Abstract
Objective:
To investigate the effect of combination of dexmedetomidine and sufentanil on patient-controlled intravenous analgesia (PCIA) in patients after abdominal operation and to assess the safety and validity of this treatment.
Methods:
This is a prospective, randomized controlled, blinded, multicenter clinical study. A total of 210 patients from 9 clinical research centers underwent selective abdominal operation with general anesthesia were enrolled in the study, including laparoscopic-assisted abdominal operation on stomach, intestines or open surgery on stomach, intestines, kidneys and liver, the American Society of Anesthesiologists status I to II. Patients were randomly assigned into 2 groups: control group (group C) sufentanil 100 μg+normal saline 100 mL in total and test group (group D) sufentanil 100 μg+ dexmedetomidine 200 μg+normal saline 100 mL in total. PCIA was set as follow: background infusion of sufentanil 2 μg/h, bolus dose of sufentanil 2 μg, lockout interval 5 minutes. Main measure indices were analgesic consumption, pressing times and effective pressing times of analgesic pump, usage count, and consumption of remedy drug. Validity indices were visual analog scale (VAS) scores and patient satisfaction. Drug safety indices were hemodynamic parameters, drug side effects, and anal exhaust time.
Results:
In total, 203 cases were analyzed. Seven cases were eliminated for incomplete data record. The total consumption of sufentanil (μg) in 24 hours after operation of group C and group D were 56.9±21.5 and 49.8±15.5, respectively, and the difference was statistically significant (P<0.05). Pressing times of analgesic pump in 24 hours after operation of group C and group D were 9.47±16.07 and 5.02±5.56 times, respectively, and the difference was statistically significant (P<0.05). Effective pressing times of analgesic pump in 24 hours after operation of group C and group D were 7.8±9.7 and 4.57±5.02 times, respectively, and the difference was statistically significant (P<0.05). Resting VAS scores and movement VAS scores at 2, 4, 8, and 24 hours postoperatively were statistically different (P<0.05). Usage times of rescue drug (pethidine) of group C and group D were 9 and 1, mean rank 118.13 and 85.71, respectively, and the difference was statistically significant (P<0.05). Mean rank of general satisfaction of group C and group D were 98.99 and 105.04, respectively, and the difference was statistically significant (P<0.05). Incidence rate of nausea in group C and group D within 24 hours after surgery was 25% and 12.5%, and of vomiting 18.2% and 6.25%, respectively and of vomiting and the difference was statistically significant.
Conclusions:
Compared with sufentanil PCIA alone, the combination of dexmedetomidine and sufentanil for PCIA after abdominal operation could reduce sufentanil consumption, decrease VAS scores, lower the rate of nausea and vomiting, and improve patient satisfaction.
Key Words: dexmedetomidine, sufentanil, abdominal surgery, patient-controlled intravenous analgesia (PCIA)
Postoperative pain is pain immediately after an operation. It may develop into chronic postsurgical pain if have not been controlled in the initial stages. Postoperative pain after abdominal surgery is influenced by various factors including pathophysiological changes and subjective feelings of patients. The safe and effective analgesia method for postoperative pain may influence the outcome of patients after major abdominal surgery. Even restricted by different factors, patient-controlled intravenous analgesia (PCIA) is still the most commonly used analgesic method.1 Opioids are the most commonly used analgesics in PCIA. An increase in the dosage of a single drug may improve the postoperative pain; however, the adverse reactions also increase, such as neusea, vomiting, pruritus, and respiratory depression.
Dexmedetomidine, a highly selective α-2 adrenergic agonist with α-1: α-2 specificity of 1:1620, has hypnotic, sedative, analgesic, and antianxiey actions, but does not cause respiratory depression. Research2 has shown that dexmedetomidine regulates the analgesic actions at the spinal cord level or above by different mechanisms. Intraoperative administration of dexmedetomidine can enhance the analgesic effect of opioids and reduce opioid requirements in the perioperative period. However, it still lacks large sample and multicenter clinical trials to evaluate the effects of dexmedetomidine on patients after abdominal operation. This prospective, randomized controlled, blinded, multicenter clinical study was designed to demonstrate the safety and validity of PCIA with dexmedetomidine on patients after abdominal operation and provide a reliable basis for pain management.
METHODS
Research Agreements
This multicenter clinical study was completed by Affiliated Hospital of Nantong University and other 8 clinical centers, all approved by the bioethics committees and registered on Chinese Clinical Trial Registry (registration number ChiCTR-IPR-16007889), and all the patients have signed informed consents. The ethical processes of the study conformed to the “Declaration of Helsinki.”
