Abstract
Background:
Cognitive fatigue can be objectively measured on tasks of sustained attention and can be defined as decreased performance as a result of sustained cognitive effort. Individuals with multiple sclerosis (MS) early in their disease are vulnerable to cognitive fatigue, although this has yet to be evaluated longitudinally. We aimed to evaluate cognitive fatigue over a 3-year interval in individuals with early-phase relapsing-remitting MS (RRMS). The sensitivity of the Paced Auditory Serial Addition Test (PASAT) at detecting cognitive fatigue was evaluated, as was the impact of scoring method.
Methods:
32 people with MS and 32 controls completed the 3- and 2-second PASAT (PASAT-3″ and -2″) as a measure of sustained attention at baseline and 3-year follow-up.
Results:
Performance on the PASAT remained stable across time, with improvement noted on the PASAT-2″ likely due to practice and the small sample size. Cognitive fatigue was noted at both times, although sensitivity varied based on scoring method. No evidence of worsening cognitive fatigue was noted over time. The MS group performed worse only when cognitive fatigue was the outcome variable.
Conclusions:
Although individuals with MS continue to be vulnerable to cognitive fatigue at follow-up, severity does not seem to increase with time. Cognitive fatigue may be a more sensitive marker of cognitive impairment than overall task performance in those with early-phase RRMS, which has important implications given that clinically only task performance is typically assessed.
Fatigue is a significant problem in multiple sclerosis (MS), occurring in as many as 90% of people diagnosed.1–3 Compared with controls, individuals with MS report more frequent and more severe levels of fatigue.4 Fatigue, considered to be one of the most debilitating symptoms of the disease, can greatly affect quality of life.5 The body of literature examining MS-related fatigue is substantial, yet the concept of fatigue is still not well understood, probably because of its multifaceted nature and limitations regarding measurement. For the most part, research has focused on the study of physical fatigue; however, cognitive fatigue is frequently just as debilitating.
Assessment of cognitive fatigue in MS has many challenges. Typically, assessment of cognitive fatigue relies on self-report measures, but these can present with limitations. Individuals are asked to rate their fatigue without adequate definition, and thus subjective measures of cognitive fatigue are inherently flawed and can be subject to recall bias.6,7 An alternative is to assess cognitive fatigue during the performance of a sustained attention task. In this way, cognitive fatigue can be objectively quantified as a breakdown in performance from the beginning to the end of the task.6,8,9 Whether cognitive fatigue can be measured objectively, however, remains a matter of debate. Although currently there is no universally accepted definition for objective cognitive fatigue, it can be defined as an inability to maintain optimal task performance throughout a sustained attention task.8,9 Because cognitive fatigue is likely a reflection of several underlying deficits (slowed processing speed, sustained attention deficits, etc.), note that this is potentially not the only way it can be operationalized. Nonetheless, we chose to remain consistent with past work6,8,9 by defining cognitive fatigue in the context of decreased performance over time to expand on the current literature.
During sustained attention tasks, people with MS demonstrate more susceptibility to the effects of cognitive fatigue compared with controls.9 This is often observed as a breakdown in performance as the task goes on. Individuals susceptible to cognitive fatigue are less able to maintain the necessary effort required to meet task demands continuously over time. Studies have shown that even during periods of substantial fatigue, healthy individuals are capable of continuously exerting effort to obtain a rewarding outcome (in this case, increased task performance).10,11 As such, Dobryakova et al.12 suggest that cognitive fatigue may be a result of an effort-reward imbalance, although to date this has yet to be evaluated in an MS sample. Despite its limitations,13 the Paced Auditory Serial Addition Test (PASAT) has been shown to serve as a sensitive and valid measure of cognitive fatigue in MS.14,15
The PASAT is generally acknowledged in the literature to be one of the most sensitive measures of information-processing speed and working memory deficits in MS.13,16 Given its demonstrated sensitivity, the PASAT is used in studies analyzing cognitive fatigue in MS where sustained attention needs to be maintained over time. Cognitive fatigue is objectively assessed by comparing early performance on the task with later performance. Cognitive fatigue has been measured using the PASAT by comparing performance on the first half versus the second half14 or on the first third versus the last third of the task.15 Individuals with MS are expected to perform worse as the task progresses, as they have a particularly difficult time processing information quickly enough. This problem increases in frequency as the task progresses, resulting in performance declines.17,18
The sensitivity of the PASAT in detecting cognitive fatigue differs depending on the scoring method.14 PASAT performance is typically evaluated by counting only the number of correct responses. Individuals may, however, use a “chunking method” whereby the first two numbers are added, the next number is skipped, the following two numbers are added, etc.16,19 This results in a reduction in the overall difficulty of the task by decreasing the need to perform simultaneous cognitive processes Although scores within normal limits may be achieved, their performance is no longer a reflection of the ability to cope with the challenging working memory demands, nor is it an accurate representation of their ability to perform the task as planned.
