Abstract
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease and a leading cause of mortality and morbidity worldwide. The aim of this study was to investigate the sex dependency of circulating metabolic profiles in COPD.
Serum from healthy never-smokers (healthy), smokers with normal lung function (smokers), and smokers with COPD (COPD; Global Initiative for Chronic Obstructive Lung Disease stages I–II/A–B) from the Karolinska COSMIC cohort (n=116) was analysed using our nontargeted liquid chromatography–high resolution mass spectrometry metabolomics platform.
Pathway analyses revealed that several altered metabolites are involved in oxidative stress. Supervised multivariate modelling showed significant classification of smokers from COPD (p=2.8×10−7). Sex stratification indicated that the separation was driven by females (p=2.4×10−7) relative to males (p=4.0×10−4). Significantly altered metabolites were confirmed quantitatively using targeted metabolomics. Multivariate modelling of targeted metabolomics data confirmed enhanced metabolic dysregulation in females with COPD (p=3.0×10−3) relative to males (p=0.10). The autotaxin products lysoPA (16:0) and lysoPA (18:2) correlated with lung function (forced expiratory volume in 1 s) in males with COPD (r=0.86; p<0.0001), but not females (r=0.44; p=0.15), potentially related to observed dysregulation of the miR-29 family in the lung.
These findings highlight the role of oxidative stress in COPD, and suggest that sex-enhanced dysregulation in oxidative stress, and potentially the autotaxin–lysoPA axis, are associated with disease mechanisms and/or prevalence.
Short abstract
Oxidative stress and the autotaxin–lysoPA axis evidence sex-associated metabotypes in the serum of COPD patients http://ow.ly/kAeE309MpdI
Introduction
Chronic obstructive pulmonary disease (COPD) is an umbrella diagnosis that is characterised by airflow obstruction and permanent reduction of the forced expiratory volume [1]. COPD-related mortality is estimated to reach 1 billion by the end of the 21st century [2]. The early diagnosis of COPD is challenging due to disease heterogeneity and lack of predictive molecular markers. The diagnosis is based solely on spirometry, while lung function, symptoms and exacerbation history are used for disease staging. Decline in lung function over time is accepted as a reliable index of disease progression; however, the mechanisms underlying different COPD subphenotypes and their relationship with prognosis are still unclear [3]. For example, evidence of sex differences, with higher mortality in females even after correction for smoking has emerged [4]. Smoking results in greater impairment in lung function in females, especially post-menopause [5, 6].
Cigarette smoking exerts extensive airway epithelial damage and is an important component driving the onset of COPD [7]. However, not all smokers develop COPD and disease severity varies among smoking COPD individuals. Other risk factors include genetics, asthma, environmental exposures, premature birth and persistent respiratory infections in early childhood [8, 9]. In addition, COPD pathogenesis may be linked to oxidative stress resulting from the overproduction of oxidants/reactive oxygen species (ROS), arising endogenously (e.g. from mitochondrial respiration or immune cells) or exogenously (e.g. tobacco smoke) [10, 11].
The aim of the current study was to employ a nontargeted high-resolution mass spectrometry (HRMS) metabolomics approach to identify molecular markers of metabolic dysregulation in COPD using the Karolinska COSMIC (Clinical & Systems Medicine Investigations of Smoking-related Chronic Obstructive Pulmonary Disease) cohort [12–15]. A particular focus of the COSMIC study is to evaluate the role of sex in the aetiology of COPD. Accordingly, our statistical analysis focused on sex-specific shifts in the observed metabolic pathways.
Materials and methods
Subjects and study design
The Karolinska COSMIC cohort (www.clinicaltrials.gov/ct2/show/NCT02627872) is a three-group cross-sectional study designed for investigating molecular sex differences in early-stage COPD, including 40 never-smokers (“healthy”), 40 smokers with normal lung function (“smokers”) and 38 individuals with COPD (Global Initiative for Chronic Obstructive Lung Disease stage I–II/A–B; forced expiratory volume in 1 s (FEV1) 51–97%; FEV1/forced vital capacity (FVC) <70%; 27 current smokers (“COPD”) and 11 ex-smokers (“COPD-ExS”)) [12–15]. Of the 118 recruited individuals, two never-smokers did not provide a blood sample and were excluded from the analysis. The remaining 116 subjects were matched for age, sex and current smoking status, and history where relevant (table 1 and online supplementary table E1). Blood was drawn from fasting individuals by venipuncture between 07:00 h and 09:00 h and allowed to stand at room temperature for ≥30 min before centrifugation at 1695×g for 10 min at room temperature, and stored at −80°C until use. During the same visit, bronchoalveolar lavage (BAL) was performed and bronchial epithelial cell (BEC) brushings were collected. Detailed methods, as well as study inclusion and exclusion criteria are provided in the online supplementary material. The study was approved by the Stockholm regional ethical board (case number 2006/959-31/1) and participants provided their informed written consent.
TABLE 1.
