Abstract
Diabetic kidney disease, commonly termed diabetic nephropathy (DN) is the most common cause of end-stage renal disease (ESRD) worldwide. The characteristic histopathology of DN includes glomerular basement membrane (GBM) thickening, mesangial expansion, nodular glomerular sclerosis, and tubulointerstitial fibrosis. Diabetes is associated with a number of metabolic derangements, such as reactive oxygen species (ROS) overproduction, hypoxic state, mitochondrial dysfunction, and inflammation. In the past few decades, our knowledge of DN has advanced considerably although much needs to be learned. The traditional paradigm of glomerulus-centered pathophysiology has expanded to the tubule-interstitium, the immune response and inflammation. Biomarkers of proximal tubule injury have been shown to correlate with DN progression, independent of traditional glomerular injury biomarkers such as albuminuria. In this review, we summarize mechanisms of increased susceptibility to acute kidney injury (AKI) in diabetes mellitus (DM) and the roles played by many kidney cell types, in facilitating maladaptive responses leading to chronic kidney disease (CKD) and end-stage kidney disease (ESKD).
Kidney Disease in Diabetes Mellitus
According to the National Diabetes Fact sheet data (www.cdc.gov), diabetes mellitus (DM) was the 7th leading cause of death in the United States in 2013, and more than 20% of health care was spent for patients with diabetes. Long-term complications derived from diabetes include both macrovascular and microvascular diseases. Diabetic nephropathy (DN) or diabetic kidney disease (DKD), is one of the main microvascular complications. After adjustment for age, sex, and race, diabetes remains the most common cause leading to end-stage renal disease (ESRD) in the United States.1 Although the number of major complications from diabetes has substantially declined in the U.S., the smallest decline was in ESRD.2 DN is characterized by glomerular basement membrane (GBM) thickening, mesangial expansion, nodular glomerular sclerosis, and tubulointerstitial fibrosis.3 While DN in individuals with diabetes is common it is important to recognize that not all patients with diabetes and impaired kidney function have DN with its characteristic pathological features as the cause of their kidney dysfunction and the renal pathology, if obtained, varies among patients.4–9. When we refer to DN in this manuscript we refer to patterns of injury that are consistent described as characteristic of nephropathy associated with diabetes without other contributors.
Acute Kidney Injury (AKI) in Diabetes Mellitus
Multiple studies have shown that diabetes alone is an independent risk for acute kidney injury (AKI).1,10,11 The incidence of AKI was found to be higher in diabetic patients undergoing surgery12–16, taking certain medications 17, with sepsis/septic shock18, and even without precipitating events.19,20 Renal insults resulting in tissue injury, including acute tubular injury, may affect kidney function and the rate of chronic functional impairment in diabetic patients, due to the fact that the subsequent maladaptive recovery fails to fully reverse the insults.21,22 There is, in general, a strong association between AKI and development of chronic kidney disease (CKD) and ESRD23–26. Thakar et al. demonstrated, in a cohort of 4082 patients with diabetes, single or repetitive episodes of AKI significantly increases the risk of developing advanced CKD27. Subsequently, a large prospective study further confirmed that AKI itself can also predict major adverse outcomes including doubling of serum creatinine or ESRD in patients with diabetes28. In the past few decades, the knowledge of the impact of DN has expanded beyond the glomerulus (podocyte) to other cell types. In this review, we will discuss the pathophysiology of increased susceptibility to AKI in diabetes and the impact of AKI in diabetes on various kidney cell types, each of which can play a role in injury and maladaptive repair. Furthermore, we discuss how repair after injury might be particularly maladaptive given the diabetic milieu and underlying chronic diabetic changes in the kidney.
Endothelial Dysfunction in Diabetic Nephropathy
NO Production and Signaling in Endothelial Cells
Glomerular endothelial cells are highly-fenestrated cells, carrying a thick layer of negatively-charged glycocalyx as a component of the glomerular filtration barrier.29 Dysfunction of endothelial cells is one of the key mechanisms in DN, and this dysfunction involves different parts of the kidneys including interstitial peritubular capillary vessels as well as the glomerular afferent and efferent arterioles.30–32 Nitric oxide (NO), an endothelium-derived relaxation factor produced by endothelial nitric oxide synthase (eNOS), is known to be reduced in diabetic kidneys. In normal physiology, NO dilates both the afferent and efferent arterioles, regulates activity of sympathetic tone in the kidneys and modulates sodium homeostasis.33 In advanced DN, although the expression of eNOS is upregulated, the production of NO is decreased due to the “uncoupling” of the synthase.34 Insulin resistance can also inhibit insulin receptor signaling leading to reduction of NO synthesis.35 Genetic polymorphisms causing reduced production of NO are reported to accelerate the progression of DN.36–38 Altogether, this disturbed NO metabolism in diabetes renders the renal vasculature more sensitive to stimuli leading to vasoconstriction. Furthermore, the level of vasoactive hormone, endothelin-1 (ET-1), is significantly elevated in DN and makes the vascular resistance even worse.39 Therefore, increased renal resistive indices and sensitivity to vasoconstrictors such as adenosine has been found in animal models,40,41 and also in patients with both metabolic syndrome and type 2 DM.42 These phenomena can explain why DN is a known risk factor for contrast-induced AKI, where vasoconstriction plays an important role, and ischemic-reperfusion injury (IRI), one of the most common causes of AKI often associated with shock, sepsis, surgery, or contrast administration. For example, the incidence of contrast-induced AKI (CIAKI) in DN is markedly higher than in the general population.43 Several potential therapeutic agents against CIAKI were investigated in patients. These include vasodilators (calcium channel blockers44–46, dopamine47), sodium bicarbonate48–51, N-acetylcysteine (NAC)52,53, and statins54–56. Unfortunately to date, none of these agents were found to be conclusively beneficial. One recent systemic review suggested that high-dose statin plus hydration with or without NAC might prevent CIAKI, but more trials targeted at specific populations such as DN are required to verify the result.57
Vascular Rarefaction and Hypoxia
Besides endothelial cell dysfunction, persistent hyperglycemia can also lead to endothelial cells apoptosis via NF-κB and c-Jun NH2-terminal pathways,58,59 and interstitial vascular rarefaction has been shown in human diabetic nephropathy.60 Vascular rarefaction then leads to significant hypoxic state in the kidneys, and the endothelial cell itself responds to chronic hypoxia with apoptosis rather than proliferation.32 In addition, peritubular capillary changes can be also observed as a long-term consequence of AKI61, which means DN can potentially aggravate the loss of perfusion in kidneys during AKI. Since oxygen (O2) supply is in high demand in kidneys, decreased O2 supply secondary to vascular rarefaction can compromise the generation of ATP, which is very important for proximal tubule functions.62 Apart from impaired O2 delivery, oxidative stress from overproduction of reactive oxygen species (ROS) in proximal tubules can damage the endothelial cells in the diabetic state. For example, Han et al. showed that, in the setting of persistent hyperglycemia, ROS such as H2O2 can be generated by proximal tubule cells via protein kinase C (PKC) and nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase activity, and ROS can directly reduce the level of available NO.63 Excessive oxidative stress has been well-validated as important pathway leading to CKD.64,65
Sustained integrity between endothelial cells and pericytes is essential for blood vessels stabilization.66,67 During AKI, the transformation of pericytes to myofibroblasts leads to series of fibrotic response,68 and pericytes detachment from endothelial cells can also trigger tubular injury and peritubular capillary rarefaction.69 Given damaged endothelial cells due to DN, the loss of endothelial-pericyte interaction may also cause fibrosis and eventually CKD. Therapy targeted on pericyte differentiation has been discussed as a potential therapeutic option for DN, but more studies are required to confirm the role of pericytes in DN model.70
Cross-talk Between Endothelial Cells and Podocytes
The cross-talk between glomerular endothelium and podocytes has been proposed to play an important role in DN. In the normal glomerulus, VEGF is mainly produced by podocytes and binds to VEGFR-2 located at endothelium71, maintaining vascular formation and differentiation. This paracrine effect is shown to be renoprotective in many different kidney diseases.72 Specific deletion of VEGF in mice podocytes can lead to renal disease characterized by proteinuria and endotheliosis.73 By contrast, increased levels of VEGF and VEGFR have been reported to aggravate DN71,74,75 due to the propagation of positive feedback loop from TGF-β/CTGF and overproduction of ROS.76 Given this observation, multiple studies have attempted to reduce the VEGF signal and demonstrated a favorable outcome.77–79 A recent study by Oltean et al. evaluated the efficacy of anti-VEGF antibody (VEGF-A165b) injection in both type 1&2 DN rodent models, and showed amelioration of renal function decline and pathological improvement.80 However, Sivaskandarajah et al. used the Cre-Loxp system to specifically delete the Vegfa gene in glomerular podocytes, and showed that diabetic mice with Vegfa deletion paradoxically have greater proteinuria and glomerular scarring.81 These seemingly conflicting results suggested a stringent balance of VEGF signaling (such as different subtypes) at different stages of DN must be maintained to prevent progression in DN82,83. It is unknown whether AKI on DN alters glomerular VEGF signaling.
