Abstract
An antiangiogenic state might constitute a terminal pathway for the multiple aetiologies of pre-eclampsia, especially those resulting from placental abnormalities. The levels of angiogenic and antiangiogenic proteins in maternal blood change prior to a diagnosis of pre-eclampsia, correlate with disease severity and have prognostic value in identifying women who will develop maternal and/or perinatal complications. Potential interventions exist to ameliorate the imbalance of angiogenesis and, hence, might provide opportunities to improve maternal and/or perinatal outcomes in pre-eclampsia. Current strategies for managing pre-eclampsia consist of controlling hypertension, preventing seizures and timely delivery of the fetus. Prediction of pre-eclampsia in the first trimester is of great interest, as early administration of aspirin might reduce the risk of pre-eclampsia, albeit modestly. Combinations of biomarkers typically predict pre-eclampsia better than single biomarkers; however, the encouraging initial results of biomarker studies require external validation in other populations before they can be used to facilitate intervention in patients identified as at increased risk. Angiogenic and antiangiogenic factors might also be useful in triage of symptomatic patients with suspected pre-eclampsia, differentiating pre-eclampsia from exacerbations of pre-existing medical conditions and performing risk assessment in asymptomatic women. This Review article discusses the performance of predictive and prognostic biomarkers for pre-eclampsia, current strategies for preventing and managing the condition and its long-term consequences.
Introduction
Current management of pre-eclampsia consists of controlling maternal hypertension, prevention of seizures and timely delivery of the fetus. Although understanding of the pathophysiology of this important obstetric syndrome remains elusive,1 efforts are underway to identify biomarkers that can predict pre-eclampsia as early as the first trimester.2 Subgroup findings from meta-analyses and small randomized controlled trials of aspirin3–6 and combinations of nitric oxide donors (L-arginine) and antioxidants (vitamins E and C)5 for the prevention of pre-eclampsia are promising. However, these preventive strategies remain investigational. An imbalance between angiogenic and antiangiogenic factors has emerged as an important pathogenetic mechanism in pre-eclampsia.7–11 The levels of these proteins in maternal blood, especially when measured in patients suspected of having pre-eclampsia <34 weeks of gestation, have prognostic value in identifying patients who will develop maternal and/or perinatal complications.12–16 Importantly, several potential interventions could ameliorate an imbalance between angiogenic and antiangiogenic factors17–22 and, hence, might provide opportunities to improve maternal and/or perinatal outcomes. Patients with pre-eclampsia are at increased risk of long-term complications, such as cardiovascular disease,23 renal disease24 and metabolic syndrome.23 This Review discusses the potential clinical value of biomarkers, focusing on the use of angiogenic and antiangiogenic factors for prediction and monitoring of pre-eclampsia. Current management, possible interventions and long-term consequences of pre-eclampsia are also discussed.
Biomarkers that predict pre-eclampsia
Considerable efforts have been made to identify biomarkers that can predict pre-eclampsia as early as the first trimester,2 as some evidence suggests that patients could benefit from early (<16 weeks of gestation) administration of aspirin or combinations of nitric oxide donors and antioxidants.3–6 Risk assessment of patients with pre-eclampsia using biochemical markers25,26 (such as pregnancy-associated plasma protein A [PAPP-A],27 inhibin A, activin A, α-fetoprotein [AFP] and free choriogonadotropin subunit β [CG-β]28,29), placental morphology and/or perfusion30 and uterine artery Doppler velocimetry (UtADV) in the first or second trimesters (Figure 1),31 has not provided encouraging results. Plasma levels of placental protein 13 (PP13, also known as galactoside-binding soluble lectin 13 or galectin-13) in the first trimester were predictive of early pre-eclampsia in two studies,32,33 but these findings were not subsequently corroborated.27,34,35
Figure 1. Uterine artery Doppler velocimetry findings in the second trimester of pregnancy.
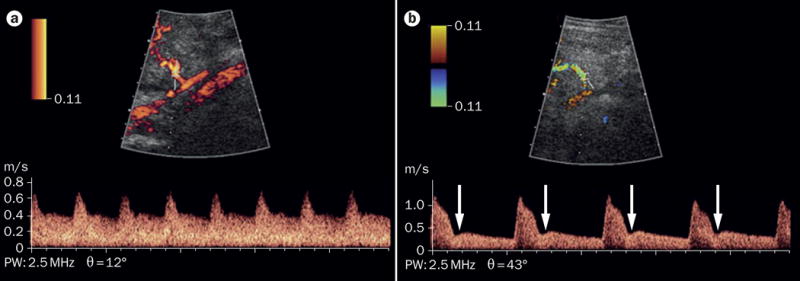
A. Normal findings. B. Abnormal findings, indicated by either the presence of bilateral uterine artery early diastolic notches (arrows) or a mean pulsatility index (calculated as [peak systolic velocity – end diastolic velocity]/time averaged velocity, averaged across both uterine arteries), above the 95th percentile for gestational age.
Combinations of biomarkers generally have better diagnostic performance than single biomarkers in predicting pre-eclampsia.2,25 In a systematic review, combinations of two or more of the seven most widely studied serum biomarkers—A disintegrin and metalloproteinase domain-containing protein 12 (ADAM 12), free CG-β, inhibin A, activin A, PP13, placental growth factor (PlGF) and PAPP-A)—in the first trimester identified 55–75% of patients with early pre-eclampsia (delivery <34 weeks) and 30–40% of all patients with pre-eclampsia, with a false-positive rate of 10%.2 Three studies have reported strong prognostic performance for multiple biomarkers in the prediction of pre-eclampsia as early as in the first trimester; however, these studies involved imputed or simulated data and are, accordingly, not directly comparable to those that used traditional analytical methods.36–38 In the first study, an estimated 91.0%, 79.4% and 60.9% of individuals with early (<34 weeks), intermediate (34–36 weeks) and late (>37 weeks) pre-eclampsia (on the basis of gestational age at delivery), respectively, could be identified with a 10% false-positive rate.36 The first study used a combination of maternal characteristics, obstetric history, UtADV measurements, mean arterial pressure and maternal serum levels of PAPP-A, PP13, inhibin A, lifeactivin A, soluble endoglin, pentraxin-3 and P-selectin, determined in the first trimester (11–13 weeks of gestation).36 The second and third studies used competing risk models (survival function) to predict pre-eclampsia. In the second, an estimated 80.0%, 54.6% and 34.9% of patients with pre-eclampsia who delivered at <34 weeks, <37 weeks and <42 weeks, respectively, could be identified using a combination of maternal characteristics, UtADV results and mean arterial pressure, with a 10% false-positive rate.37 The third study additionally incorporated serum biomarkers (PAPP-A and PlGF), with the result that an estimated 96.3%, 76.6% and 53.6% of these patients could be identified with the same false-positive rate.38
Although several marker combinations (especially those including UtADV measurements in the first trimester) have poor performance for detecting all cases of pre-eclampsia, they are much better at identifying early pre-eclampsia.39 The encouraging results of these biomarker studies nonetheless require validation in independent sets of samples.40,41 In addition, for these biomarkers to have clinical utility, effective interventions must be developed for those identified as at increased risk, before their use can be implemented in clinical practice. 42
Other systems biology approaches to identify biomarkers for pre-eclampsia include proteomics,43,44 metabolomics,45–47 measuring cell-free fetal DNA48,49 and quantifying cell-free mRNAs encoding relevant proteins, such as corticotrophin-releasing hormone, placenta-specific protein 1, P-selectin,50 vascular endothelial growth factor (VEGF) receptor 1 (VEGFR-1) and endoglin.51 Early studies that quantified cell-free fetal DNA (which is thought to originate from apoptotic trophoblasts) for the prediction of pre-eclampsia before 20 weeks of gestation yielded encouraging results.48,52 However, subsequent studies could not replicate these findings.53,54 Studies using transcriptomic,50,55,56 proteomic43,44 and metabolomic45–47 approaches have shown promising results, even in the first trimester, according to several case-control studies; however, large prospective cohort studies are required to validate these results.
