Abstract
It is extremely challenging to achieve strong adhesion in soft tissues while minimizing toxicity, tissue damage, and other side effects caused by wound sealing materials. In this study, flexible synthetic hydrogel sealants were prepared based on polyethylene glycol (PEG) materials. PEG is a synthetic material that is nontoxic and inert and, thus, suitable for use in medical products. We evaluated the in vitro biocompatibility tests of the dressings to assess cytotoxicity and irritation, sensitization, pyrogen toxicity, and systemic toxicity following the International Organization for Standardization 10993 standards and the in vivo effects of the hydrogel samples using Coloskin liquid bandages as control samples for potential in wound closure.
Keywords: hydrogel, polyethylene glycol, wound closure, biocompatibility
Introduction
One way to treat wounds is to apply a dressing, and conventional dressings such as gauze and cotton are frequently used. Over the past 2 decades, modern dressings have been used which provide a humid environment for the healing of the wound1,2.
Wound sealants can be made using natural or synthetic polymers or a combination of both. The market for surgical sealants and hemostats is growing rapidly, increasing from $4 (USA) billion in 2012 to $7 billion in 2017 worldwide3. Although several tissue adhesives are commercially available, none of them are ideal for use as a tissue sealant for wound repair4.
The currently available technologies for reconnecting and sealing tissues after surgical procedures, such as sutures, wires, and staples, have several limitations, especially in minimally invasive procedures5,6. For example, the use of sutures for wound closure is time-consuming, may cause further tissue damage, may result in infection, and does not provide immediate sealing to prevent leakage of bodily fluids or exposure to air. The application of adhesives is a convenient alternative method for wound closure because of the simple implementation procedure, shorter healing time, less pain for patients, and lack of need for removal7. It is extremely challenging to achieve strong adhesion in soft tissues while minimizing toxicity, tissue damage, and other side effects caused by the sealing materials. Another limitation is the low adhesion strength of most commercially available sealants in wet environments due to the presence of bodily fluids and the highly dynamic areas of the body5. Most of the clinically available glues and sealants do not offer both elasticity and good adhesion. For example, cyanoacrylates have high stiffness and adhesion strength but are not elastic and are toxic8. On the other hand, fibrin-based sealants are more flexible but have low stiffness and adhesion strength9. There is an unmet need for tissue sealants that can provide an effective barrier for bodily fluids with flexibility and without compromising strength10.
Recently, extensive research efforts have been made to engineer biocompatible, biodegradable, and flexible sealants that can form leak-free closures in soft tissues6,11–13. The sealant materials must be elastic and compliant to allow normal functioning and movement of elastic native tissues, such as blood flow and the tissues surrounding the wound.
In this study, flexible synthetic hydrogel sealants were prepared based on polyethylene glycol (PEG) materials. PEG is a synthetic material that is nontoxic and inert and, thus, suitable for use in medical products10. All samples were characterized and assessed in swelling studies to evaluate their performance as wound sealants.
In vitro biocompatibility tests of the dressings were performed to assess cytotoxicity and irritation, sensitization, pyrogen toxicity, and systemic toxicity following the International Organization for Standardization (ISO) 10993 standards for safety in wound closure14. We also evaluated the in vivo effects of the hydrogel samples using Coloskin liquid bandages as control samples.
Materials and Methods
Materials
PEG (M.W. 35,000) was purchased from Sigma-Aldrich (St. Louis, MO, USA). First, aqueous PEG solutions were prepared, and then, homogeneous solutions were obtained by dissolving and mixing the materials at a constant temperature. The solutions were poured into molds, and after the solutions cooled down, they formed a gel structure with high viscosity. Finally, the samples were sterilized under an appropriate dose of radiation. To assess the wound-healing effects of the hydrogel samples, the results were compared with those of the Coloskin liquid bandages (Tokyokoshisha Co., Ltd., Tokyo, Japan).
Swelling Studies and Degradation Analysis
The samples were placed in a vacuum oven at 37 °C for 24 h, and then their apparent dry weights (Wd) were measured. The samples were then placed in distilled water to determine their water uptake (Ws) after drying at 37 °C.
Mass loss was measured using a balance. At each time point, the samples were weighed after drying, and mass loss was calculated by comparing the initial mass with that at a given time point. Measurements were performed while maintaining the samples at 3 temperatures, 37 °C, 50 °C, and 65 °C, and the results are presented as the mean.