Proximal Tubules Dysfunction in Diabetic Nephropathy
Pivotal Role of Proximal Tubules in DN
As DN is historically characterized by the development of albuminuria, glomerular change has been considered as primary with tubulointerstitial disease secondary. However, growing evidence argues that tubular damage occurs early in DN and may play a key role in progression of kidney disease.84 The implications of proximal tubular injury for development of CKD has been well established in several cohort studies85. There is a good reason to extrapolate to CKD secondary to tubular injuries in diabetes. Using biomarkers of tubular injury, we performed a cross-sectional comparison on samples from the second Joslin study, and concluded that lower urinary kidney injury molecule-1 (KIM-1) and N-acetyl-β-D-glucosaminidase (NAG) at baseline, which both indicate tubular damage, were associated with regression of microalbuminuria.86 Another analysis of the same cohort revealed that elevated baseline plasma KIM-1 was significantly associated with the risk of early decline of renal function, independent of albuminuria.87 Najafian et al. studied kidney biopsies obtained from type 1 DM patients, and found that, even with normal to only moderately impaired GFR, 17% of glomeruli were atubular, and 51% were attached to atrophic tubules.88 These clinical and pathological data strongly suggested that tubular damage plays a critical role in the development of DN, and may precede and interact with functional glomerular changes.89 As our previous laboratory work revealed that repetitive proximal tubular injury can lead to serial interstitial inflammation, fibrosis and eventually CKD, more attention to proximal tubules in DN is essential.22,90
Hypoxia and Proximal Tubules
Anatomically, the proximal tubule can be divided into three different segments, S1, S2 and S3. The number of mitochondria decreases from S1 to S3, but S3 is more prone to ischemic changes due to baseline lower oxygenation, susceptibility to reduced blood flow and likely impairment in the ability of the S3 segment to switch from oxidative to glycolytic metabolism.91–93 In diabetic animal studies outer medullary hypoxia has been documented using blood oxygen level-dependent (BOLD) MRI94,95. Cortical and medullary hypoxia also have been reported in humans with DN.96,97 Since medullary hypoxia ensues in the early stages of diabetes before the presence of kidney disease measured by increased serum creatinine or albuminuria, hypoxia gets worse as kidney disease progresses. As mentioned above, capillary rarefaction with decreased O2 delivery occurs in DN, the highly-demanded aerobic metabolic process of proximal tubules can be quickly disrupted during AKI. Meanwhile, the stress from mitochondrial dysfunction or increased O2 utilization can further intensify the damage during hypoxia.98 In this setting it is not surprising that patients with diabetes are more susceptible to insults that would further impair oxygen delivery to tubules which have increased oxygen utilization due to hyperfiltration associated with diabetes. Hypoxia-inducible factor (HIF)-1α, a transcription factor activated during hypoxia which contributes to fibrogenesis, has been shown to be increased in expression and implicated in the progression of DN.99,100 Persistent HIF-1 signaling also plays an important role in development of CKD.101 Suppression of HIF-1α both in vitro and vivo by using human renal proximal tubular epithelial cells (HRPTECs) and diabetic fatty rat ameliorated tubular injury102; similarly, YC-1 (a HIF-1 inhibitor) decreased tubulointerstitial fibrosis in OVE 26 type 1 diabetic mice.103 The preexistent HIF-1 upregulation in DN might explain more proximal tubular cells damage during AKI, since HIF-1 mediated pathways have been implicated in development of apoptosis after IRI.104
Increasing O2 utilization from upregulation of glucose transporters in diabetic proximal tubules may likewise worsen the hypoxia in DN. For instance, sodium-glucose cotransporter type 2 (SGLT-2), one of the proximal tubule glucose reabsorption transporters, is upregulated by four-fold to maximize the glucose reabsorption capacity105. As such, O2 consumption increases because the influx of glucose via luminal SGLT-2 requires a sodium gradient generated by Na+/K+-ATPase, an O2-dependent process. Clinically, SGLT-2 inhibitor empagliflozin is associated with improvement in cardiovascular outcomes, reduction of GFR decline and incidence of AKI (EMPA-REG OUTCOME trial)106,107 The effects of SGLT-2 inhibitors to reduce O2 usage has been compared with the “β-blockers effect” to reduce workload on the heart.108
Diabetic proximal tubules are also more susceptible to mitochondrial dysfunction during hypoxia due to heavy aerobic metabolism, and the delicate yet complicated dynamic of mitochondrial fusion and fission might be disorganized causing delayed recovery from kidney injuries.68 Of note, the change of mitochondrial adaptation can occur at proximal tubules as early as 4 weeks of age in the diabetic rat model.109 Myo-inositol oxygenase (MIOX), an enzyme specifically expressed in tubular cells, is found to be upregulated in the persistent hyperglycemic state. MIOX upregulation is accompanied with the accumulation of dysfunctional mitochondria, and therefore increased ROS production, apoptosis and eventually progression of DN.110 Sharma et al. reported that specificity protein (Sp)-1 epigenetically modulates the expression of MIOX. Using either a Sp-1 siRNA or inhibitor can significantly reduce ROS production.111 The tubule-specific MIOX knockout mice are also protected from cisplatin-induced AKI.112
p53 and Proximal Tubules
The role of p53 in AKI has been extensively discussed in the past decade. In addition to our previous laboratory work reporting that activation of p53 during AKI leads to progressive renal fibrosis22, subsequent other studies have shown similar results both in vivo and in vitro.113–115 In 2015, Peng et al. demonstrated, in high glucose-conditioned renal proximal tubular cells (RPTCs) and Akita diabetic mice, that p53 is upregulated in azide-induced chemical anoxic injury and IRI respectively. In Akita diabetic mice pretreated with either Pifithrin-α (p53 inhibitor) or p53 siRNA, levels of blood urea nitrogen (BUN), serum creatinine, and tubular injury were reduced after IRI. The authors further tested proximal tubule-specific p53-knockout mice (PT-p53-KO) injected with streptozotocin (STZ), a commonly-used method to induce diabetes. After IRI, PT-p53-KO mice had lower BUN, serum creatinine, and apoptosis on histological analysis, supported by less cleaved/active caspase 3 and Bax in the mitochondrial fraction.116
Inflammation and Proximal Tubules
Inflammatory cytokines have been shown to play an important role in the development and progression of DN117. For example, tissue necrosis factor (TNF)-α, interleukin (IL)-1118,119, IL-6118–121 have been reported to be upregulated and lead to serial cascades of inflammation. Hyperglycemia and advanced glycation endproducts (AGE) can induce the expression of adhesion molecules and chemokines in proximal tubular cells122,123, and albuminuria itself is also a tubular stimulus to express IL-8 via the NF-κB/PKC pathway and ROS production.124 As acute tubular injury creates an inflammatory milieu attracting neutrophils, monocytes, and macrophages68,125–128, the baseline inflammatory status in DN might predispose to an aggravated inflammatory state by the acute renal insult. Other studies have used tubule-specific genetic-modified mice and showed levels of tubular inflammation can affect the progression of DN. Gremlin, a bone morphogenetic proteins (BMPs) antagonist, is related to renal fibrosis and inflammation via activation of Smad/TGF-β and VEGFR2 respectively129,130, and is found to be highly expressed in human DN biopsies predominantly in the areas of tubulointerstitial fibrosis.131 Marchant et al. used a diabetic model induced by STZ in transgenic mice overexpressing Gremlin in proximal tubular cells, and showed more interstitial fibrosis and inflammation after inducing diabetes.132 Overexpression of Netrin-1 (a regulator of leukocyte migration) in proximal tubules was shown to suppress inflammation and albuminuria in STZ-induced diabetic mice,133 and the deletion of its receptor (UNC5B) aggravated albuminuria.134 Recently, sphingosine kinase 2 global knockout in mice (Sphk2−/−) was reported to attenuate kidney fibrosis after injury produced by folic acid (FA) injection or IRI.135 Since increased sphingosine 1-phosphate has been reported to mediate inflammation and fibrosis in tubular injury in diabetic nephropathy.136, deletion of Sphk2 in proximal tubules will be informative to assess its role in enhancing susceptibility to AKI in DN.
Maladaptive proximal tubular injury with subsequent fibrosis is the key component in AKI to CKD transition137, since tubulointerstitial fibrosis is the major determinant to ESRD.138 In addition to different mechanism of injury to proximal tubules mentioned above, hyperglycemia or AGE can stimulate proximal tubules cells and lead to profibrogenic cytokine secretion. When proximal tubular epithelial cells (PTEC) are cultured in hyperglycemia media, they can secrete extracellular matrix (ECM) via a TGF-β-dependent pathway.139,140 In vivo, TGF-β is found to be significantly elevated in both diabetic mouse model and human biopsy141, and considered as the key mediator of renal fibrosis. Blocking TGF-β signaling in DN has been investigated and shown decreasing fibrosis in animal models; however, it might remain a controversial therapeutic option due to increasing production of mineralocorticoids from adrenal glands.142 Platelet-derived growth factor (PDGF)143,144 and connecting tissue growth factor (CTGF)145 are another two important profibrotic in DN.146
Cross-talk between Proximal Tubules and Podocytes
In 2013, Hasegawa et al. published an important finding on the crosstalk between proximal tubular cells and podocytes in DN. The authors used the model of PT-specific Sirt1 transgenic and Sirt1 knockout mice, and demonstrated that Sirt1 can modulate the expression of Claudin-1 in podocytes by local nicotinamide mononucleotide (NMN) concentration in DN. The induction of Claudin-1 expression in podocytes leads to podocyte foot process effacement and albuminuria89,147, possibly via destabilizing the slit diaphragm (SD), an essential cell-cell junction complex as filtration barrier.148 A recent study showed that manipulation of SIRT1 by supplementation of NMN can protect mice from cisplatin-induced AKI149, suggesting SIRT1 is another possible pathway to reduce AKI susceptibility in DN.
Podocyte Dysfunction in Diabetic Nephropathy
Apoptosis and Foot Process Effacement of Podocytes in DN
Podocytes are highly differentiated visceral epithelial cells within the glomerulus, and provide an important component of the glomerular filtration barrier (GFB) along with glomerular endothelium and glomerular basement membrane (GBM).150 Podocytes are divided into three main components: cell body, major and foot process, and the processes are characterized by various types of cytoskeletons. Foot processes are enriched in actin filaments, and play an important role in cellular contracture features and forming slit-diaphragms (SD) with adjacent processes from the same cell body or adjacent podocytes. In the past decade, many proteins have been identified on the SD, and mutation of these proteins can lead to congenital nephrotic syndrome. Various genetic disease of podocytes have been summarized in a review.151 An important study describing the correlation between podocytes pathology and CKD progression in DN demonstrated the correlation of reduced podocytes (podocytopenia) with the progression of DN and the foot processes broadening on biopsies in type 2 DM patients.152 Such podocyte injury leads to the loss of integrity of GFB in the glomerulus, finally resulting in albuminuria in DN. As mentioned previously, albuminuria itself can damage tubular cells, and therefore uncontrolled albuminuria from podocyte injuries facilitates tubular epithelial cells injury leading to interstitial fibrosis which, in turn, impairs vascular supply to the glomerulus. Many clinical trials have sought to arrive at a treatment strategy to cease progression of DN. Therapeutic approaches have included angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs).153 Currently, the Kidney Disease Outcomes Quality Initiative (KDOQI) advises measuring albuminuria annually in patients with either type 1 or 2 diabetes after 5 years of diagnosis.154
Podocytes Apoptosis in DN
Hyperglycemia can induce podocytes apoptosis via several mechanisms, such as excessive ROS, renin-angiotensin-system (RAS) activation, mammalian target of rapamycin (mTOR) pathway, tumor growth factor-β (TGF-β)/SMAD155–157, or AGE.150,158,159 Apoptosis has been observed both in a diabetic mice model160 as well as human renal biopsies in type 2 DM patients.161 Since podocytes have much lower capacity to regenerate when compared with tubular cells, podocytopenia in DN might be an irreversible process leading to permanent damage.162
Susztak et al. used both an in vitro and in vivo model to demonstrate that in podocytes ROS are generated through NAPDH oxidase (NOX) and consequently led to activation of mitogen-activated protein kinase (MAPK) and caspase 3.160 Later, it was further suggested that NOX4 is the key NADPH oxidase, and pharmacological inhibition or genetic deletion of NOX4 ameliorates albuminuria.163,164 In addition to MAPK pathway, NOX4 can also be upregulated by mTOR pathway in hyperglycemia, and inhibiting mTOR by rapamycin reveals a similar phenotype as NADPH inhibition.165 Since NOX4 is also highly expressed in other types of cells, Jha et al. used a podocyte-specific Nox4 deletion mice, and established the deleterious effect of Nox4 expression in STZ-induced DN, specifically in podocytes with resultant albuminuria and extracellular matrix accumulation.166 Recently, the epigenetic regulation by histone methyltransferase enzyme enhancer of zeste homolog 2 (EZH2) further expands the role of oxidative stress in DN podocytes.167
Using ACEIs or ARBs to reduce proteinuria has been wildly used to control albuminuria in DN.168–171 Besides the concept of decreasing intra-glomerular pressure by ACEIs/ARBs, RAS blockade could also affect podocyte pathology in DN. Angiotensin II has been shown to cause podocyte apoptosis via TRPC6 (transient receptor potential canonical) calcium channel and resultant influx of Ca2+.172,173 The authors of a recent study found that podocytes isolated from diabetic rats has increased response to Ang II, which could aggravate AII-induced damage to podocytes.174 Since the RAS is activated in AKI175,176, circulating angiotensin II levels during AKI could further enhance the apoptosis of diabetic podocytes and lead to progression of DN.
