ABSTRACT
Purpose of Review: Extracranial or intracranial large artery atherosclerosis is often identified as a potential etiologic cause for ischemic stroke and transient ischemic attack. Given the high prevalence of large artery atherosclerosis in the general population, determining whether an identified atherosclerotic lesion is truly the cause of a patient’s symptomatology can be difficult. In all cases, optimally treating each patient to minimize future stroke risk is paramount. Extracranial or intracranial large artery atherosclerosis can be broadly compartmentalized into four distinct clinical scenarios based upon the individual patient’s history, examination, and anatomic imaging findings: asymptomatic and symptomatic extracranial carotid stenosis, intracranial atherosclerosis, and extracranial vertebral artery atherosclerotic disease. This review provides a framework for clinicians evaluating and treating such patients.
Recent Findings: Intensive medical therapy achieves low rates of stroke and death in asymptomatic carotid stenosis. Evidence indicates that patients with severe symptomatic carotid stenosis should undergo carotid revascularization sooner rather than later and that the risk of stroke or death is lower using carotid endarterectomy than with carotid stenting. Specific to stenting, the risk of stroke or death is greatest among older patients and women. Continuous vascular risk factor optimization via sustained behavioral modifications and intensive medical therapy is the mainstay for stroke prevention in the setting of intracranial and vertebral artery origin atherosclerosis.
Summary: Lifelong vascular risk factor optimization via sustained behavioral modifications and intensive medical therapy are the key elements to reduce future stroke risk in the setting of large artery atherosclerosis. When considering a revascularization procedure for carotid stenosis, patient demographics, comorbidities, and the periprocedural risks of stroke and death should be carefully considered.
INTRODUCTION
Large artery atherosclerosis of the head and neck is responsible for approximately 15% of all ischemic strokes. The identification and appropriate treatment of such atherosclerotic lesions is an essential skill for all physicians diagnosing and treating patients with stroke. Large artery atherosclerotic lesions can be broadly classified into four distinct clinical scenarios as based upon the individual patient’s anatomic and clinical findings: asymptomatic and symptomatic extracranial carotid stenosis, intracranial atherosclerotic disease, and extracranial vertebral artery atherosclerotic disease. While the anatomic lesion locations differ for each of these, it is important to note they all share the same risk factor profiles and somewhat overlapping treatment options. In short, continuous vascular risk factor optimization via sustained behavioral modifications and intensive medical therapy is critical to prevent stroke in the setting of large artery atherosclerosis. In fact, specific to the settings of intracranial and vertebrobasilar atherosclerosis as well as asymptomatic carotid atherosclerosis, risk factor modification is the primary treatment option. In patients with symptomatic extracranial carotid atherosclerosis, treatment options also include revascularization procedures such as carotid endarterectomy (CEA) and carotid artery stenting, but, again, optimal medical therapy is a critical treatment modality. Appropriate patient selection and timing of such revascularization procedures must also be considered. Across each of these four clinical scenarios, the results of numerous randomized and nonrandomized clinical trials lead to periodically updated meta-analyses and consensus guidelines that provide evidence-based recommendations for practicing clinicians. While each of these four clinical scenarios could easily be (and often is) the subject of independent reviews, this article aims to provide a concise framework for clinicians evaluating and treating patients across all four scenarios, emphasizing key clinical considerations, clinical trial evidence, and the most recent professional and societal guidelines.
CONSIDERATIONS ACROSS ALL CASES OF LARGE ARTERY ATHEROSCLEROSIS
While the clinical manifestations of large artery atherosclerosis of the head and neck differ based upon the lesion location, it is important to note that they all share the same risk factor profiles, similar workups, and somewhat overlapping treatment options.
Clinical Presentation and Workup
First, it is important to determine if the identified large artery atherosclerotic lesion is proximal to a vascular territory that corresponds to the patient’s stroke on imaging or symptoms in the setting of a transient ischemic attack (TIA). To optimize anatomic localization (anterior versus posterior circulation) in the setting of both stroke and TIA, clinicians must take a detailed history, asking about symptoms (eg, weakness, sensory changes, vision changes, balance problems) and whether these occurred recently in isolation or multiple times in the past, over both the near and long term. All patients with stroke and suspected TIA warrant an expedited evaluation that can be simply defined as from heart to head. In other words, the heart, proximal aorta, and vasculature of the head and neck should be evaluated, and clinical and laboratory testing related to vascular risk factors should be performed on an inpatient basis. While it is beyond the scope of this review to provide detailed testing recommendations, at a minimum, a transthoracic echocardiogram, brain imaging via an emergent CT and then MRI, and vessel imaging of the head and neck by CT angiography (CTA) or magnetic resonance angiography (MRA) should be performed in all patients with stroke and TIA. If large artery atherosclerotic disease is identified, other techniques, such as carotid Doppler studies, contrast-enhanced MRA, and even judicious use of cerebral angiogram, can be used to better define stenosis severity. To identify patients at the greatest risk for stroke, large artery atherosclerotic plaque stability and emboli potential can be accessed via transcranial Doppler (TCD) microembolus detection and other, more research-oriented, techniques, such as plaque echolucency measurements, that have yet to be formally defined.1
Positive imaging demonstrating a clearly defined infarction can make the large artery atherosclerosis etiologic diagnosis easier, assuming the infarct is located in a vascular territory distal to a highly stenosed vessel or an irregularly calcified plaque. Large artery atherosclerosis leads to ischemic symptomatology via two mechanisms: embolic phenomena and regional brain hypoperfusion. Clearly embolic phenomena should be considered as symptomatic, necessitating clinicians to consider potential revascularization procedures as possible to the specific case. Stroke in the setting of hypoperfusional states from large artery atherosclerosis, while symptomatic, offers additional choices such as intensive medical therapy in combination with permissive hypertension, thereby allowing the individual patient time to develop improved collateral circulatory pathways and potentially reducing the need for a revascularization procedure.
Vascular Risk Factors
Across all locations of large artery atherosclerosis discussed in this article, continuous lifelong vascular risk factor optimization via sustained behavioral (lifestyle) modifications and intensive medical therapy is critical for stroke prevention. This point cannot be emphasized enough. Over the past 10 years, our understanding of the importance of medical management in the setting of atherosclerosis has markedly increased. Population-wide improved control of hypertension, dyslipidemia, and diabetes mellitus, coupled with a reduction in tobacco use, has resulted in a decline in stroke mortality from the third to the fifth leading cause of death in the United States.2 Clinicians should take pride in these facts, as these improvements are based upon their efforts in implementing professional society position statements and guidelines. As such, maintaining a working knowledge of these evolving guidelines and position statements is a critical tool for physicians and other health professionals working to reduce stroke risk. In 2014, the American Heart Association (AHA) and the American Stroke Association (ASA) released updated Guidelines for the Primary Prevention of Stroke3 and updated Guidelines for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack.4 These guidelines emphasize an individualized approach to lifestyle modification, including physical activity, diet and nutrition, smoking cessation, and management of obesity and dyslipidemia. Taken in aggregate, these guidelines offer up-to-date comprehensive evidence-based recommendations for the primary and secondary prevention of stroke, including those related to large artery atherosclerosis. While it is beyond the scope of this review to cover all the latest recommendations regarding vascular risk factor control, a few specifics as related to large artery atherosclerosis are warranted.
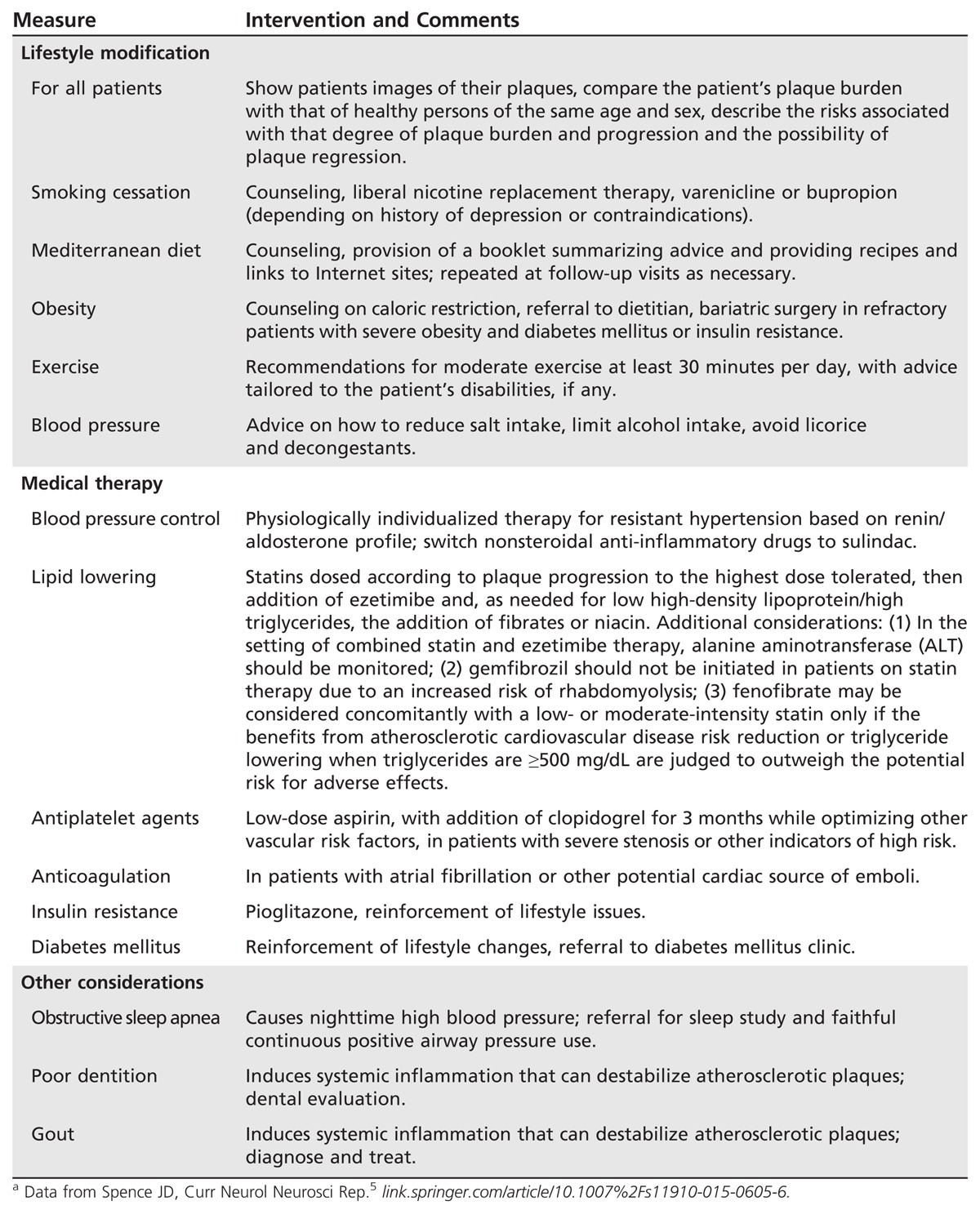
Vascular risk factor control via intensive medical therapy. Based upon the results of numerous recent clinical trials, and as incorporated into the aforementioned guidelines, intensive (or best) medical therapy is emphasized for all patients with large artery atherosclerosis. While the precise definition of intensive medical therapy can be debated, Table 7-1 summarizes the key elements.5 Intensive medical therapy includes smoking cessation, diet, exercise, control of blood pressure (including diagnosis of the physiologic drivers of resistant hypertension by measuring plasma renin and aldosterone),6 antiplatelet therapy (mono versus dual), and intensive lipid-lowering therapy, not just achieving a target level of fasting low-density lipoprotein cholesterol (LDL-C). Overall, the goals of these therapies are first to stop and then reverse large artery atherosclerotic plaque progression. Such regimens clearly are effective. One study demonstrated that by implementing a regimen similar to that outlined in Table 7-1, the risk of stroke and myocardial infarction (MI) was reduced by more than 80% among patients with asymptomatic carotid stenosis.7 Similarly, the Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial demonstrated that “aggressive” (intensive) medical therapy resulted in better outcomes than stenting among patients with intracranial stenosis.8
Table 7-1.