Cytotoxicity Analysis and 3-(4,5-Dimethyl-Thiazol-2-yl)-2,5-Diphenyltetrazolium (MTT) Assay
The cytotoxicity analysis was performed according to ISO 10993-5:2009. Using a mouse fibroblast cell line (L929; Bioresources Collection and Research Center, Hsin Chu, Taiwan), qualitative measures of cell morphology and monolayer confluency were scored under a microscope. Cryopreserved L929 cells were thawed and cultured in minimum essential media/alpha modification medium (Invitrogen, Carlsbad, CA, USA) at 37 ± 1 °C in a 5% CO2 atmosphere. When the cell monolayer reached 80% confluency as assessed with a microscope, the cells were subcultured until reaching passage 2 or 3 prior to use. The culture medium was replaced twice a week. According to ISO 10993-12, the surface ratio of the test compound/culture medium was 0.2 g/1 mL. Accordingly, 3.6 g of each test sample (S) was extracted with 18 mL of culture medium at a rotation speed of 1,000g at 37 ± 1 °C for 24 ± 2 h. A 10% (v/v) solution of dimethyl sulfoxide (DMSO Sigma-Aldrich) prepared using culture medium was used as the positive control (PC). Based on ISO 10993-12, the extraction ratio was 0.2 g/mL, and 1.0 g of high-density polyethylene (Sigma-Aldrich) was used as a negative control (NC). Each sample was immersed in 5 mL of culture medium and extracted at a rotation speed of 1,000g at 37 ± 1 °C for 24 ± 2 h. In addition, 5 mL of culture medium was used as a blank (B) and then incubated at a rotation speed of 100 rpm at 37 ± 1°C for 24 ± 2 h.
The MTT (Sigma-Aldrich) assay was performed to quantitatively assess the cytotoxicity of the test sample extracts. For the assay, 5 × 104 L929 mouse fibroblast cells were seeded in 24-well culture plates and then incubated at 37 ± 1 °C in a 5% CO2 atmosphere for 24 ± 2 h to obtain confluent cell monolayers. After cell attachment, the original culture medium in each well was removed and replaced with 0.5 mL of the respective test medium (B, PC, NC, and S). Then, the test plates were incubated at 37 ± 1 °C in a 5% CO2 atmosphere for 24 ± 2 h. The plate was incubated for 24 h. After 24 h, the cells in each well were stained with neutral red solution (Sigma-Aldrich) and scored according to the change in cell morphology and viability under an inverted microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany) in accordance with the criteria in Table 1. At the end of the incubation period, 10 μL of MTT reagent was added to each well, which contained 100 μL of medium. The reaction was performed at 37 °C in a 5% CO2 atmosphere for 2 h in the dark. Then, 0.1 mL of detergent reagent (Sigma-Aldrich) was added to each well followed by incubation in the dark for 2 h, after which the absorbance was measured. The absorbance of test samples was measured at 570 nm (reference wavelength: 630 nm) with a microplate reader (Molecular Devices, Silicon Valley, CA, USA).
Table 1.
Cytotoxicity Evaluated Using Neutral Red Stain.
| Test Item | Cell Lysis (%) | Grade |
|---|---|---|
| Blank (B) | 0 | 0 |
| Negative control (NC) | 1 | 0 |
| Positive control (PC) | 100 | 4 |
| Test sample (S) | 5 | 0 |
Skin Sensitization Study
For the study to comply with the “Good Laboratory Practice for Nonclinical Laboratory Study,” quality assurance unit audited the facility, equipment, personnel, test methods, raw data, and records regularly. All animal experiments were conducted according to a protocol approved by Master Laboratory CO., Ltd (IACUC No.: MS20160706, Hsinchu County, Taiwan, People’s Republic of China).
The skin sensitization potential of the PEG-based sealant extracts was tested on guinea pigs (body weights [BWs; gender]: 300 to 500 g [male]; age around 85 d, from the National Laboratory Animal Center, Taiwan) following ISO 10993-10: 2010. After treatment with the test compound (polar and nonpolar extracts), the extracts were applied twice in the induction phase and once in the challenge phase. Then, 24 h and 48 h after the challenge phase, the treated areas were assessed for visible changes according to the criteria of the Magnusson and Kligman scale, ISO 10993-10.
Intracutaneous Irritation Study
Intracutaneous irritation in response to the PEG-based sealant extracts was assessed in New Zealand White rabbits (BWs [gender]: >2 kg [male]; age around 65 d, from the National Laboratory Animal Center, Taiwan). The testing was performed in compliance with ISO 10993-10. The rabbits were shaved to remove the fur at 5 sites and subsequently injected with an extract of a test compound at each site. The control solution (0.9% saline and cottonseed oil [Sigma-Aldrich]) was injected into the same side of each rabbit, and dermal reactions were assessed at time points of 24, 48, and 72 h.
