Abstract
On a daily basis, planktonic organisms migrate vertically and thus experience widely varying conditions in their physico-chemical environment. In the Gulf of Finland, these changes are larger than values predicted by climate change scenarios predicted for the next century (up to 0.5 units in pH and 5°C in temperature). In this work, we are interested in how temporal variations in physico-chemical characteristics of the water column on a daily and weekly scale influence oxidative stress level and antioxidant responses in the planktonic copepod of the genus Acartia. Responses were determined from samples collected during a two-week field survey in the western Gulf of Finland, Baltic Sea. Our results showed that GST (Glutathione-S-transferase) enzyme activity increased in the surface waters between Weeks I and II, indicating antioxidant defense mechanism activation. This is most likely due to elevating temperature, pH, and dissolved oxygen observed between these two weeks. During Week II also GSSG (oxidized glutathione) was detected, indicating that copepods responded to stressor(s) in the environment. Our results suggest that Acartia copepods seem fairly tolerant to weekly fluctuations in environmental conditions in coastal and estuarine areas, in terms of antioxidant defense and oxidative stress. This could be directly connected to a very efficient glutathione cycling system acting as antioxidant defense system for neutralizing ROS and avoiding elevated levels of LPX.
Introduction
Carbon dioxide, Ultraviolet radiation (UV-B), eutrophication, hypoxia, and salinity changes are strongly modifying the pelagic and coastal marine ecosystems globally [1, 2, 3, 4]. In addition, the coastal environment can be affected by upwelling of deep-water as well as by freshwater riverine input [5]. Zooplankton, living in these habitats, are a central part of the marine food-web, between microzooplankton, primary producers and higher trophic levels, such as fish larvae, mysid shrimps and jellyfish [6]. Copepods are excellent model organisms to study as they experience large variability in their physico-chemical environment (i.e., light, temperature, salinity, pH, oxygen and chlorophyll a conditions) by performing diel vertical migration. Thus, Almén et al. [7] reported that copepods from a coastal area in the south-western Finland can experience a change in pH up to >0.5 units, and a change in temperature of 5°C on a daily basis, which means larger fluctuations than climate change scenarios propose for the coming century [7,8]. Moreover, the same species was found to experience a change in salinity of 0.24 [7]. The migration behavior is likely to affect the physiological plasticity in copepods and can have positive effects on their ability to cope with environmental fluctuations caused by climate change [9, 10, 11, 12].
Oxidative stress is becoming an increasingly important process to include in ecological work, studying global warming and other human-induced environmental changes [13, 14]. Warming, UV-B radiation, hypoxia and salinity fluctuations can cause oxidative stress in animals inhabiting the marine environment [15, 16, 17], causing an imbalance between the reactive oxygen species (ROS) and its elimination [18, 19]. In case an imbalance occurs between the level of ROS and antioxidant protection, it can result in oxidative damage to tissues and a state of oxidative stress [20]. Baltic Sea zooplankton is reported to be sensitive to both chemical contamination and changes in oxygen and temperature. Vehmaa et al. [21] found that a 3 degrees rise in temperature increased the antioxidant capacity (ORAC, Oxygen Reactive Absorbance Capacity) in Acartia copepods by almost 15%, and they measured a 2-fold increase also in oxidative damage, measured as lipid peroxidation. When involving also other stressors such as pH and toxic algae, a decrease in antioxidant was detected [21]. The glacial relict copepod Limnocalanus macrurus’ antioxidant defense and oxidative stress biomarkers showed associations with hydrographic factors and selected proxies describing organochlorine contaminant loads during a large survey covering the northern Baltic Sea basins [16]. Nevertheless, toxic cyanobacterial blooms can also increase antioxidant defense levels and improve oxidative status, as Hogfors et al. [22] showed in their work reporting ORAC: TBARS ratio (TBARS, measuring lipid peroxidation) in Acartia bifilosa. In addition, some organisms can protect themselves from oxidative damage, an event called “preparation for oxidative stress” [23], as shown in the mysid shrimps of the genus Mysis, which seem fairly tolerant to large variation in dissolved oxygen concentrations [17].
In terms of climate change, the coastal environment is essential to study due to the high variability of hydrographical variables commonly monitored in aquatic studies, such as temperature, nutrients, chlorophyll a (Chl a), pH and dissolved inorganic carbon (DIC). The Baltic Sea, in which the present study is implemented, is especially sensitive to environmental change as the area is heavily eutrophicated and suffers from large-scale deep-water anoxia [24], enhanced by global warming [25].
The main aim of the study was to obtain a more comprehensive understanding of the oxidative stress status in copepods as they experience large fluctuations in their biotic and abiotic environment on a daily basis. Moreover, copepods are known to respond on a short-time scale to fluctuations in environmental conditions in the water column [26, 27]. For this purpose, we based our study on one of the most abundant zooplankton species Acartia sp., which were collected from different depths (according to the depth of maximum copepod abundance) and times of day (night and day) during a two-week field survey in the western Gulf of Finland. We measured different biomarkers to reveal potential relationships between copepod antioxidant defense, oxidative stress and the environmental condition (Chl a, pH, DIC, salinity, temperature) at different depths. Finally, this study will help to increase our understanding concerning the cellular responses to climate change in this species.
Material and methods
Sampling
The sampling was carried out during two weeks in August 2015 at Storfjärden monitoring station (59° 52' 56" N, 23° 15' 14" E) in the western Gulf of Finland of the Baltic Sea. In 2015, water temperature varied between ~1°C in January-February and 17.5°C in August. Salinities ranged between 5.64 and 6.46 with higher salinities in deeper waters. pH values fluctuated between 7.57 and 8.57 with the highest value observed in August in the layer 0-10m (S1 Fig). The copepods were sampled with a 150 μm closing net at specific depths (in accordance with maximal distribution of copepods [7]) and time of day (Table 1). After each haul (5 per sampling time), the animals were immediately transferred into coolers to 20 L of seawater from the sampling depth. After returning to the laboratory, the samples were stored in a climate chamber until sorted for picking (<6 h in total). In this study, our main target species was adult copepods Acartia bifilosa, the most abundant copepod species in the area. However, we cannot exclude that some of the specimens collected were Acartia tonsa, which occurs in the community in late summer (hereafter Acartia sp.). The number of replicates was dependent on the copepod abundance at the considered depth. Therefore, as we usually managed to collect 3–4 replicates, only one replicate was collected on the 11 August 2015 (Table 1). Thirty adults Acartia (size <1 mm) were rapidly sorted on ice individually using forceps, and transferred to a 2 mL plastic Eppendorf microtube. Samples were stored immediately in -80°C until analyses [21]. A comparison between samples frozen in -80°C, and samples snap-frozen in liquid N and stored in -80°C were made, to ensure there were no differences caused by methodology (t-test, p > 0.05).
Table 1. Main hydrographic variables measured (mean ± SD) and the number of zooplankton samples collected during the study.
