Abstract
Objective
Identify associations between improvement in genitourinary symptoms of menopause (GSM) and vaginal microbiota, vaginal glycogen and serum estrogen.
Methods
Thirty postmenopausal women enrolled in a hot flash treatment trial (oral estradiol vs. venlafaxine vs. placebo) who reported GSM and provided vaginal swabs at 0, 4 and 8 weeks were studied. Bacterial communities were characterized using deep sequencing targeting the 16S rRNA gene V3–V4 region. Participants selected a most bothersome genitourinary symptom (dryness, discharge, pain, itch/burn or inability to have sex) and rated severity on a 10-point scale at baseline and 8 weeks. Vaginal glycogen and serum estradiol and estrone were measured at enrollment and 8 weeks. Comparisons according to improvement in most bothersome symptom (MBS) were made using chi-square, Wilcoxon signed-rank test, or Hotelling’s t test.
Results
Of 30 participants, 21 (70%) had improvement in MBS over the 8-week study, and 9 (30%) had no improvement or worsening of MBS. A higher proportion of women receiving estradiol or venlafaxine reported improvement in MBS (88%, 78%) compared to placebo (54%; p = 0.28). MBS improvement was associated with Lactobacillus-dominant vaginal microbiota at enrollment (57% vs 22%, p = 0.08). Vaginal glycogen, serum estradiol and estrone increased significantly in women whose MBS improved.
Conclusion
Improvement in GSM was more common in women receiving oral estradiol or venlafaxine and those with Lactobacillus-dominant vaginal microbiota at enrollment but differences were not statistically significant. Larger trials are need to determine whether vaginal microbiota modify or mediate treatment responses in women with GSM.
Keywords: Vaginal microbiome, genitourinary syndrome of menopause, vaginal glycogen, serum estradiol
Introduction
Genitourinary syndrome of menopause (GSM) is reported in at least 45% of postmenopausal women and can cause significant distress.1,2 The most commonly reported symptoms are vaginal dryness, pain with intercourse, and vulvovaginal itching/irritation.3 Little is known about the etiology of these symptoms, beyond the association with decreased serum estrogen levels. Atrophy of the genitourinary tissue is a common finding after menopause, but physical exam findings of atrophy do not correlate consistently with patient report of symptoms.4,5 In many studies, both symptoms and physical exam findings improve with systemic or local estrogen therapy.6,7 Recently, studies showing an association between vulvovaginal atrophy or dryness on exam and decreased vaginal detection of Lactobacillus bacteria have suggested that vaginal colonization with lactobacilli may mediate the development of genitourinary symptoms.8,9 However, our recent evaluation of patient-reported symptoms and vaginal Lactobacillus colonization in a cross-sectional analysis of postmenopausal women experiencing hot flashes did not identify an association between GSM and vaginal detection of or dominance by Lactobacillus species.10
After menopause, vaginal colonization with Lactobacillus species is much less common than in pre-menopausal women.11,12 Treatment with systemic hormone therapy is associated with increased detection of lactobacilli in the vagina.13,14 In Chinese women, those with both GSM and exam findings of atrophy (“atrophic vaginitis”) had significantly lower abundance of vaginal Lactobacillus species than women without symptoms or atrophy.15 After treatment of atrophic vaginitis with low dose oral estrogen, abundance of vaginal lactobacilli increased and symptoms improved. Abundance of Lactobacillus was significantly negatively associated with symptom scores, but there was no discussion of symptom prevalence or severity in the 6 women who did not have a Lactobacillus-dominant vaginal microbiota after 4 weeks of estrogen therapy.
We performed a longitudinal study of the association between changes in vaginal symptoms and microbiota in postmenopausal women in a placebo controlled trial of oral estradiol or venlafaxine for hot flashes. We hypothesized that women whose symptoms improved would have an increase in the detection, quantity and dominance of Lactobacillus species, either because colonization is a marker of a healthy vaginal environment, or because colonization drives postmenopausal vaginal health.
Methods
Study participants
This study used samples and data from a subset of women who participated in a 3-arm double-blind, 8-week randomized trial of oral estradiol, venlafaxine, or placebo for the alleviation of menopausal hot flashes. The study was conducted at three Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH) network sites (Boston, Philadelphia and Seattle). Details about the MsFLASH Research Network,16 study design, methods, and main trial results have been reported elsewhere.17 Women aged 40–62, in the menopausal transition (≤12 months since last menstrual period) or in menopause (> 12 months since last period), who reported ≥ 14 hot flashes per week and met other inclusion criteria participated. The protocol was approved by the appropriate institutional review board at each site. All participants provided written informed consent. Women who agreed to the ancillary vaginal microbiome study signed a second consent form. The parent study enrolled 339 women between November 2011 and October 2012. Enrollment in the vaginal health sub-study was offered between June and October 2012 and 93 of 117 (79%) women who enrolled in the parent study during that time also enrolled in the vaginal health sub-study. Of those, 30 women with vaginal symptoms at enrollment who also agreed to provide multiple vaginal samples over the course of the 8–week trial were included in a longitudinal analysis described here.
