Abstract
Background
Repeatedly pairing a tone with a brief burst of vagus nerve stimulation (VNS) results in a reorganization of primary auditory cortex (A1). The plasticity-enhancing and memory-enhancing effects of VNS follow an inverted-U response to stimulation intensity, in which moderate intensity currents yield greater effects than low or high intensity currents. It is not known how other stimulation parameters effect the plasticity-enhancing effects of VNS.
Objective
We sought to investigate the effect of pulse-width and intensity on VNS efficacy. Here, we used the extent of plasticity induced by VNS-tone pairing to assess VNS efficacy.
Methods
Rats were exposed to a 9 kHz tone paired to VNS with varying current intensities and pulse widths. Cortical plasticity was measured as changes in the percent of area of primary auditory cortex responding to a range of sounds in VNS-treated rats relative to naïve rats.
Results
We find that a combination of low current intensity (200 μA) and short pulse duration (100 μs) is insufficient to drive cortical plasticity. Increasing the pulse duration to 500 μs results in a reorganization of receptive fields in A1 auditory cortex. The extent of plasticity engaged under these conditions is less than that driven by conditions previously reported to drive robust plasticity (800 μA with 100 μs wide pulses).
Conclusion
These results suggest that the plasticity-enhancing and memory-enhancing effects of VNS follow an inverted-U response of stimulation current that is influenced by pulse width. Furthermore, shorter pulse widths may offer a clinical advantage when determining optimal stimulation current. These findings may facilitate determination of optimal VNS parameters for clinical application.
Keywords: Vagal nerve stimulation, stimulation parameters, stimulation intensity, pulse width, cortical plasticity, auditory cortex
Introduction
Repeatedly pairing short bursts of vagus nerve stimulation (VNS) with sensory or motor events causes lasting changes to the cortical circuits being activated [1–5]. Depending on the type of sound paired with VNS, it is possible to change the frequency tuning, temporal following rate, or phoneme selectivity of A1 neurons [3–5]. When a single pure tone frequency is paired with VNS, the proportion of A1 neurons that respond to that frequency is increased [4,6]. When a particular movement is paired with VNS, the proportion of motor cortex neurons that generate that movement is increased [1,2]. In addition to increasing neural plasticity in normal subjects, VNS paired with motor or sensory rehabilitation can be used to enhance recovery from nervous system damage. Numerous preclinical studies have demonstrated that pairing VNS with rehabilitative training enhances recovery in animal models of stroke, traumatic brain injury, and tinnitus [4,7–10]. Recent clinical studies indicate that VNS may be a useful therapy for the treatment of chronic stroke and tinnitus [11,12]. Determining optimal stimulation parameters to maximize clinical benefit will be critical to developing its therapeutic utility.
Several studies provide evidence that varying stimulation parameters strongly influences VNS efficacy. Intensity, frequency, and pulse width are all modifiable parameters that influence the effects of VNS [6,13–18]. Among the parameters investigated, the influence of current intensity has been best characterized. Increasing VNS intensity enhances efficacy up to a certain point, after which higher amounts of VNS begin reducing efficacy. Increasing VNS intensity results in an inverted-U function of cortical map plasticity, in which middle intensity (400 and 800 μA) stimulation drives significantly greater plasticity than strong stimulation (1200 and 1600 μA) [6]. VNS delivered post-training enhances memory retention in both humans and rats when delivered at moderate intensities, whereas VNS delivered at higher intensities exerts little or no effect on memory performance [19–21]. Similarly, moderate VNS intensities facilitate hippocampal LTP, while higher current intensity results in significantly less facilitation [22]. Taken together, these findings suggest that exceeding the optimal simulation intensity decreases VNS efficacy. However, the lower range of effective VNS intensities has not been fully explored.
The degree of nerve activation is determined by both the stimulation intensity and pulse width. Varying the pulse width of VNS while keeping the current constant results in differential activation and deactivation of distinct brain regions [18]. The vagus nerve compound action potential (cAP) displays a standard strength-duration curve demonstrating that the intensity required to elicit a cAP decreases with increasing pulse widths [23]. Consistent with this notion, studies using 500 μs pulse widths reveal a narrower range of effective VNS intensities [19,21,22] when compared with a study using 100 μs pules widths [6]. However, the effect of varying pulse width on VNS-mediated enhancement of plasticity has not been explored within a single study.
Many of the adverse side effects associated with VNS can be mitigated by reducing the stimulation intensity, either through reductions in current amplitude or pulse width [11,24,25]. Therefore, the present study aims to investigate the lower range of effective intensities as well as the influence of pulse width on VNS-dependent plasticity. The threshold for eliciting a cAP in the vagus nerve is near 200 μA [23]. However, whether this intensity is effective for the stimulation paradigm used to enhance plasticity has not been explored. The threshold for eliciting spiking activity in the locus coeruleus (LC), an area believed to be required for VNS-dependent enhancement of plasticity, is near 200 μA when delivered at 100 μs pulse widths. However, when the same intensity was delivered at 500 μs pulse widths, LC spiking activity reached levels similar to that elicited by 800 μA VNS delivered at 100 μs [17], a parameter set previously shown to drive robust plasticity in auditory cortex [6]. Here, we evaluated the effect of 200 μA VNS, an intensity near the threshold for nerve activation, on cortical rearrangement at these two commonly-used pulse widths.