Weeks I and II are separated by a thick border.
| Date | Sampling Time | Sampling depth (m) | T°C (mean ± SD) |
Salinity (mean ± SD) | Oxygen (mg l-1) | pH | Number of zooplankton samples |
|---|---|---|---|---|---|---|---|
| 10.08.2015 | 12:00 | 20–30 | 10.18 ± 0.67 | 6.03 ± 0.02 | 6.50 ± 0.31 | 7.37 | 3 |
| 11.08.2015 | 18:00 | 10–20 | 13.08 ± 0.74 | 5.98 ± 0.01 | 8.60 ± 1.30 | 7.84 | 1 |
| 13.08.2015 | 00:00 | 0–10 | 16.70 ± 2.17 | 5.76 ± 0.27 | 10.40 ± 0.90 | 8.02 | 3 |
| 14.08.2015 | 06:00 | 15–25 | 11.30 ± 0.89 | 5.99 ± 0.02 | 7.12 ± 1.15 | 7.48 | 3 |
| 17.08.2015 | 12:00 | 20–30 | 12.08 ± 0.28 | 6.00 ± 0.01 | 6.83 ± 0.75 | 7.84 | 4 |
| 18.08.2015 | 18:00 | 10–20 | 15.62 ± 1.47 | 6.02 ± 0.08 | 9.22 ± 1.21 | 8.21 | 3 |
| 20.08.2015 | 00:00 | 0–10 | 17.96 ± 0.47 | 5.94 ± 0.29 | 11.43 ± 0.90 | 8.52 | 3 |
| 21.08.2015 | 06:00 | 15–25 | 14.66 ± 1.04 | 5.94 ± 0.02 | 8.16 ± 1.37 | 7.77 | 4 |
Hydrography
Dissolved oxygen (DO) was measured in the field at 0, 5, 10, 15, 20, 25 and 30 m using an oxygen meter (ProDSS Sensor, YSI Incorporated, Yellow Springs, OH, USA, resolution 0.1, accuracy 0.1 mg L-1). The water samples for pH, Chl a and DIC analyses were collected at 5, 15 and 25 m depth using a 2 L Limnos water sampler. For pH analysis, seawater was sampled in 250 mL airtight glass bottles and kept in a cooler on board. The Jenway 3510 pH meter (Bibby Scientific, Ltd., Staffordshire, United Kingdom) was calibrated daily using pH buffers 4.0, 7.0 and 10.0, and sensor kept in pH 4.0 after calibration. The samples were measured after reaching room temperature (~20°C). Chl a analysis was determined according to Almén et al. [7]; samples were collected in 1000 mL plastic bottles and protected from light after sampling. In the laboratory, 100 mL was filtered on a Millipore vacuum filtration device using glass-fibre filters (25 mm, GF/F Whatman). The samples were dissolved with 5 mL ethanol (96%) in the dark for 24 h, and Chl a was determined by fluorometry (Varian Cary Eclipse Fluorescence Spectrophotometer, Varian Optical Spectroscopy Instruments, Mulgrave, Australia), using a 96-well microplate reader. Samples for total DIC were collected in triplicate from each of three depths into 25 mL acid-washed, airtight glass bottles, stored in dark on ice at +3°C until measured within 12 h, using the acidification/gas stripping/infrared detection method [11, 28]. Temperature, salinity and conductivity were measured with a CTD probe (Valeport Mini CTD, Valeport Limited, Devon, United Kingdom) from the surface to the bottom.
Determination of biomarkers
A number of biomarkers (described in Table 2) was assessed to reveal responses in antioxidant defense and oxidative stress of Acartia sp. to environmental conditions. Zooplankton samples (thirty adults Acartia sample-1) were entirely homogenized in 100 μL of 0.1 M K2HPO4 + 0.15 M KCl buffer (pH 7.4) using a Tissue Lyser II bead mill (Qiagen). An aliquot of raw homogenate (25 μL) was immediately frozen in liquid nitrogen and stored at -80°C for lipid peroxide determination (LPX). Then, the sample homogenate was centrifuged at 10,000g for 15 min at 4°C and the resulting supernatant was divided into aliquots for Glutathione S-transferase (GST), Glutathione reductase (GR), Catalase (CAT) and Superoxide dismutase (SOD) enzyme activity determination, Oxygen Radical Absorbance Capacity (ORAC) assay and for glutathione sample preparation.
Table 2. Definition, justification, interpretation of used biomarkers with zooplankton examples from literature.
AO = antioxidant, OD = oxidative damage, O2- = superoxide radical, H2O2 = hydrogen peroxide.
| Biomarker | Definition | Justification | Interpretation ↑ or ↓ |
Example of species and study area | Reference | |
|---|---|---|---|---|---|---|
| GSH: GSSG | Reduced: oxidised glutathione ratio | OD | Assays glutathione redox state [29] | Low ratio or increase in GGSG indicate stress | Limnocalanus macrurus, Baltic Sea, FI | [16] |
| GR | Glutathione reductase | AO | Reduces GSSG back to GSH [30] | ↑ indicates more AO | L. macrurus, Baltic Sea, FI | [16] |
| GST | Glutathione S-transferase | AO | Catalyzes reactions that detoxify harmful compounds [31] | ↑ indicates more AO | L. macrurus, Baltic Sea, FI; Eurytemora affinis, Seine estuary, FR | [16, 27] |
| SOD | Superoxide dismutase | AO | Part of enzymatic defense to remove O2- [31] | ↑ indicates more AO | L. macrurus, Baltic Sea, FI | [16] |
| CAT | Catalase | AO | Part of enzymatic defense to remove H2O2 [31] | ↑ indicates more AO | Boeckella gibbosa, Mixodiaptomus laciniatus, Lake Los Cantaros, ARG; Lake La Caldera, ESP | [32] |
| ORAC | Oxygen reactive absorbance capacity | AO | Assesses antioxidant capacity [33] | ↑ indicates more AO | Acartia bifilosa, Baltic Sea, FI | [21] |
| LPX | Lipid peroxidation | OD | Oxidative degradation of lipids in cell membrane, resulting in cell damage [33] | ↑ indicates stress | L. macrurus, Baltic Sea, FI | [16] |
The Total GSH sample was deproteinized by adding 5% sulfosalicylic acid (SSA) and subsequently incubated on ice for 10 min and centrifuged for 10 min at 10,000g at 4°C. The supernatant was divided into two different tubes for reduced (GSH) and oxidized glutathione (GSSG) and 33 mM M2VP (1-methyl-2-vinylpyridinium trifluoromethanesulfonate, Sigma Chemicals) in 0.1M HCl, a scavenger of GSH, was added to the GSSG sample. The sample homogenate aliquots and glutathione samples were frozen in liquid nitrogen and stored at -80°C until further analysis. GST and GR activities were determined as described in Vuori et al. [16]. Glutathione 384-well plate Fluorescent Detection Kit (Arbor Assays) was used to measure GSH and GSSG fluorescent emission at 510 nm, with excitation 370–410 nm. The FOXII assay, modified from the protocols in Bou et al. [34] and Eymard et al. [35], was used to measure lipid peroxidation (LPX) in the samples, as described in Vuori et al. [16]. The raw homogenates were mixed with methanol and centrifuged at 3,000g for 5 min at room temperature. FOXII reagent (450 μL) was added to the samples (50 μL), and absorbance measured at 590 nm after 2 h of incubation. The CAT activity measurement was modified from the Catalase Assay kits’ colorimetric assay (Sigma Chemicals) to a 96-well microplate, as described in Vuori et al. [36]. The reaction of catalase and H202 (total volume 7.5 μL, containing 0.133 μg μL-1 sample protein) was stopped with NaN3. Two μL of the reaction was pipetted to a microplate and the leftover H2O2 was detected with colorimetric reaction by adding 200 μL of color reagent to the wells, incubating for 15 min and measuring the absorbance at 520 nm. The inhibition rate of SOD was measured with the SOD determination kit (Sigma Chemicals). Intracellular soluble antioxidant capacity was measured with OxiSelectTM Oxygen Radical Antioxidant Capacity (ORAC) Activity Assay (Cell Biolabs) following the manufacturers’ instructions, except for adjusting the reaction volumes for 384-well plate, when needed. The enzyme activities, lipid hydroperoxides and total GSH were normalized to the protein content of the samples, which were determined with PierceTM BCA Protein Assay (Thermo Scientific) with bovine serum albumin (Sigma) as the standard.