Key Elements of Intensive Medical Therapy in the Setting of Large Artery Atherosclerotic Diseasea
Antiplatelet agents. Antiplatelet agents, including aspirin and clopidogrel, are routinely used for primary and secondary stroke prevention in the setting of large artery atherosclerosis. In individuals whose 10-year risk of stroke is greater than 10% and whose risk of stroke outweighs the risks associated with aspirin therapy, the latest guidelines for the primary prevention of stroke recommend the daily use of aspirin.3 A cardiovascular risk calculator can assist in estimating 10-year risk (my.americanheart.org/cvriskcalculator).9 Aspirin is not recommended for primary stroke prevention in individuals with lower risk or in those with diabetes mellitus who do not have other risk factors. In those for whom aspirin therapy is deemed appropriate, faithful daily use of low-dose aspirin is considered sufficient. Since coated aspirin is less efficacious than uncoated aspirin in about 40% of individuals, uncoated aspirin is recommended. Clopidogrel alone reduces stroke by only 1.7% more than aspirin10 and is thus only marginally better, whereas combined aspirin-dipyridamole is no better than clopidogrel.11 The Clopidogrel in High-risk Patients With Acute Non-disabling Cerebrovascular Events (CHANCE) study indicated that secondary stroke may be reduced by 32% (hazard ratio 0.68; 95% confidence interval 0.57–0.81; P<.001) with no increase in major hemorrhage by the short-term use of the combination of clopidogrel and aspirin, showing it to be more effective than aspirin alone.12 More recently, the CHANCE investigators demonstrated that the early benefit of clopidogrel-aspirin treatment in reducing the risk of subsequent stroke persisted after 1 year of follow-up.13 Again, no difference in moderate or severe hemorrhage was demonstrated in the combined treatment group versus the aspirin-alone group (0.3% versus 0.4%, respectively; P=.44). Dual antiplatelet therapy with aspirin and clopidogrel was also used in the SAMMPRIS trial of intracranial arterial stenosis, which demonstrated that aggressive medical management was superior to percutaneous transluminal angioplasty and stenting.8 Of note, the CHANCE study was performed in China; while the results might be generalizable to Western populations, this hypothesis is currently being evaluated in the ongoing Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trial.14
Several recent studies using TCD evaluations to assess for microemboli found that dual antiplatelet therapy is more efficacious than aspirin alone in reduction of microemboli for both intracranial15 and extracranial16 arterial stenosis. While dual antiplatelet therapy is commonly used for risk reduction in the setting of coronary disease, particularly in the setting of cardiac stenting, it is not widely used in carotid disease based on the results of one study in which an excess of bleeding was seen in the dual therapy group.17 To reduce the risk of intracranial hemorrhage in the setting of dual antiplatelet therapy, effective blood pressure control is critical, as evidenced by the North American Symptomatic Carotid Endarterectomy Trial (NASCET), in which effective blood pressure control reduced intracranial hemorrhage to 0.4% of strokes.18 Gastrointestinal hemorrhages could theoretically be reduced by the identification and treatment of Helicobacter pylori infections prior to the initiation of dual therapy.
In summary, dual antiplatelet therapy can be considered across most settings of large artery atherosclerosis, particularly in symptomatic carotid stenosis or if the patient was already on monotherapy at the time of his or her event. The optimal duration of therapy will remain a topic of debate until further informed by randomized clinical trials and their subgroup analyses. In the meantime, as consistent with SAMMPRIS, 3 months of dual antiplatelet therapy is reasonable while working to optimize vascular risk factors, including healthy lifestyle decision making.
Treatment of vascular risk factors. Treatment of vascular risk factors, including dyslipidemia, hypertension, and diabetes mellitus, over both the near and long term is of critical importance in the prevention and treatment of atherosclerotic disease. Recent changes to lipid guidelines dramatically alter the prior emphasis focusing on specific LDL-C targets.19 Instead, the guidelines now emphasize the 10-year risk for the development and progression of atherosclerotic cardiovascular disease. As based on an individual’s estimated risk, a statin at low, moderate, or high potency is now prescribed. Although these new guidelines shift the emphasis away from specific lipid targets, total cholesterol and high-density lipoprotein values are components of the cardiovascular risk calculator previously mentioned.9 Hypertension also continues to be a major well-documented and modifiable risk factor for stroke, with the treatment of hypertension being the most effective strategy for stroke prevention across all populations. The 2015 Systolic Blood Pressure Intervention Trial (SPRINT) comparing a systolic blood pressure target of less than 120 mm Hg (intensive treatment) to a target of less than 140 mm Hg (standard treatment) was stopped early as related to a significantly lower rate of vascular events in the intensive-treatment group than in the standard-treatment group (1.65% per year versus 2.19% per year; hazard ratio with intensive treatment, 0.75; 95% confidence interval 0.64–0.89; P<.001).20 While lowering blood pressure is strongly associated with reduction of stroke risk, the reduction of blood pressure to lower targets may not be beneficial in all groups of individuals, such as in patients with flow-limiting large artery atherosclerosis or diabetes mellitus or the very elderly. Diabetes mellitus is another well-established risk factor for stroke and large artery atherosclerosis. Optimal glucose control is achieved by reinforcing lifestyle changes (eg, dietary changes, regular exercise, weight loss) and through the faithful use of medications. Hypertension and diabetes mellitus remain undertreated, and personalized approaches to lifestyle changes and medical therapy are critical for successful stroke prevention.
Other Emerging Risk Factors
Other emerging risk factors for large artery atherosclerosis have been identified, including elevated homocysteine, fibrinogen, lipoprotein (a), and C-reactive protein levels. Other lesser-known risk factors implicated include obstructive sleep apnea,21 gout,22 and poor dentition.23 Future studies should work to verify the results of these preliminary reports while considering implications for preventive strategies. From a genetic standpoint, a recently published study by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) Stroke Genetics Network (SiGN) details the largest and most comprehensive genome-wide association study of stroke and its subtypes ever performed.24 This study verified several previous genetic associations with ischemic stroke and identified a new risk locus on chromosome 1p13. Of the replicated loci, it is notable that this study confirmed the association between the HDAC9 locus and large artery atherosclerotic ischemic stroke. Interestingly, this same locus (and the same specific variant) has also been reproducibly associated with coronary artery disease, suggesting a shared underlying causal gene and mechanism. The novel locus identified by the SiGN investigators was detected near TSPAN2 and was also found to be associated with large artery atherosclerosis. TSPAN2 is a scaffolding protein expressed in large arteries. This locus has not been reproducibly associated with coronary artery disease in genome-wide association studies. This suggests that TSPAN2 is potentially specific to ischemic stroke and therefore may provide insight into the pathophysiology of large artery atherosclerotic ischemic stroke rather than just generic atherosclerosis. Studies regarding the mechanistic links between both HDAC9 and TSPAN2 with large artery atherosclerotic stroke are ongoing. Given the rapid evolution of genomic medicine, it is anticipated that in the near future disease susceptibility within individuals, families, and populations will be able to be genetically evaluated, thereby allowing preventive stroke therapies as based on individualized genotype.
In summary, the most recent guidelines emphasize intensive medical therapy with a focus on optimal vascular risk factor control but now implementing a more patient-centered approach than in the past. Given that the results from multiple clinical trials drive these recommendations, the applicability of these results at the level of the individual can be confusing, particularly if an individual does not fulfill the clinical trial inclusion criteria driving the recommendations. As such, physicians should consider each patient on an individual basis, working to optimize their risk factor profile over the long term while inferring from the most recent guidelines. While the described multifaceted approach of intensive medical therapy reduces stroke risk in all patients with large artery atherosclerosis, broadly classifying patients with large artery atherosclerosis into one of four clinical scenarios as based upon the individual patient’s history, examination, and anatomic imaging findings is a useful way to help clarify treatment options. These four scenarios include asymptomatic and symptomatic extracranial carotid stenosis, intracranial atherosclerosis, and atherosclerotic vertebrobasilar disease.
EXTRACRANIAL CAROTID ATHEROSCLEROSIS
Carotid atherosclerosis accounts for approximately 10% of cases of ischemic stroke. Although carotid artery stenosis is a risk factor for stroke, not every carotid stenosis carries the same risk for future stroke. Assuming a relevant stenosis is identified, key factors to consider include the degree of stenosis and the stability of the plaque in the setting of the individual patient. Clinicians should work toward answering two questions: (1) Which patients should opt for revascularization procedures (versus intensive medical therapy alone)? and (2) Which is the appropriate revascularization procedure, CEA or carotid artery stenting?