Methods
Sixty 3–6 month old Sprague Dawley female rats were housed in a 12:12 hour reversed light-dark cycle. All handling, housing, stimulation, and surgical procedures were approved by The University of Texas at Dallas Institutional Animal Care and Use Committee. Rats receiving tone-paired VNS were implanted with cuff electrodes around the left vagus nerve and then randomly assigned to one of 3 groups which were interleaved in time. After 20 days of VNS-tone pairing, auditory cortex recordings were performed to assess changes in auditory stimulus-evoked cortical responses. Recordings were also performed in 10 additional rats that received no VNS-tone pairing to serve as naïve controls.
Vagus nerve surgery
A custom made platinum iridium bipolar cuff electrode was implanted around the left cervical vagus nerve as described previously [2–6,8–10,26,27]. In brief, rats were anesthetized with ketamine hydrochloride (80 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally and given supplemental doses as needed. Body temperature was maintained at 37° C throughout the surgery. The vagus nerve was isolated with blunt dissection and placed in the cuff electrode. Leads from the cuff electrode were tunneled subcutaneously to interface with a headcap fixed with acrylic to 4 bone screws on the skull. Immediately following implantation, activation of the vagus nerve by the cuff electrode was confirmed by a drop in blood oxygen saturation in anesthetized rats with up to ten seconds of continuous 30 Hz stimulation (800 μA, 100 μs pulse width). Nerve activation was considered to be successful if blood oxygen saturation reliably decreased by at least 5% from a stable baseline. Cefotaxime sodium (10mg) was administered subcutaneously to prevent infection. Rats received amoxicillin (5 mg) and carprofen (1 mg) tablets for 2 days following surgery and were allowed to recover for at least 5 days before beginning VNS.
Vagus Nerve Stimulation and Tone Pairing
Rats were exposed to 500 ms, 9 kHz, 50 dB tones paired with VNS 300 times per day for 20 days as in previous studies [4,6]. To eliminate acoustic transients, 5 ms ramps were used at the beginning and end of each tone. VNS consisted of a 500 ms train of biphasic pulses at 30 Hz, with a 30 s average interval between VNS events. To prevent rats from anticipating when stimulation would occur, there was a 50% chance of stimulation every 15 s. Current amplitude was either 200 μA or 800 μA and pulse width was either 100 μs or 500 μs, as appropriate for each experimental group. Cuff impedance was monitored daily and rats who had impedance values > 10 kΩ for 2 consecutive days were removed from the study. Sixteen rats were removed from the study based on this criterion.
Auditory Cortex Recordings
Auditory cortex recordings were performed according to standard procedures [4,6]. 24 – 72 hrs after the last day of pairing, rats were anesthetized with sodium pentobarbital (50 mg/kg). Depth of anesthesia was monitored throughout the procedure and supplemented with additional pentobarbital as needed. A tracheal tube and cisternal drain were used to facilitate respiration and alleviate brain swelling. A section of skull was removed to expose right auditory cortex. The dura was removed and a thin layer of silicone oil was applied to the surface of the cortex to prevent desiccation. Four parylene-coated tungsten microelectrodes (1.5–2.5 MΩ, FHC) were lowered to depths of 600 – 700 μm below the pial surface to target layer IV. Neural signals were amplified using an RA16PA preamplifier (Tucker-Davis Technologies) and digitized at 24.414 ks/s with 16-bit resolution using an RZ5 BioAmp processor (Tucker-Davis Technologies) and subsequently filtered with a 300 to 3,000 Hz bandpass filter and further amplified 20,000 times using Brainware (Jan Schnupp). A 600 mV threshold was applied to amplified voltage signals for spike detection. For electrical and acoustic isolation, recordings were conducted in a foam-lined, doubled-walled sound attenuated chamber. Pure tones spanning 81 frequencies ranging from 1 – 32 kHz and 16 intensities ranging from 0 – 75 dB were delivered via a speaker placed 10 cm from the left ear. Tones were presented every 500 ms in a randomly interleaved fashion. Multiunit neural activity was recorded using Brainware (TDT) and each recording site location was logged on a digitized photo of exposed cortex. Upon completion of the recordings, vagus nerve activation by the cuff electrode was again confirmed by observation of a decrease in blood oxygen saturation. One animal failed to exhibit a drop in oxygen saturation after mapping and was consequently excluded from analysis. Seventeen animals were excluded due to complications during auditory cortex recordings.
Data analysis
Auditory evoked neural responses were analyzed using a custom, fully-automated Matlab program to quantify receptive field and response characteristics at each site. Onset latency was defined as the time point where the response was 2 standard deviations above the spontaneous firing rate. Peak latency was defined as the timepoint of maximum firing. End of peak latency was defined as the timepoint following the response peak in which the firing rate fell below 2 standard deviations above the firing rate for 4 consecutive ms. For spike counts, tone-evoked responses were defined as the average number of spikes occurring during the 8–40 ms window following the tone presentation minus the average spontaneous rate.
The percent of area of A1 that responded to each of the 1296 combinations of tone frequency and intensity was quantified for each rat. Cortical reorganization was evaluated by subtracting the response of experimentally naïve rats from rats that received VNS paired with 9 kHz tones. The percent of area responding, extent of cortical reorganization, and response latencies were measured for each animal. Response strengths were measured for each site. Group averages were compared across conditions using one-way ANOVAs followed by Tukey’s test for multiple comparisons when appropriate to determine significant differences in receptive field organization, response latencies, and response strengths. The characteristic frequency for each site was defined as the frequency of the lowest intensity sound which elicited a response. For figure 2, a Benjamini-Hochberg correction was used to control the false discovery rate.
