Abstract
Introduction
Psychopathological origins of personally distressing, hypoactive sexual desire disorder (HSDD) in women are unknown, but are generally attributed to an inhibitory neural regulator, serotonin (5-HT). Flibanserin, a 5-HT1A agonist and 5-HT2A antagonist, shows promise as a treatment for HSDD.
Aim
To test the hypothesis that female marmoset sexual behavior is enhanced by flibanserin and diminished by 8-OH-DPAT, in order to evaluate the efficacy of serotonergic modulation of female sexual behavior in a pairmate social setting comparable to humans.
Methods
Sexual and social behavior were examined in 8 female marmoset monkeys receiving daily flibanserin (15mg/kg), 8-OH-DPAT (0.1 mg/kg) or corresponding vehicle for 15–16 weeks in a counterbalanced, within-subject design, while housed in long-term, stable male-female pairs.
Main outcome measures
Marmoset pairmate interactions, including sexual and social behavior, were scored during weeks 5–6 of daily flibanserin, 8-OH-DPAT or vehicle treatment. 24-h pharmacokinetic profiles of the drugs and their metabolites, as well as drug-induced acute symptoms of the 5-HT behavioral syndrome were also assessed.
Results
2-way analysis of variance reveals that flibanserin-treated females attract more male sexual interest (p = .020) and trigger increased grooming (p = .001) between partners. In contrast, 8-OH-DPAT-treated females show increased rejection of male sexual advances (p = .024), a tendency for decreased male sexual interest (p = .080), and increased aggression with their male pairmates (p = .049).
Conclusions
While 8-OH-DPAT-treated female marmosets display decreased sexual receptivity and increased aggressive interactions with their male pairmates, flibanserin-treated female marmosets demonstrate increased affiliative behavior with their male pairmates. Such pro-affiliation attributes may underly flibanserin’s effectiveness in treating HSDD in women.
Keywords: Flibanserin, female sexual function, HSDD, affiliative behavior, serotonin receptor, nonhuman primate
INTRODUCTION
In an estimated 10% of women (Clayton, 2010a), marked distress and interpersonal difficulty arise from unwanted, persistent or recurrent low sexual desire (hypoactive sexual desire disorder, HSDD; American Psychiatric Association’s Diagnostic and Statistical Manual, DSM-IV-TR). Psychopathogenesis of HSDD is not known, but neurotransmitter dysfunction has been proposed involving the excitatory regulators dopamine (DA) and norepinephrine (NE), as well as inhibitory serotonin (5-HT) (Pfaus, 2009; Clayton and Hamilton, 2010b). 5-HT is a key neurotransmitter involved in female sexual inhibition (Pfaus, 2009). Pharmacological manipulation of 5-HT commonly results in diminished female sexual satisfaction and activity, particularly in women prescribed selective serotonin reuptake inhibitors (SSRIs) for depression (Rosen et al, 1999). Animal studies that apply 5-HT receptor subtype specific ligands permit mechanistic examination of 5-HT-mediated effects on sexual behavior. There are 7 known 5-HT receptor families, each with its own specific brain distribution, as well as effects on behavior and physiology (Barnes and Sharp, 1999). For example, in rodents the sexually receptive female lordosis posture is inhibited by 5-HT1A receptor activation (Ahlenius et al, 1986; Uphouse et al, 1992) and 5-HT3 receptor antagonism (Maswood et al, 1997), but is facilitated by 5-HT2A/C receptor activation (Mendelson and Gorzalka, 1985).
Recently, flibanserin (2H-benzimidazol-2-one, 1,3-dihydro-1-[2-[4-[3-(trifluoromethyl) phenyl]-1-piperazinyl]ethyl]), an agonist of 5-HT1A and antagonist of 5-HT2A receptors (Borsini et al, 1995; Borsini et al, 2002), has been shown to stimulate sexual solicitation and receptivity in female rats (Gelez et al, 2010) and to improve sexual desire in women with major depression (Kennedy, 2010). Women with HSDD report increased satisfying sexual events, increased desire and decreased distress following flibanserin treatment (Stahl et al, 2010). R-(+)-8-hydroxy-2-(di-n-propylamino)-tetralin hydrobromide (8-OH-DPAT), however, a selective 5-HT1A receptor agonist (Arvidsson et al, 1981), diminishes female sexual receptivity in rats (Uphouse et al, 1991). Thus, despite the shared 5-HT1A agonist activity between flibanserin and 8-OH-DPAT, the contrasting effects of the two drugs on rodent female sexual behavior provides us with an opportunity to examine whether such contrasting behavioral outcomes translate to a nonhuman primate model, the common marmoset, in a well-established male-female pairmate social environment.
Female marmoset monkeys present an opportunity to contrast the effects of flibanserin and 8-OH-DPAT in an animal model that readily translates to humans because marmosets form stable, long-term, male-female relationships (Abbott et al, 2003) and display modest amounts of sexual behavior (Barnett et al, 2006). Unlike the multiple-mating social structures of rats and many nonhuman primates, such as macaques and baboons, marmoset sexual behavior most commonly occurs within stable male-female pairs (Evans and Poole, 1984; Abbott et al, 2003). During acceptance or rejection of a pairmate’s sexual advances, female marmosets can readily promote, prevent or terminate sexual interactions (Stevenson and Poole, 1976), and our recent development of a standardized behavioral testing paradigm permits repeatable, quantitative exploration of neurally active compounds that enhance or diminish female marmoset sexual behavior (Barnett et al, 2006).
AIMS
The aim of the present study was to test the hypothesis that female marmoset sexual behavior is enhanced by flibanserin and diminished by 8-OH-DPAT, in order to evaluate the efficacy of serotonergic modulation of female sexual behavior in a pairmate social setting comparable to humans.
METHODS
Study animals
This study was conducted in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act and its subsequent amendments. All animal procedures were reviewed and approved by the Graduate School Animal Care and Use Committee of the University of Wisconsin-Madison. The Wisconsin National Primate Research Center (WNPRC) is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care as part of the University of Wisconsin-Madison Graduate School. Sixteen adult (age 2–5 yr) nulliparous captive-born common marmoset (Callithrix jacchus) females were pair housed with similarly aged male partners at the WNPRC for 8–20 months before onset of this study. Females were housed with the same male partner for the entire study, as previously described (Hearn, 1977), and were ovariectomized and primed with either mid-follicular phase estradiol levels or no hormone (Barnett et al, 2006) before study onset. This model allows us to provide a repeatable estrogen replete (estradiol capsules implanted) or estrogen deficient (empty capsules implanted) hormonal environment in which sex hormone levels are stable and reflect, respectively, the equivalent of an estrogen-dominant, pre-ovulatory stage in the ovarian cycle or a post-menopausal stage.
Experimental design
A counterbalanced, cross-over study that applied within-subject comparisons, was designed to examine the effects of chronic (15–16 weeks) daily (12:00h–14:00h) administration of flibanserin (n=8; 15 mg/kg, orally (PO) in 1ml/kg vehicle; Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach an der Riss, Germany), 8-OH-DPAT (n=8; 0.1 mg/kg in 0.4ml/kg vehicle, injected subcutaneously (SC); Sigma-Aldrich St. Louis, MO, USA), or respective vehicles (for flibanserin, 98.5% of 0.5% hydroxycellulose solution and 1.5% of 1% polysorbate 80 solution, 1.0 ml/kg PO; for 8-OH-DPAT, 0.4 ml saline, SC). The study focused on two major behavioral outcomes: (1) sexual and social interactions between pairmates, and (2) acute manifestation of the 5-HT behavioral syndrome providing quantitative indication of continued drug efficacy.
As flibanserin was not previously administered to female marmosets, we performed an initial dose response study comparing male-female interactions observed after 5–6 weeks of oral dosing of 10 mg/kg (n=4) or 30 mg/kg (n=4) flibanserin to behavior observed during baseline male-female interactions made prior to these flibanserin treatments. We observed changes in selected behaviors (Supplementary Table S1) in our male-female testing paradigm, described below, 16–24 hours after daily flibanserin administration, compared to baseline. Blood samples assessing pharmacokinetics of both doses (Supplementary Figure S1) were obtained after 3–4 weeks. As similar results were obtained from both doses for behavioral and pharmacokinetic assessments, we decided to employ an intermediate dose of 15 mg/kg for the main study. The scaled equivalent in humans of 15 mg/kg flibanserin administered to marmosets is ~2.4 mg/kg (Sharma and McNeill, 2009) and approximately similar to ~1.7 mg/kg flibanserin administered to women in clinical trials (100 mg/day, assuming 60 kg body weight; Jolly et al, 2009).
8-OH-DPAT has previously been administered to marmosets in i.p. injections of 0.3 mg/kg, resulting in pronounced expression of the serotonin behavioral syndrome immediately following each treatment. 8-OH-DPAT at a dose of 0.3 mg/kg also induces scratching and diarrhea (Elliott et al, 1990). To minimize the latter responses and to remain within a dose range previously employed to consistently diminish female sexual behavior in rats (0.025–1.0 mg/kg, s.c. or i.p.; Ahlenius et al, 1986; Johansson and Meyerson, 1991; Uphouse et al, 1991), we selected 0.1 mg/kg s.c. for this study.