The number of correct dyads better highlights an individual's ability to correctly meet task demands.16,20 In this case, a correct score is given only when one correct response is immediately followed by another. The number of correct responses and the number of correct dyads, therefore, both provide a measure of performance accuracy. A percent dyad score may also be calculated, giving an indication of the proportion of time an individual is performing the task as instructed. Higher percent dyad scores reflect a greater ability to give correct responses in line with task demands.16 The percent dyad scoring method thus provides an indication of performance strategy.
Previous work by our group14 examined task performance in people with early-phase relapsing-remitting MS (RRMS) and controls on the PASAT to determine whether task performance is influenced by cognitive fatigue. Furthermore, we evaluated whether the PASAT scoring method influenced its sensitivity. When comparing performance on the first half of the task versus the second half, cognitive fatigue was apparent in the MS sample compared with controls on only the 3-second PASAT (PASAT-3″), and only with the percent dyad scoring method. This is consistent with previous work that found that the percent dyad method (ie, performance strategy) is more sensitive to cognitive fatigue than performance level alone.9 In addition, when performance was compared between the last third and the first third of the PASAT, an average of two to three fewer correct responses was noted for the MS group, suggesting that cognitive fatigue may be reliably detected using thirds on the PASAT as well. These results provided preliminary evidence that cognitive fatigue can be detected using the PASAT in individuals with MS; however, results varied based on the method used. Further expansion on this preliminary work involves determining whether the susceptibility of the MS group to cognitive fatigue changes over time. The primary goal of the present study, therefore, was to evaluate cognitive fatigue longitudinally.
The longitudinal evaluation of cognitive fatigue represents a novel area of research. Given that cognitive dysfunction has been shown to be progressive across longitudinal follow-up,21,22 it is important to determine whether an individual's susceptibility to cognitive fatigue also changes over time. The PASAT was chosen as the tool to measure cognitive fatigue in the present study given its demonstrated sensitivity at detecting information-processing speed deficits in MS, as well as its continued use as a measure of sustained attention. The 3-year duration of the test-retest interval was selected because longitudinal studies have suggested that a minimum test-retest interval of 2 to 3 years is necessary to detect cognitive changes.22,23 Performance on the PASAT was evaluated by comparing the first and last thirds of the task, thus allowing for the examination and comparison of performance at the extremes of the task, which may provide a more precise reflection of early task performance versus later performance.15
The primary objective was to examine task performance after a 3-year interval in individuals with RRMS and controls on the PASAT to determine whether task performance is influenced by cognitive fatigue and whether this influence changes over time. In addition, we examined whether the PASAT scoring method influenced its sensitivity. It was hypothesized that 1) individuals with MS would perform worse on the PASAT at 3-year follow-up compared with their performance at baseline. It was expected that the performance of the control group would remain consistent over time. Similarly, given the presumed progression of cognitive decline over time, it was hypothesized that 2) individuals with MS would perform worse overall than controls on the PASAT at follow-up. In terms of cognitive fatigue, it was hypothesized that 3) both groups would display evidence of cognitive fatigue at baseline and follow-up, but that the degree of cognitive fatigue would be greater in the MS group. Similarly, on the basis of disease progression, it was hypothesized that 4) the degree of cognitive fatigue in the MS group at follow-up would be greater than the degree noted at baseline.