Case Selection
Inclusion criteria consisted of patients who were able to communicate, underwent selective abdominal operation with general anesthesia, including laparoscopic-assisted abdominal operation on stomach, intestines or open surgery on stomach, intestines, kidneys, and liver, the American Society of Anesthesiologists status I to II, aged 18 to 65 years old, operation time 1 to 4 hours, awake and extubated. Exclusion criteria: (1) Allergic to dexmedetomidine or any drugs used during this study; (2) on long-term therapy with opioid analgesic, sedative, or nonsteroid anti-inflammatory drugs; (3) with neuromuscular disorders; (4) with endocrine system disease; (5) with allergic disorders; (6) with mental illness; (7) with cardiac conduction disturbances or allorhythmia, heart rate (HR)<50 bpm, systolic blood pressure (SBP)<100 mm Hg; (8) with blood loss of >600 mL or secondary operation; (9) enrolled in other clinical test within 3 months; and (10) other situations unsuitable for this study such as language communication difficulties. Researchers explained the use of visual analog scale (VAS) and analgesia pump to the patients that participated in the study.
Randomization and Blind Method
Groups were divided by random figure table, control group (group C) sufentanil 100 μg+ normal saline 100 mL in total and test group (group D) sufentanil 100 μg+ dexmedetomidine 200 μg+ normal saline 100 mL in total. The staff members, prepared the analgesic pump for the researchers, were blinded to the purpose of this study. The analgesic pump was set as followed: background infusion of sufentanil 2 μg/h, bolus dose of sufentanil 2 μg, lockout interval 5 minutes.
Methods of Anesthesia
The clinical research centers conducted a unified general anesthesia program, the 2 groups of patients with tracheal intubation anesthesia. Intraoperation, the bispectral index was applied while anesthesia was given to obtain the appropriate anesthesia depth and controlled between 40 and 60. Loading dose of sufentanil (0.15 μg/kg) was given 30 minutes before completion of the operation. Residual muscle relaxant effect was antagonized by neostigmine and atropine after the surgery. Ondansetron 8 mg or azasetron 10 mg was used before the abdominal cavity was closed to prevent nausea and vomiting. PCIA pump initiated after tracheal extubation with conscious sedation according to different groups. Administration mode: maintenance dose+patient-controlled analgesia dose, lock time as described. Patients were admitted to general wards after 1 hour in postanesthesia care unit.
Outcome Measures
Hemodynamics parameters (SBP, diastolic blood pressure, mean arterial pressure, HR), percutaneous oxygen saturation, and respiratory rate were recorded when patients entered the operative room, before the start-up of analgesic pump and 1, 2, 4, 8, 24, 48 hours after the start-up of analgesic pump. Total pressing times and effective pressing times of analgesic pump were recorded within 24 hours after the operation. Pain scores and sedation scores were recorded at 2, 4, 8, 24, and 48 hours after operation. The VAS was applied for the evaluation of postoperative pain at rest and movement. Observer’s assessment of alertness/sedation (OAA/S) scores were performed for the evaluation of sedation. The consumption of sufentanil during 24 hours after surgery was recorded. The levels of patient satisfaction regarding degree of pain relief were recorded at 24 and 48 hours after surgery, including very satisfied, satisfied, adequately satisfied, and dissatisfied. The type, dosage and usage times of rescue drugs were recorded at 24 and 48 hours after surgery. The dosage and usage times of vasoactive agents, anal exhaust time after operation and other adverse events such as nausea scores, vomiting scores, dry mouth, shivering, delirium, and pruritus were also recorded.
Statistical Analysis
According to the published literature3–5about sample size estimation, primary literature, and the results of meta-analysis, the sample size was calculated. The maximum sample size calculated by these 2 methods was considered as the minimum sample size in this research. Sample size was determined by VAS scores and the consumption of sufentanil. The 2 groups were compared with independent t test, P<0.05 as the standard for statistically significant, missing rate was 0.1, and on the basis of that, the number of samples was 216 and 108 for each group, 24 for each clinical center. The χ2 tests and the Fisher exact test were used to analyze baseline comparability, adverse events, and side effects. Mixed-effects models was used to analyze curative effect. And rank-sum test was performed for ranked data. Numerical values were performed with SPSS software, P<0.05 as statistically significant.
RESULTS
Figure 1 presents the CONSORT diagram of patient recruitment. Among the 210 patients enrolled, 7 were excluded because of incomplete data records. In total, 203 patients were statistically analyzed. There were no statistically significant differences in age, height, weight, sex, operation time, and consumption of sufentanil during surgery (Table 1).
FIGURE 1.
