Abstract
Pattern separation, the ability to independently represent and store similar experiences, is a crucial facet of episodic memory. Growing evidence suggests that the hippocampus possesses unique circuitry that is computationally capable of resolving mnemonic interference by using pattern separation. In this Review, we discuss recent advances in the understanding of this process and evaluate the caveats and limitations of linking across animal and human studies. We summarize clinical and translational studies using methods that are sensitive to pattern separation impairments, an approach that stems from the fact that the hippocampus is a major site of disruption in many brain disorders. We critically evaluate the assumptions that guide fundamental and translational studies in this area. Finally, we suggest guidelines for future research and offer ways to overcome potential interpretational challenges to increase the utility of pattern separation as a construct that can further understanding of both memory processes and brain disease.
Episodic memories—records of unique experiences and events in our lives—guide adaptive future behavior. The hippocampus is known to play a crucial role in the formation and storage of episodic memories1,2. In doing so, it is constantly faced with the challenge of resolving interference that arises from overlapping day-to-day experiences. In other words, events in people’s lives share many similar features (for example, parking a car in the same parking lot every day). Despite this overlap, humans are able to recall specific memories (for example, today’s versus yesterday’s parking spot). Thus, a key facet of episodic memory is being able to distinguish among these similar experiences. Pattern separation is one potential neurocomputational mechanism that is capable of reducing this interference by using nonoverlapping representations3–6. Although the term “pattern separation” may be used to describe any number of processes that reduce the similarity of input patterns, even in low- level sensory cortex, our use of it here is limited to its application to episodic memory.
Hippocampal features that support pattern separation
The hippocampus receives and combines information from sensory and associational cortical areas with modulatory inputs from limbic and subcortical regions. It is thought to process this multidimensional input, forming a coherent representation of the experience that is then projected back onto the cortex7 (Fig. 1a). The hippocampus consists of several subfields, each of which has unique properties and connectivity8. Input from cortical regions enters the hippocampus via the entorhinal cortex (EC), which projects to the dentate gyrus (DG) and CA3 subregions via the perforant path (PP). The EC–DG–CA3 circuit is often implicated in pattern separation, although the exact mechanisms by which this occurs remain subject to debate. Several putative mechanisms have been proposed3–6,9–15, but more empirical studies that test these alternatives are needed.
Fig. 1. Circuitry and computational properties of the hippocampus.
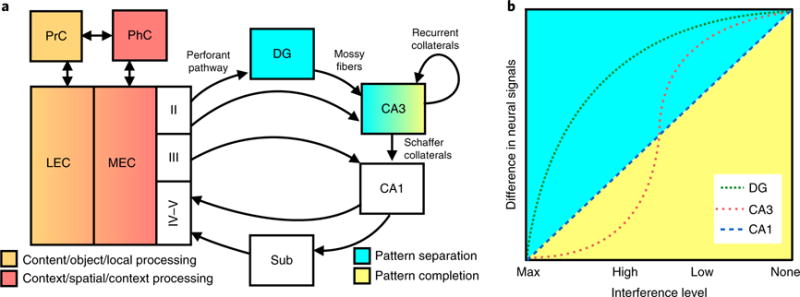
a, This simplified circuitry of the hippocampus consists of the DG, CA3, CA1 and subiculum (Sub). The EC is the major input into the hippocampus and consists of the LEC and MEC. Layer II of the EC is the main input to DG and CA3 subregions (via the perforant pathway), whereas layers IV-V are the main output from the CA1 and Sub of the hippocampus to other cortical regions. The DG projects to the CA3 via mossy fibers. The CA3’s largest projection is onto itself via recurrent collaterals145. The CA3 projects to the CA1 via Schaffer collaterals. The LEC mainly receives input from PrC (postrhinal in rodents), and the MEC mainly receives input from the PhC, although there is crosstalk between PrC and PhC, as well as between LEC and MEC. The PrC-LEC pathway is largely involved in content, object and local processing (orange), whereas the PhC-MEC pathway is largely involved in context, spatial and global processing (red). The DG is capable of performing pattern separation (blue), whereas the CA3 can perform pattern separation and pattern completion, depending on the input. b, The x axis shows interference levels (maximum, high, low and no interference), and the y axis shows the difference in neural signals (for example, BOLD fMRI contrast, decorrelation in IEG population activity or single-unit firing, or any other indicator of neural change in output). The DG shows a sharp increase in signal even with high levels of interference (pattern separation), whereas the CA3 shows lower neural signals at higher levels of interference and higher neural signals at lower levels of interference and is capable of performing pattern completion and separation. The CA1 shows a linear response function, with greater neural signals with lower levels of interference.
Empirical evidence for pattern separation in the hippocampus
A number of empirical reports across species and approaches have provided convergent evidence for the hippocampus’s involvement in pattern separation. In these experiments, subjects were exposed to experiences that systematically varied in similarity, and neural responses in hippocampal subfields were recorded. A set of three studies in 2004 provided a suitable parametric framework for the examination of pattern separation and pattern completion16–18. Results from these studies were summarized as data points along input/output transformations that described the computational bias in each subfield19 (Fig. 1b). Overall, convergent data have suggested that CA3 is capable of exhibiting pattern completion or pattern separation, depending on the magnitude of the change in sensory input. The CA1 subfield generally appears to respond linearly to incremental changes in sensory input; however, under some conditions it may respond with an abrupt nonlinear change, perhaps reflecting a switch in its dominant input from the entorhinal cortex to CA318–20 (but see Stokes et al.21). This could be a result of explicit task influences or the requirement for a mnemonic judgment instead of free exploration19,22. Consistent with this account, the CA1 region has also been characterized as a match/mismatch detector23,24, which has been proposed as a core hippocampal com- putation25–27. The CA1 receives convergent input from CA3 and EC and may be able to shift between encoding and retrieval modes based on comparison of the inputs24,28,29.
The DG is more likely than CA3 to show decorrelated patterns, even with minor distortions in the input; that is, its output is consistent with pattern separation30 and occurs when the output layer shows more distinct firing patterns than the input layer31. To determine whether this pattern was a function of subfield-specific computations or simply a reflection of upstream processing, the authors of a recent study recorded activity from EC, CA3 and DG of behaving rats as the testing environment was distorted to varying degrees31. They observed rapid decorrelation of the neural signal after any distortion of the testing enclosure in the DG, but not in the EC. The same analyses showed that in CA3, the signal remained relatively coherent over the varying levels of distortion31. Similarly, CA3 coherence was weakly represented in upstream areas, which indicated that CA3 is able to pattern-complete a previously learned neural representation given noisy inputs. This work provided additional strong evidence that DG and CA3 computational signals are transformations of upstream signals and, together with prior work showing these dissociated subfield computations30, offered crucial support for the long-standing hypotheses regarding the functional properties of these areas3,4,32.
Hippocampal pattern separation and episodic memory
The study of pattern separation and its role in episodic memory has dramatically increased in recent years. A PubMed search for “pattern separation” yielded about 400 articles on pattern separation published since the 1970s, with an exponential increase in the number of publications since 1974 (r2 = 0.708, F126 = 63.01, P < 0.001; Fig. 2). The development of suitable behavioral models in which to assess this computation has provided a tractable approach to assessing the computation’s mechanisms and implications. Performance in these models is typically characterized in terms of a ‘discrimination index’ that quantifies the subject’s ability to overcome interference across similar experiences. Importantly, in discussing this work, we here use the term “discrimination” to refer to the behavioral measures, and reserve the use of “pattern separation” for neural data. For example, a number of studies have used an object-based mnemonic discrimination task33–38 in which subjects are shown everyday objects during encoding and are then given a recognition test, where they are shown repeated images (targets), novel objects (foils), and similar, but not identical, objects (lures). Behavioral results from this task typically show a linear relationship, with lure-discrimination performance increasing with decreasing similarity of lure items39–42.