Clinical parameters of individuals from the Karolinska COSMIC cohort included in the current study
| Healthy never-smokers | Smokers | COPD | COPD ex-smokers | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Subjects | 20 | 18 | 20 | 20 | 15 | 12 | 5 | 6 |
| Age years | 62.0 (51.5–64.0) | 55.5 (47.8–62.0) | 52.5 (49.0–56.0) | 54.0 (48.0–58.0) | 61.0 (55.0–63.0) | 59.0 (57.0–63.0) | 64.0 (58.0–65.5) | 58.0 (53.8–65.0) |
| BMI kg·m-2 | 25.6 (23.5–27.9) | 26.5 (23.3–30.6) | 25.0 (21.9–26.2) | 24.2 (22.6–25.9) | 24.2 (21.3–28.7) | 23.5 (20.8–26.0) | 29.1 (24.0–31.0) | 27.6 (22.3–29.6) |
| Smoking pack-years | NA | NA | 33.5 (30.0–40.0) | 33.0 (27.3–40.0) | 42.0 (36.0–50.0) | 40.5 (35.8–47.3) | 30.0 (21.5–39.5) | 28.5 (19.3–37.8) |
| Menopause no/yes | NA | 12/6 | NA | 8/12 | NA | 0/12 | NA | 1/5 |
| GOLD stage 1/2 | NA | NA | NA | NA | 7/7 | 6/6 | 2/3 | 4/2 |
| GOLD-2011 A/B/C | NA | NA | NA | NA | 11/4/0 | 9/3/0 | 3/1/1 | 4/2/0 |
| Blood leukocytes ×109·L-1 | 5.8 (4.8–6.7) | 5.6 (5.0–6.8) | 7.4 (6.9–8.3) | 6.8 (6.3–8.0) | 7.8 (6.4–9.2) | 8.2 (5.9–10.2) | 6.6 (5.5–7.5) | 7.0 (6.5–9.3) |
| Blood platelets ×109·L-1 | 216.0 (193.3–246.8) | 267.5 (244.5–307.8) | 239.0 (209.0–272.0) | 287.5 (241.5–346.3) | 264.0 (224.0–345.0) | 280.5 (235.8–329.5) | 199.0 (196.0–285.0) | 244.5 (207.0–327.8) |
| Serum albumin g·L−1 | 40.0 (38.0–42.0) | 40.0 (37.8–41.0) | 39.0 (38.0–41.0) | 39.0 (37.3–39.8) | 38.0 (37.0–38.0) | 39.5 (38.0–41.0) | 38.0 (36.5–39.5) | 41.0 (39.3–42.0) |
| Antitrypsin g·L−1 | 1.4 (1.3–1.5) | 1.4 (1.3–1.5) | 1.4 (1.3–1.6) | 1.6 (1.4–1.7) | 1.6 (1.4–1.7) | 1.6 (1.4–1.7) | 1.4 (1.3–1.7) | 1.4 (1.2–1.6) |
| FEV1 % | 119.0 (104.0–127.5) | 120.5 (111.0–127.3) | 107.0 (103.3–118.5) | 110.0 (98.3–116.0) | 78.0 (73.0–84.0) | 78.5 (74.3–93.5) | 72.0 (58.0–91.0) | 83.5 (73.8–90.8) |
| FEV1/FVC % | 80.0 (76.3–84.8) | 82.5 (76.8–84.3) | 77.0 (74.3–80.0) | 79.0 (74.3–82.5) | 64.0 (56.0–66.0) | 61.5 (53.8–63.5) | 59.0 (48.5–68.0) | 64.0 (56.5–66.3) |
| Emphysema no/yes | NA | NA | 10/10 | 7/13 | 5/10 | 1/11 | 1/4 | 4/2 |
| Chronic bronchitis no/yes | NA | NA | 13/7 | 13/7 | 13/2 | 7/5 | 1/4 | 5/1 |
Data are presented as n or median (interquartile range). COPD: chronic obstructive pulmonary disease; BMI: body mass index; GOLD: Global Initiative for Chronic Obstructive Lung Disease; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; NA: not applicable.
Mass spectrometry analysis
Sample processing and analyses were performed as previously published [16] and are described in the online supplementary material. Briefly, for nontargeted metabolomics, 50 μL of serum was used for both hydrophilic interaction liquid chromatography (HILIC) and reversed-phase (RP) chromatography. Samples were analysed using an Ultimate 3000 UHPLC coupled to a Q-Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). Mass spectrometry (MS) data were acquired (full scan mode) in both positive and negative ionisation. Molecular features were extracted using the software XCMS (https://metlin.scripps.edu). Putative metabolite annotation was performed using the Human Metabolome Database (HMDB) [17], and output matched to an in-house accurate mass/retention time library of reference standards [18]. Metabolite identity was described as confirmed following a match to reference standards and/or MS/MS. Targeted metabolite quantification was performed using the Biocrates AbsoluteIDQ p180 kit (Biocrates Life Sciences, Innsbruck, Austria) on a Xevo TQ-S triple quadrupole (Waters Corporation, Milford, MA, USA).
miRNA profiling
miRNA from BAL cells and BECs, and exosomes from BAL fluid from a subset of the COSMIC cohort based upon sample availability (n=45; five to 13 subjects per group and sex) were analysed as described in the online supplementary material. Statistical analyses were performed on probe intensities from a subset of four miRNAs of interest, selected using TargetScan release 7.1 (June 2016): miR-29a-3p, miR-29b-3p, miR-29c-3p targeting autotaxin (ENPP2) and miR-218-5p targeting N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD).
Statistical analysis
Due to the confounding effects of smoking, stratification by smoking status was applied in both univariate and multivariate statistical analyses. Accordingly, the smoking population (smokers and COPD) and nonsmoking population (healthy and COPD-ExS) were analysed separately. Statistical analysis was applied to metabolites present in ≥70% of the samples in at least one group, with a coefficient of variation <30% in quality control samples [19]. The percentage of missing values was compared across all clinical groups prior to removal, to ensure that a metabolite was not erroneously removed due to being absent completely in one or more groups. Metabolites with a quality control relative standard deviation >25% were deemed not suitably reproducible and removed from further analysis; this value was chosen based on literature reports [20] and our choice of chromatography (RP and HILIC). Four samples were not analysed in HILIC positive mode due to lack of material, for which missing values were imputed using k-nearest neighbours (k=10) imputation [21].
Univariate statistical analysis was performed on filtered data using the Mann–Whitney test and the Storey q-value (MATLAB vR2015a; MathWorks, Natick, MA, USA). Correction for p-values with regards to age and smoking history between smokers and COPD groups was performed in STATA (v12; StataCorp, College Station, TX, USA) (online supplementary table E2).
Multivariate statistical modelling was performed on log-transformed, mean-centered and pareto-scaled data using SIMCA (v14.0; MKS Umetrics, Malmö, Sweden). Orthogonal projections to latent structures discriminant analysis (OPLS-DA) was performed using metabolites that passed quality control. OPLS-DA models were optimised using variable selection criteria of |p(corr)|≥0.4 (loadings scaled as correlation coefficient between model and original data) and variable importance in projection ≥1.0 as previously described [22]. Model statistics are reported by the cumulative correlation coefficient (R2Y), the predictive variance based on seven-fold cross-validation (Q2) and cross-validated ANOVA p-values for OPLS models. Shared-and-unique-structure (SUS) analysis correlating p(corr) values between models was performed as previously described [23]. A short tutorial on the multivariate methods is provided in the online supplementary material. Pathway enrichment analysis on structurally confirmed metabolites was performed using integrated pathway-level analysis [24]. In addition, we performed stratification by sex prior to univariate and multivariate statistical analyses to facilitate investigation of inter- and intra-group sex differences. Additionally, investigations of the effects of menopause were performed by construction of multivariate models including and excluding premenopausal females, and correlating these models through SUS-based analysis.
Results
Smokers versus COPD using nontargeted metabolomics
Univariate statistical data analysis
A metabolite is described as “putative” following an accurate mass match to the HMDB database [17]. A metabolite is described as “confirmed” following a match to reference standards and/or MS/MS spectrum. A total of 1153 putative metabolites were extracted from the nontargeted metabolomics raw data, of which 959 passed quality control. These putative metabolites were subjected to both sex-combined and sex-stratified comparisons of smokers versus COPD. Of the 959 putative metabolites, 184 were significant at p<0.05 and selected for structural confirmation. Of these 184 metabolites, 67 were structurally confirmed by MS/MS and/or matching to reference standards, and the corresponding p-value, Storey's q-value and fold change are provided in online supplementary table E2. All nontargeted metabolomics data presented in this study refer to these 67 structurally confirmed metabolites.