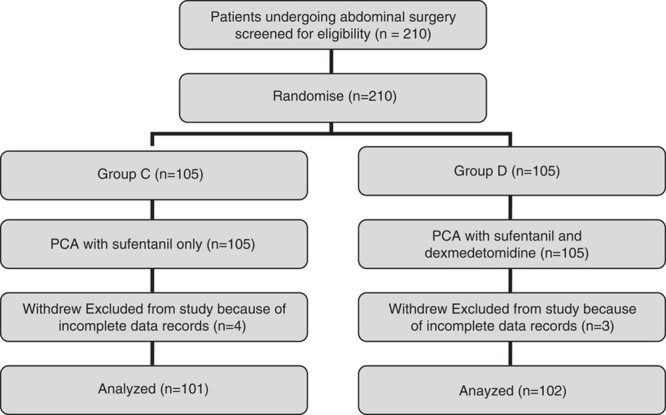
Patient enrollment flow diagram. This illustrates the flow of all patients. PCA indicate patient-controlled analgesia.
TABLE 1.
Clinical Characteristics of Patients (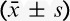 )
)
Indication of Drug’s Effectiveness
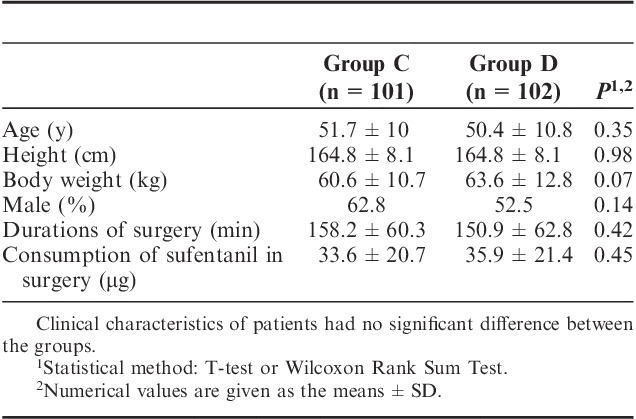
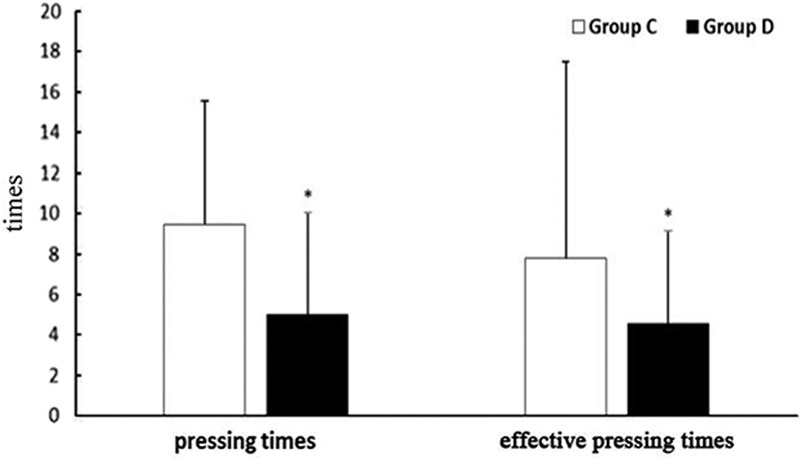
The total consumption of sufentanil within 24 hours after operation of group C and group D were 56.9±21.5 and 49.8±15.5 μg respectively, and the difference was statistically significant (P<0.05) (Fig. 2). These results indicate that PCIA with dexmedetomidine associated with opioids could reduce the demand of opioids in 24 hours after operation by the equivalent of 12.5% of sufentanil doses. Pressing times of analgesic pump in 24 hours after operation of group C and group D were 9.47±6.07 and 5.02±5.56 times, respectively, and the difference was statistically significant (P<0.05). Effective pressing times of analgesic pump in 24 hours after operation of group C and group D were 7.8±9.7 and 4.57±5.02 times, respectively, and the difference was statistically significant (P<0.05) (Fig. 3), which indicated that dexmedetomidine reduces total pressing times and effective pressing times of analgesic pump 24 hours after surgery. Resting VAS scores (VASr) and movement VAS scores (VASm) at 2, 4, 8, and 24 hours postoperatively were statistically different (P<0.05) (Figs. 4, 5), which indicated that dexmedetomidine alleviates pains in incision, which means a better analgesic effect. Usage times of rescue drug (pethidine) of group C and group D were 9 and 1, respectively, mean rank 118.13 and 85.71, respectively, and the difference was statistically significant (P<0.05) (Table 2). Mean rank of general satisfaction of group C and group D were 98.99 and 105.04, respectively, and the difference was statistically significant (P<0.05). There was no significant difference in the OAA/S scores at each timepoint between 2 groups (Fig. 6).
