Abstract
Purpose
A pilot study to validate the collection of vitreous reflux (VR) after intravitreal injection using Schirmers tear strips was carried out. We assessed its efficiency for proteomics studies by estimating the differential expression of 27 cytokines using multiplexed bead array in diabetic macular oedema and proliferative diabetic retinopathy. To set, validate and assess the efficacy of Schirmer tear strips for collecting VR in patients undergoing intravitreal injections for diabetic macular oedema (DME).
Patients and methods
VR samples were collected from 11 eyes of DME patients after intravitreal injections using Schirmer tear strips. Undiluted vitrectomy samples were obtained from six eyes of non-diabetic patients with idiopathic macular hole and seven eyes of diabetic patients with high-risk proliferative diabetic retinopathy (Hr-PDR), which were also subsampled on the Schirmer tear strips. Tear sampling was done in a subset of the DME patients. Total protein concentration between VR and vitrectomy samples was compared. Levels of the set of 27 cytokines in Schirmer tear strips samples were measured. Inter-group comparison for cytokines was done using Mann–Whitney U-test.
Results
Similar protein concentration in VR samples and vitrectomy samples (P<0.05) was obtained. Tear protein contamination was not detected in VR samples. In comparison with no-DR patients, 25 and 20 of the measured 27 cytokines were significantly elevated (P<0.05) in the Hr-PDR and DME patients, respectively. As compared with no-DR patients, vascular endothelial growth factor was only moderately elevated in DME patients (P>0.05), but significantly elevated in Hr-PDR patients (P<0.05). Interleukin 1 receptor antagonist/interleukin 1b (IL1RA/IL1b) ratio was 13 times higher in DME patients as compared with Hr-PDR group.
Conclusion
We demonstrated a simple, safe method of VR sampling. This technique provides a pure, albeit small, vitreous sample for proteomics. IL1RA/IL1b ratio was found to be 13-fold higher in the DME group as compared to the Hr-PDR.
Introduction
Diabetic retinopathy (DR), a microangiopathy causing macular oedema and neovascularization, is a leading cause of visual impairment seen in the middle-aged group worldwide.1 Although anti-vascular endothelial growth factor (anti-VEGF) therapy is the standard treatment for diabetic macular oedema (DME), an early and consistent response has been reported in only 50% of the cases, as the other 50% were either slow, partial or non-responder.2 Although the reasons are poorly understood, vitreous proteomics and efficacy of steroids in treating DME suggest additional inflammatory component in the pathogenesis of DR.3, 4, 5
The vitreous with its close proximity to the retina represents an indirect source of information on the retinal micro-environment.6 Vitreous proteomics involves the collection of undiluted vitreous at the beginning of a pars plana vitrectomy.7, 8 This limited the studies to the stages of DR requiring vitreous surgery, the advanced proliferative DR stages (PDR). Although serum and aqueous humour proteomics have been carried out in DME, it might not reliably reflect the true retinal micro-environment.9
Vitreous reflux (VR) is a common observation at the end of the intravitreal injection.10 The efficacy of VR for proteomics has been recently explored in age-related macular degeneration by Cacciamani et al.11 It is imperative to develop alternative techniques for VR collection in the DME patients, which may be used for vitreous proteomics.
We conducted a pilot study to collect the VR after intravitreal injection using Schirmer tear strips (adsorption technique). We validated its efficiency by comparing the protein concentration extracted in this novel technique with the aspirated vitreous sample obtained during vitrectomy. We also estimated the differential cytokine expression of the vitreous in DME and PDR in this pilot study.
Subjects and methods
Study population
A total of 24 consecutive patients above the age of 45 years were included in this prospective study. The no-DR group had six patients with no diabetes, who underwent pars plana vitrectomy for idiopathic macular hole. The high-risk PDR (Hr-PDR) group had seven consecutive patients, who underwent pars plana vitrectomy for vitreous haemorrhage. In the DME group, there were 11 patients who received intravitreal injections for macular oedema.
Patients with history of vitreo-retina surgery in the past and having other ocular diseases such as uveitis, infection, vascular occlusion, age-related macular degeneration were not included in this study. We excluded patients with history of intravitreal anti-VEGF and/or steroids, laser (both focal and pan retinal photocoagulation), intra-ocular surgery in the last 6 months were excluded. We also excluded patients with systemic illness like Alzheimer's diseases, connective tissue disorders, inflammatory bowel diseases, history of myocardial infarction, and patients on anti-platelets, anti-inflammatory, and immune modulatory medication.
This study was approved by the Institutional Review Board and was in compliance with the tenets of the Declaration of Helsinki. A written informed consent was obtained from the participants.
Collection of vitreous aspirate and vitreous reflux samples
In patients with DME, the VR was collected on sterile single wrapped Schirmer tear strips (Tear Touch, Med Devices and Life Science Pvt. Ltd., Dublin, Ireland). Topical anaesthesia was achieved using 0.5% proparacaine (Alcaine, Alcon Laboratories, Inc., Fort Worth, TX, USA) and 5% povidone iodine solution was applied to the per-ocular skin and conjuctival sac for 3 min, followed by drapping and insertion of the lid speculum. The supero-temporal quadrant conjunctiva was carefully dried with micro-sponges after giving a through wash of the cul-de-sac with balanced salt solution before administering the intravitreal injection. The 30-gauge needle was introduced into the midvitreous cavity. Using a single, continuous maneuver, the intravitreal drug (0.05 ml) was injected into the eye. In all the cases, a more vertical entry, rather than an oblique entry was done, so that VR was obtained in all the cases. The VR observed after the removal of the syringe was adsorbed on the Schirmer tear strip that was placed over the site of injection for 10 s to standardize the procedure. Any undue pressure was avoided. Topical antibiotic drops were instilled at the end of the procedure.
