Abstract
The water relations parameters involved in assimilate flow into developing wheat (Triticum aestivum L.) grains were measured at several points from the flag leaf to the endosperm cavity in normally watered (Ψ ≈ −0.3 MPa) and water-stressed plants (Ψ ≈ −2 MPa). These included direct measurement of sieve tube turgor and several independent approaches to the measurement or calculation of water potentials in the peduncle, grain pericarp, and endosperm cavity. Sieve tube turgor measurements, osmotic concentrations, and Ψ measurements using dextran microdrops showed good internal consistency (i.e. Ψ = Ψs + Ψp) from 0 to −4 MPa. In normally watered plants, crease pericarp Ψ and sieve tube turgor were almost 1 MPa lower than in the peduncle. This suggests a high hydraulic resistance in the sieve tubes connecting the two. However, observations concerning exudation rates indicated a low resistance. In water-stressed plants, peduncle Ψ and crease pericarp Ψ were similar. In both treatments, there was a variable, approximately 1-MPa drop in turgor pressure between the grain sieve tubes and vascular parenchyma cells. There was little between-treatment difference in endosperm cavity sucrose or osmotic concentrations or in the crease pericarp sucrose pool size. Our results re-emphasize the importance of the sieve tube unloading step in the control of assimilate import.
One of the most striking aspects of fruit and seed physiology is the independence of their water relations from other parts of the plant. For seeds, stable water relations are required for normal embryo development (Walbot, 1978; Kermode, 1990; Bradford, 1994), for example, in preventing precocious germination. Embryo growth also requires a steady supply of nutrients. Since imported assimilates make up a large proportion of the embryo's osmotic environment, it is likely that these two requirements have some controls in common.
In contrast to the embryonic environment, water relations and assimilate concentrations in the vegetative portion of the plant may vary considerably. Water potential especially may vary diurnally and/or decline progressively with the onset of drought conditions. Thus, reproductive structures, or at least their embryos, must be insulated from the variations in water potential experienced by the rest of the plant. At the same time, they typically maintain a constant growth rate (Barlow et al., 1980; Lang and Thorpe, 1989; Westgate et al., 1989; Lang, 1990) despite such variation.
In part, this independence of seed and fruit water relations is often achieved by a functional discontinuity in the xylem at or near their point of attachment to the plant. Quite large Ψ differences have been reported between seeds or fruit and vegetative portions of the plant, and even between the embryo and adjacent seed or fruit tissues (Bradford, 1994). However, as Bradford points out in his critical review of these issues, such differences have been reported even in instances where there seems to be no effective barrier to water movement (e.g. seeds in fleshy fruits and cultured embryos). As he notes, a technical problem in these studies may arise from the use of psychrometric measurements of Ψ on detached tissues, a procedure that requires several hours of equilibration time. This raises the possibility that significant changes in water relations of the tissues could occur, especially in the apoplastic solute concentration.
In a pioneering study of seed water relations, Barlow et al. (1980) found that wheat grain Ψ was about −1.0 MPa during most of grain filling in well-watered conditions, and vegetative tissues were about 0.5 MPa higher. When water was withheld, grain Ψ dropped only slightly during the next 10 d, while that in the rest of the plant declined to −3 to −4 MPa. The growth rate of the grain was unaffected. The discontinuity in the xylem at the base of the wheat grain (Zee and O'Brien, 1970) is an important factor in the independence of grain water relations from other parts of the plant.
Similar values for wheat grain pericarp Ψ and leaf Ψ, and their independence, were also measured by Fisher (1985) in well-watered plants. The measurements were not made by psychrometry, but employed the osmotic equilibration of microdrops about 10 nL in volume with plant Ψ, thus providing in situ measurements of Ψ on attached grains. At the same time, measurements of sieve tube osmotic concentrations in the grain and peduncle showed them to be similar (Fisher and Gifford, 1986). Thus, given the Ψ difference between the two sites, there appeared to be a sharp drop in sieve tube turgor from the peduncle to the grain, evidently along the phloem paralleling the xylem discontinuity at the base of the grain. However, while this is feasible for well-watered plants, it is not for the droughted plants studied by Barlow et al. (1980) if the sieve tube sap concentration was constant along the pathway. In that event, sieve tube turgor would be 2 to 3 MPa higher in the grains, with transport occurring against a gradient in turgor pressure.
To clarify this apparent anomaly and other issues regarding the driving forces for assimilate flow into the wheat grain, several independent approaches were taken for the measurement or calculation of Ψ and sieve tube turgor pressure in well-watered and droughted wheat plants. The experiments provide estimates of gradients in Ψ and sieve tube turgor between the plant and grain, for the turgor differential across the sieve element/companion cell (SE/CC) unloading step within the grain, and for Ψ differences between tissues within the grain. Issues concerning the use of psychrometric methods for the measurement of grain Ψ are also examined.