Figure 2. VNS-tone pairing drives plasticity in auditory cortex.
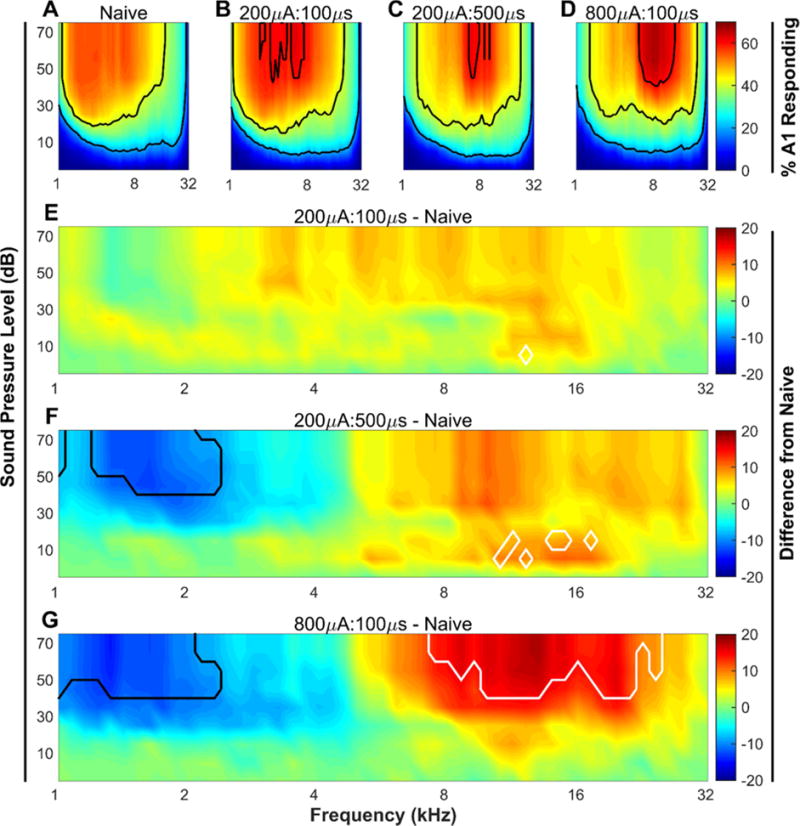
(A – D) Percent of the area of primary auditory cortex responding to each of the 1296 tones played. Black contour lines indicate 20, 40, and 60% of primary auditory cortex responding. (E – G) The group average difference in the percent of cortex responding to each tone between each of the three experimental groups and the naïve group. White contour lines surround tones that were significantly (P < 0.05) increased between the two groups, while black contour lines surround tones that were significantly (P < 0.05) decreased. N = 10, 10, 9, & 7 for Naïve, 200μA:100μs, 200μA:500μs, & 800μA:100μs groups, respectively.
Results
Pairing tones with low intensity-VNS causes receptive field plasticity if pulses are wide
A range of different pulse widths have been used in studies investigating VNS-enhanced plasticity. However, the effect of systematically varying this parameter on any one measure of plasticity has not been examined [6,22,28,29]. Here, we tested the effect of varying pulse width on VNS-dependent enhancement of plasticity in auditory cortex. As in previous studies, VNS tone-pairing consisted of 20 days of short bursts of VNS paired with a 500 ms 9 kHz, 50 dB tone [4,6] (Fig. 1). Following 20 days of VNS-tone pairing, multi-unit recordings in A1 auditory cortex were collected to characterize the responses to tones varying in frequency and intensity. All rats had a statistically significant correlation between anterior-posterior location and the binary logarithm of the characteristic frequency. Average correlation coefficients were not significantly different between groups (R2 = 0.68 ± 0.02, 0.68 ± 0.02, 0.65 ± 0.02, 0.64 ± 0.01 for naïve, 200μA:100μs, 200μA:500μs, and 800μA:100μs VNS groups, respectively). Rats that received VNS at 200 μA with 500 μs pulses (200μA:500μs) paired with high frequency (9 kHz) tones displayed receptive field reorganization characterized by a significant decrease in the percent of A1 responding to low frequency tones, compared to naïve animals (1.5 kHz, 50 dB tone; Naïve (N = 10) vs. 200μA:500μs (N = 9), 53.9 ± 2.2% vs. 41.4 ± 3.0%; Unpaired t(17) = 3.31, P = 0.008, Fig. 2F). This observation demonstrates that 200μA:500μs VNS is sufficient to drive cortical plasticity. 200μA:500μs VNS did not significantly alter the percent of A1 responding to frequencies near the tone paired with VNS (9 kHz, 50 dB tone; Naïve (N = 10) vs. 200μA:500μs (N = 9), 47.3 ± 3.1% vs. 57.8 ± 4.1%; Unpaired t(17) = −2.03, P = 0.10, Fig. 2F).
Figure 1. Schematic of VNS-tone paring paradigm and experimental groups.