FIGURE 2.
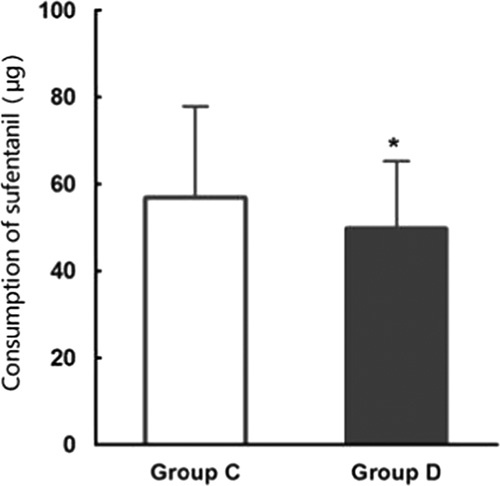
Total consumption of sufentanil in 24 hours after operation (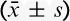 ). The total consumption of sufentanil in 24 hours after operation of group C and group D.
). The total consumption of sufentanil in 24 hours after operation of group C and group D.
FIGURE 3.
Pressing times and effective pressing times of analgesic pump within 24 hours after surgery (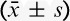 ). Pressing times and effective pressing times of analgesic pump within 24 hours after surgery, group D compared with group C (*P<0.05).
). Pressing times and effective pressing times of analgesic pump within 24 hours after surgery, group D compared with group C (*P<0.05).
FIGURE 4.
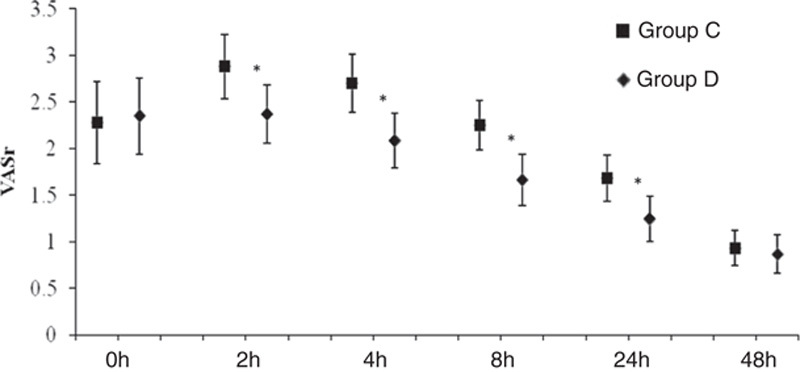
Mean (95% CI) of VAS resting. *P<0.05 group D compared with the group C.
FIGURE 5.
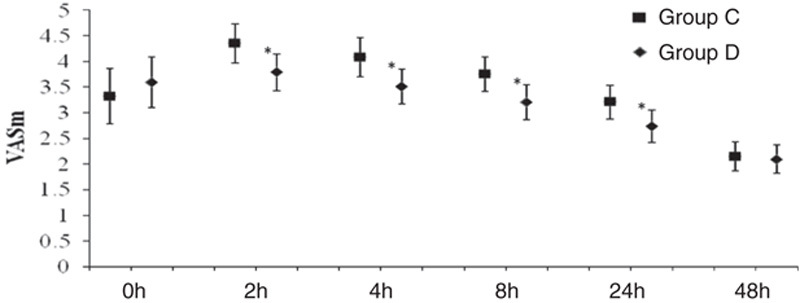
Mean (95% CI) of VAS movement. *P<0.05 group D compared with the group C.
TABLE 2.
Usage Time of Rescue Drug (Pethidine) and General Satisfaction Within 24 Hours After Surgery (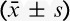 )
)
FIGURE 6.
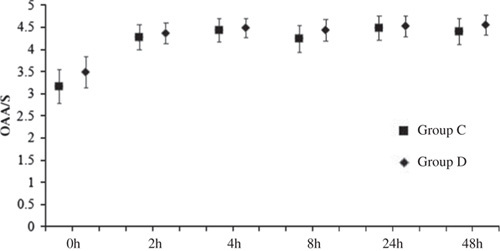
OAA/S scores (mean [95% CI]). The OAA/S scores at each timepoint have no significant difference between groups. P-value (time×group >0.05) for differences between the groups C and D over the 48-hour study period (mixed-effects models). CI indicates confidence interval; OAA/S, observer’s assessment of alertness/sedation. P>0.05 group D compared with the group C (Tukey-Kramer-adjusted).