Research in this area focuses not only on the identification of biomarkers that can predict pre-eclampsia, but also on predictors of adverse perinatal outcomes after diagnosis of pre-eclampsia. For example, the full PIERS (pre-eclampsia integrated estimate of risk) model was developed in 2011 to predict fatal or life-threatening maternal complications of pre-eclampsia within 48 h of admission, using standard clinical and laboratory information.57 This model was validated using a threshold of ≥10% predicted probability to define a positive test, and predictive variables obtained within 6 h and 24 h of admission. The full PIERS model identified 44% and 57% of the women who would later develop adverse outcomes at these two respective time points, resulting in positive predictive values (PPVs) of 24% and 26%.58
An imbalance between angiogenic and antiangiogenic factors has emerged as an important pathogenetic mechanism in pre-eclampsia,59,60 and evidence indicates that maternal plasma levels of these factors can identify the majority of patients who will develop early pre-eclampsia.61,62 Moreover, levels of these biomarkers correlate with disease severity59,60,63,64 and have prognostic value in identifying women who subsequently develop maternal and/or perinatal complications, especially in patients suspected of having pre-eclampsia at <34 weeks of gestation.12–15,65,66
An emerging debate is whether pre-eclampsia is a homogeneous disease.67 Critics argue that assessing a few biomarkers involved in only one pathway of disease (angiogenesis) is unlikely to predict or detect all women with pre-eclampsia, a disease that has multiple aetiologies.68 Some investigators counter this argument with the proposal that the forms of pre-eclampsia associated with an angiogenic imbalance are those that result in adverse outcomes.67,69 Furthermore, reported evidence of heterogeneity in the literature might be largely due to misclassification of other diseases as pre-eclampsia, as the criteria for its diagnosis (such as hypertension and proteinuria) are nonspecific.67 However, several questions should be answered prior to concluding that pre-eclampsia is a homogenous disease. For example, the criteria used to define angiogenic imbalance (including fixed cut-offs derived from receiver operating characteristic [ROC] curve analyses, multiples of median values or percentile distributions of analyte levels) have yet to be agreed. Consequently, it is difficult to ascertain what proportion of patients with pre-eclampsia truly has an angiogenic imbalance. Most women with preterm pre-eclampsia have abnormal profiles of angiogenic and/or antiangiogenic factors, but this is not the case in women with pre-eclampsia at term (≥37 weeks of gestation).64,61 It is uncertain that patients who do not have abnormal ratios of angiogenic to antiangiogenic factors at term have been misclassified as having pre-eclampsia. Most patients with pre-eclampsia are diagnosed at term, and eclampsia is also not uncommon after 37 weeks gestation. Moreover, what causes the antiangiogenic state in the first place remains unclear.
Our view is that if an imbalance between angiogenic and antiangiogenic factors represents the terminal pathway of multiple aetiologies, it is possible that assessing biomarkers of angiogenesis might identify most patients with pre-eclampsia, especially the forms of this disorder resulting from placental abnormalities (such as early pre-eclampsia), which have a major adverse effect on perinatal outcomes.61,67,70 Indeed, our research group has observed that ~80–90% of women with preterm pre-eclampsia and 40–50% of those with pre-eclampsia at term have abnormal plasma PlGF:sVEGFR-1 or PlGF:soluble endoglin ratios, (defined as being below the 10th percentile for gestational age of uncomplicated pregnancies) within the 7 days prior to delivery (Chaiworapongsa et al., unpublished work).
Clinical value of biomarker assessment
Potential interventions exist to ameliorate an imbalance between angiogenic and antiangiogenic factors, and hence provide opportunities to improve maternal and/or perinatal outcomes in patients with pre-eclampsia. Studies evaluating the potential clinical utility of measuring angiogenic and antiangiogenic factors in pre-eclampsia have focused on three scenarios: firstly, triage of symptomatic patients suspected of having pre-eclampsia; secondly, differentiation of pre-eclampsia from exacerbations of pre-existing medical conditions; and thirdly, risk assessment in asymptomatic women.
Triage of women with suspected pre-eclampsia
Patients with pre-eclampsia can present with some, but not all, of the symptoms of pre-eclampsia and/or features similar to other conditions. Accurate diagnosis of pre-eclampsia by a combination of clinical symptoms and biomarkers might improve the management of patients who are at risk of adverse outcomes. Indeed, determination of plasma levels of angiogenic and antiangiogenic factors in combination with assessment of clinical variables, in patients with suspected pre-eclampsia who present before 34–35 weeks of gestation, improves the detection of patients who are likely to require preterm delivery or develop adverse outcomes within 2 weeks, compared with standard evaluations based on clinical factors or standard laboratory tests for pre-eclampsia alone.12–15,65,66,71 Moreover, implementation of this strategy reduced costs and usage of health-care resources, according to a cost-effectiveness analysis.72 Larger studies, using appropriate algorithms for management of patients with suspected pre-eclampsia according to the results of these biomarkers, are urgently needed.
Differentiation from pre-existing conditions
The diagnosis of pre-eclampsia in patients with chronic kidney disease (CKD) is challenging, especially in patients who have proteinuria or high blood pressure before 20 weeks of gestation. Indeed, in nonpregnant women, higher plasma levels of sVEGFR-1 have been observed in patients with CKD than in healthy controls.73 This elevation correlates with serum levels of von Willebrand factor (a marker of endothelial dysfunction), suggesting that sVEGFR-1 might be associated with endothelial dysfunction and future cardiovascular risk in these patients.74
The potential clinical utility of angiogenic and antiangiogenic factors in differentiating superimposed pre-eclampsia from exacerbations of pre-existing medical conditions has been proposed in several case reports relating to pregnant women with nephrotic syndrome,75 systemic lupus erythematosus76 or those with end-stage renal disease undergoing haemodialysis.77 Subsequently, in two case–control studies, higher serum levels of sVEGFR-1 and lower serum levels of PlGF were reported in patients with CKD having superimposed pre-eclampsia than in women with CKD having normal pregnancies.78,79 However, the diagnostic performance of these biomarkers in a clinical setting has yet to be reported.