All samples, standards and blanks were analyzed in triplicate, and a positive control was included to ensure the assay worked as expected. For all assays performed in this study, the mean coefficient of variation percentage (CV%) of technical replicates ranged between 1.69 and 6.24%.
Statistical analyses
All data were tested for normal distribution using the Shapiro-Wilk normality test. The environmental data were not normally distributed and were analyzed using the k-sample Kolmogorov–Smirnov test. Differences between different samples storage methods were analysed with Independent sample t-test. Differences of environmental parameters between weeks and depths were tested using General Linear Model (GLM). The biomarker variables that were not normally distributed were log transformed. All biomarkers (response variables) were analyzed in association with the environmental conditions using a Linear Mixed Model (LLM), fitted by REML (Restricted Maximum Likelihood) estimation using the package ‘lmerTest’ in R [37]. The Welch-Satterthwaite approximation was used for p-values and degrees of freedom. Environmental variables were used as fixed factors and sampling occasion (date) was added as random factor. Some missing values were replaced with the group mean (1/25 GST and SOD values, 2/25 ORAC and 2/24 CAT values). A three-table ordination method, RLQ analysis [38] was used for visualizing the associations between measured antioxidant defense and oxidative stress variables in the samples and environmental data. First, PCA principal component analysis was done for both the biomarkers and the environment data, and then a correspondence analysis on the sample groups. GR and GSSG were indicated as present (P) when detected, and absent (A) when under detection level. These three analyses were then passed to the RLQ function of ‘ade4’ package in R [39]. SPSS 21.0. and the free statistical software R, version 3.2.3, were used for the data analyses.
Results
Hydrography and environmental parameters
The different variables measured during the present study varied between sampling depths and dates (Table 1). Neither thermocline, nor halocline was observed between 0 and 30 m depth. Temperatures were higher at the surface, and increased by almost 2°C in all depths between Weeks I and II (Table 1). Salinity varied between 5.76 ± 0.27 at 5 m depth and between 6.03 ± 0.02 at ~25 m. Dissolved oxygen concentrations showed a gradient from surface to deep water (~25 m). pH decreased with depth and ranged between 8.02 and 7.37 in Week I, and between 8.52 and 7.84 in Week II. Water temperature and salinity differed significantly between weeks and between depths (GLM, p-values < 0.001). Oxygen differed between depths (GLM, p-values < 0.001), whereas neither oxygen nor pH differed between weeks (GLM, p-values > 0.05).
Weather conditions were stable during Weeks I and II with clear (night time) or sunny skies. Winds were below 1m s-1 (personal observation).
Oxidative stress status
The oxidative stress status of the Acartia population was analysed using different biomarkers for antioxidant defense (GR, GSH, GST, CAT, SOD and ORAC), glutathione oxidation (GSSG) and oxidative stress (LPX, Tables 2 and 3).
Table 3. Antioxidant defense and oxidative stress variables measured in Acartia sp. in August 2015.
Week I and II are separated by a thick border; CAT: Catalase, ORAC: Oxygen radical absorbance capacity, SOD: Superoxide dismutase, GR: Glutathione reductase, GST: glutathione S-transferase, totGSH: total glutathione, GSSG: oxidized glutathione, LPX: Lipid peroxidation, ND: under detection level, n: number of samples.
| Antioxidant defense | Oxidative stress | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Date | Depth (m) | CAT (μmol min-1 mg-1) |
ORAC (μM trolox equivalents mg-1) |
SOD Inhibition (%) | GR (nmol min-1 mg-1) |
GST (μmol min-1 mg-1) |
totGSH (μM mg-1) |
GSSG (μM mg-1) |
LPX (μM cumene-hydroperoxide equivalents mg-1) |
|
| 10 Aug. | 20–30 | 7.59 ± 3.06 | 323.87 ± 168.32 | 57.65 ± 6.56 | 1.49 | 0.15 ± 0.04 | 26.86 ± 5.62 | ND | 33.49 ± 9.15 | |
| n | 2 | 3 | 3 | 1 | 3 | 3 | 3 | |||
| 11 Aug. | 10–20 | 98.10 | 179.72 | 69.42 | ND | 0.20 | 33.85 | ND | 53.99 | |
| n | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| 13 Aug. | 0–10 | 23.77 ± 2.08 | 353.21 ± 318.02 | 69.98 ± 4.59 | 1.69 | 0.21 ± 0.08 | 21.46 ± 2.02 | ND | 30.74 ± 7.09 | |
| n | 3 | 3 | 3 | 1 | 3 | 4 | 4 | |||
