Abstract
Recent studies suggest improved survival in patients with severe aplastic anemia receiving hematopoietic cell transplant (HCT) from unrelated donors with longer telomeres. Here, we tested whether this effect is generalizable to patients with acute leukemia. From the Center for International Blood and Marrow Transplant Research (CIBMTR®) database, we identified 1,097 patients who received 8/8 HLA matched unrelated HCT for acute myeloid leukemia (AML) or acute lymphocytic leukemia (ALL) between 2004 and 2012 with myeloablative conditioning, and had pre-HCT blood sample from the donor in CIBMTR repository. The median age at HCT for recipients was 40 years (range=<1-68), and 32 years for donors (range=18-61). We used qPCR for relative telomere length measurement, and Cox proportional hazard models for statistical analyses. In a discovery cohort of 300 patients, longer donor RTL (>25th percentile) was associated with reduced risks of relapse (HR=0.62, p=0.05) and acute graft-versus-host disease II-IV (HR=0.68, p=0.05), and possibly with a higher probability of neutrophil engraftment (HR=1.3, p=0.06). However, these results did not replicate in two validation cohorts of 297 and 488 recipients. There was one exception; a higher probability of neutrophil engraftment was observed in one validation cohort (HR=1.24, p=0.05). In a combined analysis of the three cohorts, no statistically significant associations (all p>0.1) were found between donor RTL and any outcomes.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a curative therapy for patients with acute leukemia because of the high doses of chemo- and irradiation therapy and donor-driven anti-leukemia reactions.1, 2 Acute myeloid leukemia (AML), myelodysplastic syndrome, and acute lymphoblastic leukemia (ALL) are the most common indications for allogeneic HCT.3 Despite advances in HCT regimens, cell processing, and supportive care, risks of mortality and significant morbidity remain high. The 3-year survival probabilities for patients with AML or ALL receiving HCT in first complete remission are approximately 50%, and 60%, respectively.4 Notably, HCT studies in acute leukemia have shown no differences between matched sibling and matched unrelated donors on any transplant outcome.5-7 This may be due to the contemporary use of 8/8 HLA allele-matched unrelated donors. A large retrospective study of 8003 unrelated donor HCT showed that HLA mismatch (7/8 or less) were associated with increased risks of acute and chronic graft vs host disease, transplant-related mortality, and overall mortality compared with 8/8 HLA-matched cases.8
Recent studies in patients receiving HCT for severe aplastic anemia (SAA) suggest that longer donor telomere length is associated with improved patient survival.9, 10 Telomeres, the long tandem nucleotide repeats and protein complexes at chromosome ends, are essential for maintaining genomic stability.11 They shorten with each cell division and are markers for cellular replication capacity, and aging.12 Younger donor age has been associated with improved patient survival after HCT in several large retrospective studies.13, 14 However, cellular aging is a complex process and not perfectly defined by chronological age.15 For example, results from a large population-based prospective cohort of 2,186 individuals showed that telomere length adds predictive power over chronological age in detecting measures of physical and cognitive functioning.16 Telomeres shorten early after HCT as a consequence of the high cellular replication necessary to reach engraftment.17, 18 Critical telomere shortening triggers cellular senescence, and therefore short donor leukocyte telomeres may predispose HCT recipients to graft failure, or immune dysfunction.
In this study, we evaluated whether pre-HCT donor leukocyte relative telomere length (RTL) adds prognostic information over age and other clinical characteristics in predicting outcomes of unrelated donor HCT for patients with acute leukemia.
Patients and Methods
Study population
We used data and biospecimens collected by the Center for International Blood and Marrow Transplant Research (CIBMTR®), a research collaboration between the National Marrow Donor Program (NMDP)/Be The Match® and the Medical College of Wisconsin. CIBMTR collects baseline and outcome data on allogeneic and autologous HCT from more than 450 transplant centers worldwide, and pre-HCT biospecimens from all recipients and donors of unrelated donor transplant, and more recently from related donor transplants.
We identified all patients who received unrelated HCT for AML or ALL and who fulfilled the following criteria: 1) HCT between 2004 and 2012, 2) in first or second complete remission (CR1, or CR2) prior to HCT, 3) had an available donor pre-HCT blood sample in the CIBMTR repository, 4) recipient-donor 8/8 HLA matching based on retrospective high resolution typing,19 5) received myeloablative conditioning regimen, and 6) graft source was either bone marrow (BM) or peripheral blood stem cells (PBSC).
These patients (n=1,097) were then divided into three cohorts: discovery (n=300), first validation (n=297) and a second validation (n=500) cohort. The discovery and first validation set included patients who underwent HCT between 2004-2008 and were randomly assigned, whereas the second validation set spanned HCTs between 2004-2012 and included randomly assigned patients between 2004-2008 plus all patients between 2009-2012.
All participants provided informed consent, and the study was approved by the NMDP Institutional Review Board and the NIH office of Human Subjects Research Protections.
