Abstract
Purpose
The present study examined the effect of unpredictable chronic mild stress (UCMS) on peripheral microvessel function in healthy and metabolic syndrome (MetS) rodents, and whether exercise training could prevent the vascular dysfunction associated with UCMS.
Methods
Lean and obese (model of MetS) Zucker rats (LZR; OZR) were exposed to 8 weeks of UCMS, exercise (Ex), UCMS+Ex, or control conditions. At the end of the intervention, gracilis arterioles (GAs) were isolated and hung in a pressurized myobath to assess endothelium-dependent (EDD) and -independent (EID) dilation. Levels of nitric oxide (NO) and reactive oxygen species (ROS) were measured through DAF-FM and DHE staining, respectively.
Results
Compared to LZR controls, EDD and EID was lower (p=0.0001) in LZR-UCMS. The OZR-Ex group had a higher EDD (p=0.0001) and EID (p=0.003), compared to OZR-Controls; whereas only a difference in EDD (p=0.01) was noted between LZR-Control and LZR-Ex groups. Importantly, EDD and EID were higher in the LZR (p=0.0001; p=0.02) and OZR (p=0.0001; p=0.02) UCMS+Ex groups compared to UCMS alone. Lower NO bioavailability and higher ROS were noted in the LZR-UCMS group (p=0.0001), but not OZR-UCMS, compared to controls. Ex and UCMS-Ex groups had higher NO bioavailability (p=0.0001) compared to control and UCMS groups, but ROS levels remained high.
Conclusions
The comorbidity between UCMS and MetS does not exacerbate the effects of one another on GA EDD responses, but does lead to the development of other vasculopathy adaptations, which can be partially explained by alterations in NO and ROS production. Importantly, exercise training alleviates most of the negative effects of UCMS on GA function.
Keywords: obesity, UCMS, skeletal muscle arteriole, endothelial dysfunction
Introduction
Fifty-six million American adults are diagnosed with the metabolic syndrome (MetS) (1–4), which significantly increases an individual’s risk of peripheral vascular disease (PVD). Indeed, Maksimovic et al. (5) showed that around 60% of patients with PVD also manifested with MetS. Our group, and others, have shown in a model of MetS (obese Zucker rat) there is a global reduction in vascular-derived nitric oxide (NO) bioavailability, leading to significant peripheral vascular dysfunction associated with PVD (6–9).
Depression is independent risk factor for PVD (10). Previous studies have shown that chronic stress is a major contributor to depressive illness and may be the link between depression and PVD (11–15). Stress-induced depression causes vascular dysfunction, in part, by impairing the bioavailability of dilator metabolites such as NO (16–18). Furthermore, it has been postulated that exposure to chronic psychosocial stress is a significant risk factor for the development of MetS. Indeed, approximately 43% of MetS patients present with depression (20, 23). Given that both MetS and depression result in significant vasculopathies, it remains unknown whether MetS exposed to chronic stress results in more severe vasculopathies thereby significantly increasing the risk of PVD events (myocardial infarction, heart failure, stroke, and/or limb ischemia).
Exercise, when used as an intervention, reduces the risk of PVD associated with MetS and depression, separately (19,20). Previous studies have shown that chronic exercise training can increase the expression of endothelial NO synthase (NOS) and its activity (21–24). Exercise also upregulates antioxidant activity which leads to an attenuation of reactive oxygen species (ROS) production, and therefore, decreases oxidative stress (25,26). Through these two mechanisms, aerobic exercise can increase NO bioavailability, improving vascular endothelial function (27) and perhaps reduce the risk of PVD associated with MetS and depression. However, it is unknown whether chronic exercise can limit or restore the vasculopathies involving MetS exposed to chronic stress.
The current study used the obese (fa/fa) Zucker rat, a translational model of MetS that develops significant PVD risk (in the absence of significant atherosclerosis) that can impair skeletal muscle perfusion and performance, and its lean controls (LZR) to explore the role of unpredictable chronic mild stress (UCMS), exercise (Ex), and a combination of each on peripheral microvascular function (8,28). UCMS is a protocol used to induce depressive-like symptoms by exposing animal models to daily, mild stressors (29–31). The UCMS protocol is accepted as a relevant rodent model of depression that has been shown to reproduce clinical symptoms of depression, including anhedonia and increased anxiety-like behavior (32). Our initial hypothesis was that: 1) LZRs exposed to UCMS would have peripheral microvascular dysfunction similar to that evident in the OZRs controls due to a reduction in NO bioavailability and an increase in ROS production; 2) the comorbidity between MetS exposed to UCMS would exacerbate the already existing peripheral microvascular dysfunction; and 3) exercise could limit the peripheral microvascular dysfunction by decreasing oxidative stress and improving vasodilation associated with MetS exposed to chronic stress.
