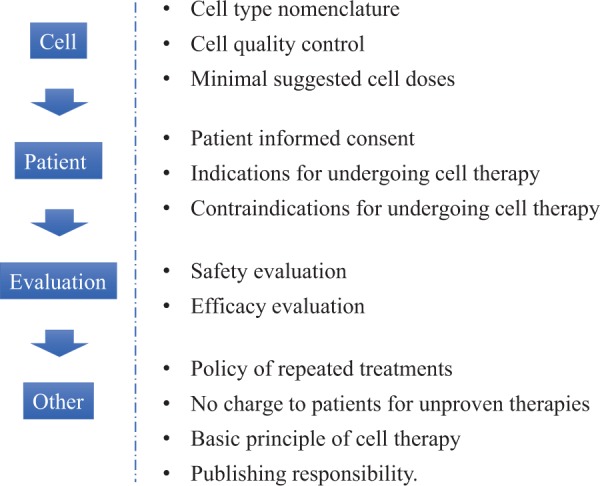
Provisions for cell therapy.
Cell Type Nomenclature
Cells are the basic unit of structure and function of organisms. A stem cell can generate itself in a process known as self-renewal and also produce the other kinds of more differentiated, committed cells with specialized functions. Progenitor or precursor cells are not able to self-renew but instead can multiply rapidly and differentiate into one or more kinds of specialized cells. Mature and functionally differentiated cells usually cannot make additional cells unless they dedifferentiate.
The nomenclature and description of cells used for therapy should specify developmental stage (e.g., embryonic, fetal, neonatal, adult, allogeneic, and autologous), tissue of origin (e.g., blood, bone marrow, umbilical cord blood, placenta, brain, spinal cord, olfactory bulb, subventricular zone, peripheral nerve, adipose, tumor, and cell line), method to isolate or expand the cells (e.g., genetically induced, expanded in culture, laser sorted, minimally manipulated, centrifugation, and type of osmotic gradient), and selection process (e.g., human leukocyte antigen [HLA]- or ABO blood group-matched, CD34+, and aldehyde dehydrogenase expressing). If nonhuman, the species must be specified. If mixtures of cells are used, the type of cells should be specified (e.g., mononuclear) together with the approximate percentages of the different types of cells present in the mixture (Fig. 3).
Fig. 3.
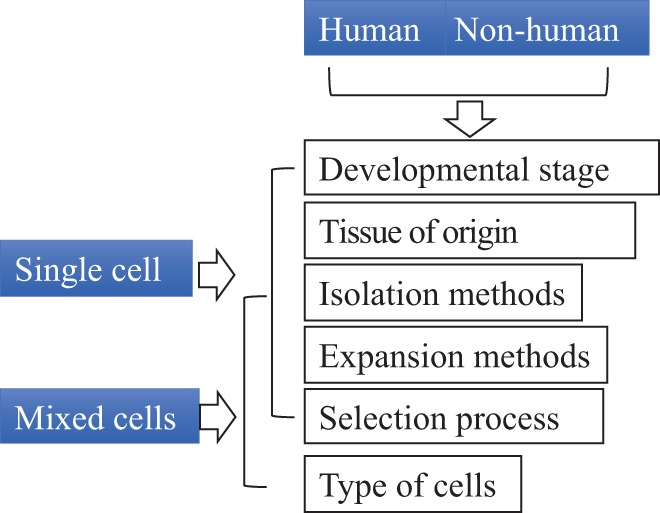
Provision for cell type nomenclature. The nomenclature and description of the cells used for therapy should specify species, developmental stage, tissue of origin, method to isolate or expand the cells, and selection process. If mixtures of cells are used, the type of cells should be specified.
Cell Quality Control
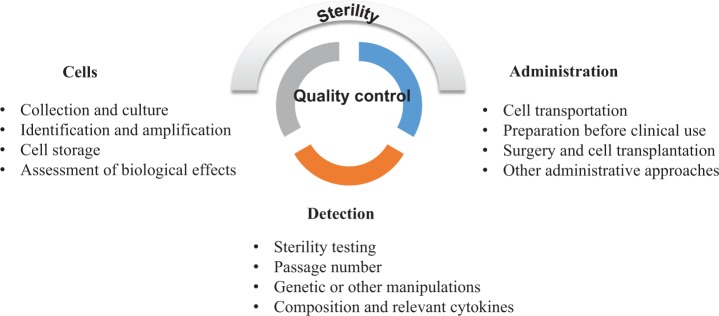
Quality control is essential for ensuring safety and efficacy of cell therapies. Quality control encompasses cell collection; culturing; identifying; amplifying; detecting composition and relevant cytokines, genetic or other manipulations, and passage number; exogenous factors; cell storage; assessment of biological effects (dynamics proliferation); cell transportation; preparation before clinical use; surgery and cell transplantation; or other administrative approaches. Use of animal serum such as fetal bovine serum (FBS) is discouraged. If FBS is used, it should be washed or otherwise removed before transplantation. If human sera are used, the source and quality of the serum must be documented.
Sterility of the cell preparation must be rigorously monitored. Because microbiological cultures may take days or even weeks to complete, the sterility of the cell preparation procedure must be carefully assessed and validated, particularly if the cells are transplanted before sterility data are available. If sterility testing reveals that the cells are contaminated with bacteria, fungus, or virus, sensitivity to antibiotics, antivirals, or fungicides should be determined. If the cells come from donor sources where infections are possible, donors should be screened for human immunodeficiency virus, hepatitis B virus, cytomegalovirus, or other prevalent contaminants. In addition, if tissues are stored at room temperature for any length of time, endotoxin levels should be determined. Before cells are frozen and stored, dimethylsulfoxide should be tested. The standards for sterility depend on the type and source of cells and route of administration. For example, administration of cells to skin or eyes may require criteria as stringent as for their administration to the CNS.
Before transplantation, certain minimal information must be obtained concerning the cells. These include the number of nucleated cells, the number or percentage of therapeutically relevant cells, and cell viability. Some information may be obtained after treatment as long as the preparation process is validated and contaminants can be treated with antibiotics, antivirals, or fungicides. The maximum time between preparation and transplantation of cells should be based on evidence115 (Fig. 4).
Fig. 4.
Cell quality control. Quality control is essential for ensuring safety and efficacy of cell therapies from the preparation of cells to cell transplantation. Sterility of the cell preparation must be rigorously monitored.
Minimal Suggested Cell Doses
Cells must be used at an effective dose. Thus, the cell dosage and injection volume must be determined and controlled based on evidence of efficacy and safety. Currently, we recommend that the maximum injection volume of cell suspensions does not exceed 200 μL per injection for brain parenchyma79,116–118, 25 μL per injection into spinal cord parenchyma74,75, 10 mL by intrathecal injection into cerebrospinal fluid119,120, and 10 to 100 mL by intravenous and intra-arterial routes120–124. The volume or number of cells being transplanted will be reformulated if further trials show stronger evidence or indicate suitable doses in terms of patient body weight.
Current recommendations for a commonly used single dose of cells are as follows (Table 1):
Table 1.
Minimal Suggested Cell Doses.
| Olfactory ensheathing cells and Schwann cells | 2–3 × 106 | 1–2 × 106 | 2–4 × 106 | — |
|---|---|---|---|---|
| Neural progenitor/precursor cells | 5–6 × 106 | 5–6 × 106 | 10 × 106 (or lateral ventricle) | — |
| Mesenchymal stromal cells derived from umbilical cord | 5–8 × 106 | — | 10 × 106 | 0.5–0.8 × 106/kg |
| Mononuclear cells derived from cord blood | 5–6 × 106 | 6–7 × 106 (above and below the injury site) | — | 1–2 × 106/kg |
| Mononuclear cells derived from bone marrow | 5–6 × 106 | — | — | 3–9 × 108 |
Note. Current recommendations of commonly used single dose of different cells, including olfactory ensheathing cells and Schwann cells, neural progenitor/precursor cells, mesenchymal stromal cells derived from umbilical cord, mononuclear cells derived from cord blood, and mononuclear cells derived from bone marrow.
OECs and Schwann cells: 2 to 3 × 106 cells for intrathecal injection, 1 to 2 × 106 cells for intraspinal spinal cord injection, and 2 to 4 × 106 cells for brain parenchymal injection74,75,79,118,125.
Neural progenitor/ precursor cells: 5 to 6 × 106 cells by intrathecal injection, 5 to 6 × 106 cells for spinal cord injection, and 10 × 106 by brain parenchymal injection118,123,125 or lateral ventricle126.
MSCs derived from umbilical cord: 0.5 to 0.8 × 106/kg body weight for intravenous infusion (the dose should be reduced by 1/3 to 1/2 for elderly and frail patients), 5 to 8 × 106 for intrathecal injection, and 10 × 106 for brain parenchymal injection127–134.