Bilateral ovariectomy
Females were injected intramuscularly (IM) with ketamine (15 mg/kg), 0.02–0.04 mg/kg atropine and 0.01 mg/kg buprenorphine, and were maintained on isofluorane (2%; 0.6 liter/min oxygen). Each ovary was isolated through a ventral midline incision and exteriorized for visualization of the fallopian tube and ovarian pedicle. Subsequent histological examination confirmed complete ovarian removal.
Estradiol replacement
One week before the start of daily treatment, females were implanted SC with silastic capsules that were either estradiol-filled (n=4 per active compound/vehicle) or empty (n=4 per active compound/vehicle). Plasma estradiol levels were determined every 2 weeks whenever capsules were implanted. Treatment with active compound/vehicle started at (1) either 7 weeks after ovariectomy or 7 weeks after removal of capsules, and (2) 1 week after implantation or re-implantation of capsules that occurred at 6 weeks after ovariectomy or 6 weeks after removal of previous capsules, resulting in a constant inter-treatment interval of 7 weeks. Estradiol status was maintained throughout treatment for each female.
Estradiol levels in blood samples that were collected by femoral puncture (Hearn, 1977) were determined using celite column chromatography and a validated estradiol radioimmunoassay (RIA) for marmoset plasma (Saltzman et al, 1998). Assay sensitivity was 4.6 pg/tube (30.4 pg/ml), and intra- and inter-assay assay coefficients of variation (CVs), respectively, were 5.0% and 14.0%.
Ovariectomized females implanted with estradiol-filled capsules (n=8) had higher (p<.003) circulating estradiol levels (396.0 ± 30.6 pg/ml) compared to females implanted with empty capsules (67.5 ± 5.2 pg/ml), and circulating estradiol values in the former females were comparable to those previously reported for female marmosets in the mid-follicular phase of the ovarian cycle (Barnett et al, 2006). Estradiol replacement is thus below the mid-cycle, peri-ovulatory levels employed in some previous studies (Kendrick and Dixson, 1983) and thus supports modest (Barnett et al, 2006) rather than maximal (Kendrick and Dixson, 1985) expression of female marmoset sexual behavior.
Pharmacokinetic assessment of flibanserin and 8-OH-DPAT administration
Treatment-induced systemic exposure to circulating levels of flibanserin and two common flibanserin metabolites, 1-(3-trifluoromethylphenyl)piperazine (TFMPP) and 6-hydroxy-flibanserin (BIMA 23 BS), and to 8-OH-DPAT, was assessed during study weeks 40 to 42 by validated high performance liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Serial blood samples were collected at 0.25, 0.5, 1, 3, 6, and 24h after flibanserin or 8-OH-DPAT administration (n=4 for each compound).
Behavioral observation of sexual and social behavior
In order to stimulate social and sexual interactions upon reunion (Barnett et al, 2006), females and males were separated for 90 min prior to each of four, 30-min behavioral tests at 07:00h–13:00h (16–24 h after daily administration of active compound/vehicle), 5–6 weeks after treatment onset. This time window for observations was chosen to assess the chronic changes induced by flibanserin and 8-OH-DPAT when circulating levels of drugs were minimal, thus avoiding potential acute effects driven by elevated circulating concentrations of the drug preparations and active metabolites. Potential behavioral changes are thus consequences of long-term adaptation to treatment that may involve changes in gene and protein expression (Allers et al, 2010a).
At the start of the behavioral test, the male was introduced to the female by remote door operation and behavior was manually and digitally recorded by two observers from behind a one-way window (Tab. 1). Behavioral tests were reanalyzed in a random fashion from the digitally stored recordings by two observers blinded with respect to treatment. The reanalyzed data were compared with those obtained on the day of the test to confirm final values and to generate behavioral data not originally scored during live observations (for aggression and self-grooming). Inter-observer reliability scores for behavioral data collection averaged 90.6%, and within-observer reliability scores averaged 96.1%.
Table 1. Ethogram and mean ± SEM values for behavioral scores during pairmate tests.
A zero value indicates complete absence of behavior. Bold numbers indicate significant changes compared to vehicle treatment. 8-OH-DPAT, R-(+)-8-hydroxy-2-(di-n-propylamino)-tetralin.
| Behavior | Flibanserin Vehicle | Flibanserin | 8-OH-DPAT Vehicle | 8-OH-DPAT |
|---|---|---|---|---|
| Proceptive female sexual behavior1) | ||||
| Proceptive tongue flicking | 0 | 0.03 ± 0.06 | 0 | 0 |
| Proceptive staring | 0 | 0 | 0 | 0 |
| Proceptive freeze posture/Sprawling position | 0.16 ± 0.28 | 0.12 ± 0.14 | 0.05 ± 0.06 | 0.06 ± 0.09 |
| Receptive female sexual behaviors1) | ||||
| Acceptance of mounts | 0.61 ± 0.58 | 0.75 ± 0.75 | 0.23 ± 0.27 | 0.64 ± 0.57 |
| Rejection of mount attempts and mountsb) | 0.19 ± 0.28 | 0.25 ± 0.29 | 0.62 ± 0.75 | 1.49 ± 1.50 |
| Receptive freeze posture | 0.29 ± 0.28 | 0.45 ± 0.56 | 0.23 ± 0.27 | 0.58 ± 0.55 |
| Receptive tongue flicking | 0 | 0 | 0 | 0 |
| Receptive head turning/biting | 0.40 ± 0.35 | 0.35 ± 0.30 | 0.20 ± 0.23 | 0.29 ± 0.24 |
| Male sexual behaviors1) | ||||
| Penile erection | 0.78 ± 0.65 | 1.12 ± 0.98 | 0.89 ± 0.73 | 1.05 ± 0.46 |
| Mounting | 0.61 ± 0.58 | 0.71 ± 0.69 | 0.23 ± 0.27 | 0.64 ± 0.57 |
| Mounting attemptsb) | 0.19 ± 0.28 | 0.26 ± 0.27 | 0.63 ± 0.83 | 1.63 ± 1.59 |
| Intromitting | 0.43 ± 0.37 | 0.48 ± 0.43 | 0.17 ± 0.20 | 0.35 ± 0.27 |
| Ejaculating | 0.28 ± 0.31 | 0.26 ± 0.27 | 0.17 ± 0.20 | 0.21 ± 0.15 |
| Social odors1) | ||||
| Genital investigation by female | 0.03 ± 0.06 | 0.06 ± 0.08 | 0.28 ± 0.66 | 0.08 ± 0.17 |
| Genital investigation by malea) | 1.76 ± 1.54 | 2.62 ± 1.89 | 1.12 ± 0.74 | 0.49 ± 0.39 |
| Scent marking by female | 2.54 ± 2.53 | 3.15 ± 2.67 | 3.50 ± 3.95 | 4.36 ± 5.17 |
| Scent marking by male | 3.52 ± 4.71 | 3.39 ± 3.07 | 5.18 ± 5.23 | 3.69 ± 2.62 |
| Social interactions1) | ||||
| Grooming by female | 1.29 ± 1.57 | 2.19 ± 2.15 | 2.77 ± 3.57 | 1.79 ± 2.60 |
| Grooming by malea) | 2.21 ± 2.02 | 3.97 ± 3.27 | 1.09 ± 0.57 | 0.76 ± 0.47 |
| Total allogroominga) | 3.94 ± 2.22 | 6.91 ± 3.20 | 4.07 ± 3.74 | 2.71 ± 2.69 |
| Aggression by female | 0.94 ± 1.17 | 0.78 ± 1.07 | 0.92 ± 1.03 | 1.66 ± 1.76 |
| Aggression by male | 0.06 ± 0.08 | 0.17 ± 0.23 | 0.18 ± 0.18 | 0.53 ± 0.62 |
| Total aggressionb) | 0.99 ± 1.21 | 0.91 ± 1.27 | 1.09 ± 1.14 | 2.23 ± 1.98 |
| Contact within arm-length | 5.54 ± 1.23 | 5.42 ± 1.04 | 7.71 ± 2.04 | 6.27 ± 1.75 |
| Direct body contact/huddling | 8.69 ± 1.72 | 10.37 ± 2.60 | 9.42 ± 2.84 | 9.77 ± 2.48 |
| Total contact | 14.30 ± 2.07 | 15.87 ± 3.07 | 17.62 ± 1.55 | 16.19 ± 3.30 |
| Initiating contact by female | 11.14 ± 5.67 | 10.99 ± 3.41 | 20.13 ± 8.08 | 16.99 ± 7.84 |
| Breaking contact by female | 15.60 ± 5.99 | 15.03 ± 7.71 | 21.09 ± 6.46 | 14.27 ± 7.20 |
| Following by female | 1.60 ± 1.66 | 1.67 ± 1.39 | 3.00 ± 2.52 | 2.60 ± 2.33 |
| Avoiding contact by female | 1.69 ± 1.15 | 1.53 ± 1.22 | 1.60 ± 1.00 | 1.15 ± 0.61 |
| Self-directed behavior by female1) | ||||
| Self-groominga) | 2.75 ± 1.86 | 3.96 ± 1.95 | 1.00 ± 0.55 | 1.89 ± 1.25 |
| Genital inspection/Genital self-groominga) | 1.87 ± 1.58 | 4.18 ± 2.95 | 0.45 ± 0.43 | 0.33 ± 0.23 |
| Scratching | 12.74 ± 8.73 | 11.50 ± 4.94 | 8.44 ± 5.09 | 10.95 ± 3.56 |
| Locomotion and movement by female1) | ||||
| Mobile in locomotion | 2.50 ± 0.74 | 1.93 ± 0.53 | 3.07 ± 1.15 | 2.39 ± 1.03 |
| Mobile while stationary | 4.45 ± 1.55 | 5.33 ± 1.64 | 4.06 ± 1.95 | 4.14 ± 1.41 |
| Mobile total | 6.97 ± 2.14 | 7.29 ± 1.92 | 7.25 ± 2.47 | 6.67 ± 1.63 |
| Immobile, incl. sprawling position | 22.72 ± 2.09 | 22.47 ± 1.77 | 22.33 ± 2.59 | 23.14 ± 1.59 |
| Acute behavioral changes2) | ||||
| Scratching1) | 7.32 ± 2.40 | 5.23 ± 2.32 | 1.50 ± 0.90 | 5.56 ± 4.49 |
| Random rapid limb movements3,b) | 0 | 0 | 0 | 6.89 ± 4.32 |
| “Wet-dog” shakes3,b) | 0.24 ± 0.16 | 0.11 ± 0.18 | 0 | 17.44 ± 11.90 |
| Latency to sprawling position4,a) | 19.47 ± 4.19 | 14.58 ± 4.71 | not scored | not scored |
Adapted from Stevenson and Poole, 1976. Behavior was recorded at 16–24 hours after daily drug/vehicle administration during weeks 5–6 of daily treatment.