Methods
Participants
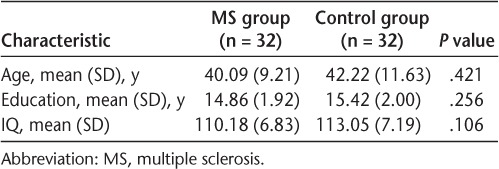
At baseline, 32 individuals with a confirmed diagnosis of RRMS24 were recruited from the MS Clinic of The Ottawa Hospital (Ontario, Canada) to complete a comprehensive battery of neuropsychological tests. All had a mild level of physical disability (mean [SD] Expanded Disability Status Scale [EDSS] score = 1.83 [1.18]) and a disease duration of less than 10 years (mean [SD] = 4.35 [3.09] years). Individuals who were experiencing an exacerbation at the time of baseline recruitment or who had had less than 28 days between the onset of improvement of all signs and symptoms attributable to an MS exacerbation were excluded. Thirty-two age-, education-, and IQ-matched controls were recruited by word of mouth from the community, as well as newspaper and website advertisements. All the participants were fluent in English and were aged 18 to 65 years. All were free of previous neurologic, medical, or psychiatric illnesses (besides MS and depression) that may have impaired cognition.
Procedure and Measures
The study was approved by The Ottawa Hospital research ethics board. After informed consent was obtained, participants completed a demographic questionnaire. At baseline, the North American Adult Reading Test was administered as an estimate of premorbid intellectual ability, with participants required to achieve an estimated IQ score of at least 90 to be considered eligible. To evaluate cognitive fatigue, the PASAT-3″ and the 2-second PASAT (PASAT-2″) were administered in the context of a larger neuropsychological battery of testing. Subjective fatigue was assessed using the Modified Fatigue Impact Scale.25
Statistical Analysis
Overview
Performance on the PASAT was compared between interstimulus intervals (ISIs) and between the first and last thirds of each trial for both groups. In addition, performance at baseline was compared with performance at 3-year follow-up. Responses were recorded, and the following scores were tallied: total number of correct responses, total dyad score, and percent dyad score. Percent dyad scores were calculated using the following formula: [1 − (total correct score − dyad score) / total correct score] × 100.9,16 To examine the degree of change in cognitive fatigue over time, a change score was calculated by subtracting performance on the first third of the PASAT from that on the last third at baseline and follow-up for all the participants. Once calculated, the change at follow-up was subtracted from the change at baseline to obtain an overall score reflective of cognitive fatigue change over time.
Each analysis was performed three times for each of the two ISIs (PASAT-3″ and PASAT-2″), once for each of the three scoring methods: total correct score, total dyad score, and percent dyad score.
Hypotheses 1 and 2
A repeated-measures analysis of variance (ANOVA) was performed to address whether the MS and control groups performed differently on the PASAT at 3-year follow-up compared with their respective performances at baseline and compared with each other.
Hypothesis 3
To evaluate cognitive fatigue, a 2 × 2 (ISI × third) repeated-measures ANOVA was performed to determine whether individuals performed differently on the last third of the PASAT compared with the first third and to examine whether this difference varied between groups or between ISIs. This analysis was repeated at baseline and follow-up.
Hypothesis 4
To evaluate whether the degree of cognitive fatigue differed between the MS and control groups and whether the degree changed over time, a 2 × 2 (group × time) repeated-measures ANOVA was performed using the cognitive fatigue change scores.
Statistical Software
A statistical software package (IBM SPSS Statistics for Windows, version 23; IBM Corp, Armonk, NY) was used for all data analyses. A significance level of α ≤ .05 was used throughout.
Results
Demographics
The MS and control groups were matched on age, education, and IQ scores. One-way ANOVAs showed no significant differences between the two groups on any of these variables (Table 1).
Table 1.
Demographic characteristics of the 64 study participants
Performance
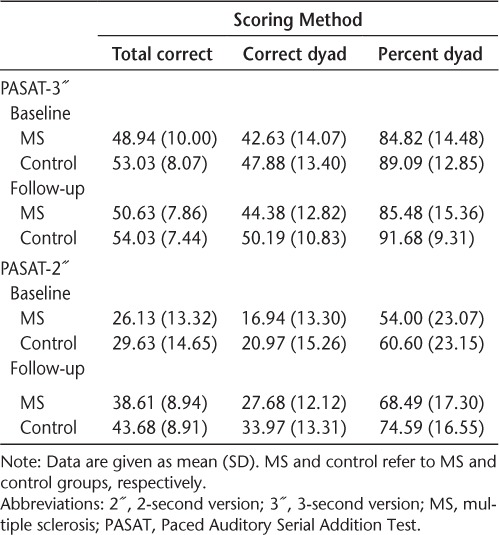
The PASAT raw performance data are listed in Table 2. At the 3″ and 2″ ISIs, no significant main effect for group was noted for either baseline or follow-up performance. Overall task performance did not differ between the MS and control groups at either time point. At the 2″ ISI, a significant main effect for time was noted (total correct: F1,62 = 125.84, P < .001; total dyad: F1,62 = 116.32, P < .001; percent dyad: F1,62 = 56.98, P < .001); however, no group × time interaction was observed. Both groups showed improved performance at follow-up. These results were consistent across all three scoring methods.