RESULTS
Grain Water Relations during Growth of Normally Watered Plants
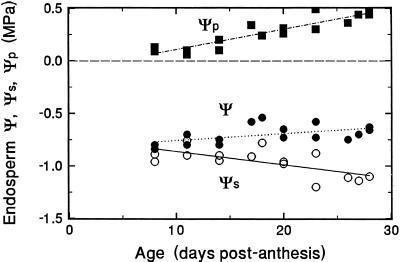
The trend in endosperm Ψ, Ψs, and Ψp during the final 3 weeks of grain filling are shown in Figure 1. Endosperm Ψ was taken to be equal to that of the endosperm cavity sap Ψ, which in turn is assumed to equal cavity sap Ψs. Some error is incurred in the latter assumption, but it is small (see below). As noted previously, (Fisher and Gifford, 1986), cavity sap Ψs and, by inference, endosperm Ψ are fairly constant during grain growth at about −0.7 MPa. Endosperm turgor, calculated as the difference between Ψ and endosperm sap Ψs, was initially quite low, but increased slowly during the remaining 3 weeks. Interestingly, the increase in turgor can be accounted for almost entirely on the basis of the expected amount of potassium imported during this period, assuming an import rate of 10 μL sieve tube sap d−1 (Fisher, 1990) and a K+ concentration of 20 mm (Fisher and Gifford, 1987).
Figure 1.
Trends in endosperm water potential and its components in wheat grains during the final 3 weeks of grain filling. Total water potential was assumed to equal endosperm cavity sap Ψs. Endosperm solute potential was determined on sap released from the endosperm by two freeze-thaw cycles. Turgor pressure was calculated as (Ψ − Ψs).
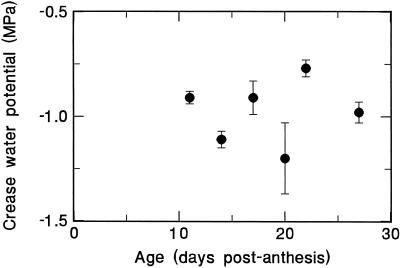
Water potential of the grain pericarp, as measured by microdrops on the crease region, were somewhat variable, but showed no evident trend during grain filling (Fig. 2), averaging about −1.0 MPa. Thus, pericarp Ψ was consistently lower, by about 0.3 MPa, than endosperm and endosperm cavity Ψ (Fig. 1).
Figure 2.
Trend in grain pericarp Ψ during the final 3 weeks of grain filling. Ψ was measured by the use of dextran microdrops sited on the crease pericarp of attached grains.
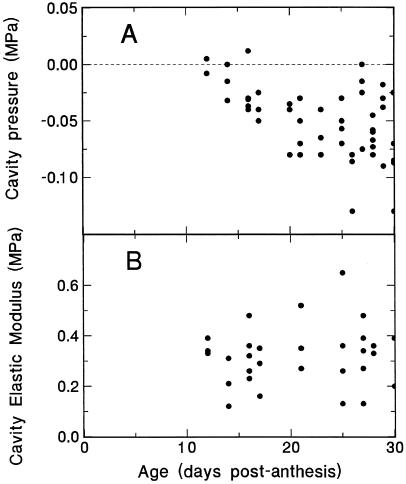
Pressure in the endosperm cavity was negligible as the grain entered the linear phase of grain filling, but declined to roughly −0.05 MPa as filling progressed (Fig. 3). Positive pressures have been observed occasionally, but not in the measurements taken for this data set.
Figure 3.
Trends in endosperm cavity pressure (A), and in cavity volumetric modulus (B) during the final 3 weeks of grain filling. Measurements were made immediately after detaching the grain, using a modified pressure probe.
The elastic modulus of the endosperm cavity wall was low, averaging about 0.3 MPa, with no evident trend during grain filling (Fig. 3). Cavity volume was 1.7 ± 0.8 μL (n = 41, averaged for all grains regardless of age). Much of the variation can be ascribed to the elasticity of the cavity wall and the range of pressures observed, since the cavity sap volume clearly decreased as tension increased. The extent to which this occurred is illustrated in the following section (see Fig. 4C), where the volumetric change that accompanied an increase in cavity tension was followed directly.
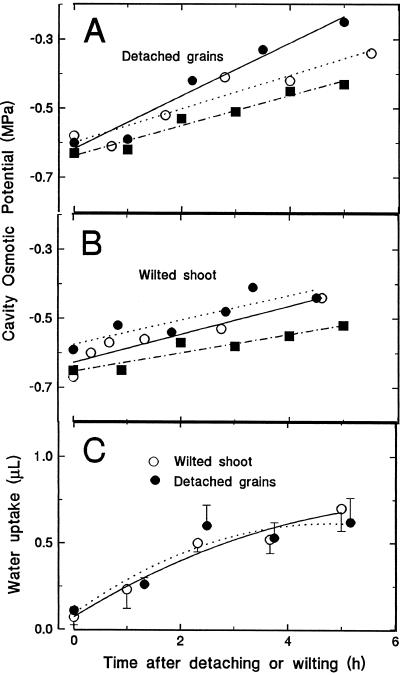
Figure 4.
The effect of detaching grains, or of rapidly wilting the shoot, on endosperm cavity sap concentration (A and B) and cavity volume (C). In grain detachment experiments, grains were removed from the ear and placed in a humid chamber for the times indicated. Each plot represents grains from the same ear (one grain per point). In wilting experiments, a tiller on an illuminated plant was excised at ground level and allowed to wilt in the light. Grains were removed at intervals after excision. Changes in the cavity volume (C) were followed by inserting a fine-tipped capillary into the distal end of the cavity and recording the volume sucked from the pipette during the next several seconds, after which movement virtually ceased.