| 14 Aug. | 15–25 | 17.69 ± 5.71 | 67.14 ± 23.32 | 59.23 ± 4.71 | ND | 0.09 ± 0.07 | 55.49 ± 24.46 | 3.81 ± 0.13 | 37.26 ± 5.38 | |
| n | 3 | 3 | 3 | 3 | 3 | 2 | 3 | |||
| 17 Aug. | 20–30 | 17.69 ± 5.14 | 122.50 ± 23.46 | 53.17 ± 13.57 | 1.41 | 0.15 ± 0.04 | 27.83 ± 16.66 | 3.53 | 39.89 ± 11.59 | |
| n | 4 | 4 | 4 | 1 | 4 | 4 | 1 | 4 | ||
| 18 Aug. | 10–20 | 13.75 ± 1.82 | 172.60 ± 99.50 | 50.45 ± 3.59 | 0.07 | 0.24 ± 0.01 | 60.42 ± 5.22 | 3.96 ± 0.49 | 29.75 ± 9.25 | |
| n | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | ||
| 20 Aug. | 0–10 | 15.75 ± 5.23 | 128.26 ± 75.77 | 41.18 ± 3.98 | 4.12 ± 1.28 | 1.14 ± 0.54 | 38.34 ± 4.91 | 4.24 ± 0.33 | 30.22 ± 8.76 | |
| n | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||
| 21 Aug. | 15–25 | 15.87 ± 4.33 | 146.63 ± 30.11 | 44.79 ± 11.40 | 4.56 ± 1.00 | 0.24 ± 0.06 | 41.65 ± 17.41 | 3.59 ± 2.04 | 34.08 ± 10.67 | |
| n | 4 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | ||
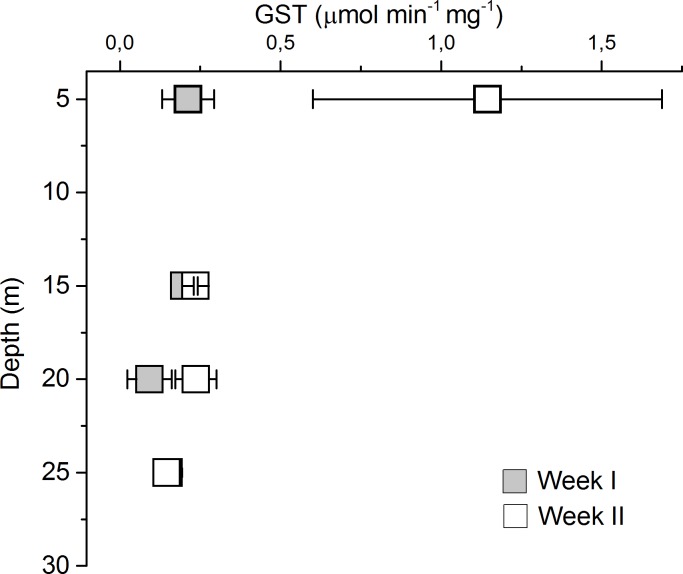
The catalase activity was quite low with values below 23.77 μmol min-1 mg-1 except at 15 m in Week I when the activity was 98.10 μmol min-1 mg-1. ORAC activities were found to be higher during Week I, except at 20 m. The SOD activity was higher in Week I (~60%) compared with Week II (~50%). GST activity was six-fold higher during Week II in the surface compared with other depths during both weeks (Table 2, Fig 1). The GR activity was below limit of quantification (≤ 0.07 nmol min-1 mg-1) for 58% of the samples especially in Week I, whereas GR was detected in the end of Week II (Table 2). Lipid peroxidation (LPX) was highest in Acartia at 15 m depth during Week I (53.99 μM mg-1 protein) and lowest during Week II at the same depth (29.75 ± 9.25 μM mg-1 protein).
Fig 1. Glutathione S-transferase activity measured in Acartia sp.
The animals were collected at different depths (5, 15, 20 and 25 m) during two weeks in August 2015.
The oxidized form of glutathione (GSSG) was below limit of quantification (≤ 0.10 μM mg-1) during most of Week I, except at 20 m, while GSSG was detected during Week II (Table 2).
Biomarkers and environmental variables
The GST activity was found to be higher in samples collected when temperature (LMM, t 5.95500 = 3.425, p = 0.014), oxygen (LMM, t5.9770 = 3.281, p = 0.017) and pH (LMM, t6.0710 = 3.815, p = 0 .009) were higher. A higher level of LPX was found to be associated with lower Chl a concentration (LMM, t23.000 = -2.307, p = 0.030), and lower salinity with higher CAT activity (LMM, t4.487 = -3.026, p = 0.034). No significant relationships were found for ORAC, SOD, total GSH and environmental variables.
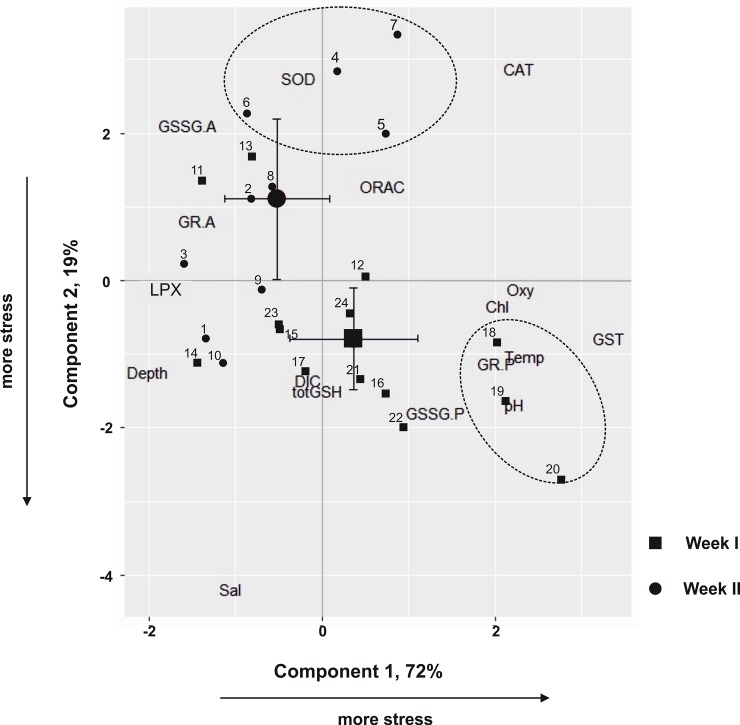
In the PCA (Fig 2, Table 4), GST, and GR.P (P = present) were associated with Component 1, whereas LPX and GR.A (A = absent) had an inverse relationship with these variables. Component 2 was positively associated with CAT, ORAC, SOD and GSSG.A and negatively associated with total GSH and GSSG.P. The two main components explained in total > 90% of the observed variation in the PCA, i.e., 72.33% of Component 1 and 18.99% of Component 2. Samples from Weeks I and II were clearly separated in the PCA score plots (Fig 2). High GST and total GSH values were recorded in Acartia during Week II, whereas specimens collected during Week I exhibited higher levels of SOD activities (Fig 2). This tendency was especially true for samples collected at the surface. Activity in both GR and GSSG were detected in samples in Week II, but not during Week I, whereas lipid peroxidation LPX levels were higher in Week I and lower in Week II. The increased CAT activity was associated with lower salinity and vice versa (Fig 2).
Fig 2. Component 1 and 2 scores from Principal Component Analysis (PCA) for Acartia sp. collected in the western Gulf of Finland during two weeks.
Samples collected during Week I (black dots 1–10), and samples collected during Week II (black squares, 11–24). The corresponding loading plots (biomarker variables and environmental variables) are superimposed on the scores plot. Abbreviations are as follows: CAT Catalase, LPX Lipid peroxidation, GST Glutathione S-transferase, ORAC Oxygen radical absorbance capacity, SOD Superoxide dismutase, totGSH total glutathione, GSSG.A oxidized glutathione Absent, GSSG.P oxidized glutathione Present, GR.A Glutathione reductase Absent, GR.P Glutathione reductase Present. One sample from 11 August was not considered in the PCA analysis as there were no replicates.