Table 2.
PASAT performance data
Cognitive Fatigue
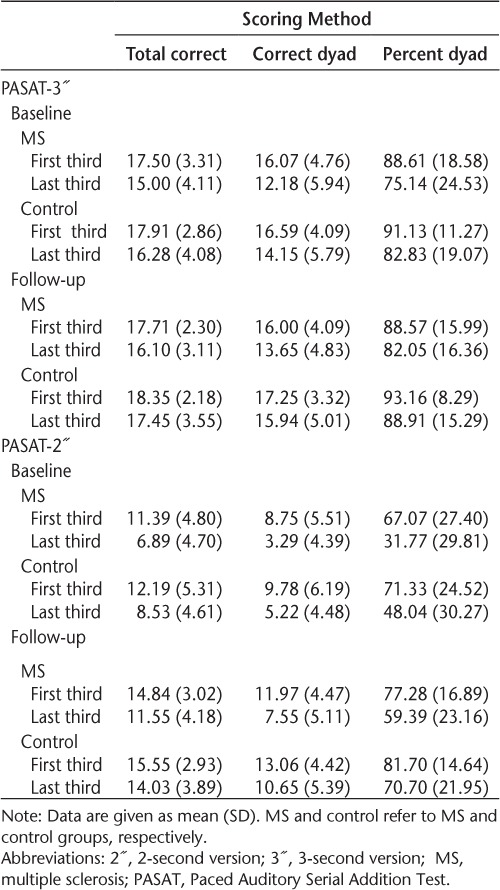
The PASAT raw performance data during the first and last third of the task are listed in Table 3. Consistent with the literature, subjective measures of fatigue (Modified Fatigue Impact Scale) and objective cognitive fatigue (PASAT) were not correlated.14,26
Table 3.
PASAT performance data on first and last thirds of task
Baseline
At baseline, a main effect for third of the task was noted across all three scoring methods (total correct: F1,58 = 151.82, P < .001; total dyad: F1,58 = 132.24, P < .001; percent dyad: F1,58 = 97.73, P < .001), indicating that individuals' performance differed between the first and last third of the task. Overall, poorer performance on the last third of the task compared with the first third was noted (ie, cognitive fatigue), although this was true for both groups. When using the percent dyad scoring method, however, a third × group interaction was observed (F1,58 = 4.48, P = .039), suggesting that the proportion of time in which the MS group met task demands was lower on the last third of the task versus the first third for the 3″ and 2″ ISIs compared with controls. In addition, an ISI × third interaction was observed across all three scoring methods (total correct: F1,58 = 17.40, P < .001; total dyad: F1,58 = 51.01, P = .015; percent dyad: F1,58 = 28.37, P < .001). Examination of the means showed poorer performance on the last third of the task at the 2″ ISI compared with the 3″ ISI for both groups, suggesting that higher-difficulty tasks are more sensitive to cognitive fatigue (although not specifically to individuals with MS).
Follow-up
Similar to baseline, at follow-up a main effect for third of the task was noted across all three scoring methods (total correct: F1,60 = 46.51, P < .001; total dyad: F1,60 = 50.43, P < .001; percent dyad: F1,60 = 43.19, P < .001). Unlike at baseline, however, a third × group interaction was observed only when using the total correct (F1,60 = 5.35, P = .024) and total dyad (F1,60 = 4.19, P = .045) scoring methods. This finding suggests that at follow-up, measures of performance accuracy rather than a measure of performance strategy yielded greater cognitive fatigue in the MS group compared with controls. Similar to baseline, an ISI × third interaction was also observed at follow-up across all three scoring methods (total correct: F1,60 = 6.56, P = .013; total dyad: F1,60 = 38.73, P = .017; percent dyad: F1,60 = 16.55, P < .001). Poorer performance continued to be noted on the last third of the task at the 2″ ISI compared with the 3″ ISI for both groups.