Pyrogen Study in White Rabbits
The guidelines of USP39/NF34(151) were followed to determine whether the test sample extracts passed the pyrogen evaluation in the New Zealand White rabbits (Hui Jun, BWs [gender]: >2 kg [male]; age around 65 d) following a single-dose injection (10 mL/kg) in an ear vein. Three to 5 d before the experiment, the body temperatures of the rabbits were measured. The criteria for selecting rabbits for the pyrogen study were a body temperature that does not exceed 39.8 °C and no more than a 1 °C difference between the highest and the lowest body temperatures among the 4 measurement times. During the study, only reverse osmosis water was provided. The control temperature of each animal was determined using a rectal thermometer (accuracy ± 0.1 °C). Every appliance used in the study was also pyrogen free. In the preliminary test, the control temperatures of the 3 animals were determined. Test samples (warmed up to 37 °C ± 2 °C) were injected into an ear vein of an animal. The administration time did not exceed 10 min. Body temperatures were measured 5 times at 30-min intervals at 1, 1.5, 2, 2.5, and 3 h after administration. The elevations in body temperature of the animals were calculated by subtracting the control temperature from the highest temperature among the 5 measurement times. The main test was performed with 5 additional animals when the results of the preliminary test indicated a fever (a high temperature).
Acute System Injection Study
A system toxicity study following the ISO 10993-11:2006 guidelines was performed to evaluate the toxicity response to the PEG-based sealant extracts in mice (ICR mice, BioLASCO Taiwan Co., Ltd, Taipei, Taiwan; BWs [gender]: 17 to 23 g [male]). The test compound extracts were injected into mice at a dose of 50 mL test compound extract per kilogram BW. Control solutions of 0.9% saline for intravenous injections and cottonseed oil for intraperitoneal injections were given at a dose level of 50 mL/kg BW. Polar extracts were used for the intravenous injections, and nonpolar extracts were used for the intraperitoneal injections.
Wound Closure Animal Study
To evaluate the bioadhesive property and the biocompatibility of the PEG-based hydrogels, rats (normal Sprague-Dawley [SD] rats, 100 to 150 g, 4 wk old, male; BioLASCO Taiwan Co., Ltd) were anesthetized using Zoletil (Virbac, Taiwan, Taipei), and their backs were shaved. Skin incisions that were 1.5 cm long and full-skin thickness deep were made on both sides of the backs of the rats. The skin incisions were quickly closed using Coloskin or the PEG-based hydrogels. The PEG-based hydrogels were sterilized by filtration using 200-nm syringe filters and prepared with a dual syringe kit. A 50-μL aliquot of a hydrogel was applied to the wound area. Immediately after wound closure and at 1 and 4 d postimplantation, the closed skin was harvested and fixed in paraformaldehyde (PFA) solution (3.7 wt.%) for histological analysis after hematoxylin and eosin (H&E) staining and Masson’s trichrome stain (Sigma-Aldrich). The growth of collagen was calculated by using Image J software (National Institutes of Health (NIH), Bethesda, MD, USA).
Statistical Analysis
All data are presented as the mean (standard deviation). The significance of differences between results was assessed via one-way analysis of variance (ANOVA) (EXCEL, Microsoft, Seattle, WA, USA). For all tests, a P value < 0.05 was considered statistically significant.