Demographic and symptom measures
Baseline demographic characteristics were assessed by questionnaire and included: smoking status, menopausal status (menopause transition, postmenopausal), and health status. Height and body weight were measured at baseline and were used to calculate body mass index (BMI). At enrollment, participants completed the Patient Health Questionnaire (PHQ9)18 to screen for depression, and the Generalized Anxiety Disorder Scale (GAD-7)19 to evaluate anxiety. Self-reported vaginal symptom measures included: 1) selection of the most bothersome symptom (MBS) for the participant, and rating of severity on a 10-point scale and 2) presence and severity of individual symptoms (vaginal dryness, vulvovaginal itch/burn, vaginal discharge, vaginal pain with intercourse) on a 5-point scale. Choices for MBS were limited to vaginal dryness, vaginal discharge, vulvovaginal pain, vulvovaginal itch/burn or inability to have sex. Women had blood drawn at enrollment and at 8 weeks. Women self-collected vaginal swabs at 0 and 8 weeks in clinic, and at 4 weeks at home and sent those swabs through mail to the Fredricks Lab in Seattle.
Characterization of the vaginal microbiota
After receipt, vaginal swabs were stored at −80°C until processed. Swabs were eluted in 400 μL sterile, filtered saline and centrifuged at 14,000 rpm for 10 minutes. DNA was extracted from the cell pellet using the MoBio Bacteremia extraction kit (MoBio, Carlsbad, CA) as previously described.20 The bacterial 16S rRNA gene was amplified using primers for the V3–V4 hypervariable regions, libraries were created using barcoded primers, and amplicons were sequenced using the Roche 454 Titanium platform (Roche, CT).21 Negative controls included sham digests that were processed in the same way as samples to assess contamination from DNA extraction or PCR reagents. Sequences were filtered for length (minimum 250 bp) and quality score (minimum 30), and reads originating from contaminants in PCR controls were removed. Sequence reads were classified using the pplacer phylogenetic placement tool and a curated reference set of vaginal bacterial sequences.21 Sequence reads have been deposited to the NCBI Short Read Archive (SRP100779). Based on the dominant bacterial genus, participants were categorized into two groups: Lactobacillus-dominant (> 50% of sequences from Lactobacillus species) and non-Lactobacillus dominant. For quantification of L. crispatus and L. iners, species-specific qPCR using a TaqMan-based assay was performed as previously described.20,22
Vaginal glycogen assay
A second vaginal swab was processed as described above, and supernatant from that eluate was further diluted in saline (1:5), and 50 μL of the diluted fluid was used in a fluorometric assay (BioVision, Milpitas, CA) to measure glycogen levels.
Serum estrogen measurements
At each clinic visit blood was drawn and serum stored at −80°C until processing. An ultrasensitive stable isotope dilution liquid chromatography/selected reaction monitoring/mass spectrometry (LC/SRM/MS) assay was used to measure total and unconjugated estradiol and estrone.23 The limit of detection for each estrogen using 0.5 mL of serum was 0.156 pg/mL and linear standard curves were obtained up to 20 pg/mL. Serum samples were not available for all participants at all time points.
Statistical analysis
Improvement in MBS was defined as a greater than 1 point decrease in the MBS severity score between enrollment and 8 weeks. Comparisons of participant characteristics between women whose MBS improved or didn’t used Student’s t-test, Kruskall-Wallis test, chi square or Fisher’s exact test, as appropriate. Comparison of laboratory values between time points within a group using Wilcoxon signed-rank test. Detection of Lactobacillus species by qPCR was compared between visits using McNemar’s test. Log10-transformed qPCR values at 0 and 8 weeks, as well as the change in value between week 8 and enrollment, were compared between groups using students t-test. Differences in Shannon diversity index were compared between groups and time points using either linear regression (adjusted for treatment arm) or Wilcoxon rank-sum test. Differences in Bray-Curtis dissimilarity at each time point were compared between groups using MiRKAT.24 Taxon-level associations with improvement in symptoms were assessed across time points using Hotelling’s T-squared test,25 adjusted for multiple comparisons using Benjamini-Hochberg equations.26
Results
Of the 30 women included in this longitudinal analysis, 21 (70%) had improvement in their most bothersome symptom (MBS) over the course of the study, and 9 (30%) had no improvement or worsening of the MBS. A higher proportion of women receiving estradiol or venlafaxine reported improvement in MBS (88%, 78%) compared to placebo (54%; p = 0.28). The mean change in MBS for the entire group was a decrease of 2 points (SD of ± 3), with a range of decrease by 8 points to an increase of 3 points. There were no significant differences in age, ethnicity, treatment arm, enrollment lab values or which symptom was the most bothersome between the two groups (Table 1). Most women reported the same MBS at enrollment and at follow-up, but 8 women reported a different MBS at the follow-up visit. In all 8, the original MBS decreased in severity and the new MBS was reported as the same or less severe than at the original visit, so these women are classified as “MBS improved.” There was no association between improvement in vulvovaginal MBS and number of hot flashes per day at enrollment, or improvement in hot flashes over the course of the trial. Women whose MBS did not improve had significantly higher depression and anxiety scores at baseline, though the median values in this group were just at or slightly above the cutoff for “mild” anxiety or depression.18,19
Table 1.