Response of Grain Water Relations to Detachment or Rapid Wilting
The effect on endosperm cavity water relations of detaching grains from the plant is shown in Figure 4A. On average, cavity Ψs increased by about 0.3 MPa in the 5 h following grain detachment. This was accompanied by a substantial loss of water (compared with the cavity volume) from the endosperm cavity (Fig. 4C). The amount of water loss was estimated in a separate experiment by inserting the end of a water-filled glass capillary drawn to a fine tip into the endosperm cavity, and recording the volume rapidly sucked into the cavity as tension was released. Because cavity tension was initially low in these grains, the volume change required to relieve the tension provided an estimate of cavity water movement into the endosperm after grain excision, which reached about 0.7 μL after 5 h.
The effect of rapid wilting on grain water relations, caused by cutting off the stem of an illuminated plant just above soil level, was quite similar to that caused by detaching the grain. Cavity Ψs increased (Fig. 4B), as did tension within the endosperm cavity (Fig. 4C).
Figure 5 illustrates an additional response of grains to excision, with important implications for psychrometric measurements of grain Ψ. In the vast majority of cases (at least 85%), grains detached from normally watered plants exuded from the surface of their broken pedicels, typically accumulating 100 to 200 nL during the course of exudation (1–2 h). Almost certainly this is phloem exudate, since Suc and hexoses account for most of the solutes present (data not shown). For ease of illustration, the accumulation is shown here under mineral oil. However, similar volumes were produced by grains in air in a closed chamber, as employed for psychrometric measurements. Exudate concentration declined with time and varied somewhat between grains, but was typically about −1.0 to −1.2 MPa. After the initial 1- to 2-h outflow, the exudate was reabsorbed by the grain over the next 2 to 4 h. Exudation did not usually occur from grains detached from droughted plants.
Figure 5.
Exudation from the broken pedicel surface of grains detached 40 min earlier and placed in mineral oil.
Comparison of Microdrop Ψ to Values Calculated from Sieve Tube Turgor and Concentration Measurements
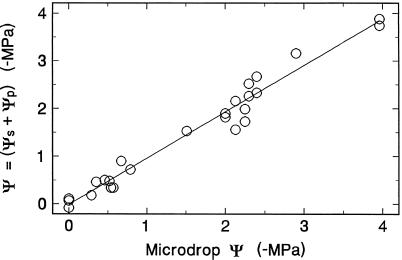
The reliability of the methods used to measure Ψ and its components (i.e. turgor and osmotic potential) is illustrated by their internal consistency in measurements taken from the peduncle of plants with Ψ ranging from 0 MPa (detached tillers in water; plant Ψ assumed to be zero) to −4.0 MPa (Fig. 6). Microdrop values for plant Ψ were in excellent agreement with values calculated from sieve tube turgor and concentration measurements. Because sieve tube exudate concentrations typically show gradual changes with time, the agreement is probably better than that shown, since some turgor measurements took 30 to 60 min or more to reach full compression.
Figure 6.
Comparison of Ψ measured with dextran microdrops with values calculated from manometric measurements on the same tiller of sieve tube Ψp and Ψs. After a turgor measurement, the manometer tip was clipped off to confirm that the stylet was still exuding. The manometer was then broken from the plant surface and its contents were ejected into mineral oil to allow measurement of exudate Ψs by freezing point depression. A Ψ of zero cannot be measured by microdrops, but was assumed for detached tillers in water.
Turgor and Ψ Gradients between the Grain and Plant Vegetative Tissues in Normal and Water-Stressed Plants
In agreement with earlier, less extensive observations (Fisher, 1985), no measurable differences in Ψ could be detected by microdrops sited on the leaves and the peduncle of the same plant. Several attempts to demonstrate a sieve tube sap concentration difference, and therefore a pressure gradient between the flag leaf and peduncle, yielded marginal results. With four to six stylets at each site, Ψs in three experiments was 0.0, 0.3, and 0.4 MPa higher in the flag leaf, with a sd in each case of approximately ±0.3 MPa. However, the sd in Ψs from different stylets at the same site was, in all cases, about ±0.1 MPa. Thus, any possible Ψs difference between the flag leaf and peduncle could not be distinguished convincingly from background variability.
In sharp contrast, manometric measurements showed large differences in sieve tube Ψ and turgor pressure between the peduncle/rachis and grains of normally watered plants (Table I). As noted previously (Fisher and Gifford, 1986), there was no evident difference in sieve tube exudate Ψs between the two sites. On average, Ψ and turgor were both about 1 MPa lower in the grain. While it was not possible to make microdrop and manometric measurements on the same grain, the values for grain sieve tube Ψ were in good agreement with microdrop measurements of about −1.0 MPa on other grains (Fig. 2). Also, they agree well with estimates of phloem Ψ based on extrapolation of stylet exudation rates on detached grains to zero (next section; Table II).
Table I.
Measurement of sieve tube turgor pressure and Ψs and their gradients between the grain and peduncle or rachis
| Measurement | Normally Watered Plants
|
Water-Stressed Plants
|
||||
|---|---|---|---|---|---|---|
| Ψp | Ψs | Ψ | Ψp | Ψs | Ψ | |
| mPa | ||||||
| Grain phloem | 1.16 ± 0.26 | −2.38 ± 0.42 | −1.17 ± 0.38 | 1.04 ± 0.27 | −2.86 ± 0.42 | −1.82 ± 0.24 |
| Peduncle phloem | 2.35 ± 0.56 | −2.56 ± 0.62 | −0.35 ± 0.30 | 1.39 ± 0.33 | −3.42 ± 0.20 | −2.05 ± 0.38 |
| Gradienta | −1.09 ± 0.63 | 0.02 ± 0.28 | −0.90 ± 0.44 | −0.39 ± 0.41 | 0.53 ± 0.40 | 0.14 ± 0.38 |
Sieve tube turgor was measured manometrically. After completing a turgor measurement, the manometer tip was clipped off to confirm that the stylet was still exuding. Stylet exudate was ejected from the manometer under mineral oil to allow Ψs determination by freezing point depression. Values for Ψ are calculated from turgor and Ψs. Paired measurements were made on the grain and peduncle (three tillers) or rachis (three tillers) of normally watered plants (Ψ ≈ −0.4 MPa) and seven tillers (grain and peduncle) of droughted plants (Ψ ≈ −2.1 MPa).