Undiluted vitreous aspirate (~0.5–1 ml) was collected in a sterile syringe connected to the vitreous cutter at the beginning of the standard three port pars plana vitrectomy from the patients with macular hole and from the Hr-PDR patients.
The collected samples were transported on ice to the laboratory within 20 min of collection. Tear samples were collected in a subset of DME patients at the time of admission using glass capillary micropipettes and were stored in sterile vials at −80 °C till further analysis.
Processing and storage of samples
The average Schirmer tear strip recording in the DME group was noted to be 9.55±3.13 mm. It was observed that 1 μl of vitreous migrated to 1 mm in a Schirmer tear strip. On the basis of this observation, 10 μl of the vitreous aspirate from the no-DR and Hr-PDR groups was loaded on to the Schirmer tear strips using micropipette (sub-sampling). The average migration in the vitreous aspirate loaded strips was noted to be 10.17±1.47 mm.
Subsequently, the aspirate samples were centrifuged at 5000 rpm for 10 min in a cooling centrifuge. The clear supernatant was aliquoted into 500 μl in DNAase- and RNAase-free vials and stored at −80 °C until further use. Vitreous that showed RBC lysis was not included in the study.
Extraction of the vitreous from Schirmer tear strips
Schirmer tear strip samples from the DME patients and Schirmer tear strip subsamples from the vitreous aspirates (no-DR and Hr-PDR group) were processed in a similar manner to standardize the procedure.
In the tube containing the Schirmer tear strip, 200 μl of 1 × phosphate buffered saline tween (pH 7.2) was added and incubated for 3 h at 4 °C on a rocker followed by centrifugation at 8000 rpm for 5 min. The strips were removed and the samples were immediately frozen at −80 °C until further analysis.
Total protein quantification
The total protein concentration was estimated by bicinchoninic acid colorimetric assay (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific, Waltham, MA, USA; Cat no. 23227) by diluting according to the assay’s detection limit. The total protein was used to assess the change in the total protein content between the sampling methods and to normalize the samples for sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and multiplexed bead cytokines analysis.
SDS–PAGE
To compare the vitreous protein profile in the aspirate and adsorption samples, 30 μg of the total protein from each group was run on 15% SDS–PAGE gels and stained with Coomassie blue stain (0.1% Coomassie R250, 10% glacial acetic acid, 40% methanol, 50% H2O). The gel was scanned using HP Scan Jet Plus scanner to assess the band density. To check for the contamination of VR samples with tear proteins, the vitreous was spiked with varying concentrations of tear and it was run on a SDS–PAGE gel.
Multiplex analysis of cytokines in Schirmer-extracted vitreous samples
A Bio-Plex multiplex assay (Bio-Plex Human Cytokine 27-plex panel, Bio-Rad Laboratories, Hercules, CA, USA; Cat no. M500KCAFOY) was used to measure the concentration of 27 cytokines in the Schirmer tear strip-extracted vitreous of all the patients. The analysis was performed according to the manufacturer’s instructions and read in Bio-Plex Reader (Bio-Rad Laboratories). Standard curves were generated using the Bio-Plex Manager System (Software version 6.0; Bio-Rad Laboratories) and were used to calculate the cytokines concentrations in the vitreous samples. The cytokines studied were interleukin 1b (IL1b), interleukin 1 receptor antagonist (IL1RA), IL2, 3, 4, 5, 6, 7, 8, 9, 10, 12p70, 13, 15, 17A, basic fibroblast growth factor (bFGF), granulocyte colony stimulating factor (GCSF), gamma interferon (IFNg), interferon gamma inducible protein 10 (IP10), monocyte chemotactic protein 1 (MCP1), macrophage inflammatory protein 1a & 1b (MIP 1a&b), platelet-derived growth factor (PDGF), regulated upon activation normally T-cell expressed and secreted (RANTES), tumour necrosis factor alpha (TNFa), VEGF.
All experiments were run in triplicate, except for cytokine analysis which was done once.
Data analysis and statistical analysis
Values of the vitreous cytokines concentration were reported as mean (pg/ml)±SD in each group. Inter-group comparison for cytokines was done using Mann–Whitney U-test (two-sided). Apart from being statistically significant (P<0.05), cytokines exhibiting a two-fold or more change in their levels across the groups were discussed.
Results
Baseline characteristics
Supplementary Table 1 shows the baseline characteristics of the study population.
Comparison of protein concentration
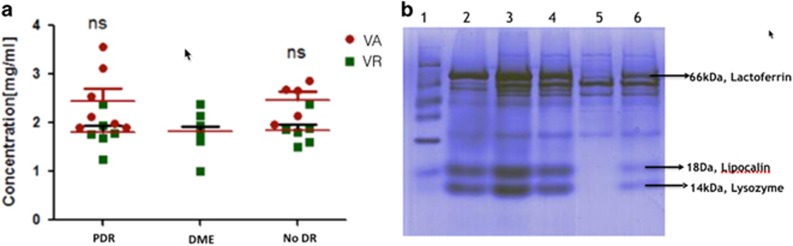
Figure 1a shows the protein concentration in the aspirate and adsorption technique samples. In the no-DR group, the mean protein concentration in the aspirate samples was 2.11±0.39 mg/ml, whereas in the Schirmer tear strip samples, it was 1.57±0.07 mg/ml (74.27% recovery; P=0.093). In the Hr-PDR group, the mean protein concentration in the aspirate samples was 3.67±0.91 mg/ml, whereas in the Schirmer tear strip samples, it was 2.65±0.19 mg/ml (72.2% recovery; P=0.075). The protein concentration from the Schirmer tear strips technique in the DME group was 1.77±0.07 mg/ml, which was comparable to the other groups.
Figure 1.