Demographic, laboratory and symptom characteristics at enrollment.
| MBS Improved (n = 21) |
MBS did not improve (n = 9) |
p valuea | |
|---|---|---|---|
| Age (years, mean ± SD) | 53 ± 4 | 54 ± 4 | 0.82 |
| BMI (kg/m2, mean ± SD) | 30 ± 7 | 27 ± 4 | 0.31 |
| Ethnicity | 0.77 | ||
| White | 9 (43%) | 5 (56%) | |
| African American | 10 (48%) | 3 (33%) | |
| Hispanic | 1 (5%) | 1 (11%) | |
| Other | 1 (5%) | 0 | |
| Study arm | 0.22 | ||
| Placebo | 7 (33%) | 6 (67%) | |
| Venlafaxine | 7 (33%) | 2 (22%) | |
| Estradiol | 7 (33%) | 1 (11%) | |
| Menopausal status | 0.84 | ||
| LMP < 1 year | 4 (19%) | 2 (22%) | |
| LMP > 1 year | 17 (81%) | 7 (78%) | |
| Female Sexual Function Index (median, IQR) | 21 (12, 27) | 20 (18, 23) | 0.82 |
| PHQ-9 (median, IQR) | 2 (0, 4) | 6 (3, 10) | 0.008 |
| GAD-7 (median, IQR) | 2 (0, 3) | 5 (2, 10) | 0.02 |
|
| |||
| Vasomotor symptoms | |||
|
| |||
| Hot flashes/day at week 0 (median, IQR) | 8 (6, 9) | 6 (5, 11) | 0.39 |
| >50% decrease in hot flashes/day at week 8 | 7 (33%) | 3 (33%) | 1.0 |
|
| |||
| Baseline laboratory values | |||
|
| |||
| Vaginal glycogen (pg/mL, median, IQR)b | 5.3 (2.7, 8.3) | 7.1 (4.3, 166) | 0.12 |
| Estradiol (pg/mL, median, IQR)c | |||
| Total | 22 (4, 30) | 14 (0.5, 20) | 0.31 |
| Unconjugated | 3 (0.5, 7) | 5 (0.5, 6) | 0.99 |
| Estrone (pg/mL, median, IQR)c | |||
| Total | 195 (146, 314) | 173 (122, 229) | 0.27 |
| Unconjugated | 26 (22, 40) | 27 (12, 35) | 0.61 |
| Lactobacillus-dominant vaginal microbiota | 12 (57%) | 2 (22%) | 0.08 |
| L. crispatus (+ qPCR) | 10 (48%) | 2 (22%) | 0.11 |
| L. iners (+ qPCR) | 14 (67%) | 5 (56%) | 0.79 |
|
| |||
| Moderate-Severe symptoms: | |||
|
| |||
| Vaginal dryness | 8 (38%) | 5 (56%) | 0.27 |
| Vulvar itch/burn | 6 (29%) | 4 (44%) | 0.33 |
| Vaginal itch/burn | 3 (14%) | 1 (11%) | 0.89 |
| Vaginal discharge | 6 (29%) | 4 (44%) | 0.40 |
| Pain | 5 (24%) | 1 (11%) | 0.58 |
|
| |||
| Most Bothersome Symptom (MBS) | |||
|
| |||
| Vaginal dryness | 7 (33%) | 4 (44%%) | 0.27 |
| Vulvar itch/burn | 7 (33%) | 1 (11%) | |
| Vaginal itch/burn | 2 (10%) | 0 | |
| Vaginal discharge | 0 | 1 (11%) | |
| Pain | 1 (5%) | 2 (22%) | |
| Inability to have sex | 3 (14%) | 1 (11%) | |
| Severity of MBS (median, IQR) | 6 (4, 7) | 3 (2, 5) | 0.14 |
PHQ-9 (Patient Health Questionnaire); GAD-7 (Generalized Anxiety Disorder Scale)
p-value calculated by chi-square or Fisher’s exact test for categorical data, Student’s t-test or Kruskall Wallis for continuous data, as appropriate.