Multivariate statistical modelling
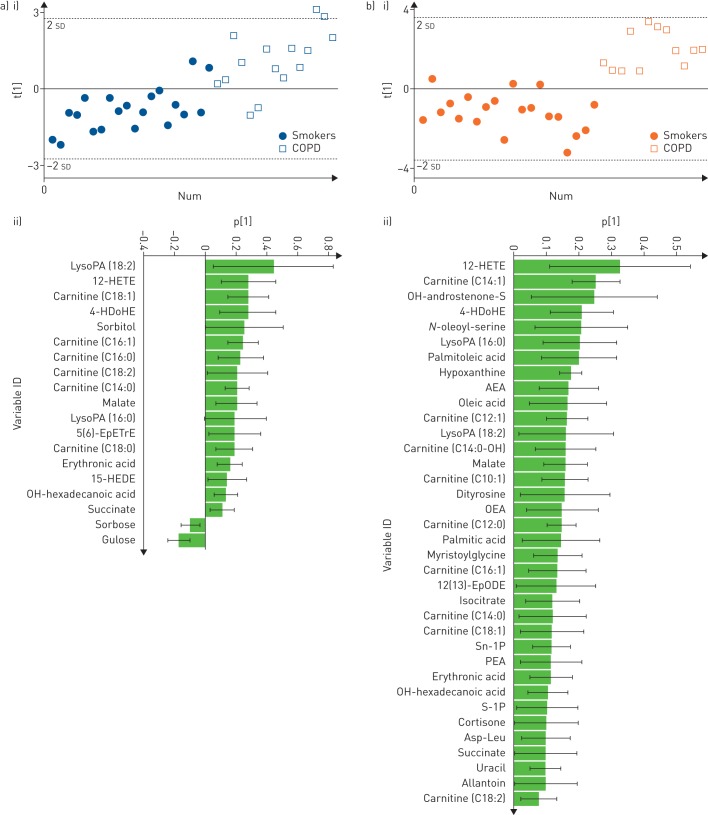
Multivariate statistical modelling of smokers versus COPD was performed using OPLS-DA on the 67 confirmed metabolites. The joint sex OPLS-DA model for classifying smokers versus COPD showed significant group separation (R2Y=0.45, Q2=0.38, p=2.8×0−7; online supplementary figure E1), with a receiver operating characteristic area under the curve (AUC) of 0.90. Sex stratification revealed that the group separation in smokers versus COPD was more robust in females (R2Y=0.73, Q2=0.65, p=2.4×0−7, AUC=1.0) relative to males (R2Y=0.49, Q2=0.38, p=4.0×0−4, AUC=0.89) (figure 1). Permutation tests confirmed the robustness of the models (Y-intercept (500 permutations): females R2Y=0.24, Q2=−0.18; males R2Y=0.20, Q2=−0.26; figure not shown). Only 13 metabolites overlapped all three models (online supplementary figure E2).
FIGURE 1.
Optimised orthogonal projections to latent structures discriminant analysis multivariate models using nontargeted metabolomics data. a) i) Scores plot of male smokers versus males with chronic obstructive pulmonary disease (COPD) (R2Y=0.49, Q2=0.38, p=4.0×10−4); ii) loadings of confirmed metabolites that were the most prominent for driving the separation between male smokers versus males with COPD. b) i) Scores plot of female smokers versus females with COPD (R2Y=0.73, Q2=0.65, p=2.4×10−7); ii) loadings of confirmed metabolites that were the most prominent for driving the separation of female smokers versus females with COPD. For ease of display, parts a ii) and b ii) exclude metabolites whose standard error crossed the x-axis. The complete list of loadings is shown in online supplementary figure E8. LysoPA: lyso-phosphatidic acid; HETE: hydroxyeicosatetraenoic acid; HDoHE: hydroxydocosahexaenoic acid; EpETrE: epoxyeicosatrienoic acid; HEDE: hydroxyeicosadienoic acid; AEA: N-arachidonoylethanolamine; OEA: N-oleoylethanolamine; EpODE: epoxyoctadecadienoic acid; PEA: N-palmitoylethanolamide; Asp-Leu: aspartic acid-leucine.
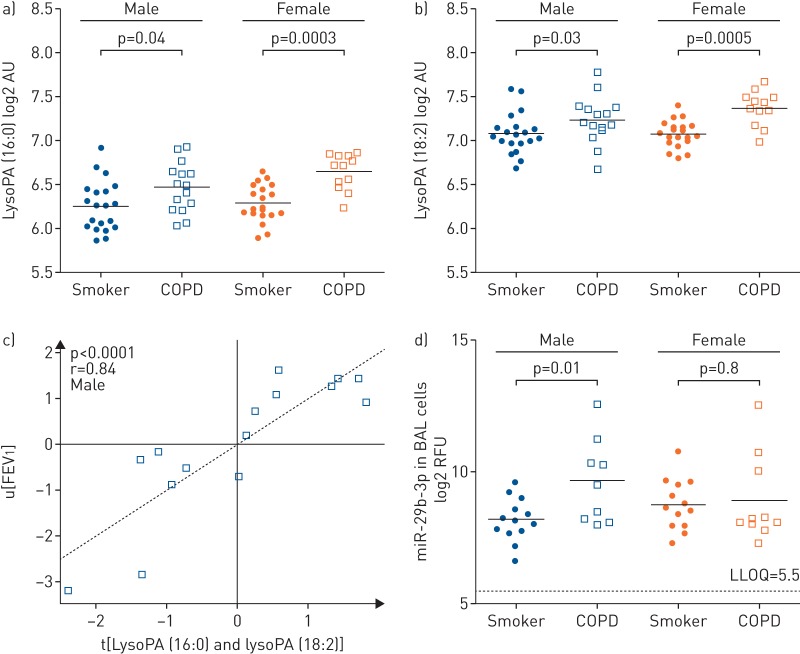
Correlations were performed for all significantly altered metabolites with lung function parameters (FEV1 (%) and FEV1/FVC) using Spearman's correlation, as well as group-wise using partial least squares multivariate correlation (online supplementary figure E3). Lysophosphatidic acid (lysoPA) (16:0) and lysoPA (18:2) were most strongly correlated with FEV1 (%), and were further stratified by sex, evidencing strong correlations in male COPD patients (partial least squares inner relation: r=0.86, p<0.0001) (figure 2), but not females (r=0.44, p=0.15). Based upon these findings, the serum levels of lysoPA (16:0) and lysoPA (18:2) were examined and found to exhibit greater increases in females with COPD relative to smokers (p=0.0003 and p=0.0005, respectively) than the corresponding males (p=0.04 and p=0.03, respectively) (figure 2).