Degree of Change in Cognitive Fatigue
At the 3″ ISI, neither a main effect of time nor a time × group interaction was noted for any scoring method, suggesting that the degree of cognitive fatigue did not differ between the MS and control groups, and neither did the degree of cognitive fatigue change over time (Table 4).
Table 4.
Degree of change in cognitive fatigue from first to last third of task
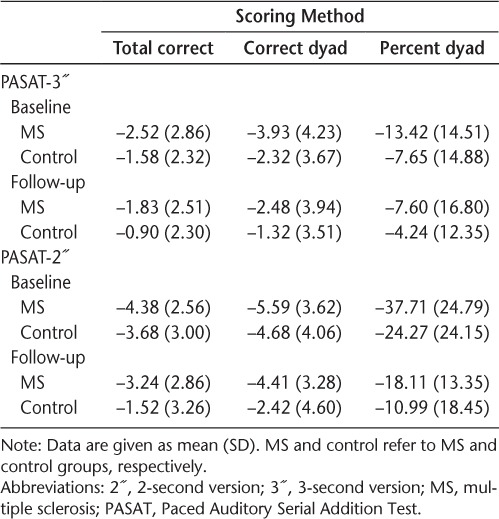
At the 2″ ISI, a main effect of group was noted on the total correct and percent dyad scoring methods (total correct: F1,58 = 5.35, P = .024; percent dyad: F1,58 = 7.04, P = .010), indicating that overall, the MS group showed a greater degree of cognitive fatigue than the control group at both time points. These results are expected given the third × group interaction seen (see earlier “Cognitive Fatigue” section). In addition, a main effect of time was also noted on all three scoring methods (total correct: F1,58 = 9.03, P = .004; total dyad: F1,58 = 6.82, P = .011; percent dyad: F1,58 = 19.73, P < .001). Examination of the means indicated that both groups showed less cognitive fatigue at follow-up compared with baseline.
Discussion
Differences in performance between the MS and control groups on the PASAT were not found at either baseline or follow-up. Although unexpected, the lack of significant group differences regarding overall PASAT performance has been observed by other groups in the past (see Johnson et al.27). Although there was a trend for the MS group in this sample to perform worse than controls at all times (Table 2), the lack of statistical significance may have been due to the small sample size and the characteristics of the MS sample. The MS group was composed of only those with RRMS who were early in their disease course (mean [SD] = 4.35 [3.09] years) and with minimal disability (mean [SD] EDSS score = 1.83 [1.18]). Although cognitive dysfunction can occur in those with early-phase RRMS,28 typically less impairment is noted than in those with more progressive subtypes.29,30
Performance across the 3-year interval remained stable at the 3″ ISI. Although improved performance was noted at the 2″ ISI at follow-up, this was true for both the MS and control groups. As such, this improvement may be attributable to practice. Past literature has demonstrated that repeated exposure to the PASAT results in improved performance. Although typically this improvement is observed over short test-retest intervals,31,32 practice effects have been noted over the span of years.33,34 It has been suggested that these practice effects are the result of experience gained during the initial administration as individuals learn what is expected during the task and develop an effective strategy to perform the task successfully. It is likely that the general procedural knowledge about the test and the development of effective strategies are retained over time, thus resulting in improved performance with subsequent exposure.13 In addition, the PASAT is often perceived as unpleasant35 and has been shown to elicit high levels of anxiety and frustration, particularly when the test is novel.36 However, the novelty of the task diminishes with repeated exposure and, thus, the anxiety effects associated with the test are likely reduced. As such, individuals may have performed better at follow-up simply because they felt less anxious throughout the task.
Evidence of objective cognitive fatigue was noted in both the MS and control groups at baseline (Table 3). Both groups demonstrated poorer performance later in the task compared with performance at the beginning. There was a trend for the more difficult task (2″ ISI) to be more sensitive to cognitive fatigue (Table 3). Although both groups demonstrated cognitive fatigue, the scoring method most sensitive to group differences at baseline was the percent dyad score (ie, performance strategy). As such, the proportion of time in which the MS group was successfully able to meet task demands at baseline diminished as the task progressed, perhaps due to a greater vulnerability to cognitive fatigue compared with controls.