Gradient = Grain − Peduncle.
Table II.
Grain sieve tube and vascular parenchyma water relations parameters estimated from the effects of grain removal on exudation from a stylet on the grain
| Measurement | Normally Watered Plants | Water-Stressed Plants |
|---|---|---|
| Pre-excision exudate Ψs | −2.21 ± 0.50 | −2.97 ± 0.42 |
| Ψ (Ψs at Ψp = 0) | −1.14 ± 0.13 | −1.68 ± 0.24 |
| Sieve tube Ψp (Ψp = Ψ − Ψs) | 1.11 ± 0.53 | 1.30 ± 0.27 |
| Parenchyma | ||
| Ψs | −1.30 ± 0.12 | −1.73 ± 0.26 |
| Ψp | 0.12 ± 0.99 | 0.08 ± 0.06 |
| ▵Ψpa | −1.00 ± 0.45b | −1.12 ± 0.42c |
Initially, exudate comes from incoming sieve tube contents (row 1). On detaching a grain, exudation slows rapidly and becomes more dilute. Extrapolation of this rate to zero as a function of exudate Ψs gives Ψ, since Ψp = 0 (row 2). Within several minutes, exudate Ψs becomes fairly constant, and is presumed to originate from surrounding vascular parenchyma cells (row 4). Sieve tube and parenchyma turgor pressures (rows 2 and 5, respectively) are calculated from Ψ and Ψs. Data are from 10 normally watered plants and 15 water-stressed plants.
▵Ψp = Parenchyma − sieve tube.
Range = 0.34 to 1.49.
Range = 0.85 to 1.66.
Similar manometric measurements on water-stressed plants showed substantial changes in all water relations parameters except sieve tube turgor in the grains, which did not differ from that in normally watered plants. In particular, there was no evidence that the gradient in sieve tube turgor between the peduncle and the grain had reversed, as might be inferred from Barlow et al.'s (1980) Ψ measurements if sieve tube sap concentrations were similar in the peduncle and grain. While there was substantial variability, only one pair of measurements indicated a higher turgor pressure in the grains (by 0.2 MPa) compared with the peduncle. The principal water relations adjustment that maintained lower turgor in the grain sieve tubes appeared to be a decline of almost 0.7 MPa in grain pericarp Ψ. Also, the sieve tube sap concentration may have been lower in the grain.
Gradients between the Grain Sieve Tubes and Surrounding Vascular Parenchyma Cells
Grain phloem Ψ, estimated by extrapolating stylet exudation rates on detached grains to zero, also indicated lower values in water-stressed plants (Table II), as did dextran microdrop measurements on the crease pericarp (−1.65 ± 0.18 MPa; n = 7). As shown previously in similar experiments with normally watered plants (Fisher, 1995, and additional measurements), turgor pressure in the vascular parenchyma surrounding the sieve tubes was quite low. As a result, there was a large turgor differential of, on average, 1.12 MPa between the two, which is similar to that in normally watered plants (1.00 MPa). However, as for normally watered plants, there was substantial variability in this gradient.
Table III compares the crease Suc pools and endosperm cavity sap Suc and osmotic concentrations for normally watered and droughted plants. Although the endosperm cavity sap Suc concentration was somewhat lower in grains from droughted plants, there was only a slight, if any, difference in crease Suc pool sizes or in cavity sap Ψs (and therefore Ψ). The decline noted in crease pericarp Ψ in water-stressed plants to approximately −1.7 MPa was highly localized, with a Ψ difference between the endosperm and crease pericarp of about 0.9 MPa. This is three times the difference in grains of unstressed plants.
Table III.
Crease Suc pools and endosperm cavity sap Suc and osmotic concentrations in normally watered and water-stressed plants
| Measurement | Normally Watered Plants | Water-Stressed Plants |
|---|---|---|
| Crease tissues (total Suc in nmol) | 299 ± 70 (n = 108) | 249 ± 53 (n = 20) |
| Endosperm cavity | ||
| Suc (mm) | 72 ± 13 (n = 35) | 50 ± 8 (n = 8) |
| Ψs (MPa) | −0.71± 0.07 (n = 53) | −0.85 ± 0.08 (n = 15) |
The presence of nearly unaltered grain Suc pools confirms the continuing import of assimilates by grains on water-stressed plants. As for grains on normally watered plants (Fisher and Gifford, 1987; Fisher and Wang, 1993), these pools are rapidly depleted when the grains are detached from the ear (data not shown).