Missing values for 4 in the improved group and 2 in the not improved group
Missing values for 6 in the improved group
Of the 21 women whose MBS improved, 12 (57%) had a Lactobacillus-dominant microbiota at enrollment, compared to 2 (22%) of those whose MBS did not improve (Table 1; p = 0.08). At 8 weeks, 11 (52%) women whose MBS improved had Lactobacillus-dominant community compared to 3 (33%) in the group that did not improve (p = 0.22)(Figure 1A). In the group whose symptoms improved, one woman lost Lactobacillus dominance (placebo treatment group), while among women whose symptoms did not improve, 2 women gained Lactobacillus dominance over 8 weeks (1 placebo, 1 estradiol) though in both women, this was primarily L. iners.(Figure 1C) In unadjusted analysis, several non-Lactobacillus bacterial species had different mean prevalence across all 3 time points between women whose MBS improved and those whose did not, largely due to a higher prevalence in women who did not improve (Figure 2a). After adjustment for multiple comparisons none of these differences were significant. When comparing alpha-diversity, there was a trend to a higher Shannon diversity index in women whose MBS did not improve, especially when adjusting for treatment assignment, but differences were not statistically significant (Figure 2b). Using MiRKAT, there was no association between beta diversity and symptom improvement at enrollment (p = 0.58) or 8 weeks (p = 0.64). There was a trend to lower detection of L. crispatus at enrollment in the group whose MBS did not improve, but this did not reach statistical significance (Table 1). There were no differences between groups in mean log10 gene copies/swab of L. crispatus as measured by qPCR at enrollment (MBS improved 4.3 ± 0.6 vs. Not improved 2.9 ± 0.7; p = 0.22), 8 weeks (4.2 ± 0.7 vs. 3.2 ± 0.9; p = 0.43) or change in quantity over time (−0.1 ± 0.4 vs. 0.3 ± 0.3; p = 0.48). The same was true for log10-transformed quantity of L. iners measured by qPCR at 0 weeks (MBS improved 5.0 ± 0.7 vs. Not improved 4.6 ± 1.1; p = 0.76), 8 weeks (4.7 ± 0.7 vs. 5.4 ± 1.2; p = 0.57) or in change between 0 and 8 weeks (−0.3 ± 0.2 vs. 0.8 ± 0.7; p = 0.06)(Figure 2C).
Figure 1.
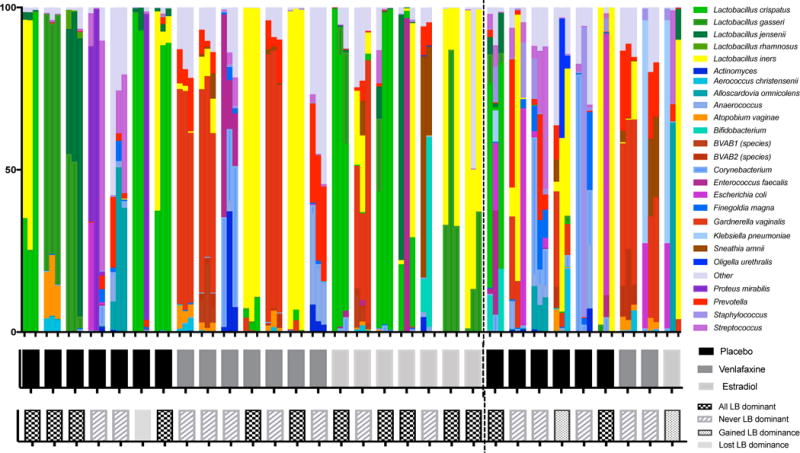
Relative abundance of vaginal bacteria at 0, 4 and 8 weeks by sequencing of the 16S rRNA gene. Each cluster of three columns represents a vaginal swab from each of the three time points from a single participant (A) and the study arm is identified below (B). Lactobacillus dominance (defined as > 50% of sequences from Lactobacillus species) was largely consistent across all visits, with few women changing to or from a Lactobacillus dominant state (C). The dotted line distinguishes women whose MBS improved (n=21) on the left and women who did not report an improvement in MBS (n=9) on the right.
Figure 2.