Risk assessment in asymptomatic women
Observational studies examining the predictive performance of measuring angiogenic and antiangiogenic factors in asymptomatic pregnant women have yielded inconsistent results.61,80–84 This observation is partly explained by differences in the timing of sample collection (first, second or third trimester), case definitions (early, late or all pre-eclampsia) and statistical methods (logistic regression, fixed specificity or ROC curve analysis).7–11 However, most data suggest that these factors are unlikely to be useful as early biomarkers of pre-eclampsia in asymptomatic patients at <16 weeks of gestation.84 The predictive performance of these biomarkers for identifying patients at risk of developing pre-eclampsia generally increases with advancing gestational age at the time of sample collection (that is, they perform best when the evaluation is done within 5 weeks before the clinical presentation), or when trying to predict early rather than late disease.61,82 The magnitude of the association between maternal plasma levels of angiogenic and antiangiogenic factors evaluated in the late second trimester (20–25 weeks) is stronger for early pre-eclampsia than for late disease.61 The predictive performance of biomarkers for late pre-eclampsia is improved if the evaluation is performed in the third trimester.85,86 However, in most studies, the majority of patients with an imbalance between angiogenic and antiangiogenic factors do not subsequently develop pre-eclampsia.81–87 Such tests, therefore, have low PPVs regardless of the timing of sample collection.81–87
Although a low PPV is often interpreted as a lack of clinical utility in biomarker studies,61,81,82,84 PPV is also a function of disease prevalence; the prevalence of pre-eclampsia is typically 3–5%.85 A test with 90% sensitivity and 90% specificity for identifying patients at risk of a condition that has this prevalence would achieve a PPV of only approximately 30%. PPV by itself is, therefore, generally a poor indicator of biomarker utility in uncommon diseases.86 Evidence supporting this view comes from the quadruple screening programme for Down syndrome, which has a prevalence of 1:800.89 Screening has a PPV <1% for a positive test result, which represents a >1:273 chance of the fetus being affected.89 Nevertheless, without safe and effective interventions, it is difficult to establish the clinical utility of screening for pre-eclampsia on the basis of angiogenic and antiangiogenic factors or other biomarkers.
Management of pre-eclampsia
The management of pre-eclampsia focuses on the control of acute hypertension, the prevention of seizures and timely delivery of the fetus. In a patient with pre-eclampsia who is near or at term (≥37 weeks gestation), when the fetus is mature, delivery is an effective way to treat the disorder and optimize pregnancy outcomes (Figure 2). In preterm gestations, the risk of continuing the pregnancy in the face of a multisystemic disorder must be balanced against the risks of premature birth.90 Delivery is indicated when life-threatening maternal complications are present or impending, such as severe hypertension refractory to treatment (which places the mother at risk of stroke), pulmonary oedema, acute renal failure, hepatic rupture or eclampsia.90 Delivery would also be indicated if a viable fetus is at risk of impending death. The mode of delivery (vaginal versus caesarean) depends on obstetric indications (such as fetal distress or previous classic caesarian deliveries). Attempted induction of labour does not seem to increase neonatal morbidity, but is rarely successful at <28 weeks in these patients.91 Detailed discussion of the expectant management of women who have pre-eclampsia with severe features at <34 weeks of gestation, including patient selection, treatment and delivery indications, can be found elsewhere.90,92
Figure 2. Management of pre-eclampsia.

Management of pre-eclampsia depends on the severity of the disease (with or without severe features) and gestational age at diagnosis.134 For pre-eclampsia without severe features, delivery is recommended at term (≥37 weeks). For pre-eclampsia with severe features, delivery is recommended if gestational age is at ≥34 weeks. Before 34 weeks of gestation, the decision to deliver should be balanced between risk of maternal or fetal complications and benefit of continuing pregnancy to fetal maturity.
*Patients with gestational hypertension or mild pre-eclampsia after 36 weeks who undergo induction of labour have a reduced rate of adverse maternal outcomes (especially the development of severe hypertension), lower incidence of caesarean delivery and a better quality of life than those who had expectant management.130–133 If preterm induction of labour is contemplated, steroids are administered between 24 weeks and 34 weeks of gestation to improve fetal lung maturity. Magnesium sulfate is administered during labour and the first 24 h after delivery for seizure prophylaxis.104
Abbreviations: BUN, blood urea nitrogen; CBC, complete blood count; GA, gestational age; HELLP, haemolysis, elevated liver transaminases, low platelets; sCr, serum creatinine.
Acute onset, severe hypertension
The primary goal of treating hypertension in patients with pre-eclampsia is to prevent an acute hypertensive crisis, which might lead to intracranial haemorrhage or stroke. Acute-onset, persistent (lasting ≥15 min) and severe hypertension (systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥110 mmHg) requires immediate treatment.90,93 This recommendation is based on a report describing 28 women with severe pre-eclampsia who developed stroke; all but one of these individuals had a systolic blood pressure ≥160 mmHg just before haemorrhagic stroke, whereas 13% had a diastolic blood pressure ≥110 mmHg within 6–12 h preceding stroke.94 Thus, the goal of antihypertensive therapy is not to normalize blood pressure, but to maintain uteroplacental perfusion and achieve a blood pressure within the range of 140–160/90–100 mmHg, above which loss of autoregulation of cerebral vasculature occurs.
Every professional organization uses different blood pressure thresholds to prompt antihypertensive treatment in women with pre-eclampsia during the nonacute setting. For example, the National Institute for Health and Clinical Excellence in the UK recommends antihypertensive medication if blood pressure is >150/100 mmHg.95 The Society of Obstetricians and Gynaecologists of Canada recommends starting antihypertensive therapy at blood pressure levels of 160/110 mmHg,96 as does the American Congress of Obstetricians and Gynecologists.90 By contrast, the Society of Obstetric Medicine of Australia and New Zealand recommends antihypertensive treatment if systolic blood pressure is >170 mmHg or diastolic blood pressure >110 mmHg.97 Additional information can be found in various professional organizations’ guidelines.90,95–97
Control of blood pressure should be achieved before delivery, even in urgent circumstances, such as eclampsia;98 endotracheal intubation for a caesarean delivery increases maternal blood pressure, sometimes to severe levels.98 Hydralazine, labetalol and nifedipine are the three most commonly used agents in the acute setting (Table 1).99 Nimodepine, ketanserin and diazoxide were not recommended for the management of severe hypertension during pregnancy because nimodepine and ketanserin were associated with more persistent high blood pressure than hydralazine and diazoxide was associated with a higher risk of hypotension than labetalol.100 Sodium nitroprusside is reserved only for the rare patients in whom hypertension is refractory to other agents, because of concerns related to cyanide and thiocyanate toxicity in the mother and baby, and potential worsening of cerebral oedema in the mother.93
Table 1.
Therapeutic agents for control of severe hypertension in pregnancy
| Agent | Mechanism | Dose | Adverse effects | Comments |
|---|---|---|---|---|
| Hydralazine | Vasodilator | 5 mg (intravenous or intramuscular), then 5–10 mg every 10–40 min, or constant infusion of 0.5–10 mg/h | Risk of delayed maternal hypotensionFetal bradycardia | Substantial experience of safety and efficacy |
| Labetalol | α- and β-blocker | 20 mg (intravenous), then 20–80 mg every 5–15 min (maximum 300 mg), or constant infusion of 1–2 mg/min | Risk of neonatal bradycardia Should be avoided in women with asthma or heart failure |
Lower risk of tachycardia and arrhythmia than vasodilators Increasingly preferred as first-line agent |
| Nifedipine | Calcium channel antagonist | 10–30 mg (oral), repeat after 45 min if needed* | Tachycardia, but is seldom associated with palpitations Flushing, headache, sweaty palms133 |
Possible interference with labour or synergistic effects with magnesium sulfate have not been proven134 |
Blood pressure falls within 5–10 min of a capsule being bitten and swallowed, and 10–30 min with oral administration.133 All agents are FDA category C. Information in this Table is used with permission and was partly obtained from Chesley’s Hypertensive Disorders in Pregnancy, Lindheimer, M. D. et al. (eds)
Prevention of seizures
The development of seizures and/or coma is a characteristic of eclampsia, and increases the risk of maternal and perinatal death, as well as other complications (such as disseminated intravascular coagulation, pulmonary oedema, acute renal failure and cardiopulmonary arrest). The onset of eclampsia can be antepartum (38–53%), during labour (18–36%) or postpartum (11–44%).101 Although most patients with postpartum eclampsia present within 48 h after delivery, some can occur as late as 23 days postpartum.101
The agent of choice for the prevention of seizures or recurrent seizure episodes in eclampsia is magnesium sulfate (Box 1),102 which reduces the rate of seizures by 52% when compared to diazepam, and by 67% when compared with phenytoin.103 Clinicians generally agree that magnesium sulfate should be administered to patients with pre-eclampsia who have severe features (number needed to treat [NNT] 63–71).104 However, whether this agent is required in the management of pre-eclampsia without severe features (NNT 109–400) is currently unknown.104 Treatment with magnesium sulfate is associated with a reduced rate of eclampsia (RR 0.4) and placental abruption (RR 0.64), but an increased rate of caesarean delivery (RR 1.05) compared with placebo or no anticonvulsant.105
Box 1. Use of magnesium sulfate for seizure prophylaxis in pre-eclampsia.