Protein concentrations in the vitreous of No-DR, DME and Hr-PDR groups. (a) The scatter plot shows the distribution levels of the protein concentration in the VA (red circle) and the Schirmers collected VR (green square) method of sampling in the PDR, DME, and no-DR group. Median (black line) of total protein concentration in the vitreous aspirate (red circle) samples and Schirmers (green squares) samples is shown. 'ns' denotes 'not significant.' (b) Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) profile of Schirmer-extracted DME vitreous and tear proteins; Lane 1: molecular weight marker; Lane 2: tear protein; Lane 3: VR from DME patient+16 μg of tear protein; Lane 4: VR from DME patient+8 μg of tear protein; Lane 5: VR from DME patient; Lane 6: VR from DME patient+4 μg of tear protein. Lactoferrin, lipocalin, and lysozyme are characteristic tear proteins. DME, diabetic macular oedema; no-DR, no diabetes; PDR, proliferative diabetic retinopathy; VA, vitreous aspirate; VR, vitreous reflex.
SDS–PAGE
Protein extracted from the VR samples was run on SDS–PAGE gel spiked with various total protein concentrations of tear (0, 4, 8, 12, and 16 μg) (Figure 1b). Tear-specific bands of lactoferrin, lipocalin, and lysozyme were detected dose-dependently in the DME group samples only when spiked with tears, which suggested that there was no significant contamination of tear in the VR collected by Schirmer tear strips in the DME cases.
Cytokines assay
No-DR vs Hr-PDR group
Compared with the no-DR group, the concentrations of the cytokines IL8 (35.1-fold increase), IP10 (29.2-fold increase), IL7 (23.6-fold increase), IL6 (12.2-fold increase), IL13 (10.2-fold increase), MCP1 (5.4-fold increase), IL1RA (3.8-fold increase), MIP-1b (3.0-fold increase), IL12p70 (2.7-fold increase), GCSF (2.5-fold increase), VEGF (2.3-fold increase), IL10 (2.1-fold increase), Eotaxin (2.0-fold increase), MIP1a (2.0-fold increase), PDGF-BB (2.0-fold increase), and TNFa (2.0-fold increase) were significantly higher in the Hr-PDR group (Table 1).
Table 1. Comparison of cytokines between subjects with no DR and subjects with Hr-PDR.
| Cytokines |
No DR |
Hr-PDR |
Folds change | P-value | ≥2-fold change | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| IL1b | 0.86 | 0.06 | 1.54 | 0.14 | 1.79 | 0.00 | |
| IL1RA | 40.55 | 7.29 | 153.09 | 149.46 | 3.78 | 0.00 | 3 (42.8%) |
| IL2 | 8762.28 | 1163.32 | 13421.50 | 2585.39 | 1.53 | 0.01 | |
| IL4 | 0.68 | 0.04 | 1.24 | 0.14 | 1.83 | 0.00 | |
| IL5 | 4.03 | 0.42 | 7.50 | 0.62 | 1.86 | 0.00 | |
| IL6 | 4.52 | 1.37 | 55.20 | 21.72 | 12.21 | 0.00 | 7 (100%) |
| IL7 | 4.52 | 1.37 | 106.60 | 49.84 | 23.57 | 0.00 | 7 (100%) |
| IL8 | 3.47 | 0.24 | 121.84 | 99.26 | 35.12 | 0.00 | 7 (100%) |
| IL9 | 10.76 | 0.81 | 20.90 | 4.91 | 1.94 | 0.00 | |
| IL10 | 17.51 | 1.71 | 37.43 | 9.42 | 2.14 | 0.00 | 3 (42.8%) |
| IL12p70 | 14.02 | 1.61 | 37.26 | 15.92 | 2.66 | 0.00 | 5 (71.4%) |
| IL13 | 651.91 | 29.03 | 6640.86 | 4000.93 | 10.19 | 0.00 | 6 (85.7%) |
| IL15 | 33.25 | 4.53 | 63.17 | 9.65 | 1.90 | 0.00 | |
| IL17 A | 1950.17 | 169.92 | 2657.03 | 529.52 | 1.36 | 0.01 | |
| bFGF | 33.99 | 2.64 | 57.56 | 18.06 | 1.69 | 0.00 | |
| Eotaxin | 8.54 | 1.45 | 16.90 | 2.89 | 1.98 | 0.00 | 4 (57.1%) |
| GCSF | 8.31 | 0.69 | 20.61 | 6.65 | 2.48 | 0.00 | 5 (71.4%) |
| GMCSF | 116.05 | 6.81 | 142.06 | 34.44 | 1.22 | 0.37 | |
| IFNg | 585.98 | 19.01 | 1074.95 | 128.63 | 1.83 | 0.00 | |
| IP10 | 104.15 | 6.44 | 3039.54 | 2308.10 | 29.19 | 0.00 | 7 (100%) |
| MCP1 | 71.55 | 7.41 | 385.57 | 147.04 | 5.39 | 0.00 | 7 (100%) |
| MIP1a | 174.78 | 8.40 | 354.77 | 79.25 | 2.03 | 0.00 | 3 (42.8%) |
| MIP1b | 4.31 | 0.43 | 12.84 | 4.22 | 2.98 | 0.00 | 6 (85.7%) |
| PDGFBB | 5922.93 | 427.59 | 11675.67 | 4109.31 | 1.97 | 0.00 | 3 (42.8%) |
| RANTES | 3801.68 | 602.74 | 3302.78 | 1238.95 | 0.87 | 0.30 | |
| TNFa | 24.51 | 1.58 | 48.33 | 4.69 | 1.97 | 0.00 | 4 (57.1%) |
| VEGF | 72.28 | 7.51 | 163.31 | 63.65 | 2.26 | 0.02 | 5 (71.4%) |
Abbreviations: bFGF, basic fibroblast growth factor; GCSF, granulocyte colony stimulating factor; Hr-PDR, high-risk proliferative diabetic retinopathy; IFNg, gamma interferon; IL1b, interleukin 1b; IL1RA, interleukin 1 receptor antagonist; IL2, 3, 4, 5, 6, 7, 8, 9, 10, interleukin 2, 3, 4, 5, 6, 7, 8, 9, 10; IL12p70, 13, 15, 17A, interleukin 12p70, 13, 15, 17A; IP10, interferon gamma inducible protein 10; MCP1, monocyte chemotactic protein 1; MIP 1a & 1b, macrophage inflammatory protein 1a & 1b; No DR, no diabetic retinopathy; PDGF, platelet-derived growth factor; RANTES, regulated upon activation normally T-cell expressed and secreted; TNFa, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor.