Insulin signaling deficiency, either from reduced insulin secretion (type 1 DM) or insulin resistance (type 2 DM), is characteristic of diabetes. Upon insulin stimulation, there is increased PI3K/MAPK signaling increases 177, as well as glucose uptake through nephrin-dependent translocation of GLUT4 in podocytes.178,179 In mice with podocyte-specific deletion of the insulin receptor albuminuria develops even under normoglycemic conditions. There is also impaired remodeling of the actin cytoskeleton which subsequently affects podocyte morphology.177 Similarly, podocytes isolated from db/db mice also showed decreased response to insulin with reduced phosphorylation of AKT, and enhanced podocyte death after exposure to TNF-α treatment.180 This result also provides a potential mechanism for increased podocyte death during AKI in diabetes, since TNF-α is an important mediator in AKI.181 Recently, Falkevall et al. demonstrated that inactivation of VEGF-B-mediated lipid accumulation in podocytes can re-sensitize podocytes to insulin signaling and further improve podocyte survival. Therefore, anti-VEGF-B antibody might be another future treatment to halt DN progression especially in the context of episodes of AKI.182,183
Foot Process Effacement in DN
In addition to occurrence of podocyte apoptosis, foot process effacement (FPE) is an anatomical feature associating with profound albuminuria in DN, and loss of nephrin expression on slit diaphragms is thought to be one of the main pathogenic features of FPE in podocytes.184 Nephrin (a 180-kDa transmembrane protein) serves as an intracellular signaling scaffold185–187, modulating podocyte cytoskeleton188–190, and mediating insulin signaling. In DN, both increased levels of angiotensin and VEGF-A have been shown to decrease expression of nephrin.191 Recently, Teng et al. demonstrated that an increased expression of ubiquitous kinase/Cbl-interacting protein of 85 kDa (Ruk/CIN85), a binding partner of nephrin, mediated nephrin endocytosis via ubiquitination in DN.192 Of note, more glomerular insults by either unilateral nephrectomy or doxorubicin were observed in even short-term RNAi-mediated nephrin knockdown, which further suggests diabetic podocytes have higher susceptibility to AKI.193
As foot processes contain a great deal of actin cytoskeleton, regulation of actin remodeling also has an essential role in FPE. Rho-family small GTPases can mediate actin cytoskeletal dynamics changes194, and dysfunctions of RhoA, Rac1 and Cdc 42 have been related to podocyte diseases such as focal segmental glomerulosclerosis (FSGS)195 and pediatric steroid-resistant nephrotic syndrome (SRNS)196. In DN, down-regulation of PTEN (phosphatase and tensin homologue) can lead to unbalanced activation of the small GTPase Rac1/Cdc42 and RhoA,197 and Rac1 activation in podocytes induces rapid FPE and proteinuria.198 Blattner et al. used podocyte-specific Rac1 knockout mice to assess the acute podocyte injury to protamine sulfate, and showed prevention of FPE in Rac1 knockout mice. However, in chronic hypertensive glomerular damage, loss of Rac1 surprisingly leads to an exacerbation of albuminuria and glomerulosclerosis199. Thus, whether Rac1 functional changes are beneficial or detrimental in chronic DN requires more research to clarify.
Other Mechanisms of Injury to Podocytes in Diabetes
Autophagy is an important intracellular catabolic process to degrade certain proteins or organelles via lysosomes under stress,200 and protects against acute kidney injury in several models.68,201 Our work recently demonstrated that KIM-1, which is upregulated with AKI, mediated phagocytosis, followed by autophagy, downregulates inflammatory response in proximal tubules.202,203 Autophagy has also gained more attention in the pathogenesis of DN in the past few years.204–206 Although initial hyperglycemia can induce autophagy in podocytes207, prolonged hyperglycemia eventually down-regulates autophagy via various pathways such as mTOR and β-arrestins.208–212 Recent work with podocyte-specific Atg5 deletion (autophagy-deficient) mice showed additional acceleration of podocyte loss in both type 1213 and 2 DM models.214 Histone deacetylate 4215 and inositol-requiring enzyme-1α were also reported to regulate autophagy in podocytes.216
As inflammation and mitochondrial dysfunction can occur during DN, several studies explored the specific role of podocytes in these processes. For example, Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) has been found to increase in early human DN,217 and unsurprisingly acute injury to the kidney can also activates the JAK-STAT pathway.218,219 Overexpression JAK2 mRNA specifically in podocytes significantly increases albuminuria and reduces podocyte density in diabetic Akita mice, and a specific JAK1/2 inhibitor partly reversed the phenotype.220 Activation of Chemokine (C-C motif) receptor 2 (CCR2) occurs with AKI221 and has been associated with altering podocyte integrity in DN, and podocyte-specific CCR2 overexpression can mediate diabetic renal injury, independent of monocyte/macrophage recruitment.222 A phase IIa clinical trial is currently under investigation evaluating CCL2 inhibition in diabetic patients.223 In podocyte-specific soluble epoxide hydrolase (sEH) knockout mice, serum levels of inflammatory cytokines and the degrees of proteinuria were significantly reduced, suggesting that expression of sEH in podocytes might mediate the inflammatory response to lipopolysaccharide (LPS)-induced AKI.224 In addition, mitochondrial dysfunction with ROS overproduction225 and the change of mitochondrial dynamics are related to hyperglycemia.226 Increasing glucolytic flux traditionally is thought to cause mitochondrial dysfunction. In this study by Qi et al., they found that through pyruvate kinase M2 (PKM2) activation, the authors can protect from progression of DN by increasing glucose metabolic flux. Worse albuminuria and glomerular pathology were observed in podocyte-specific Pkm2-KO mice with diabetes, as was more podocyte apoptosis apparent in Pkm-knockdown immortalized mouse podocytes.227,228
Mitochondria play a key role in acute injury to the kidney. The dynamic of mitochondria consists of fission and fusion, and mitochondrial fission was shown to correlate with DN.226 The authors further identified the role of dynamin-related protein 1 (Drp1) in podocytes, showing that podocyte-specific deletion of Drp1 ameliorated progression of DN and improved mitochondrial fitness.229 Taken together, these studies all point to a key role of mediators present in acute tubular injury which may affect podocytes and facilitate the progression of DN.
Cross-talk Between Podocytes and Endothelial Cells
Apart from the VEGF signaling between podocytes and endothelial cells mentioned above, other signaling such as angiopoietin-1/2230–232, angiopoietin-like 4 (ANGPTL4)233,234, and eNOS235 have been reported to be involved in cross-talk between these two cells (see review236). Another important cross talk occurs between podocytes and endothelial cells is endothelin-1 (ET-1) signaling. In AKI and DN, the expression of ET-1 by endothelial cells is increased237, and blockade of ET-1 signaling in podocytes by podocyte-specific deletion of both endothelin receptor type A (ETRA) and type B (ETRB) showed less podocyte loss and glomerulosclerosis.238 A subsequent study revealed that endothelin-1 can activate podocytes to secrete heparanse, an enzyme cleaving the heparin sulfate (HS) which is important for the integrity of glomerular filtration barrier. As endothelial glycocalyx fragmentation is shown to predict the development of AKI and mortality of critically-ill patients239,240, ET-1 signaling in DN may also augment the severity of AKI. Treatment targeted at ET-1 receptors might be a future therapeutic option for DN.241–243
Conclusion
As our understanding of pathophysiology of DN progresses in recent years, there are still few new therapeutic options approved to prevent from the incidence or renal function decline in DN. Although it is likely that AKI can facilitate progressive decline in diabetes, leading to CKD, the current definition of AKI may delay its timely diagnosis21,244, and more precise understanding of the role that AKI plays in progression as well as the factors responsible for predisposition of the diabetic patient to AKI require better definition. The use of serum or urinary biomarkers in diagnosing and predicting outcome of DN is vigorously under investigation245,246, and new transcriptomics-proteomics technology is increasingly being applied to dissect the kidney responses to AKI.247 Therapies targeted to different cell types (e.g. podocytes, tubular cells, endothelium) in DN will likely be of use to mitigate this common kidney disease with high levels of morbidity and mortality (table 1).
Table 1.
Potential Treatments of Diabetic Kidney Diseases in Different Kidney Cells
| Mechanism of Injury | Potential Treatment | |
|---|---|---|
| Endothelium |
|
|
| Tubule |
|
|
| Podocyte |
|
|
| Non Cell-type Specific |
|
|
Abbreviation Nitric oxide (NO); Vascular endothelial growth factor (VEGF); Vascular endothelial growth factor receptor (VEGF-R); Endothelin-1 (ET-1); Reactive oxygen species (ROS); Hypoxia-induced factor 1-alpha (HIF-1α); Myo-inositol oxygenase (MIOX); Foot process effacement (FPE); Extracellular matrix (ECM); Contrast-induced acute kidney injury (CIAKI); Nuclear factor erythroid 2-like 2 (Nrf2); Dipeptidyl peptidase-4 (DPP-4); Rho-associated kinase 1 (ROCK1); Sodium-glucose cotransporter-2 (SGLT-2); Specificity protein-1 (Sp-1); Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α); NAPDH oxidase (NOX); mechanistic target of rapamycin (mTOR); Renin-angiotensin-aldosterone system (RAAS); Peroxisome proliferator-activated receptor gamma (PPARγ); Chemokine (C-C motif) receptor 2 (CCR2); N-acetyl-beta-D-glucosaminidase (NAG); Angiotensin-converting-enzyme inhibitor (ACEI); protein-tyrosine phosphatase 1B (PTP1B); Histone deacetylase (HDAC); Transforming growth factor beta (TGFβ); Mitochondria-targeted ubiquinone (MitoQ)
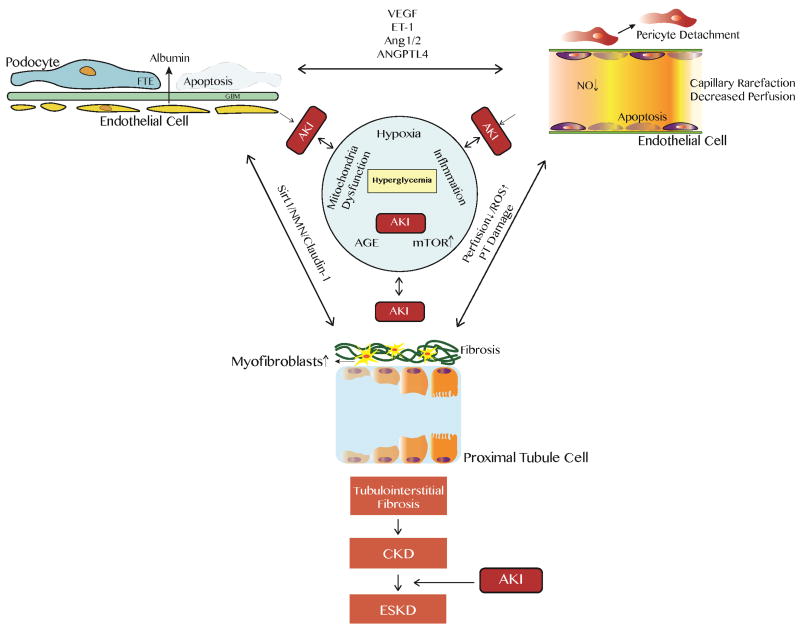
Figure. Scheme of relationship between diabetic nephropathy (DN) and acute kidney injury (AKI).
