Abstract
While the envelope glycoprotein of vesicular stomatitis virus (VSV-G) is widely used for pseudotyping of lentiviral vectors, sub-optimal gene transfer into certain cell types and its sensitivity to inactivation by human complement hinders its broader applications. To find alternative candidates, here we evaluated two serologically distinct novel viral envelopes derived from Chandipura (CNV-G) and Piry (PRV-G) vesiculoviruses. Both permitted generation of high titer psuedotyped lentiviral vectors with a capacity for high efficiency gene transfer into various cell types from different species. In human lymphoid and hematopoietic stem cells, their transduction efficiency was significantly lower than that of VSV-G. However, both novel envelopes were found to be more resistant to inactivation by human serum complement compared to VSV-G. Thus CNV-G and PRV-G envelopes can be harnessed for multiple uses in the future based on the cell type that needs to be gene transduced and possibly for in vivo gene transfer.
Keywords: Lentiviral vector pseudotyping, Chandipura and Piry viral glycoproteins, Gene transduction with pseudotyped viral vectors, Complement resistant lentiviral vectors
INTRODUCTION
Gene therapy strategies are currently being widely evaluated for the treatment of genetic disorders, cancer, neurological conditions and immuno-deficiency diseases including AIDS [1–6]. Instead of using typical drugs for treatment, gene therapy involves introduction of transgenes into host cells to enable expression of therapeutic proteins or nucleic acids, such as siRNAs to restore normal functions or mediate anti-viral protection. Viral vectors are commonly used for gene transfer, of which lentiviral vectors (LV) constitute the most popular vehicles for delivering transgenes due to their capacity to transduce non-dividing and terminally differentiated cells and the ability to maintain stable long-term expression of transgenes [5, 7, 8]. Due to the inherent fragility of their native envelopes, lentiviral viral vectors are routinely pseudotyped via transient transfection with more stable foreign viral envelope proteins with different cell tropisms [9].
During the last two decades, several heterologous viral envelopes have been tested for lentiviral vector pseudotyping to facilitate efficient gene transfer into various cell types [9–19]. Among the first to be used were the ones from amphotropic retroviruses which were soon followed by broader host tropic viral envelopes that included vesicular stomatitis virus [9, 20–22]. The preference for pseudotyping a vector with a particular envelope depends on the purported use of the vector in a particular cell type. For example, Rabies virus glycoprotein shows more efficient gene delivery to the nervous system, compared with VSV-G pseudotypes [11]. Other envelope proteins employed for lentiviral vector pseudotyping include those from the measles virus, baboon retrovirus, filovirus, baculovirus, Nipah virus, Ross river virus and Cocal virus among others [10, 15, 16, 19, 23–25]. Relative to VSV-G, some of these alternative envelope proteins achieved comparable or better gene delivery efficiencies into certain tissues or cells, such as airway epithelia and resting hematopoietic stem cells [10, 23, 26].
As the work on gene therapy strategies progress and lentiviral vectors continue to play a major role in gene transduction into diverse set of target cells, there is a growing need to identify and employ alternative viral envelopes for pseudotyping these vectors. Since VSV-G is still the most commonly used heterologous envelope for pseudotyping lentiviral vectors due to its broad host tropism and stability, here we sought to identify and develope related viral envelopes and evaluate their ability to generate high titer vector stocks and transduce cells from different species. For this reason we chose viral envelopes from the Chandipura and Piry viruses that belong to the same vesiculovirus genus [27]. Piry virus was originally isolated from an opossum in Brazil and is known to cause human generalized infection [28]. Chandipura virus is an emerging pathogen in the tropical areas of India that usually causes severe encephalitis in children [29, 30]. In contrast, VSV is primarily a livestock pathogen. Based on these viruses’ natural history, different cell tropisms are expected, with Chandipura virus to be more neurotropic. Another advantage with the use of these new envelopes is that they are immunologically non-cross reactive with VSV-G and thus permitting their use in vivo in a sequential manner as an alternative to VSV-G pseudotyped vectors for boosting gene delivery and dosage as becomes necessary [28, 31]. Here our results show that lentiviral vectors could be efficiently pseudotyped with the envelopes from both these viruses, there are tropism and transduction efficiency differences based on cell types, that the new envelopes display stability for freeze thawing and finally, they are relatively more resistant to exposure to human serum.