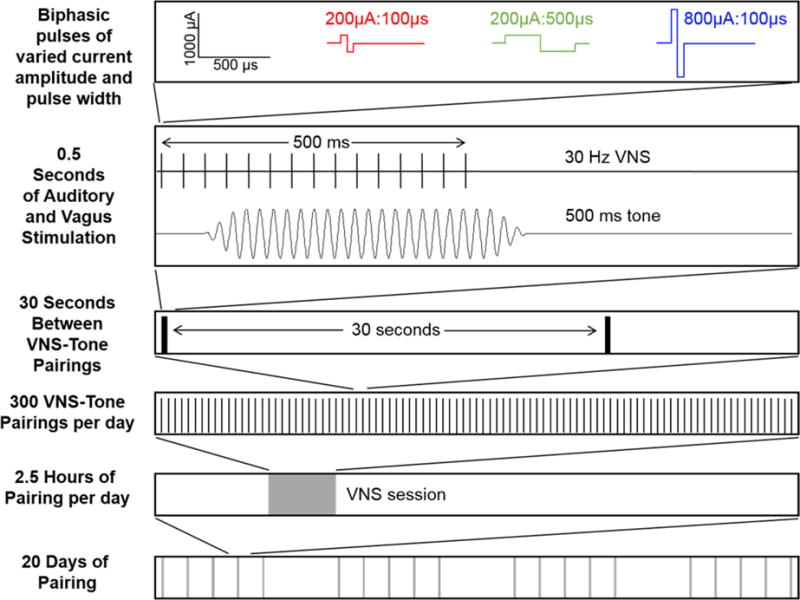
With each tone presentation, a 30 Hz, 500 ms train of electrical stimulation was delivered to the left vagus nerve via a cuff electrode. Each group received one of two current intensities (200 μA or 800 μA) and one of two biphasic pulse widths (100 μs or 500 μs per phase). Rats received VNS paired with a 9 kHz, 50 dB tone every 30 s 300 times per day for 20 days.
Low intensity, long pulse-width VNS drives sub-maximal plasticity
To assess the extent of plasticity engaged by 200μA:500μs VNS, another contemporaneous group of rats received tone-paired VNS consisting of 800 μA, 100 μs pulses (800μA:100μs), a VNS regimen previously shown to be optimally effective at driving plasticity [6], for comparison. Consistent with previous studies, we found that rats that heard tones paired with 800μA:100μs VNS showed a 22% decrease in the area of A1 responding to 1.5 kHz, 50 dB tones when compared to naïve rats (Naïve (N = 10) vs. 800μA:100μs (N = 7), 53.9± 2.2% vs. 41.9 ± 2.3%; Unpaired t(15) = 3.41, P = 0.007, Fig. 2G), similar to what was seen with 200μA:500μs. Unlike 200μA:500μs VNS, 800μA:100μs VNS caused a robust and significant increase in the area of A1 responding to 9 kHz, 50 dB tones relative to naïve animals (Naïve (N = 10) vs. 800μA:100μs (N = 7), 47.3± 3.1% vs. 62.2 ± 3.7%; Unpaired t(15) = −2.87, P = 0.02, Fig. 2G).
Earlier studies documented that pairing VNS with a 9 kHz tone can significantly increase the number of A1 neurons that respond to high tone frequencies and significantly decrease the number of A1 neurons that respond to low tone frequencies [6]. To summarize the effect of VNS on auditory cortex reorganization, we computed the difference between the percent of A1 neurons that respond to high frequency (8 – 16 kHz) tones and the percent of A1 neurons that respond to low frequency (1 – 2 kHz) tones. In naïve rats, a similar proportion of A1 responds to high and low frequency tones at 50 dB SPL, resulting in a difference of −3.8 ± 4.42% (Fig. 3). As previously reported [4,6], pairing a 9 kHz tone with VNS caused a shift in the responsiveness of A1 towards high frequency tones (One-way ANOVA, F(3, 32) = 6.58, P = 0.001, Fig. 3). After pairing 800μA:100μs VNS with a 9 kHz tone, there was a 22.2 ± 6.1% difference in the proportion of A1 neurons that respond to high compared to low frequency tones. After pairing 200μA:500μs VNS with the same tone there was a 14.8 ± 3.4% difference in the proportion of A1 neurons that respond to high compared to low frequency tones. Both 200μA:500μs and 800μA:100μs VNS generated significant reorganization in the auditory cortex compared to naïve rats (P < 0.05 for both comparisons).
Figure 3. Stimulation intensity and pulse width influence VNS-dependent rearrangement of receptive fields in primary auditory cortex.