Diabetic nephropathy ensues from chronic hyperglycemia via several mechanisms, such as worsening hypoxic status, chronic inflammation, mitochondrial dysfunction, increasing mTOR signaling, and exposure to advanced glycation endproduct (AGE). Diseased kidney cells are more susceptible to acute kidney injury (AKI), and vice versa these damaged cells from DN can hasten the damage during each AKI episode. As traditional paradigm of DN has been expanded from glomerulus (podocyte)-centered to other cell types, the crosstalk among different kidney cells are revealed to be crucial in DN development. After diabetic kidneys undergo repetitive AKI, maladaptive repair occurs from acute renal insults, and irreversible tubulointerstitial fibrosis accumulates and eventually lead to chronic kidney disease (CKD) and end-stage kidney disease (ESKD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69(3S1):A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 3.Badal SS, Danesh FR. New insights into molecular mechanisms of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 Suppl 2):S63–83. doi: 10.1053/j.ajkd.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bojestig M, Arnqvist HJ, Hermansson G, Karlberg BE, Ludvigsson J. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N Engl J Med. 1994;330(1):15–18. doi: 10.1056/NEJM199401063300103. [DOI] [PubMed] [Google Scholar]
- 5.Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26(4):1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 6.Stout LC, Kumar S, Whorton EB. Insudative lesions--their pathogenesis and association with glomerular obsolescence in diabetes: a dynamic hypothesis based on single views of advancing human diabetic nephropathy. Hum Pathol. 1994;25(11):1213–1227. doi: 10.1016/0046-8177(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa T, Tanabe K, Croker BP, et al. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol. 2011;7(1):36–44. doi: 10.1038/nrneph.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fioretto P, Stehouwer CD, Mauer M, et al. Heterogeneous nature of microalbuminuria in NIDDM: studies of endothelial function and renal structure. Diabetologia. 1998;41(2):233–236. doi: 10.1007/s001250050895. [DOI] [PubMed] [Google Scholar]
- 9.Rossing P. The changing epidemiology of diabetic microangiopathy in type 1 diabetes. Diabetologia. 2005;48(8):1439–1444. doi: 10.1007/s00125-005-1836-x. [DOI] [PubMed] [Google Scholar]
- 10.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 11.James MT, Grams ME, Woodward M, et al. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Diabetes Mellitus, and Hypertension With Acute Kidney Injury. Am J Kidney Dis. 2015;66(4):602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta RH, Grab JD, O'Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114(21):2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz 2208. [DOI] [PubMed] [Google Scholar]
- 13.Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110(3):505–515. doi: 10.1097/ALN.0b013e3181979440. [DOI] [PubMed] [Google Scholar]
- 14.Parolari A, Pesce LL, Pacini D, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg. 2012;93(2):584–591. doi: 10.1016/j.athoracsur.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 15.Ko B, Garcia S, Mithani S, Tholakanahalli V, Adabag S. Risk of acute kidney injury in patients who undergo coronary angiography and cardiac surgery in close succession. Eur Heart J. 2012;33(16):2065–2070. doi: 10.1093/eurheartj/ehr493. [DOI] [PubMed] [Google Scholar]
- 16.Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J. 2015;170(5):895–902. doi: 10.1016/j.ahj.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009;53(7):2887–2891. doi: 10.1128/AAC.01430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venot M, Weis L, Clec'h C, et al. Acute Kidney Injury in Severe Sepsis and Septic Shock in Patients with and without Diabetes Mellitus: A Multicenter Study. PLoS One. 2015;10(5):e0127411. doi: 10.1371/journal.pone.0127411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girman CJ, Kou TD, Brodovicz K, et al. Risk of acute renal failure in patients with Type 2 diabetes mellitus. Diabet Med. 2012;29(5):614–621. doi: 10.1111/j.1464-5491.2011.03498.x. [DOI] [PubMed] [Google Scholar]
- 20.Mittalhenkle A, Stehman-Breen CO, Shlipak MG, et al. Cardiovascular risk factors and incident acute renal failure in older adults: the cardiovascular health study. Clin J Am Soc Nephrol. 2008;3(2):450–456. doi: 10.2215/CJN.02610607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J Am Soc Nephrol. 2016;27(3):687–697. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543. doi: 10.1038/nm.2144. 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James MT, Ghali WA, Knudtson ML, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123(4):409–416. doi: 10.1161/CIRCULATIONAHA.110.970160. [DOI] [PubMed] [Google Scholar]
- 24.Chew ST, Ng RR, Liu W, Chow KY, Ti LK. Acute kidney injury increases the risk of end-stage renal disease after cardiac surgery in an Asian population: a prospective cohort study. BMC Nephrol. 2017;18(1):60. doi: 10.1186/s12882-017-0476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 27.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6(11):2567–2572. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monseu M, Gand E, Saulnier PJ, et al. Acute Kidney Injury Predicts Major Adverse Outcomes in Diabetes: Synergic Impact With Low Glomerular Filtration Rate and Albuminuria. Diabetes Care. 2015;38(12):2333–2340. doi: 10.2337/dc15-1222. [DOI] [PubMed] [Google Scholar]
- 29.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88(2):451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 30.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124(6):2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120(13):1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Advani A, Gilbert RE. The endothelium in diabetic nephropathy. Semin Nephrol. 2012;32(2):199–207. doi: 10.1016/j.semnephrol.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Tessari P. Nitric oxide in the normal kidney and in patients with diabetic nephropathy. J Nephrol. 2015;28(3):257–268. doi: 10.1007/s40620-014-0136-2. [DOI] [PubMed] [Google Scholar]
- 34.Goligorsky MS, Chen J, Brodsky S. Workshop: endothelial cell dysfunction leading to diabetic nephropathy : focus on nitric oxide. Hypertension. 2001;37(2 Pt 2):744–748. doi: 10.1161/01.hyp.37.2.744. [DOI] [PubMed] [Google Scholar]
- 35.Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Haring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12(12):721–737. doi: 10.1038/nrneph.2016.145. [DOI] [PubMed] [Google Scholar]
- 36.Zanchi A, Moczulski DK, Hanna LS, Wantman M, Warram JH, Krolewski AS. Risk of advanced diabetic nephropathy in type 1 diabetes is associated with endothelial nitric oxide synthase gene polymorphism. Kidney Int. 2000;57(2):405–413. doi: 10.1046/j.1523-1755.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 37.Ezzidi I, Mtiraoui N, Mohamed MB, Mahjoub T, Kacem M, Almawi WY. Association of endothelial nitric oxide synthase Glu298Asp, 4b/a, and -786T>C gene variants with diabetic nephropathy. J Diabetes Complications. 2008;22(5):331–338. doi: 10.1016/j.jdiacomp.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Ahluwalia TS, Ahuja M, Rai TS, et al. Endothelial nitric oxide synthase gene haplotypes and diabetic nephropathy among Asian Indians. Mol Cell Biochem. 2008;314(1–2):9–17. doi: 10.1007/s11010-008-9759-8. [DOI] [PubMed] [Google Scholar]
- 39.Hargrove GM, Dufresne J, Whiteside C, Muruve DA, Wong NC. Diabetes mellitus increases endothelin-1 gene transcription in rat kidney. Kidney Int. 2000;58(4):1534–1545. doi: 10.1046/j.1523-1755.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 40.Pflueger AC, Schenk F, Osswald H. Increased sensitivity of the renal vasculature to adenosine in streptozotocin-induced diabetes mellitus rats. Am J Physiol. 1995;269(4 Pt 2):F529–535. doi: 10.1152/ajprenal.1995.269.4.F529. [DOI] [PubMed] [Google Scholar]
- 41.Ishimura E, Nishizawa Y, Kawagishi T, et al. Intrarenal hemodynamic abnormalities in diabetic nephropathy measured by duplex Doppler sonography. Kidney Int. 1997;51(6):1920–1927. doi: 10.1038/ki.1997.261. [DOI] [PubMed] [Google Scholar]
- 42.Buscemi S, Verga S, Batsis JA, et al. Intra-renal hemodynamics and carotid intima-media thickness in the metabolic syndrome. Diabetes Res Clin Pract. 2009;86(3):177–185. doi: 10.1016/j.diabres.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Calvin AD, Misra S, Pflueger A. Contrast-induced acute kidney injury and diabetic nephropathy. Nat Rev Nephrol. 2010;6(11):679–688. doi: 10.1038/nrneph.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cacoub P, Deray G, Baumelou A, Jacobs C. No evidence for protective effects of nifedipine against radiocontrast-induced acute renal failure. Clin Nephrol. 1988;29(4):215–216. [PubMed] [Google Scholar]
- 45.Khoury Z, Schlicht JR, Como J, et al. The effect of prophylactic nifedipine on renal function in patients administered contrast media. Pharmacotherapy. 1995;15(1):59–65. [PubMed] [Google Scholar]
- 46.Madsen JK, Jensen JW, Sandermann J, et al. Effect of nitrendipine on renal function and on hormonal parameters after intravascular iopromide. Acta Radiol. 1998;39(4):375–380. doi: 10.1080/02841859809172448. [DOI] [PubMed] [Google Scholar]
- 47.Stone GW, McCullough PA, Tumlin JA, et al. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA. 2003;290(17):2284–2291. doi: 10.1001/jama.290.17.2284. [DOI] [PubMed] [Google Scholar]
- 48.Brar SS, Shen AY, Jorgensen MB, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300(9):1038–1046. doi: 10.1001/jama.300.9.1038. [DOI] [PubMed] [Google Scholar]
- 49.Maioli M, Toso A, Leoncini M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52(8):599–604. doi: 10.1016/j.jacc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 50.Vasheghani-Farahani A, Sadigh G, Kassaian SE, et al. Sodium bicarbonate in preventing contrast nephropathy in patients at risk for volume overload: a randomized controlled trial. J Nephrol. 2010;23(2):216–223. [PubMed] [Google Scholar]
- 51.Zoungas S, Ninomiya T, Huxley R, et al. Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast-induced nephropathy. Ann Intern Med. 2009;151(9):631–638. doi: 10.7326/0003-4819-151-9-200911030-00008. [DOI] [PubMed] [Google Scholar]
- 52.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343(3):180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 53.Fishbane S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol. 2008;3(1):281–287. doi: 10.2215/CJN.02590607. [DOI] [PubMed] [Google Scholar]
- 54.Khanal S, Attallah N, Smith DE, et al. Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am J Med. 2005;118(8):843–849. doi: 10.1016/j.amjmed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 55.Jo SH, Koo BK, Park JS, et al. Prevention of radiocontrast medium-induced nephropathy using short-term high-dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial--a randomized controlled study. Am Heart J. 2008;155(3):499, e491–498. doi: 10.1016/j.ahj.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 56.Toso A, Maioli M, Leoncini M, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am J Cardiol. 2010;105(3):288–292. doi: 10.1016/j.amjcard.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 57.Su X, Xie X, Liu L, et al. Comparative Effectiveness of 12 Treatment Strategies for Preventing Contrast-Induced Acute Kidney Injury: A Systematic Review and Bayesian Network Meta-analysis. Am J Kidney Dis. 2017;69(1):69–77. doi: 10.1053/j.ajkd.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 58.Ho FM, Liu SH, Liau CS, Huang PJ, Lin-Shiau SY. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation. 2000;101(22):2618–2624. doi: 10.1161/01.cir.101.22.2618. [DOI] [PubMed] [Google Scholar]