MATERIALS AND METHODS
Cells and envelope expression plasmids
HEK293T cell line was used to generate pseudotyped lentiviral vectors with different vesiculovirus envelopes. Different adherent cell types, namely GHOST, HeLa, BHK, MDCK and N2a cells were used for evaluating vector transductions. These cells were cultured and maintained in DMEM containing 10% FBS. Non-adherent cell lines consisted of human T lymphoid cells Sup-T1, CEM and Jurkat. These cells were maintained in RPMI media supplemented with 10% FBS. Human peripheral blood-derived mononuclear cells (PBMC) from unidentified donors were cultured in RPMI media supplemented with 10% FBS and IL-2. For hematopoietic stem cell transductions, human fetal liver derived CD34 cells were used which were cultivated in IL-3, IL-6 and SCF cytokine media. Piry and Chandipura viruses were obtained from Dr. R. E. Shope, University of Texas, Galveston. Viral G protein genes were amplified by RT-PCR and cloned into pTARGET expression plasmid containing CMV promoter. The heterologous envelope gene containing plasmids VSV-G, CNV-G and PRV-G were used to produce pseudotyped lentiviral vectors. The third generation lentiviral vector backbone contains an EGFP reporter [1, 21, 32].
Vector production, concentration and titration
A standard lentiviral vector production protocol was used as described previously [1, 21, 32]. Briefly, plasmids encoding the transfer vector with the EGFP reporter, rev, packaging proteins and the heterologous envelope were co-transfected into HEK293T cells. Vector supernatants were collected at 24, 48 and 72 hours after transfection and concentrated by ultra-centrifugation. Vector titers were determined by FACS analysis of transduced cells as described previously [1, 21, 32]. For infecting different cell types at a certain MOI, the HEK293T cell titers were used to depict the input MOI.
Determination of gene transduction efficiency of pseudotyped vectors in different cell types
For adherent cell vector transductions, HeLa, BHK, GHOST, MDCK and mouse neuroblastoma cell line N2a were used. Briefly, 1×105 cells were transduced with MOI of 0.5 and 1 in the presence of polybrene (8 µg/ml), except for N2a where an MOI of 1 and 5 were evaluated. As mentioned above, the MOIs depicted for transducing each cell type were based on TU titers obtained from vector titration on HEK293T cells. Four hours after incubation with the vector, the cells were cultured for 72 hours followed by FACS analysis for GFP expression. For nonadherent cell transductions, the human T cell lines CEM, Sup-T1 and Jurkat cells were used. MOI of 1, 5 and 10 were evaluated. For transduction of PBMC, the cells were cultured in the presence of IL-2 and PHA for 72 hours to derive lymphoblasts. Half a million expanded PBMC were exposed to the vectors at MOI of 1, 5 and 10 for 4 hours. Post-transduction, cells were cultured for 72 hours followed by FACS analysis for GFP expression. To determine the transduction efficiencies on different cell types in PBMC, various cell surface markers were used and analyzed by FACS. Briefly, cells were stained with CD3 Alexa Fluor 700, CD4 PE-Cy5, CD8 Pacific Blue, D19 PE and CD56 PE-Cy7 (all antibodies were obtained from BD Pharmingen). Stained samples were analyzed on Coulter CANTO II FACS instrument to determine GFP expression in various cell lineages. To determine gene transduction efficiency in human hematopoietic cells, human fetal liver derived CD34 cells were used. The cells were cultured in cytokine media containing IL-3, IL-6 and SCF, 10 ng/ml each. For transduction, 1×105 cells were exposed to the vector at MOI of 1, 5 and 10 in the presence of 8 µg/ml polybrene for 4 hours followed by media change. Transduced cells were analyzed by FACS for GFP expression 72 hours later.
Determination of pseudotyped vector sensitivity to human serum complement
To determine the vector sensitivity to human serum complement components, vector preparations were exposed to control and heat inactivated human sera [15, 16]. DMEM media was used as non-serum control for vector exposure. Five different human sera were used. Briefly, 1×107 TU of the vector was mixed with the respective sera and incubated for 1 hour at 37°C. Later the exposed vector suspension was titered on HEK293T cells and assayed by FACS for GFP. The titer values seen with DMEM media exposure were set as baseline and compared with those of exposure to heat-inactivated and non-inactivated sera treatment.
Statistical analysis
To assess the significance of differences seen among the three pseudotypes, statistical analysis was used to evaluate data from multiple experiments using GraphPad Prism version 6 (GraphPad Software, USA). Student’s unpaired t-test was used to compare the transduction efficiencies. Two-way ANOVA grouped test was used to evaluate the resistance to freeze/thaw cycles. Mann-Whitney u test was used to analyze the resistance to human serum complement. P values less than 0.05 were considered to be significant.