DISCUSSION
Water Relations Are Substantially Altered in Detached Grains
When a wheat grain is detached from the plant, import of assimilates clearly ceases. However, movement of assimilates into the endosperm continues, causing a steady decline of solutes in both the maternal tissues and the endosperm cavity (shown for Suc by Fisher and Gifford [1987] and by Fisher and Wang [1993]; and for total amino acids by N. Wang and D.B. Fisher [unpublished data]). Because these nutrients comprise at least half of the total solutes in the endosperm cavity (Fisher and Gifford, 1986), their absorption has a readily observable effect on total solute concentration, causing an increase in Ψs. Cavity Ψ is similarly affected, since there is little contribution from Ψp. Given the much larger volume of the endosperm (approximately 60 mm3), changes in endosperm Ψ will lag behind that in the cavity, leading to water movement from the cavity into the endosperm. These trends are clearly evident in Figure 4, A and C. Similar effects occur when illuminated shoots are rapidly wilted by cutting them off just above soil level, indicating that assimilate import ceased under these conditions as well. Thus, while Ψ was declining in the shoot, it was increasing in the grains.
In addition to effects caused by the cessation of assimilate import, detached grains often exude from the broken surface of their pedicels. Since the diffusive resistance of the rest of the grain surface will be relatively high, this exudate would likely dominate psychrometric readings. As solutes are reabsorbed by the grain, the exudate should come closer to pericarp Ψ. This may be the reason that Barlow et al.'s (1980) values for “grain” Ψ are close to ours for the pericarp on normally watered plants. However, it is not clear why their values for droughted plants were markedly higher.
Gradients along the Phloem
Aside from local variability in sieve tube Ψs and, presumably, turgor pressure, our measurements reveal fairly uniform conditions of Ψ and sieve tube turgor throughout the plant, with the exception of the grains. There, pericarp Ψ and sieve tube turgor both differed sharply from values obtained in the rest of the plant, sometimes less than 1 cm away. The methods showed good internal consistency (i.e. they satisfy the basic water relations equation, Ψ = Ψs + Ψp) and there was good agreement between methods. On the grain, three independent approaches to estimating crease pericarp Ψ gave similar values. Thus, the differences observed between the grains and the rest of the plant appear to be real. The precise location of the Ψ and turgor difference is unclear, given the anatomical complexity between even the closest measurement sites. The most likely site appears to be in the grain pedicel, in parallel with the xylem discontinuity there (Zee and O'Brien, 1970; O'Brien et al., 1985).
The large turgor differential between the grain and rachis suggests the presence of a high resistance to flow in the sieve tubes, presumably in the grain pedicel. On the basis of tritiated water experiments, Jenner (1985) has suggested that the sieve tubes may be discontinuous in this region. However, O'Brien et al.'s (1985) thorough anatomical examination provides no support for this possibility, and macromolecules up to 400 kD injected into the rachilla sieve tubes move into the grain (Fisher and Cash-Clark, 2000). Furthermore, other evidence suggests that this juncture is a site of low resistance for flow along the phloem. On breaking off a grain, phloem exudate flows from the broken pedicel surface at rates up to about 10 times the normal translocation rate (Fisher and Gifford, 1986), although the pressure differential for flow would have only doubled, from about 1 MPa with the grain attached to about 2 MPa after detaching.
Similarly, an individual cereal grain infected with ergot (Claviceps purpurea [Fr.] Tul.) may import assimilates at many times the normal grain growth rate. Although they displace the ovary, ergot hyphae penetrate the plant only to the region of the xylem discontinuity, leaving the phloem there evidently unaffected (Luttrell, 1980). Infected barley grains have been observed to produce ergot honeydew at more than 30 times the growth rate of a healthy grain (D.B. Fisher, unpublished data). While these comparisons based on exudation rates are somewhat circumstantial, they clearly do not support the view that the 1-MPa pressure drop at this juncture is due to a large hydraulic resistance. Finally, if flow through the putative resistance ceased, turgor on either side should equalize if the sieve tubes remained functional. However, when an ear is chilled to 4°C, which virtually eliminates import (D.B. Fisher, unpublished observations based on 32PO4 translocation; see Fisher, 1990), exudate concentrations from stylets on the peduncle and grain remain similar (Fisher and Gifford, 1986), suggesting that the turgor differential between the sites was unaffected by the cessation of flow.
The only source of a pressure gradient envisioned by presently available mathematical models of osmotically generated pressure flow arises from the resistance of the sieve tubes to conventional Poiseuillean viscous flow (Tyree et al., 1974). In the absence of a significant hydraulic resistance, water should be lost from the incoming translocation stream as it encounters the lower Ψ of the grain pericarp, increasing its concentration and maintaining sieve tube turgor at the same level as in the peduncle and rachis. Thus, without a high hydraulic resistance, there appears to be no satisfactory explanation for this large pressure differential. Whatever its basis, the differential appears to be less in droughted plants. Thus, part of its functional significance may be to provide a margin of insurance for maintaining assimilate import as Ψ declines in the source tissues.
Gradients along the SE/CC Unloading and Post-Phloem Transport Pathways
As noted previously for normally watered plants (Fisher, 1995), probably the most striking aspects of the SE/CC unloading step in grains from droughted plants are the low turgor of the vascular parenchyma cells and the large pressure differential, more than 1 MPa, to drive transport at this step. As with normally watered plants, there was a considerable range in this turgor differential. Since transport at this step is constant despite such a range in sieve tube sap concentrations and the driving force for SE/CC unloading, these observations re-emphasize the probable role of this juncture of assimilate movement as an important control point for assimilate import (Fisher, 1995). The contrast in conditions upstream from this step, where pressures and concentrations may vary markedly, with those downstream are emphasized by the relative constancy of the crease Suc pool size and cavity sap osmolality and Suc concentration, all of which differ slightly, if at all, between normally watered and droughted plants.