Comparison of cytokines between the no diabetic retinopathy group and high-risk proliferative diabetic retinopathy group. Fold change was calculated by dividing the mean cytokine level of Hr-PDR group by the mean cytokine level of no-DR group.
The number of the Hr-PDR group patients with a two-fold or more increase in the level of IL6, IL7, IL8, IP10, MCP1, IL13, GCSF, MIP1a, IL12p70, MIP-1b, Eotaxin, VEGF, PDGF-BB, TNFa, IL1RA, and IL10 was 7 (100%), 7 (100%), 7 (100%), 7 (100%), 7 (100%), 6 (85.7%), 6 (85.7%), 5 (71.4%), 5 (71.4%), 5 (71.4%), 4 (57.1%), 4 (57.1%), 3 (42.8%), 3 (42.8%), 3 (42.8%), and 3 (42.8%), respectively.
No-DR vs DME group
Compared with the no-DR group, the concentrations of IL1RA (57-fold increase), IL7 (13.8-fold increase), IP10 (3.8-fold increase), IL8 (2.8-fold increase), IL6 (2.7-fold increase), GCSF (2.3-fold increase), and IL1b (2.1-fold increase) were significantly higher in the DME group (Table 2).
Table 2. Comparison of cytokines between subjects with no-DR and subjects with DME.
| Cytokines |
No DR |
DME |
Folds change | P-value | ≥2-fold change N(%) | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| IL1b | 0.86 | 0.06 | 1.80 | 0.72 | 2.10 | 0.00 | 5 (45.5%) |
| IL1RA | 40.55 | 7.29 | 2313.40 | 1697.03 | 57.05 | 0.00 | 11 (100%) |
| IL2 | 8762.28 | 1163.32 | 9561.67 | 1522.06 | 1.09 | 0.08 | |
| IL4 | 0.68 | 0.04 | 1.25 | 0.41 | 1.84 | 0.00 | |
| IL5 | 4.03 | 0.42 | 6.82 | 3.03 | 1.69 | 0.00 | |
| IL6 | 4.52 | 1.37 | 12.08 | 3.33 | 2.67 | 0.00 | 10 (90.9%) |
| IL7 | 4.52 | 1.37 | 62.62 | 39.92 | 13.85 | 0.00 | 11 (100%) |
| IL8 | 3.47 | 0.24 | 9.85 | 5.40 | 2.84 | 0.00 | 6 (54.5%) |
| IL9 | 10.76 | 0.81 | 15.30 | 4.10 | 1.42 | 0.01 | |
| IL10 | 17.51 | 1.71 | 23.37 | 3.35 | 1.33 | 0.00 | |
| IL12p70 | 14.02 | 1.61 | 18.35 | 5.38 | 1.31 | 0.08 | |
| IL13 | 651.91 | 29.03 | 1196.07 | 717.95 | 1.83 | 0.00 | |
| IL15 | 33.25 | 4.53 | 36.15 | 4.15 | 1.09 | 0.26 | |
| IL17A | 1950.17 | 169.92 | 2379.33 | 366.36 | 1.22 | 0.01 | |
| bFGF | 33.99 | 2.64 | 41.59 | 8.76 | 1.22 | 0.03 | |
| Eotaxin | 8.54 | 1.45 | 12.81 | 2.51 | 1.50 | 0.00 | |
| GCSF | 8.31 | 0.69 | 19.08 | 9.81 | 2.30 | 0.00 | 5 (45.4%) |
| GMCSF | 116.05 | 6.81 | 112.66 | 16.88 | 0.97 | 0.88 | |
| IFNg | 585.98 | 19.01 | 1080.17 | 365.20 | 1.84 | 0.00 | |
| IP10 | 104.15 | 6.44 | 394.03 | 512.47 | 3.78 | 0.00 | 5 (45%) |
| MCP1 | 71.55 | 7.41 | 73.53 | 9.96 | 1.03 | 0.73 | |
| MIP1a | 174.78 | 8.40 | 278.93 | 66.25 | 1.60 | 0.00 | |
| MIP1b | 4.31 | 0.43 | 5.69 | 1.22 | 1.32 | 0.01 | |
| PDGFBB | 5922.93 | 427.59 | 8088.91 | 2081.70 | 1.37 | 0.01 | |
| RANTES | 3801.68 | 602.74 | 6086.82 | 5063.32 | 1.60 | 0.12 | |
| TNFa | 24.51 | 1.58 | 42.54 | 10.72 | 1.74 | 0.00 | |
| VEGF | 72.28 | 7.51 | 76.03 | 13.14 | 1.05 | 0.59 | |
Abbreviations: bFGF, basic fibroblast growth factor; DME, diabetic macular oedema; GCSF, granulocyte colony stimulating factor; IFNg, gamma interferon; IL1b, interleukin 1b, IL1RA, interleukin 1 receptor antagonist; IL2, 3, 4, 5, 6, 7, 8, 9, 10, interleukin 2, 3, 4, 5, 6, 7, 8, 9, 10; IL12p70, 13, 15, 17A, interleukin 12p70, 13, 15, 17A; IP10, interferon gamma inducible protein 10; MCP1, monocyte chemotactic protein 1; MIP 1a & 1b, macrophage inflammatory protein 1a & 1b; no-DR, no diabetic retinopathy; PDGF, platelet-derived growth factor; RANTES, regulated upon activation normally T-cell expressed and secreted; TNFa, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor.