Fig. 2. Exponential increase in articles on pattern separation.
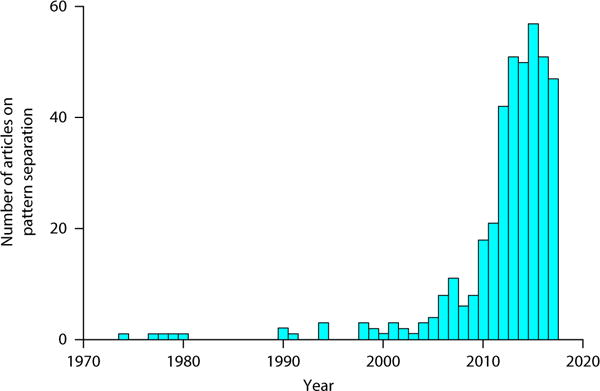
The first articles on pattern separation were published in the 1970s, but it wasn’t until ~2010 that a marked increase in pattern separation publications occurred.
Similar results have also been observed in other domains of episodic memory. For spatial tasks, the placement of objects is typically varied during retrieval across a range of spatial locations that vary in proximity from original positions during encoding. Across three different studies40,42,43, discrimination performance increased as the metric distance from the original location increased. Similar tasks have been designed for rodents, using a dry-land version of the Morris water maze44 or a touchscreen version of the tasks typically used in human studies45,46. For temporal tasks, the lag between events is typically varied, and subjects are asked to make an order judgment during retrieval47–49. Results consistently show a linear increase in performance with longer lags47,48. Thus, increasing interference in mnemonic discrimination tasks, defined as the parametric similarity along one or more domains, including visual appearance or proximity in space and/or time, poses a demand for pattern separation. Manipulation of interference along other dimensions is also possible, such as reward50 and valence51,52.
Evidence for signals consistent with pattern separation in the human hippocampus have been reported in several high-resolution functional magnetic resonance imaging (fMRI) studies in which activity during the presentation of similar items was compared with activity for novel and repeated items. The first study found that activity for lure items was on par with that for novel items rather than that for repeated items, and this was true only in the DG/CA3 subregion of the hippocampus53. This work leveraged the well-documented phenomenon of fMRI adaptation or repetition suppression (decreased response to repeated stimuli54) to establish benchmarks for novel and repeated items and assess the extent to which lures were treated as either novel or repeated. Importantly, this study could not rule out the possibility that the signals observed were reflections of match/mismatch signaling55, as similarity was not parametrically manipulated. Follow-up work examined input/output transfer functions through continuous variation of the similarity of presented items, and found a relatively more discontinuous response (step-function) in the DG and CA3 (DG/CA3) compared with that in CA1, consistent with the computational framework shown in Fig. 1b. Recent neuroimaging studies using multivariate classification approaches have also demonstrated that neural patterns are largely uncorrelated in the DG/CA3 subregion56. Recently, ultrahigh-resolution 7T fMRI was used to demonstrate that the DG, but not other hippocampal subfields or medial temporal cortices, exhibits distinct neural patterns for similar items, thus suggesting that the human DG is perhaps selectively engaged in pattern separation57. Interestingly, patient B.L., a 54-year-old man with selective bilateral ischemic lesions to the DG subregion of the hippocampus, was found to have impaired performance on a mnemonic discrimination task, which further suggests that the DG is required for pattern separation58.
Cortical contributions to pattern separation
Cortical input to the hippocampus is largely segregated into two information-processing streams, which can be thought of as ‘what/content’ and ‘where/context’ pathways59–61. The lateral entorhinal (LEC)–perirhinal cortex (PrC) pathway primarily transmits sensory cues (content) that are required for object recognition and discrimination, whereas the medial entorhinal cortex (MEC)–parahippocampal cortex (PhC) pathway primarily transmits internally guided cues (context) that are required for navigation and spatial discrimination59–61. It is becoming increasingly clear that these streams make distinct computational contributions to domain-specific pattern separation. A double dissociation was recently identified with spatial discrimination engaging the MEC–PhC pathway and object discrimination engaging the LEC–PrC pathway. This domain selectivity was not observed in the DG/CA3 region39. These findings are consistent with the representational–hierarchical perspective, which suggests that lower-level representations (cortical) are more ambiguous, whereas higher-level representations (hippocampal) are unique62. At the sensory level, interference among individual stimulus features (for example, lines and colors) may be resolved in sensory cortex. When more perceptually complex features are introduced (for example, objects and contexts), interference is resolved at the next level of processing (for example, LEC and MEC pathways). Finally, combinatorial codes (for example, conjunctive representations of objects in context) are resolved in the hippocampus. One important implication of this view is that in investigations of signals consistent with pattern separation, especially those using fMRI in humans, several levels of cortical–hippocampal processing (for example, along the ventral visual stream and into the medial temporal lobes) should be examined in order for the specificity of the computation to be accurately assessed.
In addition to medial temporal cortices, other brain networks appear to be involved in the use of pattern-separated representations in explicit memory tasks. For example, increased blood-oxygen-level-dependent (BOLD) fMRI activity during correct discrimination of similar items is observed in regions such as the bilateral occipitotemporal cortex63 and the retrosplenial cortex64, whereas activity related to recognition of similar items is seen in prefrontal cortical regions63 and thalamic nucleus reuniens64. These results suggest that cortical influences are involved in creating and using unique episodic memory traces. Future studies using multi-site neurophysiological recording or calcium imaging could potentially inform on the temporal order of hippocampal–cortical interactions that may support the use of pattern-separated representations.
Caveats in linking across human and rodent studies
It is important to note that human and rodent studies use different measures of neural activity to capture signals consistent with pattern separation, which is one of the challenges in linking results across species. Rodent studies have used firing rate in varying environments and require the measurement of inputs and outputs to the hippocampus to ensure that signals do not simply reflect downstream processes. Human studies typically measure neural signals that are thought to be consistent with pattern separation by measuring increases in hemodynamic signals (an indirect proxy for neural activity that tends to correlate with local field potentials65, although this is not always the case66–68) during viewing and/or discrimination of lure stimuli. One major difference between animal and human studies is that increased hemodynamic activity, manifesting as either reduced fMRI adaptation in incidental designs or increased contrast between lure rejections and false alarms in explicit designs, is not typically observed in neurophysiological studies of the DG region, where sparseness is thought to support pattern separation. It is worth noting that increases in hemodynamic signals may be a reflection of enhanced inhibition in the region, which is also consistent with sparse signaling. However, without direct pairing of neurophysiological recording and high-resolution fMRI, this account remains speculative. Studies using multivariate approaches such as representational similarity analyses may come closer to examining the correlated structure of activity across the region, which could allow for inferences to be made as to the degree to which similarity of the input (for example, in the EC) differs from similarity of the output (for example, in the DG and CA3). In general, although caution is warranted when generalizing across animal and human studies of pattern separation, these research approaches do appear to converge on similar findings, which can be summarized at the level of representation or input/output transformation processes regardless of the recording method.