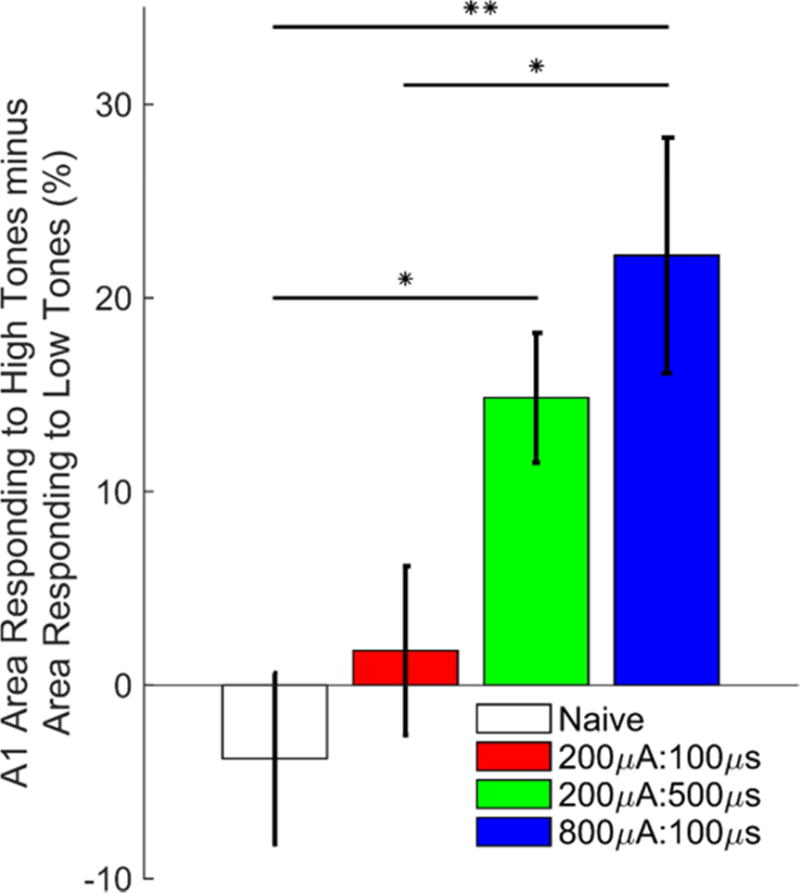
In naïve and 200μA:100μs groups, the percent of area of primary auditory cortex responding to 8–16 kHz, 50 dB tones was similar to the percent area of primary auditory cortex responding to 1–2 kHz, 50 dB tones. In subjects receiving 200μA:500μs or 800μA:100μs VNS, the responsiveness of A1 shifted towards 8–16 kHz, 50 dB tones. N = 10, 10, 9, & 7 for Naïve, 200μA:100μs, 200μA:500μs, & 800μA:100μs groups, respectively. * = P < 0.05; ** = P < 0.01.
Low intensity, short pulse-width VNS is insufficient to drive plasticity
A recent study examining electrophysiological responses from the vagus nerve reported that 200μA:100μs VNS is near the threshold for eliciting a compound action potential [23]. Therefore, we next tested whether this low level of stimulation sufficient for nerve activation could drive plasticity in auditory cortex. Rats receiving 200μA:100μs VNS showed no significant change in the percent of A1 responding to any of the individual tones tested compared to naïve rats (Fig. 2E). Similarly, there was no significant change in the difference in the proportion of A1 neurons that respond to high and low frequency tones for 200μA:100μs VNS rats compared to naïve rats (Naïve (N = 10) vs. 200μA:100μs (N = 10), −3.8 ± 4.42% vs. 1.8 ± 4.4; Unpaired t(18) = −0.90, P = 0.38, Fig. 3). The inability of 200μA:100μs VNS to alter receptive fields suggests that minimal nerve activation is insufficient to drive cortical plasticity and supports the notion that stronger activation (higher current or wider pulses) is required to adequately engage ascending structures required for enhancement of plasticity.
Low intensity, long pulse-width VNS alters spiking activity
Previous studies have observed that VNS paired with tones or speech sounds results in changes in multi-unit response strength at individual recording sites that corresponds with changes in the proportion of A1 responding [5,6]. Therefore, we next tested whether tone-paired VNS affects spiking activity in A1 at different pulse width and intensity combinations. We observed no differences in the response onset latency, peak latency, or end of peak between any groups (Two-way ANOVA main effect of VNS, F(3,102) = 1.04, P = 0.38, Fig 4). Naïve rats demonstrated a slight bias in the number of tone-driven spikes favoring low frequency tones (Fig 5). Rats receiving 200μA:500μs and 800μA:100μs VNS both show a change in the number of driven spikes that shifts towards high frequency tones, suggesting response strengths are altered in response to VNS-tone pairing (One-way ANOVA, F(3,1567) = 3.46, P = 0.02, Fig. 5). These findings are consistent with the VNS-mediated changes in receptive field size and demonstrates that receptive field response strength is similarly altered when VNS is delivered at sufficient levels.
Figure 4. Response latencies are unaffected by VNS.
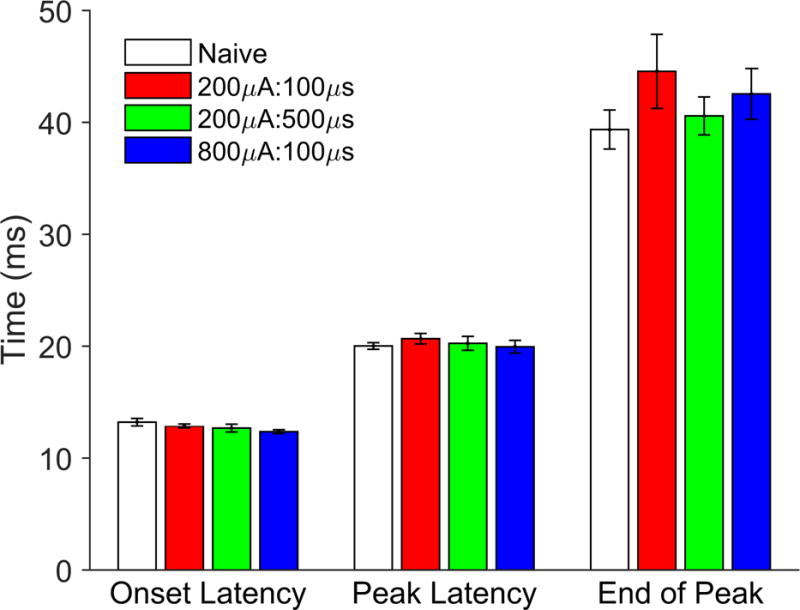
Response onset latency, peak latency, and end of peak latency are all unaffected by VNS delivered under any of the conditions tested in this paper (no significant effect of VNS revealed by two-way ANOVA or Tukey’s test for multiple comparisons).