Study outcomes
Study outcomes included: overall survival (OS); disease-free survival (DFS), defined as survival without relapse; treatment-related mortality (TRM), defined as death during continuous complete remission; leukemia relapse; neutrophil engraftment, defined as absolute neutrophil count of 0.5×109/L for 3 consecutive days; and acute and chronic graft-versus-host disease (GvHD), defined according to standard criteria.20, 21
DNA extraction and Relative Telomere Length Assay
Donor samples were collected less than a month prior to bone marrow harvest or prior to granulocyte colony stimulating factor (GCSF) stimulation. Samples consisted of whole blood collected in ACD-A stored as whole blood or separated peripheral blood mononuclear cells and maintained frozen at -80°C. All samples were processed and frozen within 3 days of collection. We used QIAamp Maxi Kit procedure (QIAGEN Inc., Valencia, CA) to extract DNA from donor whole blood samples collected before HCT. To ensure reproducibility of the results, RTL was measured in two laboratories using quantitative real-time PCR (qPCR) assay adapted from methods described elsewhere.22, 23 Samples from the discovery and first validation cohorts were measured at Telomere Diagnostics laboratory (TDx; http://telomeredx.com), and samples from the second validation cohort were measured at the NCI Cancer Genomics Research (CGR) laboratory. RTL measurements were completed on total of 1,085 HCT donors.
RTL measurement at TDx used the following primers for telomeric PCR: Tel1b: [5′-CGGTTT(GTTTGG)5GTT-3′], and Tel2b [5′-GGCTTG(CCT TAC)5CCT-3′]. Their primers for single-copy gene (human beta-globin; Hbg) PCR were Hbg1: 5′ GCTTCTGACA-CAACTGTGTTCACTAGC-3′, and Hbg2: 5′-CACCAACTTCATCCACGTTCA-CC-3′. Details of the TDx method are published elsewhere.24 The NCI's CGR telomeric PCR primers were Telo_FP [5′-CGGTTT(GTTTGG)5GTT-3′] and Telo_RP [5′-GGCTTG(CCTTAC)5CCT-3′]25. Primers for the single-copy gene (36B4) PCR were 36B4_FP [5′-CAGCAAGTGGGAAGGTGTAATCC-3′] and 36B4_RP [5′-CCCATTCTATCATCAACGGGTACAA-3′]22. Details for the NCI assay were previously described.10 Both laboratories calculated the ratio between the concentration of telomere (T) signal and that of the single copy gene (S) yielding a T/S ratio that was then standardized in relation to an internal QC calibrator samples within each plate, and final measurements were exponentiated to assure normality. For quality control, all telomeric and single copy gene reactions were measured in triplicate, and the average was used for final calculations. The mean coefficient of variation (CV) for the standardized T/S measure from replicate samples for TDx laboratory was 2.5% and for the NCI laboratory was 8.6%
Statistical Analysis
We used Kaplan-Meier estimators to calculate the univariate probabilities of overall survival (OS) and disease-free survival (DFS). Probabilities of relapse and transplant-related mortality (TRM) were estimated based on the cumulative incidence method.26 For analysis of OS, death from any cause was considered an event. For analysis of DFS, relapse and death were considered events. For analysis of TRM, relapse was treated as a competing event. For analysis of leukemia relapse, TRM was treated as a competing event.
In multivariable analyses, we used Cox proportional hazard models to adjust for significant clinical and therapeutic factors. All factors were examined for proportional hazards using a time-dependent approach. Factors violating the proportional hazard assumption were adjusted for through stratification. To select variables included in the final models, a stepwise forward-backward procedure was used for each endpoint with a p-threshold of 0.05 for entry and retention in the model. All models were adjusted for donor age to account for the association between RTL and age. Each set (discovery, 1st and 2nd validation) was treated as a separate cohort for model variable selection. Follow-up started at date of HCT and ended on event of interest, death, or end of study (November 30, 2014).
Based on the goodness-of-fit from the discovery cohort, we identified a RTL of 0.93 (25th percentile) as the optimal cut-off point for the OS outcome for the discovery cohort. We then categorized RTL, based on cohort-specific distributions, into short (≤25th percentile) or longer (>25th percentile), and compared those 2 categories for all other outcomes in the validation cohorts. Analyses were performed using SAS version 9.3 (Carey, NC).
Results
Patient Characteristics
Table 1 describes the demographics and clinical characteristics of the acute leukemia patients and their matched donors by their assignment cohort. Briefly, 52.7% of the recipients and 73.9% of the donors were younger than 40 years, the majority were white (86.9%, and 85% in the recipients and donors, respectively), and 52.9% of the recipients, and 68.4% of the donors were male. More patients had AML (68.9 %), 63.3% were in first complete remission, and 66.5% received PBSC grafts. No differences between cohorts were noted except for graft type (p=0.008), regimes for GvHD prophylaxis (p=0.05), and calendar year of HCT (p<0.001). As expected, donor RTL inversely correlated with age in all cohorts (r = - 0.21, p=0.0003 in the discovery cohort, r = - 0.26, p<0.0001 in the 1st validation, and r = - 0.37, p<0.0001 in the 2nd validation).