Mononuclear cells derived from cord blood: 1 to 2 × 106/kg body weight by intravenous infusion (the dose should be reduced by 1/3 to 1/2 for elderly and frail patients), 5 to 6 × 106 by intrathecal injection81,87,90,94, and a total of 6 to 7 × 106 cells injected into spinal cord above and below the injury site135.
Mononuclear cells derived from bone marrow: 3 to 9 × 108 by intravenous infusion and 5 to 6 × 106 by intrathecal injection84,86,121,122,124,126.
Patient-Informed Consent
Two types of informed consent must be obtained. The first is from donors or parents who must give consent for the cells to be used to treat other patients. For example, if cells are obtained from aborted fetuses, the parent must understand what the cells will be used for and give informed consent. The second is informed consent of the recipient of the cells. Patients and their families have the right to know all the possible benefits and potential risks of matters related with the cell transplantation and procedures. Physicians should continue to learn and master the latest cell therapy–related knowledge in order to give objective answers and explanations. All participants must complete and sign a consent form that is approved by the appropriate institutional review board or ethical committee before the clinical study or cell therapy is applied.
Indications for Undergoing Cell Therapy
Animal studies suggest that cell therapies may be beneficial for a variety of neurological diseases and injury including neurotraumatic injury, neurodegeneration, ischemic/hypoxic brain injury, demyelination, sensory motor disorders, neuropathic pain, and nerve damage caused by intoxication, physical/chemical factors, immune and infectious, inflammatory, hereditary, congenital or developmental factors, and so on. However, until formal regulatory approval is obtained for use of cell therapies for specific indications, cell therapy must be administered only under the auspices of clinical trials and studies approved by appropriate institutional review boards, ethical committees, and regulatory agencies.
The relevant committees for each type of neurorestorative treatment should document the special indications for each treatment and the disease categories.
Contraindications for Undergoing Cell Therapy
Patients with poor health or dysfunction of major organs may not tolerate surgery or cell therapy procedures. The presence of infections, pressure sores, bleeding tendency, coagulation disorders, and emotional disturbance likewise may introduce undesirable complications. Patients with active neoplastic diseases, hypersensitivity, or pregnancy likewise should be excluded unless the cell therapy is specifically intended for these conditions. Clinicians should consider the likelihood that a high incidence of complications risks creating unnecessarily negative and undesirable issues for cell therapy or transplantation procedures.
Documentation of the Procedure and Therapy
The operative procedures, cell therapy, and outcomes must be rigorously documented. The documented data include anesthesia methods, cell quality and source, surgical procedures, transplantation method and site, cell preparation and dose, treatment timing at different stages, and both short- and long-term outcomes.
More preclinical and clinical treatment studies are needed to establish the best doses and therapeutic routes for different cell therapies and conditions. Randomized double-blind clinical trials are needed to establish and validate the safest and most effective clinical practices74,75,79,118,128–130,136–139. Specific sites of transplantation should be compared and assessed. For example, in local brain disorders (trauma or stroke), should the cells be injected into the lesion edge? For nonspecial or diffuse disorders (cerebral palsy, amyotrophic lateral sclerosis), what is the best site to inject cells? Should they be injected into the key points for neural network restoration75,79? Some experience suggests that these key points are located anterior to the lateral ventricle and 23 to 27 mm from the midline, where the frontal corona radiata and pyramidal tract pass through and represent a nexus where numerous projection fibers, association fibers, and commissural fibers converge. Where is the best place to inject into the spinal cord? Some data suggest that cells should be injected into the spinal cord below and above the injury site, at the junction of normal and damaged tissue. For peripheral nerve disorders, should the cells be injected into the damaged site? For brain or spinal cord injury, when is the optimal time window for cell therapy, which mode of cell transplantation is better and what kind of cells should be selected at different stages? All these questions need to be answered in future clinical studies.
Safety Evaluation
Detailed records must be kept for cell therapy–related adverse events by using standardized terminology such as fever, headache, nausea, vomiting, anorexia, infection, rash, poor wound healing, dyspnea, increased/decreased blood pressure, increased/decreased heart rate, neurological deterioration, cerebrospinal fluid leakage, twitch, and so on. In the case of mortality, autopsies should be carried out to determine the cause of death and disposition of the transplanted cells.
Efficacy Evaluation
Efficacy of cell therapies should be evaluated by validated and established standards or scales currently used in the international community to assess the patients’ functions for different diseases (referred to as neurorestoratology138 or CNS neurorestoratology139). Many organizations, including IANR, regularly hold training courses for physicians to learn specific standards and assessments of patients, to test and evaluate proficiency and consistency, such as volitional control which is evaluated by a motor task involving single and multijoint movement performed repetitively at the same rate and amplitude and provide professional certification. Independent third-party examiners are recommended for clinical trials. Randomized double-blind controlled trials are preferred and required for regulatory approval. Whenever possible, detailed imaging information should be obtained before and after therapy, including magnetic resonance imaging (MRI) scans, such as functional MRI, and diffusion tensor MRI and electrophysiological examination such as transcranial magnetic stimulation (TMS), evoked potential (EP), and electromyogram (EMG) to document the presence of the cells or therapeutic effects. Patients should be scheduled for long-term follow-up examinations to determine whether beneficial effects are lasting. Functional and quality-of-life scales should be used to assess the impact of treatment effects. Natural recovery can occur over a period of time before it stops. This time may differ in different pathological conditions. It is therefore important to record the time elapsed between the pathological event and the intervention and to document other previous treatments (surgical, medical, or pharmacological) and the time prior to the cellular intervention.
Policy of Repeated Treatments
Repeated cell therapies should be based on convincing evidence of efficacy. In the absence of such evidence, patients should not be told that repeated transplants are more effective. If such evidence is not available, clinicians should do randomized trials to obtain such evidence. For example, one possible approach is to use a randomized crossover trial design, where patients are randomized to early or late repeated therapy. To rule out potential placebo effects, some trials should involve sham transplant procedures.
Do Not Charge Patients for Unproven Therapies
There should be agreement within the treatment community that if clinical trials do not show convincing evidence of benefit, practice of the therapy should be discontinued. Patients should not pay for experimental therapies. While there may be differing standards for proof of safety and efficacy, charging patients for unproven cell therapies without regulatory approval is not only illegal but may give cell therapy a bad reputation with regulatory agencies and delay acceptance of cell therapy by the mainstream clinical community. Approved medical treatments including cell therapies will not be fit for this agreement. Many countries and areas such as the United States, India, and Europe140, however, are adopting the practice of allowing compassionate use of therapies shown to be safe and with some evidence of efficacy to treat disorders for which there is no known effective therapy. Regulatory agencies will often negotiate with the clinician or company for the patient to pay the cost of the therapy.
Basic Principles of Cell Therapy
A number of clinical studies concerning cell therapies with a positive outcome have led to the suggestion that combination cell therapies and/or certain transplantation approaches with rehabilitation can be more effective107,123,128–130,136,138–200. IANR/CANR will evaluate such data and actively organize multicenter studies to test these practices for different cells and diseases. Randomized, double-blind, and controlled clinical studies should be carried out, if at all possible.
Publishing Responsibility
All groups that practice clinical cell therapies must promptly analyze and publish their data in peer-reviewed journals, so that other physicians can have access to the information.
Summary
Clinical cell therapies for neurological diseases and damage have shown promise for functional neurorestoration. These guidelines will help to promote the development of clinical cell therapies. Although currently multiple cell types have being used or have continued use for neurorestoration201,202, additional studies are necessary to determine the best type, doses, route, and timing window for administration. Further investigations in human patients will enhance our comprehension of this up-and-coming treatment.