- 59.Ho FM, Lin WW, Chen BC, et al. High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cell Signal. 2006;18(3):391–399. doi: 10.1016/j.cellsig.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Lindenmeyer MT, Kretzler M, Boucherot A, et al. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18(6):1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- 61.Kramann R, Tanaka M, Humphreys BD. Fluorescence microangiography for quantitative assessment of peritubular capillary changes after AKI in mice. J Am Soc Nephrol. 2014;25(9):1924–1931. doi: 10.1681/ASN.2013101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aksu U, Demirci C, Ince C. The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contrib Nephrol. 2011;174:119–128. doi: 10.1159/000329249. [DOI] [PubMed] [Google Scholar]
- 63.Han HJ, Lee YJ, Park SH, Lee JH, Taub M. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am J Physiol Renal Physiol. 2005;288(5):F988–996. doi: 10.1152/ajprenal.00327.2004. [DOI] [PubMed] [Google Scholar]
- 64.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009;297(2):F461–470. doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 65.Basile DP, Leonard EC, Beal AG, Schleuter D, Friedrich J. Persistent oxidative stress following renal ischemia-reperfusion injury increases ANG II hemodynamic and fibrotic activity. Am J Physiol Renal Physiol. 2012;302(11):F1494–1502. doi: 10.1152/ajprenal.00691.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diaz-Flores L, Gutierrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24(7):909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 67.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013;24(4):559–572. doi: 10.1681/ASN.2012080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zuk A, Bonventre JV. Acute Kidney Injury. Annu Rev Med. 2016;67:293–307. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1+ Pericyte Loss Induces Capillary Rarefaction and Proximal Tubular Injury. J Am Soc Nephrol. 2017;28(3):776–784. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Humphreys BD. Targeting pericyte differentiation as a strategy to modulate kidney fibrosis in diabetic nephropathy. Semin Nephrol. 2012;32(5):463–470. doi: 10.1016/j.semnephrol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooper ME, Vranes D, Youssef S, et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48(11):2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- 72.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 73.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111(5):707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsuchida K, Makita Z, Yamagishi S, et al. Suppression of transforming growth factor beta and vascular endothelial growth factor in diabetic nephropathy in rats by a novel advanced glycation end product inhibitor, OPB-9195. Diabetologia. 1999;42(5):579–588. doi: 10.1007/s001250051198. [DOI] [PubMed] [Google Scholar]
- 75.Saito D, Maeshima Y, Nasu T, et al. Amelioration of renal alterations in obese type 2 diabetic mice by vasohibin-1, a negative feedback regulator of angiogenesis. Am J Physiol Renal Physiol. 2011;300(4):F873–886. doi: 10.1152/ajprenal.00503.2010. [DOI] [PubMed] [Google Scholar]
- 76.Tufro A, Veron D. VEGF and podocytes in diabetic nephropathy. Semin Nephrol. 2012;32(4):385–393. doi: 10.1016/j.semnephrol.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12(5):993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- 78.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol. 2006;17(11):3093–3104. doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- 79.Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51(10):3090–3094. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- 80.Oltean S, Qiu Y, Ferguson JK, et al. Vascular Endothelial Growth Factor-A165b Is Protective and Restores Endothelial Glycocalyx in Diabetic Nephropathy. J Am Soc Nephrol. 2015;26(8):1889–1904. doi: 10.1681/ASN.2014040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE. Vegfa protects the glomerular microvasculature in diabetes. Diabetes. 2012;61(11):2958–2966. doi: 10.2337/db11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maezawa Y, Takemoto M, Yokote K. Cell biology of diabetic nephropathy: Roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig. 2015;6(1):3–15. doi: 10.1111/jdi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goligorsky MS. Vascular endothelium in diabetes. Am J Physiol Renal Physiol. 2017;312(2):F266–F275. doi: 10.1152/ajprenal.00473.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol. 2012;32(5):452–462. doi: 10.1016/j.semnephrol.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waikar SS, Sabbisetti V, Arnlov J, et al. Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule-1 in five cohort studies. Nephrol Dial Transplant. 2016;31(9):1460–1470. doi: 10.1093/ndt/gfw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaidya VS, Niewczas MA, Ficociello LH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int. 2011;79(4):464–470. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nowak N, Skupien J, Niewczas MA, et al. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int. 2016;89(2):459–467. doi: 10.1038/ki.2015.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Najafian B, Kim Y, Crosson JT, Mauer M. Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J Am Soc Nephrol. 2003;14(4):908–917. doi: 10.1097/01.asn.0000057854.32413.81. [DOI] [PubMed] [Google Scholar]
- 89.Hasegawa K, Wakino S, Simic P, et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19(11):1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82(2):172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fenton RAPJ. Anatomy of the kidney. In: Taal MWCG, Marsden PA, Skorecki K, Yu SL, Brener BM, editors. Brenner and Rector's The Kidney. Elsevier; 2016. [Google Scholar]
- 92.Bonventre JV. Molecular response to cytotoxic injury: role of inflammation, MAP kinases, and endoplasmic reticulum stress response. Semin Nephrol. 2003;23(5):439–448. doi: 10.1016/s0270-9295(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 93.Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol. 1998;275(5 Pt 2):F623–631. doi: 10.1152/ajprenal.1998.275.5.F623. [DOI] [PubMed] [Google Scholar]
- 94.Ries M, Basseau F, Tyndal B, et al. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging. 2003;17(1):104–113. doi: 10.1002/jmri.10224. [DOI] [PubMed] [Google Scholar]
- 95.Edlund J, Hansell P, Fasching A, et al. Reduced oxygenation in diabetic rat kidneys measured by T2* weighted magnetic resonance micro-imaging. Adv Exp Med Biol. 2009;645:199–204. doi: 10.1007/978-0-387-85998-9_31. [DOI] [PubMed] [Google Scholar]
- 96.Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22(8):1429–1434. doi: 10.1681/ASN.2010111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yin WJ, Liu F, Li XM, et al. Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur J Radiol. 2012;81(7):1426–1431. doi: 10.1016/j.ejrad.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 98.Gilbert RE. Proximal Tubulopathy: Prime Mover and Key Therapeutic Target in Diabetic Kidney Disease. Diabetes. 2017;66(4):791–800. doi: 10.2337/db16-0796. [DOI] [PubMed] [Google Scholar]
- 99.Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008;4(4):216–226. doi: 10.1038/ncpneph0757. [DOI] [PubMed] [Google Scholar]
- 100.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291(2):F271–281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takiyama Y, Harumi T, Watanabe J, et al. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1alpha expression and oxygen metabolism. Diabetes. 2011;60(3):981–992. doi: 10.2337/db10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nayak BK, Shanmugasundaram K, Friedrichs WE, et al. HIF-1 Mediates Renal Fibrosis in OVE26 Type 1 Diabetic Mice. Diabetes. 2016;65(5):1387–1397. doi: 10.2337/db15-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhatt K, Wei Q, Pabla N, et al. MicroRNA-687 Induced by Hypoxia-Inducible Factor-1 Targets Phosphatase and Tensin Homolog in Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2015;26(7):1588–1596. doi: 10.1681/ASN.2014050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54(12):3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 106.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 107.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 108.Gilbert RE. SGLT2 inhibitors: beta blockers for the kidney? Lancet Diabetes Endocrinol. 2016;4(10):814. doi: 10.1016/S2213-8587(16)30237-6. [DOI] [PubMed] [Google Scholar]
- 109.Coughlan MT, Nguyen TV, Penfold SA, et al. Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin Sci (Lond) 2016;130(9):711–720. doi: 10.1042/CS20150838. [DOI] [PubMed] [Google Scholar]
- 110.Zhan M, Usman IM, Sun L, Kanwar YS. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol. 2015;26(6):1304–1321. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharma I, Dutta RK, Singh NK, Kanwar YS. High Glucose-Induced Hypomethylation Promotes Binding of Sp-1 to Myo-Inositol Oxygenase: Implication in the Pathobiology of Diabetic Tubulopathy. Am J Pathol. 2017;187(4):724–739. doi: 10.1016/j.ajpath.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dutta RK, Kondeti VK, Sharma I, Chandel NS, Quaggin SE, Kanwar YS. Beneficial Effects of Myo-Inositol Oxygenase Deficiency in Cisplatin-Induced AKI. J Am Soc Nephrol. 2017;28(5):1421–1436. doi: 10.1681/ASN.2016070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ying Y, Kim J, Westphal SN, Long KE, Padanilam BJ. Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. J Am Soc Nephrol. 2014;25(12):2707–2716. doi: 10.1681/ASN.2013121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Samarakoon R, Dobberfuhl AD, Cooley C, et al. Induction of renal fibrotic genes by TGF-beta1 requires EGFR activation, p53 and reactive oxygen species. Cell Signal. 2013;25(11):2198–2209. doi: 10.1016/j.cellsig.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 115.Overstreet JM, Samarakoon R, Meldrum KK, Higgins PJ. Redox control of p53 in the transcriptional regulation of TGF-beta1 target genes through SMAD cooperativity. Cell Signal. 2014;26(7):1427–1436. doi: 10.1016/j.cellsig.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peng J, Li X, Zhang D, et al. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int. 2015;87(1):137–150. doi: 10.1038/ki.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19(3):433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 118.Navarro JF, Milena FJ, Mora C, Leon C, Garcia J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26(6):562–570. doi: 10.1159/000098004. [DOI] [PubMed] [Google Scholar]
- 119.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 120.Sekizuka K, Tomino Y, Sei C, et al. Detection of serum IL-6 in patients with diabetic nephropathy. Nephron. 1994;68(2):284–285. doi: 10.1159/000188281. [DOI] [PubMed] [Google Scholar]
- 121.Suzuki D, Miyazaki M, Naka R, et al. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes. 1995;44(10):1233–1238. doi: 10.2337/diab.44.10.1233. [DOI] [PubMed] [Google Scholar]
- 122.Tang SC, Leung JC, Chan LY, Tsang AW, Lai KN. Activation of tubular epithelial cells in diabetic nephropathy and the role of the peroxisome proliferator-activated receptor-gamma agonist. J Am Soc Nephrol. 2006;17(6):1633–1643. doi: 10.1681/ASN.2005101113. [DOI] [PubMed] [Google Scholar]