Table 1. Characteristics of acute leukemia patients by cohort assignment.
| Variable | Discovery N (%) | 1st Validation N (%) | 2nd Validation N (%) | p-valuea |
|---|---|---|---|---|
| Number of Recipients | 300 | 297 | 488 | |
| Number of centers | 86 | 88 | 107 | |
| Recipient age at transplant | 0.53 | |||
| 0-9 years | 18 (6) | 19 (6) | 40 (8) | |
| 10-19 years | 41 (14) | 46 (15) | 46 (9) | |
| 20-29 years | 57 (19) | 50 (17) | 84 (17) | |
| 30-39 years | 45 (15) | 47 (16) | 82 (16) | |
| 40-49 years | 66 (22) | 65 (22) | 115 (24) | |
| 50-59 years | 73 (24) | 70 (24) | 121 (25) | |
| Median (Range) | 37 (1-66) | 37 (1-67) | 39 (0-68) | 0.53 |
| Recipient race/ethnicity | 0.11 | |||
| Caucasian, non-Hispanic | 267 (92) | 248 (86) | 427 (90) | |
| Other/unknown | 33 (8) | 49 (14) | 61 (10) | |
| Recipient sex | 0.70 | |||
| Male | 157 (52) | 151 (51) | 263 (54) | |
| Female | 143 (48) | 146 (49) | 225 (46) | |
| Karnofsky score | 0.76 | |||
| 10-80 | 73 (24) | 67 (23) | 123 (25) | |
| 90-100 | 206 (69) | 205 (69) | 335 (69) | |
| Missing | 21 (7) | 25 (8) | 30 (6) | |
| Disease at transplant | 0.12 | |||
| AML | 199 (66) | 197 (66) | 352 (72) | |
| ALL | 101 (34) | 100 (34) | 136 (28) | |
| Disease status at transplant | 0.11 | |||
| Early | 189 (63) | 173 (58) | 321 (67) | |
| Intermediate | 111 (37) | 124 (42) | 167 (33) | |
| Stem cell source | 0.01 | |||
| Marrow | 100 (33) | 120 (40) | 147 (30) | |
| PBSC | 200 (67) | 177 (60) | 341 (70) | |
| BM Nucleated cell count | ||||
| Median (Range)×10ˆ9 | 18.6 (5.54-36.3) | 19.98 (0.67-38.1) | 17.32 (0.16-37.3) | 0.21 |
| BM CD34 cell count | ||||
| Median (Range) ×10ˆ8 | 1.65 (0.43-3.4) | 2.48 (0.003-4.7) | 1.8 (0.0015-51.8) | 0.65 |
| PB CD34 cell count | ||||
| Median (Range) ×10ˆ8 | 4.5 (1.1-11.7) | 4.3 (0.0038-19.4) | 5.6 (0.034-28.4) | 0.26 |
| GvHD Prophylaxis | 0.06 | |||
| CSA+/-others | 71 (23.7) | 76 (25.6) | 98 (20) | |
| Tacrolimus+/-others | 229 (76.3) | 218 (73.4) | 390 (80) | |
| Othersb | 0 | 3 (1) | 0 | |
| ATG Given | 0.33 | |||
| Yes | 58 (19) | 70 (24) | 115 (24) | |
| No | 242 (81) | 227 (76) | 373 (76) | |
| Campath Given | 0.94 | |||
| Yes | 8 (3) | 10 (3) | 12 (2) | |
| No | 289 (96) | 285 (96) | 472 (97) | |
| Unknown | 3 (1) | 2 (1) | 4 (1) | |
| TBI Regimen | 0.27 | |||
| No | 140 (47) | 139 (47) | 252 (52) | |
| Yes | 160 (53) | 158 (53) | 236 (48) | |
| Donor/Recipient sex matching | 0.3 | |||
| Male/Male | 113 (38) | 102 (34) | 201 (41) | |
| Male/Female | 85 (28) | 89 (30) | 151 (31) | |
| Female /Male | 44 (15) | 49 (16) | 62 (13) | |
| Female / Female | 58 (19) | 57 (19) | 74 (15) | |
| Donor/Recipient CMV serostatus | 0.22 | |||
| Negative/Negative | 84 (28) | 81 (27) | 166 (34) | |
| Negative/Positive | 113 (38) | 103 (35) | 163 (33) | |
| Positive/Negative | 34 (11) | 47 (16) | 65 (13) | |
| Positive/Positive | 69 (23) | 66 (22) | 94 (19) | |
| Donor age at donation | 0.66 | |||
| 18-19 y | 6 (2) | 9 (3) | 12 (2) | |
| 20-29 y | 104 (35) | 117 (39) | 209 (42) | |
| 30-39 y | 102 (34) | 97 (33) | 147 (30) | |
| 40-49 y | 70 (23) | 59 (20) | 93(19) | |
| 50 y and older | 18 (6) | 15 (5) | 30 (6) | |
| Median (Range) | 33 (19-60) | 32 (19-60) | 32 (18-61) | 0.13 |
| Donor race/ethnicity | 0.27 | |||
| Caucasian, non-Hispanic | 257 (90) | 248 (90) | 422 (91) | |
| Other/unknown | 43 (10) | 49 (10) | 66 (9) | |
| Year of transplant | <0.001 | |||
| 2004 | 38 (13) | 41 (14) | 47 (9) | |
| 2005 | 73 (24) | 46 (15) | 62 (13) | |
| 2006 | 70 (23) | 65 (22) | 73 (15) | |
| 2007 | 63 (21) | 72 (24) | 71 (15) | |
| 2008 | 56 (19) | 73 (25) | 56 (11) | |
| 2009 | 0 | 0 | 79 (16) | |
| 2010 | 0 | 0 | 63 (13) | |
| 2011 | 0 | 0 | 18 (4) | |
| 2012 | 0 | 0 | 20 (4) | |
| Follow-up among survivors, Months | ||||
| Median (Range) | 73 (8-121) | 72 (13-123) | 60.1 (5.2-123) | <0.001 |
The Pearson chi-square test was used for comparing discrete variables; the Kruskal-Wallis test was used for comparing continuous variables.