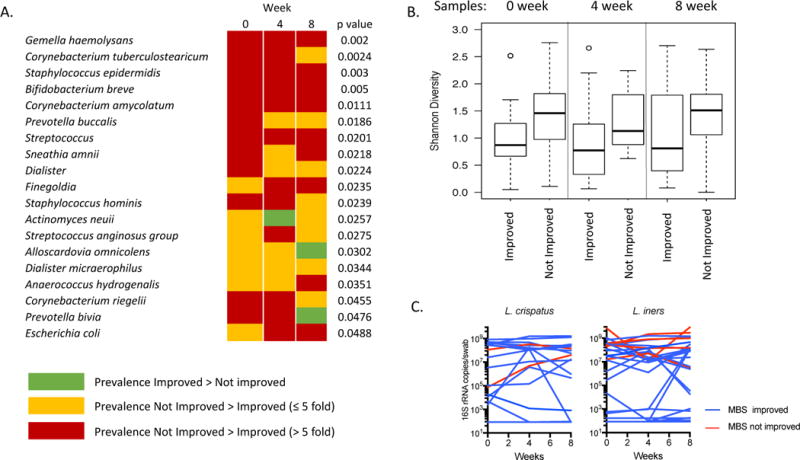
(A) Differentially abundant bacteria between women whose most bothersome symptom did or did not improve, in unadjusted analysis. After adjustment for multiple comparisons, no statistically significant results remained. (B) Comparison of Shannon diversity index between women whose symptoms did and did not improve at 0, 4 and 8 weeks. (C) Quantity of L. crispatus and L. iners measured by qPCR at 0, 4, 8 weeks. Undetectable values were recorded as half the lower limit of detection (83 16S rRNA gene copies/swab)
Vaginal free glycogen significantly increased over the 8 weeks in women whose MBS improved (p = 0.03), and showed a trend to decrease between enrollment and week 8 in women whose MBS did not improve, though this did not reach statistical significance (Figure 3). Serum unconjugated and total estradiol significantly increased in the women whose MBS improved, and while there was a trend to increase in those with no improvement, the magnitude was smaller (Figure 3). Of the 12 women with an increase in total serum estradiol, 5 (42%) were in the estradiol treatment group, 5 (42%) were in the venlafaxine group and 2 (16%) were in the placebo group. Comparisons between treatment arm were significantly confounded by race, as 7/8 (88%) women from the estradiol treatment group were African American compared to 3/13 (23%) in the placebo group and 3/9 (33%) in the venlafaxine group (Table 2). Serum estrogens increased most, as expected, in the oral estradiol treatment group (Table 2).
Figure 3.
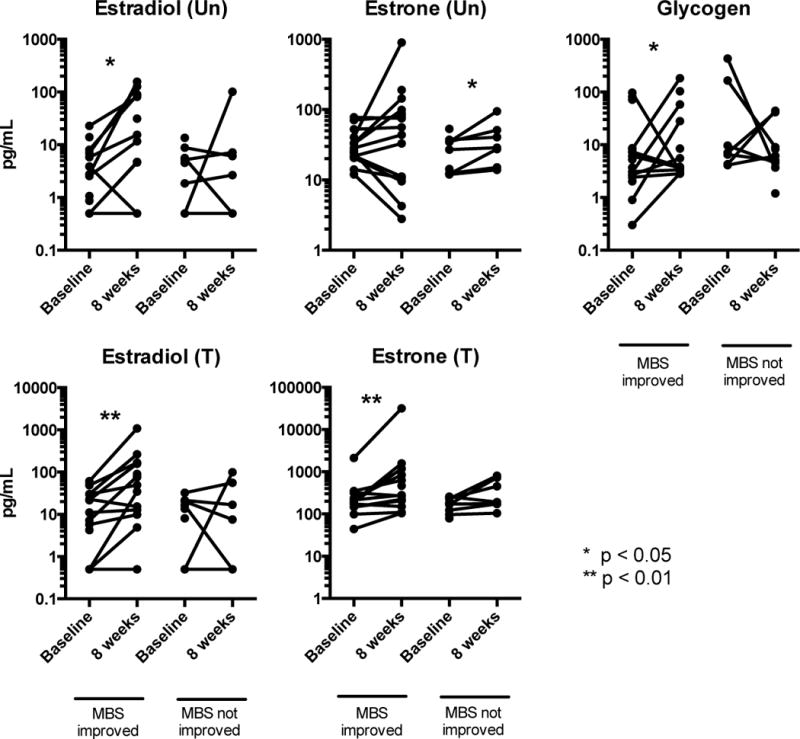
Change in serum estrogen levels and vaginal glycogen levels over 8 weeks in women whose symptoms did and did not improve. Serum estrogen values were missing for 9 participants in the MBS improved group and 2 from the not improved group. * indicates a significant change (p < 0.05) within a group between values at enrollment and at 8 weeks.
Table 2.