Results
Formation and Characterization of Adhesive Sealants Composed of PEG-Based Hydrogels
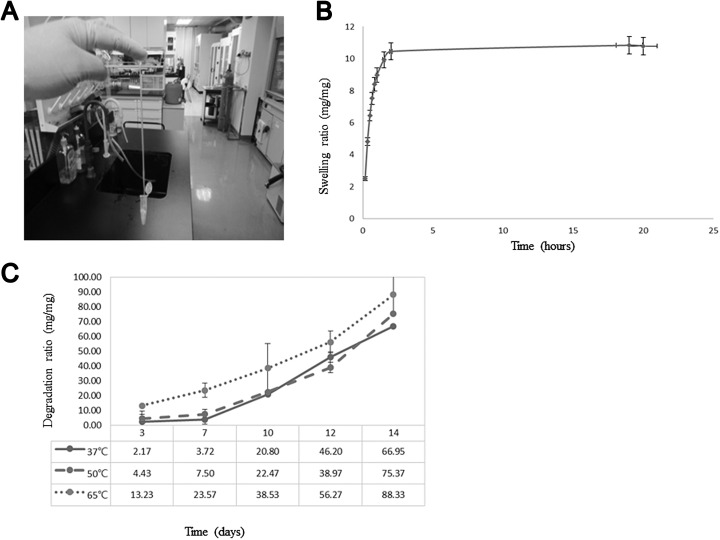
For optimal gelation, 10% to 35% weight/volume (w/v) solutions of the PEG-based hydrogel were used because a solution of less than 5% (w/v) would not solidify. Figure 1a presents the adhesion and stretching results of a line-shaped hydrogel attached to a 1.5 mL Eppendorf tube. The elongated hydrogel adhered easily to skin even when part of it was removed. Figure 1b shows the hydrogel before and after immersion in water. The hydrogel swelled to 11 times its initial weight in the first 2 h (Fig. 1b).
Fig. 1.
The characteristics of polyethylene glycol (PEG)-based hydrogel. (A) The picture displays the elasticity of PEG-based hydrogel containing 30% PEG-based hydrogel. A hydrogel strip can be easily stretched to about 10 times its initial length without visible or permanent deformation. (B) Swelling ratios of PEG-based hydrogel at different time points. (C) The relative mass of PEG-based hydrogel decreased over time due to degradation in the difference temperature.
The mass loss data for hydrogels degraded at temperatures of 37 °C, 50 °C, and 65 °C are summarized in Fig. 1C. During the first 3 d, similar mass losses were observed for hydrogels maintained at temperatures of 37 °C and 50 °C. At day 7, samples maintained at a temperature of 65 °C exhibited faster rates of mass loss, losing a total of 20% of their original mass. At day 14, samples maintained at 65 °C showed a faster mass loss rate at day 12 of degradation, losing more than 95% of their original weight. The mass loss rate of hydrogels maintained at 65 °C was higher than those of hydrogels maintained at 37 °C and 50 °C.
In Vitro Cell Viability and MTT Assay
To evaluate the cytotoxicity of the PEG-based hydrogels, the effects of the samples on cell growth, morphology, and viability were evaluated. After cells were exposed to the extracts for 24 h, the following items were evaluated:
Qualitative determination
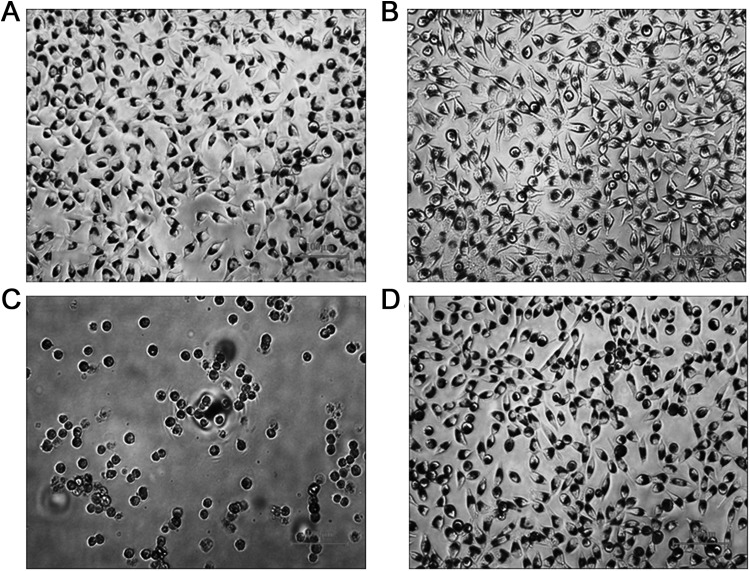
A morphological assessment of L929 cells using an inverted microscope (100×) was performed after treating them with B, NC, PC, or S for 3 h and staining them with neutral red. The morphology of B- and NC-treated cells revealed a long spindle shape with obvious lamellipodia and filopodia instead of a lysed, rounded shape and inhibited growth. However, PC-treated cells showed a nearly complete rounded lysed morphology; cell layers were almost completely destroyed, and growth inhibition was observed. The cells treated with the test samples showed the same long spindle shape as cells treated with B and NC. According to the results of the microscopic assay, the percentages of rounded or lysed cells in the B, NC, PC, and S groups were 0%, 1%, 100%, and 5%, respectively. Therefore, the cytotoxicity of B, NC, PC, and S was graded at 0, 0, 4, and 0, respectively (Table 1 and Fig. 2).