Assessment of Carotid Stenosis
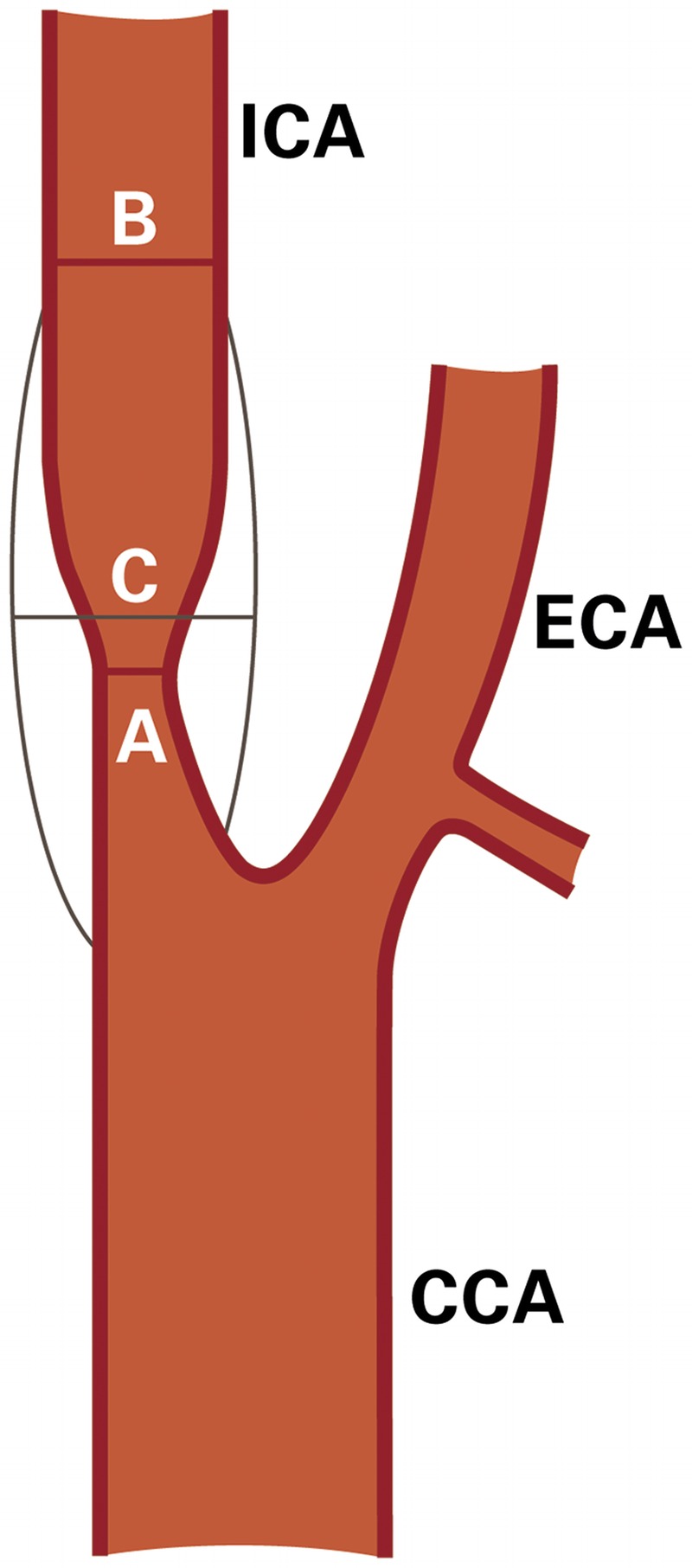
Hemodynamically significant carotid stenosis corresponds to a 60% diameter-reducing stenosis and produces a pressure drop across the lesion or a flow reduction distal to the lesion. Using the North American measurement method (Figure 7-1), the minimal residual lumen at the level of the stenotic lesion is compared to the diameter of the more distal internal carotid artery (ICA) where the walls of the artery first become parallel using the formula: stenosis = (1−A/B) × 100%, where A is the diameter at the point of maximum stenosis and B is the diameter of the arterial segment distal to the stenosis where the arterial walls first become parallel.3 In contrast, the European method (Figure 7-1) directly estimates the stenosis at the internal carotid bulb using the formula: stenosis = (1−A/C) × 100%, where C is the estimated diameter of the disease-free carotid bulb. Catheter angiography is considered the gold standard for assessing stenosis but carries a small risk of causing a stroke.
FIGURE 7-1.
Schematic contrasting internal carotid artery stenosis measurement methods. The North American measurement method: % stenosis = (1–A/B) × 100%. The European method: % stenosis = (1–A/C) × 100%.
Duplex ultrasound is the most commonly used method to screen the extracranial carotid artery for atherosclerotic stenosis and carries the lowest risks and costs. Of note, duplex ultrasound may not accurately differentiate between high-grade stenosis and complete occlusion, with additional testing required in such situations. MRA noninvasively provides images of both the cervical and intracranial vasculature. Notably, time-of-flight MRA without contrast may overestimate the degree of stenosis; thus, a gadolinium-enhanced MRA may be useful, particularly when working to differentiate high-grade stenosis from total occlusion. Clinicians should be mindful that nephrogenic systemic fibrosis is a rare complication among patients with poor renal function in the setting of gadolinium use.
CTA is yet another method that can be used to evaluate both the extracranial and intracranial carotid circulation. CTA disadvantages include radiation exposure and the need for IV injection of contrast material, with a creatinine higher than 1.7 or a glomerular filtration rate less than 45 mL/min/1.73 m2 being common limiting factors. Additionally, atherosclerotic calcifications with similar density to the contrast material may confound accurate measurements of the stenosis. On physical examination, a carotid bruit can reflect an underlying carotid stenosis; however, sensitivity can be limited.
Asymptomatic Extracranial Carotid Stenosis
All patients with carotid stenosis have atherosclerosis that warrants the implementation of intensive medical therapy as soon as possible. While several methods exist to identify those patients with carotid stenosis who are at greater risk for future events, probably the best validated methodology is TCD embolus detection. In one study evaluating 319 patients with asymptomatic carotid stenosis, it was found that the 10% of patients with two or more microemboli in 1 hour of monitoring had a 15.6% 1-year risk of stroke, while patients without microemboli had only a 1% 1-year risk of stroke.25 Similar findings were demonstrated in follow-up studies of 468 patients7 and in the Asymptomatic Carotid Emboli Study (ACES) among 467 patients (3.62% annual ipsilateral stroke risk with embolic signals versus 0.70% without embolic signals).26 As a general guideline, population screening for asymptomatic carotid artery stenosis is not recommended by the US Preventive Services Task Force, which found “no direct evidence that screening adults with duplex ultrasonography for asymptomatic stenosis reduces stroke.”27 In general, since about 2005, the risk of ipsilateral stroke with intensive medical therapy has been much lower than the risk of CEA or carotid artery stenting, even in the carefully controlled clinical trials discussed in this article. The studies of risk stratification using TCD suggest this may be a useful tool to identify those patients with the highest near-term risk of ipsilateral stroke.
Endarterectomy for asymptomatic carotid stenosis. The Asymptomatic Carotid Atherosclerosis Study (ACAS), which included 1662 patients, was the first large-scale study comparing CEA plus best medical therapy to medical therapy alone.28 A composite primary outcome of any stroke or death occurring in the perioperative period and ipsilateral cerebral infarction thereafter was evaluated. A clear benefit was seen in those undergoing CEA, leading the study to be stopped early. Patients were randomly assigned to surgery as based on contrast angiography that demonstrated diameter-reducing lesions of 60% or greater using the North American measurement method. Both treatment groups received what was considered the best medical management at the time. The aggregate risk over 5 years for any perioperative stroke, ipsilateral stroke, and death was 5.1% among the surgical patients and 11% among the medical patients (relative risk reduction 53%; 95% confidence interval 22% to 72%).
The Asymptomatic Carotid Surgery Trial (ACST) included 3120 patients with asymptomatic carotid stenoses of 60% or greater, as measured by duplex ultrasonography.29 Subjects were randomly assigned to immediate CEA versus indefinite deferral of the operation. The trial end points were perioperative mortality and morbidity (stroke and MI) and the incidence of non-perioperative stroke. Excluding perioperative events and nonstroke mortality, stroke risks (immediate versus deferred CEA) were 4.1% versus 10.0% at 5 years (gain 5.9%; 95% confidence interval 4.0% to 7.8%) and 10.8% versus 16.9% at 10 years (gain 6.1%; 95% confidence interval 2.7% to 9.4%). Subgroup analysis demonstrated the benefits of CEA were confined to patients younger than 75 years of age.
Some caveats regarding these trials should be considered. First, it should be noted that both ACAS and ACST were conducted at a time when best medical management was far less than the intensive medical therapy implemented today. Second, careful screening of surgeons participating in these clinical trials potentially led to results that cannot be generalized to the community. This is particularly evident when the complications from angiography are removed from the surgical groups; when this is done, the 30-day rate of stroke and death for CEA in ACAS drops to 1.52%.30 Study complication rates are often lower than what is seen in standard practice. In general, current surgical best practice restricts CEA for asymptomatic carotid stenosis to patients with 70% or greater carotid stenosis if the surgery can be performed with a 3% or less risk of perioperative complications. Of note, one recent review suggested that for patients who are medically stable and have a life expectancy of at least 5 years and a high-grade (80% or greater) asymptomatic carotid stenosis at baseline or have progression to 80% or greater stenosis despite intensive medical therapy while under observation, CEA is reasonable, provided the combined perioperative risk of stroke and death is less than 3%.31 Further research regarding this topic is ongoing, with the NINDS-sponsored Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Study (CREST-2) comparing centrally managed intensive medical therapy alone to intensive medical therapy with CEA or carotid artery stenting.32
Endovascular treatment for asymptomatic carotid stenosis. Carotid angioplasty and stenting was initially evaluated in patients thought to be at high risk for CEA in the Stenting and Angioplasty With Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial.33 Using a composite outcome of stroke, MI, or death within 30 days or death resulting from neurologic cause or ipsilateral stroke between 31 and 365 days, it was demonstrated that carotid artery stenting was not inferior (within 3%; P=.004) to CEA. Rates of stroke, MI, or death were 10.2% with CEA (P=.20) and 5.4% with carotid artery stenting at 30 days and 21.5% among patients who received CEA and 9.9% among patients who received carotid artery stenting (P=.02) at 1 year. Of note, 3-year outcomes among patients receiving carotid artery stenting demonstrated a significantly higher death rate (20.0%) than stroke rate (10.1%).34 Further, there was no medically treated control group in SAPPHIRE. The high complication rates in both treatment groups raised concerns about the benefit of either intervention over medical therapy alone.
The Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) enrolled patients with both symptomatic and asymptomatic carotid stenosis who were eligible for either CEA or carotid artery stenting.35 Patients with asymptomatic carotid stenosis could be included in the study if their stenosis was 60% or greater on angiography, 70% or greater on ultrasound, or 80% or greater on CTA or MRA if the stenosis on ultrasound was 50% to 69%.3,35 Patients were randomly assigned based upon symptom status. The primary end point was a composite of stroke, MI, or death resulting from any cause during the periprocedural period or any ipsilateral stroke within 4 years after randomization.3,35 No statistically significant difference was shown in the 4-year occurrence rates of the composite primary end point between carotid artery stenting (7.2%) and CEA (6.8%; hazard ratio, 1.11; 95% confidence interval 0.81–1.51; P=.51), without any significant heterogeneity based on symptom status. Notably, the primary end point in CREST demonstrated an interaction with age, with age older than 70 years showing a significant benefit for CEA over carotid artery stenting. Therefore, it is important to consider patient age when considering the two procedures in any specific patient. Periprocedural stroke occurred more often in patients undergoing carotid artery stenting than CEA (4.1% versus 2.3%; P=.01), with periprocedural MI occurring less frequently in those undergoing carotid artery stenting than CEA (1.1% versus 2.3%; P=.03). Unfortunately, as consistent with several of these trials, the lack of medically treated control groups in CREST complicates the interpretation of these results. The ongoing CREST-2 trial will compare centrally managed intensive medical therapy with or without carotid artery stenting or CEA (Case 7-1).
In practice, carotid artery stenting should be reserved for patients who are at high risk of stroke and have anatomic features that would make CEA difficult. Such considerations include severe comorbidities (eg, congestive heart failure, angina, coronary artery disease, ejection fraction 30% or less, recent MI, severe lung or renal disease) and anatomic factors (eg, prior neck operation or irradiation, post-CEA restenosis, surgically inaccessible lesions above C2 or below the clavicle, contralateral carotid occlusion, contralateral vocal cord palsy, or the presence of a tracheostomy).4 As consistent with recent guidelines, patients with asymptomatic carotid stenosis should be prescribed a daily aspirin and statin and screened for other treatable risk factors with appropriate medical therapies and lifestyle changes instituted. It is reasonable to consider performing CEA in patients who are asymptomatic and have greater than 70% stenosis of the ICA if the risk of perioperative stroke, MI, and death is low (less than 3%). In patients with greater than 50% stenosis, it is reasonable to perform annual duplex ultrasonography; however, screening low-risk populations for asymptomatic carotid artery stenosis is not recommended.3,4
Case 7-1
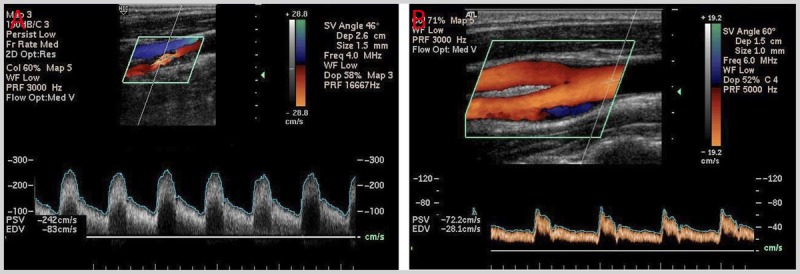
A 77-year-old woman presented for a neurologic evaluation after “some blockage” was detected in her “right neck artery” during ultrasound screening at a local mall. She had a history of dyslipidemia and hypertension and was a former heavy smoker, and her mother had a stroke in her late fifties. She denied prior stroke or transient ischemic attack symptoms. She was on aspirin 325 mg/d, atorvastatin 20 mg/d, amlodipine 10 mg/d, and a diuretic. Her blood pressure was 130/80 mm Hg, and her heart rate was 70 beats/min and regular. Neurologic examination was normal, except for the presence of a right carotid bruit. Carotid duplex ultrasonography was ordered and revealed bilateral plaques, with 70% or greater stenosis (peak systolic velocity = 242 cm/s) on the right and less than 50% (peak systolic velocity = 72 cm/s) on the left (Figure 7-2). CT angiography (CTA) was performed and interpreted as showing approximately 70% stenosis on the right and approximately 30% stenosis on the left. Low-density lipoprotein was 77 mg/dL. The patient was counseled regarding the uncertain benefit of revascularization in her age group.
FIGURE 7-2.
Carotid duplex ultrasonography of the patient in Case 7-1. A, Right internal carotid artery: greater than 70% stenosis (peak systolic velocity = 242 cm/s). B, Left internal carotid artery: less than 50% stenosis (peak systolic velocity = 72 cm/s).
Comment. This patient should be placed on intensive medical therapy, including her current agents and increasing her moderate-intensity statin therapy of atorvastatin to 40 mg/d as based upon her age and low-density lipoprotein level. This type of patient could be considered for enrollment in a clinical trial such as the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Study (CREST-2), which is comparing outcomes with intensive medical therapy alone versus intensive medical therapy plus carotid endarterectomy or carotid artery stenting.
Symptomatic Extracranial Carotid Stenosis
Over the past half century, numerous clinical trials have compared CEA plus medical therapy to medical therapy alone in the setting of symptomatic carotid stenosis. Many of these studies predate the intensive medical therapy now recommended. Further, CEA techniques have evolved and carotid artery stenting has emerged as an alternative preventive treatment.
Endarterectomy for symptomatic carotid stenosis. Three important randomized clinical trials established the superiority of CEA plus medical therapy over medical therapy alone in the setting of symptomatic high-grade (greater than 70% angiographic stenosis) carotid stenosis: the European Carotid Surgery Trial (ECST),36 NASCET,18 and the US Department of Veterans Affairs Cooperative Study Program (CSP).37 Patients with symptomatic carotid stenosis included those who had both greater than 70% ipsilateral carotid stenosis and TIAs, transient monocular blindness, or nondisabling strokes. A pooled analysis of these three randomized trials included more than 3000 patients with symptomatic carotid stenosis and demonstrated a 30-day stroke and death rate of 7.1% in patients who were surgically treated.38 In patients with 70% to 99% (severe) stenosis, NASCET criteria found that for every six patients treated, one major stroke would be prevented at 2 years (ie, a number needed to treat of 6). Additionally, all three trials demonstrated that for patients with less than 50% (mild) stenoses, surgical intervention did not reduce stroke risk. The role of CEA was less clear among patients with symptomatic stenosis in the 50% to 69% (moderate) range. Among the 858 patients who were symptomatic with 50% to 69% stenosis in NASCET, the 5-year rate of any ipsilateral stroke was 15.7% in those surgically treated versus 22.2% in those medically treated (P=.045).18 Therefore, 15 patients would have to undergo CEA to prevent one ipsilateral stroke during the 5-year follow-up period. As such, CEA is only justifiable when the risk-benefit ratio favors the patient when evaluating surgical and anesthetic risks. Given that medical management has improved since NASCET, current guidelines advise proceeding with CEA in the setting of symptomatic stenosis only if the surgeon’s rate for perioperative stroke or death is less than 6%.
In summary, for patients with a TIA or ischemic stroke within the past 6 months and ipsilateral severe (70% to 99%) carotid artery stenosis, CEA is recommended; for those with moderate (50% to 69%) carotid stenosis, CEA is recommended depending on patient-specific factors, such as age, sex, and comorbidities; and for those with a degree of stenosis of less than 50%, CEA and carotid artery stenting are not recommended.3,4
Patient-selection criteria influencing surgical risk should include sex and age. Subgroup analyses of the NASCET trial raised concerns regarding the benefit of CEA in women with symptomatic carotid stenosis, suggesting that women are more likely to have less favorable outcomes, including surgical mortality, neurologic morbidity, and recurrent carotid stenosis (14% in women versus 3.9% in men; P=.008).39 Notably, CREST was designed with preplanned subgroup analysis intended to evaluate the effects of sex and age on the primary outcome end point and found no significant interaction in the primary end point by sex. However, CREST did identify a significant interaction in relation to age, with superior results for CEA in patients older than 70 years of age.35,40
The optimal timing of carotid revascularization via CEA after a completed nondisabling stroke has been defined to be within 2 weeks if no contraindications exist. This time period is driven by data from the three major randomized clinical trials mentioned previously, among others. In these trials, the median time from randomization to surgery was 2 to 14 days, with approximately one-third of the perioperative strokes occurring within this time interval. Among the patients who were medically treated, the risk of stroke was also greatest in the first 2 weeks. After 2 to 3 years, the annual rate of stroke among the patients who were medically treated approached that of patients with asymptomatic carotid stenosis. Also of note, these three trials included only patients with TIA or nondisabling strokes, further reporting low intracerebral hemorrhage rates as associated with CEA (0.2%). Perioperative intracerebral hemorrhage risk may be increased in patients with large cerebral infarctions undergoing early surgery via a hyperperfusion or reperfusion syndrome because of the sudden increase in perfusion of the vasculature distal to stenosis. Optimal control of blood pressure before, during, and after the procedure is emphasized. As a general rule, before elective CEA, a systolic pressure of 160 mm Hg or less should be targeted, with therapy continuing up to the morning of surgery, with the possible exceptions of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists, with normal therapy restarting as soon as possible after surgery with a goal systolic pressure of 140 mm Hg or less. However, it is important to emphasize that therapy should be tailored to the individual patient.