Clinical and translational applications of the pattern-separation framework
In recent years, mnemonic discrimination tasks have become an important component of cognitive testing in clinical populations, with the hope of detecting subtle changes in hippocampal memory function early in the disease process. Figure 3 summarizes alterations in the hippocampus and surrounding medial temporal cortices across various clinical disorders.
Fig. 3. Medial temporal lobe circuitry alterations in disease.
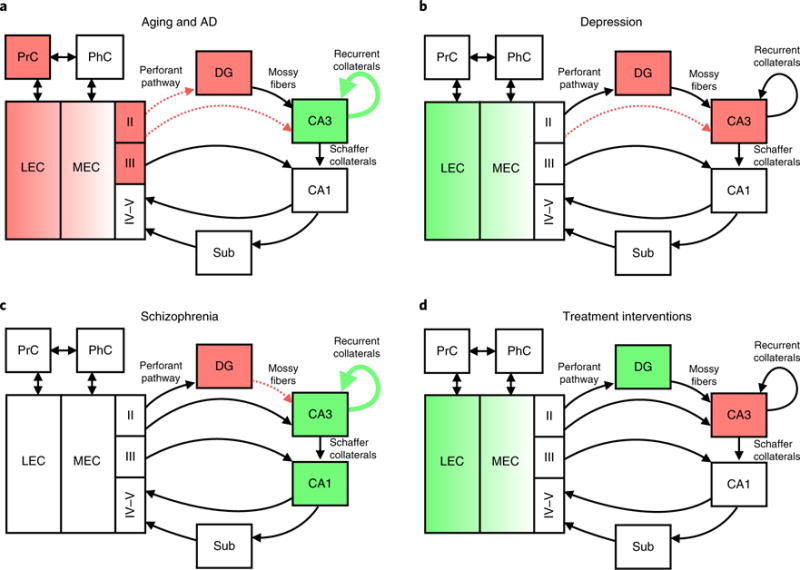
a, In aging and AD, there is a reduction of the perforant path, hyperactivity in CA3, reduced inhibition of CA3, hypoactivity in the EC, reduced reelin and tau deposition in the LEC, decreased EC thickness, and impaired object versus spatial processing depending on PrC. b, In depression, there is a retraction of the CA3 dendrites, decreased DG neurogenesis and decreased DG/CA3 BOLD activity. In late-life depression, there is altered DG/CA3 activity and LEC hyperactivity. c, In schizophrenia, there is reduced DG and mossy fiber glutamate transmission, as well as increased CA3 and CA1 activity. d, DG neurogenesis increases with exercise, environmental enrichment and SSRI treatment. Antiepileptic treatment in MCI patients reduces CA3 activity and increases the level of LEC activity.
Age-related cognitive decline and dementia
There is extensive evidence that declining memory function is present with increasing age69–71 and is a major symptom of mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Convergent data across animal and human studies have suggested that a key neural substrate for this decline is a shift in hippocampal network dynamics away from pattern separation and toward pattern com- pletion72, which appears to be mediated by CA3 hyperactivity35,73–76 and representational rigidity35,72,75,77—a failure to remap or manifest a novelty signal when stimuli are similar but not identical. These alterations may be closely linked to changes in the perforant path78–81 and to disinhibition in the DG and CA3 subregions resulting from the loss of inhibitory tone in GABAergic interneurons72,82. These changes in excitation/inhibition balance in hippocampal circuitry may act to strengthen CA3’s recurrent collaterals, potentially biasing the network toward reactivation of prior experiences (that is, pattern completion) at the expense of learning new information71,72. A recent study found that the LEC contributes to CA3 hyperactivity in aged rats with object-discrimination deficits76. However, studies have reported that hippocampal activity is eventually reduced during memory task performance in those with mild AD83,84. It is likely that the reduced hippocampal volume typically seen in aging subjects is a result of these small synaptic changes rather than of morphological cell loss85,86; however, a meta-analysis has suggested that the relationship between hippocampal volume and memory is weak in healthy older adults87. This suggests that the examination of more subtle hippocampal alterations may be a more sensitive method for detecting early memory change compared with examination of gross hippocampal volume.
A number of studies have shown that, compared with young adults, older adults show impairments in the ability to discriminate highly similar items across object34,35,37, spatial40,42,43, temporal48 and emotional88,89 domains. It is important to recognize that older adults and even amnesic patients can still recognize repeated stimuli90, but they have difficulty discriminating among highly similar items43, which highlights the importance of dissociating between general recognition memory and mnemonic discrimination. When object and spatial memory were directly compared, older adults showed greater impairment of object discrimination than spatial discrimination relative to that in young adults91. This is taken as evidence of impaired processing in the LEC–PrC pathway, which forms one of the earliest sites of tau pathology in aging92 and AD93,94.
A high-resolution fMRI study tested patients with amnestic MCI on an object-discrimination task and found that patients showed impaired performance on trials that taxed their pattern-separation abilities. In addition, the authors observed hyperactive BOLD signals in the DG/CA3 and hypoactive signals in the EC during discrimination36. Additional evidence suggests that lure-discrimination performance may be linked to ApoE4 status as well as cerebrospinal fluid β-amyloid burden95. A recent study found that cognitively normal older adults showed increased amyloid and tau as measured by in vivo positron emission tomography imaging and that this was associated with aberrant activity in the medial temporal lobes during an object-discrimination task96.
Overall, although these studies have demonstrated clear age- related deficits in these tasks (Fig. 3a), even after accounting for age-related deficits in perceptual and working memory processing, cognitive aging remains a complex condition that is not easily deconstructed. Tasks are rarely, if ever, process-pure and will therefore undoubtedly be contaminated by additional components such as impairments in pattern completion97, which may also be subject to the effect of aging and AD. An important future direction will be to validate the neural basis of each behavioral deficit and manipulate task conditions such that other variables can be systematically eliminated (perceptual or attentional influences, overt instructions during encoding, continuous recognition versus study/test blocks, etc.)98.
Neuropsychiatric disease
A role for hippocampal pattern separation has been suggested in psychiatric disorders such as depression, anxiety, schizophrenia, autism and post-traumatic stress disorder99–102. Depression is characterized by anhedonia, fatigue, changes in sleep and eating behavior, and alterations in memory and mood103,104, with impairment in episodic memory as well as a negativity memory bias. Postmortem human studies of depression have found that synaptic loss, rather than morphological cell loss, occurs mostly in the DG and CA3 subregions of the hippocampus105. Chronic stress manipulations have been used in animal models of depression to examine many of the core features of depression such as anhedonia, despair, appetite changes and anxious behavior106, and these models typically result in reduced hippocampal volume, which has been attributed to CA3 dendritic retraction and suppressed DG neurogenesis107–109. Recent work has shown that individuals with depressive symptoms have a diminished capacity to discriminate highly similar neutral objects110–112 and scene stimuli52. Furthermore, discrimination of negative scenes is enhanced in individuals with depressive symptoms in a manner that corresponds to the severity of the depressive phenotype52. This behavioral difference is accompanied by increased amygdala activity and decreased DG/CA3 activity in high-resolution fMRI. Furthermore, the level of DG/CA3 activity is negatively correlated with depressive symptom severity, which indicates that reduced DG/CA3 activity may be a pathological condition51,113. Older adults experiencing late-life depression also show enhanced discrimination of negative scenes, in addition to alterations in their amygdala–entorhinal–hippocampal network114 (Fig. 3b). Thus, overall results from studies of patients with depressive symptoms suggest the presence of impairments in pattern separation for neutral information and enhanced pattern separation for negatively valenced information, which is consistent with the negative rumination common to depression.