Others include CD34 selection or cyclophosphamide
Donor RTL and HCT outcomes
In the discovery cohort, univariate analysis showed that longer donor RTL (i.e., >25th percentile) was associated with improved post-HCT overall survival at one (OS= 71% vs. 54%, p=0.01) and three years (OS=56% vs. 38%, p=0.005), and there was higher probability of disease free survival at one (DFS=63% vs. 44%, p=0.005) and three years (50% vs. 35%, p=0.02) (Table 2). These associations were not statistically significant in multivariable models adjusted for donor and recipient age in OS models (HR=0.73, 95% CI=0.52-1.03, p=0.08), and donor age, recipient-donor cytomegalovirus match, and Karnofsky performance score in DFS models (HR=0.73, 95% CI=0.52-1.03, p=0.07) (Table 3).
Table 2. Discovery cohort univariate probability of HCT outcomes for patients with acute leukemia by donor telomere length.
| Short Donor RTL (≤25th percentile) | Longer Donor RTL (>25th percentile) | Pointwise | |||
|---|---|---|---|---|---|
| Outcome | N | Prob (95% CI) | N | Prob (95% CI) | p-value |
| Survival | 76 | 224 | |||
| 1 year | 54 (42-65) | 71 (64-76) | 0.01 | ||
| 3 years | 38 (27-49) | 56 (49-62) | 0.005 | ||
| 5 years | 36 (26-47) | 48 (41-54) | 0.08 | ||
| DFS | 75 | 222 | |||
| 1 year | 44 (33-56) | 63 (57-69) | 0.005 | ||
| 3 years | 35 (24-46) | 50 (44-57) | 0.02 | ||
| 5 years | 33 (23-44) | 43 (37-50) | 0.11 | ||
| TRM | 75 | 222 | |||
| 1 year | 27 (18-38) | 18 (14-24) | 0.14 | ||
| 3 years | 31 (21-42) | 24 (19-30) | 0.24 | ||
| 5 years | 33 (23-44) | 30 (24-36) | 0.61 | ||
| Relapse | 75 | 222 | |||
| 1 year | 29 (19-39) | 18 (14-24) | 0.09 | ||
| 3 years | 34 (24-45) | 25 (20-31) | 0.17 | ||
| 5 years | 34 (24-45) | 27 (21-33) | 0.25 | ||
| Neutrophil Engraftment | 76 | 224 | |||
| 24 days | 88 (80-94) | 94 (91-97) | 0.13 | ||
| 42 days | 97 (93-100) | 98 (96-100) | 0.68 | ||
| 100 days | 97 (93-100) | 98 (96-100) | 0.68 | ||
| Grades II-IV aGvHD | 76 | 224 | |||
| 100 days | 49 (38-60) | 38 (32-44) | 0.10 | ||
| Grades III-IV aGvHD | 76 | 224 | |||
| 100 days | 21 (13-31) | 14 (10-19) | 0.20 | ||
| cGvHD | 74 | 216 | |||
| 1 year | 49 (37-60) | 56 (49-63) | 0.28 | ||
| 2 years | 51 (40-63) | 62 (55-69) | 0.12 | ||
Abbreviations: Prob = Probability (%); CI = Confidence interval
DFS: disease free survival, TRM: transplant related mortality, aGvHD: acute graft-versus-host disease, cGvHD: chronic graft-versus-host disease
Table 3. Multivariable associations between longer donor RTL (using the 25th percentile as a cut-off point) and HCT outcomes in the discovery and validation cohorts.