Endovascular treatment for symptomatic carotid stenosis. The theoretical advantages of carotid artery stenting being a less invasive procedure resulting in decreased patient discomfort and a shorter recovery period were indeed demonstrated in CREST, with an improved health-related quality of life in the perioperative period, although this difference was not sustained at 1 year.41 As mentioned previously, in the past carotid artery stenting was typically reserved for patients who were considered high risk for CEA. The majority of the published trials evaluating carotid artery stenting have been industry sponsored, focusing on the efficacy of a single stent/neuroprotection system. The previously described SAPPHIRE trial had the primary objective of comparing the safety and efficacy of carotid artery stenting with an embolic protection device to CEA in 334 high-risk patients with symptomatic and asymptomatic carotid stenosis.33 In the periprocedural period (up to 30 days), the cumulative incidence of stroke, MI, or death was 4.8% among patients assigned to receive a stent and 9.8% among those assigned to undergo endarterectomy. One-year rates of the primary end point (death, stroke, or MI at 30 days plus ipsilateral stroke or death of neurologic causes within 31 days to 1 year) were 12.2% for carotid artery stenting versus 20.1% for CEA (P=.05) and were primarily driven by differences in the periprocedural MI rates. Overall, the primary conclusion from this trial was that carotid artery stenting was noninferior to CEA in this high-risk cohort. However, outcome analyses raised concerns that neither procedure was beneficial as compared with medical management in patients with asymptomatic carotid stenosis.
Several other randomized controlled trials have compared CEA and carotid artery stenting for patients with symptomatic carotid stenosis, including the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS),42 Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S), Stent-Supported Percutaneous Angioplasty of the Carotid Artery Versus Endarterectomy (SPACE), and the International Carotid Stenting Study (ICSS). These trials have been analyzed in isolation and the latter three in aggregate,43,44 with a preplanned meta-analysis demonstrating the rate of stroke and death at 120 days after randomization to be 8.9% for carotid artery stenting and 5.8% for CEA (P=.0006). Notably, in subgroup analyses, among patients 70 years of age or older, the rate of stroke or death at 120 days was 12.0% with carotid artery stenting compared with 5.9% with CEA (P=.0053). In patients younger than 70 years of age, no significant difference in outcome was shown between carotid artery stenting and CEA.44
CREST compared the efficacy of carotid artery stenting versus CEA among 2502 asymptomatic and symptomatic participants with carotid stenosis (greater than 50% by angiography or greater than 70% by ultrasonography).35 No significant difference was demonstrated in the composite primary outcome (30-day rate of stroke, death, and MI and 4-year ipsilateral stroke) among those treated with carotid artery stenting versus CEA (7.2% versus 6.8%; P=.51). Among patients with asymptomatic carotid stenosis, the 4-year primary outcome rate was 5.6% with carotid artery stenting versus 4.9% with CEA (P=.56), and among patients with symptomatic carotid stenosis, the rates were 8.6% with carotid artery stenting versus 8.4% with CEA (P=.69). When analyzing all patients, an interaction between age and treatment efficacy was shown (P=.02), demonstrating increased risk of carotid artery stenting in patients who were older. The risk of MI did not increase with age in either treatment group. The effects of age were primarily driven by stroke risk, which showed greater increase with age in the carotid artery stenting group than in the CEA group. The age at which the hazard ratio was 1.0 was approximately 70 years for the primary outcomes and 64 years for stroke. No difference in periprocedural events was shown between carotid artery stenting and CEA among men, but a nonstatistically significant trend toward fewer events was demonstrated with women and CEA. Periprocedural complications were lower in CREST compared with older trials. An analysis for the type of periprocedural complication identified important distinctions. Patients who had carotid artery stenting had lower rates of MI than patients who had CEA (1.1% versus 2.3%; 95% confidence interval 0.26–0.94) but higher rates of stroke (4.1% versus 2.3%; 95% confidence interval 1.14–2.82).35
In summary and as consistent with published guidelines, carotid artery stenting can be considered as an alternative to CEA for patients with symptomatic carotid stenosis with greater than 70% stenosis if the anticipated rate of periprocedural stroke or death is less than 6%; age should be considered when choosing between CEA and carotid artery stenting. For older patients (70 years of age or older), CEA may be associated with improved outcome compared with carotid artery stenting, in particular when arterial anatomy is unfavorable for endovascular intervention (Case 7-2). For younger patients, carotid artery stenting is equivalent to CEA in terms of risk for periprocedural complications (eg, stroke, MI, or death) and long-term risk for ipsilateral stroke.3
Follow-up imaging and restenosis after carotid endarterectomy or carotid artery stenting. In ACAS, the risk for restenosis after CEA, defined as 60% or greater narrowing of the lumen, was greatest in the first 18 months following surgery (7.6%), with a low incidence of 1.9% over the next 42 months. Of note, these 18-month estimates are similar to the findings from the CEA arm of the CREST trial, which demonstrated a 6.3% risk of restenosis greater than 70% after 24 months of observation. In a 2012 review representing the most reliable data on this topic, 2191 CREST patients were evaluated using standardized ultrasonography to examine the incidence of restenosis.45 At 2 years, no difference in the incidence of restenosis was shown between the two groups, 6% among patients who received carotid artery stenting and 6.3% among patients who received CEA (P=.58). Diabetes mellitus, hypertension, and female sex were independent predictors of restenosis. Smoking was an independent predictor for restenosis with CEA but not with carotid artery stenting. Therefore, unless a patient has recurrent symptoms, the need for repeat or surveillance ultrasonography after carotid revascularization is not well established.
Extracranial-intracranial bypass. The International Cooperative Study of Extracranial-Intracranial (EC-IC) Bypass Study was the first major trial of EC-IC bypass surgery, randomly assigning 1377 participants within 3 months of a TIA or minor ischemic stroke to surgery or best medical management.46 Eligible patients had severe stenosis of the (surgically inaccessible) ipsilateral distal ICA, occlusion of the ipsilateral midcervical ICA, or severe narrowing or occlusion of the ipsilateral middle cerebral artery (MCA). After approximately 5 years of follow-up, the primary outcome (fatal or nonfatal stroke) occurred more often in the surgical patients. EC-IC bypass was more recently evaluated for ipsilateral stroke prevention in 195 patients with evidence of hemodynamic cerebral ischemia distal to a symptomatic ipsilateral carotid occlusion using positron emission tomography (PET).47 The trial was stopped early because of futility. The 30-day rate of ipsilateral stroke was 14.4% in the surgical arm and 2.0% in the nonsurgical arm. However, the 2-year rate for the primary outcome (30-day stroke or death or subsequent ipsilateral stroke) was similar (P=.78), 21.0% in the surgical group and 22.7% in the nonsurgical group. As such, EC-IC bypass surgery is not recommended for patients with a recent TIA or ischemic stroke ipsilateral to a stenosis or occlusion of the middle cerebral or carotid artery and is considered investigational for those with progressive symptoms despite optimal medical management.
Case 7-2
A 78-year-old man with a history of diabetes mellitus and current smoking presented to the emergency department because of several episodes of transient speech difficulty and right hand weakness occurring over the previous 2 days. He also reported flulike symptoms, including a productive and persistent cough that had worsened recently. He stated he would not have come in, but “he couldn’t hold his cigarettes.” He was on aspirin 81 mg/d and metformin but was not on a statin. His blood pressure was 135/75 mm Hg. Examination was notable for coarse breath sounds bilaterally and decreased fine finger strength in the right hand rated at 3/5, but was otherwise normal. His initial head CT was read as normal, with a subsequent brain MRI demonstrating a small cortical infarct in the left frontal lobe on the diffusion sequence.Magnetic resonance angiography (MRA) demonstrated severe left internal carotid artery (ICA) stenosis just distal to the bulb (Figures 7-3A and 7-3B). Carotid duplex ultrasonography demonstrated severe left ICA stenosis (80% to 99%) and less than 40% stenosis on the right side. Catheter-based angiography confirmed a focal high-grade ICA stenosis (Figure 7-3C). Given the clinical transient ischemic attacks, the small infarct consistent with the proximal large artery atherosclerosis lesion, and the large amount of brain at risk, he was scheduled for an urgent carotid endarterectomy (CEA) to occur the following day.
FIGURE 7-3.
Imaging of the patient in Case 7-2 with severe atherosclerotic high-grade stenosis in the left internal carotid artery just distal to the bifurcation as shown onmagnetic resonance angiography (MRA) sequences (A, time-of flight; B, noncontrast arterial spin labeling); trickle flow seen on conventional catheter angiogram (C).
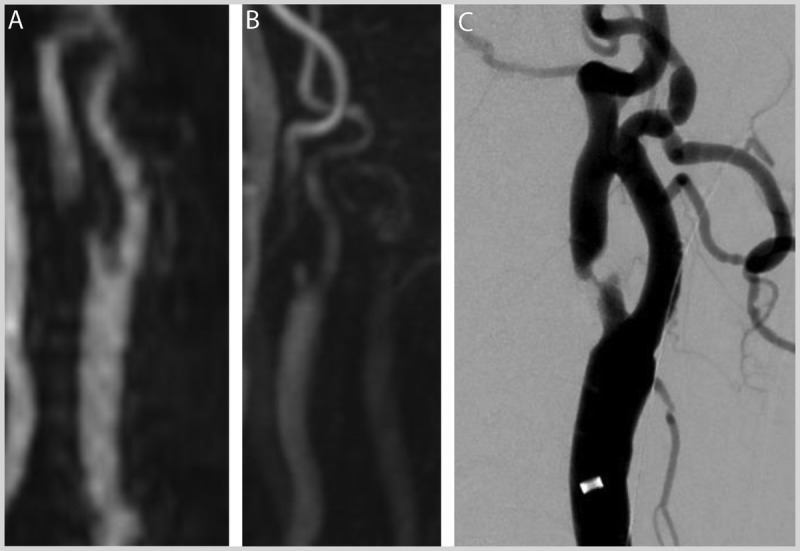
Comment. Because of this patient’s age and symptoms, urgent carotid revascularization was recommended, with CEA preferred. Because of the higher complication rate with carotid artery stenting in patients 70 years of age or older, CEA is preferred over carotid artery stenting. His blood pressure was normal, lowering his risks associated with reperfusion. He should be counseled to stop smoking and provided with aggressive medical therapy and close outpatient follow-up.
INTRACRANIAL ATHEROSCLEROSIS
Intracranial atherosclerosis is a common cause of stroke carrying a high risk for recurrence. To date, only a few large multicenter randomized trials evaluating stroke preventive therapies for this disease have been conducted; the two primary trials are the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study48 and the previously mentioned SAMMPRIS study.8 Notably, the results of these studies infer on the management of both symptomatic and asymptomatic intracranial atherosclerotic disease.