FIGURE 2.
The lyso-phosphatidic acid (lysoPA)–autotaxin axis was attenuated in males with chronic obstructive pulmonary disease (COPD). a) Serum lysoPA (16:0) levels in smokers versus COPD; (b) serum lysoPA (18:2) levels in smokers versus COPD; (c) lysoPA (16:0) and lysoPA (18:2) metabolites correlated with lung function (forced expiratory volume in 1 s (FEV1)) in male COPD patients (r=0.84, p<0.0001). No correlation was observed in the corresponding female COPD population (r=0.44, p=0.15); (d) levels of miR-29b in bronchoalveolar lavage (BAL) cells from male and female smokers and COPD patients. Values for the other members of the miR-29 family are shown in online supplementary figure E6. LysoPA data are from the nontargeted metabolomics platform and are presented as log2 of arbitrary units (AU). RFU: relative fluorescence units; LLOQ: lower limit of quantification.
All females in the COPD group were postmenopausal, while 40% (n=8) female smokers were premenopausal. To investigate the role of menopausal status, OPLS-DA models including only postmenopausal subjects were constructed, and correlated with the original model based on all female subjects using SUS analyses. The high correlation between the two models (R2=0.92) indicates that no substantial differences in metabolite levels were observed due to menopausal status (online supplementary figure E4).
Pathway enrichment analysis
Pathway analysis of the COPD-associated metabolic perturbations from the nontargeted metabolomics data identified significant shifts (p≤0.05) in eight biochemical pathways (table 2), with COPD-associated increase in metabolites of the tricarboxylic acid (TCA) cycle, glycerophospholipids, cAMP signalling, endocannabinoids, sphingolipid and fatty acid metabolism. Sex-stratified pathway analyses established that the fatty acid and sphingolipid pathways were enhanced in females, whereas shifts in cAMP signalling and endocannabinoid and tryptophan metabolism pathways were enhanced in males. The altered metabolic changes based upon the pathway analysis also highlight a strong state of oxidative stress in COPD.
TABLE 2.
Metabolic pathways significantly altered in chronic obstructive pulmonary disease (COPD)#
| Pathway name(s) | Number of metabolites in pathway | Smokers versus COPD | |||||
| Both sexes | Female | Male | |||||
| Hits | p-value (FDR¶) | Hits | p-value (FDR¶) | Hits | p-value (FDR¶) | ||
| Alterations in both sexes | |||||||
| Citrate (tricarboxylic acid) cycle | 3 | 0.0009 (0.005) | 3 | 0.0006 (0.005) | 2 | 0.005 (0.01) | |
| Glycerophospholipid metabolism | 52 | 3 | 0.002 (0.009) | 3 | 0.002 (0.007) | 4 | <0.0001 (0.0001) |
| Pyruvate metabolism | 31 | 2 | 0.03 (0.05) | 2 | 0.03 (0.04) | 2 | 0.01 (0.02) |
| Sex-enhanced: female COPD | |||||||
| Fatty acid biosynthesis | 50 | 3 | 0.0002 (0.002) | 2 | 0.006 (0.01) | 0 | 1.0 (1.0)+ |
| Sphingolipid metabolism | 25 | 2 | 0.02 (0.05) | 2 | 0.02 (0.03) | 0 | 1.0 (1.0)+ |
| Sex-enhanced: male COPD | |||||||
| cAMP signalling pathway | 40 | 2 | 0.03 (0.05) | 2 | 0.02 (0.03) | 2 | 0.009 (0.02) |
| Retrograde endocannabinoid signalling | 19 | 2 | 0.02 (0.04) | 2 | 0.01 (0.02) | 2 | 0.005 (0.02) |
| Tryptophan metabolism | 80 | 2 | 0.5 (1.0)+ | 0 | 1.0 (1.0)+ | 2 | 0.04 (0.05) |
FDR: false discovery rate. #: pathway analysis was performed using integrated pathway-level analysis [24]; ¶: calculated using Benjamini–Hochberg method with a cut-off value of p<0.3; +: pathways did not pass the FDR cut-off value.
Confirmation of oxidative stress results by targeted MS
Metabolites related to oxidative stress were identified as one of the primary drivers for differentiating the smokers and COPD groups (online supplementary table E2). A targeted MS platform (Biocrates AbsoluteIDQ p180 kit) was applied to confirm this finding. Among the 188 metabolites analysed, nine were excluded from further statistical analysis due to ≥70% missing values and/or values below the limit of detection. Measured concentrations of each metabolite as well as the corresponding p-value and q-value are shown in online supplementary table E3. The greatest differences were observed for the female comparisons (smokers versus COPD, 26 metabolites p<0.05) relative to the males (smokers versus COPD, 11 metabolites p<0.05), confirming the results of the nontargeted metabolomics platform.
OPLS-DA analysis based on the targeted data confirmed significant separation between the smokers and COPD groups (R2Y=0.29, Q2=0.19, p=2.0×10−3). Sex stratification confirmed that the separation between smokers and COPD was driven by the female population (R2Y=0.45, Q2=0.34, p=3.0×10−3), with no significant model for males (p=0.10) (online supplementary figure E5).
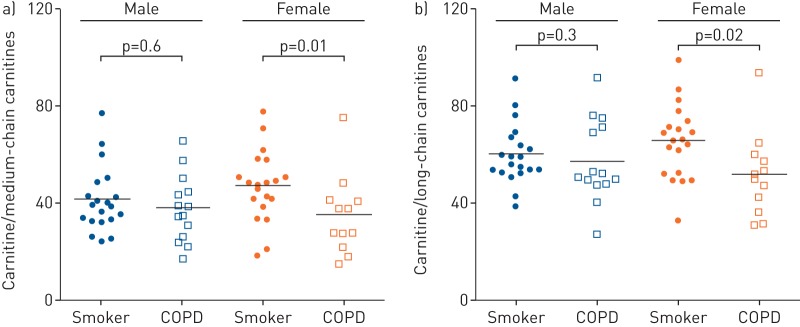
The relative level of fatty acid β-oxidation was estimated by the ratio of carnitine to acylcarnitine using the sum of short-, medium- and long-chain carnitines. The ratios between medium- and long-chain carnitines were significantly downregulated in the female COPD group versus smokers (p=0.01 and p=0.02, respectively), but not in the corresponding male population (figure 3a and b).
FIGURE 3.