Continuous intravenous infusion
Loading dose of magnesium sulfate (4–6 g administered over 15–20 min)
Maintenance infusion with 1–2 g/h
Maintain magnesium sulfate concentration between 480 mg/l and 840 mg/l
Monitor urinary output and if <25–30 ml over 2 consecutive hours, oliguria is diagnosed; fluid status and magnesium level should be assessed and magnesium infusion should be decreased or discontinued
Monitor magnesium toxicity by combination of assessment of deep tendon reflexes and respiratory rate
Discontinue infusion 24 h after delivery
Intermittent intramuscular injection (when intravenous administration is not possible)
20% magnesium sulfate solution (4 g intravenously at rate not exceeding 1 g/min)
50% magnesium sulfate solution (10 g, 5 g injected deeply in the upper outer quadrant of both buttocks). If convulsions persist after 15 min, give up to 20% solution of magnesium sulfate (2 g) intravenously at a rate not exceeding 1 g/min
Every 4 h thereafter give 50% magnesium sulfate solution (5 g) injected deeply in the upper outer quadrant of alternate buttocks, but only after confirming that the patellar reflex is present, respiration is not depressed and urinary output in the previous 4 h exceeds 100 ml
Discontinue treatment 24 h after delivery
Adverse effects
-
Flushing, nausea and vomiting, muscle weakness, thirst, headache, drowsiness, confusion and respiratory depression
Adapted with permission obtained from Chesley’s Hypertensive Disorders in Pregnancy, Lindheimer, M. D. et al. (eds)
Prevention of pre-eclampsia
A broad range of interventions has been tested for the prevention of pre-eclampsia, including low-salt diets, diuretics, fish oil, calcium supplementation, antioxidants, aspirin and heparin.106 However, most of these interventions have not been proven effective.
Antiplatelet agents
An imbalance between prostacyclin and thromboxane has been proposed to be one of the mechanisms mediating pre-eclampsia;107 thus, antiplatelet agents (in particular, aspirin, which blocks platelet production of thromboxane B2)108 have been extensively tested in randomized clinical trials to prevent pre-eclampsia. In a multicentre randomized clinical trial of low-dose aspirin for the prevention and treatment of pre-eclampsia in 9,364 pregnant women, the use of aspirin was associated with a nonsignificant reduction (12%) in the incidence of proteinuric pre-eclampsia.109 This treatment had no significant effect on the incidence of intrauterine growth restriction, stillbirth or neonatal death. Aspirin did, however, significantly reduce the likelihood of preterm delivery: 19.7% in patients receiving aspirin versus 22.2% in controls, an absolute reduction of 2.5 events per 100 women treated (P = 0.003).109 Subsequently, a meta-analysis of data from 32,217 women showed that patients receiving aspirin for the prevention of pre-eclampsia had a significant (10%) reduction in the incidence of pre-eclampsia, preterm birth (<34 weeks of gestation) and a composite of serious adverse pregnancy outcomes (pre-eclampsia, small for gestational age infant, fetal death or maternal death).3
In another meta-analysis, the incidence of early pre-eclampsia was reduced by 50% (RR 0.47, 95% CI 0.34–0.65) in women considered to be at risk of pre-eclampsia owing to a history of pre-eclampsia or abnormal UtADV findings, who started taking low-dose aspirin ≤16 weeks of gestation. 4 Expanded meta-analyses have confirmed these findings, and also suggest that this intervention is associated with a decrease in the rate of severe pre-eclampsia.6,110,111 However, these findings were obtained in subgroup analyses, and remain to be confirmed by randomized controlled trials.
Antioxidants
Although oxidative stress has been implicated in the pathophysiology of pre-eclampsia, a meta-analysis of randomized controlled trials of vitamins C and E failed to show a beneficial effect for preventing pre-eclampsia and might increase risk of gestational hypertension and prelabour rupture of membranes.112 However, pre-eclampsia was reduced by 63% in patients considered to be at risk of developing the condition (owing to a personal or family history of pre-eclampsia) who received L-arginine (5.4 g daily) in combination with vitamin C (500 mg daily) and vitamin E (400 IU daily) before 24 weeks of gestation.5 Treatment after 24 weeks of gestation with antioxidant or vitamins alone was ineffective in preventing pre-eclampsia.5 The results from this study are promising, and further investigation is warranted to confirm these interesting findings.
Calcium supplementation
As calcium deficiency has been implicated in the pathogenesis of pre-eclampsia,113 several randomized controlled trials have examined whether calcium supplementation can prevent its development.114–116 One such trial in the USA included 4,589 pregnant women who received either calcium supplementation (2 g daily) or placebo.117 The results showed no significant reduction in the incidence or severity of pre-eclampsia, or delay in its onset, in the women receiving supplemental calcium.117 By contrast, a Cochrane systematic review and meta-analysis concluded that women who received calcium supplementation (≥1 g daily) had a reduced incidence of pre-eclampsia (RR 0.45, 95% CI 0.31–0.65).118,119 The beneficial effect was greatest for patients with low baseline calcium intake (RR 0.36, 95% CI 0.20–0.65), and those with a high risk of pre-eclampsia (RR 0.22, 95% CI 0.12–0.42).118 Currently, calcium supplementation for prevention of pre-eclampsia is only considered in pregnant women from populations with low calcium intake (<600 mg daily).90
Other potential interventions
Considerable efforts are underway to identify treatments that can reverse the imbalance of angiogenic and antiangiogenic factors associated with pre-eclampsia. Statins are cholesterol-lowing agents that have the potential to reverse this angiogenic imbalance through their pleiotropic effects, which include stimulating trophoblast production of PlGF, improving endothelial function, upregulating haeme oxygenase 1, decreasing oxidative stress or inflammation and inhibiting complement as well as tissue factor activation.120 Statins might, therefore, represent a suitable intervention for patients at risk of pre-eclampsia or those with an imbalance between angiogenic and antiangiogenic factors. Pravastatin (as opposed to other statins with lipophilic properties) is a water-soluble agent that crosses the placenta slowly and, consequently, might have fewer adverse effects for the fetus than do the lipophilic statins.121 The reported congenital anomalies of statins include isolated anomalies, such as central nervous system or limb defects and the VACTERL association.122 However, abnormal pregnancy outcomes were not reported following exposure to pravastatin or fluvastatin.122 In an experiment conducted in a dually perfused at-term human placental lobule, 14% of pravastatin was retained in placental tissue, 68% remained in the maternal circulation and only 18% was transferred to the fetal circulation.123 These favourable findings support the use of pravastatin during pregnancy.