Gradients along the post-phloem symplastic pathway are markedly steeper in grains on water-stressed plants. Because crease pericarp Ψ is lower, by about 0.6 MPa, whereas cavity Ψ is only marginally different compared with normally watered plants, there is a substantially larger Ψ difference between the crease pericarp and endosperm of grains on droughted plants. In normally watered plants, this difference is about 0.3 MPa, and is countered by a similar but oppositely directed assimilate gradient (Fisher and Wang, 1995). This should result in little if any turgor gradient along the post-phloem pathway, leaving diffusion as the basis for symplastic solute movement, a conclusion supported by the movement of fluorescent tracers against that of incoming assimilates (Wang and Fisher, 1994b; Fisher and Cash-Clark, 2000).
However, since the crease Suc pool in droughted plants is no higher than in normally watered plants, it seems unlikely that a Ψ gradient of approximately 0.8 MPa could be similarly countered by an assimilate gradient in droughted plants. If turgor is constant along the symplast, solutes other than assimilates, perhaps confined to vacuoles, might contribute to the required balancing osmotic gradient.
The sharp Ψ difference between the endosperm and crease pericarp indicates a high resistance to apoplastic water movement between the two. Although solute movement is greatly restricted by secondary deposits in the chalazal cell walls of grains on normally watered plants, limited solute movement does occur (Wang and Fisher, 1994b). This indicates that the barrier is not absolute, but approximates a semipermeable membrane, allowing some water movement (also, see Bradford, 1994). Presumably, the resistance is increased in droughted plants by the increased lipid deposition that occurs in chalazal cell walls during water stress (Barlow et al., 1980).
The crease pericarp, which contains only about 5% of the grain's water, is further isolated by the xylem discontinuity in the grain pedicel (Zee and O'Brien, 1970). However, while hydraulic isolation is a necessary condition for the insulation of a tissue from changes in overall plant water status, it alone cannot ensure stability in local water relations. Some local controls, perhaps apoplast-symplast solute exchange, must also be involved.
MATERIALS AND METHODS
Plant Material
Wheat (Triticum aestivum L. cv SUN 9E) plants were grown in a growth chamber on a 16-h photoperiod at a PPFD of 450 μmol m−2 s−1. The temperature was 22°C during the day and 16°C at night. For experiments with normally watered plants, one plant per pot was grown in 1 L of a mixture of equal volumes of perlite, vermiculite, and potting soil. The pots were irrigated with water at 16-h intervals, and a complete nutrient solution was supplied once a week. Plants were used in the linear phase of grain filling, 15 to 25 DPA. Grain filling was complete at about 30 DPA.
Plants to be droughted were grown two to a pot in 3 L of soil. About 1 week after anthesis, all but two tillers were removed from each plant and droughting was initiated about 1 week later. Water relations parameters were measured 5 to 7 d later.
Aphids and Stylet Cutting
Aphids (Rhopalosiphum padi L.) were caged on the ear and peduncle or ear rachis overnight, and their stylets cut by radio frequency microcautery the next morning (Fisher and Frame, 1984). Because the grain phloem is not normally accessible for feeding, structures overlying the grain were removed. All but the basal (“a” or “b”) grain of each spikelet was removed, as well as the palea of the remaining basal grain. This exposed the groove, or crease, of the grain, at the bottom of which lies the single vascular bundle supplying the grain. To minimize water loss from the ears, they were kept in a humid environment except during experimental manipulations.
For measurements of concentrations and exudation rates, an oil well was constructed using quick-setting epoxy to seal a small O-ring, approximately 1 mm i.d., around an exuding stylet and filling the well formed by the O-ring with mineral oil. Exudation rates were measured by removing previously accumulated exudate and timing the interval taken for new exudate to reach a predetermined diameter, monitored under a dissecting microscope. Typically, this amounted to volumes of about 0.1 to 0.5 nL, accumulating over a period of 0.5 to 10 min. Only about 0.1 nL was required for the measurement of osmotic pressure.
Measurement of Ψs and Ψ
Osmotic concentrations were measured by freezing point depression using a nanoliter osmometer (Clifton Technical Physics, Hartford, NY). The endosperm cavity sap was sampled by slicing off the distal end of a grain and transferring about 1 μL of the liquid cavity contents to mineral oil, where about 0.1 nL was removed for measurement. Sap was obtained from the endosperm itself by first removing the outer pericarp and crease tissues from a grain and then transferring it to a 0.5-mL cappable centrifuge tube, where it was alternately crushed with a glass rod and frozen and thawed for two cycles. After centrifuging for 5 min at 16,000g, some of the resulting sap was transferred to mineral oil and subsampled for freezing point determination.
Plant Ψ was measured by the dextran microdrop method (Fisher, 1985). In brief, this consists of establishing an approximate 10-nL droplet of dextran solution (molecular mass = 9 kD) under mineral oil in an oil well on a small, lightly abraded area (approximately 10−2 mm2) of the plant surface. Osmotic concentration was measured periodically on subsamples to follow the approach of the droplet to equilibrium with plant Ψ. Droplets for the measurement of peduncle Ψ were established on the sclerenchyma overlying a vein. For measurements on grains, the microdrop was established on the surface of the outer pericarp of the crease region near the base of the grain.