Similar to baseline, cognitive fatigue was noted in the MS and control groups at follow-up. Unlike at baseline, the total correct and total dyad scoring methods were most sensitive at follow-up. This finding suggests that at follow-up, measures of performance accuracy are more sensitive to cognitive fatigue in the MS group than are measures of performance strategy. Taken together, these results support the notion that at baseline individuals are still in the process of refining their performance strategy, while the task is still novel. At follow-up, however, their strategy is presumed to be well developed given their previous exposure to the task and, as such, measures of performance accuracy are better able to detect cognitive fatigue.
The same degree of cognitive fatigue from the beginning to the end of the task was noted for both groups at baseline and follow-up with the 3″ ISI. At the 2″ ISI, however, the MS group showed a greater degree of cognitive fatigue than did controls at both time points, although these results were inconsistent across all scoring methods. In addition, results showed that both groups had less cognitive fatigue at follow-up. Although cognitive fatigue was noted in both groups at both times, there was a greater trend for both groups to produce more correct responses at the end of the task at follow-up. As such, although both groups displayed diminishing performance from the start of the task to the end, the magnitude of this decline (ie, the degree of cognitive fatigue) was smaller at follow-up. Given that the task is more familiar at follow-up, future research should examine whether novel tasks are more sensitive to cognitive fatigue than are more rote or practiced tasks. Whether subjective ratings of cognitive fatigue differed between the MS and control groups is an important factor to consider, as Sandry et al.26 showed that these subjective reports tended to be higher in the MS group compared with controls and were greater with increased task lengths. Although this was not explicitly examined in this study, future research should take these subjective reports into consideration.
Overall, little evidence of progression of cognitive impairment over time was noted in this sample of patients with early-phase RRMS. Performance on the PASAT remained stable across the 3-year interval, with improvement noted at the 2″ ISI likely due to practice. Cognitive fatigue was noted at both time points in the present MS sample, although the sensitivity of the PASAT in detecting cognitive fatigue at each time point varied based on the scoring method used. At baseline, a measure of performance strategy was more sensitive to cognitive fatigue (ie, percent dyad), whereas at follow-up measures of performance accuracy were more sensitive (ie, total correct and total dyad). As such, researchers should consider the importance of scoring method when designing and conducting studies investigating cognitive fatigue because sensitivity seems to vary. No evidence of worsening cognitive fatigue was noted across the 3-year interval, with an improvement in vulnerability to cognitive fatigue noted in both the MS and control groups at the 2″ ISI.
Interestingly, group differences were noted only when one considers cognitive fatigue as the outcome measure. This finding suggests that cognitive fatigue, rather than measures of performance, may be a more sensitive marker of cognitive impairment in those with early-phase RRMS. People may be able to compensate for a time regarding overall performance levels, but subtle deficits are revealed only with a finer-grained analysis of the qualitative aspects of their performance. These results support the subjective claims by those with the disease who state that they can perform their jobs or day-to-day tasks quite well (ie, have high performance), although they feel that they have to work harder to achieve an adequate level of performance and, thus, are more mentally exhausted toward the end of the day (ie, experience cognitive fatigue). This has important implications given that clinically only task performance is typically assessed. As such, clinicians should be aware that a finer-grained analysis of the qualitative aspects of that performance, as in the case of cognitive fatigue, may be more prudent given the real-world implications; this is particularly important for individuals with MS who are early in their disease course.
PRACTICE POINTS
Cognitive fatigue can be defined as decreased performance as a result of sustained cognitive effort. Individuals with MS early in their disease are vulnerable to cognitive fatigue, although this has yet to be evaluated longitudinally.
We administered an objective measure of cognitive fatigue to 32 individuals with relapsing-remitting MS and 32 controls at baseline and 3-year follow-up. Although individuals with MS continued to be vulnerable to cognitive fatigue over time, the severity does not seem to increase.
Cognitive fatigue may be a more sensitive marker of cognitive impairment than overall task performance in those early in their disease course.
Acknowledgments:
The authors acknowledge the time and effort put forth by all the study participants. Their contributions are much appreciated.
Financial Disclosures:
The authors have no conflicts of interest to disclose.
Funding/Support:
This study was financially supported by the Multiple Sclerosis Society of Canada.