Drug Safety Indices
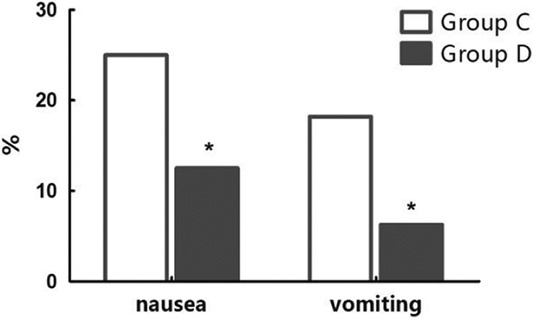
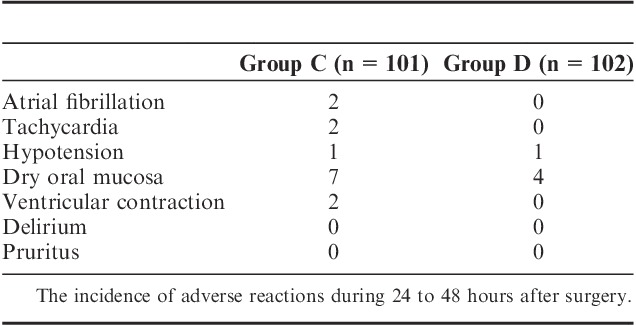
There were no significant differences in hemodynamics parameters results (SBP, diastolic blood pressure, mean arterial pressure, HR) and percutaneous oxygen saturation between 2 groups when patients entered the operative room (T0), before the start-up of analgesic pump (T1) and 1 (T2), 2 (T3), 4 (T4), 8 (T5), 24 (T6), 48 (T7) hours after surgery (Table 3). During postoperative 24 hours, Incidence rate of nausea in group C and group D, respectively, was 25% and 12.5%, and the incidence rate of vomiting was 18.2% and 6.25%. The differences were statistically significant (both P<0.05) (Figs. 7, 8). There were no significant differences in the incidence rate of other adverse reactions within 48 hours after surgery between the groups (χ2 test, P>0.05) (Tables 4, 5). Anal exhaust time (h) after surgery of group C and group D was 59.4±30.3 and 54.5±31, respectively, and there was no significant difference between the 2 groups (Fig. 8). One case of bradycardia (HR <50 bpm) was observed in test group, relieved by atropine, and 1 case of lung infection relieved after antibiotic treatment.
TABLE 3.
Changes of SBP, DBP, MAP, SpO2, HR (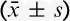 )
)
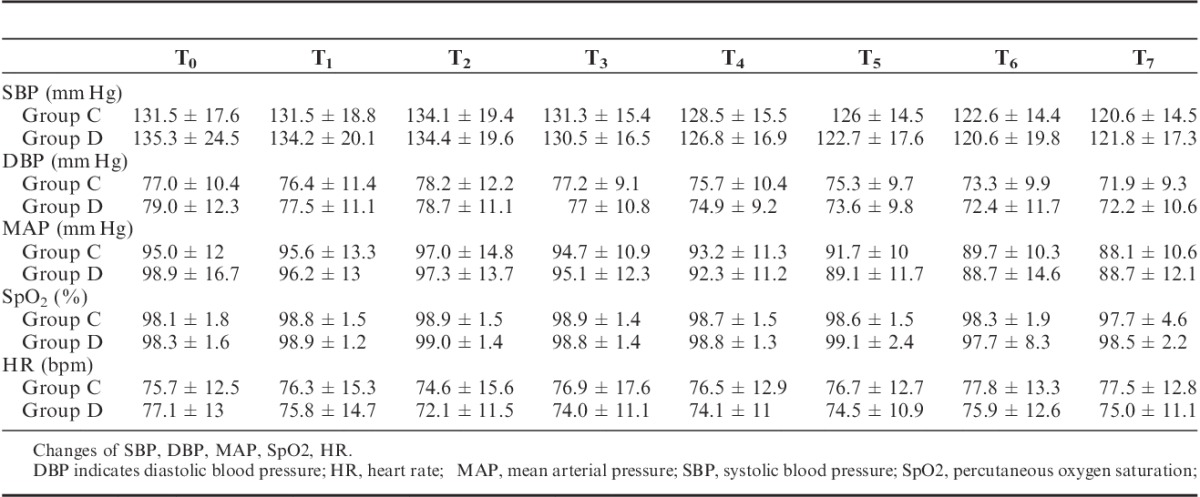
FIGURE 7.
The incidence of nausea vomiting within 24 hours after surgery. Incidence of nausea in group C and group D within 24 hours after surgery was 25% and 12.5%, and vomiting 18.2%, and 6.25%, respectively (*P < 0.05).