Ψ of the grain phloem was also estimated by following the exudation rate and exudate Ψs from exuding stylets on grains just before and immediately after detaching the grain from the plant (Fisher, 1995). Both the solute concentration and exudation rate drop sharply after detachment, after which exudation usually continues for long times (hours to days) at a slow rate. Extrapolation of the exudation rate versus Ψs to zero gives the Ψs at zero turgor, where Ψs = Ψ. Because phloem exudate from stylets on detached grains must originate from surrounding parenchyma cells, exudate collected after the initial sharp drop in concentration also provides a value for Ψs in the vascular parenchyma (Fisher, 1995). Thus, the experiment allows the determination of pericarp Ψ, sieve tube and vascular parenchyma Ψs, and, by calculation, their turgor pressures and the turgor differential between them.
Endosperm Cavity Pressure and Elastic Modulus
The contents of the endosperm cavity are typically under a negative pressure (tension). To quantify this component of the cavity sap Ψ, and to determine its contribution to changes in the cavity volume, a modified pressure probe was used to measure cavity pressure and the volumetric elastic modulus (ε). The probe consists of thermoelectrically controlled expansion chamber filled with about 200 μL of silicone fluid and attached via flexible silica tubing to a fine-tipped glass capillary. Pressure was measured to ±0.001 MPa by a transducer sealed into the expansion chamber. To make a pressure measurement, a grain was removed from the plant, and the capillary tip was inserted into the endosperm cavity through the single layer of living cells (the testa) covering the cavity at the distal end of the grain. (for an illustration of relevant grain anatomy, see Wang and Fisher, 1994a). Because the volume of the probe tip entering the cavity was less than 1% of the cavity volume, which also had a low ε (see below), insertion of the probe tip had a negligible effect on cavity pressure.
To allow measured volume changes of the endosperm cavity, and to compensate for the small volume sucked from the probe tip on entering the cavity, the terminal approximately 2 cm of the glass capillary was filled with water. After entering the cavity, the water-silicone interface was drawn back to its marked pre-entry position and the pressure was recorded. Following this, the meniscus was withdrawn another 5 to 10 mm (0.02–0.04 μL), and the change in pressure, usually 0.01 to 0.02 MPa, was recorded. To measure the cavity volume, the probe was withdrawn and the cavity contents were squeezed from the grain and sucked into a preweighed micropipette. Squeezing was continued until starch grains appeared, indicating that the cavity contents had emptied, and the collecting pipette was reweighed to determine the cavity volume. The ε of the endosperm cavity was calculated as:
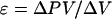 |
Manometric Measurements of Sieve Tube Turgor Pressure
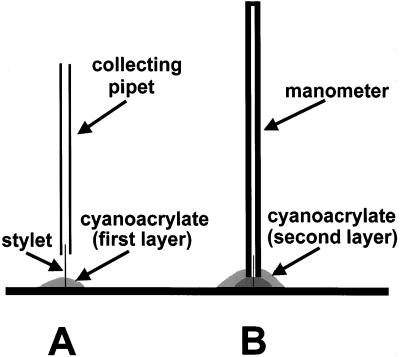
A manometric method, modified from the approach of Wright and Fisher (1980) for measurements on exuding willow aphid stylets, was used to measure sieve tube turgor pressure. The modifications, necessitated by the higher pressures, slick cuticular surface, and slower exudation rates from stylets on wheat, consisted of using smaller diameter capillary tubing and a two-step sealing procedure to obtain a stronger seal to the exuding stylet (Fig. 7). In the first step, a collecting pipette was used to collect exudate and to support the end of the stylet, while a pool of cyanoacrylate adhesive (Loctite, Rocky Hill, CT) was formed around the base of the stylet and polymerized with the vapors of an aromatic amine accelerator (Pacer Technology, Campbell, CA). A length of 20-μm i.d. tubing (Polymicro Technologies, Phoenix, AZ), approximately 15 mm long and sealed at the opposite end with epoxy, was slipped over the stylet and sealed to the first layer of cyanoacrylate with a second application of adhesive. When the gas column in the manometer was fully compressed, as indicated by the cessation of exudate movement, its length was measured by an ocular micrometer and used to calculate sieve tube turgor pressure. The calculation includes an approximate 5% correction for local altitude, as noted by Wright and Fisher (1983).
Figure 7.
A two-step procedure was used to seal a manometer to an exuding stylet. During the first step (A), a fine-tipped pipette was used to collect exudate and to support the stylet while cyanoacrylate was polymerized around the stylet base. Following this, the manometer was slipped over the stylet and sealed to the surface of the first cyanoacrylate layer (B).
After measuring the final length of the air pocket, the end of the manometer was clipped off to verify that exudation had ceased because of counterbalancing pressure in the air column and not because of plugging of the stylet or sieve tube. With few exceptions, exudation resumed, confirming sieve tube functionality. The manometer was then broken from the plant and its contents were ejected as several droplets under mineral oil to determine exudate Ψs by freezing point depression. Ψ was calculated from the turgor and osmotic measurements and compared with values obtained from microdrop measurements and from stylet exudation rates in grain detachment experiments (above).
In making measurements to be used for calculating gradients, measurements were paired, with values being determined at one site (peduncle/rachis or grain) and, within 1 to 2 h, at the other site on the same plant.
Suc Assays
Suc assays were performed by the direct fluorometric assay of Jones et al. (1977). Endosperm cavity sap was collected by cutting the distal one-fourth of a grain and sucking approximately 1 μL of cavity sap into a preweighed fine-tipped micropipette, which was reweighed on a microbalance. The Suc pool in the crease tissues was determined by removing the tissues with two parallel cuts along either side of the crease, and extracting with 1 mL of boiling water.