| Outcome | Discovery cohort* N=300 | 1st Validation* N=297 | 2nd validation# N=488 |
|---|---|---|---|
| HR (95% CI) P | |||
| OS | 0.73 (0.52-1.03) 0.08 | 0.94 (0.65-1.38) 0.76 | 1.25 (0.91-1.71) 0.17 |
| DFS | 0.73 (0.52-1.03) 0.07 | 0.92 (0.63-1.34) 0.66 | 1.18 (0.87-1.59) 0.29 |
| TRM | 0.80 (0.49-1.32) 0.38 | 0.82 (0.48-1.43) 0.49 | 1.23 (0.77-1.98) 0.38 |
| Relapse | 0.62 (0.38-1.00) 0.05 | 1.20 (0.69-2.07) 0.51 | 1.12 (0.76-1.66) 0.56 |
| aGVHD II-IV | 0.68 (0.46-1.00) 0.05 | 1.00 (0.66-1.51) 0.99 | 1.00 (0.73-1.38) 0.98 |
| aGVHD III-IV | 0.62 (0.34-1.14) 0.13 | 1.10 (0.53-2.30) 0.79 | 1.24 (0.71-2.16) 0.46 |
| cGVHD | 1.06 (0.72-1.56) 0.77 | 0.98 (0.67-1.45) 0.93 | 0.99 (0.74-1.34) 0.97 |
| Neutrophil engraftment | 1.30 (0.99-1.71) 0.06 | 0.84 (0.63-1.11) 0.21 | 1.24 (1.00-1.54) 0.05 |
OS models are adjusted for: recipient age, donor age; DFS adjusted for CMV match, KPS, donor age; TRM for time from diagnosis to HCT, KPS, recipient age, year of HCT, donor age; Relapse for donor age, TBI, DPB1 TCE match, Sex; aGVHD II-IV for ATG, graft type, donor age; aGVHD III-IV for ATG, disease status, donor age; cGVHD for ethnicity, graft type, recipient blood type, year of HCT, donor age; Engraftment for graft type, TBI, donor age
OS models are adjusted for: recipient age, donor age and use of TBI; stratified by graft type and ABO match; DFS: recipient age, graft type, donor age and use of TBI; stratified by patient ABO type; TRM: recipient age, graft type, donor age and use of TBI; stratified by graft type and ABO match; Relapse: donor age; aGVHD II-IV: ATG, use of alemtuzumab, donor age, GVHD prophylaxis, donor age and recipient age; aGVHD III-IV: donor age; cGVHD: ABO match, ATG, use of alemtuzumab, graft type, TCE match, use of TBI, donor age and year of transplant; Engraftment: ABO match, donor age and use of TBI; stratified by graft type
In two validation cohorts, longer donor RTL was not statistically significantly associated with any HCT outcome in the first validation cohort of 297 patients, or second cohort of 488 patients. However, we did observe a possible improved neutrophil engraftment with longer donor RTL (HR=1.24, 95% CI=1.0-1.54, p=0.05) after adjusting for ABO blood type match, donor age, use of TBI, and stratified by graft type (Table 3).
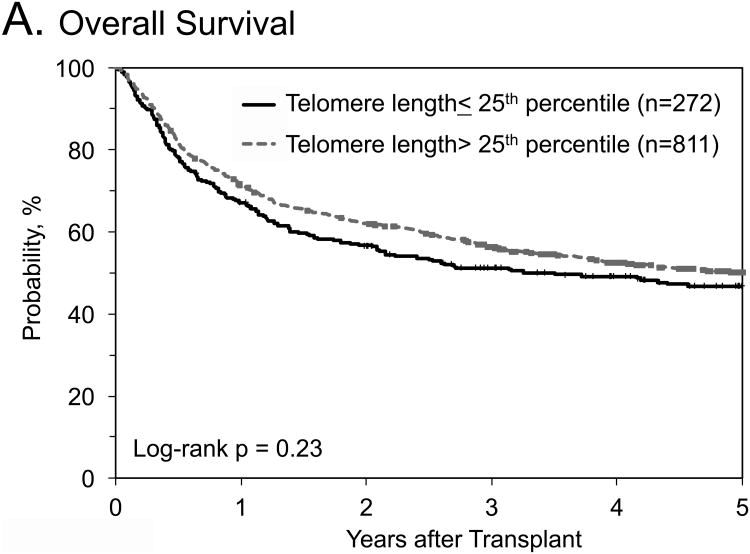
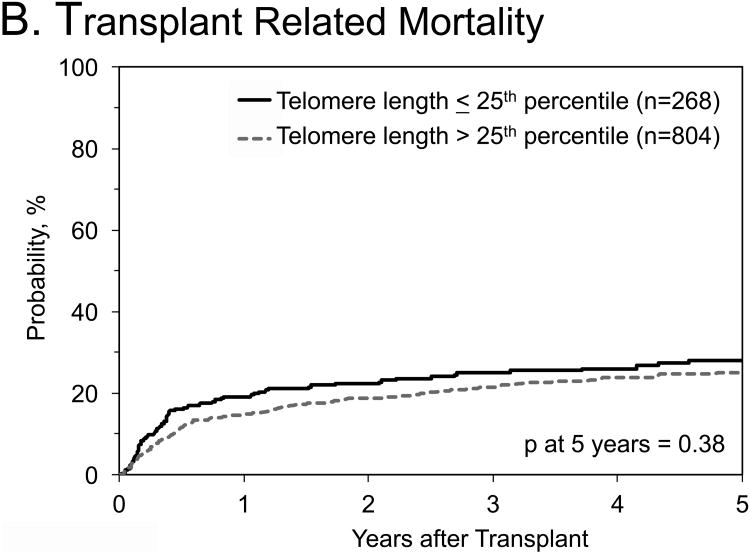
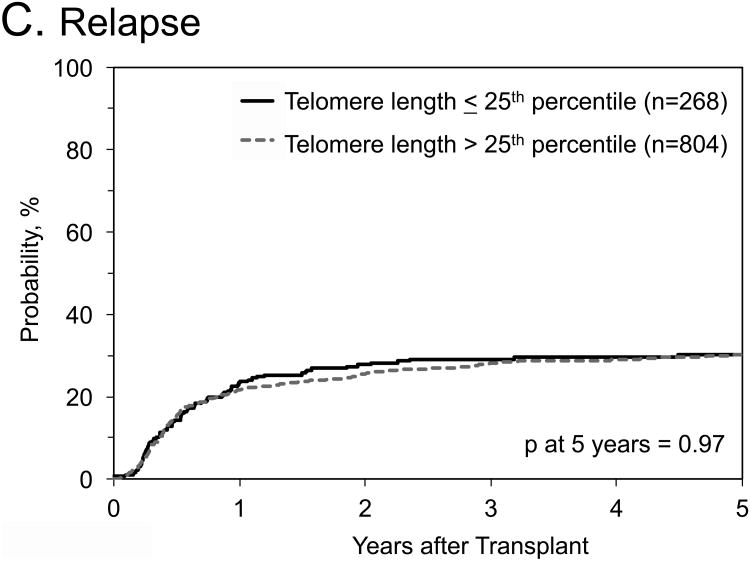