Materials and Methods
Animals
Male LZR and OZRs (Harlan) arrived at the West Virginia University Health Sciences Center (WVUHSC) animal facility at 7–8 weeks of age. After 1 week of acclimation to the local environment, LZRs (n = 8 per protocol) and OZRs (n = 8 per protocol) were randomly assigned to a specific protocol group for the subsequent 8–9 weeks including: 1) sedentary controls, 2) UCMS, 3) exercise (Ex), and 4) a combination of UCMS+Ex, resulting in 8 groups in total. All animals were fed standard chow and tap water ad libitum for all experiments. Protocols received prior approval from the WVUHSC Animal Care and Use Committee.
UCMS Protocol
Previous investigators developed the UCMS model for developing depression-like behaviors in rodents (29–31,33). The UCMS model is considered to be the most appropriate rodent model for clinical depression, based on its ability to reproduce the development of many clinical human depressive symptoms, including anhedonia and learned helplessness (32).
All rats were singly housed. In UCMS groups, rats were exposed to the following mild environmental stressors in randomly chosen sequences for 8 hours each day, 5 days/week, over the course of 8 weeks:
Damp bedding – 10 oz. of water was added to each standard cage
Bath – all bedding was removed and ~0.5 inches of water was added to empty cage. Water temperature was room temperature, ~24°C
Cage Tilt - cage was tilted to 45 degrees without bedding
Social stress – each rat was switched into a cage of a neighboring rat
No bedding – all bedding was removed from the cage
Alteration of light/dark cycles –turning lights off/on in random increments for scheduled period.
Exercise Training Protocol
LZR and OZR underwent 8 weeks of treadmill running, either concurrent with UCMS or as a standalone treatment. Animals ran 5 days/week on multi-lane motor driven treadmills set at a 5% grade. During the first week, animals were acclimatized to the treadmill by running for 20 min, then increasing by 10 min/day until sustainable duration of 60 minutes daily was achieved. A maximum speed test was performed on each animal and target-running speed was set for 60–70% of that maximum. After acclimatization, the first 15 minutes of the total 60 minutes consisted of a gradual increase until reaching target-running speed. Rats ran at this speed for the remaining 45 minutes. Mild electrical stimulus (≤0.3 mA) was used at the rear of the treadmill to discourage rats from stopping. There was a 48-hour wash out period between the last Ex bout and the terminal surgery at the end of 8-week treatment.
UCMS and Exercise Combination Protocol
OZRs and LZRs assigned to this group performed treadmill running first thing in the morning (8–9am) and then were immediately subjected to the UCMS protocol as described previously.
Coat Score
The rodents coats were evaluated throughout the duration of the 8-week protocol. Each week, the rats were weighed and inspected for grooming habits (34). The total cumulative coat score was computed by giving an individual score of 0 (clean) or 1 (dirty) to eight different body parts (i.e. head, neck, back, forelimbs, stomach, hindlimbs, tail, genitals).
Circulating Cortisol
Corticosterone is a glucocorticoid produced by the adrenal cortex in response to ACTH (corticotropic hormone) and is the precursor to aldosterone. Corticosterone is the main glucocorticoid in rodents as cortisol is in humans. The production of glucorcorticoids is increased by stress. Using a commercially available ELISA Kit (Cayman Chemical, Item #501320) serum samples, collected at time of terminal surgery, were examined for corticosterone levels in duplicate accordingly to the manufacturer’s instructions
Plasma Clinical Markers
Fasting blood was drawn intravenously from anesthetized rats into lithium-heparin coated blood tubes and transported immediately to the laboratory for analysis. Levels of triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL) were measured by the clinical laboratory service at Ruby Memorial Hospital (Morgantown, WV). Blood glucose was measured using a commercially available glucometer (FreeStyle, Abbott), and insulin was measured using a rat ELISA kit (Cayman Chemical, item #589501).
Isolation of the Gracilis Arteriole
After completion of the treatment period and at 17–18 weeks of age, each rat was anesthetized with pentobarbital sodium (50 mg/kg ip) and was intubated via the trachea to facilitate maintenance of a patent airway. In all rats, a carotid artery and an external jugular vein were cannulated for determination of arterial pressure and for infusion of heparin. Under anesthesia, an aliquot of blood was drawn from the inferior vena cava to be used for further analysis.
With specific attention to the skeletal muscle circulation and its role in the development of PVD we selected the gracilis arterioles (GA) to determine the effects of MetS and UCMS on peripheral vascular function. Under deep anesthesia, both right and left gracilis arterioles (GA) were isolated from their origin in the skeletal muscle of the thigh then placed in cold (4°C) physiological salt solution (PSS; in mM: 119 NaCL, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.026 EDTA, and 5.5 glucose). Each GA was doubly cannulated in a heated (37°C) chamber that allowed perfusion and superfusion of the lumen and exterior of the vessel, respectively, with PSS from separate reservoirs. The PSS was equilibrated with a 21% O2-5% CO2-74% N2 gas mixture. Vessel diameter was measured using microscopy and an on-screen video micrometer.