FIGURE 8.
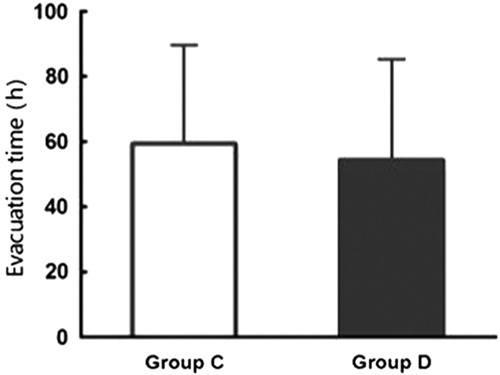
Anal exhaust time of group C and group D (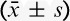 ).
).
TABLE 4.
The Incidence of Adverse Reactions Within 24 Hours After Surgery
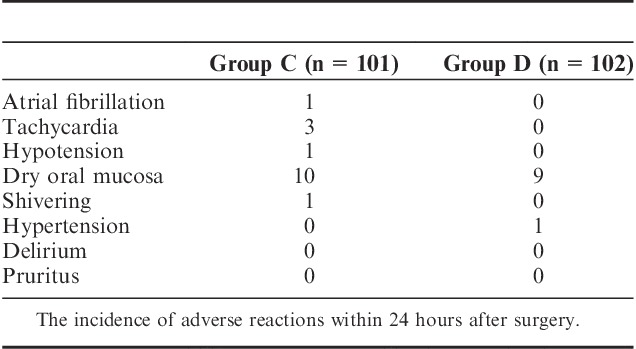
TABLE 5.
The Incidence of Adverse Reactions During 24 to 48 Hours After Surgery
DISCUSSION
This study indicated that PCIA with dexmedetomidine after abdominal operation could reduce the consumption of sufentanil, VAS pain scores, incidence of nausea and vomiting, improve patient satisfaction, without clinically relevant bradycardia, excessive sedation, and respiratory depression.
Adequate pain relief facilitates early mobilization and decreases stress reaction and organ dysfunction caused by postoperative pain, which is a prerequisite to postoperative rehabilitation.6–9 Although epidural analgesia could reduce the incidence of chronic pain,10 which has convincing side effects, potential disastrous risks, and a 17% to 37% failure rate.11–13 Nonepidural analgesia such as intravenous analgesia, transversus abdominis plane block,3,14 local anesthetic wound infiltration techniques15 are also used for analgesia after abdominal operation. A comparison between epidural analgesia and nonepidural analgesia found that patients with nonepidural analgesia had higher pain scores, which were still in the acceptable limits (VAS scores <4).16 According to the restrictions and other conditions, the authors think that intravenous analgesia is still most commonly used in patients after abdominal operation.
A systemic research suggests that dexmedetomidine can produce pain attenuation in Sprague-Dawley rats when applied >0.06 μg/kg (equivalent of 0.01 μg/kg in adults) and significant analgesia when it is 1∼10 μg/kg (equivalent of 0.16 to 1.6 μg/kg in adults). The analgesia mechanism differs as the dosage changes: low dose systemic dexmedetomidine facilitates spinal inhibitory postsynaptic potentials by disinhibiting a noradrenergic pathway. In contrast, high-dose systemic dexmedetomidine inhibits the locus coeruleus, perhaps leading to sedation, and also directly activates α2- adreneoceptors on the spinal glial neurons to induce outward potassium currents that hyperpolarize and inhibit the cells producing analgesia by a different mechanism. Many studies show that intravenous infusion of dexmedetomidine before, during or after operation reduces the postoperative consumption of opioids in PCIA,5,17,18 which confirms the analgesic effect of dexmedetomidine. Peng et al19 demonstrates in a meta-analysis that PCIA with dexmedetomidine (200 to 500 μg, not used before or during operation) reduces the consumption of opioids such as sufentanil, fentanyl, and morphine.
In most clinical trials, weight or weight index are considered as an important determinant index for drug doses.5,17,18 However, we find an interesting result that low-weight patients need more analgesic drugs while obese patients have lower VAS scores, which may be due to redistribution, or the phenomenon that individual drugs can be distributed to organs with greater blood flow first, then to organs with the lesser blood flow, but more fat soluble tissues. This is merely a guess, which needs a large sample of clinical trials to confirm.
Age, anxiety, and depression are important risk factors, which have significant effects on the level of postoperative pain in patients undergoing abdominal operation.20 Excluding sinus bradycardia, arrhythmia, myocardial ischemia, or above potential risk cases, the main consideration of cases selecting is more representative, but the actual ages of patients were 45 to 60 years old, this is mainly related to the predilection age of patients with liver, stomach, intestines, and other tumor patients, there is no difference in age between the 2 groups. Patients with mental illnesses such as anxiety and depression were excluded in the study to avoid the effect of mental state on the results.