In the WASID study, 569 patients were randomly assigned to aspirin 1300 mg or warfarin (target international normalized ratio [INR] 2 to 3) following a TIA or stroke that was attributable to 50% to 99% intracranial stenoses of the MCA, intracranial ICA, intracranial vertebral artery, or basilar artery. The trial was stopped early because of higher rates of death and major hemorrhage in the warfarin arm. Over a mean follow-up of 1.8 years, the primary end point (ischemic stroke, brain hemorrhage, or nonstroke vascular death) occurred in 22% of patients in both treatment arms. The 1- and 2-year rates of stroke in the territory of the stenotic artery were 11% and 13% in the warfarin arm and 12% and 15% in the aspirin arm, respectively. In a combined analysis of both arms, the rates of stroke in the territory of the stenotic artery at 1 year were approximately 7% in patients with 50% to 69% stenosis and 18% in patients with 70% or greater stenosis.49 Follow-up analyses did not identify any subgroup that benefited from warfarin, including patients who had their qualifying event while taking aspirin. As such, current guidelines recommend that patients with a stroke or TIA caused by 50% to 99% stenosis of a major intracranial artery be treated with aspirin 325 mg/d in preference to warfarin. Further, while concern existed that blood pressure lowering could lead to reduced cerebral blood flow, thereby causing an increased stroke risk in patients with large vessel stenosis, follow-up analysis demonstrated that patients with a mean systolic blood pressure less than 140 mm Hg had a significantly reduced risk of recurrent stroke compared with patients with a mean systolic blood pressure of 140 mm Hg or higher (P=.01).50 Patients with a mean LDL-C less than 100 mg/dL had a significantly reduced risk of recurrent stroke as compared with patients with a mean LDL-C of 100 mg/dL or higher (P=.03).
The SAMMPRIS trial compared endovascular therapy versus medical therapy for the prevention of recurrent stroke among patients with symptomatic intracranial arterial stenosis.8 In SAMMPRIS, patients with TIA or stroke within the past 30 days related to 70% to 99% stenosis of a major intracranial artery were randomly assigned to aggressive medical management alone or aggressive medical management plus percutaneous transluminal angioplasty and stenting (PTAS) using a self-expanding stent. Intensive medical therapy in both arms was the same and consisted of aspirin 325 mg/d and clopidogrel 75 mg/d for 90 days after enrollment, intensive risk factor management that primarily targeted systolic blood pressure lower than 140 mm Hg (lower than 130 mm Hg in patients with diabetes mellitus) and LDL-C lower than 70 mg/dL, and a lifestyle modification program.8 Enrollment in SAMMPRIS was stopped early after 451 patients had been assigned because the 30-day rate of stroke and death (primary end point) was significantly higher in the PTAS arm. Within 30 days of enrollment, a statistically significant difference in stroke or death was seen between the two arms, occurring in 13 patients (5.8%) in the medical arm and in 33 patients (14.7%) in the PTAS arm (P=.002). Also, at 1 year, the primary end point rate was significantly higher in the PTAS arm (20.0%) versus the medical arm (12.2%; P=.009), primarily driven by the increased 30-day events in the PTAS arm. A subsequent analysis of the 30-day events in the SAMMPRIS PTAS arm revealed that a large number of the ischemic strokes occurred from occlusion of perforators (basilar perforators to the pons or lenticulostriate perforators from the MCA) with the PTAS occluding the perforator takeoffs (ie, ostium). Of the strokes that occurred within 30 days, 10 of 33 (30.3%) in the PTAS arm and none of 12 (0%) in the medical arm were symptomatic brain hemorrhages (P=.04). The results of the medical arm demonstrated better than expected 1-year event rates as compared with WASID (12.2% observed versus 25% expected) and were thought to be associated with the intensive medical therapy used in the trial. A key distinction is that patients in the WASID study were treated with aspirin 1300 mg/d, while the SAMMPRIS medical arm used aspirin 325 mg/d (in combination with clopidogrel 75 mg/d) while achieving favorable rates of stroke as compared with the intervention arm. Lower doses of aspirin have also been effective in other large trials of secondary prevention, many of which enrolled patients with more heterogeneous stroke subtypes. Notably, of the 451 patients enrolled in SAMMPRIS, 284 (63%) had their qualifying event while undergoing antithrombotic therapy. In this large subgroup of the SAMMPRIS cohort, the rates of the primary end point were 16.0% and 4.3% at 30 days and 20.9% and 12.9% at 1 year in the stenting and medical arms, respectively (P=.03).51 Overall, these results indicate that stenting (with the tested system) is not a safe or effective rescue treatment for patients who experience a TIA or stroke while already being treated with antithrombotic therapy. As such, current guidelines recommend that in patients with a recent stroke or TIA (within 30 days) attributable to severe stenosis (70% to 99%) of a major intracranial artery, it is reasonable to add clopidogrel 75 mg/d to aspirin for 90 days, along with the initiation of high-potency statin therapy and a goal systolic blood pressure below 140 mm Hg (Case 7-3).3 For patients with a stroke or TIA attributable to stenosis (greater than 50%) of a major intracranial artery, angioplasty or stenting is not recommended given the low rate of stroke with medical management and the inherent periprocedural risk of endovascular treatment, even among those already taking an antithrombotic agent at the time of the stroke or TIA.3 Notably, the current guidelines emphasizing maximal medical therapy after a stroke or TIA also apply to asymptomatic intracranial atherosclerotic disease.
One other notable study in the setting of intracranial stenosis is the previously described EC-IC Bypass Study.46 While the focus of this study was patients who were symptomatic with extracranial carotid occlusion, it also included patients with MCA stenosis and patients with ICA stenosis above the second cervical vertebra (C2). Specifically, 109 patients with 70% or greater MCA stenosis and 149 patients with 70% or greater ICA stenosis were randomly assigned to bypass surgery or medical treatment with aspirin 1300 mg/d and followed for a mean of about 4.5 years. A statistically significant difference was demonstrated in the rates of stroke during follow-up in patients with 70% or greater MCA stenosis, favoring the medical arm (23.7%; 14 of 59) as compared to the bypass arm (44%; 22 of 50). Among patients with 70% or greater ICA stenosis above C2, the stroke rates during follow-up were 36.1% (26 of 72) in the medical arm and 37.7% (29 of 77) in the bypass arm. Given these results, EC-IC bypass has largely been discontinued as a treatment for intracranial stenosis.
Case 7-3
A 69-year-old man presented with multiple episodes of transient dizziness, feeling off balance, and double vision occurring over the previous 2 weeks. His double vision persisted, prompting him to come to the emergency department. He rarely saw doctors and appeared to have a history of uncontrolled hypertension, dyslipidemia, and diabetes mellitus. He reported remote cigarette smoking and continued to smoke one cigar per day. His initial head CT was negative for acute ischemia; however, the accompanying head and neck CT angiogram demonstrated severe but nonYflow-limiting stenosis in the distal third of the basilar artery. Brain MRI demonstrated a small region of ischemia in the superior right pons in the distribution of a perforator originating from the region of the stenosed distal basilar artery. The patient was placed on aspirin 81 mg/d, clopidogrel 75 mg/d, and rosuvastatin 40 mg/d, with blood pressure and diabetes mellitus medications instituted. After 3 months of dual antiplatelet therapy, clopidogrel was discontinued, and he continued on the aspirin and his other medications.
Comment. This patient was appropriately placed on a Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS)-style regimen. At present, no proven role exists for endovascular intervention in this type of patient. The importance of medication compliance and lifestyle modifications over the long term should be emphasized and periodically reinforced through close outpatient monitoring.
EXTRACRANIAL VERTEBRAL ARTERY ATHEROSCLEROTIC DISEASE
Extracranial vertebral artery atherosclerotic disease is a well-established cause of posterior circulation stroke. Proximal vertebral (V1 segment) lesions account for approximately 9% of all posterior circulation strokes,52 while vertebral artery ostial lesions may account for another one-third.53 Consistent with the anterior circulation, the two primary stroke mechanisms include plaque rupture with subsequent artery-to-artery thromboembolism and hemodynamic insufficiency. Treatment options for symptomatic extracranial vertebral artery atherosclerotic disease include intensive medical therapy, endovascular stenting, and, in rare cases, open surgical revascularization; while maximal medical therapy is the mainstay of treatment in asymptomatic extracranial vertebral artery atherosclerotic disease. Unfortunately, scant randomized trial results exist specific to this setting, although analyses of some participants in the previously mentioned CAVATAS trial42 as well as the Oxford Vascular Study (OXVASC)54 indicate that treatment should focus on vascular risk factor reduction. The most relevant study performed on this topic, the 2015 phase 2 Vertebral Artery Stenting Trial (VAST), was conducted in the Netherlands and identified patients with a recent TIA or minor stroke associated with an extracranial (or intracranial) vertebral artery stenosis of at least 50%.55 Patients were randomly assigned to stenting plus best medical treatment or best medical treatment alone. All patients received best medical treatment at the discretion of the treating neurologist, including antithrombotic agents, a statin, and rigorous control of other vascular risk factors. The primary outcome was the composite of vascular death, MI, or any stroke within 30 days after the start of treatment. The trial was stopped after the inclusion of 115 patients because of altered regulatory requirements, with 57 patients assigned to stenting and 58 to medical treatment alone. Three patients in the stenting group experienced the primary outcome (5%, 95% confidence interval 0% to 11%) versus one patient in the medical treatment group (2%, 95% confidence interval 0% to 5%). During the planned 4 years of follow-up, 60 serious adverse events (eight strokes) occurred in the stenting group and 56 serious adverse events (seven strokes) in the medical treatment group. The investigators concluded that stenting of symptomatic vertebral artery stenosis was associated with a major periprocedural vascular complication in about 1 in 20 patients and the risk of recurrent vertebrobasilar stroke under best medical treatment alone was low. Based upon these results, a phase 3 study was deemed unwarranted. Another study that completed enrollment in February 2015 is the Vertebral Artery Ischaemia Stenting Trial (VIST).56 This is a UK multiple-center randomized controlled trial comparing vertebral artery stenting/angioplasty versus the best medical therapy alone in patients with symptomatic vertebral artery stenosis greater than 50%. Recruitment was stopped early at 182 patients because of a cessation of funding as related to low recruitment. The primary end points are perioperative risk and long-term efficacy, not further specified; the final results are pending. One can also infer from SAMMPRIS,8 which evaluated the similar condition of recently symptomatic large vessel intracranial stenosis, that an aggressive medical therapy strategy, including dual antiplatelet therapy for 3 months, statin therapy, blood pressure and glycemic control, and risk factor modification, is highly effective for secondary prevention of stroke. It remains unclear if aggressive medical therapy is as effective for patients with symptoms caused by hemodynamic compromise from extracranial vertebral artery atherosclerotic disease.