- 123.Tang SC, Chan LY, Leung JC, et al. Differential effects of advanced glycation end-products on renal tubular cell inflammation. Nephrology (Carlton) 2011;16(4):417–425. doi: 10.1111/j.1440-1797.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 124.Tang S, Leung JC, Abe K, et al. Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest. 2003;111(4):515–527. doi: 10.1172/JCI16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ysebaert DK, De Greef KE, Vercauteren SR, et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15(10):1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 126.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22(2):317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rabb H, Griffin MD, McKay DB, et al. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J Am Soc Nephrol. 2016;27(2):371–379. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodrigues-Diez R, Rodrigues-Diez RR, Lavoz C, et al. Gremlin activates the Smad pathway linked to epithelial mesenchymal transdifferentiation in cultured tubular epithelial cells. Biomed Res Int. 2014;2014:802841. doi: 10.1155/2014/802841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lavoz C, Alique M, Rodrigues-Diez R, et al. Gremlin regulates renal inflammation via the vascular endothelial growth factor receptor 2 pathway. J Pathol. 2015;236(4):407–420. doi: 10.1002/path.4537. [DOI] [PubMed] [Google Scholar]
- 131.Dolan V, Murphy M, Sadlier D, et al. Expression of gremlin, a bone morphogenetic protein antagonist, in human diabetic nephropathy. Am J Kidney Dis. 2005;45(6):1034–1039. doi: 10.1053/j.ajkd.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 132.Marchant V, Droguett A, Valderrama G, et al. Tubular overexpression of Gremlin in transgenic mice aggravates renal damage in diabetic nephropathy. Am J Physiol Renal Physiol. 2015;309(6):F559–568. doi: 10.1152/ajprenal.00023.2015. [DOI] [PubMed] [Google Scholar]
- 133.Mohamed R, Jayakumar C, Ranganathan PV, Ganapathy V, Ramesh G. Kidney proximal tubular epithelial-specific overexpression of netrin-1 suppresses inflammation and albuminuria through suppression of COX-2-mediated PGE2 production in streptozotocin-induced diabetic mice. Am J Pathol. 2012;181(6):1991–2002. doi: 10.1016/j.ajpath.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ranganathan P, Mohamed R, Jayakumar C, Brands MW, Ramesh G. Deletion of UNC5B in Kidney Epithelium Exacerbates Diabetic Nephropathy in Mice. Am J Nephrol. 2015;41(3):220–230. doi: 10.1159/000381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bajwa A, Huang L, Kurmaeva E, et al. Sphingosine Kinase 2 Deficiency Attenuates Kidney Fibrosis via IFN-gamma. J Am Soc Nephrol. 2017;28(4):1145–1161. doi: 10.1681/ASN.2016030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yaghobian D, Don AS, Yaghobian S, Chen X, Pollock CA, Saad S. Increased sphingosine 1-phosphate mediates inflammation and fibrosis in tubular injury in diabetic nephropathy. Clin Exp Pharmacol Physiol. 2016;43(1):56–66. doi: 10.1111/1440-1681.12494. [DOI] [PubMed] [Google Scholar]
- 137.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26(8):1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20(1):1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 139.Ziyadeh FN, Snipes ER, Watanabe M, Alvarez RJ, Goldfarb S, Haverty TP. High glucose induces cell hypertrophy and stimulates collagen gene transcription in proximal tubule. Am J Physiol. 1990;259(4 Pt 2):F704–714. doi: 10.1152/ajprenal.1990.259.4.F704. [DOI] [PubMed] [Google Scholar]
- 140.Rocco MV, Chen Y, Goldfarb S, Ziyadeh FN. Elevated glucose stimulates TGF-beta gene expression and bioactivity in proximal tubule. Kidney Int. 1992;41(1):107–114. doi: 10.1038/ki.1992.14. [DOI] [PubMed] [Google Scholar]
- 141.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993;90(5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang AS, Hathaway CK, Smithies O, Kakoki M. Transforming growth factor-beta1 and diabetic nephropathy. Am J Physiol Renal Physiol. 2016;310(8):F689–F696. doi: 10.1152/ajprenal.00502.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lassila M, Jandeleit-Dahm K, Seah KK, et al. Imatinib attenuates diabetic nephropathy in apolipoprotein E-knockout mice. J Am Soc Nephrol. 2005;16(2):363–373. doi: 10.1681/ASN.2004050392. [DOI] [PubMed] [Google Scholar]
- 144.Fraser D, Brunskill N, Ito T, Phillips A. Long-term exposure of proximal tubular epithelial cells to glucose induces transforming growth factor-beta 1 synthesis via an autocrine PDGF loop. Am J Pathol. 2003;163(6):2565–2574. doi: 10.1016/s0002-9440(10)63611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chung AC, Zhang H, Kong YZ, et al. Advanced glycation end-products induce tubular CTGF via TGF-beta-independent Smad3 signaling. J Am Soc Nephrol. 2010;21(2):249–260. doi: 10.1681/ASN.2009010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang SC, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol Dial Transplant. 2012;27(8):3049–3056. doi: 10.1093/ndt/gfs260. [DOI] [PubMed] [Google Scholar]
- 147.Nihalani D, Susztak K. Sirt1-Claudin-1 crosstalk regulates renal function. Nat Med. 2013;19(11):1371–1372. doi: 10.1038/nm.3386. [DOI] [PubMed] [Google Scholar]
- 148.Gong Y, Sunq A, Roth RA, Hou J. Inducible Expression of Claudin-1 in Glomerular Podocytes Generates Aberrant Tight Junctions and Proteinuria through Slit Diaphragm Destabilization. J Am Soc Nephrol. 2017;28(1):106–117. doi: 10.1681/ASN.2015121324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guan Y, Wang SR, Huang XZ, et al. Nicotinamide Mononucleotide, an NAD+ Precursor, Rescues Age-Associated Susceptibility to AKI in a Sirtuin 1-Dependent Manner. J Am Soc Nephrol. 2017;28(8):2337–2352. doi: 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol (Lausanne) 2014;5:151. doi: 10.3389/fendo.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brinkkoetter PT, Ising C, Benzing T. The role of the podocyte in albumin filtration. Nat Rev Nephrol. 2013;9(6):328–336. doi: 10.1038/nrneph.2013.78. [DOI] [PubMed] [Google Scholar]
- 152.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johnson SA, Spurney RF. Twenty years after ACEIs and ARBs: emerging treatment strategies for diabetic nephropathy. Am J Physiol Renal Physiol. 2015;309(10):F807–820. doi: 10.1152/ajprenal.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kdoqi. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 155.Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108(6):807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abe Y, Sakairi T, Beeson C, Kopp JB. TGF-beta1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway. Am J Physiol Renal Physiol. 2013;305(10):F1477–1490. doi: 10.1152/ajprenal.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Das R, Xu S, Nguyen TT, et al. Transforming Growth Factor beta1-induced Apoptosis in Podocytes via the Extracellular Signal-regulated Kinase-Mammalian Target of Rapamycin Complex 1-NADPH Oxidase 4 Axis. J Biol Chem. 2015;290(52):30830–30842. doi: 10.1074/jbc.M115.703116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chuang PY, Yu Q, Fang W, Uribarri J, He JC. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int. 2007;72(8):965–976. doi: 10.1038/sj.ki.5002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou LL, Cao W, Xie C, et al. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82(7):759–770. doi: 10.1038/ki.2012.184. [DOI] [PubMed] [Google Scholar]
- 160.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. [PubMed] [Google Scholar]
- 161.Verzola D, Gandolfo MT, Ferrario F, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72(10):1262–1272. doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- 162.Anders HJ. Immune system modulation of kidney regeneration--mechanisms and implications. Nat Rev Nephrol. 2014;10(6):347–358. doi: 10.1038/nrneph.2014.68. [DOI] [PubMed] [Google Scholar]
- 163.Jha JC, Gray SP, Barit D, et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol. 2014;25(6):1237–1254. doi: 10.1681/ASN.2013070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thallas-Bonke V, Jha JC, Gray SP, et al. Nox-4 deletion reduces oxidative stress and injury by PKC-alpha-associated mechanisms in diabetic nephropathy. Physiol Rep. 2014;2(11) doi: 10.14814/phy2.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eid AA, Ford BM, Bhandary B, et al. Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes. 2013;62(8):2935–2947. doi: 10.2337/db12-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jha JC, Thallas-Bonke V, Banal C, et al. Podocyte-specific Nox4 deletion affords renoprotection in a mouse model of diabetic nephropathy. Diabetologia. 2016;59(2):379–389. doi: 10.1007/s00125-015-3796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Siddiqi FS, Majumder S, Thai K, et al. The Histone Methyltransferase Enzyme Enhancer of Zeste Homolog 2 Protects against Podocyte Oxidative Stress and Renal Injury in Diabetes. J Am Soc Nephrol. 2016;27(7):2021–2034. doi: 10.1681/ASN.2014090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Parving HH, Hommel E, Jensen BR, Hansen HP. Long-term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int. 2001;60(1):228–234. doi: 10.1046/j.1523-1755.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- 169.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 170.Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45(2):281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 171.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 172.Zhang H, Ding J, Fan Q, Liu S. TRPC6 up-regulation in Ang II-induced podocyte apoptosis might result from ERK activation and NF-kappaB translocation. Exp Biol Med (Maywood) 2009;234(9):1029–1036. doi: 10.3181/0901-RM-11. [DOI] [PubMed] [Google Scholar]
- 173.Eckel J, Lavin PJ, Finch EA, et al. TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol. 2011;22(3):526–535. doi: 10.1681/ASN.2010050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ilatovskaya DV, Levchenko V, Lowing A, Shuyskiy LS, Palygin O, Staruschenko A. Podocyte injury in diabetic nephropathy: implications of angiotensin II-dependent activation of TRPC channels. Sci Rep. 2015;5:17637. doi: 10.1038/srep17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Efrati S, Berman S, Abu Hamad R, et al. Hyperglycaemia, inflammation, RAS activation: three culprits to blame for acute kidney injury emerging in healthy rats during general anaesthesia. Nephrology (Carlton) 2012;17(7):591–602. doi: 10.1111/j.1440-1797.2012.01638.x. [DOI] [PubMed] [Google Scholar]
- 176.Rosa RM, Colucci JA, Yokota R, et al. Alternative pathways for angiotensin II production as an important determinant of kidney damage in endotoxemia. Am J Physiol Renal Physiol. 2016;311(3):F496–504. doi: 10.1152/ajprenal.00121.2014. [DOI] [PubMed] [Google Scholar]
- 177.Welsh GI, Hale LJ, Eremina V, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12(4):329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Coward RJ, Welsh GI, Yang J, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54(11):3095–3102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 179.Coward RJ, Welsh GI, Koziell A, et al. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56(4):1127–1135. doi: 10.2337/db06-0693. [DOI] [PubMed] [Google Scholar]
- 180.Tejada T, Catanuto P, Ijaz A, et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73(12):1385–1393. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- 181.Vielhauer V, Mayadas TN. Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin Nephrol. 2007;27(3):286–308. doi: 10.1016/j.semnephrol.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 182.Falkevall A, Mehlem A, Palombo I, et al. Reducing VEGF-B Signaling Ameliorates Renal Lipotoxicity and Protects against Diabetic Kidney Disease. Cell Metab. 2017;25(3):713–726. doi: 10.1016/j.cmet.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 183.Lieben L. Diabetic nephropathy: Lipid toxicity drives renal disease. Nat Rev Nephrol. 2017;13(4):194. doi: 10.1038/nrneph.2017.22. [DOI] [PubMed] [Google Scholar]