References
- 1. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC.. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006; 12: 24– 38. [DOI] [PubMed] [Google Scholar]
- 2. Brassington JC, Marsh NV.. Neuropsychological aspects of multiple sclerosis. Neuropsychol Rev. 1998; 8: 43– 47. [DOI] [PubMed] [Google Scholar]
- 3. Kinkel PR. Fatigue in multiple sclerosis: reducing the impact through comprehensive management. Int J MS Care. 2000; 2: 3– 10. [Google Scholar]
- 4. Paul RH, Beatty WW, Schneider R, Blanco CR, Hames KA.. Cognitive and physical fatigue in multiple sclerosis: relations between self-report and objective performance. Appl Neuropsychol. 1998; 5: 143– 148. [DOI] [PubMed] [Google Scholar]
- 5. Opara JA, Jaracz K, Brola W.. Quality of life in multiple sclerosis. J Med Life. 2010; 3: 352– 358. [PMC free article] [PubMed] [Google Scholar]
- 6. Schwid SR, Tyler CM, Scheid EA, Weinstein A, Goodman AD, McDermott MP.. Cognitive fatigue during a test requiring sustained attention: a pilot study. Mult Scler. 2003; 9: 503– 508. [DOI] [PubMed] [Google Scholar]
- 7. Cohen JA, Fischer JS, Bolibrush DM, . et al. Intrarater and interrater reliability of the MS functional composite outcome measure. Neurology. 2000; 54: 802– 806. [DOI] [PubMed] [Google Scholar]
- 8. Schwid SR, Covington M, Segal BM, Goodman AD.. Fatigue in multiple sclerosis: current understanding and future directions. J Rehabil Res Dev. 2002; 39: 211– 224. [PubMed] [Google Scholar]
- 9. Bryant D, Chiaravalloti ND, DeLuca J.. Objective measurement of cognitive fatigue in multiple sclerosis. Rehabil Psychol. 2004; 49: 114– 122. [Google Scholar]
- 10. Boksem MA, Meijman TF, Lorist MM.. Mental fatigue, motivation and action monitoring. Biol Psychol. 2006; 72: 123– 132. [DOI] [PubMed] [Google Scholar]
- 11. Lorist MM, Bezdan E, Ten Caat M, Span MM, Roerdink JB, Maurits NM.. The influence of mental fatigue and motivation on neural network dynamics: an EEG coherence study. Brain Res. 2009; 1270: 95– 106. [DOI] [PubMed] [Google Scholar]
- 12. Dobryakova E, DeLuca J, Genova HM, Wylie GR.. Neural correlates of cognitive fatigue: cortico-striatal circuitry and effort-reward imbalance. J Int Neuropsychol Soc. 2013; 19: 849– 853. [DOI] [PubMed] [Google Scholar]
- 13. Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch Clin Neuropsychol. 2006; 21: 53– 76. [DOI] [PubMed] [Google Scholar]
- 14. Walker LAS, Berard JA, Berrigan LI, Rees LM, Freedman MS.. Detecting cognitive fatigue in multiple sclerosis: method matters. J Neurol Sci. 2012; 316: 86– 92. [DOI] [PubMed] [Google Scholar]
- 15. Morrow SA, Rosehart H, Johnson AM.. Diagnosis and quantification of cognitive fatigue in multiple sclerosis. Cogn Behav Neurol. 2015; 28: 27– 32. [DOI] [PubMed] [Google Scholar]
- 16. Fisk JD, Archibald CJ.. Limitations of the Paced Auditory Serial Addition Test (PASAT) as a measure of working memory in patients with multiple sclerosis. J Int Neuropsychol Soc. 2001; 7: 363– 372. [DOI] [PubMed] [Google Scholar]
- 17. DeLuca J, Johnson S, Natelson BH.. Information processing efficiency in chronic fatigue syndrome and multiple sclerosis. Arch Neurol. 1993; 50: 301– 304. [DOI] [PubMed] [Google Scholar]
- 18. Forn C, Belenguer A, Pacet-Ibars MA, Ávila C.. Information-processing speed is the primary deficit underlying poor performance of multiple sclerosis patients in the Paced Auditory Serial Addition Test (PASAT). J Clin Exp Neuropsychol. 2008; 30: 789– 796. [DOI] [PubMed] [Google Scholar]
- 19. Snyder PJ, Aniskiewicz AS, Synder AM.. Quantitative MRI correlates and diagnostic utility of multi-modal measures of executive control in multiple sclerosis. J Clin Exp Neuropsychol. 1993; 15: 18. [Google Scholar]