Footnotes
Authors’ Note: This manuscript was approved by the International Association of Neurorestoratology and Chinese Association of Neurorestoratology.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Paul R. Sanberg is Co-Editor-in-Chief of Cell Transplantation. Neither Paul R. Sanberg nor any of his colleagues were involved in the peer review or decision making processes for this manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chinese Branch of International Association of Neurorestoratology and Preparatory Committee of Chinese Association of Neurorestoratology. Chinese clinical standard of neurorestorative cell therapy (2011 first vision). Chin J Clin Physicians. 2011;5(19):5710–5714. [Google Scholar]
- 2. Chinese Branch of the International Association of Neurorestoratology and Preparatory Committee of Chinese Association of Neurorestoratology. Standard recommendation for the application of Chinese clinical cell therapy for neurorestoration. Cell Transplant. 2013;22(Suppl 1):S5–S10. [DOI] [PubMed] [Google Scholar]
- 3. Chinese Association of Neurorestoratology and Chinese Branch of International Association of Neurorestoratology. Clinical application guideline of neurorestorative cell therapy in China (2015 version). Chin J Cell Stem Cell. 2016;6(1):1–7. [Google Scholar]
- 4. Huang H, Chen L, Zou Q, Han F, Sun T, Mao G, He X. Clinical cell therapy guidelines for neurorestoration (China version 2016). J Neurorestoratology. 2017;5:39–46. [Google Scholar]
- 5. Young W, AlZoubi Z, Saberi H, Sharma A, Muresanu D, Feng S, Chen L, Huang H. Beijing declaration of International Association of Neurorestoratology (IANR). J Neurorestoratology. 2015;3:121–122. [Google Scholar]
- 6. Döbrössy M, Busse M, Piroth T, Rosser A, Dunnett S, Nikkhah G. Neurorehabilitation with neural transplantation. Neurorehabil Neural Repair. 2010;24(8):692–701. [DOI] [PubMed] [Google Scholar]
- 7. Ghanizadeh A. Non-neuronal cell transplantation as a possible therapeutic approach for epilepsy treatment. Brain Res Bull. 2010;83(5):194–195. [DOI] [PubMed] [Google Scholar]
- 8. Waldau B, Hattiangady B, Kuruba R, Shetty AK. Medial ganglionic eminence–derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells. 2010;28(7):1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang H, Chen L, Sanberg PR. Cell therapy from bench to bedside translation in CNS neurorestoratology era. Cell Med. 2010;1(1):15–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi JH, Chung JY, Yoo DY, Hwang IK, Yoo KY, Lee CH, Yan BC, Ahn JO, Youn HY, Won MH. Cell proliferation and neuroblast differentiation in the rat dentate gyrus after intrathecal treatment with adipose-derived mesenchymal stem cells. Cell Mol Neurobiol. 2011;31(8):1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011;6(3):e17899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, Yoshimura S, Iwama T. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13(6):675–685. [DOI] [PubMed] [Google Scholar]
- 13. Yang YC, Liu BS, Shen CC, Lin CH, Chiao MT, Cheng HC. Transplantation of adipose tissue-derived stem cells for treatment of focal cerebral ischemia. Curr Neurovasc Res. 2011;8(1):1–13. [DOI] [PubMed] [Google Scholar]
- 14. Yalvac ME, Rizvanov AA, Kilic E, Sahin F, Mukhamedyarov MA, Islamov RR, Palotás A. Potential role of dental stem cells in the cellular therapy of cerebral ischemia. Curr Pharm Des. 2009;15(33):3908–3916. [DOI] [PubMed] [Google Scholar]
- 15. Dell’Anno MT, Caiazzo M, Leo D, Dvoretskova E, Medrihan L, Colasante G, Giannelli S, Theka I, Russo G, Mus L, Pezzoli G, Gainetdinov RR, Benfenati F, Taverna S, Dityatev A, Broccoli V. Remote control of induced dopaminergic neurons in parkinsonian rats. J Clin Invest. 2014;124(7):3215–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dyson SC, Barker RA. Cell–based therapies for Parkinson’s disease. Expert Rev Neurother. 2011;11(6):831–844. [DOI] [PubMed] [Google Scholar]
- 17. Lasala GP, Minguell JJ. Vascular disease and stem cell therapies. Br Med Bull. 2011;98:187–197. [DOI] [PubMed] [Google Scholar]
- 18. Rhee YH, Ko JY, Chang MY, Yi SH, Kim D, Kim CH, Shim JW, Jo AY, Kim BW, Lee H, Lee SH, Suh W, Park CH, Koh HC, Lee YS, Lanza R, Kim KS, Lee SH. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011;121(6):2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunt CJ. Cryopreservation of human stem cells for clinical application: a review. Transfus Med Hemother. 2011;38(2):107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Wang D, Chen M, Yang B, Zhang F, Cao K. Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart. PLoS One. 2011;6(4):e19012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tomaskovic-Crook E, Crook JM. Human embryonic stem cell therapies for neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10(4):440–448. [DOI] [PubMed] [Google Scholar]
- 22. Hernándeza J, Torres-Espína A, Navarro X. Adult stem cell transplants for spinal cord injury repair: current state in preclinical research. Curr Stem Cell Res Ther. 2011;6(3):273–287. [DOI] [PubMed] [Google Scholar]
- 23. Loewenbrück K, Storch A. Stem cell-based therapies in Parkinson’s disease: future hope or current treatment option? J Neurol. 2011;258(Suppl 2):S346–S353. [DOI] [PubMed] [Google Scholar]
- 24. Lunn JS, Sakowski SA, Federici T, Glass JD, Boulis NM, Feldman EL. Stem cell technology for the study and treatment of motor neuron diseases. Regen Med. 2011;6(2):201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396(4):489–497. [DOI] [PubMed] [Google Scholar]
- 26. Gaspard N, Vanderhaeghen P. From stem cells to neural networks: recent advances and perspectives for neurodevelopmental disorders. Dev Med Child Neurol. 2011;53(1):13–17. [DOI] [PubMed] [Google Scholar]
- 27. Pellegrini KL, Beilharz MW. The survival of myoblasts after intramuscular transplantation is improved when fewer cells are injected. Transplantation. 2011;91(5):522–526. [DOI] [PubMed] [Google Scholar]
- 28. Chang YK, Chen MH, Chiang YH, Chen YF, Ma WH, Tseng CY, Soong BW, Ho JH, Lee OK. Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells. J Biomed Sci. 2011;18(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yalvaç ME, Yarat A, Mercan D, Rizvanov AA, Palotás A, Şahin F. Characterization of the secretome of human tooth germ stem cells (hTGSCs) reveals neuro-protection by fine-tuning micro–environment. Brain Behav Immun. 2013;32:122–130. [DOI] [PubMed] [Google Scholar]
- 30. Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle. 2008;7(12):1865–1869. [DOI] [PubMed] [Google Scholar]
- 31. Lee JM, Bae JS, Jin HK. Intracerebellar transplantation of neural stem cells into mice with neurodegeneration improves neuronal networks with functional synaptic transmission. J Vet Med Sci. 2010;72(8):999–1009. [DOI] [PubMed] [Google Scholar]
- 32. Sotelo C, Alvarado-Mallart RM. Reconstruction of the defective cerebellar circuitry in adult Purkinje cell degeneration mutant mice by Purkinje cell replacement through transplantation of solid embryonic implants. Neuroscience. 1987;20(1):1–22. [DOI] [PubMed] [Google Scholar]
- 33. Novikova LN, Lobov S, Wiberg M, Novikov LN. Efficacy of olfactory ensheathing cells to support regeneration after spinal cord injury is influenced by method of culture preparation. Exp Neurol. 2011;229(1):132–142. [DOI] [PubMed] [Google Scholar]
- 34. Su L, Xu J, Ji BX, Wan SG, Lu CY, Dong HQ, Yu YY, Lu DP. Autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Int J Hematol. 2006;84(3):276–281. [DOI] [PubMed] [Google Scholar]
- 35. Yarygin KN, Kholodenko IV, Konieva AA, Burunova VV, Tairova RT, Gubsky LV, Cheglakov IB, Pirogov YA, Yarygin VN, Skvortsova VI. Mechanisms of positive effects of transplantation of human placental mesenchymal stem cells on recovery of rats after experimental ischemic stroke. Bull Exp Biol Med. 2009;148(6):862–868. [DOI] [PubMed] [Google Scholar]