Anxiety is characterized by feelings of restlessness, alterations in fight-or-flight responses, difficulty concentrating and memory problems. Many patients with anxiety disorders display an overgeneralization of fear responses to emotional stimuli. In post-traumatic stress disorder, there is an overgeneralization of memory for the stimuli associated with the aversive event. Anxiety states have also been tested via mnemonic discrimination procedures in rodents and humans. In rodents, a contextual fear discrimination task has been used in which mice are trained to fear an aversive context and then to discriminate between the aversive context and a highly similar safe (no shock) environment. Mice freeze when exposed to the similar context, and thus generalize across the two contexts101. When humans are placed in an induced anxious state (that is, when subjected to the threat of unpredictable shock) during encoding, mnemonic discrimination improves when retrieval occurs in a safe context. However, when retrieval occurs in an unsafe environment, the benefit of improved pattern separation is lost, and this provides a putative mechanism for overgeneralization115. Thus, it appears that impaired pattern separation underlies the overgeneralization that is often seen in anxiety disorders.
Schizophrenia has been associated with impairments in cognitive functioning such as poor executive functioning, inability to sustain attention, and episodic memory deficits116. Postmortem studies have demonstrated a selective reduction in glutamate transmission in the DG and in its efferent mossy fiber pathway117,118, as well as increased neuronal activity in CA3119 and CA1120 (Fig. 3c). Individuals with schizophrenia have impaired object discrimination but not impaired general recognition memory compared with healthy controls121,122. Similar deficits have been shown in a ketamine-administration model of schizophrenia, which suggests that NMDA-receptor-mediated mechanisms might underlie the deficit122. Overall, these studies suggest that pattern separation may be impaired in schizophrenia, although neural recording studies (for example, using fMRI) would be needed to draw this conclusion. In addition, the behavioral deficits observed in schizophrenia may be partially explained by visual and perceptual deficits123.
Individuals with autism spectrum disorder (ASD) exhibit cognitive dysfunction and impaired emotion regulation124. They tend to overidentify objects as more different from previously viewed objects in object mnemonic discrimination tasks125. Discrimination accuracy also correlates with multiple measures of negative emotionality in those with ASD. Although it is still very early to say for certain, it is possible that the hyperdiscrimination observed in ASD is related to the negativity-related hyperdiscrimination observed in depression52 and may stem from limbic imbalance. However, neural studies are needed to formally test these hypotheses.
Across clinical conditions that involve hippocampal impairment, it appears that the computational capacity of the DG is compromised in largely nonspecific ways and, as a result, deficits in mnemonic discrimination are characteristic of a number of these conditions. Although the use of tasks sensitive to pattern-separation deficits is informative with regard to the pathophysiological mechanisms of different diseases, the phenotype does not have the specificity for differential diagnosis and seems to be generally sensitive to hippocampal impairment, but not specific to condition. Notable exceptions are the behavioral enhancements observed in ASD and depression (for negative stimuli), and it is possible that these enhancements stem from nonhippocampal modulations such as the amygdala or prefrontal cortex that may shift attention toward differences or bias the hippocampus toward a more discriminative encoding procedure. We caution, however, against simple interpretations in clinical disorders that tend to be quite complex, affecting not only memory but also a swath of other cognitive functions. Other factors, such as perception, attention and executive functioning, must be examined and/or controlled to determine whether impairments or enhancements in discrimination are a result of memory or other non-mnemonic effects.
Physical activity and exercise
Voluntary running has been shown to enhance the ability of adult mice to discriminate between the locations of two adjacent identical stimuli. More recent work has shown that running increases hippocampal neurogenesis and significantly improves memory for similar objects, whereas different objects can be distinguished by both running and sedentary mice126 (Fig. 3d). Age-related impairments in contextual discrimination are also reversed by running, which may be supported by mechanisms other than neurogenesis127. In humans, long-term aerobic exercise has been associated with improved discrimination of similar lures in an object mnemonic discrimination task111. A brief (10-min) bout of moderate exercise (50% of VO2 max) was shown to improve mnemonic discrimination of similar lures, but did not alter performance with either identical targets or novel foils128. Recent work has examined the effect of aerobic exercise in healthy older adults on vascular plasticity in the hippocampus. Changes in fitness and in hippocampal perfusion and volume were positively associated with changes in recognition memory and early recall for complex spatial objects, which requires discrimination among similar complex objects129. Overall, it appears that the benefits of both acute and long-term exercise for memory may be mediated in part by effects on hippocampal pattern separation, which enhances performance on discrimination tasks130. Understanding the neural mechanisms for these effects remains a significant challenge, especially because exercise is multifaceted and probably targets several mechanisms to enhance cognition.
Environmental enrichment
The exploration of visually stimulating virtual environments in video games can be used as a model of environmental enrichment, which has been associated with increased hippocampal neurogenesis, synaptogenesis, neurotrophic factors and dramatic improvement on hippocampus-dependent learning and memory tasks131,132 (Fig. 3d). A recent study showed that video gamers who specifically favor complex 3D video games performed better in object discrimination. In addition, after 2 weeks of training on the 3D video game (Super Mario 3D World), naive video gamers showed improved discrimination ability. Training on a comparable 2D video game (Angry Birds) showed no such improvements. Furthermore, individual performance in both hippocampal-associated behaviors correlated with performance in the 3D game, but not in the 2D game, which suggests that how individuals explore a virtual environment may influence hippocampal computational abilities133, although this account remains speculative in the absence of neural data.
Psychostimulants and pharmacological agents
Post-training caffeine administration (compared with placebo) was shown to improve consolidation on a 24-h object-discrimination test in caffeine-naive individuals134. Although the mechanism for this effect is not clear, it is possible that it is mediated at least partly by noradrenergic modulation, or possibly through action on adenosine receptors in the hippocampus. In patients with MCI, low doses of an anti-epileptic drug (levetiracetam) have been shown to reduce dysfunctional DG/CA3 hyperactivity and EC hypoactivity and rescue memory deficits on a mnemonic discrimination task74,135. Notably, the same therapy may also reduce hippocampal hyperactivity and rescue memory deficits in aged rodents136 and in mouse models of schizophrenia137 and AD138. This suggests that this interventional route may restore the excitation–inhibition balance in a host of conditions that involve hippocampal pathology.
Recent data have suggested that one mechanism by which selective serotonin-reuptake inhibitors (SSRIs) may reduce memory symptoms in those with depression is by improving DG neurogenesis139,140 (Fig. 3d). A recent review suggests that an improvement in pattern separation, particularly for situations that are emotionally arousing, may affect mood and anxiety symptoms as well141. The effect of DG neurogenesis on pattern-separation computations in healthy and disease- affected populations is an important avenue of future investigation, particularly as rodent studies have identified a role for adult-born neurons in population-based coding in DG14 and CA3142. Recent studies using nuclear-bomb-test-derived 14C suggest high levels of turnover in the human DG143, indicative of a continuous role for adult neurogenesis. However, assessing neurogenesis in humans is far from trivial. Some attempts have been made, but the applicability and specificity of these techniques has yet to be determined144.