Footnotes
This work was supported by the National Science Foundation (grant no. IBN–9514188).
LITERATURE CITED
- Barlow EWR, Lee JW, Munns R, Smart MG. Water relations of developing wheat grains. Aust J Plant Physiol. 1980;7:519–525. [Google Scholar]
- Bradford KJ. Water stress and the water relations of seed development: a critical review. Crop Sci. 1994;34:1–11. [Google Scholar]
- Fisher DB. In situ measurement of plant water potentials by equilibration with microdroplets of polyethylene glycol 8000. Plant Physiol. 1985;79:270–273. doi: 10.1104/pp.79.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB. Measurement of phloem transport rates by an indicator-dilution technique. Plant Physiol. 1990;94:455–462. doi: 10.1104/pp.94.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB. Phloem unloading in developing wheat grains. In: Pontis HG, Salerno GL, Echeverria EJ, editors. Sucrose Metabolism, Biochemistry, Physiology and Molecular Biology. Rockville, MD: American Society of Plant Physiologists; 1995. pp. 206–215. [Google Scholar]
- Fisher DB, Cash-Clark CE. Sieve tube unloading and post-phloem transport of fluorescent tracers and proteins injected into sieve tubes via severed aphid stylets. Plant Physiol. 2000;123:125–137. doi: 10.1104/pp.123.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB, Frame JM. A guide to the use of the exuding-stylet technique in phloem physiology. Planta. 1984;161:385–393. doi: 10.1007/BF00394567. [DOI] [PubMed] [Google Scholar]
- Fisher DB, Gifford RM. Accumulation and conversion of sugars by developing wheat grains: VI. Gradients along the transport pathway from the peduncle to the endosperm cavity during grain filling. Plant Physiol. 1986;82:1024–1030. doi: 10.1104/pp.82.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB, Gifford RM. Accumulation and conversion of sugars by developing wheat grains: VII. Effects of sieve tube and endosperm cavity sap concentrations on the grain filling rate. Plant Physiol. 1987;84:341–347. doi: 10.1104/pp.84.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB, Wang N. A kinetic and microautoradiographic analysis of 14C-sucrose import by developing wheat grains. Plant Physiol. 1993;101:391–398. doi: 10.1104/pp.101.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB, Wang N. Sucrose concentration gradients along the post-phloem transport pathway in the maternal tissues of developing wheat grains. Plant Physiol. 1995;109:587–592. doi: 10.1104/pp.109.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner CF. Transport of tritiated water and 14C-labeled assimilate into grains of wheat: III. Diffusion of THO through the stalk. Aust J Plant Physiol. 1985;12:595–608. [Google Scholar]
- Jones MGK, Outlaw WH, Jr, Lowry OH. Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol. 1977;60:379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermode AR. Regulatory mechanisms involved in the transition from seed development to germination. CRC Crit Rev Plant Sci. 1990;9:155–195. [Google Scholar]
- Lang A. Xylem, phloem and transpiration flows in developing apple fruits. J Exp Bot. 1990;41:645–651. [Google Scholar]
- Lang A, Thorpe MR. Xylem, phloem and transpiration flows in a grape: application of a technique for measuring the volume of attached fruits to high resolution using Archimedes' principle. J Exp Bot. 1989;40:1069–1078. [Google Scholar]
- Luttrell ES. Host-parasite relationships and development of the ergot sclerotium in Claviceps purpurea. Can J Bot. 1980;58:942–958. [Google Scholar]
- O'Brien TP, Sammut ME, Lee JW, Smart MG. The vascular system of the wheat spikelet. Aust J Plant Physiol. 1985;12:487–511. [Google Scholar]
- Tyree MT, Christy AL, Ferrier JM. A simpler iterative steady-state solution of Munch pressure-flow systems applied to long and short translocation paths. Plant Physiol. 1974;54:589–560. doi: 10.1104/pp.54.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbot V. Control mechanisms for plant embryogeny. In: Clutter ME, editor. Dormancy and Developmental Arrest. New York: Academic Press; 1978. pp. 114–167. [Google Scholar]
- Wang N, Fisher DB. Monitoring phloem unloading and post-phloem transport by microperfusion of attached wheat grains. Plant Physiol. 1994a;104:7–16. doi: 10.1104/pp.104.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Fisher DB. The use of fluorescent tracers to characterize the post-phloem transport pathway in maternal tissues of developing wheat grains. Plant Physiol. 1994b;104:17–27. doi: 10.1104/pp.104.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate ME, Schussler JR, Reicosky DC, Brenner ML. Effect of water deficits on seed development in soybean: II. Conservation of seed growth rate. Plant Physiol. 1989;91:980–985. doi: 10.1104/pp.91.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JP, Fisher DB. Direct measurement of sieve tube turgor pressure using severed aphid stylets. Plant Physiol. 1980;65:1133–1135. doi: 10.1104/pp.65.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JP, Fisher DB. Estimation of the volumetric elastic modulus and membrane hydraulic conductivity of willow sieve tubes. Plant Physiol. 1983;73:1042–1047. doi: 10.1104/pp.73.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee SY, O'Brien TP. A special type of tracheary element associated with ‘xylem discontinuity’ in the floral axis of wheat. Aust J Biol Sci. 1970;23:783–791. [Google Scholar]