Although minimally invasive surgery is a trend for the treatment of abdominal surgery disease in clinic, both the cases undergoing laparoscopic-assisted abdominal operation (the length of incision was 6∼8 cm) or open surgery were representative. Considering the different degrees of pain for these patients, the patients randomly assigned into groups. There were no significant differences in the operation type between these 2 groups. The concentration of dexmedetomidine and sufentanil is 2 and 1 μg/mL in patient-controlled analgesia, the equivalent of 0.05∼0.09 and 0.025∼0.045 μg.kg−1.h−1 for background dose. More, the same starting dose could avoid excessive analgesia and interference with the experimental results.
The application of dexmedetomidine in PCIA in patients after abdominal operation reduced the consumption of sufentanil, PCIA pressing times, and effective pressing times of analgesic pump, and the results were in accordance with reported literatures about application of dexmedetomidine in PCIA.5,17–19 The analgesia pump capacity was 100 mL, for most patients, it cannot last for 48 hours, hence the total consumption of sufentanil in postoperative 48 hours was not analyzed. Instead, we assessed pain intensity in postoperative 48 hours. The results indicated that peak response of pain intensity at 2, 4, 8 hours and relieved at 24 hours after operation, VASr and VASm below 4 scores in both groups at 48 hours, and there was no significant difference between the groups. VASm is more meaningful for early mobilization and recovery than VASr. Even though there was a small but positive effect on pain scores, other important outcomes such as reduced consumption of sufentanil, reduced effective pressing times, and reduced side effects were seen. So we think this difference of VAS between the groups still has clinical value.
Dexmedetomidine produces sedative effect in Sprague-Dawley rats when applied in a concentration higher than 1 μg/kg (equivalent of 0.16 μg/kg in adults). In this study there was no significant difference in the OAA/S scores between the 2 groups, which is in accordance with the reported literatures.5,17–19 It is possible that the small dose of dexmedetomidine does not produce a very strong sedative effect, which may be related to the awakening sedation of dexmedetomidine.
Compared with group C, incidence of nausea and vomiting was significantly decreased in group D. This might be related to the reduced dose of sufentanil. Previous studies have demonstrated that dexmedetomidine causes dose-dependent sedation, hypotension, and bradycardia.17,21 The large dose of dexmedetomidine leads to the decrease of HR and BP by decreasing plasma levels of norepinephrine and epinephrine.22–24 Al-Zaben et al25 reported that dexmedetomidine-related bradycardia was found in 6.67% of the patients in their study. Wu et al26 found that dexmedetomidine as an anesthetic adjuvant could cause bradycardia but no severe hypotension. Other researches27 showed that incidence of these side effects were increased in the patients that receive dexmedetomidine >0.7 μg.kg−1.h−1. In our study, the incidence of bradycardia was <1% in group D and was lower than that reported in the foreign literature.25
There was no significant difference in safety indices such as hemodynamic parameters between the groups, possibly reason might be the small dose of dexmedetomidine. and the 1 case of lung infection cannot rule out the effect of sufentanil. Although it is not statistically significant, much more attention should be paid to the potential risk of severe bradycardia caused by dexmedetomidine and the high sensitivity to sufentanil among some patients.
CONCLUSIONS
Compared with sufentanil PCIA alone, the combination of dexmedetomidine and sufentanil for PCIA after abdominal operation could reduce sufentanil consumption, decrease VAS scores, and the incidence of nausea and vomiting, improve patient satisfaction.
ACKNOWLEDGMENT
Professor Xinming Wu, MD, Department of anesthesiology, Peking university first hospital, Beijing, P.R. China, for valuable comments on the article.
Footnotes
Y.G. completed the design and clinical trials of this subject, who wrote the paper. X.W. guided the design, the other authors completed the clinical trials of each research center and participated in the project design.
The authors declare no conflict of interest.