No statistical significant associations with any outcomes were identified when we combined data from the three cohorts, and compared longer (>25th percentile of their cohort) with short (≤25th percentile) donor RTL (Table 4; Figure 1). Similar results were noted in OS analyses stratified by: 1) graft type (HR=1.11, 95% CI=0.76-1.63 in bone marrow patients, and HR=0.96, 95% CI=0.76-1.22 in peripheral blood stem cell patients); 2) recipient age (HR=0.9, 95% CI=0.84-1.41 in patients ≤35 years old, and HR=1.09, 95% CI=0.84-1.41 in patients >35 years old); 3) disease subtype (HR=1.08, 95% CI=0.85-1.38 for AML, and HR=0.81, 95% CI=0.58-1.14 for ALL); or 4) disease status at HCT (HR=1.14, 95% CI=0.88-1.47 for patients in 1st complete remission, and HR=0.83, 95% CI=0.61-1.13 for patients in 2nd complete remission). When restricting the OS analysis to patients with ALL in 2nd complete remission, the HR -comparing donor RTL > 25th percentile with those ≤25th percentile- was 0.62, 95% CI=0.38-1.0, p=0.06)
Table 4.
Multivariate association between donor RTL and HCT outcome in the combined cohorts comparing long to short RTL (using the 25th percentile as a cut-off point).
| Outcome | N event/N total | HR* | 95% CI | p-value |
|---|---|---|---|---|
| OS | 564/1077 | 0.99 | 0.81-1.20 | 0.91 |
| DFS | 577/1072 | 0.99 | 0.82-1.21 | 0.95 |
| TRM | 265/1072 | 1.01 | 0.76-1.34 | 0.95 |
| Relapse | 312/1072 | 0.99 | 0.76-1.29 | 0.59 |
| aGVHD II-IV | 472/1067 | 0.89 | 0.72-1.11 | 0.30 |
| aGVHD III-IV | 168/1080 | 1.06 | 0.74-1.52 | 0.75 |
| cGVHD | 582/1032 | 0.97 | 0.79-1.18 | 0.75 |
| Neutrophil engraftment | 1040/1065 | 1.13 | 0.97-1.31 | 0.11 |
Hazard ratio; models are adjusted for the following covariates:
OS: cytogenetics, donor age, recipient age, use of TBI; stratified by graft type
DFS: cytogenetics, donor age, recipient age, disease status, interval from diagnosis to transplant; stratified by graft type
TRM: cytogenetics, donor age, recipient age, graft type and use of TBI; stratified by graft type
Relapse: cytogenetics, disease status, interval from diagnosis to transplant, donor age
aGVHD II-IV: ATG, use of alemtuzumab, recipient-donor sex match; stratified by permissive mismatches and year of transplant, donor age
aGVHD III-IV: ATG, graft type and recipient-donor sex match; stratified by TCE match, donor age
cGVHD: ATG, use of alemtuzumab, conditioning regimen, graft type and year of transplant, donor age
Neutrophil engraftment: ABO match, donor age, GvHD prophylaxis; stratified on graft type, and conditioning regimen
Figure 1. Outcomes after hematopoietic cell transplant for patients with acute leukemia by donor relative leukocyte telomere length.