Comparison of cytokines between the no diabetic retinopathy group and diabetic macular oedema group. Fold change was calculated by dividing the mean cytokine level of DMO group by the mean cytokine level of no-DR group.
The number of DME group patients with a two-fold or more increase in the level of IL1RA, IL7, IL6, IL8, IL1b, GCSF, and IP10 was 11 (100%), 11 (100%), 10 (90.9%), 6 (54.5%), 5 (45.5%), 5 (45.5%), and 5 (45.5%), respectively.
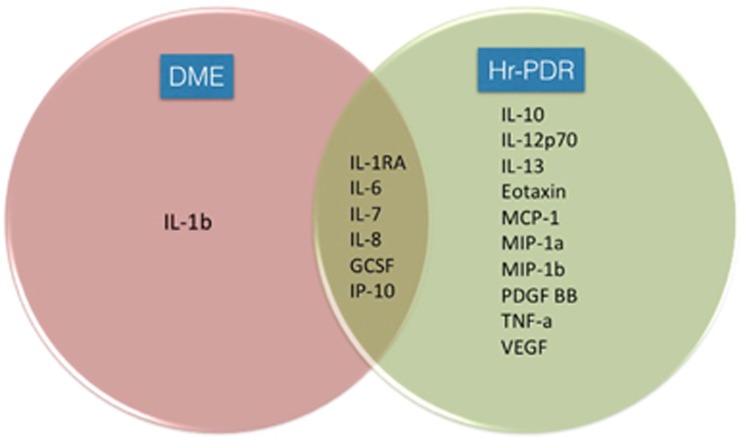
We found that the cytokines IL1RA, IL6, IL7, IL8, GCSF, and IP10 were unregulated in both the groups (Figure 2). IL1b was selectively more unregulated in the DME group, whereas IL10, IL12p70, IL13, Eotaxin, MCP1, MIP1a, MIP-1b, PDGF-BB, TNFa, and VEGF were selectively more unregulated in the Hr-PDR group.
Figure 2.
Venn diagram showing the differential cytokine expression in the DME and high-risk proliferative diabetic retinopathy (Hr-PDR) groups. The enlisted cytokines showed a >2-fold increase (P<0.05) >50% of the cases compared to the no-DR group. DME, diabetic macular oedema; GCSF, granulocyte colony stimulating factor; Hr-PDR, high-risk proliferative diabetic retinopathy; IL1b, interleukin 1b; IL1RA, interleukin 1 receptor antagonist; IL6, 7, 8, 10, 12p70, 13, interleukin 6, 7, 8, 10, 12p70, 13; IP10, interferon gamma inducible protein 10; MCP1, monocyte chemotactic protein 1; MIP1a &1b, macrophage inflammatory protein 1a & 1b; PDGF, platelet-derived growth factor; TNFa, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor.
DME vs Hr-PDR group
Compared with the DME group, the concentrations of IL8 (12.4-fold increase), IP10 (7.7-fold increase), IL13 (5.5-fold increase), MCP1 (5.2-fold increase), IL6 (4.6-fold increase), MIP-1b (2.2-fold increase), VEGF (2.1-fold increase), and IL12p70 (2.0-fold increase) were significantly higher in the Hr-PDR group (Supplementary Table 2). However, the concentration of IL1RA (15.1-fold decrease) was significantly lower in the Hr-PDR group as compared with the DME group.
The number of Hr-PDR group patients with a two-fold or more increase in the level of IL6, IL8, MCP1, IL13, MIP-1b, IP10, VEGF, and IL12p70 was 7 (100%), 7 (100%), 7 (100%), 6 (85.7%), 5 (71.4%), 4 (67.1%), 4 (57.1%), and 3 (42.8%), respectively. All seven (100%) patients in the Hr-PDR group showed a two-fold or more decrease in the levels of IL1RA.
VEGF, IL1b, and IL1RA levels
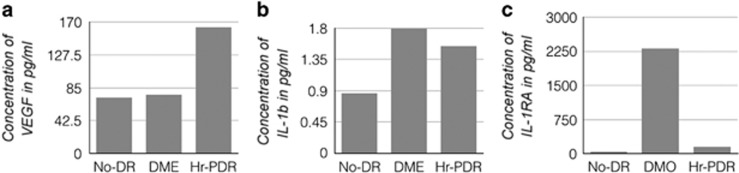
The mean vitreous concentrations of VEGF were 72.28±7.51, 76.02±13.14, and 163.31±63.64 pg/ml in the no-DR, DME, and Hr-PDR groups, respectively (Figure 3a). We compared the VEGF levels between the groups: no-DR vs Hr-PDR (P=0.022), DME vs Hr-PDR (P=0.008), and no-DR vs DME (P=0.591).
Figure 3.
Vitreous concentrations of VEGF, IL-1b and IL-1RA in No-DR, DME and Hr-PDR groups. (a) The comparison of vascular endothelial growth factors levels in vitreous reflux across no-DR, DME, and Hr-PDR. (b) The comparison of IL1b levels across no-DR, DME, and Hr-PDR groups. (c) The comparison of IL1RA levels across no-DR, DME, and Hr-PDR groups. DME, diabetic macular oedema; Hr-PDR, high-risk proliferative diabetic retinopathy groups; IL1b, interleukin 1b; IL1RA, interleukin 1 receptor antagonist; no-DR, no diabetic retinopathy.
The mean vitreous concentrations of IL1b were 0.86±0.62, 1.80±0.72, and 1.54±0.13 pg/ml in the no-DR, DME, and Hr-PDR groups, respectively (Figure 3b).
The mean vitreous concentrations of IL1RA were 40.55±7.29, 2313.40±1697.03, and 153.09±149.46 pg/ml in the no-DR, DME, and Hr-PDR groups, respectively (Figure 3c).
We compared the IL1RA levels between the groups: no-DR vs Hr-PDR (P=0.001), DME vs Hr-PDR (P=0.001), and no-DR vs DME (P<0.001). IL1RA/IL1b ratio was 1285.2 and 99.4 in the DME and Hr-PDR groups, respectively.