REFERENCES
- 1.Niraj G, Kelkar A, Jeyapalan I, et al. Comparison of analgesic efficacy of subcostal transverses abdominis plane blocks with epidural analgesia following upper abdominal surgery. Anaesthesia. 2011;66:465–471. [DOI] [PubMed] [Google Scholar]
- 2.Yusuke F, Anthony EP, Daisuke U, et al. Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: an in vivo patch-clamp analysis of analgesic mechanisms. Pain. 2014;155:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin TF, Yeh YC, Lin FS, et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth. 2009;102:117–122. [DOI] [PubMed] [Google Scholar]
- 4.Lee W, Shin JD, Choe K, et al. Comparison of dexmedetomidine and ketamine for the analgesic effect using intravenous patient-controlled analgesia after gynecological abdominal surgery. Korean J Anesthesiol. 2013;65:S132–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nie Y, Liu Y, Luo Q, et al. Effect of dexmedetomidine combined with sufentanil for post-caesarean section intravenous analgesia: a randomized, placebo-controlled study. Eur J Anaesthesiol. 2014;31:197–203. [DOI] [PubMed] [Google Scholar]
- 6.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–198. [DOI] [PubMed] [Google Scholar]
- 7.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–1928. [DOI] [PubMed] [Google Scholar]
- 8.Carli F, Kehlet H, Baldini G, et al. Evidence basis for regional anesthesia in multidisciplinary fast-track surgical care pathways. Reg Anesth Pain Med. 2011;36:63–72. [DOI] [PubMed] [Google Scholar]
- 9.White PF, Kehlet H. Improving postoperative pain management: what are the unresolved issues? Anesthesiology. 2010;112:220–225. [DOI] [PubMed] [Google Scholar]
- 10.Esther A, Bouman EA, Theunissen M, et al. Reduced incidence of chronic postsurgical pain after epidural analgesia for abdominal surgery. Pain Pract. 2014;14:E76–E84. [DOI] [PubMed] [Google Scholar]
- 11.Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: evidence from published data. Br J Anaesth. 2002;89:409–423. [PubMed] [Google Scholar]
- 12.Scott DA, Chamley DM, Mooney PH, et al. Epidural ropivacaine infusion for postoperative analgesia after major lower abdominal surgery—a dose finding study. Anesth Analg. 1995;81:982–986. [DOI] [PubMed] [Google Scholar]
- 13.Stenseth R, Sellevold O, Breivik H. Epidural morphine for postoperative pain: experience with 1085 patients. Acta Anaesthesiol Scand. 1985;29:148–156. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet F, Berger J, Aveline C. Transversus abdominis plane block: what is its role in postoperative analgesia? Br J Anaesth. 2009;103:468–470. [DOI] [PubMed] [Google Scholar]
- 15.Ventham NT, Hughes M, O’Neill S, et al. Systematic review and meta-analysis of continuous local anaesthetic wound infiltration versus epidural analgesia for postoperative pain following abdominal surgery. Br J Surg. 2013;100:1280–1289. [DOI] [PubMed] [Google Scholar]
- 16.Joshi GP, Bonnet F, Kehlet H. Evidence-based postoperative pain management after laparoscopic colorectal surgery. Colorectal Dis. 2012;15:146–155. [DOI] [PubMed] [Google Scholar]
- 17.Schnabel A, Meyer-Friebem CH, Reichi SU, et al. Is intra-operative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain. 2013;154:1140–1149. [DOI] [PubMed] [Google Scholar]
- 18.Ren C, Chi M, Zhang Y, et al. Dexmedetomidine in postoperative analgesia in patients undergoing hysterectomy. Medicine. 2015;94:e1348–e1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng K, Liu HY, Wu SR, et al. Effects of combining dexmedetomidine and opioids for postoperative intravenous patient controlled analgesia: a systematic review and meta-analysis. Clin J Pain. 2015;31:1097–1104. [DOI] [PubMed] [Google Scholar]
- 20.Caumo W, Schmidt AP, Schneider CN, et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand. 2002;46:1265–1271. [DOI] [PubMed] [Google Scholar]
- 21.Blaudszun G, Lysakowski C, Elia N, et al. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. [DOI] [PubMed] [Google Scholar]
- 22.Talke P, Richardson CA, Scheinin M, et al. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–1142. [DOI] [PubMed] [Google Scholar]
- 23.Ebert TJ, Hall JE, Barney JA, et al. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. [DOI] [PubMed] [Google Scholar]
- 24.Bloor BC, Ward DS, Belleville JP, et al. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–1142. [DOI] [PubMed] [Google Scholar]
- 25.Al-Zaben KR, Qudaisat IY, Abu-Halaweh SA, et al. Comparison of caudal bupivacaine alone with bupivacaine plus two doses of dexmedetomidine for postoperative analgesia in pediatric patients undergoing infra-umbilical surgery: a randomized controlled double-blinded study. Paediatr Anaesth. 2015;25:883–890. [DOI] [PubMed] [Google Scholar]
- 26.Wu HH, Wang HT, Jin JJ, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9:e93114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36:926–939. [DOI] [PubMed] [Google Scholar]