Comparison of ethnicity, symptom improvement and change in biologic measurements over 8 weeks by treatment arm
| Placebo (n = 13) |
Venlafaxine (n = 9) |
Estradiol (n = 8) |
P valuea | |
|---|---|---|---|---|
| Ethnicity | 0.02 | |||
| Black | 3 (23%) | 3 (33%) | 7 (88%) | |
| Non-black | 8 (77%) | 6 (67%) | 1 (12%) | |
| MBS improved | 7 (54%) | 7 (78%) | 7 (88%) | 0.28 |
|
| ||||
| Median change (IQR) in: | P valueb | |||
|
| ||||
| Glycogen | 0.6 (−3.7, 1.7) | 2.1 (−1.0, 32.7) | 1.2 (−2.5, 4.6) | 0.58 |
| Estradiol (T)c | −4.7 (−12.8, 19.5) | 38 (1.7, 99) | 119 (23, 235) | 0.03 |
| Estradiol (Un) | 1.4 (−2.1, 3.1) | 10 (9, 100) | 58 (4.2, 79) | 0.13 |
| Estrone (T) | 50 (7.8, 220) | 65 (−22, 553) | 616 (578, 1376) | 0.05 |
| Estrone (Un) | 1.8 (−6.3, 5.8) | 11.1 (−10.7, 59.9) | 15.4 (3.5, 66.5) | 0.17 |
Fisher’s exact test,
Kruskall Wallis
Serum estrogen values not available for 6 participants from placebo group, 2 from venlafaxine group and 3 from estradiol group.
MBS = most bothersome symptom, (T) = Total, (Un) = Unconjugated
Conclusions
Our pilot analysis of this small cohort of postmenopausal women recruited from a randomized placebo controlled trial evaluating systemic therapy for hot flashes showed an association between decreasing severity of vulvovaginal symptoms and increased serum estrogen and vaginal glycogen levels. In addition, this small pilot study suggested that vaginal Lactobacillus dominance might predispose to a better treatment response, though we did not see a shift to greater Lactobacillus presence or dominance in women whose symptoms improved. This suggests that the presence of vaginal lactobacilli may be more a marker of a healthy vagina than a driver of vulvovaginal symptom severity.
In premenopausal women, absence of vaginal Lactobacillus colonization is associated with bacterial vaginosis (BV), a condition that can present with vaginal discharge and odor. However, half of women with BV are asymptomatic. Mechanisms underlying vulvovaginal symptoms are not well understood. One study linked detection of Gardnerella and report of pain through the metabolite diethylene glycol.27 Another study linked bacteria such as Prevotella timonensis, Leptotrichia amnionii (now Sneathia amnii), Eggerthella, Parvimonas micra with vaginal discharge.21 A study of postmenopausal, Chinese women with atrophic vaginitis showed a negative correlation between abundance of vaginal Lactobacillus and severity of vaginitis symptoms.15
Vaginal colonization with lactobacilli in postmenopausal women has been associated with vaginal fluid glycogen, which is presumed to be driven by serum estrogen levels.28 However, while quantities of vaginal free glycogen and vaginal lactobacilli appear to be correlated, no association was seen between serum estradiol and vaginal glycogen or lactobacilli in premenopausal women28 or in our cross-sectional analysis of post-menopausal women.10 We did see an increase in vaginal free glycogen in women whose MBS improved, without a corresponding change in Lactobacillus detection or quantity. Our glycogen measurements were significantly lower than in other reports,28 thus the magnitude of change may have been too small to induce changes in the microbiota. It is also possible that we did not follow women long enough to see a change in Lactobacillus, however, this observation also supports the hypothesis that lactobacilli are a marker of health, rather than a driver of health.
The most commonly reported MBS in our cohort was vaginal dryness, followed by vulvar itch/burn. Post-menopausal Canadian women with vaginal dryness on exam were found to have increased expression of genes for inflammatory cytokines in the vaginal mucosa.9 We have shown that in premenopausal women vaginal colonization with Lactobacillus is associated with lower levels of the pro-inflammatory cytokine IL1b.29 Preliminary data suggest that estrogen decreases neutrophil activity in the vaginal mucosa by altering cytokine gradients,30 and inhibits Th17 differentiation and proliferation.31 However, a separate study suggested that vaginal pro-inflammatory cytokines were not associated with GSM.32 Our pilot results suggest that decrease in symptom severity is associated more with increased serum estrogen than change in vaginal Lactobacillus colonization. However, treatment with low-dose vaginal preparations of estradiol that do not significantly increase serum estrogen has also been shown to decrease genitourinary symptoms of menopause, suggesting that other mechanisms may also be important. It is possible that changes in the hormonal environment facilitate changes in Lactobacillus function that would not be captured by the quantitative methods used in this study.