Behavior was recorded at 0–0.5 hours after drug/vehicle administration during weeks 1–4 of daily treatment.
Behavior indicative of the 5-HT behavioral syndrome. Elliott et al, 1990.
Behavior resembling the rodent flat-body posture. Tricklebank et al, 1984.
Significantly altered by flibanserin administration (p < 0.05).
Significantly altered by 8-OH-DPAT administration (p < 0.05).
Monitoring of the 5-HT behavioral syndrome
Locomotor behaviors indicative of the 5-HT behavioral syndrome, i.e. “random rapid limb movements” and “wet-dog shakes” (Arvidsson et al, 1981; Elliott et al, 1990), were monitored once per week (0.5h) at 0–0.5 h and at 16–24 h after administration of active compound/vehicle treatment, during weeks 1 to 4 of treatment (Tab. 1). Females were placed in a test cage for the duration of the test (8-OH-DPAT/vehicle), or returned to their home cage (flibanserin/vehicle). In addition, sprawling behavior (females lying down in prone position, monitored during flibanserin or respective vehicle treatment only), a possible non-locomotor component of the 5-HT behavioral syndrome (Borsini et al, 2001), and self-scratching behavior, a locomotor behavior not specifically linked to 5-HT neurotransmission, were scored.
Data analysis
Observed levels of female proceptive sexual behavior (Tab. 1) were too infrequent to permit statistical analysis. Female sexual receptivity was quantified by frequency of female acceptance of male ejaculatory mounts. Female refusal of male sexual advances was quantified by frequency of rejection of male mounts and mount attempts. Sexual arousal of the male was quantified by frequency of penile erection. Analyses of all behavior were performed on transformed frequency data [square root (1+x)] to achieve homogeneity of variance and to increase linearity of data. This transformation generates positive numbers, permitting appropriate analysis of behavioral frequency data as square root transformation in which the variance is independent of the mean (Bland and Altman, 1996). Mean values of the frequencies were analyzed by 2-way ANOVA incorporating repeated measures design, with Treatment (active compound, vehicle) and Observations (observation 1–4) as within-subject factors. Data are presented as backtransformed mean values (95% confidence intervals). A p-value less than 0.05 was considered significant.
Initial analyses of behavioral data were performed using the same mixed design ANOVA with Estradiol supplementation and Order of treatment as between-subject factors, and Treatment as within-subject factor. As Estradiol supplementation and Order of treatment consistently failed to affect (p>0.05) any behavioral variable, both factors were omitted in the final analyses reported here.
MAIN OUTCOME MEASURES
Marmoset pairmate interactions, including sexual and social behavior, were scored at weeks 5–6 of daily flibanserin, 8-OH-DPAT or vehicle treatment. In addition, 24-h pharmacokinetic profiles of flibanserin, TFMPP, BIMA 23 BS and 8-OH-DPAT, as well as drug-induced acute symptoms of the 5-HT behavioral syndrome, were assessed.
RESULTS
Systemic exposure to flibanserin, flibanserin metabolites and 8-OH-DPAT during chronic drug treatment
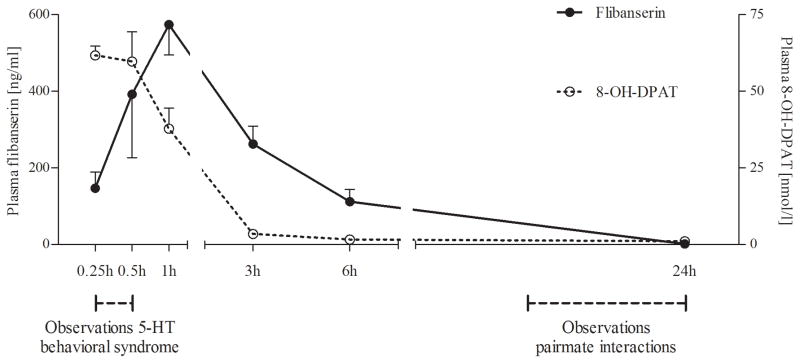
Maximal plasma concentrations of flibanserin, TFMPP and BIMA 23 BS (C(max)) of 628 ± 225 ng/ml (mean ± SEM), 46.0 ± 17.0 ng/ml and 149 ± 38.7 ng/ml, respectively, were reached at 0.5–1h after oral administration, decreasing rapidly to low levels by 24h, providing a circulating half-life for flibanserin of 2.8h (Fig. 1). For 8-OH-DPAT, C(max) of 69.7 ± 19.8 nmol/l was reached by 0.25–0.5h after SC injection, and values dropped rapidly to 3.4 ± 2.6 nmol/l after 3h, providing a circulating half-life of 8-OH-DPAT of 0.6h (Fig. 1). No cumulative effects of chronic 8-OH-DPAT and flibanserin administration were apparent. The results confirm a substantial systemic exposure to flibanserin, BIMA 23 BS, TFMPP and 8-OH-DPAT during observations for 5-HT behavioral syndrome, but not during pairmate observations. Thus, treatment-induced changes during pairmate observations are most likely attributable to the long-term consequences of flibanserin and 8-OH-DPAT exposure, and not to acute effects elicited by elevated circulating levels of active drug.
Figure 1. 24-hour pharmacokinetic profiles of flibanserin and 8-OH-DPAT in female marmoset monkeys.
Pairmate observations were performed at 16–24 hours after administration, when exposure to circulating drug concentrations was low or absent, while observation of the 5-HT behavioral syndrome was performed at 0–0.5 hours after administration, when circulating drug concentrations were high. 8-OH-DPAT, R-(+)-8-hydroxy-2-(di-n-propylamino)-tetralin; 5-HT, serotonin.
Chronic effects of 8-OH-DPAT and flibanserin on sexual, social and self-directed behavior
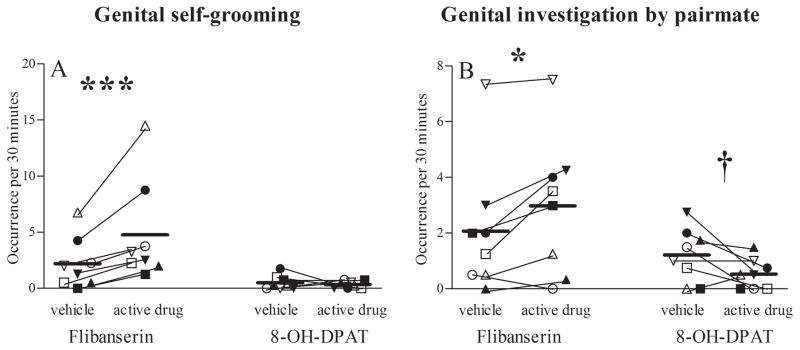
Five to six weeks of daily oral administration of flibanserin to female marmosets noticeably increased female genital area self-grooming (F(1,7) = 31.28, p = .001; Fig. 2A) and male pairmate sniffing/licking of their female’s genital area (F(1,7) = 8.91, p = .020; Fig. 2B). No other sexually-related behavior was altered by flibanserin (Tab. 1). In contrast, 5–6 weeks of daily SC administration of 0.1 mg/kg 8-OH-DPAT strikingly increased female rejection of male pairmate mount attempts and mounts compared to corresponding vehicle (F(1,7) = 8.24, p = .024; Fig. 3). 8-OH-DPAT-induced female rejection of male sexual advances also increased male attempts to mount their female pairmate (F(1,7) = 6.93, p = .034). The male pairmates of 8-OH-DPAT treated females also tended to sniff/lick their female pairmate’s genital area less (F(1,7) = 4.20, p = .080; Fig. 2B), and decreased male genital sniffing was correlated with female rejection of male mount attempts and mounts (r = −0.747, p = 0.033). No other sexually-related behavior was altered (Tab. 1).