RESULTS
Lentiviral vectors can be pseudotyped with Chandipura and Piry virus envelope glycoproteins to generate high titer vector stocks
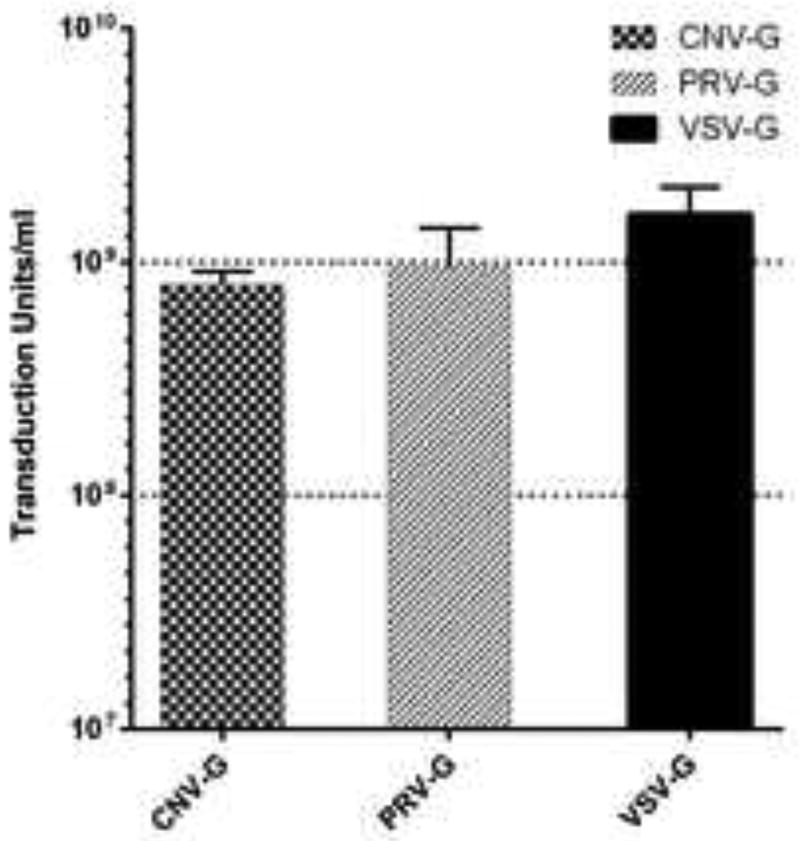
To determine if the envelope proteins of vesiculoviruses Chandipura and Piry can pseudotype lentiviral vectors, we produced vector particles by a standard plasmid transfection protocol. For comparison VSV-G envelope was also used in parallel. Cell culture supernatants (titers around 1 × 106 TU/ml) were concentrated approximately 500–1000 fold by ultra-centrifugation. The concentrated viral stocks were assayed by titration in HEK293T cells. The titers ranged from 6.9 × 108 to 9.1 × 108 TU/ml for CNV-G pseudotypes and 5.0 × 108 to 1.4 × 109 TU/ml for PRV-G pseudotypes (Figure 1).The titers observed for the VSV-G pseudotypes were in the range of 1.1 × 109 to 2.0 × 109 TU/ml (Figure 1). These results showed that high titer pseudotyped lentiviral vectors can be produced with both the novel vesiculovirus envelopes.
Figure 1. Determination of vector titers.
Vector titers were assayed on HEK293T cells. Dilutions of the vector preparations were used to infect 5×105 cells/well and the cells were later assayed for GFP expression at 72 hours post transduction to determine the titer.
CNV-G and PRV-G pseudotyped lentiviral vectors can transduce different cell types at varying efficiencies
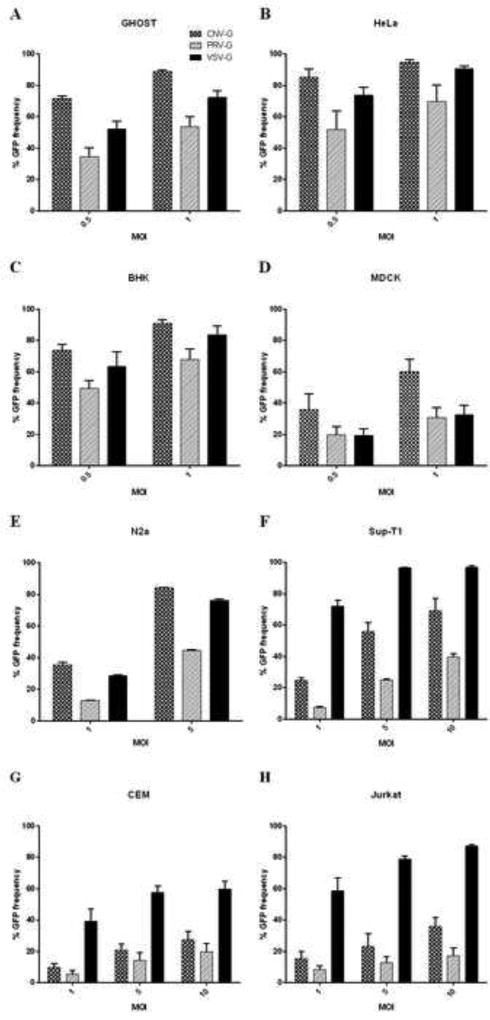
To determine the gene-delivery efficacies of the novel CNV and PRV glycoprotein pseudotyped lentiviral vectors, we employed various lab adapted cell lines from different species that encompassed both adherent and non-adherent cells. VSV-G pseudotyped vector was also used in parallel for comparison. The adherent cells consisted of epithelial, fibroblast or neuroblast origin (GHOST, HeLa, BHK, MDCK and N2a cells) whereas the non-adherent cells were of lymphocytic lineage (Sup-T1, CEM, Jurkat cells). The HeLa, GHOST and T lymphocyte cell lines are of human origin whereas the BHK, MDCK and N2a cells are of hamster, canine and murine origin, respectively. MOI equivalents, as based on HEK293T titers, of 0.5, 1 or 5 were used to transduce adherent cells. Varying efficacies of transduction with different viral envelopes could be seen with increased transduction with increased MOI. Relative to the VSV-G envelope, CNV-G was more efficient in transducing GHOST (88% transduction) and MDCK cells (60% transduction) with 1.2 to 2 fold increase whereas similar efficiency were seen with HeLa and BHK cells (85 to 95% transduction) at an MOI of 1 (Figure 2A–D). By comparison, the PRV-G was less efficient in transducing all adherent cells than VSV-G (30 to 80% transduction efficiency, depending