- 36. Loftis JM. Sertoli cell therapy: a novel possible treatment strategy for treatment–resistant major depressive disorder. Med Hypotheses. 2011;77(1):35–42. [DOI] [PubMed] [Google Scholar]
- 37. Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476(7359):224–227. [DOI] [PubMed] [Google Scholar]
- 38. Xu X, Geremia N, Bao F, Pniak A, Rossoni M, Brown A. Schwann cell co-culture improves the therapeutic effect of bone marrow stromal cells on recovery in spinal cord-injured mice. Cell Transplant. 2010;20(7):1065–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwartz M. “Tissue-repairing” blood-derived macrophages are essential for healing of the injured spinal cord: from skin-activated macrophages to infiltrating blood-derived cells? Brain Behav Immun. 2010;24(7):1054–1057. [DOI] [PubMed] [Google Scholar]
- 40. Vaquero J, Zurita M. Functional recovery after severe CNS trauma: current perspectives for cell therapy with bone marrow stromal cells. Prog Neurobiol. 2010;93(3):341–349. [DOI] [PubMed] [Google Scholar]
- 41. Roshal LM, Tzyb AF, Pavlova LN, Soushkevitch GN, Semenova JB, Javoronkov LP, Kolganova OI, Konoplyannikov AG, Shevchuk AS, Yujakov VV, Karaseva OV, Ivanova TF, Chernyshova TA, Konoplyannikova OA, Bandurko LN, Marey MV, Sukhikh GT. Effect of cell therapy on recovery of cognitive functions in rats during the delayed period after brain injury. Bull Exp Biol Med. 2009;148(1):140–147. [DOI] [PubMed] [Google Scholar]
- 42. Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, Hsu JY, Fu YS. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42(7):2045–2053. [DOI] [PubMed] [Google Scholar]
- 43. Chehrehasa F, Windus LC, Ekberg JA, Scott SE, Amaya D, Mackay-Sim A, St John JA. Olfactory glia enhance neonatal axon regeneration. Mol Cell Neurosci. 2010;45(3):277–288. [DOI] [PubMed] [Google Scholar]
- 44. Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31(12):4675–4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nagai N, Kawao N, Okada K, Okumoto K, Teramura T, Ueshima S, Umemura K, Matsuo O. Systemic transplantation of embryonic stem cells accelerates brain lesion decrease and angiogenesis. Neuroreport. 2010;21(8):575–579. [DOI] [PubMed] [Google Scholar]
- 46. Ronaghi M, Erceg S, Moreno-Manzano V, Stojkovic M. Challenges of stem cell therapy for spinal cord injury: human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells. 2010;28(1):93–99. [DOI] [PubMed] [Google Scholar]
- 47. Salehi M, Pasbakhsh P, Soleimani M, Abbasi M, Hasanzadeh G, Modaresi MH, Sobhani A. Repair of spinal cord injury by co-transplantation of embryonic stem cell-derived motor neuron and olfactory ensheathing cell. Iran Biomed J. 2009;13(3):125–135. [PubMed] [Google Scholar]
- 48. Park BW, Kang DH, Kang EJ, Byun JH, Lee JS, Maeng GH, Rho GJ. Peripheral nerve regeneration using autologous porcine skin-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2012;6(2):113–124. [DOI] [PubMed] [Google Scholar]
- 49. Rosenkranz K, Meier C. Umbilical cord blood cell transplantation after brain ischemia—from recovery of function to cellular mechanisms. Ann Anat. 2011;193(4):371–379. [DOI] [PubMed] [Google Scholar]
- 50. Xiong N, Cao X, Zhang Z, Huang J, Chen C, Zhang Z, Jia M, Xiong J, Liang Z, Sun S, Lin Z, Wang T. Long-term efficacy and safety of human umbilical cord mesenchymal stromal cells in rotenone-induced hemiparkinsonian rats. Biol Blood Marrow Transplant. 2010;16(11):1519–1529. [DOI] [PubMed] [Google Scholar]
- 51. Dongmei H, Jing L, Mei X, Ling Z, Hongmin Y, Zhidong W, Li D, Zikuan G, Hengxiang W. Clinical analysis of the treatment of spinocerebellar ataxia and multiple system atrophy-cerebellar type with umbilical cord mesenchymal stromal cells. Cytotherapy. 2011;13(8):913–917. [DOI] [PubMed] [Google Scholar]
- 52. Wang Y, Piao JH, Larsen EC, Kondo Y, Duncan ID. Migration and remyelination by oligodendrocyte progenitor cells transplanted adjacent to focal areas of spinal cord inflammation. J Neurosci Res. 2011;89(11):1737–1746. [DOI] [PubMed] [Google Scholar]
- 53. Xu XX, Shao XM, Yu F, Liu LM, Zhang MX, Gao XL. Effects of tanycytes transplantation on the motor function score and rubrospinal motor evoked potentials of adult rats after spinal cord completely transected. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2010;26(4):433–435. [PubMed] [Google Scholar]
- 54. Michel-Monigadon D, Brachet P, Neveu I, Naveilhan P. Immunoregulatory properties of neural stem cells. Immunotherapy. 2011;3(Suppl 4):39–41. [DOI] [PubMed] [Google Scholar]
- 55. Obenaus A, Dilmac N, Tone B, Tian HR, Hartman R, Digicaylioglu M, Snyder EY, Ashwal S. Long-term magnetic resonance imaging of stem cells in neonatal ischemic injury. Ann Neurol. 2011;69(2):282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eaton MJ, Widerström-Noga E, Wolfe SQ. Subarachnoid transplant of the human neuronal hNT2.19 serotonergic cell line attenuates behavioral hypersensitivity without affecting motor dysfunction after severe contusive spinal cord injury. Neurol Res Int. 2011;2011:891605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Minnerup J, Kim JB, Schmidt A, Diederich K, Bauer H, Schilling M, Strecker JK, Ringelstein EB, Sommer C, Schöler HR, Schäbitz WR. Effects of neural progenitor cells on sensorimotor recovery and endogenous repair mechanisms after photothrombotic stroke. Stroke. 2011;42(6):1757–1763. [DOI] [PubMed] [Google Scholar]
- 58. Wang R, Zhang J, Guo Z, Shen L, Shang A, Chen Y, Yao S, He T, Yin D, Tian J. In-vivo PET imaging of implanted human retinal pigment epithelium cells in a Parkinson’s disease rat model. Nucl Med Commun. 2008;29(5):455–461. [DOI] [PubMed] [Google Scholar]
- 59. Narantuya D, Nagai A, Sheikh AM, Wakabayashi K, Shiota Y, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S. Microglia transplantation attenuates white matter injury in rat chronic ischemia model via matrix metalloproteinase-2 inhibition. Brain Res. 2010;1316:145–152. [DOI] [PubMed] [Google Scholar]
- 60. Davies SJ, Shih CH, Noble M, Mayer-Proschel M, Davies JE, Proschel C. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PLoS One. 2011;6(3):e17328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang X, Song L, Wu N, Liu Z, Xue S, Hui G. An experimental study on intracerebroventricular transplantation of human amniotic epithelial cells in a rat model of Parkinson’s disease. Neurol Res. 2010;32(10):1054–1059. [DOI] [PubMed] [Google Scholar]
- 62. Cipriani S, Bonini D, Marchina E, Balgkouranidou I, Caimi L, Grassi Zucconi G, Barlati S. Mesenchymal cells from human amniotic fluid survive and migrate after transplantation into adult rat brain. Cell Biol Int. 2007;31(8):845–850. [DOI] [PubMed] [Google Scholar]
- 63. Huang H, Chen L. Questions and discussion of clinical cell therapy in Neurorestoratology. Chin J Cell Stem Cell. 2012;2(3):154–159. [Google Scholar]
- 64. Huang H, Mao G, Chen L, Liu A. Progress and challenges with clinical cell therapy in neurorestoratology. J Neurorestoratology. 2015;3:91–95. [Google Scholar]
- 65. Buzanska L, Jurga M, Stachowiak EK, Stachowiak MK, Domanska-Janik K. Neural stem-like cell line derived from a nonhematopoietic population of human umbilical cord blood. Stem Cells Dev. 2006;15(3):391–406. [DOI] [PubMed] [Google Scholar]
- 66. Janowski M, Lukomska B, Domanska-Janik K. Migratory capabilities of human umbilical cord blood-derived neural stem cells (HUCB–NSC) in vitro. Acta Neurobiol Exp. 2011;71(1):24–35. [DOI] [PubMed] [Google Scholar]
- 67. Vertès A. 2010 world stem cell summit—part 2. IDrugs. 2010;13(12):822–824. [PubMed] [Google Scholar]
- 68. Schwarz SC, Schwarz J. Translation of stem cell therapy for neurological diseases. Transl Res. 2010;156(3):155–160. [DOI] [PubMed] [Google Scholar]
- 69. Baker M. Stem-cell pioneer bows out. Nature. 2011;479(7374):459. [DOI] [PubMed] [Google Scholar]
- 70. Reardon S, Cyranoski D. Japan stem-cell trial stirs envy. Nature. 2014;513(7518):287–288. [DOI] [PubMed] [Google Scholar]