Fig. 2.
In vitro cell viability and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The observed cell morphology of L929 cells (mouse fibroblast cell line) after being treated for 24 h under 100× inverted microscope. (A) Blank (B): culture medium. (B) Negative control (NC): high-density polyethylene. (C) Positive control (PC): 10% dimethyl sulfoxide (DMSO). (D) Test sample (S): polyethylene glycol (PEG)–based hydrogel.
Quantitative determination
L929 cells were treated with B, NC, PC, and S for 24 h, and cell viability was evaluated with an MTT cell proliferation/viability assay. The absorbance values of the B, NC, PC, and S groups at 570 nm were 1.065 ± 0.071, 1.051 ± 0.056, 0.294 ± 0.038, and 0.861 ± 0.053, respectively; the respective cell viability values were 100%, 99%, 28%, and 81%, and the respective mortality values were 0%, 1%, 72%, and 19%.
The qualitative and quantitative assay results (Tables 1 and 2) indicated zero reactivity. Therefore, the extract solutions of the PEG-based hydrogels were considered to have no in vitro cytotoxicity.
Table 2.
The Results of MTT Assay for Evaluation of Cell Viability.
| Test Item | Absorbance (OD570 nm) | Viability (%) | Mortality (%) |
|---|---|---|---|
| Blank (B) | 1.065 ± 0.071 | 100 | 0 |
| Negative control (NC) | 1.051 ± 0.056 | 99 | 1 |
| Positive control (PC) | 0.294 ± 0.038 | 28 | 72 |
| Test sample (S) | 0.861 ± 0.053 | 81 | 19 |
Abbreviation: MTT, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium.
Skin Sensitization Study
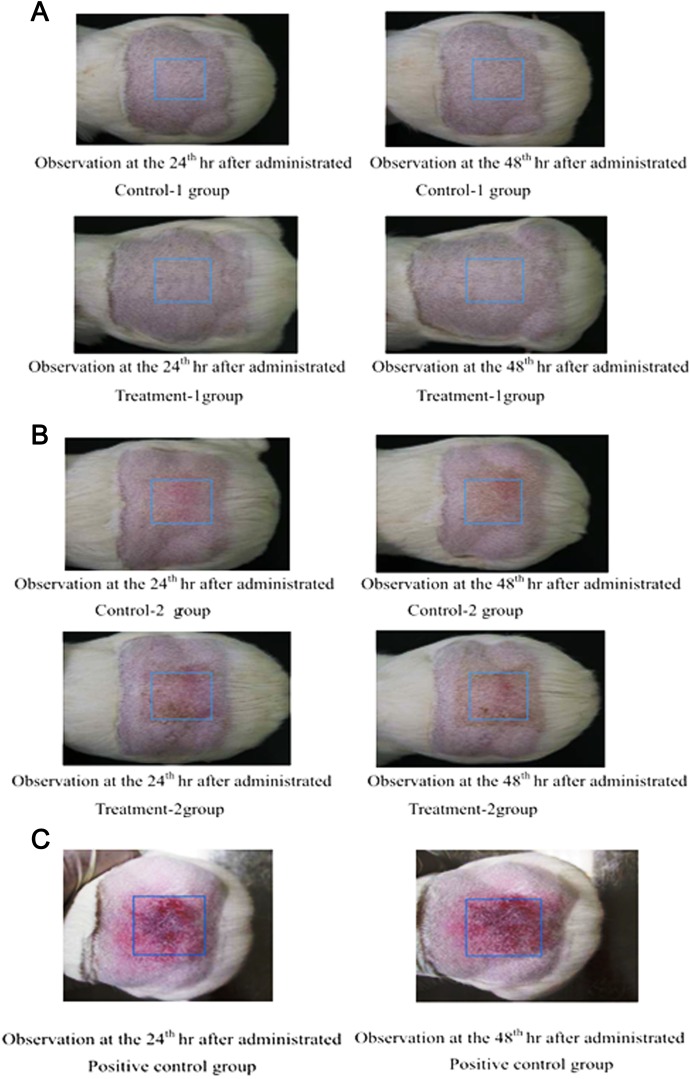
The skin sensitization potential of the PEG-based hydrogels was tested on guinea pigs. Following extraction of the test samples, the extracts were applied twice in the induction phase and once in the challenge phase. Then, 24 h and 48 h after the challenge phase, neither the control nor the treatment group showed visible changes in the skin response on the treated areas (Table 3 and Fig. 3). The results indicated that the test samples (polar or nonpolar) did not cause delayed hypersensitivity on the skin of the tested guinea pigs.