Figure 5. Stimulation intensity and pulse width influence VNS-dependent changes in tone-evoked response strength.
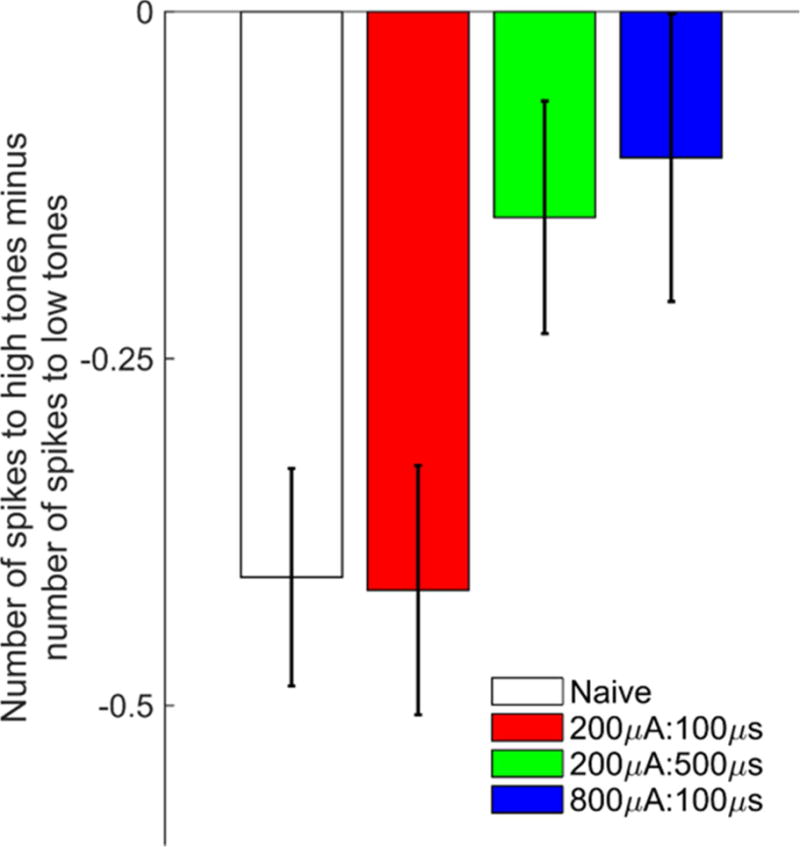
Response strength to high frequency tones (8–16 kHz, 45–75 dB) minus the response strength to low frequency tones (1–2 kHz, 45–75 dB) in naïve and VNS treated animals. In naïve and 200μA:100μs VNS groups, A1 neurons fired more robustly in response to low frequency tones. This bias was reduced in rats who received 200μA:500μs or 800μA:100μs VNS due to a shift in response strength to favor high frequency tones.
Discussion
Previous findings have demonstrated that repeatedly pairing VNS with tones leads to plasticity in the auditory cortex [4,6]. Studies examining electrophysiological responses of the vagus nerve have suggested that the intensity threshold for vagus nerve activation is 200 μA [23,30]. Here, we tested if repeatedly pairing 200 μA VNS with a 9 kHz tone is sufficient to generate cortical plasticity. VNS delivered at 200μA:500μs caused significant changes in A1 receptive fields, although the extent of this plasticity is weaker than that elicited by 800μA:100μs, the most effective stimulation strength in previous reports [1,4,6]. VNS delivered at 200μA:100μs, an intensity shown to be sufficient to activate some vagus nerve fibers [23] and weakly drive spiking in neurons of the locus coeruleus [17], was insufficient to drive cortical plasticity. Response strengths to a range of tones were similarly affected by VNS. Taken together, these findings suggest that while vagus nerve activation is required for enhancement of plasticity, the level of activation must exceed a minimum threshold between 200μA:100μs and 200μA:500μs to elicit a sufficient response from CNS structures mediating cortical reorganization.
Pairing VNS with a 9 kHz tone caused both an expansion in the percent of A1 responding to the 8–16 kHz frequency range as well as suppression in the percent responding to low frequencies, corroborating previous findings [6]. While VNS delivered at 800μA:100μs caused a more robust expansion at the targeted frequency range between 8 – 16 kHz than VNS delivered at 200μA:500μs, the degree of suppression in the low frequency range (1 – 2 kHz) was not different between these groups. One explanation for this observation is that expansion and suppression may occur in parallel, but the mechanisms underlying reorganization of low frequency neurons are engaged at lower stimulation thresholds. Alternatively, the shift in receptive fields may be a sequential process in which low frequency suppression precedes the expansion at the targeted range. A more detailed understanding of the mechanisms underlying VNS-dependent cortical reorganization may be useful in developing selective stimulation strategies for VNS-based therapies.