Figure 2. Investigation of female genital area.
Frequencies (backtransformed mean [+95% confidence limit]) of (A) female genital area self-grooming and (B) male inspection of female genital area per 30 minutes during 5–6 weeks following the onset of flibanserin, flibanserin vehicle, 8-OH-DPAT or 8-OH-DPAT vehicle administration. *** p=0.001 vs. flibanserin vehicle (F(1,7) = 31.3), * p=0.020 vs. flibanserin vehicle (F(1,7) = 8.9), † p=0.080 vs. 8-OH-DPAT vehicle (F(1,7) = 4.2). Each symbol indicates the same individual female marmoset receiving estradiol (solid symbols) or no estradiol (open symbols). 8-OH-DPAT, R-(+)-8-hydroxy-2-(di-n-propylamino)-tetralin.
Figure 3. Female sexual rejection.
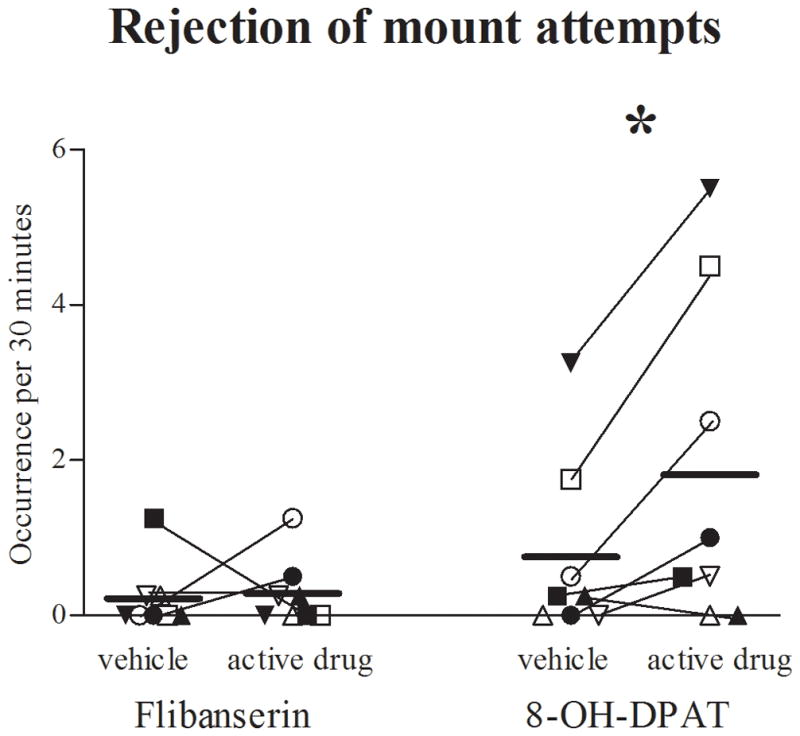
Frequency (backtransformed mean [+95% confidence limit]) of female rejection of male mounts and mount attempts per 30 minutes during 5–6 weeks of flibanserin, flibanserin vehicle, 8-OH-DPAT or 8-OH-DPAT vehicle administration. * p=0.024 vs. 8-OH-DPAT vehicle (F(1,7) = 8.2). Each symbol indicates the same individual female marmoset receiving estradiol (solid symbols) or no estradiol (open symbols). 8-OH-DPAT, R-(+)-8-hydroxy-2-(di-n-propylamino)-tetralin.
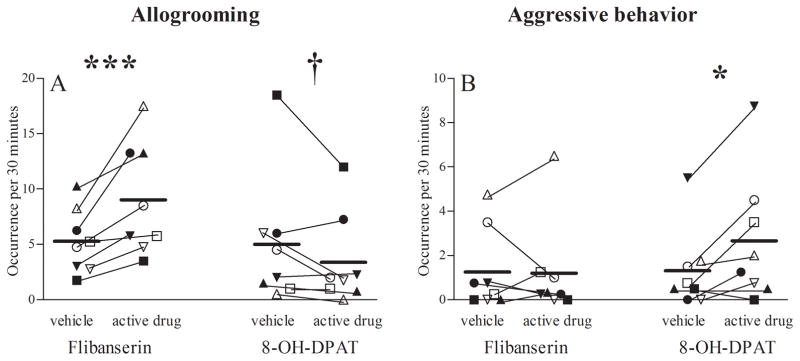
Such opposing effects of flibanserin and 8-OH-DPAT were also observed in social interactions between male and female pairmates. Flibanserin increased allogrooming between pairmates (F(1,7) = 34.25, p = .001; Fig. 4A), mostly male grooming of their female pairmates (F(1,7) = 6.25, p = .041). 8-OH-DPAT, in contrast, tended to diminish allogrooming between males and females (F(1,7) = 4.23, p = .079; Fig. 4A), including a trend towards diminished male grooming of female pairmates (F(1,7) = 4.92, p = .062). Frequency of aggressive interactions between the pairmates was increased by 8-OH-DPAT (F(1,7) = 5.65, p = .049; Fig. 4B), but not by flibanserin (F(1,7) = 0.09, p = .778). This 8-OH-DPAT induced increase in aggression positively correlated with increased female rejection of male mount attempts and mounts (r = 0.941, p < 0.001), and correlated negatively with male genital sniffing (r = −0.834, p = 0.010). No other changes in social behavior were induced by flibanserin or 8-OH-DPAT (Tab. 1).
Figure 4. Pairmate allogrooming and aggression.
Frequencies (backtransformed mean [+95% confidence limit]) of (A) allogrooming and (B) aggressive interactions between pairmates per 30 minutes during 5–6 weeks of flibanserin, flibanserin vehicle, 8-OH-DPAT or 8-OH-DPAT vehicle administration. *** p=0.001 vs. flibanserin vehicle (F(1,7) = 34.2), † p=0.079 vs. 8-OH-DPAT vehicle (F(1,7) = 4.2), * p=0.049 vs. 8-OH-DPAT vehicle (F(1,7) = 5.6). Each symbol indicates the same individual female marmoset receiving estradiol (solid symbols) or no estradiol (open symbols). 8-OH-DPAT, R-(+)-8-hydroxy-2-(di-n-propylamino)-tetralin.
Female self-grooming behavior was increased by flibanserin (F(1,7) = 7.13, p = .032), but not by 8-OH-DPAT (F(1,7) = 3.02, p = .126). There were no other behavioral effects of either drug on self-directed behaviors (Tab. 1). Estradiol supplementation, in both 8-OH-DPAT and flibanserin groups, was without effect on pairmate behavior.
Effects of flibanserin and 8-OH-DPAT on acute induction of the 5-HT behavioral syndrome
During pairmate observations to assess sexual and social behavior (0700h–1300h, 16–24 h following daily drug administration), neither 8-OH-DPAT nor flibanserin resulted in females displaying symptoms of the potentially disruptive 5-HT behavioral syndrome.
Directly (0–0.5h) following administration, however, flibanserin shortened the latency to the first occurrence of sprawling or prone behavior (Fig. 5; F(1,7) = 11.90, p = .011), without affecting the frequency of this behavior (F(1,7) = 0.44, p = .528). Furthermore, in contrast to 8-OH-DPAT, flibanserin first increased (weeks 1–3) female scratching behavior 0–0.5h after administration (F(3,21) = 5.64, p = .005), and then diminished scratching behavior over the remaining weeks of flibanserin treatment compared to corresponding vehicle treatment (Treatment x Week interaction (F(3,21) = 7.21, p = .002)).
Figure 5. Sprawling behavior.
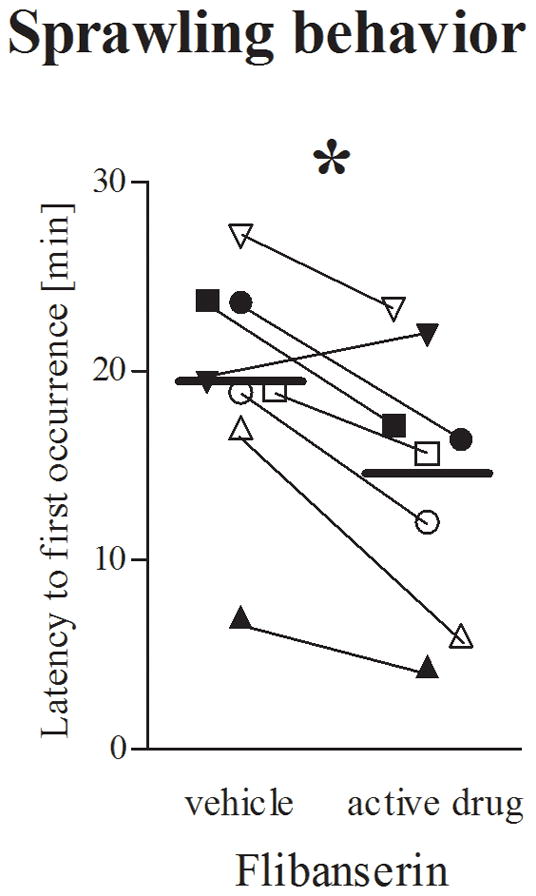
Latency (minutes; backtransformed mean [+95% confidence limit]) to the first occurence of sprawling behavior by female marmosets during pairmate observation after 5–6 weeks of flibanserin or flibanserin vehicle. * p=0.011 vs. flibanserin vehicle (F(1,7) = 11.90). Each symbol indicates the same individual female marmoset receiving estradiol (solid symbols) or no estradiol (open symbols).