Cautionary notes for clinical applications of pattern separation
The application of knowledge about the computational properties of the hippocampus in order to better characterize memory impairment and clinical populations can be a fruitful avenue of investigation. However, caution is warranted when making claims about the links between hippocampal computations and cognitive deficits. Absent neurobiological data, it is very difficult to make strong claims about hippocampal pattern separation or contributing mechanisms such as DG neurogenesis. For example, there are numerous other neurobiological processes outside of pattern separation that can contribute to discrimination-task performance. Thus, inferences about underlying neurobiology made purely on the basis of task performance and in the absence of neurobiological evidence may be premature. We suggest that, to avoid confusion and potentially misleading inferences, the term “pattern separation” should specifically refer to neurobiological processes, whereas the term “mnemonic discrimination” should be used to refer to the behavioral correlate of pattern separation. Translation from basic neurobiology and computational principles to clinical disorders is a crucial avenue of research and should be encouraged. Box 1 contains suggested guidelines for experimental design. Below, we also outline conditions for behavioral and neurobiological validation, intended to improve the quality of clinical and translational science in this arena.
Box 1. Guidelines for designing clinical research studies on pattern separation.
Although making direct claims about pattern-separation deficits solely on the basis of performance in mnemonic discrimination tasks is not recommended, it is important to note that it is critical to demonstrate a deficit in discrimination before asking neurobiological questions about the underlying mechanisms. We suggest that this might be considered a first step in the line of inquiry, and further delineate the conditions necessary to demonstrate relative specificity to hippocampal computations.
Condition 1: A parametric manipulation of similarity (at least two levels, but preferably more) is needed in which performance is impaired in conditions that have elevated interference (for example, high similarity), but improves when this interference is minimized. This type of behavior is suggestive of impairment in pattern separation.
Condition 2: Control conditions in which there is no interference (such as traditional recognition in human tasks or simple object recognition in the absence of similarity in animal tasks) are required to dissociate deficits in pattern separation from general memory deficits. These tasks should show minimal or no difference between groups.
Condition 3: Control conditions in which there is interference but no mnemonic load (for example, no-delay or short-delay discrimination tasks with no buildup of proactive interference—that is, each trial is tested immediately after encoding) are also necessary to demonstrate the specificity of the deficit to mnemonic rather than sensory, perceptual or attentional confounds.
Condition 4: If task-performance profiles satisfy all three of the conditions listed above, a tentative argument can be made that the mnemonic deficit may stem from an impairment in hippocampal computations and, in particular, pattern separation. However, in order for definitive conclusions about this to be drawn, the task results must be coupled with neurobiological data. Neurobiological data from in vivo recording (for example, electrophysiology, optical imaging, IEG imaging and fMRI) in the same subjects or the same population can be used to discover brain–behavior relationships that provide mechanistic support for the deficit. Several types of neurobiological evidence can be considered here, but the most powerful demonstrations will involve observations of deficits in coding properties as a function of interference.
As research in this field has flourished in recent years, meeting these conditions has become an important focus. However, it may be difficult to meet all of these conditions in any single study. There are numerous examples of studies that meet either condition 134,37,38,49,52,53 or condition 234–36,38,43,52,123,146. A smaller number of studies have met condition 335,52,123, and we find that this condition is often overlooked in clinical samples where performance could be altered on a number of untested conditions. Condition 4 has often been met20,31,35,36,51,57,114, but perhaps not in the same studies that satisfy conditions 1-3. One reason that concurrently satisfying all four conditions may be difficult is that imposition of an explicit task will sometimes lead to a diminished ability to record subfield-specific evidence of pattern separation33. We suggest that convergent data across more than one study may be used to make the case that a particular clinical deficit can be ascribed to pattern separation. In the absence of neurobiological evidence in a particular clinical population, however, it is difficult to justify the claim that pattern separation is specifically impaired and to rule out the alternative that the observed behavioral deficit is a result of another type of neural dysfunction.
Behavioral validation
Mnemonic discrimination tasks are largely similar to traditional object-recognition tasks, with the exception of the use of similar lure stimuli. The lures (in particular, the parametric manipulation of lure similarity) offer a unique opportunity to test the individual’s ability to resolve mnemonic interference. Thus, characterization of performance on just one level of similarity is not sufficient to draw conclusions about underlying mechanisms. Rather, performance should be considered as a function of interference. Figure 4 illustrates this approach and the types of patterns that can be detected both behaviorally (Fig. 4a) and neurally (Fig. 4b).
Fig. 4. Behavioral and neural predictions of discrimination performance and pattern separation.
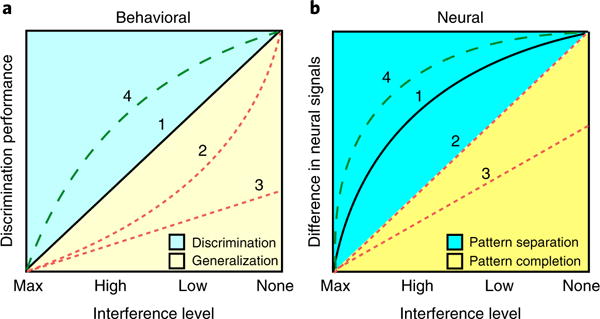
a, The x axis shows the interference level, from maximum interference to no interference, and the y axis shows discrimination performance (typically measured as a lure-discrimination index). The typically observed pattern of discrimination performance as a function of decreasing interference is largely linear (pattern 1). In clinical conditions, one might expect to see variations in this pattern, such that for certain interference conditions discrimination performance is lower or higher than that expected on the basis of control performance. Deviations from this linear pattern may be suggestive of impairments in pattern separation; however, the exact pattern of deviation will dictate whether certain conclusions can be drawn. For example, a clinical sample may show worse discrimination than that in healthy controls at high or low interference, but performance may not differ from that of controls in discrimination of targets or foils (pattern 2). This would suggest an impairment that is selective to items with interference and perhaps some specificity to pattern separation. This type of behavioral deficit has been characterized as a form of mnemonic rigidity. A selective deficit on the high, but not the low, interference stimuli could be stronger evidence in favor of this argument. In contrast, a more generalized impairment that includes even the items that induce little or no interference cannot be interpreted as selective to pattern separation (pattern 3). In some cases, it is possible to observe an enhanced discrimination profile that is selective to items with interference (pattern 4). b, Difference in neural signals (on the y axis) is more sharply tuned, such that normative samples typically show a curvilinear pattern (pattern 1). In this case, an impairment profile could appear linear (pattern 2). A linear ‘flattening’ of the neural tuning function can be interpreted as impairment in pattern separation. A more generalized impairment even on the items without interference (pattern 3) would be more suggestive of a general memory impairment that is not specific to pattern separation. Finally, enhanced tuning of the input/output neural transformation function is also possible (pattern 4) and would be expected under conditions that enhance memory, although evidence for this type of tuning remains scarce, with exercise studies possibly being an exception. Note the correspondence between numbers 1-4 in a and b, which indicates that overall curves in a are detuned versions of the curves in b, probably as a result of nonhippocampal influences, which introduce additional variability that influences the decision-making process. These hypothetical curves are based on a combination of observations from extant data and computational predictions.