- 184.Doublier S, Salvidio G, Lupia E, et al. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52(4):1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 185.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T. Interaction with podocin facilitates nephrin signaling. J Biol Chem. 2001;276(45):41543–41546. doi: 10.1074/jbc.C100452200. [DOI] [PubMed] [Google Scholar]
- 186.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T. SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol. 2004;15(12):3006–3015. doi: 10.1097/01.ASN.0000146689.88078.80. [DOI] [PubMed] [Google Scholar]
- 187.Harita Y, Kurihara H, Kosako H, et al. Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2. J Biol Chem. 2008;283(14):9177–9186. doi: 10.1074/jbc.M707247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhu J, Sun N, Aoudjit L, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73(5):556–566. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 189.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116(5):1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jones N, Blasutig IM, Eremina V, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440(7085):818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 191.Veron D, Reidy KJ, Bertuccio C, et al. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 2010;77(11):989–999. doi: 10.1038/ki.2010.64. [DOI] [PubMed] [Google Scholar]
- 192.Teng B, Schroder P, Muller-Deile J, et al. CIN85 Deficiency Prevents Nephrin Endocytosis and Proteinuria in Diabetes. Diabetes. 2016;65(12):3667–3679. doi: 10.2337/db16-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li X, Chuang PY, D'Agati VD, et al. Nephrin Preserves Podocyte Viability and Glomerular Structure and Function in Adult Kidneys. J Am Soc Nephrol. 2015;26(10):2361–2377. doi: 10.1681/ASN.2014040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 195.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011;22(9):1621–1630. doi: 10.1681/ASN.2010111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gee HY, Saisawat P, Ashraf S, et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest. 2013;123(8):3243–3253. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lin J, Shi Y, Peng H, et al. Loss of PTEN promotes podocyte cytoskeletal rearrangement, aggravating diabetic nephropathy. J Pathol. 2015;236(1):30–40. doi: 10.1002/path.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yu H, Suleiman H, Kim AH, et al. Rac1 activation in podocytes induces rapid foot process effacement and proteinuria. Mol Cell Biol. 2013;33(23):4755–4764. doi: 10.1128/MCB.00730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Blattner SM, Hodgin JB, Nishio M, et al. Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int. 2013;84(5):920–930. doi: 10.1038/ki.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82(12):1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Brooks CR, Yeung MY, Brooks YS, et al. KIM-1-/TIM-1-mediated phagocytosis links ATG5-/ULK1-dependent clearance of apoptotic cells to antigen presentation. EMBO J. 2015;34(19):2441–2464. doi: 10.15252/embj.201489838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang L, Brooks CR, Xiao S, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125(4):1620–1636. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kume S, Yamahara K, Yasuda M, Maegawa H, Koya D. Autophagy: emerging therapeutic target for diabetic nephropathy. Semin Nephrol. 2014;34(1):9–16. doi: 10.1016/j.semnephrol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 205.Kume S, Koya D. Autophagy: A Novel Therapeutic Target for Diabetic Nephropathy. Diabetes Metab J. 2015;39(6):451–460. doi: 10.4093/dmj.2015.39.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lenoir O, Tharaux PL, Huber TB. Autophagy in kidney disease and aging: lessons from rodent models. Kidney Int. 2016;90(5):950–964. doi: 10.1016/j.kint.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 207.Ma T, Zhu J, Chen X, Zha D, Singhal PC, Ding G. High glucose induces autophagy in podocytes. Exp Cell Res. 2013;319(6):779–789. doi: 10.1016/j.yexcr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Godel M, Hartleben B, Herbach N, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121(6):2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Inoki K, Mori H, Wang J, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121(6):2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fang L, Zhou Y, Cao H, et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One. 2013;8(4):e60546. doi: 10.1371/journal.pone.0060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Audzeyenka I, Rogacka D, Piwkowska A, Angielski S, Jankowski M. Viability of primary cultured podocytes is associated with extracellular high glucose-dependent autophagy downregulation. Mol Cell Biochem. 2017;430(1–2):11–19. doi: 10.1007/s11010-017-2949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu J, Li QX, Wang XJ, et al. beta-Arrestins promote podocyte injury by inhibition of autophagy in diabetic nephropathy. Cell Death Dis. 2016;7:e2183. doi: 10.1038/cddis.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lenoir O, Jasiek M, Henique C, et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy. 2015;11(7):1130–1145. doi: 10.1080/15548627.2015.1049799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tagawa A, Yasuda M, Kume S, et al. Impaired Podocyte Autophagy Exacerbates Proteinuria in Diabetic Nephropathy. Diabetes. 2016;65(3):755–767. doi: 10.2337/db15-0473. [DOI] [PubMed] [Google Scholar]
- 215.Wang X, Liu J, Zhen J, et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014;86(4):712–725. doi: 10.1038/ki.2014.111. [DOI] [PubMed] [Google Scholar]
- 216.Kaufman DR, Papillon J, Larose L, Iwawaki T, Cybulsky AV. Deletion of inositol-requiring enzyme-1alpha in podocytes disrupts glomerular capillary integrity and autophagy. Mol Biol Cell. 2017;28(12):1636–1651. doi: 10.1091/mbc.E16-12-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes. 2009;58(2):469–477. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang J, Ouyang C, Chen X, Fu B, Lu Y, Hong Q. Effect of Jak2 kinase inhibition on Stat1 and Stat3 activation and apoptosis of tubular epithelial cells induced by ATP depletion/recovery. J Nephrol. 2008;21(6):919–923. [PubMed] [Google Scholar]
- 219.Yang N, Luo M, Li R, et al. Blockage of JAK/STAT signalling attenuates renal ischaemia-reperfusion injury in rat. Nephrol Dial Transplant. 2008;23(1):91–100. doi: 10.1093/ndt/gfm509. [DOI] [PubMed] [Google Scholar]
- 220.Zhang H, Nair V, Saha J, et al. Podocyte-specific JAK2 overexpression worsens diabetic kidney disease in mice. Kidney Int. 2017 doi: 10.1016/j.kint.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.You H, Gao T, Raup-Konsavage WM, et al. Podocyte-specific chemokine (C-C motif) receptor 2 overexpression mediates diabetic renal injury in mice. Kidney Int. 2017;91(3):671–682. doi: 10.1016/j.kint.2016.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Menne J, Eulberg D, Beyer D, et al. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol Dial Transplant. 2017;32(2):307–315. doi: 10.1093/ndt/gfv459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bettaieb A, Koike S, Chahed S, et al. Podocyte-specific soluble epoxide hydrolase deficiency in mice attenuates acute kidney injury. FEBS J. 2017;284(13):1970–1986. doi: 10.1111/febs.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 226.Wang W, Wang Y, Long J, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15(2):186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Qi W, Keenan HA, Li Q, et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med. 2017;23(6):753–762. doi: 10.1038/nm.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Allison SJ. Diabetic nephropathy: Glucose metabolic flux in DN. Nat Rev Nephrol. 2017;13(7):384. doi: 10.1038/nrneph.2017.70. [DOI] [PubMed] [Google Scholar]
- 229.Ayanga BA, Badal SS, Wang Y, et al. Dynamin-Related Protein 1 Deficiency Improves Mitochondrial Fitness and Protects against Progression of Diabetic Nephropathy. J Am Soc Nephrol. 2016;27(9):2733–2747. doi: 10.1681/ASN.2015101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dessapt-Baradez C, Woolf AS, White KE, et al. Targeted glomerular angiopoietin-1 therapy for early diabetic kidney disease. J Am Soc Nephrol. 2014;25(1):33–42. doi: 10.1681/ASN.2012121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rizkalla B, Forbes JM, Cao Z, Boner G, Cooper ME. Temporal renal expression of angiogenic growth factors and their receptors in experimental diabetes: role of the renin-angiotensin system. J Hypertens. 2005;23(1):153–164. doi: 10.1097/00004872-200501000-00026. [DOI] [PubMed] [Google Scholar]
- 232.Gnudi L. Angiopoietins and diabetic nephropathy. Diabetologia. 2016;59(8):1616–1620. doi: 10.1007/s00125-016-3995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chugh SS, Mace C, Clement LC, Del Nogal Avila M, Marshall CB. Angiopoietin-like 4 based therapeutics for proteinuria and kidney disease. Front Pharmacol. 2014;5:23. doi: 10.3389/fphar.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Clement LC, Avila-Casado C, Mace C, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17(1):117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yuen DA, Stead BE, Zhang Y, et al. eNOS deficiency predisposes podocytes to injury in diabetes. J Am Soc Nephrol. 2012;23(11):1810–1823. doi: 10.1681/ASN.2011121170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fu J, Lee K, Chuang PY, Liu Z, He JC. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Renal Physiol. 2015;308(4):F287–297. doi: 10.1152/ajprenal.00533.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fukui M, Nakamura T, Ebihara I, et al. Gene expression for endothelins and their receptors in glomeruli of diabetic rats. J Lab Clin Med. 1993;122(2):149–156. [PubMed] [Google Scholar]