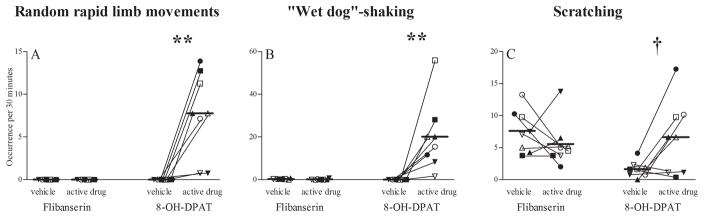
In contrast to flibanserin, 8-OH-DPAT consistently induced locomotor components of an acute, transient 5-HT behavioral syndrome in female marmosets 0–0.5 h after administration, which persisted over the entire time course (15–16 weeks) of chronic treatment. In 8-OH-DPAT-treated female marmosets, the acute 5-HT behavioral syndrome involved displays of “random rapid limb movements” (Fig. 6A; F(1,7) = 16.67, p = .005) and “wet-dog shakes” (Fig. 6B; F(1,7) = 19.81, p = .003). In addition, frequency of scratching behavior tended to be elevated 0–0.5 h after 8-OH-DPAT administration (Fig. 6C; F(1,7) = 5.46, p = .052), but there was a Treatment x Week interaction (F(1,7) = 15.39, p < .001). Post-hoc analysis indicated that female marmosets demonstrated an acute onset of scratching behavior during the first week of 8-OH-DPAT treatment (week 1), but not during subsequent weeks. As found with 5-HT behavioral syndrome behaviors, there was no effect of 8-OH-DPAT treatment on scratching behavior at 16–24 h following drug administration (F(1,7) = 1.75, p = .227) when pairmate behavioral observations were conducted.
Figure 6. Acute induction of the 5-HT behavioral syndrome.
Frequency (backtransformed mean [+95% confidence limit]) of female (A) “rapid, random limb movements” behavior, (B) “wet dog shake” behavior, and (C) scratching behavior per 30 minutes during 5–6 weeks of flibanserin, flibanserin vehicle, 8-OH-DPAT or 8-OH-DPAT vehicle and following 0–0.5 hours of treatment administration. ** p<0.01 vs. 8-OH-DPAT vehicle (“rapid, random limb movements”: F(1,7) = 16.7; “wet dog shakes”: F(1,7) = 19.8), † p=0.052 vs. 8-OH-DPAT vehicle (F(1,7) = 5.46). Each symbol indicates the same individual female marmoset receiving estradiol (solid symbols) or no estradiol (open symbols). 8-OH-DPAT, R-(+)-8-hydroxy-2-(di-n-propylamino)-tetralin.
DISCUSSION
Despite the prevalence of HSDD among women (Clayton, 2010a; Clayton and Hamilton, 2010b), its psychopathogenesis is unknown. Pharmacological manipulation of 5-HT, however, commonly induces an HSDD-like condition, especially in women chronically treated with SSRIs for depression (Rosen et al, 1999), likely involving 5-HT1A receptors (Guptarak et al, 2010). Flibanserin, a 5-HT1A post-synaptic receptor agonist and 5-HT2A antagonist, presents a pharmacological opportunity to ameliorate psychosexual distress in women. Flibanserin improves sexual desire in women with major depression (Kennedy, 2010), and in women with HSDD it has been demonstrated to increase satisfying sexual events, sexual desire and decreases distress (Stahl et al, 2010). Female marmosets, already established as a mechanistic model for neural regulation of female sexual behavior (Kendrick and Dixson, 1983; Barnett et al, 2006), form stable, long-term, male-female relationships (Abbott et al, 2003) that allow for the examination of the pharmacological efficacy of flibanserin with regard to female sexual behavior.
Chronic administration of flibanserin to female marmoset pairmates stimulates male inspection of female genital area and increases female genital self-grooming. Female sexual behavior, however, is otherwise unaltered, possibly because we employ well-established male-female pairs without a history of infrequent-to-absent sexual behavior. Enhancement of proceptive and receptive aspects of female marmoset sexual behavior can be quantified using our behavioral testing paradigm, as previously shown following administration of gonadotropin releasing-hormone II and respective analogues (Barnett et al., 2006). In contrast, 8-OH-DPAT (a 5-HT1A pre- and postsynaptic receptor agonist) administered to female marmoset pairmates tends to diminish male inspection of female genitalia and increases female rejection of male-initiated sexual advances, thus diminishing female sexual receptivity towards their long-standing male pairmate. The comparability of behavioral responses in both estrogen and ‘no hormone replaced’ ovariectomized female marmosets used in this study suggests applicability of our results to both pre- and post-menopausal conditions in women, as found in a previous marmoset study (Barnett et al, 2006).
The differential effects of flibanserin and 8-OH-DPAT on female marmoset sexual behavior resemble findings in female rats. Female rats given flibanserin increase expression of proceptive and receptive sexual behavior and receive increased genital sniffing by the male pairmate (Gelez et al, 2010), whereas female rats given 8-OH-DPAT exhibit diminished female sexual receptivity (Uphouse et al, 1991). These contrasting effects of flibanserin and 8-OH-DPAT on marmoset and rodent behavior are surprising since both compounds are described as 5-HT1A agonists. However, while both flibanserin and 8-OH-DPAT activate postsynaptic 5-HT1A receptors, flibanserin is functional only as a postsynaptic receptor agonist (Stahl et al, 2011), whereas 8-OH-DPAT activates both pre- and postsynaptic 5-HT1A receptors. This difference in biological action results in fundamental differences in pharmacology and the abilities of flibanserin and 8-OH-DPAT to induce functional changes in a brain region-specific manner. For example, flibanserin inhibits forskolin-stimulated cAMP formation in the cortex, while 8-OH-DPAT does not affect cortical cAMP accumulation (Borsini et al, 1995a). Flibanserin decreases neuronal firing rate in the rat cortex regardless of whether the presynaptic receptor-containing dorsal raphe nucleus is intact, while the effects of 8-OH-DPAT are dependent upon intact raphé serotonergic neurons (Borsini et al, 1995b). Taken together, flibanserin and 8-OH-DPAT display a different regional selectivity, and they differentially affect neuronal function in 5-HT projection sites.
These functional differences are particularly evident in pyramidal neurons in the prefrontal cortex, a key site for flibanserin’s mode of action in affecting female sexual behavior, as these neurons are an important part of regulatory networks that coordinate the release of 5-HT, DA and NE in a brain region-specific manner (Stahl et al, 2011). Additional 5-HT2A antagonist effects of flibanserin, which 8-OH-DPAT lacks, have been shown to enhance 5-HT1A agonist effects on pyramidal neurons of the prefrontal cortex, thus creating a biochemical environment in flibanserin-exposed prefrontal cortex that is clearly different from 8-OH-DPAT exposure. 8-OH-DPAT is thus not likely to directly affect cortical neurocircuitries (Borsini et al, 1995a), but will suppress female sexual behavior by acting on 5-HT1A receptors in postsynaptic hypothalamic areas, such as the ventromedial hypothalamus (Uphouse et al, 1993a) and medial preoptic area (Uphouse et al, 1993b), or in presynaptic raphé nuclei (Uphouse et al, 1994). Thus, flibanserin’s effects on female sexual behavior are likely mediated by altering cortical neurocircuitries, while 8-OH-DPAT likely inhibits female sexual behavior by activation of hypothalamic or midbrain 5-HT1A receptor populations. The inhibitory effects of 8-OH-DPAT occur immediately and do not require chronic administration in rats (Ahlenius et al, 1986). Chronic administration seems not to lead to either tolerance or sensitization of sexual behavior, at least in male rats (Johansson et al, 1990). Flibanserin, in contrast, might regulate female sexual behavior by affecting pyramidal neurons in the prefrontal cortex and establishing a new monoamine neurotransmitter balance in a brain region-specific manner (Stahl et al, 2011). Chronic elevations in prefrontal cortex levels of DA and NE in rats are reported after 21 days of repeated flibanserin administration, consistent with the duration needed for flibanserin to facilitate female sexual behavior in rats (Gelez et al, 2010).