Other proposed therapeutic interventions to reverse an antiangiogenic state in pregnant women at risk of pre-eclampsia include the administration of VEGF121 17,18,21 or extracorporeal removal of soluble VEGFR-1 (sVEGFR-1).20 Supplemental choline intake during the third trimester of pregnancy can reduce maternal serum levels of sVEGFR-1 and placental expression of total (soluble and membrane-bound) VEGFR-1. However, whether this approach can reduce the incidence of pre-eclampsia remains to be determined.22 These preventive interventions probably will not reverse established pre-eclampsia, as the antiangiogenic state is an adaptive response to various insults, rather than being the primary abnormality leading to pre-eclampsia. However, as delivery of the placenta remains the only therapeutic option available for women with established pre-eclampsia, any interventions that enable the safe prolongation of pregnancy might result in improved perinatal outcomes.
Long-term sequelae of pre-eclampsia
Although pre-eclampsia is a pregnancy-specific disorder that resolves on delivery of the placenta, women with pre-eclampsia are at increased risk of subsequent cardiovascular disease. The results of a systematic review and meta-analysis support prior observations that women with pre-eclampsia are more likely than those without pre-eclampsia to develop long-term sequelae, including chronic hypertension (OR 3.13), cardiovascular disease (OR 2.28), stroke (OR 1.76), diabetes (OR 1.80)23 and end-stage renal disease (RR 4.70).24 The risk of end-stage renal disease seems to increase progressively with the number of pregnancies affected by pre-eclampsia.24 Moreover, women with pre-eclampsia are more likely to have microalbuminuria 3–5 years after delivery than women with a normal pregnancy.124 These findings might reflect undiagnosed renal disease,125 which is common in patients with pre-eclampsia,126 or, alternatively, might suggest that pre-eclampsia compromises renal function. Glomerular endotheliosis, a typical renal lesion observed in pre-eclampsia and previously thought to resolve after delivery, can be detected long after pregnancy in some women affected by pre-eclampsia.127
Receiving a diagnosis of pre-eclampsia could have profound long-term health-care implications, as it identifies a group of women at risk of adverse health outcomes and potentially enables the early implementation of preventative interventions (for example diet, exercise and/or pharmacological treatment).128,129
Conclusions
Current management of pre-eclampsia consists of controlling hypertension, preventing seizures and timely delivery of the fetus. Considerable effort has been made to identify biomarkers that can predict pre-eclampsia. In the absence of safe and effective interventions, however, the low prevalence of pre-eclampsia (which necessarily results in low PPVs for identified biomarkers) means that establishing the clinical utility of any biomarker as a screening test for pre-eclampsia is difficult. A few preventive interventions for pre-eclampsia—such as low-dose aspirin or the combination of nitric oxide donors and antioxidant vitamins—remain to be fully investigated. Potential interventions exist to ameliorate the imbalance between angiogenic and antiangiogenic factors observed in some patients with pre-eclampsia, and hence might provide opportunities to improve maternal and/or perinatal outcomes. The diagnosis of pre-eclampsia can have profound long-term health-care implications, as it identifies a subgroup of women at increased risk of adverse health outcomes. Further studies are required to improve screening strategies to identify women at risk of pre-eclampsia who could benefit from targeted preventive interventions. Evaluation of the cost-effectiveness of any intervention should also be considered.
Review criteria
A search for original research and review articles published in English between 1840 and 2013 focusing on pre-eclampsia was performed in PubMed using the following search terms alone or in combination: “pre-eclampsia,” “toxaemia,” “pregnancy-induced hypertension” and “eclampsia.” The bibliographies of pertinent articles were also examined to identify further relevant papers.
Key points.
Combinations of biomarkers perform better than single biomarkers for predicting pre-eclampsia, but require external validation before they can be used routinely.
Potentially effective interventions to prevent pre-eclampsia in patients at risk include early administration of low-dose aspirin or l-arginine in combination with oral antioxidants (vitamins C and E).
Maternal plasma levels of angiogenic and antiangiogenic factors identify most patients who will develop early pre-eclampsia, correlate with disease severity and have prognostic value for maternal and/or perinatal complications.
Management of pre-eclampsia includes control of hypertension, prevention of seizures and timely delivery; steroids are administered to enhance fetal lung maturity if induction of labour before 34 weeks is contemplated.
In pre-eclampsia at ≥37 weeks of gestation, delivery effectively optimizes pregnancy outcomes; for preterm gestations, the risk of continued pregnancy must be balanced against that of premature birth.
Women with pre-eclampsia are at increased risk of developing cardiovascular disease, including chronic hypertension, stroke, coronary artery disease, diabetes and end-stage renal disease later in life.
Acknowledgments
Funding
This research was supported, in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS) and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
Disclosure
The authors report no conflicts of interest.
Author contributions
All authors researched data for the article, made substantial contributions to discussion of the content, wrote, reviewed and edited the manuscript before submission.
References
- 1.Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. doi: 10.1038/nrneph.2014.102. http://dx.doi.org/10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed]
- 2.Kuc S, et al. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: a systematic review. Obstet Gynecol Surv. 2011;66:225–239. doi: 10.1097/OGX.0b013e3182227027. [DOI] [PubMed] [Google Scholar]
- 3.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–1798. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 4.Bujold E, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 5.Vadillo-Ortega F, et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ. 2011;342:d2901. doi: 10.1136/bmj.d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberge S, et al. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. Am J Perinatol. 2012;29:551–556. doi: 10.1055/s-0032-1310527. [DOI] [PubMed] [Google Scholar]
- 7.Goel A, Rana S. Angiogenic factors in preeclampsia: potential for diagnosis and treatment. Curr Opin Nephrol Hypertens. 2013;22:643–650. doi: 10.1097/MNH.0b013e328365ad98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagmann H, Thadhani R, Benzing T, Karumanchi SA, Stepan H. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem. 2012;58:837–845. doi: 10.1373/clinchem.2011.169094. [DOI] [PubMed] [Google Scholar]
- 9.Silasi M, Cohen B, Karumanchi SA, Rana S. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin North Am. 2010;37:239–253. doi: 10.1016/j.ogc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Smith GC, Wear H. The perinatal implications of angiogenic factors. Curr Opin Obstet Gynecol. 2009;21:111–116. doi: 10.1097/GCO.0b013e328328cf7d. [DOI] [PubMed] [Google Scholar]