- 71. Brown SA, Levi B, Lequeux C, Wong VW, Mojallal A, Longaker MT. Basic science review on adipose tissue for clinicians. Plast Reconstr Surg. 2010;126(6):1936–1946. [DOI] [PubMed] [Google Scholar]
- 72. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 73. Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, Dominici M, Fibbe WE, Gee AP, Gimble JM, Hematti P, Koh MB, LeBlanc K, Martin I, McNiece IK, Mendicino M, Oh S, Ortiz L, Phinney DG, Planat V, Shi Y, Stroncek DF, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang H, Chen L, Wang H, Xiu B, Li B, Wang R, Zhang J, Zhang F, Gu Z, Li Y, Song Y, Hao W, Pang S, Sun J. Influence of patients’ age on functional recovery after transplantation of olfactory ensheathing cells into injured spinal cord injury. Chin Med J. 2003;116(10):1488–1491. [PubMed] [Google Scholar]
- 75. Huang H, Chen L, Xi H, Wang H, Zhang J, Zhang F, Liu Y. Fetal olfactory ensheathing cells transplantation in amyotrophic lateral sclerosis patients: a controlled pilot study. Clin Transplant. 2008;22(60):710–718. [DOI] [PubMed] [Google Scholar]
- 76. Raisman G, Carlstedt T, Choi D, Li Y. Clinical prospects for transplantation of OECs in the repair of brachial and lumbosacral plexus injuries: opening a door. Exp Neurol. 2011;229(1):168–173. [DOI] [PubMed] [Google Scholar]
- 77. Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G, Maia CA, Capucho C, Hasse-Ferreira A, Peduzzi JD. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair. 2010;24(1):10–22. [DOI] [PubMed] [Google Scholar]
- 78. Mackay-Sim A, Féron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, Fronek P, Gray C, Kerr G, Licina P, Nowitzke A, Perry C, Silburn PA, Urquhart S, Geraghty T. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131(Pt 9):2376–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen L, Huang H, Xi H, Xie Z, Liu R, Jiang Z, Zhang F, Liu Y, Chen D, Wang Q, Wang H, Ren Y, Zhou C. Intracranial transplant of olfactory ensheathing cells in children and adolescents with cerebral palsy: a randomized controlled clinical trial. Cell Transplant. 2010;19(2):185–191. [DOI] [PubMed] [Google Scholar]
- 80. Mizuno H. Adipose-derived stem and stromal cells for cell-based therapy: current status of preclinical studies and clinical trials. Curr Opin Mol Ther. 2010;12(4):442–449. [PubMed] [Google Scholar]
- 81. Sanberg PR, Eve DJ, Willing AE, Garbuzova-Davis S, Tan J, Sanberg CD, Allickson JG, Cruz LE, Borlongan CV. The treatment of neurodegenerative disorders using umbilical cord blood and menstrual blood-derived stem cells. Cell Transplant. 2011;20(1):85–94. [DOI] [PubMed] [Google Scholar]
- 82. Richardson RM, Freed CR, Shimamoto SA, Starr PA. Pallidal neuronal discharge in Parkinson’s disease following intraputamenal fetal mesencephalic allograft. J Neurol Neurosurg Psychiatry. 2011;82(3):266–271. [DOI] [PubMed] [Google Scholar]
- 83. Mendez I, Dagher A, Hong M, Gaudet P, Weerasinghe S, McAlister V, King D, Desrosiers J, Darvesh S, Acorn T, Robertson H. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: a pilot study. Report of three cases. J Neurosurg. 2002;96(3):589–596. [DOI] [PubMed] [Google Scholar]
- 84. Liao GP, Harting MT, Hetz RA, Walker PA, Shah SK, Corkins CJ, Hughes TG, Jimenez F, Kosmach SC, Day MC, Tsao K, Lee DA, Worth LL, Baumgartner JE, Cox CS., Jr Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children. Pediatr Crit Care Med. 2015;16(3):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Walker PA, Harting MT, Shah SK, Day MC, El Khoury R, Savitz SI, Baumgartner J, Cox CS. Progenitor cell therapy for the treatment of central nervous system injury: a review of the state of current clinical trials. Stem Cells Int. 2010;2010:369578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Attar A, Ayten M, Ozdemir M, Ozgencil E, Bozkurt M, Kaptanoglu E, Beksac M, Kanpolat Y. An attempt to treat patients who have injured spinal cords with intralesional implantation of concentrated autologous bone marrow cells. Cytotherapy. 2011;13(1):54–60. [DOI] [PubMed] [Google Scholar]
- 88. Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H, Scolding N, Slavin S, Le Blanc K, Uccelli A; MSCT Study Group. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. 2010;16(4):503–510. [DOI] [PubMed] [Google Scholar]
- 89. Forthofer M, Wirth ED III. Coordination of a neural tissue transplantation study in patients with posttraumatic syringomyelia. SCI Nurs. 2001;18(1):19–29. [PubMed] [Google Scholar]
- 90. Yang WZ, Zhang Y, Wu F, Zhang M, Cho SC, Li CZ, Li SH, Shu GJ, Sheng YX, Zhao N, Tang Y, Jiang S, Jiang S, Gandjian M, Ichim TE, Hu X. Human umbilical cord blood-derived mononuclear cell transplantation: case series of 30 subjects with hereditary ataxia. J Transl Med. 2011;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. López-Lozano JJ, Bravo G, Brera B, Dargallo J, Salmeán J, Uría J, Insausti J, Millán I. Long-term follow-up in 10 Parkinson’s disease patients subjected to fetal brain grafting into a cavity in the caudate nucleus: the Clinica Puerta de Hierro experience. CPH Neural Transplantation Group. Transplant Proc. 1995;27(1):1395–1400. [PubMed] [Google Scholar]
- 92. Appel SH, Engelhardt JI, Henkel JS, Siklos L, Beers DR, Yen AA, Simpson EP, Luo Y, Carrum G, Heslop HE, Brenner MK, Popat U. Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology. 2008;71(17):1326–1334. [DOI] [PubMed] [Google Scholar]
- 93. Sharma A, Gokulchandran N, Sane H, Gokulchandran N, Sane H, Badhe P, Kulkarni P, Lohia M, Nagrajan A, Thomas N. Detailed analysis of the clinical effects of cell therapy for thoracolumbar spinal cord injury: an original study. J Neurorestoratology. 2013;1:13–22. [Google Scholar]
- 94. Gong D, Yu H, Wang W, Yang H, Han F. Human umbilical cord blood mononuclear cell transplantation for delayed encephalopathy after carbon monoxide intoxication. J Neurorestoratology. 2013;1:23–29. [Google Scholar]
- 95. Sych N, Klunnik M, Ivankova O, Matyaschuk I, Demchuk M, Novytska A, Arkhipenko I, Shalita I, Siniscalco D. Efficacy of fetal stem cells in Duchenne muscular dystrophy therapy. J Neurorestoratology. 2014;2:37–46. [Google Scholar]
- 96. Tsolaki M, Zygouris S, Tsoutsikas V, Anestakis D, Koliakos G. Treatment with adipose stem cells in a patient with moderate Alzheimer’s disease: case report. J Neurorestoratology. 2015;3:115–120. [Google Scholar]
- 97. Huang H, Sun T, Chen L, Moviglia G, Chernykh E, von Wild K, Deda H, Kang KS, Kumar A, Jeon SR, Zhang S, Brunelli G, Bohbot A, Soler MD, Li J, Cristante AF, Xi H, Onose G, Kern H, Carraro U, Saberi H, Sharma HS, Sharma A, et al. Consensus of clinical neurorestorative progress in patients with complete chronic spinal cord injury. Cell Transplant. 2014;23(Suppl 1):S5–S17. [DOI] [PubMed] [Google Scholar]
- 98. Qiao L, Huang H, Muresanu DF. Clinical neurorestorative progress in Alzheimer’s disease. J Neurorestoratology. 2015;3:1–9. [Google Scholar]
- 99. Huang H, Chen L, Huang H. Clinical neurorestorative progress in traumatic brain injury. J Neurorestoratology. 2015;3:57–62. [Google Scholar]
- 100. Qiao L, Lu J, Huang H. Clinical neurorestorative progress in stroke. J Neurorestoratology. 2015;3:63–71. [Google Scholar]
- 101. Geng TC, Mark VW. Clinical neurorestorative progress in multiple sclerosis. J Neurorestoratology. 2015;3:83–90. [Google Scholar]
- 102. Chen L, Huang H, Duan WM, Mao GS. Clinical neurorestorative progress in Parkinson’s disease. J Neurorestoratology. 2015;3:101–107. [Google Scholar]
- 103. Chen L, Huang H, Xi H, Mao G. Clinical neurorestorative progress in amyotrophic lateral sclerosis. J Neurorestoratology. 2015;3:109–114. [Google Scholar]
- 104. Sharma A, Geng T, Sane H, Kulkarni P. Clinical neurorestorative progresses in cerebral palsy. J Neurorestoratology. 2017;5:51–57. [Google Scholar]
- 105. Moviglia GA, Varela G, Brizuela JA, Moviglia Brandolino MT, Farina P, Etchegaray G, Piccone S, Hirsch J, Martinez G, Marino S, Deffain S, Coria N, Gonzáles A, Sztanko M, Salas–Zamora P, Previgliano I, Aingel V, Farias J, Gaeta CA, Saslavsky J, Blasseti N. Case report on the clinical results of a combined cellular therapy for chronic spinal cord injured patients. Spinal Cord. 2009;47(6):499–503. [DOI] [PubMed] [Google Scholar]