β-oxidation-related metabolite ratio of carnitine with acylcarnitines in relation to sex and disease status for smoking subjects. a) Ratio of carnitine with sum of the medium-chain carnitines; (b) ratio of carnitine with sum of the long-chain carnitines. Subjects are divided into smokers with normal lung function and smokers with chronic obstructive pulmonary disease (COPD). Significance was calculated using the nonparametric Mann–Whitney test. Data are from the targeted metabolomics method (Biocrates).
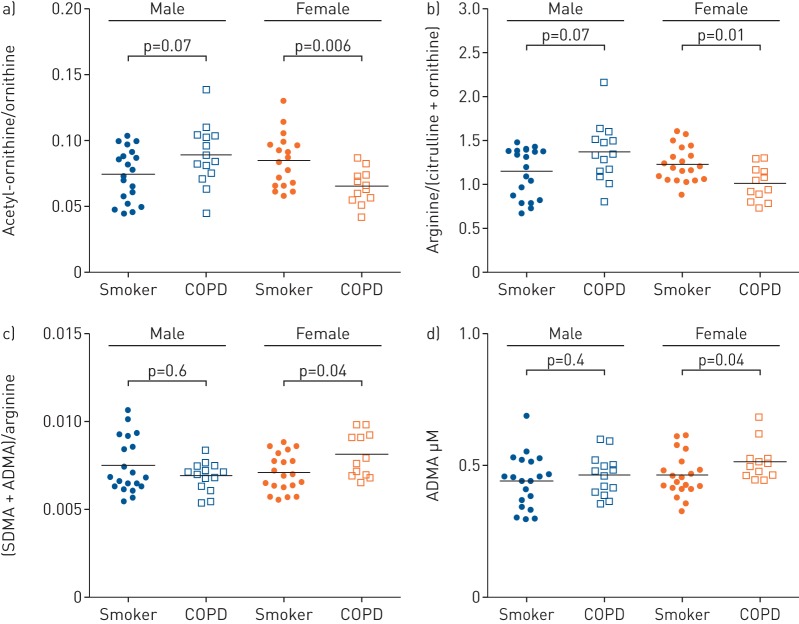
Perturbations in nitric oxide synthesis were examined via metabolites of the arginine pathway. The ratios of acetyl–ornithine/ornithine and arginine/(citrulline+ornithine) were significantly lower in females with COPD versus smokers (p=0.006 and p=0.01, respectively; figure 4a and b), but not the corresponding male subjects. Conversely, the ratio of asymmetric (ADMA) and symmetric dimethylarginine (SDMA) to arginine as well as ADMA alone was significantly upregulated in females with COPD (p=0.04 and p=0.04, respectively; figure 4c and d), with no differences in male subjects.
FIGURE 4.
Serum levels of analytes involved in the arginine/nitric oxide pathway. a) Ratio of acetyl-ornithine to ornithine; (b) ratio of total arginine to the inferred activity of the nitric oxide synthase (NOS) enzyme expressed as arginine/(ornithine+citrulline); (c) ratio of endogenous NOS inhibitors (sum of asymmetric and symmetric dimethylarginine (ADMA + SDMA)) with arginine; and (d) concentration of the endogenous NOS inhibitor ADMA. Significance was calculated using the nonparametric Mann–Whitney test. Subjects are divided into smokers with normal lung function and smokers with chronic obstructive pulmonary disease (COPD). Data are from the targeted metabolomics method (Biocrates).
Correlation to miRNA expression in the lung
Aberrant expression of miRNAs has been associated with several pulmonary disorders, including COPD [25–27]. We therefore performed microarray profiling of the miR-29-3p family (-29a, -29b and -29c), which are putative regulators of autotaxin (lysophospholipase D, ENPP2). We found that these miRNAs were present at levels substantially above the limit of detection in BAL cells and BECs in both the smokers and COPD groups (average expression level 28–211), but not in exosomes isolated from BAL fluid. The miR-29 family was significantly upregulated in male COPD patients compared to smokers both in BAL cells (p=0.004–0.056, fold change 1.5–2.7; figure 2d and online supplementary figure E6) and BECs (p=0.03–0.06, fold-change 2.0–2.8, online supplementary figure E6), while no alteration was detected in the corresponding female cohort (BAL p=0.78–0.90; BEC p=0.22–0.29). Levels of miR-218-5p, a putative regulator of NAPE-PLD (an alternative route of lysoPA production) previously reported to be involved in the pathogenesis of COPD [27], were below the lower limit of quantification in all three lung compartments (BAL cells, BECs and BAL fluid exosomes).
Healthy versus COPD-ExS
An OPLS-DA model comparing the nonsmoking population (healthy versus COPD-ExS) was correlated to the OPLS-DA model of smokers versus COPD groups described earlier, to investigate whether the metabolite shifts related to COPD were independent from current smoking status. SUS correlation analysis between the models describing the nonsmoking and smoking populations was highly correlated (R2=0.73), suggesting that the alterations observed due to COPD in the smoking population are independent of current smoking status (online supplementary figure E7).
Discussion
The objective of the current study was to investigate systemic shifts in metabolism in early-stage COPD. Using our suite of HRMS-based nontargeted and targeted metabolomics platforms, we observed systemic molecular shifts in serum from smokers and early-stage COPD patients. Further stratification revealed sex-associated metabotypes, with a subset of metabolites significantly separating female smokers and female COPD patients (p=2×10−7). This corresponds well with our previous findings of a female-associated molecular subphenotype of COPD in this cohort [12, 15].
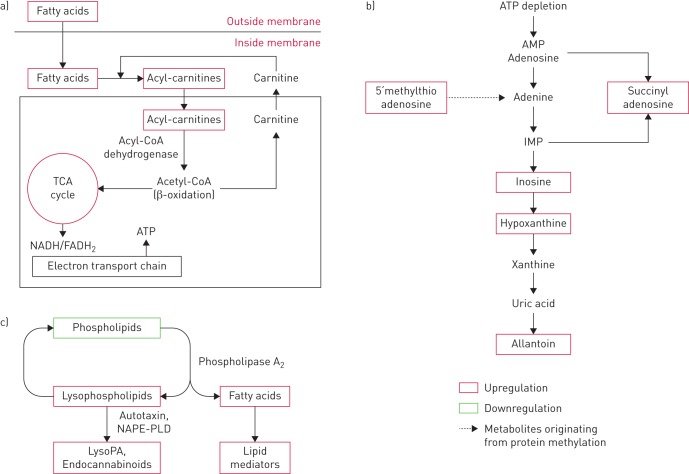
The majority of the observed COPD-related metabolic shifts were associated with oxidative stress (figure 5). The lungs are constantly exposed to ROS, and dysregulation of oxidative stress related pathways has been implicated in airway disease [28, 29]. The observed elevation in circulating levels of acylcarnitines in COPD suggests an amplified energy demand that is reflected in the increased transfer of acetyl coenzyme A to the TCA cycle (figure 5a). In this capacity, free carnitine acts as a fatty acid carrier between the mitochondria and cytosol, and reduced levels of free carnitine in lung tissue have been reported to associate with progressive emphysema [30]. Upregulation of the TCA cycle leads to increased ATP production (figure 5a), and increased extracellular ATP levels in the airway lumen have been associated with COPD pathogenesis via the recruitment and activation of inflammatory cells, accelerating inflammation and tissue degradation [31].