on cell type). For transducing neuroblastoma cells CNV-G was found to be better than VSV-G (85% for CNV-G compared to 75% for VSV-G, at an MOI of 5), whereas PRV-G showed only moderate transduction ability (45% transduction at an MOI of 5) (Figure 2E). With regard to the cells of lymphocytic origin tested at MOI of 1, 5 and 10, both CNV-G and PRV-G were less efficient than VSV-G (60 to 95% for VSV-G compared to 30 to 70% for CNV-G and 15 to 40% for PRV-G, at an MOI of 10), although CNV-G was found to be relatively more efficient than PRV-G (1.3 to 2.4 fold) (Figure 2F–H).
Figure 2. Transduction efficiencies of CNV-G, PRV-G and VSV-G pseudotyped lentiviral vectors in different cell types.
(A–E) Transduction of adherent cells GHOST, HeLa, BHK, MDCK and N2a. Cells were exposed to MOI of 0.5, 1 or 5 for 4 hours and cultured for 72 hours before assaying for GFP expression. Percent transduction of different cell types are indicated. Transduction efficiency of CNV-G was significantly higher than VSV-G in GHOST, MDCK and N2a cells (p<0.05) (F–H) Transduction of non-adherent cells. Human lymphoid cells (Sup-T1, CEM and Jurkat cells) were transduced at MOI of 1, 5 and 10 for 4 hours and evaluated for GFP expression at 72 hours. Percent transduction of different cell types are indicated. Note that the MOIs depicted for input vector here are based on titers in HEK293T cells.
Gene transduction efficiencies of CNV-G and PRV-G in human PBMC
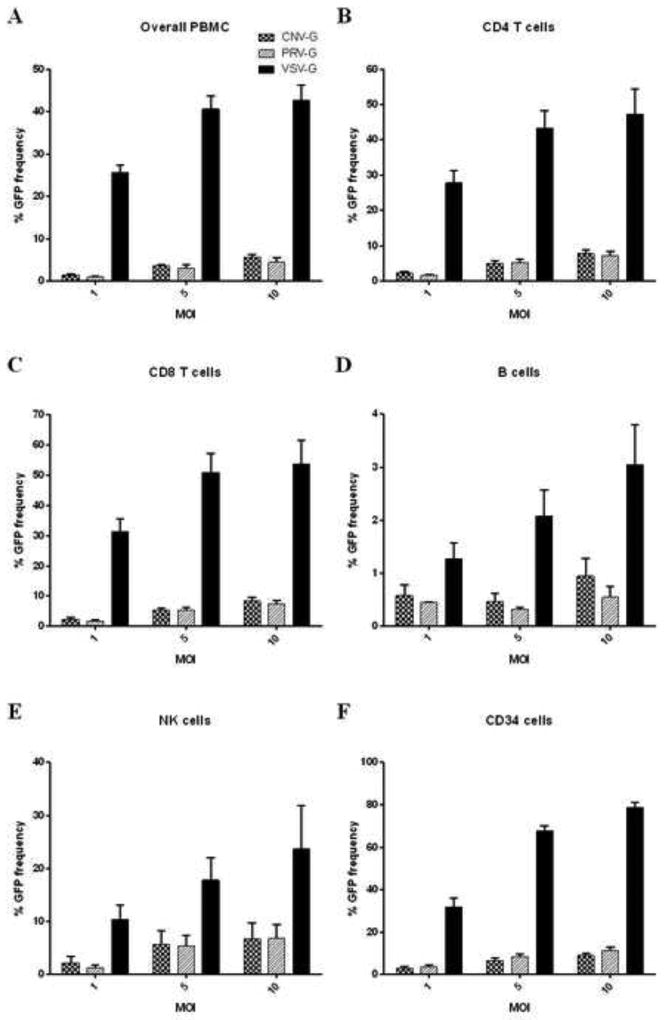
While most current gene therapy strategies involve ex vivo gene transduction with lentiviral vectors on purified target cells, future outlook is focused on direct systemic injection of vectors for in vivo application. In this case, the PBMC would be the major frontier cells to come in contact with these lentiviral vectors. PBMC is a mixed population of cell types that include T and B lymphocytes, monocytes/macrophages and NK cells. Here we evaluated the transduction efficiency of the new pseudotyped vectors on the mixed population of PBMC to determine which cell type is most efficiently transduced. PBMC from four different donors were tested. When the total PBMC were evaluated for gene transduction by FACS for GFP expression, VSV-G was found to be more efficient than that of either CNV-G or PRV-G (42% for VSV-G, compared to 5 to 7% for PRV-G and CNV-G at an MOI of 10- 6 to 8 fold increase) (Figure 3A). When individual cell types were analyzed, VSV-G was again found to be more efficient in all cell types analyzed, namely, CD4 T, CD8 T, NK and B cells, with B cells being the least efficient cell type for gene transduction (Figure 3B–E). In general, higher MOI resulted in higher transduction levels.