Effective behavioral tests of pattern separation require multiple levels of stimulus similarity in order for inferences to be made about the computation. Performance should be evaluated as a function of these different levels of stimulus similarity, which range from maximum interference (essentially no discernable difference) to no interference (very different stimuli). Along this continuum are stimuli that possess intermediate levels of similarity and interference, which can be broadly categorized as high and low interference. The resolution and range of the x axis will vary across experiments and designs, but a minimum of two intermediate levels of interference are recommended to characterize the input/output function. Figure 4a describes several stereotypical patterns of behavior that have been observed in past studies in healthy and clinical populations.
In healthy adults, the expected pattern of discrimination performance as a function of decreasing interference is largely linear (pattern 1). In clinical conditions, one might expect to see variations in this pattern such that for certain interference conditions, discrimination performance is lower or higher than expected compared with controls. Thus, an important first step in developing a task would be to show a linear relationship between interference level and task performance in a healthy sample. Deviations from this linear pattern may be suggestive of impairments in pattern separation, but the exact pattern of deviation will dictate whether certain conclusions can be drawn.
For example, a clinical sample may show worse discrimination than healthy controls at high or low interference, but may not differ from controls in the discrimination of targets or foils (pattern 2). This would suggest an impairment that is selective to items with interference and perhaps some specificity to pattern separation. This type of behavioral deficit has been characterized as a form of ‘mnemonic rigidity’71. A selective deficit on the high, but not the low, interference stimuli could be stronger evidence in favor of this argument. In contrast, a more generalized impairment that includes even the items that induce little or no interference cannot be interpreted as selective to pattern separation (pattern 3).
It is interesting to note that in some cases, it is possible to observe an enhanced discrimination profile that is selective to items with interference (pattern 4). This tuning would be suggestive of more effective processing and resolution of interference and can be thought of as a form of ‘mnemonic flexibility’. We suggest that this is possible under conditions that enhance pattern separation, such as emotional arousal, caffeine use, or physical or cognitive exercise. Only one report thus far has observed this phenomenon130, which is associated with higher levels of physical fitness.
Neurobiological validation
Although behavioral tasks that appropriately parametrically manipulate interference are necessary for the examination of pattern separation, behavioral results are not a sufficient basis for claims about altered neural computations. Neurobiological validation is still required for such claims. We suggest that the most robust neurobiological validation uses a procedure analogous to that recommended for behavioral analyses, namely, the examination of data in terms of input/output transformations.
For example, given the same four levels of interference we used for behavioral analyses, the y axis can be switched from discrimination performance to ‘difference in neural signals’ (Fig. 4b). This can be BOLD fMRI contrast, decorrelated immediate early gene (IEG) population activity or single-unit firing, or any other indicator of neural change (Δ) in output. These measures reflect different scales of pattern-separation measurement, but their expected input/output transformations are similar (that is, linear versus curvilinear patterns as interference decreases). Unlike behavior, however, neural signals are more sharply tuned such that normative samples typically show a curvilinear pattern (pattern 1). In this case, an impairment profile could appear to be linear (pattern 2). A linear ‘flattening’ of the neural tuning function can be interpreted as impairment in pattern separation. A more generalized impairment even on the items without interference (pattern 3) would be more suggestive of a general memory impairment that is not specific to pattern separation.
Finally, an enhanced tuning of the input/output neural transformation function is also possible (pattern 4) and would be expected under conditions that enhance memory, although evidence reporting this type of tuning remains lacking.
It is important to note that with any neural recording technique, including fMRI, IEG and unit recordings, it is still difficult to directly measure hippocampal pattern separation, as most studies do not have the capability to measure the inputs and outputs simultaneously and with sufficient resolution to evaluate the input/output transformation directly. Some studies have come close to this31, but the assessment requires recording from multiple different regions and different types of cells simultaneously, which is challenging even with the most modern techniques. Instead, we rely on convergent data across studies and species to make inferences about the role of different brain regions in pattern separation and the linking of behavioral discrimination impairments to regionally specific pattern separation impairments.
Summary and conclusions
Over the past decade, there has been renewed interest in investigations of hippocampal pattern separation. We argue that although this has certainly been a healthy expansion of the field and has substantially informed efforts to understand memory computations, it has also led to some growing pains that are typical of the early stages of expansion of any young enterprise. As we reflect on the 13 years of empirical studies since the seminal demonstrations of hippocampal pattern separation in 2004, it behooves us to critically evaluate our approaches thus far to chart the path forward. In this Review, we not only summarized the current literature on the topic, but also put forth guidelines for future research, as well as some boundary conditions for making claims about pattern separation. We hope that this provides some guidance to improve the quality of the collective science and allow for careful fundamental investigation and even more careful clinical and translational application.
Acknowledgments
We thank M. Tsai, E. Murray and J. Noche for their helpful feedback on earlier versions of this manuscript. We also acknowledge our sources of support. S.L.L. is supported by NIA F32 AG054116. M.A.Y. is supported by NIA R01 AG053555, P50 AG16573 (Alzheimer’s Disease Research Center), NIMH R01 MH102392 and P50 MH096889 (Conte Center at UC Irvine).
Footnotes
Competing interests
The authors declare no competing financial interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- 2.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 3.Marr D. Simple memory: a theory for archicortex. Phil Trans R Soc Lond B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 4.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 5.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 6.Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J. The Hippocampus Book. Oxford University Press; Oxford, UK: 2007. [Google Scholar]
- 9.Deng W, Mayford M, Gage FH. Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife. 2013;2:e00312. doi: 10.7554/eLife.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavlis S, Petrantonakis PC, Poirazi P. Dendrites of dentate gyrus granule cells contribute to pattern separation by controlling sparsity. Hippocampus. 2017;27:89–110. doi: 10.1002/hipo.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharfman HE. The enigmatic mossy cell of the dentate gyrus. Nat Rev Neurosci. 2016;17:562–575. doi: 10.1038/nrn.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GoodSmith D, et al. Spatial representations of granule cells and mossy cells of the dentate gyrus. Neuron. 2017;93:677–690.e5. doi: 10.1016/j.neuron.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danielson NB, et al. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron. 2016;90:101–112. doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAvoy KM, et al. Modulating neuronal competition dynamics in the dentate gyrus to rejuvenate aging memory circuits. Neuron. 2016;91:1356–1373. doi: 10.1016/j.neuron.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adlaf EW, et al. Adult-born neurons modify excitatory synaptic transmission to existing neurons. eLife. 2017;6:e19886. doi: 10.7554/eLife.19886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 17.Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- 18.Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2010;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokes J, Kyle C, Ekstrom AD. Complementary roles of human hippocampal subfields in differentiation and integration of spatial context. J Cogn Neurosci. 2015;27:546–559. doi: 10.1162/jocn_a_00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leutgeb JK, et al. Progressive transformation of hippocampal neuronal representations in “morphed” environments. Neuron. 2005;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus. Hippocampus. 2012;22:389–398. doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J Neurosci. 1994;14:3898–3914. doi: 10.1523/JNEUROSCI.14-06-03898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumaran D, Maguire EA. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 2006;4:e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumaran D, Maguire EA. Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci. 2007;27:8517–8524. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumaran D, Maguire EA. Which computational mechanisms operate in the hippocampus during novelty detection? Hippocampus. 2007;17:735–748. doi: 10.1002/hipo.20326. [DOI] [PubMed] [Google Scholar]