Table 3.
Skin Reaction in Guinea Pigs.
| Group (0.9% saline) | Control | Treatment |
|---|---|---|
| Gender | Male | Male |
| Number of animals | 5 | 10 |
| Erythema and eschar | 0/5 | 0/10 |
| Edema | 0/5 | 0/10 |
| Group (cottonseed oil) | Control | Treatment |
| Gender | Male | Male |
| Number of animals | 5 | 10 |
| Erythema and eschar | 0/5 | 0/10 |
| Edema | 0/5 | 0/10 |
Note. n/n, no. of guinea pigs with abnormal clinical signs/no. of guinea pigs per group.
Fig. 3.
Skin sensitization study. (A) Pictures for observation of skin reaction (0.9% saline). (B) Pictures for observation of skin reaction (cottonseed oil). (C) Pictures for observation of skin reaction (positive control).
Intracutaneous Irritation Study
The intracutaneous irritation results showed that there were no significant clinical signs or gross findings in the control or treatment groups, and there was no mortality in any of the tested groups. Therefore, a single topical application of 0.2 mL of the test compound extracts did not cause intracutaneous irritation in New Zealand White rabbits. The intracutaneous irritation results showed that a single application of the test compound extracts induced neither observable clinical signs nor dermal gross changes in New Zealand White rabbits at any time point. Therefore, a single topical application of a PEG-based hydrogel did not cause observable irritation in New Zealand White rabbits (Fig. 4).
Fig. 4.
Intracutaneous irritation study. (A) Observation at the 24th h of administration. (B) Observation at the 48th h of administration. (C) Observation at the 72nd h of administration.
Pyrogen Study in White Rabbits
The BWs of 3 New Zealand White rabbits were above 1.5 kg, qualifying them for the study. The control temperatures of the 3 animals were 39.42 °C, 39.37 °C, and 39.25 °C. Any elevation of body temperature for the 3 rabbits was below 0.5 °C (Table 5; Animal No.79-1001: −0.46 °C, Animal No.79-1002: −0.10 °C, and Animal No.79-1003: −0.05 °C; Table 5).
Table 5.
Pyrogen Study in White Rabbits.
| (A) Control Temperature of Rabbits | |
|---|---|
| Animal number | Control temperature (°C) |
| 79-1001 | 39.42 |
| 79-1002 | 39.37 |
| 79-1003 | 39.25 |
| (B) Temperature Elevation | |
| Animal number | Elevation (body temperature after administration) |
| 79-1001 | −0.46 °C |
| 79-1002 | −0.10 °C |
| 79-1003 | −0.05 °C |
Note. Temperature elevation = the highest body temperature (among 5 times measurement) subtract control temperature.
The body temperatures of the 3 qualifying White rabbits were measured 5 times after a single dose (10 mL/kg) of a compound was injected in an ear vein. The pyrogen response was negative for all samples; therefore, the samples were considered to pass the pyrogen study.
Acute System Injection Study
The toxicity responses to the PEG-based hydrogels were assessed in mice after injecting the test samples and control solutions. The results showed that there were no significant clinical signs or gross findings in the control or treatment groups. Therefore, the test samples did not cause toxicity reactions or death after injection (Table 4). The study results showed that a single application of the hydrogels or the controls induced neither observable clinical signs nor gross findings in the mice at any time point. Therefore, the PEG-based hydrogels did not cause a toxicity reaction or death after injection (Table 4).
Table 4.
Acute System Injection Study.
| (A) Incidence of Clinical Observation in Mice | ||
|---|---|---|
| Polar group | ||
| Number of animals | 5 male | 5 male |
| Treated article | 0.9% saline | PEG-based hydrogel |
| Toxicity reaction | 0/5 | 0/5 |
| Death | 0/5 | 0/5 |
| Nonpolar group | ||
| Number of animals | 5 male | 5 male |
| Treated article | Cottonseed oil | PEG-based hydrogel |
| Toxicity reaction | 0/5 | 0/5 |
| Death | 0/5 | 0/5 |
| (B) Incidence of Gross Finding | ||
| Polar group | ||
| Dose level (mL/kg) | 50 | 50 |
| Gender | Male | Male |
| Animal number | 5 | 5 |
| Symptom | 0/5 | 0/5 |
| Nonpolar group | ||
| Dose level (mL/kg) | 50 | 50 |
| Gender | Male | Male |
| Animal number | 5 | 5 |
| Symptom | 0/5 | 0/5 |
Note. n/n, no. of mice with gross signs/no. of mice per group.