Flibanserin’s enhancement of interest in treated female marmosets’ genitals is reminiscent of its effects when administered twice daily to female rats (45 mg/kg, PO) for 2–3 weeks. Flibanserin-treated female rats attract increased male inspection of their genital area (Gelez et al, 2010). In both rats and marmosets, female genital odor is important for activation of male sexual arousal [rat: (Kannan and Archunan, 2001); marmoset: (Ferris et al, 2004)], particularly female genital odor from the peri-ovulatory period when female sexual proceptivity and receptivity are maximal [rat: (Ferreira-Nuño et al, 2005); marmoset: (Dixson and Lunn, 1987)]. 5-HT regulates likely mediators of genital olfactory attractiveness, such as oxytocinergic (OT) and vasopressinergic (AVP) neurons (Ho et al, 2007) that innervate female external genitalia, possibly in conjunction with the central DA neurotransmitter system (Keverne and Curley, 2004). Neuro-regulated changes in vaginal odor may also be a consequence of 5-HT-mediated alterations in genital vasodilatation (Keverne and Curley, 2004) or salt-water regulation (de Arruda Camargo et al, 2010). Increased female interest in genital self-grooming and self-grooming, in general, could involve 5-HT mediated changes in OT, as central administration of OT (and AVP) to female rats increases self-grooming, particularly of the genital area (Pedersen et al, 1992).
Perhaps the most intriguing finding of this marmoset study, the opposing effects of flibanserin and 8-OH-DPAT on the quality of male-female pair interactions, raises the possibility that female sexuality is strongly influenced by the perceived quality of the relationship with their partner [e.g., (Bancroft et al, 2003; Clayton and Hamilton, 2010b)]. Social attachment and maintenance of proximity, allogrooming and pair-bonding, function to facilitate reproduction (Carter, 1998) and sexual behavior may contribute towards maintenance of a close male-female relationship (Snowdon et al, 2006). In marmosets, affiliative behavior between females and males co-occurs with sexual behavior, while aggressive behavior between pairmates impedes sexual interaction (Evans, 1983; Anzenberger, 1985). Thus, the aggression-inducing effect of 8-OH-DPAT and the affiliation-enhancing effect of flibanserin may create relationship environments that either diminish or facilitate sexual interactions between marmoset pairmates, respectively, likely mediated by neurotransmitter systems implicated in the regulation of social behavior, such as 5-HT (Insel and Winslow, 1998), DA (Young et al, 2011), AVP and OT (Ferris, 1996; Snowdon et al, 2010). Flibanserin selectively activates postsynaptic 5-HT1A receptors while antagonizing 5-HT2A receptors (Borsini et al, 1995). Gerretsen et al (2010) recently reported a negative association between the desire for social relationships in humans and 5-HT2A binding in the prefrontal cortex. Furthermore, flibanserin’s distinct receptor binding profile induces long-lasting increases in basal DA in the prefrontal cortex, while not changing basal levels in other tested brain areas, including the hypothalamus and nucleus accumbens (Allers et al, 2010b; Ferger et al, 2010). If replicated in flibanserin-treated female marmosets, such increases in prefrontal cortex DA would be expected to enhance prefrontal cortex-guided attention to male behavioral cues and to more readily elicit female responses based on previous experience (Ferger et al, 2010). Improved efficiency of DA-mediated neural processing is proposed as one aspect of therapeutic efficacy for a variety of drugs that improve disorders of PFC dysfunction, possibly including HSDD (Arnow et al, 2009; Holstege et al, 2009).
Beyond neurobiological factors that influence sexual function, several studies, including the National and Social Life Survey (Laumann et al, 1999), point out that sexual behavior in women is closely linked to psychosocial factors and quality of their relationship with a partner (Bancroft et al, 2003). The complex interplay of these factors with hormonal and neural environments is a challenge for any animal model of female sexual function. The translation of rodent findings to humans, for example, is difficult in light of their strict circadian hormonal control of female sexual function (Pfaff, 1980). Studies in non-primate mammals are thus not likely able to capture the multifactorial environment in which sexual behavior is expressed in nonhuman primates and humans (Wallen and Zehr, 2004). In contrast, our observations in the marmoset monkey emulate the human situation more closely and highlight the importance of pair-bond quality in female sexual behavior. Our findings are likely based within an important set of marmoset-typical characteristics, including well-developed social behavior, long-term female-male pairings and a degree of emancipation of female sexual behavior from strict hormonal control (Kendrick and Dixson, 1983) that permit a significant influence of the social environment on the shaping of sexual behavior.
The strong and consistent, but transient, expression of a 5-HT behavioral syndrome induced in female marmosets by 8-OH-DPAT administration is likely mediated by activation of postsynaptic 5-HT1A receptors (Tricklebank et al, 1985;Larsson et al, 1990). Flibanserin, in contrast, does not elicit shaking or rapid limb movements despite its postsynaptic 5-HT1A agonist characteristic. This may be due to flibanserin’s 5-HT2A antagonist property, as 5-HT1A and 5-HT2A receptors are functionally linked and show reciprocal modulation (Arnt and Hyttel, 1989). Indeed, pretreatment with 5-HT2A antagonists reduces 8-OH-DPAT-induced 5-HT behavioral syndrome in rats (Tricklebank et al, 1984). It is possible that flibanserin increases the expression of brain-derived neurotrophic factor (BDNF) in the cerebral cortex and hippocampus, which, in rats, diminishes the 5-HT behavioral syndrome induced by 8-OH-DPAT (Rogóz et al, 2008). A shortened latency to sprawl induced by flibanserin may be equivalent to the flat-body posture component of the 5-HT behavioral syndrome (Borsini et al, 2001). Sprawling, however, is also commonly observed when marmosets solicit allogrooming from a partner (Stevenson and Poole, 1976). Thus, a shortened latency to sprawling behavior may indicate another pro-social effect of flibanserin rather than a flibanserin-induced component of the 5-HT behavioral syndrome. Neither sprawling in flibanserin treated females, nor the 5-HT behavioral syndrome displayed by 8-OH-DPAT treated females, are likely related to 5-HT toxicity (or 5-HT syndrome) described in humans, a potentially life-threatening condition induced by excess 5-HT (Dunkley et al, 2003). 5-HT toxicity can result from combinations of antidepressant treatments, such as monoamine oxidase inhibitors (MAOIs) and SSRIs, causing clonus (involuntary muscle contraction and relaxation) and hyperthermia (Dunkley et al, 2003) apparently due to 5-HT2 receptor-mediated effects (Isbister and Buckley, 2005). Activation of 5-HT1A receptors, in contrast, commonly leads to hypothermia in rodents and humans (Hjorth, 1985; Anderson et al, 1990). Flibanserin, a 5-HT1A agonist and 5-HT2A receptor antagonist, and 8-OH-DPAT, a 5-HT1A agonist, are thus both unlikely to cause hyperthermic responses. Furthermore, both flibanserin and 8-OH-DPAT administered to rodents selectively decrease 5-HT synthesis in several brain regions (Brambilla et al, 1999), further indicating the unlikelihood of either contributing to 5-HT toxicity.
Similar to a previous observation indicating that low-level estradiol supplementation is not required for the efficacy of gonadotropin-releasing hormone II to stimulate sexual behavior in ovariectomized female marmosets (Barnett et al, 2006), our present study, applying the same design in regard to estradiol replacement, demonstrates that behavioral changes due to chronic flibanserin and 8-OH-DPAT treatments are independent of estradiol status. This finding might be somewhat surprising in light of well-documented interactions of estrogens with the central serotonin system (Bethea et al, 2002) and contrast with a previous study conducted by Kendrick and Dixson (Kendrick and Dixson, 1985). Kendrick and Dixson (1985), however, applied high levels of estradiol supplementation equivalent to preovulatory peak levels (~940 pg/ml). The much lower circulating estradiol levels in our study (average with estradiol supplementation: 396 pg/ml), chosen to facilitate a low to modest baseline of sexual behavior, may be responsible for the lack of obvious estradiol-induced behavioral changes in the present study.
CONCLUSIONS
Our findings are the first to demonstrate differential and potentially bi-directional regulation of female sexual behavior (diminished versus unchanged) and social behavior (diminished versus enhanced) in a nonhuman primate through prolonged serotonergic modulation, supporting the central 5-HT system as a promising target for pharmacotherapy of sexual dysfunction in women. Our model applies species-appropriate settings in which female partners can control sexual and social interactions. We show that female marmoset sexual behavior is suppressed by selective chronic stimulation of 5-HT1A receptors by 8-OH-DPAT. In contrast, through its distinctively different 5-HT receptor binding profile, and potential additional abilities to enhance DA levels (and possibly other neurotransmitters) in a brain-region specific manner (Allers et al, 2010b; Ferger et al, 2010), flibanserin increases pro-social interactions in male and female marmoset pairmates without obvious enhancement of female sexual behavior. While these findings suggest that flibanserin’s effects may not translate from female marmosets to women in every respect, they do show that flibanserin enhances species-specific, intimate aspects of partner interactions. These are manifest by increased intimate affiliative engagement between marmoset pairmates and improved sexual relationships between women and their partners (Kennedy, 2010). Thus, the putative beneficial effect of flibanserin on sexual well-being in women, otherwise distressed about low sexual desire, may arise from its ability to positively influence the quality of relationship with long-term partners. Our observations of female marmosets may therefore suggest the need to integrate mechanistic, neurochemical explanations of female sexual behavior with holistic views of health psychology, including emotional and relational aspects of female sexuality, in order to successfully contribute novel therapeutic approaches to female sexual well-being.