Discussion
We described a new and simple technique for collecting VR in the DME patients requiring intravitreal injections, which did not affect the patient comfort. To further assess its efficacy for proteomics, a set of 27 cytokines was quantified in the study population.
Cacciamani et al11 found that the millipore and micropipette techniques yielded high protein extracts, whereas the Schirmer tear strips and micro-sponge techniques yielded low-protein extracts. Unlike this study, we obtained a good quality protein extract from the Schirmer tear strip method (Figure 1a). The protein profile of 1D gel showed no evidence of tear contamination, excluding the possibility of tear proteins confounding our results. (Figure 1b), implying that our technique provides a pure, albeit small, vitreous sample for proteomics. Good protein extraction in our study was probably because of the following reasons: (1) We kept the Schirmer strips for 10 s at the site of injection after the syringe withdrawal vs 5 s in the previous study, which would have resulted in more vitreous adsorption; (2) We used phosphate buffered saline tween buffer for the protein extraction from the Schirmer tear strips vs the modified radioimmunoprecipitation assay buffer used in the previous study, which could have resulted in the difference in protein extraction; (3) We used the bicinchoninic acid assay method of total protein quantification vs the digital spectrophotometer in the previous study, which could have resulted in the difference in protein estimation. Our previous studies on tear proteomics had shown capillary vs Schirmer strips technique of collection of tear had a similar protein profile in two-dimensional gel electrophoresis, wherein a similar Schirmers extraction protocol was used.12
Recently, Ghodasra et al13 in their pilot study had shown that the office-based vitreous aspiration samples can be used for proteomics. This technique would possibly increase the risk of retinal break or detachment from the vitreoretinal traction during aspiration. Moreover, the sample collection and intravitreal injection are two separate invasive procedures. This translates into more patient discomfort and increases the rate of complications associated with the intravitreal injections. Larger randomized studies with a longer followup are necessary to quantify the risk involved before its transition to a standardized diagnostic procedure. Our simple technique overcomes the above limitations; thus, this technique will have a higher acceptance in the clinical practice.
ELISA and western blot limit the number of cytokines assayed. The recent development of multiplexed cytometric bead analysis has allowed the simultaneous quantification of multiple proteins with a small volume of the sample.14 Apart from PDR, for the first time, we have provided a broader insight into the DME pathogenesis using vitreous samples collected non-invasively using multiplexed bead analysis.
Similar to the previous reports, we found that the Hr-PDR is a highly pro-inflammatory state.13, 15, 16, 17, 18, 19, 20, 21, 22, 23 Of the 27 cytokines tested, we found that 25 were significantly elevated in the Hr-PDR group (P<0.05; Table 1). Of the above 25 cytokines, 13 had a two-fold or more increase in their levels. We found that the cytokines IL13, IP10, IL7, IL6, IL8, and MCP1 showed a 35.2-, 29.2-, 23.6-, 12.2-, 10.2-, and 5.4-fold increase, respectively, in comparison with the no-DR group.
We found that DME is also a pro-inflammatory state. Of the 27 cytokines tested, we found that 20 were significantly elevated in the DME group (P<0.05; Table 2). Of these, seven had a two-fold or more increase in their levels. Interestingly, we found that the cytokines IL1RA, IL7, and IP10 showed 57.0-, 13.8-, and 3.8-fold increase, respectively, in comparison with the no-DR group.
However, when compared with Hr-PDR, we found that DME is less pro-inflammatory. Of the 27 measured cytokines, 17 were significantly elevated in the Hr-PDR group (Supplementary Table 2). Interestingly, three cytokines were elevated in DME as compared with Hr-PDR: IL1b (1.17-fold, P=0.724), IL1RA (15.1-fold, P=0.01) and RANTES (1.84-fold elevated, P=0.044).
Increased levels of VEGFs have been reported in vitreous of patients with PDR.24, 25 We found the levels of VEGFs in Hr-PDR were significantly higher when compared with the other two groups (P<0.05; Figure 3a). Interestingly, we found that the levels of VEGFs in DME were little higher as compared with the no-DR state (P=0.591). There are two possible reasons for this observation of ours. First, VEGF alone is not ‘the key player’ in the DME pathogenesis as has been previously believed.2, 3, 4, 26 Currently, it is known that the inflammatory process contributes to the breakdown of the vascular barriers in DME.27, 28 Previous proteomics in DME have shown that the angiogenic factors, inflammatory cytokines, chemokine, and growth factors are involved in the disease process (Supplementary Table 3).29, 30, 31, 32, 33, 34, 35, 36 The vitreous cytokine profiling in the DME group in this study showed multiple pro-inflammatory cytokines to be significantly raised as compared with the no-DR group, and showed differential expression with respect to Hr-PDR.
Secondly, as it is known that the VR contains a very small reflux of the anti-VEGF injected, we believe that the neutralization of a fraction of the VEGF molecules by the drug molecules contributes partially to the observation of relatively lower VEGF levels in the DME samples.10, 37, 38 The reason being, the VR represents the peripheral liquified vitreous that instantly oozes out rather than the core vitreous, where the anti-VEGF drug is injected.
We found IL1b to be 2.1- and 1.8-fold elevated as compared with the no-DR group in the DME and Hr-PDR group, respectively (Figure 3b). We found IL1RA to be 57.0- and 3.8-fold elevated as compared with the no-DR group in the DME and Hr-PDR group, respectively (Figure 3c). What was more interesting to note was the 15.1-fold (P=0.01) decrease in the levels of IL1RA in the Hr-PDR group as compared with the DME group. Animal and human study models of DR have shown that IL1b- and IL1b-converting enzymes are overexpressed in the retina cells.39, 40 Increased IL1b levels have been detected in the vitreous of the Hr-PDR patients.41, 42 Kowluru and Odenbach43 in animal model showed that IL1b acting via the activation of NF-κB and an increase in the oxidative stress accelerate the apoptosis of the retinal capillary cells, and the antioxidants inhibit diabetes-induced increases in the retinal IL1beta. Gerhardinger et al44 in animal model showed that the chronic overexpression of IL1RA prevents the excessive vascular cell death and the loss of capillaries in the diabetic rat retina.