- 11.Roberts JM, Bell MJ. If we know so much about preeclampsia, why haven’t we cured the disease? J Reprod Immunol. 2013;99:1–9. doi: 10.1016/j.jri.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaiworapongsa T, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187–1207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore AG, et al. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651–2657. doi: 10.3109/14767058.2012.713055. [DOI] [PubMed] [Google Scholar]
- 14.Rana S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaiworapongsa T, et al. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2013;7:132–144. doi: 10.3109/14767058.2013.806905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore Simas TA, et al. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197:244.e1–244.e8. doi: 10.1016/j.ajog.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert JS, et al. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55:380–385. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumasawa K, et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA. 2011;108:1451–1455. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thadhani R, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124:940–950. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]
- 21.Woods AK, et al. Adenoviral delivery of VEGF121 early in pregnancy prevents spontaneous development of preeclampsia in BPH/5 mice. Hypertension. 2011;57:94–102. doi: 10.1161/HYPERTENSIONAHA.110.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, et al. A higher maternal choline intake among third-trimester pregnant women lowers placental and circulating concentrations of the antiangiogenic factor fms-like tyrosine kinase-1 (sFLT1) FASEB J. 2013;27:1245–1253. doi: 10.1096/fj.12-221648. [DOI] [PubMed] [Google Scholar]
- 23.Brown MC, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1–19. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 24.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 25.Forest JC, et al. Candidate biochemical markers for screening of pre-eclampsia in early pregnancy. Clin Chem Lab Med. 2012;50:973–984. doi: 10.1515/cclm.2011.820. [DOI] [PubMed] [Google Scholar]
- 26.Anderson UD, Olsson MG, Kristensen KH, Akerstrom B, Hansson SR. Review: Biochemical markers to predict preeclampsia. Placenta. 2012;33(Suppl):S42–S47. doi: 10.1016/j.placenta.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Spencer K, Cowans NJ, Chefetz I, Tal J, Meiri H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;29:128–134. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 28.Ong CY, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG. 2000;107:1265–1270. doi: 10.1111/j.1471-0528.2000.tb11618.x. [DOI] [PubMed] [Google Scholar]
- 29.Spencer K, Yu CK, Cowans NJ, Otigbah C, Nicolaides KH. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free β-hCG and with second-trimester uterine artery Doppler. Prenat Diagn. 2005;25:949–953. doi: 10.1002/pd.1251. [DOI] [PubMed] [Google Scholar]
- 30.Odibo AO, et al. Placental volume and vascular flow assessed by 3D power Doppler and adverse pregnancy outcomes. Placenta. 2011;32:230–234. doi: 10.1016/j.placenta.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plasencia W, Maiz N, Poon L, Yu C, Nicolaides KH. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks and 21 + 0 to 24 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:138–146. doi: 10.1002/uog.5402. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, et al. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Am J Obstet Gynecol. 2008;199:122.e121–122.e111. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 33.Nicolaides KH, et al. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27:13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]
- 34.Akolekar R, Syngelaki A, Beta J, Kocylowski R, Nicolaides KH. Maternal serum placental protein 13 at 11–13 weeks of gestation in preeclampsia. Prenat Diagn. 2009;29:1103–1108. doi: 10.1002/pd.2375. [DOI] [PubMed] [Google Scholar]
- 35.Stamatopoulou A, Cowans NJ, Matwejew E, von Kaisenberg C, Spencer K. Placental protein-13 and pregnancy-associated plasma protein-A as first trimester screening markers for hypertensive disorders and small for gestational age outcomes. Hypertens Pregnancy. 2011;30:384–395. doi: 10.3109/10641955.2010.484081. [DOI] [PubMed] [Google Scholar]
- 36.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat Diagn. 2011;31:66–74. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- 37.Wright D, Akolekar R, Syngelaki A, Poon LC, Nicolaides KH. A competing risks model in early screening for preeclampsia. Fetal Diagn Ther. 2012;32:171–178. doi: 10.1159/000338470. [DOI] [PubMed] [Google Scholar]
- 38.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33:8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 39.Pedrosa AC, Matias A. Screening for pre-eclampsia: a systematic review of tests combining uterine artery Doppler with other markers. J Perinat Med. 2011;39:619–635. doi: 10.1515/jpm.2011.077. [DOI] [PubMed] [Google Scholar]
- 40.Herraiz I, et al. Application of a first-trimester prediction model for pre-eclampsia based on uterine arteries and maternal history in high-risk pregnancies. Prenat Diagn. 2009;29:1123–1129. doi: 10.1002/pd.2383. [DOI] [PubMed] [Google Scholar]
- 41.Farina A, et al. Prospective evaluation of ultrasound and biochemical-based multivariable models for the prediction of late pre-eclampsia. Prenat Diagn. 2011;31:1147–1152. doi: 10.1002/pd.2849. [DOI] [PubMed] [Google Scholar]
- 42.Smith GC. Researching new methods of screening for adverse pregnancy outcome: lessons from pre-eclampsia. PLoS Med. 2012;9:e1001274. doi: 10.1371/journal.pmed.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasanen J, et al. Comprehensive maternal serum proteomic profiles of preclinical and clinical preeclampsia. J Proteome Res. 2010;9:4274–4281. doi: 10.1021/pr100198m. [DOI] [PubMed] [Google Scholar]
- 44.Carty DM, et al. Urinary proteomics for prediction of preeclampsia. Hypertension. 2011;57:561–569. doi: 10.1161/HYPERTENSIONAHA.110.164285. [DOI] [PubMed] [Google Scholar]
- 45.Kenny LC, et al. Robust early pregnancy prediction of later preeclampsia using metabolomic biomarkers. Hypertension. 2010;56:741–749. doi: 10.1161/HYPERTENSIONAHA.110.157297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahado-Singh RO, et al. First-trimester metabolomic detection of late-onset preeclampsia. Am J Obstet Gynecol. 2013;208:58.e1–58.e7. doi: 10.1016/j.ajog.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Bahado-Singh RO, et al. Metabolomics and first-trimester prediction of early-onset preeclampsia. J Matern Fetal Neonatal Med. 2012;25:1840–1847. doi: 10.3109/14767058.2012.680254. [DOI] [PubMed] [Google Scholar]
- 48.Leung TN, Zhang J, Lau TK, Chan LY, Lo YM. Increased maternal plasma fetal DNA concentrations in women who eventually develop preeclampsia. Clin Chem. 2001;47:137–139. [PubMed] [Google Scholar]
- 49.Farina A, et al. Cell-free fetal DNA (SRY locus) concentration in maternal plasma is directly correlated to the time elapsed from the onset of preeclampsia to the collection of blood. Prenat Diagn. 2004;24:293–297. doi: 10.1002/pd.863. [DOI] [PubMed] [Google Scholar]
- 50.Purwosunu Y, et al. Cell-free mRNA concentrations of CRH, PLAC1, and selectin-P are increased in the plasma of pregnant women with preeclampsia. Prenat Diagn. 2007;27:772–777. doi: 10.1002/pd.1780. [DOI] [PubMed] [Google Scholar]
- 51.Purwosunu Y, et al. Prediction of preeclampsia by analysis of cell-free messenger RNA in maternal plasma. Am J Obstet Gynecol. 2009;200:386.e1–386.e7. doi: 10.1016/j.ajog.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 52.Levine RJ, et al. Two-stage elevation of cell-free fetal DNA in maternal sera before onset of preeclampsia. Am J Obstet Gynecol. 2004;190:707–713. doi: 10.1016/j.ajog.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 53.Crowley A, et al. Free fetal DNA is not increased before 20 weeks in intrauterine growth restriction or pre-eclampsia. Prenat Diagn. 2007;27:174–179. doi: 10.1002/pd.1645. [DOI] [PubMed] [Google Scholar]