- 106. Moviglia GA, Moviglia-Brandolino MT, Varela GS, Albanese G, Piccone S, Echegaray G, Martinez G, Blasseti N, Farias J, Farina P, Perusso A, Gaeta CA. Feasibility, safety, and preliminary proof of principles of autologous neural stem cell treatment combined with T-cell vaccination for ALS patients. Cell Transplant. 2012;21(Suppl 1):S57–S63. [DOI] [PubMed] [Google Scholar]
- 107. Huang H, Xi H, Chen L, Zhang F, Liu Y. Long-term outcome of olfactory ensheathing cell therapy for patients with complete chronic spinal cord injury. Cell Transplant. 2012;21(Suppl 1):S23–S31. [DOI] [PubMed] [Google Scholar]
- 108. Tabakow P, Jarmundowicz W, Czapiga B, Fortuna W, Miedzybrodzki R, Czyz M, Huber J, Szarek D, Okurowski S, Szewczyk P, Gorski A, Raisman G. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013;22(9):1591–1612. [DOI] [PubMed] [Google Scholar]
- 109. Tabakow P, Raisman G, Fortuna W, Czyz M, Huber J, Li D, Szewczyk P, Okurowski S, Miedzybrodzki R, Czapiga B, Salomon B, Halon A, Li Y, Lipiec J, Kulczyk A, Jarmundowicz W. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014;23(12):1631–1655. [DOI] [PubMed] [Google Scholar]
- 110. Riley J, Glass J, Feldman EL, Polak M, Bordeau J, Federici T, Johe K, Boulis NM. Intraspinal Stem Cell Transplantation in ALS: a phase I trial, cervical microinjection and final surgical safety outcomes. Neurosurgery. 2012;71(2):405–416. [DOI] [PubMed] [Google Scholar]
- 111. Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, Polak M, Bordeau J, Sakowski SA, Glass JD. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75(3):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, Mareschi K, Testa L, Stecco A, Tarletti R, Miglioretti M, Fava E, Nasuelli N, Cisari C, Massara M, Vercelli R, Oggioni GD, Carriero A, Cantello R, Monaco F, Fagioli F. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol. 2010;223(1):229–237. [DOI] [PubMed] [Google Scholar]
- 113. Mazzini L, Gelati M, Profico DC, Sgaravizzi G, Projetti Pensi M, Muzi G, Ricciolini C, Rota Nodari L, Carletti S, Giorgi C, Spera C, Domenico F, Bersano E, Petruzzelli F, Cisari C, Maglione A, Sarnelli MF, Stecco A, Querin G, Masiero S, Cantello R, Ferrari D, et al. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med. 2015;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Seledtsova GV, Rabinovich SS, Belogorodtsev SN, Parlyuk OV, Seledtsov VI, Kozlov VA. Delayed results of transplantation of fetal neurogenic tissue in patients with consequences of spinal cord trauma. Bull Exp Biol Med. 2010;149(4):530–533. [DOI] [PubMed] [Google Scholar]
- 115. Gobbel GT, Kondziolka D, Fellows-Mayle W, Uram M. Cellular transplantation for the nervous system: impact of time after preparation on cell viability and survival. J Neurosurg. 2010;113(3):666–672. [DOI] [PubMed] [Google Scholar]
- 116. Savitz SI, Dinsmore J, Wu J, Henderson GV, Stieg P, Caplan LR. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovasc Dis. 2005;20(2):101–107. [DOI] [PubMed] [Google Scholar]
- 117. Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, Elder EM, Zhang PJ, Jacobs A, McGrogan M, Lee VM, Trojanowski JQ. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160(4):1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Huang H, Chen L, Xi H, Wang Q, Zhang J, Liu Y, Zhang F. Olfactory ensheathing cells transplantation for central nervous system diseases in 1,255 patients. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(1):14–20. [PubMed] [Google Scholar]
- 119. Syková E, Rychmach P, Drahorádová I, Konrádová Š, Růžičková K, Voříšek I, Forostyak S, Homola A, Bojar M. Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transplant. 2017;26(4):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mehta T, Feroz A, Thakkar U, Vanikar A, Shah V, Trivedi H. Subarachnoid placement of stem cells in neurological disorders. Transplant Proc. 2008;40(4):1145–1147. [DOI] [PubMed] [Google Scholar]
- 121. Battistella V, de Freitas GR, da Fonseca LM, Mercante D, Gutfilen B, Goldenberg RC, Dias JV, Kasai-Brunswick TH, Wajnberg E, Rosado-de-Castro PH, Alves-Leon SV, Mendez-Otero R, Andre C. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med. 2011;6(1):45–52. [DOI] [PubMed] [Google Scholar]
- 122. Friedrich MA, Martins MP, Araújo MD, Klamt C, Vedolin L, Garicochea B, Raupp EF, Sartori El Ammar J, Machado DC, Costa JC, Nogueira RG, Rosado-de-Castro PH, Mendez-Otero R, Freitas GR. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012;21(Suppl 1):S13–S21. [DOI] [PubMed] [Google Scholar]
- 123. Moviglia GA, Fernandez Viña R, Brizuela JA, Saslavsky J, Vrsalovic F, Varela G, Bastos F, Farina P, Etchegaray G, Barbieri M, Martinez G, Picasso F, Schmidt Y, Brizuela P, Gaeta CA, Costanzo H, Moviglia Brandolino MT, Merino S, Pes ME, Veloso MJ, Rugilo C, et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy. 2006;8(3):202–209. [DOI] [PubMed] [Google Scholar]
- 124. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY; STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem cells. 2010;28(6):1099–1106. [DOI] [PubMed] [Google Scholar]
- 125. Chen L, Huang HY, Jiang Z, Wang YC, Wang QM, Xi HT, Bao JL, Wang HM. Electromyogram evaluation in 389 patients with amyotrophic lateral sclerosis following olfactory ensheathing cell intracranial transplantation. J Clin Rehabil Tissue Eng Res. 2008;12(43):8422–8425. [Google Scholar]
- 126. Luan Z, Liu W, Qu S, Du K, He S, Wang Z, Yang Y, Wang C, Gong X. Effects of neural progenitor cell transplantation in children with severe cerebral palsy. Cell Transplant. 2012;21(Suppl 1):S91–S98. [DOI] [PubMed] [Google Scholar]
- 127. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. [DOI] [PubMed] [Google Scholar]
- 128. Chen L, Xi H, Huang H, Zhang F, Liu Y, Chen D, Xiao J. Multiple cell transplantation based on an intraparenchymal approach for patients with chronic phase stroke. Cell Transplant. 2013;22 (Suppl 1):S83–S91. [DOI] [PubMed] [Google Scholar]
- 129. Xi H, Chen L, Huang H, Zhang F, Liu Y, Chen D, Xiao J. Preliminary report of multiple cell therapy for patients with multiple system atrophy. Cell Transplant. 2013;22(Suppl 1):S93–S99. [DOI] [PubMed] [Google Scholar]
- 130. Chen L, Huang H, Xi H, Zhang F, Liu Y, Chen D, Xiao J. A prospective randomized double–blind clinical Ttial using a combination of olfactory ensheathing cells and Schwann cells for the treatment of chronic complete spinal cord injuries. Cell Transplant. 2014;23(Suppl 1):S35–S44. [DOI] [PubMed] [Google Scholar]
- 131. Wang S, Cheng H, Dai G, Wang X, Hua R, Liu X, Wang P, Chen G, Yue W, An Y. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013;1532:76–84. [DOI] [PubMed] [Google Scholar]
- 132. Li JF, Zhang DJ, Geng T, Chen L, Huang H, Yin HL, Zhang YZ, Lou JY, Cao B, Wang YL. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014;23(Suppl 1):S113–S122. [DOI] [PubMed] [Google Scholar]
- 133. Wang X, Hu H, Hua R, Yang J, Zheng P, Niu X, Cheng H, Dai G, Liu X, Zhang Z, An Y. Effect of umbilical cord mesenchymal stromal cells on motor functions of identical twins with cerebral palsy: pilot study on the correlation of efficacy and hereditary factors. Cytotherapy. 2015;17(2):224–231. [DOI] [PubMed] [Google Scholar]
- 134. Li P, Cui K, Zhang B, Wang Z, Shen Y, Wang X, Zhang J, Tong F, Li S. Transplantation of human umbilical cord-derived mesenchymal stems cells for the treatment of Becker muscular dystrophy in affected pedigree members. Int J Mol Med. 2015;35(4):1051–1057. [DOI] [PubMed] [Google Scholar]
- 135. Zhu H, Poon W, Liu Y, Leung GK, Wong Y, Feng Y, Ng SC, Tsang KS, Sun DT, Yeung DK, Shen C, Niu F, Xu Z, Tan P, Tang S, Gao H, Cha Y, So KF, Fleischaker R, Sun D, Chen J, Lai J, et al. Phase I-II Clinical trial assessing safety and efficacy of umbilical cord blood mononuclear cell transplant therapy of chronic complete spinal cord injury. Cell Transplant. 2016;25(11):1925–1943. [DOI] [PubMed] [Google Scholar]