FIGURE 5.
Representative pathway outline for the altered metabolites involved in oxidative stress metabolism in chronic obstructive pulmonary disease (COPD). (a) Fatty acid β-oxidation pathway; (b) purine degradation pathway; (c) Land's cycle/phospholipid metabolism. TCA: tricarboxylic acid; NADH: nicotinamide adenine dinucleotide; FADH2: flavin adenine dinucleotide; IMP: inosine monophosphate; NAPE-PLD: N-acyl phosphatidylethanolamine phospholipase D; lysoPA: lysophosphatidic acid.
Following sex stratification, we observed that the majority of the oxidative stress related shifts were more pronounced in females with COPD. These findings were confirmed by targeted analysis, identifying sex-associated metabotypes of COPD. It has been postulated that antioxidant genes are downregulated in smoking-induced COPD in females. In an elegant mouse study, Tam et al. [32] showed that long-term exposure to smoking was associated with increased small airway remodelling and distal airway resistance, as well as downregulation of a range of antioxidant genes and increased oxidative stress in female, but not male or ovariectomised mice. These effects were attenuated by tamoxifen treatment, indicating that female sex hormones play an important role in the sensitivity to smoking, with impairment of antioxidant defence being a contributing factor. In our study, enhanced β-oxidation, purine degradation and endocannabinoid production, as well as the ratios of free carnitine to medium- and long-chain acylcarnitines were significantly increased in females relative to males (figure 3a and b). These findings provide a strong molecular signature that substantiates the findings of Tam et al. [32], further supporting the theory of systemic dysregulation of the antioxidant defence in a female-dominated COPD subphenotype [12, 15]. Reactive nitrogen species (RNS) also contribute to oxidative damage in COPD. The arginine pathway is one of the major sources of RNS and is involved in maintaining airway tone [33]. ADMA and SDMA are endogenous nitric oxide synthase (NOS) inhibitors that are associated with COPD prognosis and airway remodelling [34, 35] as well as airway obstruction in asthma [36]. The observed sex-selective alterations in the arginine pathway metabolites (figure 4) suggest that in female COPD, oxidative damage is both ROS- and RNS-mediated. These findings further support the hypothesis that nitrosative stress may be involved in the progression of COPD, with endothelial NOS expression previously reported to increase in the bronchial submucosa of smokers [37].
While a number of metabolites shifted between smokers and COPD, both lysoPA species correlated strongest with lung function (online supplementary figure E3). The enzyme autotaxin (lysophospholipase D) is the primary source of lysoPA lipid mediators in blood [38], and has been suggested as a promising target for COPD treatment [39]. The serum lysoPA levels only correlated with lung function in male COPD patients (figure 2c), suggesting a sex-associated dysregulation in the autotaxin–lysoPA pathway. These findings were supported by greater increases in the levels of autotaxin-regulating miRNA in BAL cells and BECs of male COPD patients relative to females (figure 2d). Interestingly, levels of miR-29b-3p, the family member with the highest alterations, correlated with FEV1 in male COPD patients (r=0.62, p=0.07; data not shown), but not female COPD patients (r=0.48, p=0.16), male smokers (r=0.33, p=0.33) or female smokers (r=0.25, p=0.75). The miR-29 family was selected for investigation based upon a TargetScan query for autotaxin; however, there are no reports of miR-29 interacting with autotaxin in the literature, suggesting that this is a new area of investigation. While we observed a strong upregulation in BAL cells and BECs in male COPD patients for the entire miR-29 family, a decrease in miR-29-b has been previously reported in BAL cells from COPD patients [25]. However, the previous study did not register or control for glucocorticoid treatment and the authors reported that it is likely that the COPD patients in their cohort were taking inhaled corticoids (ICS). In the Karolinska COSMIC cohort ICS use was not permitted, with a minimum 3-month washout period. Solberg et al. [40] reported 1.7–2.8-fold decreases of all three miR-29s in ICS-using asthmatics compared to healthy controls. Accordingly, the observed discrepancies in miR-29 levels are probably due to differences in ICS treatment. While the role of miR-29 in COPD is unclear, it has been shown to have important functions in pulmonary fibrosis [41] and lung cancer [42, 43], suggesting that it plays a role in lung injury and highlighting the interest in targeting this pathway.
Upregulation of the autotaxin–lysoPA axis has been associated with a number of inflammatory lung conditions, including hyperoxic lung injury [44], fibrosis [45] and asthma [46, 47]. Sex differences in the autotaxin–lysoPA axis have been reported, with both autotaxin [48] and lysoPA [49] plasma levels higher in females relative to males. Platelet activation can also lead to increased serum lysoPA levels via autotaxin activity [38], and platelet involvement is supported by the observed increased in serum levels of the 12-lipoxygenase product 12-hydroxyeicosatetraenoic acid (12-HETE; q=0.01 in females, q=0.3 in males). 12-HETE plays a critical role in platelet aggregation and thrombosis [50, 51]. Based on these observations, it is possible that the upregulation of miR-29 exhibits a protective role against oxidative stress-mediated shifts in the autotaxin–lysoPA axis in male, but not female COPD patients, potentially in combination with increased platelet activation in females. However, the potential interaction between miR-29 levels in the lung and circulatory lysoPA levels is unclear. Exosomal regulation is one potential mechanism; however, miR-29 exosome levels were below the limit of quantification. In order to investigate the potential mechanism, further work should determine autotaxin levels in the circulation and the airways as well as quantify the full panel of lysoPA species in both compartments. It would be of particular interest to examine the autotaxin mRNA levels as well as protein levels in BAL cells in order to better understand the relationship between message, protein and metabolite profiles. The current findings suggest that the autotaxin–lysoPA should be further investigated in the pathobiology of COPD, but studies should be designed for sex-stratification.