- 54.Poon LC, Musci T, Song K, Syngelaki A, Nicolaides KH. Maternal plasma cell-free fetal and maternal DNA at 11–13 weeks’ gestation: relation to fetal and maternal characteristics and pregnancy outcomes. Fetal Diagn Ther. 2013;33:215–223. doi: 10.1159/000346806. [DOI] [PubMed] [Google Scholar]
- 55.Sekizawa A, et al. Prediction of pre-eclampsia by an analysis of placenta-derived cellular mRNA in the blood of pregnant women at 15–20 weeks of gestation. BJOG. 2010;117:557–564. doi: 10.1111/j.1471-0528.2010.02491.x. [DOI] [PubMed] [Google Scholar]
- 56.Farina A, et al. Performance of messenger RNAs circulating in maternal blood in the prediction of preeclampsia at 10-14 weeks. Am J Obstet Gynecol. 2010;203:575.e1–575.e7. doi: 10.1016/j.ajog.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 57.von Dadelszen P, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the full PIERS model. Lancet. 2011;377:219–227. doi: 10.1016/S0140-6736(10)61351-7. [DOI] [PubMed] [Google Scholar]
- 58.Payne B, et al. Performance of the fullPIERS model in predicting adverse maternal outcomes in pre-eclampsia using patient data from the PIERS (Pre-eclampsia Integrated Estimate of RiSk) cohort, collected on admission. BJOG. 2013;120:113–118. doi: 10.1111/j.1471-0528.2012.03496.x. [DOI] [PubMed] [Google Scholar]
- 59.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkatesha S, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 61.Kusanovic JP, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore Simas TA, et al. Angiogenic biomarkers for prediction of early preeclampsia onset in high-risk women. J Matern Fetal Neonatal Med. 2014;27:1038–1048. doi: 10.3109/14767058.2013.847415. [DOI] [PubMed] [Google Scholar]
- 63.Levine RJ, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 64.Chaiworapongsa T, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1550. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 65.Rana S, et al. Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS ONE. 2012;7:e48259. doi: 10.1371/journal.pone.0048259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chappell LC, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128:2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 67.Rana S, et al. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens Pregnancy. 2013;32:189–201. doi: 10.3109/10641955.2013.784788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powers RW, et al. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertension. 2012;60:239–246. doi: 10.1161/HYPERTENSIONAHA.112.191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rana S, Karumanchi SA, Lindheimer MD. Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension. 2014;63:198–202. doi: 10.1161/HYPERTENSIONAHA.113.02293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaiworapongsa T, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 71.Sibiude J, et al. Placental growth factor for the prediction of adverse outcomes in patients with suspected preeclampsia or intrauterine growth restriction. PLoS ONE. 2012;7:e50208. doi: 10.1371/journal.pone.0050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schnettler W, et al. Cost and resource implications with serum angiogenic factor estimation in the triage of pre-eclampsia. BJOG. 2013;120:1224–1232. doi: 10.1111/1471-0528.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rolfo A, et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 2013;83:177–181. doi: 10.1038/ki.2012.348. [DOI] [PubMed] [Google Scholar]
- 74.Di Marco GS, et al. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J Am Soc Nephrol. 2009;20:2235–2245. doi: 10.1681/ASN.2009010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams WW, Jr, Ecker JL, Thadhani RI, Rahemtullah A. Case records of the Massachusetts General Hospital. Case 38–2005. A 29-year-old pregnant woman with the nephrotic syndrome and hypertension. N Engl J Med. 2005;353:2590–2600. doi: 10.1056/NEJMcpc059031. [DOI] [PubMed] [Google Scholar]
- 76.Qazi U, Lam C, Karumanchi SA, Petri M. Soluble Fms-like tyrosine kinase associated with preeclampsia in pregnancy in systemic lupus erythematosus. J Rheumatol. 2008;35:631–634. [PubMed] [Google Scholar]
- 77.Shan HY, et al. Use of circulating antiangiogenic factors to differentiate other hypertensive disorders from preeclampsia in a pregnant woman on dialysis. Am J Kidney Dis. 2008;51:1029–1032. doi: 10.1053/j.ajkd.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 78.Masuyama H, et al. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006;194:551–556. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 79.Masuyama H, et al. Superimposed preeclampsia in women with chronic kidney disease. Gynecol Obstet Invest. 2012;74:274–281. doi: 10.1159/000339935. [DOI] [PubMed] [Google Scholar]
- 80.Widmer M, et al. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol. 2007;109:168–180. doi: 10.1097/01.AOG.0000249609.04831.7c. [DOI] [PubMed] [Google Scholar]
- 81.Sibai BM, et al. Serum inhibin A and angiogenic factor levels in pregnancies with previous preeclampsia and/or chronic hypertension: are they useful markers for prediction of subsequent preeclampsia? Am J Obstet Gynecol. 2008;199:268.e1–268.e9. doi: 10.1016/j.ajog.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 82.McElrath TF, et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012;207:407.e1–407.e7. doi: 10.1016/j.ajog.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 83.Myatt L, et al. Can changes in angiogenic biomarkers between the first and second trimesters of pregnancy predict development of pre-eclampsia in a low-risk nulliparous patient population? BJOG. 2013;120:1183–1191. doi: 10.1111/1471-0528.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Myers J, et al. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: a predictive test accuracy study. BJOG. 2013;120:1215–1223. doi: 10.1111/1471-0528.12195. [DOI] [PubMed] [Google Scholar]
- 85.Chaiworapongsa T, et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol. 2013;208:287.e1–287.e15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lai J, Syngelaki A, Poon LC, Nucci M, Nicolaides KH. Maternal serum soluble endoglin at 30–33 weeks in the prediction of preeclampsia. Fetal Diagn Ther. 2013;33:149–155. doi: 10.1159/000343220. [DOI] [PubMed] [Google Scholar]
- 87.Kleinrouweler CE, et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG. 2012;119:778–787. doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 88.Powers RW, et al. Soluble fms-Like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PLoS ONE. 2010;5:e13263. doi: 10.1371/journal.pone.0013263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Driscoll DA, Gross S. Clinical practice. Prenatal screening for aneuploidy. N Engl J Med. 2009;360:2556–2562. doi: 10.1056/NEJMcp0900134. [DOI] [PubMed] [Google Scholar]
- 90.American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy, Hypertension in Pregnancy [online] 2013 doi: 10.1097/GRF.0000000000000247. http://www.acog.org/resources_and_publications/task_force_and_work_group_reports/hypertension_in_pregnancy. [DOI] [PubMed]
- 91.Blackwell SC, et al. Labor induction for the preterm severe pre-eclamptic patient: is it worth the effort? J Matern Fetal Med. 2001;10:305–311. doi: 10.1080/714904348. [DOI] [PubMed] [Google Scholar]