Wound Closure Animal Study
After applying the PEG-based hydrogels, bleeding from the incisions on the dorsum of SD rats immediately stopped, and the wound openings closed within minutes. The wound openings did not close after applying Coloskin. Furthermore, visual examinations were performed at different time points to compare the PEG-based hydrogel-treated incisions and Coloskin (a commercial product)-treated incisions. The results indicated that the PEG-based hydrogels enhanced the wound closure process (Fig. 5A and B). The histological evaluation (H&E staining) showed that after 14 d, there was more collagen at the wound sites in the hydrogel-treated groups than in the Coloskin-treated group (Fig. 5C). The average collagen in the Coloskin and PEG-based hydrogel groups were 65.2 and 77.2% (Image J), respectively (Fig. 5D). We also calculated the wound area between 2 groups, and the average wound area in the Coloskin and PEG-based hydrogel groups were 0.4 cm2 and 0.2 cm2, respectively, after 7 d of treatment.
Fig. 5.
Animal experiment for wound closure. Photographs of wound closures. Skin incisions on back of the rats were treated by Coloskin and hydrogel, (A) 1 and (B) 4 d postimplantation. The closure skin was harvested and fixed in p-formaldehyde solution (3.7 wt.%) for histological analysis by hematoxylin and eosin and Masson’s trichrome stain (C) after 14 d. (D) The statistical results of collagen assay after 14 d treatment (P < 0.01). (E) The differences in wound area between the 2 treatments after 7 d (P < 0.01).
Discussion
In this study, the biocompatibility of hydrogel samples was compared with that of control samples by assessing cytotoxicity, irritation, sensitization, pyrogen toxicity, and systemic toxicity, which are the most important factors in in vitro tests for wounds care14. According to the results of these analyses, the PEG-based hydrogel showed good biocompatibility and safety15. The animal study confirmed the effectiveness and convenience of using PEG-based hydrogels for wound closure and bleeding control and their enhanced performance over a commercial product.
After applying the hydrogel solution to the full-skin thickness cutaneous incisions on the backs of SD rats, the bleeding immediately ceased, and the wound opening closed within a few minutes, whereas this did not occur with the Coloskin-treated wounds. In addition, the PEG-based hydrogels led to improved and accelerated wound closure compared to Coloskin. Interestingly, 14 d after application, no trace of hydrogel was observed at the location of the healed tissues, indicating the complete in vivo degradation of the hydrogel. In vivo degradation rates of polymers could be faster than in vitro; the higher in vivo degradation rate has been explained by the effects caused by cellular and enzymatic activities found in the body16. Traditionally, it is believed that PEG-based hydrogels degrade due to ester hydrolysis17 and hydroxyl radicals18.
The statistical results of the collagen assay showed the statistically significant difference between the 2 groups, but reepithelialization of PEG-based treatment was better than the Coloskin treatment after 14 d (Fig. 5). Healing is a systematic process, traditionally explained in terms of 4 overlapping classic phases: hemostasis, inflammation, proliferation, and maturation. The balance of fibrogenesis and fibrinolysis affects the formation of scars. Scar tissue is composed of the same protein (collagen) as the tissue that it replaces, but the fiber composition of the protein is different; instead of a random basketweave formation of the collagen fibers found in normal tissue, in fibrosis, the collagen cross-links and forms a pronounced alignment in a single direction. In this study, the structure of wounds treated by PEG-based sealant was more like the normal skin in Fig. 5.
These results demonstrate that this bioadhesive hydrogel is a suitable candidate for biological applications, particularly in cases in which the employed biomaterial needs to be degraded and absorbed after its intended time of service with minimal toxicity to the host body.
Our results also demonstrate that this biodegradable adhesive has convenient handling procedures, good tissue adhesion, controlled degradability, and elastomeric mechanical properties, indicating the potential of the PEG-based hydrogel as a tissue adhesive sealant.
Acknowledgments
The authors acknowledge the support of the Industrial Technology Research Institute (ITRI).
Authors’ Note: Sen-Lu Chen and Ru-Huei Fu provided equal contribution to this work.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All animal experiments were conducted according to a protocol approved by Master Laboratory CO., Ltd (IACUC No.: MS20160706, Hsinchu County, Taiwan, People’s Republic of China). Statement of Human rights is not applicable.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Diligence G. Wound Closure and Anti-Adhesion Market. MedMarket LWSS; 2012.