This study highlights a number of interesting metabolic shifts with COPD and sex; however, there are limitations that should be considered when interpreting the results. While the Karolinska COSMIC cohort is a large study with regards to the invasive sampling through bronchoscopy, from a statistical standpoint the group sizes are relatively small. Accordingly, even though the cross-validated multivariate models gave robust classification models, an independent validation cohort is required to confirm our findings. Furthermore, given our choice to only include confirmed metabolites in the analysis, it is likely that other metabolic shifts occur in the pathobiology of COPD that are not observed with the current metabolite panel. In addition, the longitudinal stability of these metabolite signatures needs to be confirmed. Importantly, the relationship between miRNA levels in the lung compartment and lysoPA levels in circulation is unclear, and as lysoPA can also be released from platelets during the clotting process, this mechanistic information should be interpreted with caution.
To summarise, this study highlights the role of oxidative stress in the pathobiology of COPD. Of particular interest is that even in early-stage COPD, strong systemic alterations were observed in oxidative stress associated metabolic pathways. These findings further highlight the sex differences in COPD, emphasising the importance of sex-stratification in future studies. While oxidative stress appears to be more strongly upregulated in females with COPD, as previously reported [32] the effects may be due to an increase in the antioxidant pathways in the corresponding male population. For example, the selective increase in miR-29b in two lung compartments in males could potentially account for the observed sex differences in the autotaxin–lysoPA axis and its associated pathology. In addition, it has recently been reported that autotaxin binds to steroids [52], further opening the potential for interactions between sex hormones and the autotaxin–lysoPA axis. Finally, as with the previous studies from the Karolinska COSMIC cohort, the majority of the observed alterations were more pronounced in the female population, providing further molecular evidence of a female-driven subphenotype of COPD.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02322-2016_Supplement (2.5MB, pdf)
Disclosures
A. Wheelock ERJ-02322-2016_A_Wheelock (1.2MB, pdf)
Acknowledgements
We thank the research nurses Heléne Blomqvist, Margitha Dahl and Gunnel de Forest, as well as biomedical analyst Benita Engvall (Respiratory Medicine Unit, Dept of Medicine Solna and Center for Molecular Medicine, Karolinska Instituet, Stockholm, Sweden), for sample collection and preparation, and all of the COSMIC volunteers for making the study possible. We thank Chuan-Xing Li (Respiratory Medicine Unit, Dept of Medicine Solna and Center for Molecular Medicine, Karolinska Instituet) for performing the k-nearest neighbour imputations.
Footnotes
This article has supplementary material available from erj.ersjournals.com
Support statement: This study was funded by the Swedish Heart-Lung Foundation (HLF 20140469, HLF20130486 and HLF20100463), the Swedish Research Council (2016-02798, K2014-58X-22502-01-5 and K2014-58X-22502-01-5), the Swedish Foundation for Strategic Research (SSF), VINNOVA (VINN-MER), EU FP6 Marie Curie, Karolinska Institutet, AFA Insurance, the King Oscar II Jubilee Foundation, the King Gustaf V and Queen Victoria's Freemasons Foundation, the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, and the Karolinska Institutet and AstraZeneca Joint Research Program in Translational Science (ChAMP; Centre for Allergy Research Highlights Asthma Markers of Phenotype). S.N. Reinke was supported by a Canadian Institutes of Health Research Fellowship (MFE-135481). C.E. Wheelock was supported by the Swedish Heart Lung Foundation (HLF 20150640). Å.M. Wheelock was supported by the Swedish Heart-Lung Foundation (HLF20120801 and HLF20160427). Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: Disclosures can be found alongside this article at erj.ersjournals.com
References
- 1.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370: 765–773. [DOI] [PubMed] [Google Scholar]
- 2.Adamko DJ, Nair P, Mayers I, et al. . Metabolomic profiling of asthma and chronic obstructive pulmonary disease: a pilot study differentiating diseases. J Allergy Clin Immunol 2015; 136: 571–580. [DOI] [PubMed] [Google Scholar]
- 3.Snoeck-Stroband JB, Lapperre TS, Gosman MME, et al. . Chronic bronchitis sub-phenotype within COPD: inflammation in sputum and biopsies. Eur Respir J 2008; 31: 70–77. [DOI] [PubMed] [Google Scholar]
- 4.Han MK, Postma D, Mannino DM, et al. . Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med 2007; 176: 1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sørheim IC, Johannessen A, Gulsvik A, et al. . Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010; 65: 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camp PG, Coxson HO, Levy RD, et al. . Sex differences in emphysema and airway disease in smokers. Chest 2009; 136: 1480–1488. [DOI] [PubMed] [Google Scholar]
- 7.Eisner MD, Anthonisen N, Coultas D, et al. . An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 182: 693–718. [DOI] [PubMed] [Google Scholar]
- 8.To T, Zhu J, Larsen K, et al. . Progression from asthma to chronic obstructive pulmonary disease. Is air pollution a risk factor? Am J Respir Crit Care Med 2016; 194: 429–438. [DOI] [PubMed] [Google Scholar]
- 9.Kurt OK, Zhang J, Pinkerton KE. Pulmonary health effects of air pollution. Curr Opin Pulm Med 2016; 22: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valko M, Leibfritz D, Moncol J, et al. . Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84. [DOI] [PubMed] [Google Scholar]
- 11.Vestbo J, Hurd SS, Agustí AG, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 12.Kohler M, Sandberg A, Kjellqvist S, et al. . Gender differences in the bronchoalveolar lavage cell proteome of patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2013; 131: 743–751. [DOI] [PubMed] [Google Scholar]
- 13.Forsslund H, Mikko M, Karimi R, et al. . Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest 2014; 145: 711–722. [DOI] [PubMed] [Google Scholar]
- 14.Karimi R, Tornling G, Forsslund H, et al. . Lung density on high resolution computer tomography (HRCT) reflects degree of inflammation in smokers. Respir Res 2014; 15: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balgoma D, Yang M, Sjödin M, et al. . Linoleic acid-derived lipid mediators increase in a female-dominated subphenotype of COPD. Eur Respir J 2016; 47: 1645–1656. [DOI] [PubMed] [Google Scholar]