- 238.Lenoir O, Milon M, Virsolvy A, et al. Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J Am Soc Nephrol. 2014;25(5):1050–1062. doi: 10.1681/ASN.2013020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schmidt EP, Overdier KH, Sun X, et al. Urinary Glycosaminoglycans Predict Outcomes in Septic Shock and Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2016;194(4):439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li L, Bonventre JV. Endothelial Glycocalyx: Not Just a Sugar Coat. Am J Respir Crit Care Med. 2016;194(4):390–393. doi: 10.1164/rccm.201603-0624ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.de Zeeuw D, Coll B, Andress D, et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25(5):1083–1093. doi: 10.1681/ASN.2013080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Komers R, Plotkin H. Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2016;310(10):R877–884. doi: 10.1152/ajpregu.00425.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.De Miguel C, Speed JS, Kasztan M, Gohar EY, Pollock DM. Endothelin-1 and the kidney: new perspectives and recent findings. Curr Opin Nephrol Hypertens. 2016;25(1):35–41. doi: 10.1097/MNH.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Barasch J, Zager R, Bonventre JV. Acute kidney injury: a problem of definition. Lancet. 2017;389(10071):779–781. doi: 10.1016/S0140-6736(17)30543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Park M, Hsu CY, Go AS, et al. Urine Kidney Injury Biomarkers and Risks of Cardiovascular Disease Events and All-Cause Death: The CRIC Study. Clin J Am Soc Nephrol. 2017;12(5):761–771. doi: 10.2215/CJN.08560816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Niewczas MA, Mathew AV, Croall S, et al. Circulating Modified Metabolites and a Risk of ESRD in Patients With Type 1 Diabetes and Chronic Kidney Disease. Diabetes Care. 2017;40(3):383–390. doi: 10.2337/dc16-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Xu K, Rosenstiel P, Paragas N, et al. Unique Transcriptional Programs Identify Subtypes of AKI. J Am Soc Nephrol. 2017;28(6):1729–1740. doi: 10.1681/ASN.2016090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57(10):2809–2817. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shahzad K, Bock F, Al-Dabet MM, et al. Stabilization of endogenous Nrf2 by minocycline protects against Nlrp3-inflammasome induced diabetic nephropathy. Sci Rep. 2016;6:34228. doi: 10.1038/srep34228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kanasaki K, Shi S, Kanasaki M, et al. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63(6):2120–2131. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 251.Peng H, Li Y, Wang C, et al. ROCK1 Induces Endothelial-to-Mesenchymal Transition in Glomeruli to Aggravate Albuminuria in Diabetic Nephropathy. Sci Rep. 2016;6:20304. doi: 10.1038/srep20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee SY, Kang JM, Kim DJ, et al. PGC1alpha Activators Mitigate Diabetic Tubulopathy by Improving Mitochondrial Dynamics and Quality Control. J Diabetes Res. 2017;2017:6483572. doi: 10.1155/2017/6483572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Xiao L, Xu X, Zhang F, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hou Y, Li S, Wu M, et al. Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am J Physiol Renal Physiol. 2016;310(6):F547–559. doi: 10.1152/ajprenal.00574.2014. [DOI] [PubMed] [Google Scholar]
- 255.Gangadharan Komala M, Gross S, Zaky A, Pollock C, Panchapakesan U. Linagliptin Limits High Glucose Induced Conversion of Latent to Active TGFss through Interaction with CIM6PR and Limits Renal Tubulointerstitial Fibronectin. PLoS One. 2015;10(10):e0141143. doi: 10.1371/journal.pone.0141143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang Q, Shi Y, Wada J, et al. In vivo delivery of Gremlin siRNA plasmid reveals therapeutic potential against diabetic nephropathy by recovering bone morphogenetic protein-7. PLoS One. 2010;5(7):e11709. doi: 10.1371/journal.pone.0011709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kolling M, Kaucsar T, Schauerte C, et al. Therapeutic miR-21 Silencing Ameliorates Diabetic Kidney Disease in Mice. Mol Ther. 2017;25(1):165–180. doi: 10.1016/j.ymthe.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bai X, Geng J, Zhou Z, Tian J, Li X. MicroRNA-130b improves renal tubulointerstitial fibrosis via repression of Snail-induced epithelial-mesenchymal transition in diabetic nephropathy. Sci R ep. 2016;6:20475. doi: 10.1038/srep20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li J, Gui Y, Ren J, et al. Metformin Protects Against Cisplatin-Induced Tubular Cell Apoptosis and Acute Kidney Injury via AMPKalpha-regulated Autophagy Induction. Sci Rep. 2016;6:23975. doi: 10.1038/srep23975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gorin Y, Cavaglieri RC, Khazim K, et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol. 2015;308(11):F1276–1287. doi: 10.1152/ajprenal.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gray SP, Jha JC, Kennedy K, et al. Combined NOX1/4 inhibition with GKT137831 in mice provides dose-dependent reno- and atheroprotection even in established micro- and macrovascular disease. Diabetologia. 2017;60(5):927–937. doi: 10.1007/s00125-017-4215-5. [DOI] [PubMed] [Google Scholar]
- 262.Cha JJ, Min HS, Kim KT, et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab Invest. 2017;97(4):419–431. doi: 10.1038/labinvest.2017.2. [DOI] [PubMed] [Google Scholar]
- 263.Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepinski JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun. 2010;393(2):268–273. doi: 10.1016/j.bbrc.2010.01.119. [DOI] [PubMed] [Google Scholar]
- 264.Kim J, Shon E, Kim CS, Kim JS. Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp Diabetes Res. 2012;2012:210821. doi: 10.1155/2012/210821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN, Lasaridis AN. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis. 2010;55(5):835–847. doi: 10.1053/j.ajkd.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 266.Liu Y, Li H, Liu J, et al. Variations in MicroRNA-25 Expression Influence the Severity of Diabetic Kidney Disease. J Am Soc Nephrol. 2017;28(12):3627–3638. doi: 10.1681/ASN.2015091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cao AL, Wang L, Chen X, et al. Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab Invest. 2016;96(6):610–622. doi: 10.1038/labinvest.2016.44. [DOI] [PubMed] [Google Scholar]
- 268.Navarro JF, Mora C, Muros M, Maca M, Garca J. Effects of pentoxifylline administration on urinary N-acetyl-beta-glucosaminidase excretion in type 2 diabetic patients: a short-term, prospective, randomized study. Am J Kidney Dis. 2003;42(2):264–270. doi: 10.1016/s0272-6386(03)00651-6. [DOI] [PubMed] [Google Scholar]
- 269.Kelly DJ, Aaltonen P, Cox AJ, et al. Expression of the slit-diaphragm protein, nephrin, in experimental diabetic nephropathy: differing effects of anti-proteinuric therapies. Nephrol Dial Transplant. 2002;17(7):1327–1332. doi: 10.1093/ndt/17.7.1327. [DOI] [PubMed] [Google Scholar]
- 270.Wang XX, Levi J, Luo Y, et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: SGLT2 PROTEIN INHIBITION DECREASES RENAL LIPID ACCUMULATION, INFLAMMATION, AND THE DEVELOPMENT OF NEPHROPATHY IN DIABETIC MICE. J Biol Chem. 2017;292(13):5335–5348. doi: 10.1074/jbc.M117.779520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Chang YP, Sun B, Han Z, et al. Saxagliptin Attenuates Albuminuria by Inhibiting Podocyte Epithelial- to-Mesenchymal Transition via SDF-1alpha in Diabetic Nephropathy. Front Pharmacol. 2017;8:780. doi: 10.3389/fphar.2017.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Xu XH, Ding DF, Yong HJ, et al. Resveratrol transcriptionally regulates miRNA-18a-5p expression ameliorating diabetic nephropathy via increasing autophagy. Eur Rev Med Pharmacol Sci. 2017;21(21):4952–4965. [PubMed] [Google Scholar]
- 273.Ito Y, Hsu MF, Bettaieb A, et al. Protein tyrosine phosphatase 1B deficiency in podocytes mitigates hyperglycemia-induced renal injury. Metabolism. 2017;76:56–69. doi: 10.1016/j.metabol.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Guo H, Wang Y, Zhang X, et al. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKalpha-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci Rep. 2017;7(1):6852. doi: 10.1038/s41598-017-07061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jin Y, Liu S, Ma Q, Xiao D, Chen L. Berberine enhances the AMPK activation and autophagy and mitigates high glucose-induced apoptosis of mouse podocytes. Eur J Pharmacol. 2017;794:106–114. doi: 10.1016/j.ejphar.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 276.Sun J, Li ZP, Zhang RQ, Zhang HM. Repression of miR-217 protects against high glucose-induced podocyte injury and insulin resistance by restoring PTEN-mediated autophagy pathway. Biochem Biophys Res Commun. 2017;483(1):318–324. doi: 10.1016/j.bbrc.2016.12.145. [DOI] [PubMed] [Google Scholar]
- 277.Li D, Lu Z, Xu Z, et al. Spironolactone promotes autophagy via inhibiting PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity damage in podocytes under mechanical stress. Biosci Rep. 2016;36(4) doi: 10.1042/BSR20160086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Quiroga B, Arroyo D, de Arriba G. Present and future in the treatment of diabetic kidney disease. J Diabetes Res. 2015;2015:801348. doi: 10.1155/2015/801348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163(13):1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 280.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK, Investigators AS. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358(23):2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 281.Zheng H, Whitman SA, Wu W, et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60(11):3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 283.Davila-Esqueda ME, Martinez-Morales F. Pentoxifylline diminishes the oxidative damage to renal tissue induced by streptozotocin in the rat. Exp Diabesity Res. 2004;5(4):245–251. doi: 10.1080/154386090897974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Navarro JF, Mora C, Muros M, Garcia J. Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: a short-term, randomized, controlled trial. J Am Soc Nephrol. 2005;16(7):2119–2126. doi: 10.1681/ASN.2005010001. [DOI] [PubMed] [Google Scholar]
- 285.McCormick BB, Sydor A, Akbari A, Fergusson D, Doucette S, Knoll G. The effect of pentoxifylline on proteinuria in diabetic kidney disease: a meta-analysis. Am J Kidney Dis. 2008;52(3):454–463. doi: 10.1053/j.ajkd.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 286.Shan D, Wu HM, Yuan QY, Li J, Zhou RL, Liu GJ. Pentoxifylline for diabetic kidney disease. Cochrane Database Syst Rev. 2012;(2):CD006800. doi: 10.1002/14651858.CD006800.pub2. [DOI] [PubMed] [Google Scholar]
- 287.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015;26(1):220–229. doi: 10.1681/ASN.2014010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.John A, Kundu S, Pushpakumar S, et al. GYY4137, a Hydrogen Sulfide Donor Modulates miR194-Dependent Collagen Realignment in Diabetic Kidney. Sci Rep. 2017;7(1):10924. doi: 10.1038/s41598-017-11256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kanamori H, Matsubara T, Mima A, et al. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun. 2007;360(4):772–777. doi: 10.1016/j.bbrc.2007.06.148. [DOI] [PubMed] [Google Scholar]
- 290.de Zeeuw D, Bekker P, Henkel E, et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3(9):687–696. doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
- 291.RamachandraRao SP, Zhu Y, Ravasi T, et al. Pirfenidone is renoprotective in diabetic kidney disease. J Am Soc Nephrol. 2009;20(8):1765–1775. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sharma K, Ix JH, Mathew AV, et al. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol. 2011;22(6):1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Jung GS, Jeon JH, Choe MS, et al. Renoprotective Effect of Gemigliptin, a Dipeptidyl Peptidase-4 Inhibitor, in Streptozotocin-Induced Type 1 Diabetic Mice. Diabetes Metab J. 2016;40(3):211–221. doi: 10.4093/dmj.2016.40.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bhatt K, Lanting LL, Jia Y, et al. Anti-Inflammatory Role of MicroRNA-146a in the Pathogenesis of Diabetic Nephropathy. J Am Soc Nephrol. 2016;27(8):2277–2288. doi: 10.1681/ASN.2015010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ka SM, Yeh YC, Huang XR, et al. Kidney-targeting Smad7 gene transfer inhibits renal TGF-beta/MAD homologue (SMAD) and nuclear factor kappaB (NF-kappaB) signalling pathways, and improves diabetic nephropathy in mice. Diabetologia. 2012;55(2):509–519. doi: 10.1007/s00125-011-2364-5. [DOI] [PubMed] [Google Scholar]
- 296.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol. 2012;23(3):458–469. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chacko BK, Reily C, Srivastava A, et al. Prevention of diabetic nephropathy in Ins2(+/)(−)(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J. 2010;432(1):9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]