A) Probability of overall survival; B) probability of transplant related mortality; C) Cumulative incidence of relapse
Discussion
This study evaluated whether donor telomere length was associated with outcomes after HCT in 1,085 acute leukemia patients. The results from the discovery cohort suggested possible associations between longer donor telomeres and lower risk of leukemia relapse and acute GvHD II-IV, as well as possible rapid neutrophil engraftment after HCT. However, these findings were not replicated in the two validation cohorts, with the exception of an observed association with neutrophil engraftment in the second validation set. Our combined analysis showed no statistically significant association between donor pre-HCT RTL and any HCT outcomes.
Previous studies in patients with SAA showed improved OS for patients receiving HCT from donors with longer RTL.9,10 The inconsistency between results of the current acute leukemia study and those previously published in SAA may be explained, in part, by differences in the underlying disease biology for which the recipients received HCT. Successful HCT in SAA depends primarily on correcting for the underlying marrow defect; while in acute leukemia, successful HCT mainly depends on the donor-driven anti-leukemia reaction. Patients with SAA typically have an underlying immune-mediated process, germline telomere biology disorder, or other inherited condition affecting the stem cell niche.27 In this setting, longer donor telomeres may be helpful in correcting for some of the underlying marrow defect and hence improving patient overall survival. In the leukemia setting, our data suggested that longer donor telomere length may result in faster engraftment but have no effect on survival, possibly because it doesn't reduce patient risk of relapse. Another possibility could be related to the differences in recipient-donor ages. In the current acute leukemia study, donors were younger than recipients; the opposite was true in SAA. It is possible that selecting young donors for older patients may obscure the possible advantage of longer donor TL. This hypothesis may be justified, in part, by our previous study which showed no association between donor RTL and post-HCT survival in SAA patients older than 40 years of age.10 Other explanations could include the small but important design differences between studies, as the current study included more recent transplants (2004-2012), and was restricted to myeloablative regimens as well as 8/8 HLA matching.
Although not consistent between the validation sets, our data suggest a possible association between longer donor RTL and improved neutrophil engraftment in 2 of the 3 cohorts. Similarly, a recent report from the Children's Oncology Group showed that longer patient telomeres after induction chemotherapy was associated with faster neutrophil recovery in subsequent chemotherapy courses.28 This finding suggests a role for telomere length in hematopoietic reconstitution capacity after chemotherapy in patients with AML. The role that donor telomere length may play in hematological recovery after HCT is still unclear. A small study of 19 HCT pediatric patients, showed that longer donor RTL was associated with faster post-HCT hematological recovery.29 In contrast, donor telomere length was not associated with hematological recovery in patients receiving HCT for SAA.9
The strengths of this study include its large sample size, and the availability of pre-HCT donor blood samples and comprehensive transplant and outcome information. The study was restricted to acute leukemia patients undergoing unrelated donor HCT, 8/8 HLA matching, and myeloablative regimens; therefore results may not be generalizable to all patients receiving HCT for acute leukemia. The qPCR telomere length measurement method is a high throughput technique, but prone to measurement errors30 due to its high sensitivity to pre-analytic conditions, such as DNA extraction methods.31 In the current study, we used the same method to extract DNA from all samples to reduce the likelihood of qPCR RTL variability. Inter-laboratory assay variability could also contribute to differences between cohorts but this was likely minimal as the correlation coefficient (r) between 100 blinded samples measured in both laboratories was 0.70, P<0.0001. Since qPCR RTL provides an average measure of telomere length in all white blood cell subsets, results could be affected by the cellular composition of the samples. A future study with a cell-specific method, such as flow cytometry with fluorescence in-situ hybridization may be warranted.
In conclusion, our study showed no association between donor telomere length and HCT outcomes in patients who received unrelated, 8/8 HLA allele-matched HCT and myeloablative regimen for AML or ALL. These results may not be generalizable to other HCT indications or procedures; more studies are warranted to answer this question.
Acknowledgments
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute; by a Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases, Heath Resources, and Services Administration (HHSH234200637015C); and by two Grants N00014-14-1-0028 and N00014-15-1-0848 from the Office of Naval Research.
Footnotes
Conflict of Interest: David Loftus, Damjan Krstajic, and Ljubomir Buturovic are paid consultant for Telomere Diagnostics, Inc. Lyssa Friedman, Marsha Blauwkamp, and Jason Shelton are employees at Telomere Diagnostics, Inc.