- 20. Snyder PJ, Cappelleri JC, Archibald CJ, Fisk JD.. Improved detection of differential information-processing speed deficits between two disease-course types of multiple sclerosis. Neuropsychology. 2001; 15: 617– 625. [PubMed] [Google Scholar]
- 21. Amato MP, Ponziani G, Siracusa G, Sorbi S.. Cognitive dysunction in early-onset multiple sclerosis: a reappraisal after 10 years. Arch Neurol. 2001; 58: 1602– 1606. [DOI] [PubMed] [Google Scholar]
- 22. Kujala P, Portin R, Ruutiainen J.. The progress of cognitive decline in multiple sclerosis: a controlled 3-year follow-up. Brain. 1997; 120: 289– 297. [DOI] [PubMed] [Google Scholar]
- 23. Bernardin L, Rao S, Luchetta TL.. A prospective, long-term, longitudinal study of cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol. 1993; 15: 17. [Google Scholar]
- 24. McDonald WI, Compston A, Edan G, . et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001; 50: 121– 127. [DOI] [PubMed] [Google Scholar]
- 25. Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RW.. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci. 2013; 331: 102– 107. [DOI] [PubMed] [Google Scholar]
- 26. Sandry J, Genova HM, Dobryakova E, DeLuca J, Wylie G.. Subjective cognitive fatigue in multiple sclerosis depends on task length. Front Neurol. 2014; 5: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson SK, Lange G, DeLuca J, Korn LR, Natelson B.. The effects of fatigue on neuropsychological performance in patients with chronic fatigue syndrome, multiple sclerosis, and depression. Appl Neuropsychol. 1997; 4: 145– 153. [DOI] [PubMed] [Google Scholar]
- 28. Ruggieri RM, Palermo R, Vitello G, Gennuso M, Settipani N, Piccoli F.. Cognitive impairment in patients suffering from relapsing-remitting multiple sclerosis with EDSS ≤ 3.5. Acta Neurol Scand. 2003; 108: 323– 326. [DOI] [PubMed] [Google Scholar]
- 29. Huijbregts SCJ, Kalkers NF, de Sonneville LMJ, De Groot V, Reuling IEW, Polman CH.. Differences in cognitive impairment of relapsing remitting, secondary, and primary progressive MS. Neurology. 2000; 63: 335– 339. [DOI] [PubMed] [Google Scholar]
- 30. Ghajarzadeh M, Jalilian R, Eskandari G, Sahraian MA, Aszimi A, Mohammadifar M.. Fatigue in multiple sclerosis: relationship with disease duration, physical disability, disease pattern, age and sex. Acta Neurol Belg. 2013; 113: 411– 414. [DOI] [PubMed] [Google Scholar]
- 31. Feinstein A, Brown R, Ron M.. Effects of practice of serial tests of attention in healthy subjects. J Clin Exp Neuropsychol. 1994; 16: 436– 447. [DOI] [PubMed] [Google Scholar]
- 32. McCaffrey RJ, Westervelt HJ, Haase RF.. Serial neuropsychological assessment with the National Institute of Mental Health (NIMH) AIDS Abbreviated Neuropsychological Battery. Arch Clin Neuropsychol. 2001; 16: 9– 18. [PubMed] [Google Scholar]
- 33. Di Stefano G, Radanov BP.. Course of attention and memory after common whiplash: a two-years prospective study with age, education and gender pair-matched patients. Acta Neurol Scand. 1995; 91: 346– 352. [DOI] [PubMed] [Google Scholar]
- 34. Fischer JS, Priore RL, Jacobs LD, . et al. ; Multiple Sclerosis Collaborative Research Group Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Ann Neurol. 2000; 48: 885– 892. [PubMed] [Google Scholar]
- 35. Walker LAS, Cheng A, Berard J, Berrigan LI, Ress LM, Freedman MS.. Tests of information processing speed: what do people with multiple sclerosis think about them? Int J MS Care. 2012; 14: 92– 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holdwick DJ, Wingengeld SA.. The subjective experience of PASAT testing: does the PASAT induce negative mood? Arch Clin Neuropsychol. 1999; 14: 273– 284. [DOI] [PubMed] [Google Scholar]