The observation that 200 μA was insufficient to drive any plasticity with 100 μs pulse widths but was able to drive receptive field reorganization when delivered with 500 μs pulse widths is consistent with electrophysiological examinations demonstrating the vagus nerve displays a typical strength-duration function [23,31]. Therefore, to the extent that nerve activation is correlated with the inverted U response of VNS-mediated enhancement of plasticity and memory, VNS delivered with a longer pulse width may yield a narrower range of effective VNS intensities compared to a shorter pulse width. In concert with this notion, studies using 500 μs pulse width VNS achieved maximal efficacy at 400 μA, but changing the intensity level by a factor of 2 in either direction resulted in a > 50% reduction in efficacy [19,21,22]. In contrast, 100 μs pulse width VNS doubles the range of effective VNS intensities, with 400 μA and 800 μA VNS being equally effective [6]. However, the timing and amount of VNS delivered was different across studies, which may account for the enhanced effectiveness of VNS delivered at 200μA:500μs in the current study relative to previous studies. Future studies exploring a narrower distribution of intensity and pulse width combinations will be required to confirm whether increasing pulse widths leads to a refined range of effective VNS intensities.
VNS has been shown to influence the transmission of several neuromodulator systems involved with memory and plasticity. Activation of these systems is thought to underlie the neurophysiological mechanisms contributing to the plasticity-enhancing and memory-enhancing effects of VNS [17,32–35]. The wide ranging extent of neuromodulator systems influenced by VNS, which include norepinephrine, serotonin, dopamine, and acetylcholine, would be expected to generate a complex cortical response. It is likely, therefore, that the mechanisms underlying the inverted U will be revealed through the understanding of the activation profiles and dose-responses unique to each neuromodulator system as well as the interactions between these systems in response to varying VNS. A recent study characterizing the activity of noradrenergic neurons in the locus coeruleus (LC) in response to short trains (500 ms) of VNS observed that VNS evokes acute phasic activity in LC [17]. The difference between the activation threshold intensity and the intensity at which LC activation saturated was greater when the pulse width was shorter (100 μs vs. 500 μs), suggesting a wider pulse width results in a narrower range of VNS intensities that give rise to a dynamic activation range. Moreover, the difference in on-target LC activation and off-target activation is greater at shorter pulse widths than longer pulse widths. These findings are consistent with the notion that VNS delivered with a shorter pulse width yields a broader range of effective stimulation intensities. Future studies detailing the direct and indirect activation of other neuromodulator systems by varied VNS parameters will be vital to understanding the biomechanisms of VNS.
In summary, we find that pairing tones with VNS delivered at 200μA:500μs results in significant changes in auditory cortex receptive fields, but only to a level of ~50% of that reached with VNS delivered at 800μA:100μs. In contrast, pairing tones with VNS delivered at 200μA:100μs is insufficient to drive cortical plasticity, providing a description of the lower limit of the inverted-U effect of plasticity. Taken together, these findings provide insight into the influence of varying pulse width on VNS-dependent plasticity in auditory cortex.
Highlights.
The interaction of pulse width and current intensity on VNS-directed cortical plasticity is unclear.
200 μA VNS with 100 μs pulses is insufficient for driving cortical reorganization.
200 μA VNS with 500 μs pulses drives sub-maximal plasticity.
Pulse width influences the range of effective VNS intensities.
Clinical application of VNS should consider the interaction of pulse width and intensity.
Acknowledgments
We would like to thank Elizabeth Buell, John Buell, Alan Carroll, Natasha Houshmand, Emily Jensen, Corinne Kelly, Aisha Khan, Irfath Khan, and Shen Xian for help with electrophysiological recordings, running VNS pairing sessions, and electronics construction.
This work was supported by NIH R01NS085167, R01NS094384, and the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr. Doug Weber and Eric Van Gieson through the Space and Naval Warfare Systems Center, Pacific Cooperative Agreement No. HR0011-15-2-0017 and N66001-15-2-4057 and the DARPA BTO Targeted Neuroplasticity Training (TNT) program under the auspices of Dr. Doug Weber and Dr. Tristan McClure-Begley through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001-17-2-4011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
MPK is a consultant for, and has a financial interest in, MicroTransponder, Inc., which is developing therapies using VNS. KWL, MSB, RLR, and SAH report no biomedical financial interests or potential conflicts of interest.