Figure 3. Transduction efficiencies of CNV-G, PRV-G and VSV-G vector pseudotypes in human peripheral blood-derived mononuclear cells (PBMC) and CD34 hematopoietic stem cells.
(A–E) PBMC and (F) CD34 cells were transduced with different vector pseudotypes at MOI of 1, 5 and 10 in the presence of polybrene for 4 hours. After media change, the cells were cultured for 72 hours, stained for CD4, CD8, D19, CD56 and GFP expression assayed by FACS to determine the levels of overall transduction and that of in respective cell types. Percent transduction for each pseudotype are indicated. Note that the MOIs depicted for input vector here are based on titers in HEK293T cells.
Transduction efficiencies on human hematopoietic stem cells (CD34 cells)
For sustained transgene expression in the cells of hematopoietic lineage, VSV-G pseudotyped lentiviral vectors are routinely used to transducer CD34 hematopoietic stem cells. Here we evaluated CNV-G and PRV-G to determine their efficiency on these cells at MOI of 1, 5 and 10. Cells from different donors were used in separate set of experiments to verify the results. Increasing transduction efficiencies were seen as expected with increased MOI with all three pseudotypes (Figure 3F). Whereas VSV-G pseudotype showed an impressive transduction efficiency reaching up to 80%, the CNV-G and PRV-G pseudotypes showed much lower efficiencies of 10% and 15% respectively, at an MOI of 10. These data demonstrate that CNV-G and PRV-G have a narrower cell tropism compared to the broad cell tropic VSV-G.
Pseudotyped vector stability during freeze/thaw cycles
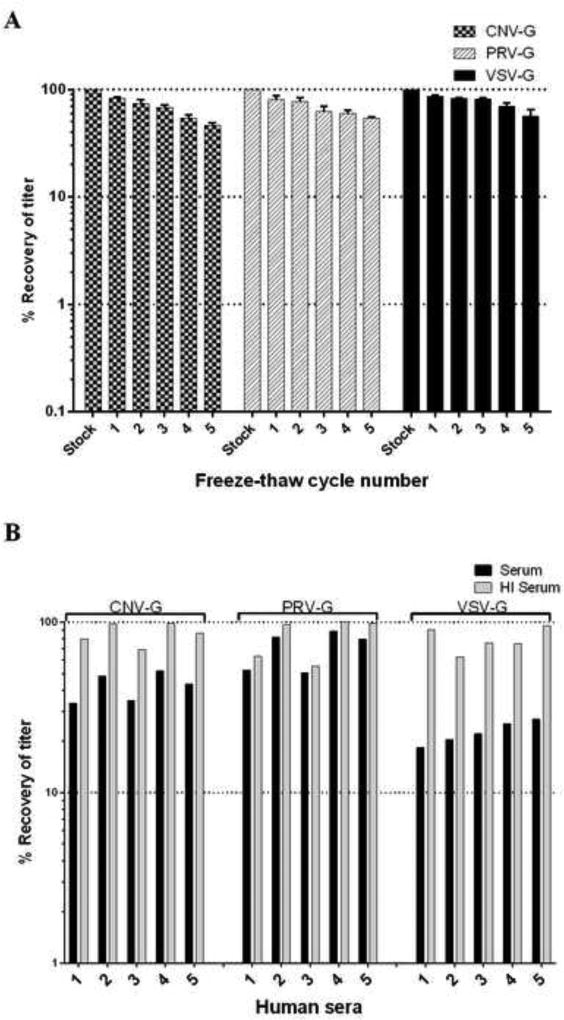
One of the practical considerations for lentiviral-based gene therapy is the stability of pseudotyped HIV-1 vectors during storage and freeze/thaw cycles [13, 21]. This aspect was examined by vector titration on HEK293T cells, after cycling between −80°C and 37°C successively for five times. The relative titer at each freeze/thaw cycle was compared to the starting point titer for each vector pseudotype and is presented in Figure 4A. All three pseudotypes behaved similarly, with moderate resistance to freeze/thaw cycles showing about a 10% decrease in titer after each cycle. There was no significant difference in the stability among the three pseudotypes (p>0.05). After five freeze/thaw cycles, all pseudotypes still maintained 50% of their original titers, indicating their relative resistance to the damage from freeze/thaw cycling.