Our study did show greater improvement in MBS in the two groups of women treated with an active agent compared to placebo. Oral estradiol has been shown to improve GSM in many studies.6,33,34 MBS improvement with venlafaxine correlates with MsFLASH trial findings from all 335 women when vaginal dryness was assessed as part of the MENQOL questionnaire. At baseline, 37.9%, 37.5% and 41.7% of women in the estradiol, venlafaxine and placebo groups, respectively, reported vaginal dryness. At 8- weeks, this decreased to 26.4%, 18.2%, and 35.6%, respectively. In the larger cohort, only the venlafaxine group had statistically significant improvement in the proportion of women reporting vaginal dryness, compared with placebo (p=0.006).35
This pilot study is limited by its small size, which precludes more complex analyses controlling for or stratifying by treatment arm or other participant characteristics. However, the sample size was adequate to show a change in serum estrogens and vaginal glycogen levels over 8 weeks. Women were recruited from a trial for which the inclusion criteria were based on hot flashes, not genitourinary symptoms, thus symptom severity was moderate, which may also have limited our ability to assess associations between symptoms and microbiota. However, we did ask about a wide range of genitourinary symptoms, as opposed to the single question about dryness present in the MENQOL.
Many studies of GSM use exam findings of atrophy, vaginal pH, vaginal maturation index or other exam findings to measure response to treatment. However, the true goal of treating GSM is improving quality of life for postmenopausal women. Results from this pilot analysis suggest that laboratory-based evaluation of treatment responses may not correlate as expected with change in symptom severity, highlighting our lack of understanding of the underlying cause of GSM, and the need for mechanistic studies in this area. These results support the possibility that vaginal microbiota affect changes in GSM in response to treatment. However larger trials are need to determine whether vaginal microbiota modify or mediate treatment responses in women with GSM.
Acknowledgments
This project was funded by the National Institutes of Health as a cooperative agreement issued by the National Institute of Aging: #U01 AG032656, U01AG032659, U01AG032669, U01AG032682, U01AG032699, U01AG032700. Dr. Mitchell is supported by a Clinical Scientist Development Award from the Doris Duke Foundation.
Disclosures: Dr. Mitchell serves as a consultant for Symbiomix Therapeutics. Dr. Reed has received grant funding from Bayer. Dr. Joffe is a consultant for Merck & Co, Inc, NeRRe Therapeutics, Mitsubishi Tanabe Pharma America Inc, SAGE Therapeutics. Dr. Joffe has received grant funding from Merck & Co, Inc, and SAGE Therapeutics. Dr. Cohen has received research funding from Cephalon, Inc, JayMac Pharmaceuticals, Takeda/Lundbeck Pharmaceuticals.
Footnotes
Clinical trials registry: Clinicaltrials.gov NCT01418209
These data were presented in part at the annual meeting of the Infectious Disease Society of Obstetrics & Gynecology, August 11–13, 2016.
References
- 1.Minkin MJ, Reiter S, Maamari R. Prevalence of postmenopausal symptoms in North America and Europe. Menopause. 2015;22(11):1231–1238. doi: 10.1097/GME.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 2.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142. doi: 10.1111/j.1743-6109.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- 3.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799. doi: 10.1111/jsm.12190. [DOI] [PubMed] [Google Scholar]
- 4.Davila GW, Singh A, Karapanagiotou I, et al. Are women with urogenital atrophy symptomatic? Am J Obstet Gynecol. 2003;188(2):382–388. doi: 10.1067/mob.2003.23. [DOI] [PubMed] [Google Scholar]
- 5.Greendale GA, Zibecchi L, Petersen L, Ouslander JG, Kahn B, Ganz PA. Development and validation of a physical examination scale to assess vaginal atrophy and inflammation. Climacteric. 1999;2(3):197–204. doi: 10.3109/13697139909038062. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause. 2009;16(4):719–727. doi: 10.1097/gme.0b013e3181a48c4e. [DOI] [PubMed] [Google Scholar]
- 7.Ekin M, Yasar L, Savan K, et al. The comparison of hyaluronic acid vaginal tablets with estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Arch Gynecol Obstet. 2011;283(3):539–543. doi: 10.1007/s00404-010-1382-8. [DOI] [PubMed] [Google Scholar]