References
- 1.Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex. Cereb Cortex. 2012;22:2365–74. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 2.Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, et al. Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul. 2016;9:174–81. doi: 10.1016/j.brs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shetake JA, Engineer ND, Vrana WA, Wolf JT, Kilgard MP. Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp Neurol. 2012;233:342–9. doi: 10.1016/j.expneurol.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–4. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP. Pairing speech sounds with vagus nerve stimulation drives stimulus-specific cortical plasticity. Brain Stimul. 2015;8:637–44. doi: 10.1016/j.brs.2015.01.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borland MS, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Sharma P, et al. Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul. 2016;9:117–23. doi: 10.1016/j.brs.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hays Sa, Rennaker RL, Kilgard MP. Prog Brain Res. 1st. Vol. 207. Elsevier B.V.; 2013. Targeting Plasticity with Vagus Nerve Stimulation to Treat Neurological Disease; pp. 275–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, et al. Vagus Nerve Stimulation During Rehabilitative Training Improves Functional Recovery After Intracerebral Hemorrhage. Stroke. 2014;45:3097–100. doi: 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, et al. Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats. Neurorehabil Neural Repair. 2016;30:676–84. doi: 10.1177/1545968315616494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, et al. Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J Neurotrauma. 2016;33:871–9. doi: 10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Ridder D, Vanneste S, Engineer ND, Kilgard MP. Safety and Efficacy of Vagus Nerve Stimulation Paired With Tones for the Treatment of Tinnitus: A Case Series. Neuromodulation Technol Neural Interface. 2014;17:170–9. doi: 10.1111/ner.12127. [DOI] [PubMed] [Google Scholar]
- 12.Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, et al. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke. 2016;47:143–50. doi: 10.1161/STROKEAHA.115.010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zabara J. Inhibition of Experimental Seizures in Canines by Repetitive Vagal Stimulation. Epilepsia. 1992;33:1005–12. doi: 10.1111/j.1528-1157.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 14.Manta S, Dong J, Debonnel G, Blier P. Optimization of vagus nerve stimulation parameters using the firing activity of serotonin neurons in the rat dorsal raphe. Eur Neuropsychopharmacol. 2009;19:250–5. doi: 10.1016/j.euroneuro.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Chase MH, Nakamura Y. Cortical and subcortical EEG patterns of response to afferent abdominal vagal stimulation: Neurographic correlates. Physiol Behav. 1968;3:605–10. doi: 10.1016/0031-9384(68)90121-2. [DOI] [Google Scholar]
- 16.Jiao J, Harreby KR, Sevcencu C, Jensen W. Optimal Vagus Nerve Stimulation Frequency for Suppression of Spike-and-Wave Seizures in Rats. Artif Organs. 2016;40:E120–7. doi: 10.1111/aor.12669. [DOI] [PubMed] [Google Scholar]
- 17.Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol. 2017;289:21–30. doi: 10.1016/j.expneurol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu Q, Bohning DE, Nahas Z, Walker J, Anderson B, Johnson KA, et al. Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biol Psychiatry. 2004;55:816–25. doi: 10.1016/j.biopsych.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training Unilateral Vagal Stimulation Enhances Retention Performance in the Rat. Neurobiol Learn Mem. 1995;63:213–6. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- 20.Clark KB, Naritoku DK, Smith DC, Browning Ra, Jensen Ra. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–8. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 21.Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining Electrical Stimulation of Vagal Afferents with Concomitant Vagal Efferent Inactivation Enhances Memory Storage Processes in the Rat. Neurobiol Learn Mem. 1998;70:364–73. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- 22.Zuo Y, Smith DC, Jensen RA. Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol Behav. 2007;90:583–9. doi: 10.1016/j.physbeh.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollet L, Raedt R, Delebke J, El Tahry R, GRimonprez A, Dauwe I, et al. Electrophysiological Responses from vagus nerve stimulation in rats. Int J Neural Syst. 2013;23:1350027. doi: 10.1142/S0129065713500275. [DOI] [PubMed] [Google Scholar]
- 24.Liporace J, Hucko D, Morrow R, Barolat G, Nei M, Schnur J, et al. Vagal nerve stimulation: Adjustments to reduce painful side effects. Neurology. 2001;57:885–6. doi: 10.1212/WNL.57.5.885. [DOI] [PubMed] [Google Scholar]
- 25.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: A randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/WNL.51.1.48. [DOI] [PubMed] [Google Scholar]
- 26.Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, et al. Vagus Nerve Stimulation Delivered During Motor Rehabilitation Improves Recovery in a Rat Model of Stroke. Neurorehabil Neural Repair. 2014;28:698–706. doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, et al. The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. Neuroreport. 2014;25:682–8. doi: 10.1097/WNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biggio F, Gorini G, Utzeri C, Olla P, Marrosu F, Mocchetti I, et al. Chronic vagus nerve stimulation induces neuronal plasticity in the rat hippocampus. Int J Neuropsychopharmacol. 2009;12:1209. doi: 10.1017/S1461145709000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol. 2008;214:259–65. doi: 10.1016/j.expneurol.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Grimonprez A, Raedt R, De Taeye L, Larsen LE, Delbeke J, Boon P, et al. A Preclinical Study of Laryngeal Motor-Evoked Potentials as a Marker Vagus Nerve Activation. Int J Neural Syst. 2015;25:1550034. doi: 10.1142/S0129065715500343. [DOI] [PubMed] [Google Scholar]
- 31.Koo B, Ham SD, Sood S, Tarver B. Human vagus nerve electrophysiology: a guide to vagus nerve stimulation parameters. J Clin Neurophysiol. 2001;18:429–33. doi: 10.1097/00004691-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci. 2009;34:272–80. [PMC free article] [PubMed] [Google Scholar]
- 33.Manta S, El Mansari M, Debonnel G, Blier P. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol. 2013;16:459–70. doi: 10.1017/S1461145712000387. [DOI] [PubMed] [Google Scholar]
- 34.Raedt R, Clinckers R, Mollet L, Vonck K, El Tahry R, Wyckhuys T, et al. Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. 2011;117:461–9. doi: 10.1111/j.1471-4159.2011.07214.x. [DOI] [PubMed] [Google Scholar]
- 35.Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–14. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]