- 2. Scognamiglio F, Travan A, Rustighi I, Tarchi P, Palmisano S, Marsich E, Borgogna M, Donati I, de Manzini N, Paoletti S. Adhesive and sealant interfaces for general surgery applications. J Biomed Mater Res B Appl Biomater. 2015;104(3):626–639. [DOI] [PubMed] [Google Scholar]
- 3. Colombo GL, Caruggi M, Ottolini C, Di Matteo S, Valentino MC, Bruno GM. Economic evaluation of fibrin sealant patch: a proposal to assess the economic value in hemostasis and as a surgical sealant. Value Health. 2015;18(7):A533. [Google Scholar]
- 4. Annabi N, Tamayol A, Shin SR, Ghaemmaghami AM, Peppas NA, Khademhosseini A. Surgical materials: current challenges and nano-enabled solutions. Nano Today. 2014;9(5):574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albala DM, Riebman JB, Kocharian R, Ilie B, Albanese J, Shen J, Ovington L, Batiller J. Hemostasis during urologic surgery: fibrin sealant compared with absorbable hemostat. Rev Urol. 2015;17(1):25–30. [PMC free article] [PubMed] [Google Scholar]
- 6. Lang N, Pereira MJ, Lee Y, Friehs I, Vasilyev NV, Feins EN, Ablasser K, O’Cearbhaill ED, Xu C, Fabozzo A, Padera R, Wasserman S, Freudenthal F, Ferreira LS, Langer R, Karp JM, del Nido PJ. A blood-resistant surgical glue for minimally invasive repair of vessels and heart defects. Sci Transl Med. 2014;6(218):218ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jamous N, Carter S, Ferko N, Hogan A, Corral M. Economic analysis of Evarrest® sealant matrix compared with standard of care in severe soft tissue surgical bleeding: a United Kingdom hospital perspective. Value Health. 2015;18(7):A368. [Google Scholar]
- 8. Tseng YC, Tabata Y, Hyon SH, Ikada Y. In vitro toxicity test of 2-cyanoacrylate polymers by cell culture method. J Biomed Mater Res. 1990;24(10):1355–1367. [DOI] [PubMed] [Google Scholar]
- 9. Watson JT, Webb DL, Stoikes NF, Voeller GR. Fibrin sealant: a review of the history, biomechanics, and current applications for prosthetic fixation in hernia repair. Surg Technol Int. 2015;27:140–145. [PubMed] [Google Scholar]
- 10. Yesilirmak N, Diakonis VF, Battle JF, Yoo SH. Application of a hydrogel ocular sealant to avoid recurrence of epithelial ingrowth after LASIK enhancement. J Refract Surg. 2015;31(4):275–277. [DOI] [PubMed] [Google Scholar]
- 11. Behrens AM, Lee NG, Casey BJ, Srinivasan P, Sikorski MJ, Daristotle JL, Sandler AD, Kofinas P. Biodegradable-polymer-blend-based surgical sealant with body-temperature-mediated adhesion. Adv Mater. 2015;27(48):8056–8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim KD, Wright NM. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized controlled study. Spine (Phila Pa 1976). 2011;36(23):1906–1912. [DOI] [PubMed] [Google Scholar]
- 13. Liu Z, Guan L, Sun K, Wu X, Su L, Hou J, Ye M, Huang W, He H. In vivo study of novelly formulated porcine-derived fibrinogen as an efficient sealant. J Mater Sci Mater Med. 2015;26(3):146. [DOI] [PubMed] [Google Scholar]
- 14. ISO 10993: Biological evaluation of medical devices. International Organization for Standardization; 2009. [Google Scholar]
- 15. Ho VY, Shah GK, Liu EM. ReSure sealant for pars plana vitrectomy wound closure. Ophthalmic Surg Lasers Imaging Retina. 2015;46(10):1042–1044. [DOI] [PubMed] [Google Scholar]
- 16. Patenaude M, Hoare T. Injectable, mixed natural-synthetic polymer hydrogels with modular properties. Biomacromolecules. 2012;13(2):369–378. [DOI] [PubMed] [Google Scholar]
- 17. Metters AT, Anseth KS, Bowman CN. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer. 2000;41(11):3993–4004. [Google Scholar]
- 18. Reid B, Gibson M, Singh A, Taube J, Furlong C, Murcia M, Elisseeff J. PEG hydrogel degradation and the role of the surrounding tissue environment. J Tissue Eng Regen Med. 2015;9(3):315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]