Table 4. Component matrix for Fig 2.
(A). Biomarker abbreviations are as follows: CAT: Catalase, LPX: Lipid peroxidation, GST: glutathione S-transferase, ORAC: Oxygen radical absorbance capacity, SOD: Superoxide dismutase, totGSH: total glutathione, GSSG.A: oxidized glutathione Absent, GSSG.P: oxidized glutathione Present, GR.A: Glutathione Reductase Absent, GR.P: Glutathione Reductase Present. (B). Environmental variables. Loadings highlighted in bold represent the highest absolute value of the parameter when considering different PCs.
| (A) | Component | |
| 1 | 2 | |
| GST | 0.663 | -0.156 |
| GR.P | 0.404 | -0.226 |
| LPX | -0.364 | -0.019 |
| GR.A | -0.288 | 0.162 |
| CAT | 0.456 | 0.575 |
| SOD | -0.051 | 0.549 |
| GSSG.A | -0.309 | 0.423 |
| GSSG.P | 0.262 | -0.358 |
| totGSH | -0.008 | -0.298 |
| ORAC | 0.141 | 0.257 |
| (B) | Component | |
| 1 | 2 | |
| Temperature | 0.469 | -0.206 |
| Oxygen | 0.458 | -0.022 |
| pH | 0.445 | -0.337 |
| Chlorophyl a | 0.406 | -0.069 |
| Depth | -0.401 | -0.249 |
| Salinity | -0.211 | -0.839 |
| DIC | -0.030 | -0.270 |
Discussion
The site in the western Gulf of Finland, where the copepods of the genus Acartia were sampled for oxidative stress and antioxidant defense measurements, is characterized by a strong thermocline in late summer, shown as low temperatures and low pH at the bottom, but also occasionally higher salinity, due to seasonal upwellings. In the present study (August 2015), no sharp clines were found. However, the temperature elevated by almost 2°C during the two-week field survey and the study area also suffered from cyanobacteria blooms (mainly Aphanizomenon and Nodularia, personal observation).
We showed that higher temperature, pH and oxygen were associated with increased activities of Glutathione S-transferase (GST) in Acartia sp., which is among the first enzymes to respond to stressors, such as temperature [27]. GSTs are multifunctional proteins, being important phase II enzymes [40], and that in association with Glutathione (GSH) transform xenobiotics (i.e., foreign elements) into other conjugates as part of a detoxification route, playing major roles in the metabolic ‘purification’ and in the defense against ROS [41, 42]. Considering time of response, GSTs are known to react on a short-time scale (within an hour) to both temperature and salinity in copepods [27]. The antioxidant defenses (i.e., GST, CAT, SOD) are known to activate due to warming, both in cladocerans [43, 44], mussels [45] and crabs [46]. Moreover, Vehmaa et al. [21] showed that a rise by 3°C was affecting copepods’ oxidative balance by increasing ORAC and TBARS levels, whereas toxic cyanobacteria promoted antioxidative defenses (ORAC) and decreased oxidative damage (TBARS). In the amphipod Gammarus, Turja et al. [47] measured elevated antioxidative defenses (GST, GP glutathione peroxidase and SOD) as a response to nodularin, a common cyanobacteria toxin in the Baltic Sea. In our study area, surface pH and oxygen increased (Table 1), most likely due to microalgae (Chl a and cyanobacteria increased, personal observation), taking up DIC for growth and photosynthesis [48, 49]. The high dissolved oxygen (DO) concentrations during Week II (from 6.83 ± 0.75 to 11.43 ± 0.90 mg l-1, in deep layers and the surface, respectively) could also have influenced GST to respond. Our results are supported by measurements from the glacial relict Limnocalanus macrurus, exhibiting higher GSTs in elevated DO concentration [14]. Hermes-Lima et al. [50] also suggest that redox-sensitive transcription factors and antioxidant defense are activated under low oxygen levels or even hypoxia, in order to prepare the organisms for oxidative stress during reoxygenation phase, in which a sharp overproduction of ROS is expected to take place. Despite fast changing conditions [51], copepods experience widely varying conditions in their physico-chemical environment during diel vertical migration [7]. Nevertheless, vertical migration is an important way of escaping harmful conditions, as shown in euphausiid krill [42], whose redox status is positively affected by migration [52]. ORAC and SOD did not respond to environmental variables, and considering that they were fluctuating on a weekly basis, it suggests that the copepods were already facing oxidative stress, potentially as a response to differential allocation of resources to growth or reproduction. When antioxidants rise it indicates either a decrease in reproduction with no accelerating senescence, or a reduced investment in functions, such as immunity, causing increased risk of mortality [18]. In addition, CAT activity had an inverse relationship with salinity, with higher activity when the salinity was low and vice versa (Fig 2). This response in Acartia is interesting as the salinity changes in our study site are relatively small (Table 1). Whitfield et al. [53] showed that salinities 5–8 are critical for brackish-water animals inhabiting low salinity areas, including that even small salinity changes can be important for animal’s general performance. In the mud crab Scylla serrata, increasing salinity (from 10 to 35) induces an increase and/or decrease in the antioxidant enzyme activities depending on the tissue (abdominal muscle, hepatopancreas, or gills) considered [54]. In the white shrimp Litopenaeus vannamei, the SOD and CAT activities were increased at low salinity (3‰) in both the muscle and the hepatopancreas [55].