In summary and as per current guidelines, routine preventive therapy with an emphasis on antithrombotic therapy, lipid lowering, blood pressure control, and lifestyle optimization is recommended for all patients with asymptomatic or recently symptomatic extracranial vertebral artery stenosis (Case 7-4).3
Numerous retrospective nonrandomized case series specific to stenting in the setting of extracranial vertebral artery atherosclerotic disease have been published. One review including 980 patients from 27 studies demonstrated a technical success rate of 99%, with a periprocedural risk of 1.2% for stroke and 0.9% for TIA.57 In this study, participants were followed for an average of 21 months perioperatively, with vertebrobasilar territory stroke occurring in 1.3% and TIA occurring in 6.5%. In a different prospectively maintained database of 114 patients undergoing stenting for 127 vertebral ostial lesions, of which 88% were considered to be either “highly likely” or “probably” the cause of the patient’s posterior circulation symptoms, recurrence of symptoms at 1 year was just 2% after stenting.53 In another review of 300 endovascular interventions in symptomatic vertebral artery origin stenosis, periprocedural neurologic complications occurred in 5.5%, and the restenosis rate was 26%.58 Based upon these results, current guidelines3,4 indicate that endovascular stenting of patients with extracranial vertebral stenosis may be considered when patients are having symptoms despite optimal medical treatment.
Symptomatic restenosis rates in the setting of extracranial vertebral artery atherosclerotic disease stenting remain uncertain and are a topic of study. A 2016 pooled analysis59 of five studies comparing drug-eluting versus bare-metal stents found no significant difference in the technical success (odds ratio = 1.53, P=.62), clinical success (odds ratio = 1.92, P=.27), and periprocedural complications (odds ratio = 0.74, P=.61) between the two stent types. A significantly higher restenosis rate in the bare metal stent group (33.57%) as compared to the drug-eluting stent group (15.49%) was identified (P=.001). When compared with the drug-eluting stent group, the bare metal stent group also had a significantly higher rate of recurrent symptoms (2.76% versus 11.26%; odds ratio = 3.32, P=.01).
Open surgical procedures for revascularization of extracranial vertebral artery atherosclerotic disease include vertebral artery transposition and vertebral artery endarterectomy. While such procedures are performed rarely, they can be considered in patients with persistent symptoms despite intensive medical therapy. In one older series of 27 patients, no perioperative stroke or death was seen and two permanent neurologic complications occurred (one case of Horner syndrome and one case of hoarseness); in addition, two patients developed posterior circulation neurologic symptoms after the perioperative period.60 Larger randomized trials are required to better define evidence-based recommendations for patients with extracranial vertebral artery atherosclerotic disease and to determine if vertebral artery stenting is a viable primary treatment option for patients with symptomatic disease.
Case 7-4
A 69-year-old woman presented to the emergency department with acute dizziness and difficulty walking. She reported a history of hypertension, dyslipidemia, and former smoking. She was obese and also reported chronic fatigue and poor sleep. Further, she stated she had been noncompliant with her medications as she had run out of them a few weeks previously. At presentation, her blood pressure was 167/88 mm Hg and she was in normal sinus rhythm. Initial head CT demonstrated diffuse periventricular white matter changes but no areas of obvious ischemia. MRI demonstrated a small left cerebellar ischemic stroke, and magnetic resonance angiography (MRA) of the head and neck demonstrated diffuse non–flow-limiting atherosclerosis throughout the anterior and posterior head and neck vasculature. Notably, the left vertebral artery takeoff was poorly visualized. A subsequent CT angiogram (CTA) of the neck demonstrated stenosis at the origin of both vertebral arteries, left greater than right as associated with calcific plaques. The patient was placed on daily aspirin, clopidogrel, and 40 mg rosuvastatin, and her blood pressure medication was reinstituted with good control. After discharge, an outpatient sleep study demonstrated obstructive sleep apnea, and she was placed on nighttime continuous positive airway pressure. After 3 months of dual antiplatelet therapy, the clopidogrel was discontinued while she continued on daily aspirin and a statin as well as her other medications.
Comment. Similar to the patient in Case 7-3, this patient was placed on a Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS)-style regimen. At present, endovascular intervention has no proven role in symptomatic extracranial vertebral artery disease. The importance of medication compliance and lifestyle modifications over the long term should be reinforced. Stenting and open surgical procedures can be considered if she experiences further ischemia despite optimal medical therapy.
CONCLUSION
Extracranial and intracranial large artery atherosclerosis are common causes of ischemic stroke and TIA. Lifelong vascular risk factor optimization via sustained behavioral modifications and intensive medical therapy are the key elements to reduce future stroke risk. Intensive medical therapy achieves low rates of stroke and death in asymptomatic carotid stenosis. Evidence indicates that patients with moderate to severe symptomatic carotid stenosis should undergo carotid revascularization sooner rather than later and that, in most settings, the risk of stroke or death is lower using carotid endarterectomy than carotid stenting. The risk of stroke or death with stenting is greatest among patients who are older and women. When considering a revascularization procedure for carotid stenosis, patient demographics, comorbidities, and the periprocedural risks of stroke and death should be carefully considered. In the settings of intracranial atherosclerotic disease or extracranial vertebral artery atherosclerotic disease, the mainstay of stroke prevention is continuous vascular risk factor optimization via sustained behavioral modifications and intensive medical therapy.
KEY POINTS
Continuous vascular risk factor optimization via sustained behavioral modifications and intensive medical therapy is critical to prevent stroke in the setting of large artery atherosclerosis.
It is important to determine if the identified large artery atherosclerotic lesion is proximal to a vascular territory that corresponds to the patient’s stroke on imaging or symptoms in the setting of a transient ischemic attack.
Population-wide improved control of hypertension, dyslipidemia, and diabetes mellitus, coupled with a reduction in tobacco use, has resulted in a decline in stroke mortality from the third to the fifth leading cause of death in the United States.
Hypertension and diabetes mellitus remain undertreated, and personalized approaches to lifestyle changes and medical therapy are critical for successful stroke prevention.
Physicians should consider each patient on an individual basis, working to optimize their risk factor profile over the long term while inferring upon the most recent professional society statement recommendations.
Current surgical best practice restricts carotid endarterectomy for asymptomatic carotid stenosis to patients with 70% or greater carotid stenosis if the surgery can be performed with a 3% or less risk of perioperative complications.
As consistent with recent guidelines, patients with asymptomatic carotid stenosis should be prescribed a daily aspirin and statin and screened for other treatable risk factors with appropriate medical therapies and lifestyle changes instituted.
For patients with a transient ischemic attack or ischemic stroke within the past 6 months and ipsilateral severe (70% to 99%) carotid artery stenosis, carotid endarterectomy is recommended; for those with moderate (50% to 69%) carotid stenosis, carotid endarterectomy is recommended depending on patient-specific factors, such as age, sex, and comorbidities; and for those with a degree of stenosis of less than 50%, carotid endarterectomy and carotid artery stenting are not recommended.
The optimal timing of carotid revascularization via carotid endarterectomy after a completed nondisabling stroke has been defined to be within 2 weeks if no contraindications exist.
Carotid artery stenting can be considered as an alternative to carotid endarterectomy for patients who are symptomatic with greater than 70% stenosis if the anticipated rate of periprocedural stroke or death is less than 6%; age should be considered when choosing between carotid endarterectomy and carotid artery stenting.
For patients who are older (70 years of age or older), carotid endarterectomy may be associated with improved outcome compared with carotid artery stenting, in particular when arterial anatomy is unfavorable for endovascular intervention. For patients who are younger, carotid artery stenting is equivalent to carotid endarterectomy in terms of risk for periprocedural complications (eg, stroke, myocardial infarction, or death) and long-term risk for ipsilateral stroke.
Extracranial-intracranial bypass surgery is not recommended for patients with a recent transient ischemic attack or ischemic stroke ipsilateral to a stenosis or occlusion of the middle cerebral or carotid artery and is considered investigational for those with progressive symptoms despite optimal medical management.
Current guidelines recommend that patients with a stroke or transient ischemic attack caused by 50% to 99% stenosis of a major intracranial artery be treated with aspirin 325 mg/d in preference to warfarin.
Current guidelines recommend that in patients with a recent stroke or transient ischemic attack (within 30 days) attributable to severe stenosis (70% to 99%) of a major intracranial artery, it is reasonable to add clopidogrel to aspirin for 90 days, along with the initiation of high-potency statin therapy and a goal systolic blood pressure below 140 mm Hg.
For patients with a stroke or transient ischemic attack attributable to stenosis (greater than 50%) of a major intracranial artery, angioplasty or stenting is not recommended given the low rate of stroke with medical management and the inherent periprocedural risk of endovascular treatment, even among those already taking an antithrombotic agent at the time of the stroke or transient ischemic attack.
Routine preventive therapy with an emphasis on antithrombotic therapy, lipid lowering, blood pressure control, and lifestyle optimization is recommended for all patients with recently symptomatic extracranial vertebral artery stenosis.
Current guidelines indicate that endovascular stenting of patients with extracranial vertebral stenosis may be considered when patients are having symptoms despite optimal medical treatment.
While vertebral artery transposition and vertebral artery endarterectomy are performed rarely, they can be considered in patients with persistent symptoms despite intensive medical therapy.
ACKNOWLEDGMENTS
This work was supported by grants from the American Heart Association (Cardiovascular Genome Phenome Study ID number 15GPSPG237700000), the National Institutes of Health (1U01NS069208), and the US Department of Veterans Affairs.
REFERENCES
- 1.Gounis MJ, van der Marel K, Marosfoi M, et al. Imaging inflammation in cerebrovascular disease. Stroke 2015;46(10):2991–2997. doi:10.1161/STROKEAHA.115.008229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC 2013. Detailed Tables for the National Vital Statistics Report (NVSR) “Deaths: Final Data for 2013.”, authors. cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed December 6, 2016.