Supplementary Material
Acknowledgments
We thank the veterinary staff and animal care staff at the WNPRC including Dr. Kevin Brunner, Vicky Carter, Megan Sosa and Marilina Vazquez for assistance with animal care, and Amber Edwards, Kristie Barnick-Snyder, Nicole Diol, Alison Parker-Cole and Brian Pisula for technical assistance with marmoset observation and blood sample collection. Fritz Wegner and Dan Wittwer provided excellent technical assistance with hormone assays. We gratefully acknowledge Laurie Poast for graphic design.
This study was supported by Boehringer Ingelheim (to D.H.A.) and was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
Footnotes
DISCLOSURE/CONFLICTS OF INTEREST
Dr. Abbott reports having received a grant from Boehringer Ingelheim that supported the work of Yves Aubert, Morgan Gustison, Lindsey Gardner, Michael Bohl and Jason Lange. Dr. Allers and Dr. Sommer are employed by Boehringer Ingelheim. Dr. Datson currently holds a grant from Boehringer Ingelheim to perform molecular follow up studies of the work described here.
References
- 1.Clayton AH. The pathophysiology of hypoactive sexual desire disorder in women. Int J Gynaecol Obstet. 2010a;110:7–11. doi: 10.1016/j.ijgo.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6:1506–1533. doi: 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- 3.Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2010b;33:323–338. doi: 10.1016/j.psc.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Rosen RC, Lane RM, Menza M. Effects of SSRIs on sexual function: A critical review. J Clin Psychopharmacology. 1999;19:67–85. doi: 10.1097/00004714-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Barnes NM, Sharp T. A review of central 5-HT receptors and their functions. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Ahlenius S, Fernandez-Guasti A, Hjorth S, Larsson K. Suppression of lordosis behavior by the putative 5-HT receptor agonist 8-OH-DPAT in the rat. Eur J Pharmacol. 1986;124:361–363. doi: 10.1016/0014-2999(86)90241-4. [DOI] [PubMed] [Google Scholar]
- 7.Uphouse L, Caldarola-Pastuszka M, Montanez S. Intracerebral actions of the 5-HT1A agonists, 8-OH-DPAT and buspirone and of the 5-HT1A partial agonist/antagonist, NAN-190, on female sexual behavior. Neuropharmacology. 1992;31:969–981. doi: 10.1016/0028-3908(92)90097-9. [DOI] [PubMed] [Google Scholar]
- 8.Maswood N, Caldarola-Pastuszka M, Uphouse L. 5-HT3 receptors in the ventromedial nucleus of the hypothalamus and female sexual behavior. Brain Res. 1997;769:13–20. doi: 10.1016/s0006-8993(97)00670-7. [DOI] [PubMed] [Google Scholar]
- 9.Mendelson SD, Gorzalka BB. A facilitatory role for serotonin in the sexual behavior of the female rat. Pharmacol Biochem Behav. 1985;22:1025–1033. doi: 10.1016/0091-3057(85)90313-2. [DOI] [PubMed] [Google Scholar]
- 10.Borsini F, Ceci A, Bietti G, Conetti A. BIMT 17, a 5-HT1A receptor agonist/5-HT2A receptor antagonist, directly activates postsynaptic 5-HT inhibitory responses in the rat cerebral cortex. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:283–290. doi: 10.1007/BF00168558. [DOI] [PubMed] [Google Scholar]
- 11.Borsini F, Evans K, Jason K, Rohde F, Alexander B, Pollentier S. Pharmacology of flibanserin. CNS Drug Rev. 2002;8:117–142. doi: 10.1111/j.1527-3458.2002.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelez H, Allers K, Sommer B, Giuliano F. Chronic Flibanserin treatment increases solicitations in the female rat. J Sex Med. 2010;7(Suppl 3):118. [Google Scholar]
- 13.Kennedy S. Flibanserin: Initial evidence of efficacy on sexual dysfunction, in patients with major depressive disorder. J Sex Med. 2010;7:3449–3459. doi: 10.1111/j.1743-6109.2010.01938.x. [DOI] [PubMed] [Google Scholar]
- 14.Stahl SM, Sommer B, Allers KA. Multifunctional pharmacology of flibanserin: Possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med. 2010;8:15–27. doi: 10.1111/j.1743-6109.2010.02032.x. [DOI] [PubMed] [Google Scholar]
- 15.Arvidsson LE, Hacksell U, Nilsson JL, Hjorth S, Carlsson A, Lindberg P, et al. 8-Hydroxy-2-(di-n-propylamino)tetralin, a new centrally acting 5-hydroxytryptamine receptor agonist. J Med Chem. 1981;24:921–923. doi: 10.1021/jm00140a002. [DOI] [PubMed] [Google Scholar]
- 16.Uphouse L, Montanez S, Richards-Hill R, Caldarola-Pastuszka M, Droge M. Effects of the 5-HT1A agonist, 8-OH-DPAT, on sexual behaviors of the proestrous rat. Pharmacol Biochem Behav. 1991;39:635–640. doi: 10.1016/0091-3057(91)90139-s. [DOI] [PubMed] [Google Scholar]
- 17.Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med. 2003;53:339–350. [PubMed] [Google Scholar]
- 18.Barnett DK, Bunnell TM, Millar RP, Abbott DH. Gonadotropin-releasing hormone II stimulates female sexual behavior in marmoset monkeys. Endocrinology. 2006;147:615–623. doi: 10.1210/en.2005-0662. [DOI] [PubMed] [Google Scholar]
- 19.Evans S, Poole TB. Long-term changes and maintenance of the pair-bond in common marmosets, Callithrix jacchus jacchus. Folio Primatologica. 1984;42:33–41. [Google Scholar]
- 20.Stevenson MF, Poole TB. An ethogram of the common marmoset (Calithrix jacchus jacchus): general behavioural repertoire. Anim Behav. 1976;24:428–451. doi: 10.1016/s0003-3472(76)80053-x. [DOI] [PubMed] [Google Scholar]
- 21.Hearn JP. Restraining device for small monkeys. Lab Anim. 1977;11:261–262. doi: 10.1258/002367777780936459. [DOI] [PubMed] [Google Scholar]
- 22.Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol. 2009;157:907–921. doi: 10.1111/j.1476-5381.2009.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolly E, Clayton AH, Thorp J, Kimura T, Sand M, Pyke R. Efficacy of flibanserin 100 mg qhs as a potential treatment for hypoactive sexual desire disorder in North American premenopausal women. J Sex Med. 2009;6(suppl 5):465. [Google Scholar]
- 24.Johansson CE, Meyerson BJ. The effects of long-term treatment with 8-OH-DPAT on the lordosis response and hypothermia in female rats. Eur J Pharmacol. 1991;196:143–147. doi: 10.1016/0014-2999(91)90420-u. [DOI] [PubMed] [Google Scholar]
- 25.Saltzman W, Schultz-Darken NJ, Wegner FH, Wittwer DJ, Abbott DH. Suppression of cortisol levels in subordinate female marmosets: reproductive and social contributions. Horm Behav. 1998;33:58–74. doi: 10.1006/hbeh.1998.1436. [DOI] [PubMed] [Google Scholar]
- 26.Kendrick KM, Dixson AF. The effect of the ovarian cycle on the sexual behaviour of the common marmoset (Callithrix jacchus) Physiol Behav. 1983;30:735–742. doi: 10.1016/0031-9384(83)90171-3. [DOI] [PubMed] [Google Scholar]
- 27.Kendrick KM, Dixson AF. Effects of oestradiol 17B, progesterone and testosterone upon proceptivity and receptivity in ovariectomized common marmosets (Callithrix jacchus) Physiol Behav. 1985;34:123–128. doi: 10.1016/0031-9384(85)90089-7. [DOI] [PubMed] [Google Scholar]
- 28.Allers KA, Gelez H, Sommer B, Guiliano F. Activation of the immediate early gene c-fos by acute and repeated treatment with flibanserin. ISSM. 2010 Abstr 002. [Google Scholar]
- 29.Elliott PJ, Walsh DM, Close SP, Higgins GA, Hayes AG. Behavioural effects of serotonin agonists and antagonists in the rat and marmoset. Neuropharmacology. 1990;29:949–956. doi: 10.1016/0028-3908(90)90146-i. [DOI] [PubMed] [Google Scholar]
- 30.Borsini F, Brambilla A, Cesana R, Grippa N. Lack of interaction between flibanserin and antidepressants in inducing serotonergic syndrome in rats. Int J Neuropsychopharmacol. 2001;4:9–15. doi: 10.1017/S1461145701002206. [DOI] [PubMed] [Google Scholar]
- 31.Bland JM, Altman DG. Statistics notes: transforming data. BMJ. 1996;312:770–771. doi: 10.1136/bmj.312.7033.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guptarak J, Sarkar J, Hiegel C, Uphouse L. Role of 5-HT(1A) receptors in fluoxetine-induced lordosis inhibition. Horm Behav. 2010;58:290–296. doi: 10.1016/j.yhbeh.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uphouse L, Caldarola-Pastuszka M, Moore N. Inhibitory effects of the 5-HT1A agonists, 5-hydroxy- and 5-methoxy-(3-di-n-propylamino)chroman, on female lordosis behavior. Neuropharmacology. 1993a;32:641–651. doi: 10.1016/0028-3908(93)90077-g. [DOI] [PubMed] [Google Scholar]