- 8.Brotman RM, Shardell MD, Gajer P, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21(5):450–458. doi: 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hummelen R, Macklaim JM, Bisanz JE, et al. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS One. 2011;6(11):e26602. doi: 10.1371/journal.pone.0026602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell CM, Srinivasan S, Zhan X, et al. Vaginal microbiota and genitourinary menopausal symptoms: a cross-sectional analysis. Menopause. 2017;24(10) doi: 10.1097/GME.0000000000000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petricevic L, Domig KJ, Nierscher FJ, et al. Characterisation of the oral, vaginal and rectal Lactobacillus flora in healthy pregnant and postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2012;160(1):93–99. doi: 10.1016/j.ejogrb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Daroczy K, Xiao B, Yu L, Chen R, Liao Q. Qualitative and semiquantitative analysis of Lactobacillus species in the vaginas of healthy fertile and postmenopausal Chinese women. J Med Microbiol. 2012;61(Pt 5):729–739. doi: 10.1099/jmm.0.038687-0. [DOI] [PubMed] [Google Scholar]
- 13.Pabich WL, Fihn SD, Stamm WE, Scholes D, Boyko EJ, Gupta K. Prevalence and determinants of vaginal flora alterations in postmenopausal women. J Infect Dis. 2003;188(7):1054–1058. doi: 10.1086/378203. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura T, Okamura H. Short term oral estriol treatment restores normal premenopausal vaginal flora to elderly women. Maturitas. 2001;39(3):253–257. doi: 10.1016/s0378-5122(01)00212-2. [DOI] [PubMed] [Google Scholar]
- 15.Shen J, Song N, Williams CJ, et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci Rep. 2016;6:24380. doi: 10.1038/srep24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton KM, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network. Menopause. 2014;21(1):45–58. doi: 10.1097/GME.0b013e31829337a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med. 2014;174(7):1058–1066. doi: 10.1001/jamainternmed.2014.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J Clin Microbiol. 2009;47(3):721–726. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Mesaros C, Blair IA. Ultra-high sensitivity analysis of estrogens for special populations in serum and plasma by liquid chromatography-mass spectrometry: Assay considerations and suggested practices. J Steroid Biochem Mol Biol. 2016;162:70–79. doi: 10.1016/j.jsbmb.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao N, Chen J, Carroll IM, et al. Testing in Microbiome-Profiling Studies with MiRKAT, the Microbiome Regression-Based Kernel Association Test. Am J Hum Genet. 2015;96(5):797–807. doi: 10.1016/j.ajhg.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotelling H. The generalization of student’s ratio. The Annals of Mathematical Statistics. 1931;2(3):360–378. [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 27.Yeoman CJ, Thomas SM, Miller ME, et al. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PLoS One. 2013;8(2):e56111. doi: 10.1371/journal.pone.0056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirmonsef P, Modur S, Burgad D, et al. Exploratory comparison of vaginal glycogen and Lactobacillus levels in premenopausal and postmenopausal women. Menopause. 2015;22(7):702–709. doi: 10.1097/GME.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell C, Fredricks D, Agnew K, Hitti J. Hydrogen Peroxide-Producing Lactobacilli Are Associated With Lower Levels of Vaginal Interleukin-1beta, Independent of Bacterial Vaginosis. Sex Transm Dis. 2015;42(7):358–363. doi: 10.1097/OLQ.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lasarte S, Samaniego R, Salinas-Munoz L, et al. Sex Hormones Coordinate Neutrophil Immunity in the Vagina by Controlling Chemokine Gradients. J Infect Dis. 2016;213(3):476–484. doi: 10.1093/infdis/jiv402. [DOI] [PubMed] [Google Scholar]
- 31.Chen RY, Fan YM, Zhang Q, et al. Estradiol inhibits Th17 cell differentiation through inhibition of RORgammaT transcription by recruiting the ERalpha/REA complex to estrogen response elements of the RORgammaT promoter. J Immunol. 2015;194(8):4019–4028. doi: 10.4049/jimmunol.1400806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kollmann Z, Bersinger N, von Wolff M, Thurman AR, Archer DF, Stute P. Vaginal cytokines do not correlate with postmenopausal vulvovaginal symptoms. Gynecol Endocrinol. 2015;31(4):317–321. doi: 10.3109/09513590.2014.995080. [DOI] [PubMed] [Google Scholar]
- 33.Bachmann GA, Schaefers M, Uddin A, Utian WH. Microdose transdermal estrogen therapy for relief of vulvovaginal symptoms in postmenopausal women. Menopause. 2009;16(5):877–882. doi: 10.1097/gme.0b013e3181a15606. [DOI] [PubMed] [Google Scholar]
- 34.Cardozo L, Bachmann G, McClish D, Fonda D, Birgerson L. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: second report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol. 1998;92(4 Pt 2):722–727. doi: 10.1016/s0029-7844(98)00175-6. [DOI] [PubMed] [Google Scholar]
- 35.Reed SD, Mitchell CM, Joffe H, et al. Sexual function in women on estradiol or venlafaxine for hot flushes: a randomized controlled trial. Obstet Gynecol. 2014;124(2 Pt 1):233–241. doi: 10.1097/AOG.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]