Recently, Stahel et al45 in their first prospective pilot human study found the systemic IL1b inhibition to have a promising effect on DME. We presumed that the preferential overexpression of IL1b in DME as compared with the Hr-PDR, as observed in our study (Figure 2), might be the underlying mechanism.
The anti-inflammatory and the pro-inflammatory role of IL1RA and IL1b are well known.46, 47 The balance between IL1RA and IL1β plays a decisive role (IL1RA/IL1b ratio), which was found to be 13-fold higher in the DME group as compared to the Hr-PDR group in our study. Despite our relatively small cohort, this novel finding has a significant implication. At the molecular level, it denotes that the downturn of this protective cytokines leads to disease progression. From a clinical perspective, they represent potential therapeutic targets for the treatment of DR. The study raises the possibility of exploring the imbalance between IL1b and IL1RA as therapeutic targets to prevent the progression of DR in future research. Similar imbalance exists in rheumatoid arthritis, gout, and other IL1b-associated autoimmunopathies, where IL1b blockers such as anakinra, rilonacept, and canakinumab have shown great promise.48, 49, 50
This study has some limitations. It is a pilot study that involved a small number of patients. Our finding needs to be validated in larger cohorts. The VR may not represent the true vitreous. VR that appears after intravitreal injections is the liquified peripheral part and may differ in the cytokine profile as compared with the formed central vitreous. Likewise, the VR collected at the pars plans region may not reflect the retinal micro-environment as correctly as the undiluted core vitrectomy sample. However, the significant differential expression of the cytokines observed in the conditions studied indicates a high likelihood of VR samples to be an excellent alternative. Finally, though only idiopathic macular holes cases were included in our study as controls, cytokines profile in them might be different as compared with the healthy subjects. Use of donor eye vitreous samples is also not without limitations. Degenerative changes set in soon after death and can appreciably alter the profile of the cytokines. Similar to our study, previous studies on ocular cytokines assays have used pre-operative povidine iodine and have shown that it does not interfere with cytokines assays. We in our study gave a through cul-de-sac wash with balanced salt solution before injection ensuring it complete removal. Moreover, all the groups compared in our study were treated with povidine iodine similarly, therefore, its interference, if any, would have happened in all the groups. We found RANTES was relatively higher in DME than Hr-PDR. RANTES has been associated with both pro and anti-inflammatory effects and reportedly regulates VEGF levels.51 Detailed studies are required to evaluate this differential role in DME as against Hr-PDR. In our pilot study, all the patients had a VR, probably due to a more vertical entry of the needle during intravitreal injection as compared to the commonly practiced oblique entry.
In conclusion, our study shows the feasibility of VR sampling using Schirmer tear strips for functional analysis. As an alternative to invasive procedure, this would offer widespread application in many vitreoretinal pathologies. It represents a step forward in understanding the pathogenesis, exploring new therapeutic targets, identifying potential diagnostic and prognostic markers, customising personalized therapy, and monitoring the disease therapy. To the best of our knowledge, this is the first study of VR sampling in human DR. Further studies have been conducted to validate the expression of IL1b and IL1RA in DME cases in a larger cohort.

Acknowledgments
We acknowledge ICER (NIH), NIRT, Chennai for the permission to use multiplex reader facility.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
The authors declare no conflict of interest.
Supplementary Material
References
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35(3): 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoaku WM, Saker S, Stewart EA. A review of therapies for diabetic macular oedema and rationale for combination therapy. Eye 2015; 29(9): 1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem 2016; 117(11): 2443–2453. [DOI] [PubMed] [Google Scholar]
- Kaštelan S, Tomić M, Gverović Antunica A, Salopek Rabatić J, Ljubić S. Inflammation and pharmacological treatment in diabetic retinopathy. Mediators Inflamm 2013; 2013: 213130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totan Y, Güler E, Gürağaç FB. Dexamethasone intravitreal implant for chronic diabetic macular edema resistant to intravitreal bevacizumab treatment. Curr Eye Res 2016; 41(1): 107–113. [DOI] [PubMed] [Google Scholar]
- Murthy KR, Goel R, Subbannayya Y, Jacob HK, Murthy PR, Manda SS et al. Proteomic analysis of human vitreous humor. Clin Proteomics 2014; 11(1): 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Liu F, Cui SJ, Liu Y, Song ZY, Cao H et al. Vitreous proteomic analysis of proliferative vitreoretinopathy. Proteomics 2008; 8(17): 3667–3678. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim SJ, Kim K, Kang UB, Lee C, Park KS et al. Profiling of vitreous proteomes from proliferative diabetic retinopathy and nondiabetic patients. Proteomics 2007; 7(22): 4203–4215. [DOI] [PubMed] [Google Scholar]
- Ecker SM, Hines JC, Pfahler SM, Glaser BM. Aqueous cytokine and growth factor levels do not reliably reflect those levels found in the vitreous. Mol Vis 2011; 17: 2856–2863. [PMC free article] [PubMed] [Google Scholar]
- Brodie FL, Ruggiero J, Ghodasra DH, Hui JZ, VanderBeek BL, Brucker AJ. Volume and composition of reflux after intravitreal injection. Retina 2014; 34(7): 1473–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciamani A, Parravano M, Scarinci F, Esposito G, Varano M, Micera A. A simple spontaneous vitreal reflux collecting procedure during intravitreal injection: set-up and validation studies. Curr Eye Res 2016; 41(7): 971–976. [DOI] [PubMed] [Google Scholar]