The oxidized glutathione (GSSG), which is an indication of oxidative stress, is generally maintained at a level of 1–10% of the total glutathione through reduction by glutathione reductase (GR) [56]. In the current work, the GSSG level was below the limit of quantification during most of Week I (except at 20 m), whereas it was detected during Week II, suggesting increased oxidative stress, due to changes in hydrography between Weeks I and II. In line with increased GSSG level and oxidative stress, GR activity was detected during Week II in the surface and at 15–25 m depth, and suggests oxidative stress in Acartia, as GR is activated upon accumulation of GSSG [57]. We measured elevated temperature in the surface during Week I, which probably caused increased antioxidants, in general (Table 3, 0–10 m). In terms of oxidative damage, differences in LPX levels between both weeks can at least partially be explained by increasing GST during Week II, as this enzyme is known to metabolize organic hydroperoxides by using reduced glutathione [58, 59]. Moreover, we showed that Acartia copepods residing in the deep during daytime responded by elevated LPX activity when the Chl a concentration was low. We are not totally sure what is the reason behind rising LPX; low food quantity and/or low food quality could be factors to consider. Long-term elevated oxidative stress status is harmful for the animal activity level, health status and general condition, and can result in decreased growth, reproduction, early ageing and even death [13]. Studies in Chesapeake Bay reported high numbers of dead Acartia [60], and the question arises whether copepod mortality is high, in general, in turbulent estuaries, or if the environmental quality of the site was bad [51]. Also in the current study site, in situ mortality of Acartia varies between 10–40% [61]. LPX is known to correlate with high age and mortality [62], and can cause both protein and ion channel inactivation, as well as protein damage through generation of reactive aldehydes, resulting in secondary protein carbonylation [63]. One effect of LPX is to increase membrane fluidity by introducing hydro peroxide groups, which open pores in the membrane causing leakiness, allowing substances to enter that do not normally pass it otherwise than via specific channels [64]. The oxidative stress levels can also be affected by nutritional conditions, because some of the antioxidant defenses are acquired from the food source [18]. The most important antioxidants are considered to be vitamin E and various carotenoids [65]. However, there is some contradicting opinions on how important dietary antioxidants are for combating ROS (cf. Monaghan et al. [18]), as some studies have over-emphasized their importance for ROS destruction [66]. Rodríguez-Graña et al. [67] and Saiz et al. [62] demonstrated that ageing copepods exhibit lower feeding and egg production, and higher oxidative damage rates than younger copepods. Even though we did not measure feeding and reproduction in the present study, ageing is a potential factor bringing variability into the results, and it is most likely a factor to be considered in further studies. We do not have age data of the copepods in the current work, sampled from a wild population, which probably consisted of individuals of various age. It also means that we collected copepods with different degrees of maturation and development of gonads, a process which is known to be associated with changes in biochemical composition, including levels of lipid peroxidation and antioxidants (see [68, 69] and references therein). Apart from ageing, another factor that may add to the variability of the results is the background (i.e., previous life history) of the copepods. In addition, salinity, both temperature, pH and oxygen varied substantially during the study. Considering that fluctuations keep the acclimation process up in animals residing in the natural environment [70], one must note that we do not account for how large the fluctuations were prior to the work in the present study.
Conclusions
In the present study, we found that copepods were quite robust in regards to pH encountered in the deep layers, and to values, similar as expected in oceans by the end of this century. This finding is in accordance with other studies showing that copepods seem fairly tolerant to hydrographical coastal variability. Copepods perform vertical migration on a daily basis, and could be adapted to extremely variable environment. One of these adaptations could be a highly efficient glutathione cycling system, functioning as an antioxidant defense system for neutralizing ROS and avoiding elevated levels of LPX. However, GST activities responded to elevated temperature, oxygen and pH experienced by the animals near the surface of Week II, suggesting that copepods were enhancing their antioxidant capacity to counterbalance the harmful effect of reactive oxygen species produced in response to warming and algal growth. This study highlights the importance of evaluating the antioxidant defense, as well as the oxidative stress in copepods. Future studies should consider the effect of environmental stressors on the trade-off between oxidative balance and reproductive success of Acartia.
Supporting information
(XLSX)
(TIF)
Acknowledgments
Hanna Halonen, Veijo Kinnunen, Jaana Koistinen and Mervi Sjöblom at Tvärminne Zoological Station are thanked for help with practical issues. Special thanks to Maria Kihlström who contributed to the field work both day and night. Many thanks to Prof. Mikko Nikinmaa (University of Turku) for his kind hospitality. We also express our warmest thanks for excellent collaboration with his lab staff and employees. Lastly, we would like to thank Victor Cubillos M. and the anonymous reviewers for constructive comments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Academy of Finland (project nr. 276947, http://www.aka.fi/en) to Jonna Engström-Öst and Societas pro Fauna et Flora Fennica (http://www.societasfff.fi/?lang=en) to Anni Rein. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321: 926–929. doi: 10.1126/science.1156401 [DOI] [PubMed] [Google Scholar]
- 2.Feely RA, Doney SC, Cooley SR. Ocean acidification: present conditions and future changes in a high CO2 world. Oceanography. 2009;22: 36–47. [Google Scholar]
- 3.Fujii T. Climate change, sea-level rise and implications for coastal and estuarine shoreline management with particular reference to the ecology of intertidal benthic macrofauna in NW Europe. Biology (Basel). 2012;1: 597–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Häder DP. Effects of solar UV-B radiation on aquatic ecosystems. Adv Space Res. 2000;26: 2029–2040. [DOI] [PubMed] [Google Scholar]
- 5.Waldbusser GG, Salisbury JE. Ocean acidification in the coastal zone from an Organism’s perspective: Multiple system parameters, frequency domains, and habitats. Annu Rev Mar Sci.2014;6: 221–247. [DOI] [PubMed] [Google Scholar]
- 6.Mauchline J. The biology of calanoid copepods. San Diego: Academic Press; 1998. [Google Scholar]
- 7.Almén AK, Vehmaa A, Brutemark A, Engström-Öst J. Coping with climate change? Copepods experience drastic variations in their physicochemical environment on a diurnal basis. J Exp Mar Bio Ecol. 2014;460: 120–128. [Google Scholar]