- 136. Chen L, Chen D, Xi H, Wang Q, Liu Y, Zhang F, Wang H, Ren Y, Xiao J, Wang Y, Huang H, Olfactory ensheathing cell neurorestorotherapy for amyotrophic lateral sclerosis patients: benefits from multiple transplantations. Cell Transplant. 2012;21(Suppl 1):S65–S77. [DOI] [PubMed] [Google Scholar]
- 137. Li Y, Chen L, Zhao Y, Bao J, Xiao J, Liu J, Jiang X, Zhou C, Wang H, Huang H. Intracranial transplant of olfactory ensheathing cells can protect both upper and lower motor neurons in amyotrophic lateral sclerosis. Cell Transplant. 2013;22(Suppl 1):S51–S65. [DOI] [PubMed] [Google Scholar]
- 138. Huang H, Raisman G, Sanberg PR, Sharma H, Chen L. Neurorestoratology. New York (NY): Nova Biomedical; 2015;V1:93–102. [Google Scholar]
- 139. Huang H. CNS neurorestoratology. Beijing, China: Science Press; 2009. [Google Scholar]
- 140. Siniscalco D, Sych N. Stem cell transplantation for nervous system disorders in Italy, European Union, and Ukraine: Clinical approach and governmental policies. Transl Neurosci Clin. 2015;1(2):125–127. [Google Scholar]
- 141. Zhou Q, Zhang SZ, Xu RX, Xu K. Neural stem cell transplantation and postoperative management: report of 70 cases. Di Yi Jun Yi Da Xue Xue Bao. 2004;24(10):1207–1209. [PubMed] [Google Scholar]
- 142. Guzman R, Schubert M, Keller-Lang D, Huhn SL, Curt A. Human neural stem cell transplantation in chronic SCI: interim results of a phase I/II trial. Neurosurgery. 2013;60(Suppl 1):185. [Google Scholar]
- 143. Shin JC, Kim KN, Yoo J, Kim IS, Yun S, Lee H, Jung K, Hwang K, Kim M, Lee IS, Shin JE, Park KI. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015;2015:630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Curtis E, Gabel BC, Marsala M, Ciacci JD. A phase I, open-label, single-site, safety study of human spinal cord-derived neural stem cell transplantation for the treatment of chronic spinal cord injury. Neurosurgery. 2016;63(Suppl 1):168–169. [Google Scholar]
- 145. Knoller N, Auerbach G, Fulga V, Zelig G, Attias J, Bakimer R, Marder JB, Yoles E, Belkin M, Schwartz M, Hadani M. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine. 2005;3(3):173–181. [DOI] [PubMed] [Google Scholar]
- 146. Callera F, do Nascimento RX. Delivery of autologous bone marrow precursor cells into the spinal cord via lumbar puncture technique in patients with spinal cord injury: a preliminary safety study. Exp Hematol. 2006;34(2):130–131. [DOI] [PubMed] [Google Scholar]
- 147. Syková E, Homola A, Mazanec R, Lachmann H, Konrádová SL, Kobylka P, Pádr R, Neuwirth J, Komrska V, Vávra V, Stulík J, Bojar M. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15(8–9):675–687. [DOI] [PubMed] [Google Scholar]
- 148. Chernykh ER, Stupak VV, Muradov GM, Sizikov MY, Shevela EY, Leplina OY, Tikhonova MA, Kulagin AD, Lisukov IA, Ostanin AA, Kozlov VA. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull Exp Biol Med. 2007;143(4):543–547. [DOI] [PubMed] [Google Scholar]
- 149. Deda H, Inci MC, Kürekçi AE, Kayihan K, Ozgün E, Ustünsoy GE, Kocabay S. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy. 2008;10(6):565–574. [DOI] [PubMed] [Google Scholar]
- 150. Stokic DS, Curt A. Stem cells in the treatment of chronic spinal cord injury: evaluation of somatosensitive-evoked potentials in 39 patients. Spinal Cord. 2010;48(8):649. [DOI] [PubMed] [Google Scholar]
- 151. Jones LA, Lammertse DP, Charlifue SB, Kirshblum SC, Apple DF, Ragnarsson KT, Poonian D, Betz RR, Knoller N, Heary RF, Choudhri TF, Jenkins AL, 3rd, Falci SP, Snyder DA. A phase 2 autologous cellular therapy trial in patients with acute, complete spinal cord injury: pragmatics, recruitment, and demographics. Spinal Cord. 2010;48(11):798–807. [DOI] [PubMed] [Google Scholar]
- 152. Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007;16(3):461–466. [DOI] [PubMed] [Google Scholar]
- 153. Syková E, Jendelová P, Urdzíková L, Lesný P, Hejcl A. Bone marrow stem cells and polymer hydrogels-two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26(7–8):1113–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kishk NA, Gabr H, Hamdy S, Afifi L, Abokresha N, Mahmoud H, Wafaie A, Bilal D. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 2010;24(8):702–708. [DOI] [PubMed] [Google Scholar]
- 155. Bhanot Y, Rao S, Ghosh D, Balaraju S, Radhika CR, Satish Kumar KV. Autologous mesenchymal stem cells in chronic spinal cord injury. Br J Neurosurg. 2011;25(4):516–522. [DOI] [PubMed] [Google Scholar]
- 156. Park JH, Kim DY, Sung IY, Choi GH, Jeon MH, Kim KK, Jeon SR. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70(5):1238–1247. [DOI] [PubMed] [Google Scholar]
- 157. Saito F, Nakatani T, Iwase M, Maeda Y, Murao Y, Suzuki Y, Fukushima M, Ide C. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor Neurol Neurosci. 2012;30(2):127–136. [DOI] [PubMed] [Google Scholar]
- 158. Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114(7):935–939. [DOI] [PubMed] [Google Scholar]
- 159. Frolov AA, Bryukhovetskiy AS. Effects of haematopoietic autologous stem cell transplantation to the chronically injured human spinal cord evaluated by motor and somatosensory evoked potentials methods. Cell Transplant. 2012;21(Suppl 1):S49–S55. [DOI] [PubMed] [Google Scholar]
- 160. Geffner LF, Santacruz P, Izurieta M, Flor L, Maldonado B, Auad AH, Montenegro X, Gonzalez R, Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant. 2008;17(12):1277–1293. [DOI] [PubMed] [Google Scholar]
- 161. Kumar AA, Kumar SR, Narayanan R, Arul K, Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: a phase I/II clinical safety and primary efficacy data. Exp Clin Transplant. 2009;7(4):241–248. [PubMed] [Google Scholar]
- 162. Jiang PC, Xiong WP, Wang G, Ma C, Yao WQ, Kendell SF, Mehling BM, Yuan XH, Wu DC. A clinical trial report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp Ther Med. 2013;6(1):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Dai G, Liu X, Zhang Z, Yang Z, Dai Y, Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. [DOI] [PubMed] [Google Scholar]
- 164. Mendonça MV, Larocca TF, Souza BS, Villarreal CF, Silva LF, Matos AC, Novaes MA, Bahia CM, Martinez AC, Kaneto CM, Furtado SB, Sampaio GP, Soares MB, Dos Santos RR. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5(6):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Montilla J, Bustamante S, Carballido J, Marin E, Martinez F, Parajon A, Fernandez C, Reina LD; Neurological Cell Therapy Group. An approach to personalized cell therapy in chronic complete paraplegia: the Puerta de Hierro phase I/II clinical trial. Cytotherapy. 2016;18(8):1025–1036. [DOI] [PubMed] [Google Scholar]
- 166. Kakabadze Z, Kipshidze N, Mardaleishvili K, Chutkerashvili G, Chelishvili I, Harders A, Loladze G, Shatirishvili G, Kipshidze N, Chakhunashvili D, Chutkerashvili K. Phase 1 trial of autologous bone marrow stem cell transplantation in patients with spinal cord injury. Stem Cells Int. 2016;2016:6768274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Satti HS, Waheed A, Ahmed P, Ahmed K, Akram Z, Aziz T, Satti TM, Shahbaz N, Khan MA, Malik SA. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a phase I pilot study. Cytotherapy. 2016;18(4):518–522. [DOI] [PubMed] [Google Scholar]
- 168. Chhabra HS, Sarda K, Arora M, Sharawat R, Singh V, Nanda A, Sangodimath GM, Tandon V. Autologous bone marrow cell transplantation in acute spinal cord injury-an Indian pilot study. Spinal Cord. 2016;54(1):57–64. [DOI] [PubMed] [Google Scholar]
- 169. Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH, Rahimi-Movaghar V, Raza M, Firouzi M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes. Neurosci Lett. 2008;443(1):46–50. [DOI] [PubMed] [Google Scholar]
- 170. Saberi H, Firouzi M, Habibi Z, Moshayedi P, Aghayan HR, Arjmand B, Hosseini K, Razavi HE, Yekaninejad MS. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J Neurosurg Spine. 2011;15(5):515–525. [DOI] [PubMed] [Google Scholar]