Figure 4. Resistance of CNV-G, PRV-G and VSV-G pseudotyped lentiviral vectors to freeze/thaw cycles and human serum complement inactivation.
(A) To determine the stability of different pseudotyped vectors during freeze-thaw cycles, the vector stocks were frozen and thawed 5 times and later titrated on HEK293T cells as described in Methods. Results presented are from three different experiments. (B) To determine the vector sensitivity to human serum complement, vector preparations were exposed to control and heat inactivated human sera for 1 hour at 37°C. Later, the exposed vector suspensions were titered on HEK293T cells. Titer values obtained from DMEM media exposure alone were set as baseline and compared with those of exposure to heat inactivated and non-inactivated sera treatment.
Resistance to inactivation by human sera
VSV-G pseudotyped vectors are prone to inactivation by human serum complement, thus limiting their effectiveness in vivo. Here we evaluated the serum complement sensitivity of CNV-G and PRV-G and compared it to that of VSV-G. Five sera from different donors were used for testing (Figure 4B). While VSV-G pseudotype was inactivated significantly with a 70% to 80% reduction in the overall titer, the PRV-G pseudotype showed only 10% to 45% drop and CNV-G pseudotype decreased in titer by 45% to 65%. Overall, the resistance of CNV-G and PRV-G to serum inactivation relative to that of VSV-G is significant (p<0.05), thus indicating the relative stability of these for in vivo use.
DISCUSSION
Gene transduction with lentiviral vectors is among the most efficient methods for gene delivery due to the capacity of these vectors to transduce a variety of both dividing and non-diving cells. Heterologous viral envelope glycoprotein G from the Vesiculovirus, vesicular stomatitis virus, due to its broad host cell tropism has greatly facilitated transduction of many cell types. However, the relative susceptibility of VSV-G to serum complement inactivation and other drawbacks such as cell toxicity has somewhat hampered its utility for broader applications that include direct in vivo gene delivery [15, 16, 33]. Here in an effort to identify alternative heterologous viral envelopes for vector pseudotyping, we evaluated two related viral envelopes from Chandipura and Piry viruses that belong to the same Vesiculovirus genus like VSV. While both these two viruses are phylogenetically related to VSV, they are serologically distinct and with different cell tropisms [28, 31].
Here we showed that lentiviral vectors can be pseudotyped with either of these heterologous viral envelopes. High titer vector stocks yielding more than 5×108 TU/ml can be prepared by concentration via ultra-centrifugation, suggesting that these envelopes are relatively stable compared to retroviral envelopes [34]. When the transduction efficiencies were compared with that of VSV-G pseudotyped lentiviral vectors, CNV-G pseudotyped vectors were found to be relatively more efficient in transducing GHOST and MDCK cells whereas PRV-G was less efficient than both CNV-G and VSV-G. Confirming its neurotropism, CNV-G showed higher transduction into neuroblastoma cells than either VSV-G or PRV-G. In human T cell lines, transduction levels achieved with CNV-G were higher than that of PRV-G, while transduction of these cell lines with VSV-G was found to be far superior. In a mixed PBMC population, the transduction efficiencies of both the CNV-G and PRV-G were found to be far lower than that of VSV-G in transducing CD4T, CD8T, NK and B cells. For the treatment of blood disorders, CD34 hematopoietic stem cells are a main target for lentiviral vector mediated transduction with VSV-G pseudotyped vectors [26, 35]. Here we found that both CNV-G and PRV-G, while capable of transducing CD34 cells, their levels of transduction were much lower than that of VSV-G. Transducing the cells twice at an MOI of 10 or increasing the MOI to 20 resulted in only a marginal increase in gene transfer (data not shown). We also determined that the lower transduction level seen in CD34 cells was not due to possible toxicity of these envelopes (data not shown). It is interesting to note that, with regard to gene transduction of hematopoietic CD34 progenitor cells with yet another related vesiculovirus, higher transduction levels were reported with a lentiviral vector pseudotyped with Cocal virus glycoprotein compared to that of VSV-G [16]. The higher transduction observed with Cocal viral envelope on CD34 cells than with either CNV-G or PRV-G is most likely due to the reason that Cocal virus is also a broad host tropic virus like VSV that can infect a much wider species of animals including livestock [16].