- 8.IPCC. The Physical Science Basis: Working Group I Contribution to the IPCC Fifth Assessment Report. Cambridge University Press, Cambridge, UK; 2014. [Google Scholar]
- 9.Kurihara H, Ishimatsu A. Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Mar Pollut Bull. 2008;56: 1086–1090. doi: 10.1016/j.marpolbul.2008.03.023 [DOI] [PubMed] [Google Scholar]
- 10.Whiteley NM. Physiological and ecological responses of crustaceans to ocean acidification. Mar Ecol Prog Ser. 2011;430: 257–271. [Google Scholar]
- 11.Vehmaa A, Brutemark A, Engström-Öst J. Maternal effects may act as an adaptation mechanism for copepods facing pH and temperature changes. PLoS ONE. 2012;7(10): e48538 doi: 10.1371/journal.pone.0048538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConville K, Halsband C, Fileman ES, Somerfield PJ, Findlay HS, Spicer JI. Effects of elevated CO2 on the reproduction of two calanoid copepods. Mar Pollut Bull. 2013;73: 428–434. doi: 10.1016/j.marpolbul.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 13.Lesser MP. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol. 2006;68: 253–278. doi: 10.1146/annurev.physiol.68.040104.110001 [DOI] [PubMed] [Google Scholar]
- 14.Abele D, Vásquez-Medina JP, Zenteno-Savín T. Eds. Oxidative stress in aquatic ecosystems. 1st ed Chichester, UK: John Wiley & Sons; 2012. [Google Scholar]
- 15.Puthumana J, Lee M-C, Park JC, Kim H-S, Hwang D-S, Han J, et al. Ultraviolet B radiation induces impaired life cycle traits and modulates expression of cytochrome P450 (CYP) genes in the copepod Tigriopus japonicus. Aquat Toxicol. 2017;184: 116–122. doi: 10.1016/j.aquatox.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 16.Vuori KA, Lehtonen KK, Kanerva M, Peltonen H, Nikinmaa M, Berezina NA, et al. Oxidative stress biomarkers in the copepod Limnocalanus macrurus from the northern Baltic Sea: effects of hydrographic factors and chemical contamination. Mar Ecol Prog Ser. 2015;538: 131–144. [Google Scholar]
- 17.Webster CN, Hansson S, Didrikas T, Gorokhova E, Peltonen H, Brierley AS, et al. Stuck between a rock and hard place: zooplankton vertical distribution and hypoxia in the Gulf of Finland, Baltic Sea. Mar Biol. 2015;162: 1429–1440. [Google Scholar]
- 18.Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett. 2009;12: 75–92. doi: 10.1111/j.1461-0248.2008.01258.x [DOI] [PubMed] [Google Scholar]
- 19.Lushchak VI. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol. 2011;101: 13–30. doi: 10.1016/j.aquatox.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 20.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74: 139–162. doi: 10.1152/physrev.1994.74.1.139 [DOI] [PubMed] [Google Scholar]
- 21.Vehmaa A, Hogfors H, Gorokhova E, Brutemark A, Holmborn T, Engström-Öst J. Projected marine climate change: effects on copepod oxidative status and reproduction. Ecol Evol. 2013;3: 4548–4557. doi: 10.1002/ece3.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogfors H, Motwani NH, Hadju S, El-Shehawy R, Holmborn T, Vehmaa A, et al. Bloom-forming cyanobacteria support copepod reproduction and development in the Baltic Sea. PLoS ONE.2014; 9(11): e112692 doi: 10.1371/journal.pone.0112692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira DC, Oliveira MF, Liz-Guimarães L, Diniz-Rojas N, Campos ÉG, Hermes-Lima M. Current trends and research challenges regarding “preparation for oxidative stress”. Front Physiol. 2017;8: 702 doi: 10.3389/fphys.2017.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havenhand JN. How will ocean acidification affect Baltic Sea ecosystems? An assessment of plausible impacts on key functional groups. Ambio. 2012;41: 637–644. doi: 10.1007/s13280-012-0326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabel K, Moros M, Porsche C, Neumann T, Adolphi F, Andersen TJ, et al. Impact of climate change on the Baltic Sea ecosystem over the past 1,000 years. Nat Clim Chang. 2012;2: 871–874. [Google Scholar]
- 26.Abele D, Großpietsch H, Pörtner HO. Temporal fluctuations and spatial gradients of environmental PO2, temperature, H2O2 and H2S in its intertidal habitat trigger enzymatic anti-oxidant protection in the capitellid worm Heteromastus filiformis. Mar Ecol Progr Ser. 1998;163: 179–191. [Google Scholar]
- 27.Cailleaud K., Maillet G, Budzinski H, Souissi S, Forget-Leray J. Effects of salinity and temperature on the expression of enzymatic biomarkers in Eurytemora affinis (Calanoida, Copepoda). Comp Biochem Physiol A. 2007;147: 841–849. [DOI] [PubMed] [Google Scholar]
- 28.Salonen K. Rapid and precise determination of total inorganic carbon and some gases in aqueous solutions. Water Res. 1981;15: 403–406. [Google Scholar]
- 29.Gorokhova E, Löf M, Reutgard M, Lindström M, Sundelin B. Exposure to contaminants exacerbates oxidative stress in amphipod Monoporeia affinis subjected to fluctuating hypoxia. Aquat Toxicol. 2013;127: 46–53. doi: 10.1016/j.aquatox.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 30.Kanerva M. The role of oxidative stress in environmental responses of Fennoscandian animals. PhD Dissert. University of Turku. 2014. Available from: http://www.doria.fi/handle/10024/101916
- 31.Turja R, Soirinsuo A, Budzinski H, Devier MH, Lehtonen KK. Biomarker responses and accumulation of hazardous substances in mussels (Mytilus trossulus) transplanted along a pollution gradient close to an oil terminal in the Gulf of Finland (Baltic Sea). Comp Biochem Physiol C Toxicol Pharmacol. 2013;157: 80–92. doi: 10.1016/j.cbpc.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 32.Souza MS, Modenutti BE, Carrillo P, Villar-Argaiz M, Medina-Sánchez JM, Bullejos F, et al. Stoichiometric dietary constraints influence the response of copepods to ultraviolet radiation-induced oxidative stress. Limnol Oceanogr. 2010;55: 1024–1032. [Google Scholar]
- 33.Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC)) of plasma and other biological and food samples. J. Agric Food Chem. 2003;51: 3273–3279. doi: 10.1021/jf0262256 [DOI] [PubMed] [Google Scholar]
- 34.Bou R, Codony R, Tres A, Decker EA, Guardiola F. Determination of hydroperoxides in foods and biological samples by the ferrous oxidation–xylenol orange method: A review of the factors that influence the method’s performance. Anal Biochem. 2008;377: 1–15. doi: 10.1016/j.ab.2008.02.029 [DOI] [PubMed] [Google Scholar]
- 35.Eymard S, Genot C. A modified xylenol orange method to evaluate formation of lipid hydroperoxides during storage and processing of small pelagic fish. Eur J Lipid Sci Technol. 2003;105: 497–501. [Google Scholar]
- 36.Vuori KA, Kiljunen M, Kanerva M, Koljonen ML, Nikinmaa M. Stock‐specific variation of trophic position, diet and environmental stress markers in Atlantic salmon Salmo salar during feeding migrations in the Baltic Sea. J Fish Biol. 2012;81: 1815–1833. doi: 10.1111/j.1095-8649.2012.03386.x [DOI] [PubMed] [Google Scholar]
- 37.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests in Linear Mixed Effects Models. R package version 2.0–33.2016. Available from: http://CRAN.R-project.org/package=lmerTest. [Google Scholar]
- 38.Dolédec S, Chessel D, ter Braak CJF, Champely S. Matching species traits to environmental variables: a new three-table ordination method. Environ Ecol Stat. 1996;3: 143–166. [Google Scholar]