- 28.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Meeter M, Murre JMJ, Talamini LM. Mode shifting between storage and recall based on novelty detection in oscillating hippocampal circuits. Hippocampus. 2004;14:722–741. doi: 10.1002/hipo.10214. [DOI] [PubMed] [Google Scholar]
- 30.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 31.Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81:416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 33.Kirwan CB, Stark CEL. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yassa MA, et al. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yassa MA, et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- 38.Holden HM, Toner C, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation varies in older adults. Learn Mem. 2013;20:358–362. doi: 10.1101/lm.030171.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reagh ZM, Yassa MA. Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc Natl Acad Sci USA. 2014;111:E4264–E4273. doi: 10.1073/pnas.1411250111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stark SM, Yassa MA, Stark CEL. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holden HM, Hoebel C, Loftis K, Gilbert PE. Spatial pattern separation in cognitively normal young and older adults. Hippocampus. 2012;22:1826–1832. doi: 10.1002/hipo.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holden HM, Gilbert PE. Less efficient pattern separation may contribute to age-related spatial memory deficits. Front Aging Neurosci. 2012;4:9. doi: 10.3389/fnagi.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reagh ZM, et al. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus. 2014;24:303–314. doi: 10.1002/hipo.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. J Neurosci. 1998;18:804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oomen CA, et al. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc. 2013;8:2006–2021. doi: 10.1038/nprot.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- 47.Tolentino JC, Pirogovsky E, Luu T, Toner CK, Gilbert PE. The effect of interference on temporal order memory for random and fixed sequences in nondemented older adults. Learn Mem. 2012;19:251–255. doi: 10.1101/lm.026062.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts JM, Ly M, Murray E, Yassa MA. Temporal discrimination deficits as a function of lag interference in older adults. Hippocampus. 2014;24:1189–1196. doi: 10.1002/hipo.22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert PE, Kesner RP. The amygdala but not the hippocampus is involved in pattern separation based on reward value. Neurobiol Learn Mem. 2002;77:338–353. doi: 10.1006/nlme.2001.4033. [DOI] [PubMed] [Google Scholar]
- 51.Leal SL, Tighe SK, Jones CK, Yassa MA. Pattern separation of emotional information in hippocampal dentate and CA3. Hippocampus. 2014;24:1146–1155. doi: 10.1002/hipo.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leal SL, Tighe SK, Yassa MA. Asymmetric effects of emotion on mnemonic interference. Neurobiol Learn Mem. 2014;111:41–48. doi: 10.1016/j.nlm.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Kumaran D, Maguire EA. Novelty signals: a window into hippocampal information processing. Trends Cogn Sci. 2009;13:47–54. doi: 10.1016/j.tics.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Kyle CT, Stokes JD, Lieberman JS, Hassan AS, Ekstrom AD. Successful retrieval of competing spatial environments in humans involves hippocampal pattern separation mechanisms. eLife. 2015;4:415–445. doi: 10.7554/eLife.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berron D, et al. Strong evidence for pattern separation in human dentate gyrus. J Neurosci. 2016;36:7569–7579. doi: 10.1523/JNEUROSCI.0518-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker S, et al. The human dentate gyrus plays a necessary role in discriminating new memories. Curr Biol. 2016;26:2629–2634. doi: 10.1016/j.cub.2016.07.081. [DOI] [PubMed] [Google Scholar]
- 59.Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knierim JJ, Neunuebel JP. Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics. Neurobiol Learn Mem. 2016;129:38–49. doi: 10.1016/j.nlm.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knierim JJ, Neunuebel JP, Deshmukh SS. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Phil Trans R Soc Lond B. 2013;369:20130369. doi: 10.1098/rstb.2013.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kent BA, Hvoslef-Eide M, Saksida LM, Bussey TJ. The representational-hierarchical view of pattern separation: Not just hippocampus, not just space, not just memory? Neurobiol Learn Mem. 2016;129:99–106. doi: 10.1016/j.nlm.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Pidgeon LM, Morcom AM. Cortical pattern separation and item-specific memory encoding. Neuropsychologia. 2016;85:256–271. doi: 10.1016/j.neuropsychologia.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 64.Reagh ZM, Murray EA, Yassa MA. Repetition reveals ups and downs of hippocampal, thalamic, and neocortical engagement during mnemonic decisions. Hippocampus. 2016;27:169–183. doi: 10.1002/hipo.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 66.Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev. 2010;62:233–244. doi: 10.1016/j.brainresrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gong L, Li B, Wu R, Li A, Xu F. Brain-state dependent uncoupling of BOLD and local field potentials in laminar olfactory bulb. Neurosci Lett. 2014;580:1–6. doi: 10.1016/j.neulet.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 68.Tyler CW, Likova LT, Nicholas SC. Analysis of neural-BOLD coupling through four models of the neural metabolic demand. Front Neurosci. 2015;9:419. doi: 10.3389/fnins.2015.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Craik FIM, Simon E. Age differences in memory: the roles of attention and depth of processing. In: Poon LW, Fozard J, Cermak LS, Arenberg D, Thompson LW, editors. New Directions in Memory and Aging. Psychology Press; New York: 1980. pp. 95–112. [Google Scholar]
- 70.Glisky E. Changes in cognitive function in human aging. In: Riddle D, editor. Brain Aging: Models, Methods, and Mechanisms. CRC Press; Boca Raton, FL, USA: 2007. pp. 1–10. [PubMed] [Google Scholar]
- 71.Leal SL, Yassa MA. Neurocognitive aging and the hippocampus across species. Trends Neurosci. 2015;38:800–812. doi: 10.1016/j.tins.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gallagher M, et al. Individual differences in neurocognitive aging of the medial temporal lobe. Age. 2006;28:221–233. doi: 10.1007/s11357-006-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bakker A, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maurer AP, et al. Age-related changes in lateral entorhinal and CA3 neuron allocation predict poor performance on object discrimination. Front Syst Neurosci. 2017;11:49. doi: 10.3389/fnsys.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson IA, et al. Cognitive aging and the hippocampus: how old rats represent new environments. J Neurosci. 2004;24:3870–3878. doi: 10.1523/JNEUROSCI.5205-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalus P, et al. Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia. Neuroimage. 2006;30:713–720. doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 79.Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci USA. 2010;107:12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barnes CA, Rao G, Houston FP. LTP induction threshold change in old rats at the perforant path-granule cell synapse. Neurobiol Aging. 2000;21:613–620. doi: 10.1016/s0197-4580(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 81.Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spiegel AM, Koh MT, Vogt NM, Rapp PR, Gallagher M. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol. 2013;521:3508–3523. doi: 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dickerson BC, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol. 1999;45:466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 85.West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 88.Leal SL, Yassa MA. Effects of aging on mnemonic discrimination of emotional information. Behav Neurosci. 