- Saijyothi AV, Angayarkanni N, Syama C, Utpal T, Shweta A, Bhaskar S et al. Two dimensional electrophoretic analysis of human tears: collection method in dry eye syndrome. Electrophoresis 2010; 31(20): 3420–3427. [DOI] [PubMed] [Google Scholar]
- Ghodasra DH, Fante R, Gardner TW, Langue M, Niziol LM, Besirli C et al. Safety and feasibility of quantitative multiplexed cytokine analysis from office-based vitreous aspiration. Invest Ophthalmol Vis Sci 2016; 57(7): 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods 2006; 38(4): 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-White JL, Glazer L, Downer R, Furge K, Boguslawski E, Duesbery NS. Identification of VEGF-independent cytokines in proliferative diabetic retinopathy vitreous. Invest Ophthalmol Vis Sci 2013; 54(10): 6472–6480. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res 2012; 37(5): 416–420. [DOI] [PubMed] [Google Scholar]
- Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and Eales' disease. Retina 2008; 28(6): 817–824. [DOI] [PubMed] [Google Scholar]
- Maier R, Weger M, Haller-Schober EM, El-Shabrawi Y, Wedrich A, Theisl A et al. Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis 2008; 14: 637–643. [PMC free article] [PubMed] [Google Scholar]
- Chernykh VV, Varvarinsky EV, Smirnov EV, Chernykh DV, Trunov AN. Proliferative and inflammatory factors in the vitreous of patients with proliferative diabetic retinopathy. Indian J Ophthalmol 2015; 63(1): 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One 2009; 4(12): e8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res 2011; 30(5): 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol 2002; 86(4): 363–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res 1995; 14(11): 1045–1053. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994; 118(4): 445–450. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331(22): 1480–1487. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004; 18(12): 1450–1452. [DOI] [PubMed] [Google Scholar]
- Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 2013; 34: 19–48. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zeng H, Bao S, Wang N, Gillies MC. Diabetic macular edema: new concepts in patho-physiology and treatment. Cell Biosci 2014; 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Xu B, Chu L, Tang X. Study of 27 aqueous humor cytokines in type 2 diabetic patients with or without macular edema. PLoS One 2015; 10(4): e0125329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Xu B, Wang B, Chu L, Tang X. Aqueous cytokines as predictors of macular edema in patients with diabetes following uncomplicated phacoemulsification cataract surgery. Biomed Res Int 2015; 2015: 126984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Jonas RA, Neumaier M, Findeisen P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina 2012; 32(10): 2150–2157. [DOI] [PubMed] [Google Scholar]
- Sohn HJ, Han DH, Kim IT, Oh IK, Kim KH, Lee DY et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol 2011; 152(4): 686–694. [DOI] [PubMed] [Google Scholar]
- Roh MI, Kim HS, Song JH, Lim JB, Kwon OW. Effect of intravitreal bevacizumab injection on aqueous humor cytokine levels in clinically significant macular edema. Ophthalmology 2009; 116(1): 80–86. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009; 116(1): 73–79. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Kang MH, Seong M, Cho HY. Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch retinal vein occlusion. Br J Ophthalmol 2012; 96(11): 1426–1430. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol 2002; 133(1): 70–77. [DOI] [PubMed] [Google Scholar]
- Usman Saeed M, Batra R, Qureshi F, Clark D. Reflux of drug during intra-vitreal anti-VEGF therapies. Semin Ophthalmol 2011; 26(6): 357–360. [DOI] [PubMed] [Google Scholar]
- Brodie FL, Ruggiero J, Ghodasra DH, Eftekhari K, Hui JZ, Brucker AJ et al. A novel method for the measurement of reflux from intravitreal injections: data from 20 porcine eyes. Curr Eye Res 2014; 39(7): 752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo A, Cunha-Vaz JG, Carvalho AP, Lopes MC. L-arginine transport in retinas from streptozotocin diabetic rats: correlation with the level of IL-1 beta and NO synthase activity. Vis Res 1999; 39(23): 3817–3823. [DOI] [PubMed] [Google Scholar]
- Li W, Yanoff M, Jian B, He Z. Altered mRNA levels of antioxidant enzymes in pre-apoptotic pericytes from human diabetic retinas. Cell Mol Biol 1999; 45(1): 59–66. [PubMed] [Google Scholar]
- Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye 2006; 20(12): 1366–1369. [DOI] [PubMed] [Google Scholar]
- Abuel Asrar AM, Maimone D, Morse PH, Gregory S, Reder AT. Cytokines in the vitreous of patients with proliferative diabetic retinopathy. Am J Ophthalmol 1992; 114(6): 731–736. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci 2004; 45(11): 4161–4166. [DOI] [PubMed] [Google Scholar]
- Gerhardinger C, Liu Y, Dagher Z. Overexpression of IL-1 receptor antagonist in the rat retina by AAV2-mediated gene transfer prevents capillary loss in experimental diabetes. Invest Ophthalmol Vis Sci 2012; 53: 5764. [Google Scholar]
- Stahel M, Becker M, Graf N, Michels S. Systemic interleukin 1β inhibition in proliferative diabetic retinopathy: a prospective open-label study using Canakinumab. Retina 2016; 36(2): 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Gabay C. Physiologic role of interleukin-1 receptor antagonist. Arthritis Res 2000; 2(4): 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP. Interleukin-1 receptor antagonist. Adv Immunol 1993; 54: 167–227. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011; 117(14): 3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. N Engl J Med 2006; 355(6): 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff MH. Role of interleukin 1 and interleukin 1 receptor antagonist in the mediation of rheumatoid arthritis. Ann Rheum Dis 2000; 59: i103–i108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffee N, Richard B, Hlawaty H, Oudar O, Charnaux N, Sutton A. Angiogenic properties of the chemokine RANTES/CCL5. Biochem Soc Trans 2011; 39(6): 1649–1653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.