- 39.Dray S, Dufour AB. The ade4 package: implementing the duality diagram for ecologists. J Stat Softw. 2007;22: 1–20. [Google Scholar]
- 40.Wang MJ, Wang WX. Cadmium in three marine phytoplankton: Accumulation, subcellular fate and thiol induction. Aquat Toxicol. 2009;95: 99–107. doi: 10.1016/j.aquatox.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 41.Frova C. Glutathione transferases in the genomics era: New insights and perspectives. Biomol Eng. 2006;23: 149–169. doi: 10.1016/j.bioeng.2006.05.020 [DOI] [PubMed] [Google Scholar]
- 42.Tremblay N., Gómez-Gutiérrez J, Zenteno-Savín T, Robinson CJ, Sánchez-Velasco L. Role of oxidative stress in seasonal and daily vertical migration of three krill species in the Gulf of California. Limnol Oceanogr. 2010;55: 2570–2584. [Google Scholar]
- 43.Borgeraas J, Hessen DO. UV-B induced mortality and antioxidant enzyme activities in Daphnia magna at different oxygen concentrations and temperatures. J Plankton Res. 2000;22: 1167–1183. [Google Scholar]
- 44.Wolinski L, Modenutti B, Souza MS, Balseiro E. Interactive effects of temperature, ultraviolet radiation and food quality on zooplankton alkaline phosphatase activity. Environ Pollut. 2016;213: 135–142. doi: 10.1016/j.envpol.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 45.Wilhelm Filho D, Tribess T, Gáspari C, Claudio FD, Torres MA, Magalhães ARM. Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture. 2001;203: 149–158. [Google Scholar]
- 46.Kong X, Wang G, Li S. Seasonal variations of ATPase activity and antioxidant defenses in gills of the mud crab Scylla serrata (Crustacea, Decapoda). Mar Biol. 2008;154: 269–276. [Google Scholar]
- 47.Turja R., Guimarães L, Nevala A, Kankaanpää H, Korpinen S, Lehtonen KK. Cumulative effects of exposure to cyanobacteria bloom extracts and benzo[a]pyrene on antioxidant defense biomarkers in Gammarus oceanicus (Crustacea: Amphipoda). Toxicon. 2014; 78: 68–77. doi: 10.1016/j.toxicon.2013.11.015 [DOI] [PubMed] [Google Scholar]
- 48.Hansen PJ. Effect of high pH on the growth and survival of marine phytoplankton; implications for species succession. Aquat Microb Ecol. 2002;28: 279–288. [Google Scholar]
- 49.Brutemark A., Engström-Öst J, Vehmaa A. Long term monitoring data reveal pH dynamics, trends and variability in the western Gulf of Finland. Oceanol Hydrobiol Stud. 2011;40: 91–94. [Google Scholar]
- 50.Hermes-Lima M, Moreira DC, Rivera-Ingraham GA, Giraud-Billoud M, Genaro-Mattos TC, Campos ÉG. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic Biol Med. 2015;89: 1122–1143. doi: 10.1016/j.freeradbiomed.2015.07.156 [DOI] [PubMed] [Google Scholar]
- 51.Martínez M, Rodríguez-Graña L, Santos L, Denicola A, Calliari D. Oxidative damage and vital rates in the copepod Acartia tonsa in subtropical estuaries with contrasting anthropogenic impact. J Exp Mar Biol Ecol. 2017;487: 79–85. [Google Scholar]
- 52.Tremblay N, Zenteno-Savín T, Gómez-Gutiérrez J, Maeda-Martínez AN. Migrating to the oxygen minimum layer: euphausiids In Abele D, Vásquez-Medina JP, Zenteno-Savín T, editors. Oxidative stress in aquatic ecosystems. Chichester, UK: John Wiley & Sons; 2012. pp. 89–98. [Google Scholar]
- 53.Whitfield AK, Elliott M, Basset A, Blaber SJM, West RJ. Paradigms in estuarine ecology—A review of the Remane diagram with a suggested revised model for estuaries. Estuar Coast Shelf Sci. 2012;97: 78–90 [Google Scholar]
- 54.Paital B, Chainy GBN. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp Biochem Physiol C. 2010;151: 142–151. [DOI] [PubMed] [Google Scholar]
- 55.Li E, Chen L, Zeng C, Yu N, Xiong Z, Chen X, et al. Comparison of digestive and antioxidant enzymes activities, haemolymph oxyhemocyanin contents and hepatopancreas histology of white shrimp, Litopenaeus vannamei, at various salinities. Aquaculture. 2008;274: 80–86. [Google Scholar]
- 56.De Almeida EA, Humberto Silva DG, Dias Bainy AC, Freitas FP, Motta FD, Gomes OF, et al. Evaluation of glutathione status in aquatic organisms In Abele D, Vásquez-Medina JP, Zenteno-Savín T, editors. Oxidative stress in aquatic ecosystems. Chichester, UK: John Wiley & Sons; 2012. pp. 381–388 [Google Scholar]
- 57.Vuori KA, Kanerva M, Ikonen E, Nikinmaa M. Oxidative stress during Baltic salmon feeding migration may be associated with yolk-sac fry mortality. Env Sci Technol. 2008;42: 2668–2673. [DOI] [PubMed] [Google Scholar]
- 58.Halliwell B, Gutteridge JMC. 2007. Free radicals in biology and medicine. Oxford: Oxford University Press; 2007. [Google Scholar]
- 59.Raja-aho S, Kanerva M, Eeva T, Lehikoinen E, Suorsa P, Gao K, et al. Seasonal variation in the regulation of redox state and some biotransformation enzyme activities in the barn swallow (Hirundo rustica L.). Physiol Biochem Zool. 2012;85: 148–158. doi: 10.1086/664826 [DOI] [PubMed] [Google Scholar]
- 60.Elliott DT, Tang KW. Influence of carcass abundance on estimates of mortality and assessment of population dynamics in Acartia tonsa. Mar Ecol Prog Ser. 2011;427: 1–12. [Google Scholar]
- 61.Engström-Öst J, Brutemark A, Vehmaa A, Motwani NH, Katajisto T. Consequences of a cyanobacteria bloom for copepod reproduction, mortality and sex ratio. J Plankton Res. 2015;37: 388–398. [Google Scholar]
- 62.Saiz E, Calbet A, Griffel K, Bersano JGF, Isari S, Solé M, et al. Ageing and caloric restriction in a marine planktonic copepod. Sci Rep. 2015;5: 14962 doi: 10.1038/srep14962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fritz KS, Petersen DR. Exploring the Biology of Lipid Peroxidation-Derived Protein Carbonylation. Chem Res Toxicol. 2011;24: 1411–1419. doi: 10.1021/tx200169n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong-ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys J. 2007;93: 4225–4236. doi: 10.1529/biophysj.107.112565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Catoni C, Peters A, Schaefer HM. Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Animal Behav. 2008;76: 1107–1119. [Google Scholar]
- 66.Caramujo MJ., de Carvalho CC, Silva SJ, Carman KR. Dietary carotenoids regulate astaxanthin content of copepods and modulate their susceptibility to UV light and copper toxicity. Mar Drugs. 2012; 10: 998–1018. doi: 10.3390/md10050998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodríguez-Graña L, Calliari D, Tiselius P, Hansen BW, Sköld HN. Gender-specific ageing and non-Mendelian inheritance of oxidative damage in marine copepods. Mar Ecol Prog Ser. 2010;401: 1–13. [Google Scholar]
- 68.Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Chastel O, et al. An experimental manipulation of life history trajectories and resistance to oxidative stress. Evolution. 2006;60: 1913–1924. [PubMed] [Google Scholar]
- 69.Pinheiro LC, Oliveira GT. Oxidative status profile in different tissues of Parastacus brasiliensis promatensis (Crustacea, Decapoda, Parastacidae) over a seasonal cycle. J Exp Zool A Ecol Genet Physiol. 2016;325: 318–328. doi: 10.1002/jez.2019 [DOI] [PubMed] [Google Scholar]
- 70.Lewis C.N., Kristina A.B., Edwards L.A., Cooper G., Findlay H.S., 2013. Sensitivity to ocean acidification parallels natural pCO2 gradients experienced by arctic copepods under winter sea ice. Proc Natl Acad Sci U S A. 2013;110: E4960–E4967. doi: 10.1073/pnas.1315162110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.