- 16.Reinke SN, Gallart-Ayala H, Gomez C, et al. . Metabolomics analysis identifies different metabotypes of asthma severity. Eur Respir J 2017; 49: 1601740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wishart DS, Jewison T, Guo AC, et al. . HMDB 3.0 – the Human Metabolome Database in 2013. Nucleic Acids Res 2013; 41: D801–D807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamleh MA, Snowden SG, Grapov D, et al. . LC-MS metabolomics of psoriasis patients reveals disease severity-dependent increases in circulating amino acids that are ameliorated by anti-TNFα treatment. J Proteome Res 2015; 14: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bijlsma S, Bobeldijk I, Verheij ER, et al. . Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal Chem 2006; 78: 567–574. [DOI] [PubMed] [Google Scholar]
- 20.Dunn WB, Wilson ID, Nicholls AW, et al. . The importance of experimental design and QC samples in large-scale and MS-driven untargeted metabolomic studies of humans. Bioanalysis 2012; 4: 2249–2264. [DOI] [PubMed] [Google Scholar]
- 21.Gromski PS, Xu Y, Kotze HL, et al. . Influence of missing values substitutes on multivariate analysis of metabolomics data. Metabolites 2014; 4: 433–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheelock ÅM, Wheelock CE. Trials and tribulations of ’omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol Biosyst 2013; 9: 2589–2596. [DOI] [PubMed] [Google Scholar]
- 23.Wiklund S, Johansson E, Sjöström L, et al. . Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem 2008; 80: 115–122. [DOI] [PubMed] [Google Scholar]
- 24.Kamburov A, Cavill R, Ebbels TM, et al. . Integrated pathway-level analysis of transcriptomics and metabolomics data with IMPaLA. Bioinformatics 2011; 27: 2917–2918. [DOI] [PubMed] [Google Scholar]
- 25.Molina-Pinelo S, Pastor MD, Suarez R, et al. . MicroRNA clusters: dysregulation in lung adenocarcinoma and COPD. Eur Respir J 2014; 43: 1740–1749. [DOI] [PubMed] [Google Scholar]
- 26.Osei ET, Florez-Sampedro L, Timens W, et al. . Unravelling the complexity of COPD by microRNAs: it's a small world after all. Eur Respir J 2015; 46: 807–818. [DOI] [PubMed] [Google Scholar]
- 27.Conickx G, Mestdagh P, Avila Cobos F, et al. . MicroRNA profiling reveals a role for microRNA-218-5p in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 195: 43–56. [DOI] [PubMed] [Google Scholar]
- 28.Benipal B, Feinstein SI, Chatterjee S, et al. . Inhibition of the phospholipase A2 activity of peroxiredoxin 6 prevents lung damage with exposure to hyperoxia. Redox Biol 2015; 4: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben Moussa S, Rouatbi S, Saad HB. Incapacity, handicap, and oxidative stress markers of male smokers with and without COPD. Respir Care 2016; 61: 668–679. [DOI] [PubMed] [Google Scholar]
- 30.Conlon TM, Bartel J, Ballweg K, et al. . Metabolomics screening identifies reduced l-carnitine to be associated with progressive emphysema. Clin Sci 2016; 130: 273–287. [DOI] [PubMed] [Google Scholar]
- 31.Lommatzsch M, Cicko S, Müller T, et al. . Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181: 928–934. [DOI] [PubMed] [Google Scholar]
- 32.Tam A, Churg A, Wright JL, et al. . Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2016; 193: 825–834. [DOI] [PubMed] [Google Scholar]
- 33.Aydin M, Altintas N, Cem Mutlu L, et al. . Asymmetric dimethylarginine contributes to airway nitric oxide deficiency in patients with COPD. Clin Respir J 2017; 11: 318–327. [DOI] [PubMed] [Google Scholar]
- 34.Ruzsics I, Nagy L, Keki S, et al. . l-Arginine pathway in COPD patients with acute exacerbation: a new potential biomarker. COPD 2016; 13: 139–145. [DOI] [PubMed] [Google Scholar]
- 35.Scott JA, Duongh M, Young AW, et al. . Asymmetric dimethylarginine in chronic obstructive pulmonary disease (ADMA in COPD). Int J Mol Sci 2014; 15: 6062–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott JA, North ML, Rafii M, et al. . Asymmetric dimethylarginine is increased in asthma. Am J Respir Crit Care Med 2011; 184: 779–785. [DOI] [PubMed] [Google Scholar]
- 37.Ricciardolo FL, Caramori G, Ito K, et al. . Nitrosative stress in the bronchial mucosa of severe chronic obstructive pulmonary disease. J Allergy Clin Immunol 2005; 116: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 38.van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res 2007; 46: 145–160. [DOI] [PubMed] [Google Scholar]
- 39.Van der Aar E, Fagard L, Desrivot J, et al. . Favorable human safety, pharmacokinetics and pharmacodynamics of the autotaxin inhibitor GLPG1690, a potential new treatment in COPD. Eur Respir J 2015; 46: Suppl. 59, OA484. [Google Scholar]
- 40.Solberg OD, Ostrin EJ, Love MI, et al. . Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med 2012; 186: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cushing L, Kuang P, Lü J. The role of miR-29 in pulmonary fibrosis. Biochem Cell Biol 2015; 93: 109–118. [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Zhang G, Wu JH, et al. . Diverse roles of miR-29 in cancer (review). Oncol Rep 2014; 31: 1509–1516. [DOI] [PubMed] [Google Scholar]
- 43.Wu DW, Hsu NY, Wang YC, et al. . c-Myc suppresses microRNA-29b to promote tumor aggressiveness and poor outcomes in non-small cell lung cancer by targeting FHIT. Oncogene 2015; 34: 2072–2082. [DOI] [PubMed] [Google Scholar]
- 44.Nowak-Machen M, Lange M, Exley M, et al. . Lysophosphatidic acid generation by pulmonary NKT cell ENPP-2/autotaxin exacerbates hyperoxic lung injury. Purinergic Signal 2015; 11: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu X, Wei X, Lu S, et al. . Autotaxin-LPA receptor axis in the pathogenesis of lung diseases. Int J Clin Exp Med 2015; 8: 17117–17122. [PMC free article] [PubMed] [Google Scholar]
- 46.Park GY, Lee YG, Berdyshev E, et al. . Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med 2013; 188: 928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ackerman SJ, Park GY, Christman JW, et al. . Polyunsaturated lysophosphatidic acid as a potential asthma biomarker. Biomark Med 2016; 10: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pleli T, Martin D, Kronenberger B, et al. . Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis – a prospective cohort study. PLoS One 2014; 9: e103532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knowlden S, Georas SN. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J Immunol 2014; 192: 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katoh A, Ikeda H, Murohara T, et al. . Platelet-derived 12-hydroxyeicosatetraenoic acid plays an important role in mediating canine coronary thrombosis by regulating platelet glycoprotein IIb/IIIa activation. Circulation 1998; 98: 2891–2898. [DOI] [PubMed] [Google Scholar]
- 51.Maskrey BH, Rushworth GF, Law MH, et al. . 12-hydroxyeicosatetraenoic acid is associated with variability in aspirin-induced platelet inhibition. J Inflamm 2014; 11: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keune WJ, Hausmann J, Bolier R, et al. . Steroid binding to autotaxin links bile salts and lysophosphatidic acid signalling. Nat Commun 2016; 7: 11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02322-2016_Supplement (2.5MB, pdf)
A. Wheelock ERJ-02322-2016_A_Wheelock (1.2MB, pdf)