- 34.Uphouse L, Caldarola-Pastuszka M. Female sexual behavior following intracerebral infusion of the 5-HT1A agonist, 8-OH-DPAT, into the medial preoptic area. Brain Res. 1993b;601:203–208. doi: 10.1016/0006-8993(93)91711-z. [DOI] [PubMed] [Google Scholar]
- 35.Uphouse L, Maswood S, Caldarola-Pastuszka M. Agonist activation of 5-HT1A receptors in the median raphe nucleus and female rat lordosis behavior. Brain Res. 1994;668:271–275. doi: 10.1016/0006-8993(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 36.Kannan S, Archunan G. Chemistry of clitoral gland secretions of the laboratory rat: assessment of behavioural response to identified compounds. J Biosci. 2001;26:247–252. doi: 10.1007/BF02703648. [DOI] [PubMed] [Google Scholar]
- 37.Ferris CF, Snowdon CT, King JA, Sullivan JM, Jr, Ziegler TE, Olson DP, et al. Activation of neural pathways associated with sexual arousal in non-human primates. J Magn Reson Imaging. 2004;19:168–175. doi: 10.1002/jmri.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira-Nuño A, Morales-Otal A, Paredes RG, Velázquez-Moctezuma J. Sexual behavior of female rats in a multiple-partner preference test. Horm Behav. 2005;47:290–296. doi: 10.1016/j.yhbeh.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Dixson AF, Lunn SF. Post-partum changes in hormones and sexual behaviour in captive groups of marmosets (Callithrix jacchus) Physiol Behav. 1987;41:577–583. doi: 10.1016/0031-9384(87)90314-3. [DOI] [PubMed] [Google Scholar]
- 40.Ho SS, Chow BK, Yung WH. Serotonin increases the excitability of the hypothalamic paravenricular nucleus magnocellular neurons. Eur J Neurosci. 2007;25:2991–3000. doi: 10.1111/j.1460-9568.2007.05547.x. [DOI] [PubMed] [Google Scholar]
- 41.de Arruda Camargo GM, de Arruda Camargo LA, Saad WA. Role of serotonergic 5-HT1A and oxytocinergic receptors of the lateral septal area in sodium intake regulation. Behav Brain Res. 2010;209:260–266. doi: 10.1016/j.bbr.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen CA, Caldwell JD, Peterson G, Walker CH, Mason GA. Oxytocin activation of maternal behavior in the rat. Ann N Y Acad Sci. 1992;652:58–69. doi: 10.1111/j.1749-6632.1992.tb34346.x. [DOI] [PubMed] [Google Scholar]
- 44.Bancroft J, Loftus J, Long JS. Distress about sex: a national survey of women in heterosexual relationships. Arch Sex Behav. 2003;32:193–208. doi: 10.1023/a:1023420431760. [DOI] [PubMed] [Google Scholar]
- 45.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 46.Snowdon CT, Ziegler TE, Schultz-Darken NJ, Ferris CF. Social odours, sexual arousal and pairbonding in primates. Philos Trans R Soc Lond B Biol Sci. 2006;361:2079–2089. doi: 10.1098/rstb.2006.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans S. The pair-bond of the common marmoset, Callithrix jacchus jacchus: An experimental investigation. Animal Behav. 1983;31:651–658. [Google Scholar]
- 48.Anzenberger G. How stranger encounters of common marmosets (callithrix jacchus jacchus) are influenced by family members: The quality of behavior. Folia Primatologica. 1985;45:204–224. [Google Scholar]
- 49.Insel TR, Winslow JT. Serotonin and neuropeptides in affiliative behaviors. Biol Psychiatry. 1998;44:207–219. doi: 10.1016/s0006-3223(98)00094-8. [DOI] [PubMed] [Google Scholar]
- 50.Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferris CF. Serotonin diminishes aggression by suppressing the activity of the vasopressin system. Ann NY Acad Sci. 1996;794:98–103. doi: 10.1111/j.1749-6632.1996.tb32513.x. [DOI] [PubMed] [Google Scholar]
- 52.Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm Behav. 2010;58:614–618. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerretsen P, Graff-Guerrero A, Menon M, Pollock BG, Kapur S, Vasdev N, Houle S, Mamo D. Is desire for social relationships mediated by the serotonergic system in the prefrontal cortex? An [(18)F]setoperone PET study. Soc Neurosci. 2010;5:375–383. doi: 10.1080/17470911003589309. [DOI] [PubMed] [Google Scholar]
- 54.Allers KA, Dremencov E, Ceci A, Flik G, Ferger B, Cremers TI, et al. Acute and repeated flibanserin administration in female rats modulates monoamines differentially across brain areas: a microdialysis study. J Sex Med. 2010b;7:1757–1767. doi: 10.1111/j.1743-6109.2010.01763.x. [DOI] [PubMed] [Google Scholar]
- 55.Ferger B, Shimasaki M, Ceci A. Flibanserin, a drug intended for treatment of hypoactive sexual desire disorder in pre-menopausal women, affects spontaneous motor activity and brain neurochemistry in female rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:573–579. doi: 10.1007/s00210-010-0515-8. [DOI] [PubMed] [Google Scholar]
- 56.Arnow BA, Millheiser L, Garrett A, Lake Polan M, Glover GH, Hill KR, et al. Women with hypoactive sexual desire disorder compared to normal females: a functional magnetic resonance imaging study. Neuroscience. 2009;158:484–502. doi: 10.1016/j.neuroscience.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 57.Holstege G, Willemsen A, Beers C, Lont E, Schultz WW, Jansen M, Dierck R. Differences in brain activity in premenopausal women with hyopoactive sexual desire disorder (HSDD) compared to women without sexual dysfunction. J Sex Med. 2009;6(Suppl 5):407. [Google Scholar]
- 58.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;28:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 59.Pfaff DW. Estrogens and brain function. Springer; New York: 1980. [Google Scholar]
- 60.Wallen K, Zehr JL. Hormones and history: the evolution and development of primate female sexuality. J Sex Res. 2004;41:101–112. doi: 10.1080/00224490409552218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tricklebank MD, Forler C, Middlemiss DN, Fozard JR. Subtypes of the 5-HT receptor mediating the behavioural responses to 5-methoxy-N,N-dimethyltryptamine in the rat. Eur J Pharmacol. 1985;117:15–24. doi: 10.1016/0014-2999(85)90467-4. [DOI] [PubMed] [Google Scholar]
- 62.Larsson LG, Rényi L, Ross SB, Svensson B, Angeby-Möller K. Different effects on the responses of functional pre- and postsynaptic 5-HT1A receptors by repeated treatment of rats with the 5-HT1A receptor agonist 8-OH-DPAT. Neuropharmacology. 1990;29:85–91. doi: 10.1016/0028-3908(90)90047-u. [DOI] [PubMed] [Google Scholar]
- 63.Johansson CE, Meyerson BJ, Höglund AU. The long-term effects of 8-hydroxy-2-(di-n-propyl-amino)tetralin (8-OH-DPAT) on copulatory and exploratory behaviour in male rats. Eur J Pharmacol. 1990;178:1–9. doi: 10.1016/0014-2999(90)94787-x. [DOI] [PubMed] [Google Scholar]
- 64.Arnt J, Hyttel J. Facilitation of 8-OH-DPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur J Pharmacol. 1989;161:45–51. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- 65.Tricklebank MD, Forler C, Fozard JR. The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur J Pharmacol. 1984;106:271–282. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- 66.Rogóz Z, Skuza G, Legutko B. Repeated co-treatment with fluoxetine and amantadine induces brain-derived neurotrophic factor gene expression in rats. Pharmacol Rep. 2008;60:817–826. [PubMed] [Google Scholar]
- 67.Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnosis decision rules for serotonin toxicity. Q J Med. 2003;96:635–642. doi: 10.1093/qjmed/hcg109. [DOI] [PubMed] [Google Scholar]
- 68.Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol. 2005;28:205–214. doi: 10.1097/01.wnf.0000177642.89888.85. [DOI] [PubMed] [Google Scholar]
- 69.Hjorth S. Hypothermia in the rat induced by the potent serotonergic agent 8-OH-DPAT. J Neural Transm. 61:131–135. doi: 10.1007/BF01253058. [DOI] [PubMed] [Google Scholar]
- 70.Anderson IM, Cowen PJ, Grahame-Smith DG. The effects of gepirone on neuroendocrine function and temperature in humans. Psychopharmacology (Berl) 1990;100:498–503. doi: 10.1007/BF02244002. [DOI] [PubMed] [Google Scholar]
- 71.Brambilla A, Baschirotto A, Grippa N, Borsini F. Effect of flibanserin (BIMT 17), fluoxetine, 8-OH-DPAT and buspirone on serotonin synthesis in rat brain. Eur Neuropsychopharmacol. 1999;10:63–67. doi: 10.1016/s0924-977x(99)00056-5. [DOI] [PubMed] [Google Scholar]
- 72.Bethea CL, Ku NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.