- 3.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(12):3754–3832. doi:10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(7):2160–2236. doi:10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 5.Spence JD. Management of patients with an asymptomatic carotid stenosis—medical management, endovascular treatment, or carotid endarterectomy? Curr Neurol Neurosci Rep 2016;16(1):3 doi:10.1007/s11910–015–0605–6. [DOI] [PubMed] [Google Scholar]
- 6.Spence JD. Lessons from Africa: the importance of measuring plasma renin and aldosterone in resistant hypertension. Can J Cardiol 2012;28(3):254–257. doi:10.1016/j.cjca.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010;67(2):180–186. doi:10.1001/archneurol.2009.289. [DOI] [PubMed] [Google Scholar]
- 8.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365(11):993–1003. doi:10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Heart Association Professional Heart Daily. 2013 prevention guidelines tools: CV risk calculator. my.americanheart.org/cvriskcalculator. Accessed December 6, 2016.
- 10.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348(9038):1329–1339. doi:10.1016/S0140–6736(96)09457–3. [DOI] [PubMed] [Google Scholar]
- 11.Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008;359(12):1238–1251. doi:10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369(1):11–19. doi:10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Pan Y, Zhao X, et al. Clopidogrel With Aspirin in Acute Minor Stroke or Transient Ischemic Attack (CHANCE) Trial: One-Year Outcomes. Circulation 2015;132(1):40–46. doi:10.1161/CIRCULATIONAHA.114.014791. [DOI] [PubMed] [Google Scholar]
- 14. Platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trial. pointtrial.org. Accessed December 6, 2016.
- 15.Wang X, Lin WH, Zhao YD, et al. The effectiveness of dual antiplatelet treatment in acute ischemic stroke patients with intracranial arterial stenosis: a subgroup analysis of CLAIR study. Int J Stroke 2013;8(8):663–668. doi:10.1111/j.1747–4949.2012.00828.x. [DOI] [PubMed] [Google Scholar]
- 16.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 2005;111(17):2233–2240. doi:10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 17.Diener HC, Bogousslavsky J, Brass LM, et al. MATCH Investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high risk patients (MATCH): randomised, double-blind, placebo controlled trial. Lancet 2004;364(9431):331–337. doi:10.1016/S0140–6736(04)16721–4. [DOI] [PubMed] [Google Scholar]
- 18.Barnett HJM, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339(20):1415–1425. doi:10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 19.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 suppl 2):S1–45. doi:10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 20.SPRINT Research Group, Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373(22):2103–2116. doi:10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo BB, Bravata DM, Tobias LA, et al. Observational Study of Obstructive Sleep Apnea in Wake-Up Stroke: The SLEEP TIGHT Study. Cerebrovasc Dis 2016;41(5–6):233–241. doi:10.1159/000440736. [DOI] [PubMed] [Google Scholar]
- 22.Seminog OO, Goldacre MJ. Gout as a risk factor for myocardial infarction and stroke in England: evidence from record linkage studies. Rheumatology (Oxford) 2013;52(12):2251–2259. doi:10.1093/rheumatology/ket293. [DOI] [PubMed] [Google Scholar]
- 23.Stewart R, West M. Increasing evidence for an association between periodontitis and cardiovascular disease. Circulation 2016;133(6):549–551. doi:10.1161/CIRCULATIONAHA.115.020869. [DOI] [PubMed] [Google Scholar]
- 24.NINDS Stroke Genetics Network (SiGN); International Stroke Genetics Consortium (ISGC). Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study. Lancet Neurol 2015. doi:10.1016/S1474–4422(15)00338–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spence JD, Tamayo A, Lownie SP, et al. Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke 2005;36(11):2373–2378. doi:10.1161/01.STR.0000185922.49809.46. [DOI] [PubMed] [Google Scholar]
- 26.Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010;9(7):663–671. doi:10.1016/S1474–4422(10)70120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007;147(12):854–859. doi:10.7326/0003–4819–147–12–200712180–00005 [DOI] [PubMed] [Google Scholar]
- 28.Endarterectomy for asymptomatic carotid artery stenosis: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273(18):1421–1428. [PubMed] [Google Scholar]
- 29.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet 2004;363(9420):1491–1502. doi:10.1016/S0140–6736(04)16146–1. [DOI] [PubMed] [Google Scholar]
- 30.Moore WS, Young B, Baker WH, et al. Surgical results: a justification of the surgeon selection process for the ACAS trial. The ACAS Investigators. J Vasc Surg 1996;23(2):323–328. doi:10.1016/S0741–5214(96)70277-X. [DOI] [PubMed] [Google Scholar]
- 31.Mohler ER, Fairman RM. Management of asymptomatic carotid atherosclerotic disease. UpToDate. uptodate.com/contents/management-of-asymptomatic-carotid-atherosclerotic-disease. Updated June 24, 2016. Accessed December 6, 2016. [Google Scholar]
- 32.The carotid revascularization and medical management for asymptomatic carotid stenosis study. crest2trial.org. Accessed December 6, 2016. [Google Scholar]
- 33.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351(15):1493–1501. doi:10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 34.Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. 2008;358(15):1572–1579. doi:10.1056/NEJMoa0708028. [DOI] [PubMed] [Google Scholar]
- 35.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;363(1):11–23. doi:10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. European Carotid Surgery Trialists’ Collaborative Group. Lancet 1991;337(8752):1235–1243. doi:10.1016/0140–6736(91)92916-P. [PubMed] [Google Scholar]
- 37.Mayberg MR, Wilson SE, Yatsu F, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA 1991;266:3289–3294. [PubMed] [Google Scholar]
- 38.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361(9352):107–116. doi:10.1016/S0140–6736(03)12228–3. [DOI] [PubMed] [Google Scholar]
- 39.Hugl B, Oldenburg WA, Neuhauser B, Hakaim AG. Effect of age and gender on restenosis after carotid endarterectomy. Ann Vasc Surg 2006;20(5):602–608. doi:10.1007/s10016–006–9028–9. [DOI] [PubMed] [Google Scholar]
- 40.Howard VJ, Lutsep HL, Mackey A, et al. Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Lancet Neurol 2011;10(6):530–537. doi:10.1016/S1474–4422(11)70080–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen DJ, Stolker JM, Wang K, et al. Health-related quality of life after carotid stenting versus carotid endarterectomy: results from CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial). J Am Coll Cardiol 2011;58(15):1557–1565. doi:10.1016/j.jacc.2011.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 2001;357(9270):1729–1737. doi:10.1016/S0140–6736(00)04893–5. [PubMed] [Google Scholar]
- 43.International Carotid Stenting Study Investigators, Ederle J, Dobson J, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 2010;375(9719):985–997. doi:10.1016/S0140–6736(10)60239–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carotid Stenting Trialists’ Collaboration, Bonati LH, Dobson J, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010;376(9746):1062–1073. doi:10.1016/S0140–6736(10)61009–4. [DOI] [PubMed] [Google Scholar]
- 45.Lal BK, Beach KW, Roubin GS, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol 2012;11(9):755–763. doi:10.1016/S1474–4422(12)70159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med 1985;313(19):1191–1200. doi:10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 47.Powers WJ, Clarke WR, Grubb RL, Jr, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 2011;306(18):1983–1992. doi:10.1001/jama.2011.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352(13):1305–1316. doi:10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 49.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113(4):555–563. doi:10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 50.Turan TN, Cotsonis G, Lynn MJ, et al. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 2007;115(23):2969–2975. doi:10.1161/CIRCULATIONAHA.106.622464. [DOI] [PubMed] [Google Scholar]
- 51.Fiorella D, Derdeyn CP, Lynn MJ, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS). Stroke 2012;43:2682–2688. doi:10.1161/STROKEAHA.112.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wityk RJ, Chang HM, Rosengart A, et al. Proximal extracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol 1998;55(4):470–478. doi:10.1001/archneur.55.4.470. [DOI] [PubMed] [Google Scholar]
- 53.Al-Ali F, Barrow T, Duan L, et al. Vertebral artery ostium atherosclerotic plaque as a potential source of posterior circulation ischemic stroke: result from Borgess Medical Center Vertebral Artery Ostium Stenting Registry. Stroke 2011;42(9):2544–2549. doi:10.1161/STROKEAHA.110.610451. [DOI] [PubMed] [Google Scholar]
- 54.Marquardt L, Kuker W, Chandratheva A, et al. Incidence and prognosis of ≥50% symptomatic vertebral or basilar artery stenosis: prospective population-based study. Brain 2009;132(pt 4):982–988. doi:10.1093/brain/awp026. [DOI] [PubMed] [Google Scholar]
- 55.Compter A, van der Worp HB, Schonewille WJ, et al. Stenting versus medical treatment in patients with symptomatic vertebral artery stenosis: a randomised open-label phase 2 trial. Lancet Neurol 2015;14(6):606–614. doi:10.1016/S1474–4422(15)00017–4. [DOI] [PubMed] [Google Scholar]
- 56. University of Cambridge. VIST: the vertebral ischaemia stenting trial. www.vist.org.uk. Accessed November 2, 2016.
- 57.Stayman AN, Nogueira RG, Gupta R. A systematic review of stenting and angioplasty of symptomatic extracranial vertebral artery stenosis. Stroke 2011;42(8):2212–2216. doi:10.1161/STROKEAHA.110.611459. [DOI] [PubMed] [Google Scholar]
- 58.Eberhardt O, Naegele T, Raygrotzki S, et al. Stenting of vertebrobasilar arteries in symptomatic atherosclerotic disease and acute occlusion: case series and review of the literature. J Vasc Surg 2006;43(6):1145–1154. doi:10.1016/j.jvs.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 59.Tank VH, Ghosh R, Gupta V, et al. Drug eluting stents versus bare metal stents for the treatment of extracranial vertebral artery disease: a meta-analysis. J Neurointerv Surg 2016;8(8):770–774. doi:10.1136/neurintsurg-2015–011697. [DOI] [PubMed] [Google Scholar]
- 60.Berguer R, Bauer RB. Vertebral artery reconstruction. A successful technique in selected patients. Ann Surg 1981;193(4):441–447. doi:10.1097/00000658–198104000–00008. [DOI] [PMC free article] [PubMed] [Google Scholar]