2014;128:539–547. doi: 10.1037/bne0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leal SL, Noche JA, Murray EA, Yassa MA. Age-related individual variability in memory performance is associated with amygdala-hippocampal circuit function and emotional pattern separation. Neurobiol Aging. 2017;49:9–19. doi: 10.1016/j.neurobiolaging.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aggleton JP, et al. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43:1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 91.Reagh ZM, et al. Greater loss of object than spatial mnemonic discrimination in aged adults. Hippocampus. 2016;26:417–422. doi: 10.1002/hipo.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stranahan AM, Haberman RP, Gallagher M. Cognitive decline is associated with reduced reelin expression in the entorhinal cortex of aged rats. Cereb Cortex. 2011;21:392–400. doi: 10.1093/cercor/bhq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 94.Khan UA, et al. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wesnes KA, Annas P, Basun H, Edgar C, Blennow K. Performance on a pattern separation task by Alzheimer’s patients shows possible links between disrupted dentate gyrus activity and apolipoprotein E ∈4 status and cerebrospinal fluid amyloid-β42 levels. Alzheimers Res Ther. 2014;6:20. doi: 10.1186/alzrt250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marks SM, Lockhart SN, Baker SL, Jagust WJ. Tau and β-amyloid are associated with medial temporal lobe structure, function and memory encoding in normal aging. J Neurosci. 2017;37:3192–3201. doi: 10.1523/JNEUROSCI.3769-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vieweg P, Stangl M, Howard LR, Wolbers T. Changes in pattern completion: a key mechanism to explain age-related recognition memory deficits? Cortex. 2015;64:343–351. doi: 10.1016/j.cortex.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stark SM, Stevenson R, Wu C, Rutledge S, Stark CEL. Stability of age-related deficits in the mnemonic similarity task across task variations. Behav Neurosci. 2015;129:257–268. doi: 10.1037/bne0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 100.Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Besnard A, Sahay A. Adult hippocampal neurogenesis, fear generalization and stress. Neuropsychopharmacology. 2016;41:24–44. doi: 10.1038/npp.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Airaksinen E, Wahlin A, Forsell Y, Larsson M. Low episodic memory performance as a premorbid marker of depression: evidence from a 3-year follow-up. Acta Psychiatr Scand. 2007;115:458–465. doi: 10.1111/j.1600-0447.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 104.Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behav Brain Res. 2010;215:162–171. doi: 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 105.Stockmeier CA, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 107.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 108.Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 110.Shelton DJ, Kirwan CB. A possible negative influence of depression on the ability to overcome memory interference. Behav Brain Res. 2013;256:20–26. doi: 10.1016/j.bbr.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 111.Dery N, et al. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front Neurosci. 2013;7:66. doi: 10.3389/fnins.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Semenova A. BS thesis. Lund University; Lund, Sweden: 2015. Higher Depression Scores are Associated with Lower Pattern Separation Performance in Humans. [Google Scholar]
- 113.Fujii T, Saito DN, Yanaka HT, Kosaka H, Okazawa H. Depressive mood modulates the anterior lateral CA1 and DG/CA3 during a pattern separation task in cognitively intact individuals: a functional MRI study. Hippocampus. 2014;24:214–224. doi: 10.1002/hipo.22216. [DOI] [PubMed] [Google Scholar]
- 114.Leal SL, Noche JA, Murray EA, Yassa MA. Disruption of amygdala-entorhinal-hippocampal network in late-life depression. Hippocampus. 2017;27:464–476. doi: 10.1002/hipo.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Balderston NL, et al. Effect of anxiety on behavioural pattern separation in humans. Cogn Emot. 2017;31:238–248. doi: 10.1080/02699931.2015.1096235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McKenna P, Ornstein T, Baddeley AD. Schizophrenia. In: Baddeley AD, Kopelman MD, Wilson BA, editors. The Handbook of Memory Disorders. John Wiley & Sons; Chichester, UK: 2003. pp. 413–435. [Google Scholar]
- 117.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 118.Gao XM, et al. Ionotropic glutamate receptors and expression of N-methyl-d-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 119.Li W, et al. Synaptic proteins in the hippocampus indicative of increased neuronal activity in CA3 in schizophrenia. Am J Psychiatry. 2015;172:373–382. doi: 10.1176/appi.ajp.2014.14010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schobel SA, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Das T, Ivleva EI, Wagner AD, Stark CEL, Tamminga CA. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophr Res. 2014;159:193–197. doi: 10.1016/j.schres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kraguljac NV, et al. Mnemonic discrimination deficits in first episode psychosis and a ketamine model suggests dentate gyrus pathology linked to NMDA-receptor hypofunction. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017 doi: 10.1016/j.bpsc.2021.09.008. https://doi.org/10.1016/j.bpsc.2017.02.005. [DOI] [PMC free article] [PubMed]
- 123.Martinelli C, Shergill SS. Clarifying the role of pattern separation in schizophrenia: the role of recognition and visual discrimination deficits. Schizophr Res. 2015;166:328–333. doi: 10.1016/j.schres.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 124.White SW, et al. Social-cognitive, physiological, and neural mechanisms underlying emotion regulation impairments: understanding anxiety in autism spectrum disorder. Int J Dev Neurosci. 2014;39:22–36. doi: 10.1016/j.ijdevneu.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.South M, et al. Overactive pattern separation memory associated with negative emotionality in adults diagnosed with autism spectrum disorder. J Autism Dev Disord. 2015;45:3458–3467. doi: 10.1007/s10803-015-2547-x. [DOI] [PubMed] [Google Scholar]
- 126.Bolz L, Heigele S, Bischofberger J. Running improves pattern separation during novel object recognition. Brain Plast. 2015;1:129–141. doi: 10.3233/BPL-150010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu MV, Luna VM, Hen R. Running rescues a fear-based contextual discrimination deficit in aged mice. Front Syst Neurosci. 2015;9:114. doi: 10.3389/fnsys.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suwabe K, et al. Acute moderate exercise improves mnemonic discrimination in young adults. Hippocampus. 2017;27:229–234. doi: 10.1002/hipo.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maass A, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20:585–593. doi: 10.1038/mp.2014.114. [DOI] [PubMed] [Google Scholar]
- 130.Suwabe K, et al. Aerobic fitness associates with mnemonic discrimination as a mediator of physical activity effects: evidence for memory flexibility in young adults. Sci Rep. 2017;7:5140. doi: 10.1038/s41598-017-04850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 132.Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 133.Clemenson GD, Stark CEL. Virtual environmental enrichment through video games improves hippocampal-associated memory. J Neurosci. 2015;35:16116–16125. doi: 10.1523/JNEUROSCI.2580-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borota D, et al. Post-study caffeine administration enhances memory consolidation in humans. Nat Neurosci. 2014;17:201–203. doi: 10.1038/nn.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688–698. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smucny J, Stevens KE, Tregellas JR. The antiepileptic drug levetiracetam improves auditory gating in DBA/2 mice. NPJ Schizophr. 2015;1:15002. doi: 10.1038/npjschz.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sanchez PE, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci USA. 2012;109:E2895–E2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adam Samuels B, Leonardo ED, Hen R. Hippocampal subfields and major depressive disorder. Biol Psychiatry. 2015;77:210–211. doi: 10.1016/j.biopsych.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Treadway MT, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77:285–294. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Castren E, Hen R. Neuronal plasticity and antidepressant actions. Trends Neurosci. 2013;36:259–267. doi: 10.1016/j.tins.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Niibori Y, et al. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun. 2012;3:1253. doi: 10.1038/ncomms2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- 146.Ally BA, Hussey EP, Ko PC, Molitor RJ. Pattern separation and pattern completion in Alzheimer’s disease: evidence of rapid forgetting in amnestic mild cognitive impairment. Hippocampus. 2013;23:1246–1258. doi: 10.1002/hipo.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]