- 92.Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205:191–198. doi: 10.1016/j.ajog.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 93.Committee Opinion no. 514: emergent therapy for acute-onset, severe hypertension with preeclampsia or eclampsia. Obstet Gynecol. 2011;118:1465–1468. doi: 10.1097/AOG.0b013e31823ed1ef. [DOI] [PubMed] [Google Scholar]
- 94.Martin JN, Jr, et al. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105:246–254. doi: 10.1097/01.AOG.0000151116.84113.56. [DOI] [PubMed] [Google Scholar]
- 95.Redman CW. Hypertension in pregnancy: the NICE guidelines. Heart. 2011;97:1967–1969. doi: 10.1136/heartjnl-2011-300949. [DOI] [PubMed] [Google Scholar]
- 96.Magee LA, Helewa M, Moutquin JM, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30(Suppl. 1):S1–S48. doi: 10.1016/S1701-2163(16)32870-5. [DOI] [PubMed] [Google Scholar]
- 97.Lowe SA, et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust NZ J Obstet Gynaecol. 2009;49:242–246. doi: 10.1111/j.1479-828X.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 98.Gogarten W. Preeclampsia and anaesthesia. Curr Opin Anaesthesiol. 2009;22:347–351. doi: 10.1097/ACO.0b013e32832a1d05. [DOI] [PubMed] [Google Scholar]
- 99.Umans JG, Abalos EJ, Lindheimer MD. In: Chesley’s Hypertensive Disorders in Pregnancy. Lindheimer MD, Roberts JM, Cunningham GC, editors. Elsevier; 2009. pp. 369–388. [Google Scholar]
- 100.Duley L, Meher S, Jones L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews. (3) doi: 10.1002/14651858.CD001449.pub3. Art. No.: CD001449. http://dx.doi.org/10.1002/14651858.CD001449.pub2. [DOI] [PMC free article] [PubMed]
- 101.Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402–410. doi: 10.1097/01.AOG.0000152351.13671.99. [DOI] [PubMed] [Google Scholar]
- 102.Smith JM, et al. An integrative review of the side effects related to the use of magnesium sulfate for pre-eclampsia and eclampsia management. BMC Pregnancy Childbirth. 2013;13:34. doi: 10.1186/1471-2393-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345:1455–1463. [PubMed] [Google Scholar]
- 104.Altman D, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomized placebo-controlled trial. Lancet. 2002;359:1877–1890. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 105.Duley L, Gulmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database of Systematic Reviews. (2) doi: 10.1002/14651858.CD000025. Art. No.: CD000025. http://dx.doi.org/10.1002/14651858.CD000025. [DOI] [PubMed]
- 106.Sibai BM, Cunningham FG. In: Chesley’s Hypertensive Disorders in Pregnancy. Lindheimer MD, Roberts JM, Cunningham GC, editors. Elsevier; 2009. pp. 214–225. [Google Scholar]
- 107.Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152:335–340. doi: 10.1016/s0002-9378(85)80223-4. [DOI] [PubMed] [Google Scholar]
- 108.Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin Perinatol. 1988;12:302–323. [PubMed] [Google Scholar]
- 109.CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. Lancet. 1994;343:619–629. [No authors listed] [PubMed] [Google Scholar]
- 110.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database of Systematic Reviews. (2) doi: 10.1002/14651858.CD004659.pub2. Art. No.: CD004659. http://dx.doi.org/10.1002/14651858.CD004659.pub2. [DOI] [PubMed]
- 111.Villa PM, et al. Aspirin in the prevention of pre-eclampsia in high-risk women: a randomized placebo-controlled PREDO Trial and a meta-analysis of randomized trials. BJOG. 2013;120:64–74. doi: 10.1111/j.1471-0528.2012.03493.x. [DOI] [PubMed] [Google Scholar]
- 112.Conde-Agudelo A, Romero R, Kusanovic JP, Hassan SS. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204:503.e1–503.e12. doi: 10.1016/j.ajog.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Belizan JM, Villar J. The relationship between calcium intake and edema-, proteinuria-, and hypertension-getosis: an hypothesis. Am J Clin Nutr. 1980;33:2202–2210. doi: 10.1093/ajcn/33.10.2202. [DOI] [PubMed] [Google Scholar]
- 114.Lopez-Jaramillo P, Narvaez M, Felix C, Lopez A. Dietary calcium supplementation and prevention of pregnancy hypertension. Lancet. 1990;335:293. doi: 10.1016/0140-6736(90)90112-i. [DOI] [PubMed] [Google Scholar]
- 115.Villar J, Repke JT. Calcium supplementation during pregnancy may reduce preterm delivery in high-risk populations. Am J Obstet Gynecol. 1990;163:1124–1131. doi: 10.1016/0002-9378(90)90669-x. [DOI] [PubMed] [Google Scholar]
- 116.Belizan JM, Villar J, Gonzalez L, Campodonico L, Bergel E. Calcium supplementation to prevent hypertensive disorders of pregnancy. N Engl J Med. 1991;325:1399–1405. doi: 10.1056/NEJM199111143252002. [DOI] [PubMed] [Google Scholar]
- 117.Levine RJ, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337:69–76. doi: 10.1056/NEJM199707103370201. [DOI] [PubMed] [Google Scholar]
- 118.Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews. (8) doi: 10.1002/14651858.CD001059.pub3. Art. No.: CD001059. http://dx.doi.org/10.1002/14651858.CD001059.pub3. [DOI] [PubMed]
- 119.Patrelli TS, et al. Calcium supplementation and prevention of preeclampsia: a meta-analysis. J Matern Fetal Neonatal Med. 2012;25:2570–2574. doi: 10.3109/14767058.2012.715220. [DOI] [PubMed] [Google Scholar]
- 120.Costantine MM, Cleary K. Pravastatin for the prevention of preeclampsia in high-risk pregnant women. Obstet Gynecol. 2013;121:349–353. doi: 10.1097/aog.0b013e31827d8ad5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33:328–343. doi: 10.2165/00003088-199733050-00002. [DOI] [PubMed] [Google Scholar]
- 122.Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A. 2004;131:287–298. doi: 10.1002/ajmg.a.30386. [DOI] [PubMed] [Google Scholar]
- 123.Nanovskaya TN, et al. Transplacental transfer and distribution of pravastatin. Am J Obstet Gynecol. 2013;209:373.e1–373.e5. doi: 10.1016/j.ajog.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bar J, et al. Microalbuminuria after pregnancy complicated by pre-eclampsia. Nephrol Dial Transplant. 1999;14:1129–1132. doi: 10.1093/ndt/14.5.1129. [DOI] [PubMed] [Google Scholar]
- 125.Reiter L, Brown MA, Whitworth JA. Hypertension in pregnancy: the incidence of underlying renal disease and essential hypertension. Am J Kidney Dis. 1994;24:883–887. doi: 10.1016/s0272-6386(12)81055-9. [DOI] [PubMed] [Google Scholar]
- 126.Williams D, Davison J. Chronic kidney disease in pregnancy. BMJ. 2008;336:211–215. doi: 10.1136/bmj.39406.652986.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fisher KA, Luger A, Spargo BH, Lindheimer MD. Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine (Baltimore) 1981;60:267–276. [PubMed] [Google Scholar]
- 128.Carty DM, Delles C, Dominiczak AF. Preeclampsia and future maternal health. J Hypertens. 2010;28:1349–1355. doi: 10.1097/HJH.0b013e32833a39d0. [DOI] [PubMed] [Google Scholar]
- 129.Williams D. Long-term complications of preeclampsia. Semin Nephrol. 2011;31:111–122. doi: 10.1016/j.semnephrol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 130.Koopmans CM, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374:979–988. doi: 10.1016/S0140-6736(09)60736-4. [DOI] [PubMed] [Google Scholar]
- 131.Tajik P, et al. Should cervical favourability play a role in the decision for labour induction in gestational hypertension or mild pre-eclampsia at term? An exploratory analysis of the HYPITAT trial. BJOG. 2012;119:1123–1130. doi: 10.1111/j.1471-0528.2012.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pauli JM, et al. Management of gestational hypertension—the impact of HYPITATa. J Perinat Med. 2013;41:415–420. doi: 10.1515/jpm-2012-0179. [DOI] [PubMed] [Google Scholar]
- 133.Smith P, Anthony J, Johanson R. Nifedipine in pregnancy. BJOG. 2000;107:299–307. doi: 10.1111/j.1471-0528.2000.tb13222.x. [DOI] [PubMed] [Google Scholar]
- 134.Hypertension in pregnancy: executive summary. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]