With regard to stability, both CNV-G and PRV-G pseudotypes showed comparable results to VSV-G during successive freeze/thaw cycles wherein a 10% drop in titer was observed with each cycle. A common drawback with the VSV-G pseudotyped vectors is their sensitivity to inactivation by human complement present in the human sera. By comparison, PRV-G pseudotype was highly resistant to human sera complement components, registering only a slight decline in its titer after incubation with sera from five different donors. CNV-G pseudotype showed some level of inactivation by human sera, but still had much higher titer than VSV-G pseudotype. A recent study determined that VSV-G neutralization or inactivation by human sera is mediated by concerted actions of natural IgM and complement and that a related vesiculovirus, Maraba virus, G protein is relatively resistant to this phenomenon [36]. The marked resistance shown here by PRV-G and CNV-G to human serum exposure might be similar to that of Maraba virus G protein and thus confers an advantage over VSV-G pseudotyped vectors for their future use in in vivo applications.
For sustained gene expression and boosting gene dosage in vivo, it may be necessary to employ repeated administration of vectored genes. Use of a single pseudotyped vector for multiple injections will invariably lead to immune response to the envelope thus eventually precluding its utility. In such a scenario, using CNV-G and PRV-G pseudotyped vectors sequentially will help overcome the immune response mounted against VSV-G pseudotyped lentiviral vectors for subsequent vector administration.
In conclusion, Chandipura and Piry virus envelope glycoproteins mediated efficient transduction of several target cell types including fibroblastic and epithelial from different species. CNV-G pseudotyped vectors transduced these cells at a similar or better efficiency than VSV-G pseudotypes. While relatively less efficient, PRV-G pseudotyped vectors also showed gene delivery efficiencies at levels adequate for experimentation and thus provide a good alternative when necessary. However, the new envelopes were sub-optimal in mediating gene transduction into non-adherent cell types such as lymphoid cells and hematopoietic stem cells. Nevertheless, the CNV-G and PRV-G pseudotyped vectors are expected to fill a gap when alternative envelope proteins are needed to transduce a particular cell type and when neutralizing immune responses preclude the use of the standard VSV-G pseudotyped vectors for in vivo gene delivery.
RESEARCH HIGHLIGHTS.
Pseudotyping of lentiviral vectors with novel viral envelopes from Piry and Chandipura viruses
Efficient transduction of many cell types including neuronal cells
Human serum resistance of novel G protein pseudotyped lentiviral vectors
Use of new serotype viral envelops permitting multiple injections for in vivo application
Acknowledgments
Work reported here was supported by NIH grant RO1 AI073255 to R. A. We thank the NIH AIDS Research and Reference Reagents Program for supplying some of the reagents used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson J, et al. Safety and efficacy of a lentiviral vector containing three anti-HIV genes--CCR5 ribozyme, tat-rev siRNA, and TAR decoy--in SCID-hu mouse-derived T cells. Mol Ther. 2007;15(6):1182–8. doi: 10.1038/sj.mt.6300157. [DOI] [PubMed] [Google Scholar]
- 2.Nagabhushan Kalburgi S, Khan NN, Gray SJ. Recent gene therapy advancements for neurological diseases. Discov Med. 2013;15(81):111–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Cicalese MP, Aiuti A. Clinical applications of gene therapy for primary immunodeficiencies. Hum Gene Ther. 2015;26(4):210–9. doi: 10.1089/hum.2015.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89(1):21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Matrai J, Chuah MK, VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther. 2010;18(3):477–90. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emeagi PU, et al. Lentiviral vectors: a versatile tool to fight cancer. Curr Mol Med. 2013;13(4):602–25. doi: 10.2174/1566524011313040011. [DOI] [PubMed] [Google Scholar]
- 7.Buchschacher GL, Jr, Wong-Staal F. Development of lentiviral vectors for gene therapy for human diseases. Blood. 2000;95(8):2499–504. [PubMed] [Google Scholar]
- 8.Sakuma T, Barry MA, Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443(3):603–18. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- 9.Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5(4):387–98. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobinger GP, et al. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat Biotechnol. 2001;19(3):225–30. doi: 10.1038/85664. [DOI] [PubMed] [Google Scholar]
- 11.Mazarakis ND, et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet. 2001;10(19):2109–21. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- 12.Sandrin V, et al. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002;100(3):823–32. doi: 10.1182/blood-2001-11-0042. [DOI] [PubMed] [Google Scholar]