- 171. Zhou XH, Ning GZ, Feng SQ, Kong XH, Chen JT, Zheng YF, Ban DX, Liu T, Li H, Wang P. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow-up. Cell Transplant. 2012;21(Suppl 1):S39–S47. [DOI] [PubMed] [Google Scholar]
- 172. Cristante AF, Barros-Filho TE, Tatsui N, Mendrone A, Caldas JG, Camargo A, Alexandre A, Teixeira WG, Oliveira RP, Marcon RM. Stem cells in the treatment of chronic spinal cord injury: evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord. 2009;47(10):733–738. [DOI] [PubMed] [Google Scholar]
- 173. Al-Zoubi A, Jafar E, Jamous M, Al-Twal F, Al-Bakheet S, Zalloum M, Khalifeh F, Radi SA, El-Khateeb M, Al-Zoubi Z. Transplantation of purified autologous leukapheresis-derived CD34+ and CD133+ stem cells for patients with chronic spinal cord injuries: long-term evaluation of safety and efficacy.Cell Transplant. 2014;23(Suppl 1):S25–S34. [DOI] [PubMed] [Google Scholar]
- 174. Bryukhovetskiy AS, Bryukhovetskiy IS. Effectiveness of repeated transplantations of hematopoietic stem cells in spinal cord injury. World J Transplant. 2015;5(3):110–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20(8):1297–1308. [DOI] [PubMed] [Google Scholar]
- 176. Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39(6):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Liu J, Han D, Wang Z, Xue M, Zhu L, Yan H, Zheng X, Guo Z, Wang H. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15(2):185–191. [DOI] [PubMed] [Google Scholar]
- 178. Hua R, Li P, Wang X, Yang J, Zheng P, Niu X, Li Y, An Y. Evaluation of somatosensory evoked potential and pain rating index in a patient with spinal cord injury accepted cell therapy. Pain Physician. 2016;19(4):E659–E666. [PubMed] [Google Scholar]
- 179. Shroff G, Gupta R. Human embryonic stem cells in the treatment of patients with spinal cord injury. Ann Neurosci. 2015;22(4):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Shroff G. Human embryonic stem cell therapy in chronic spinal cord injury: a retrospective study. Clin Transl Sci. 2016;9(3):168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. El–Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA, El Maadawi ZM, Ewes I, Sabaawy HE. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23(6):729–745. [DOI] [PubMed] [Google Scholar]
- 182. Yazdani SO, Hafizi M, Zali AR, Atashi A, Ashrafi F, Seddighi AS, Soleimani M. Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy. 2013;15(7):782–791. [DOI] [PubMed] [Google Scholar]
- 183. Goni VG, Chhabra R, Gupta A, Marwaha N, Dhillon MS, Pebam S, Gopinathan NR, Bangalore Kantharajanna S. Safety profile, feasibility and early clinical outcome of cotransplantation of olfactory mucosa and bone marrow stem cells in chronic spinal cord injury patients. Asian Spine J. 2014;8(4):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Xi HT, Chen D. Cell-based neurorestorative therapy for postpoliomyelitis syndrome: a case report. J Neurorestoratology. 2016;4:45–50. [Google Scholar]
- 185. Chen L, Xi HT, Xiao J, Zhang F, Chen D, Huang HY. Chromaffin cell transplantation for neuropathic pain after spinal cord injury: a report of two cases. J Neurorestoratology. 2017;5:47–50. [Google Scholar]
- 186. Zhang F, Meng XZ, Lu F, Liu AX, Huang HY. Olfactory ensheathing cell transplantation for a patient with chronic sciatic nerve injury. J Neurorestoratology. 2017;5:1–4. [Google Scholar]
- 187. Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388(10046):787–796. [DOI] [PubMed] [Google Scholar]
- 188. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47(7):1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Chernykh ER, Shevela EY, Starostina NM, Morozov SA, Davydova MN, Menyaeva EV, Ostanin AA. Safety and therapeutic potential of M2 macrophages in stroke treatment. Cell Transplant. 2016;25(8):1461–1471. [DOI] [PubMed] [Google Scholar]
- 190. Kakabadze Z, Kipshidze N, Mardaleishvili K, Chutkerashvili G, Chelishvili I, Harders A, Loladze G, Shatirishvili G, Kipshidze N, Chakhunashvili D, Chutkerashvili K. Phase 1 trial of autologous bone marrow stem cell transplantation in patients with spinal cord injury. Stem Cells Int. 2016;2016:6768274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Montilla J, Bustamante S, Carballido J, Marin E, Martinez F, Parajon A, Fernandez C, Reina LD; Neurological Cell Therapy Group. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy. 2016;18(8):1025–1036. [DOI] [PubMed] [Google Scholar]
- 192. Thakkar UG, Vanikar AV, Trivedi HL, Shah VR, Dave SD, Dixit SB, Tiwari BB, Shah HH. Infusion of autologous adipose tissue derived neuronal differentiated mesenchymal stem cells and hematopoietic stem cells in post-traumatic paraplegia offers a viable therapeutic approach. Adv Biomed Res. 2016;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Wang S, Lu J, Li YA, Zhou H, Ni WF, Zhang XL, Zhu SP, Chen BB, Xu H, Wang XY, Xiao J, Huang H, Chi YL, Xu HZ. Autologous olfactory lamina propria transplantation for chronic spinal cord injury: three-year follow-up outcomes from a prospective double-blinded clinical trial. Cell Transplant. 2016;25(1):141–157. [DOI] [PubMed] [Google Scholar]
- 194. Nafissi S, Kazemi H, Tiraihi T, Beladi-Moghadam N, Faghihzadeh S, Faghihzadeh E, Yadegarynia D, Sadeghi M, Chamani-Tabriz L, Khanfakhraei A, Taheri T. Intraspinal delivery of bone marrow stromal cell-derived neural stem cells in patients with amyotrophic lateral sclerosis: a safety and feasibility study. J Neurol Sci. 2016;362:174–181. [DOI] [PubMed] [Google Scholar]
- 195. Petrou P, Gothelf Y, Argov Z, Gotkine M, Levy YS, Kassis I, Vaknin-Dembinsky A, Ben-Hur T, Offen D, Abramsky O, Melamed E, Karussis D. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016;73(3):337–344. [DOI] [PubMed] [Google Scholar]
- 196. Atkins HL, Bowman M, Allan D, Anstee G, Arnold DL, Bar-Or A, Bence-Bruckler I, Birch P, Bredeson C, Chen J, Fergusson D, Halpenny M, Hamelin L, Huebsch L, Hutton B, Laneuville P, Lapierre Y, Lee H, Martin L, McDiarmid S, O’Connor P, Ramsay T, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388(10044):576–585. [DOI] [PubMed] [Google Scholar]
- 197. Canesi M, Giordano R, Lazzari L, Isalberti M, Isaias IU, Benti R, Rampini P, Marotta G, Colombo A, Cereda E, Dipaola M, Montemurro T, Viganò M, Budelli S, Montelatici E, Lavazza C, Cortelezzi A, Pezzoli G. Finding a new therapeutic approach for no–option Parkinsonisms: mesenchymal stromal cells for progressive supranuclear palsy. J Transl Med. 2016;14(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Cox CS, Jr, Hetz RA, Liao GP, Aertker BM, Ewing-Cobbs L, Juranek J, Savitz SI, Jackson ML, Romanowska-Pawliczek AM, Triolo F, Dash PK, Pedroza C, Lee DA, Worth L, Aisiku IP, Choi HA, Holcomb JB, Kitagawa RS. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells. 2017;35(4):1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Vaquero J, Zurita M, Bonilla C, Fernández C, Rubio JJ, Mucientes J, Rodriguez B, Blanco E, Donis L. Progressive increase in brain glucose metabolism after intrathecal administration of autologous mesenchymal stromal cells in patients with diffuse axonal injury. Cytotherapy. 2017;19(1):88–94. [DOI] [PubMed] [Google Scholar]
- 200. Bradstreet JJ, Sych N, Antonucci N, Klunnik M, Ivankova O, Matyashchuk I, Demchuk M, Siniscalco D. Efficacy of fetal stem cell transplantation in autism spectrum disorders: an open-labeled pilot study. Cell Transplant. 2014;23(Suppl 1):S105–S112. [DOI] [PubMed] [Google Scholar]
- 201. Yang H, Zhang Y, Wang ZY, Lu W, Liu F, Yu X, Zheng XY, Yang YX, Luan Z, Qu SQ. Combined transplantation of neural precursor cells and olfactory ensheathing cells for the treatment of X-linked adrenoleukodystrophy in children. J Neurorestoratology. 2017;5:93–101. [Google Scholar]
- 202. Huang H, Mao G, Feng S, Chen L. 2016 yearbook of neurorestoratology. J Neurorestoratology. 2017;5:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]