References
- 1.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 2.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 3.Pasquini M, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR Summary Slides. Available from: URL (Accessed n Date Accessed Year) [Google Scholar]
- 4.Baerlocher GM, Lansdorp PM. Telomere length measurements using fluorescence in situ hybridization and flow cytometry. Methods Cell Biol. 2004;75:719–750. doi: 10.1016/s0091-679x(04)75031-1. [DOI] [PubMed] [Google Scholar]
- 5.Peters C, Schrappe M, von Stackelberg A, Schrauder A, Bader P, Ebell W, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: A prospective international multicenter trial comparing sibling donors with matched unrelated donors-The ALL-SCT-BFM-2003 trial. J Clin Oncol. 2015;33(11):1265–1274. doi: 10.1200/JCO.2014.58.9747. [DOI] [PubMed] [Google Scholar]
- 6.Robin M, Porcher R, Ades L, Boissel N, Raffoux E, Xhaard A, et al. Matched unrelated or matched sibling donors result in comparable outcomes after non-myeloablative HSCT in patients with AML or MDS. Bone Marrow Transplant. 2013;48(10):1296–1301. doi: 10.1038/bmt.2013.50. [DOI] [PubMed] [Google Scholar]
- 7.Solomon SR, Sizemore CA, Zhang X, Brown S, Holland HK, Morris LE, et al. Impact of Donor Type on Outcome after Allogeneic Hematopoietic Cell Transplantation for Acute Leukemia. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Pidala J, Lee SJ, Ahn KW, Spellman S, Wang HL, Aljurf M, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124(16):2596–2606. doi: 10.1182/blood-2014-05-576041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadalla SM, Wang T, Haagenson M, Spellman SR, Lee SJ, Williams KM, et al. Association between donor leukocyte telomere length and survival after unrelated allogeneic hematopoietic cell transplantation for severe aplastic anemia. JAMA: the journal of the American Medical Association. 2015;313(6):594–602. doi: 10.1001/jama.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadalla SM, Wang T, Dagnall C, Haagenson M, Spellman SR, Hicks B, et al. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2016. Effect of Recipient Age and Stem Cell Source on the Association between Donor Telomere Length and Survival after Allogeneic Unrelated Hematopoietic Cell Transplantation for Severe Aplastic Anemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336(6081):593–597. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greider CW. Regulating telomere length from the inside out: the replication fork model. Genes & development. 2016;30(13):1483–1491. doi: 10.1101/gad.280578.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 14.Kollman C, Spellman SR, Zhang MJ, Hassebroek A, Anasetti C, Antin JH, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127(2):260–267. doi: 10.1182/blood-2015-08-663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marioni RE, Harris SE, Shah S, McRae AF, von Zglinicki T, Martin-Ruiz C, et al. The epigenetic clock and telomere length are independently associated with chronological age and mortality. International journal of epidemiology. 2016 doi: 10.1093/ije/dyw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Der G, Batty GD, Benzeval M, Deary IJ, Green MJ, McGlynn L, et al. Is telomere length a biomarker for aging: cross-sectional evidence from the west of Scotland? PloS one. 2012;7(9):e45166. doi: 10.1371/journal.pone.0045166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiyama M, Asai O, Kuraishi Y, Urashima M, Hoshi Y, Sakamaki H, et al. Shortening of telomeres in recipients of both autologous and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2000;25(4):441–447. doi: 10.1038/sj.bmt.1702144. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama M, Hoshi Y, Sakurai S, Yamada H, Yamada O, Mizoguchi H. Changes of telomere length in children after hematopoietic stem cell transplantation. Bone Marrow Transplant. 1998;21(2):167–171. doi: 10.1038/sj.bmt.1701060. [DOI] [PubMed] [Google Scholar]
- 19.Spellman S, Setterholm M, Maiers M, Noreen H, Oudshoorn M, Fernandez-Vina M, et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14(9 Suppl):37–44. doi: 10.1016/j.bbmt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 21.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematology/oncology clinics of North America. 1999;13(5):1091–1112. doi: 10.1016/s0889-8588(05)70111-8. viii-ix. [DOI] [PubMed] [Google Scholar]
- 22.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1-2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Callicott RJ, Womack JE. Real-time PCR assay for measurement of mouse telomeres. Comp Med. 2006;56(1):17–22. [PubMed] [Google Scholar]
- 26.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Savage SA, Alter BP. The role of telomere biology in bone marrow failure and other disorders. Mechanisms of ageing and development. 2008;129(1-2):35–47. doi: 10.1016/j.mad.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerbing RB, Alonzo TA, Sung L, Gamis AS, Meshinchi S, Plon SE, et al. Shorter Remission Telomere Length Predicts Delayed Neutrophil Recovery After Acute Myeloid Leukemia Therapy: A Report From the Children's Oncology Group. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.66.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangerini R, Lanino E, Terranova P, Faraci M, Pistillo MP, Gaetani GF, et al. Telomere length of donors influences granulocyte recovery in children after hematopoietic stem cell transplantation. Ann Hematol. 2009;88(10):1029–1031. doi: 10.1007/s00277-009-0712-z. [DOI] [PubMed] [Google Scholar]
- 30.Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic acids research. 2011;39(20):e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham JM, Johnson RA, Litzelman K, Skinner HG, Seo S, Engelman CD, et al. Telomere length varies by DNA extraction method: implications for epidemiologic research. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(11):2047–2054. doi: 10.1158/1055-9965.EPI-13-0409. e-pub ahead of print 2013/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]