- 13.Strang BL, et al. Characterization of HIV-1 vectors with gammaretrovirus envelope glycoproteins produced from stable packaging cells. Gene Ther. 2004;11(7):591–8. doi: 10.1038/sj.gt.3302189. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Buchholz CJ. Cell type specific gene delivery by lentiviral vectors: New options in immunotherapy. Oncoimmunology. 2013;2(1):e22566. doi: 10.4161/onci.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schauber CA, et al. Lentiviral vectors pseudotyped with baculovirus gp64 efficiently transduce mouse cells in vivo and show tropism restriction against hematopoietic cell types in vitro. Gene Ther. 2004;11(3):266–75. doi: 10.1038/sj.gt.3302170. [DOI] [PubMed] [Google Scholar]
- 16.Trobridge GD, et al. Cocal-pseudotyped lentiviral vectors resist inactivation by human serum and efficiently transduce primate hematopoietic repopulating cells. Mol Ther. 2010;18(4):725–33. doi: 10.1038/mt.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kneissl S, et al. CD19 and CD20 targeted vectors induce minimal activation of resting B lymphocytes. PLoS One. 2013;8(11):e79047. doi: 10.1371/journal.pone.0079047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q, et al. Exclusive Transduction of Human CD4+ T Cells upon Systemic Delivery of CD4-Targeted Lentiviral Vectors. J Immunol. 2015 doi: 10.4049/jimmunol.1500956. [DOI] [PubMed] [Google Scholar]
- 19.Witting SR, Vallanda P, Gamble AL. Characterization of a third generation lentiviral vector pseudotyped with Nipah virus envelope proteins for endothelial cell transduction. Gene Ther. 2013;20(10):997–1005. doi: 10.1038/gt.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns JC, et al. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993;90(17):8033–7. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akkina RK, et al. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996;70(4):2581–5. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–7. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 23.Girard-Gagnepain A, et al. Baboon envelope pseudotyped LVs outperform VSV-G-LVs for gene transfer into early-cytokine-stimulated and resting HSCs. Blood. 2014;124(8):1221–31. doi: 10.1182/blood-2014-02-558163. [DOI] [PubMed] [Google Scholar]
- 24.Frecha C, et al. Measles virus glycoprotein-pseudotyped lentiviral vector-mediated gene transfer into quiescent lymphocytes requires binding to both SLAM and CD46 entry receptors. J Virol. 2011;85(12):5975–85. doi: 10.1128/JVI.00324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang Y, et al. In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins. J Virol. 2002;76(18):9378–88. doi: 10.1128/JVI.76.18.9378-9388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagemaker G. Lentiviral hematopoietic stem cell gene therapy in inherited metabolic disorders. Hum Gene Ther. 2014;25(10):862–5. doi: 10.1089/hum.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyles DS, Kuzmin IV, Rupprecht CE, Rhabdoviridae . In: FIELDS VIROLOGY. 6. Knipe David M., editor. Vol. 2. LIPPINCOTT WILLIAMS & WILKINS; 2013. pp. 885–922. [Google Scholar]
- 28.Brun G, Bao X, Prevec L. The relationship of Piry virus to other vesiculoviruses: a re-evaluation based on the glycoprotein gene sequence. Intervirology. 1995;38(5):274–82. doi: 10.1159/000150451. [DOI] [PubMed] [Google Scholar]
- 29.Dhanda V, Rodrigues FM, Ghosh SN. Isolation of Chandipura virus from sandflies in Aurangabad. Indian J Med Res. 1970;58(2):179–80. [PubMed] [Google Scholar]
- 30.Baquero E, et al. Structure of the low pH conformation of Chandipura virus G reveals important features in the evolution of the vesiculovirus glycoprotein. PLoS Pathog. 2015;11(3):e1004756. doi: 10.1371/journal.ppat.1004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cartwright B, Brown F. Serological relationships between different strains of vesicular stomatis virus. J Gen Virol. 1972;16(3):391–8. doi: 10.1099/0022-1317-16-3-391. [DOI] [PubMed] [Google Scholar]
- 32.Li MJ, et al. Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-HIV shRNA, anti-CCR5 ribozyme, and a nucleolar-localizing TAR decoy. Mol Ther. 2005;12(5):900–9. doi: 10.1016/j.ymthe.2005.07.524. [DOI] [PubMed] [Google Scholar]
- 33.Yao Y, et al. Membrane fusion activity of vesicular stomatitis virus glycoprotein G is induced by low pH but not by heat or denaturant. Virology. 2003;310(2):319–32. doi: 10.1016/s0042-6822(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 34.Stitz J, et al. Lentiviral vectors pseudotyped with envelope glycoproteins derived from gibbon ape leukemia virus and murine leukemia virus 10A1. Virology. 2000;273(1):16–20. doi: 10.1006/viro.2000.0394. [DOI] [PubMed] [Google Scholar]
- 35.Salmon P, et al. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood. 2000;96(10):3392–8. [PubMed] [Google Scholar]
- 36.Tesfay MZ, et al. Vesiculovirus neutralization by natural IgM and complement. J Virol. 2014;88(11):6148–57. doi: 10.1128/JVI.00074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]