Measurements of vascular reactivity in isolated GA
After cannulation, GAs were extended to their in-situ length and equilibrated at 80% of the animal’s mean arterial pressure (MAP) to approximate in vivo perfusion pressure. Active tone at the equilibration pressure was calculated as (ΔD/Dmax) × 100, where ΔD is the diameter increase from rest in response to Ca2+-free PSS and Dmax is the maximum diameter measured at the equilibration pressure in Ca2+-free PSS.
After equilibration, the dilator reactivity of GA was assessed in response to increasing concentrations (10−10–10−6M) of acetylcholine (ACh) to determine endothelium-dependent dilation (EDD) and sodium nitroprusside (SNP, 10−10–10−6M) to determine endothelium-independent dilation (EID). Constriction was assessed by exposing the GA to increasing doses of phenylephrine (PE, 10−10–10−7M). Vascular responses to ACh were also measured following acute (45–60 min) incubation with nitro-L- arginine methyl ester (L-NAME, a NO synthase inhibitor, Sigma-Aldrich N5751) (10−4M) and 4-Hydroxy-TEMPO (TEMPOL, Sigma-Aldrich 176141) (10−4M) to assess the contributions of NO and oxidative stress, respectively, to modulation of vascular reactivity.
Following the experimental procedures for measuring ex-vivo reactivity, the perfusate and superfusate PSS were replaced with Ca2+-free PSS containing the metal chelators EDTA (0.03 mM) and EGTA (2.0 mM). Vessels were stimulated with 10−7 M (PE) to facilitate Ca2+ release and eliminate active tone. Subsequently, intraluminal pressure within the isolated vessel was altered, in 20 mmHg increments, between 0 and 160 mmHg. To ensure that a negative intraluminal pressure was not exerted on the vessel, 5 mmHg was used as the “0 mmHg” intraluminal pressure point. After 7 mins at each intraluminal pressure, the inner and outer diameters of the passive GA were determined.
Measurement of Reactive Oxygen Species
Dihydroethidium (DHE, Invitrogen D1168) assays were performed on unfixed femoral arteries to evaluate superoxide and hydrogen peroxide levels in situ. Femorals were used rather than GAs because the entire section of isolated GA was used for reactivity. Femoral arteries were placed in individual wells of a 96 well plate containing 200μl of HEPES buffer. Femorals were incubated in control/drug treatment for 30 minutes at 37°C. Following incubation, 2μl of stock DHE solution was added to each well to a concentration of 10μM and incubated at 37°C for another 30 minutes. After completion of DHE incubation, arteries were washed in HEPES buffer, placed separately in Optimal Cutting Temperature compound (OCT, Fisher Healthcare™ Tissue-Plus™ O.C.T Compound), and flash frozen in liquid nitrogen to be stored at −80°C. DHE OCT blocks were then cut into 8μm slices using a cryostat at −22°C and transferred to charged slides (Fisherbrand® Superfrost® plus microscope slides) and stained/mounted with DAPI mounting media (VECTORSHEILD antifade mounting media with DAPI, Vector laboratories). Four slices per animal were imaged with an EVOS fluorescent microscope (Invitrogen EVOS FL Auto Cell Imaging System) and then analyzed in ImageJ as fluorescent density/nucleus.
Measurement of NO Bioavailability
Aortic NO levels were measured by 4-Amino-5-Methylamino-2′,7′- Difluorofluorescein Diacetate (DAF-FM-DA, Invitrogen) according to manufacturer’s instructions. As stated previously, aortas were used because GA reactivity required the entire section of vessel. 3mm aortic rings were placed in individual wells of a 96 well plate containing 200μl of HEPES buffer supplemented with L-Arginine (100μM, MP Biomedical Inc. 100736). L-NAME was used as a negative control. After 30-minutes incubation with treatment, DAF-FM 10μM was added to each well and the vessel was stimulated with Acetyl-β-methylcholine chloride (methacholine (MCh), 1×10−6, Sigma-Aldrich A2251). After 10 minutes, the aorta was removed and the conditioned solution was read in a plate reader excitation/emission at 495/515nm wavelength (BioTek Synergy HT). Fluorescence was normalized to aorta length and the L-NAME value to account for the reaction of the DAF assay with other molecules (i.e., hydrogen peroxide).
Data and Statistical Analyses
Data are presented as mean ± SD. Normality was evaluated by the Kolmogorov–Smirnov test. The maximal reactivity or the remodeling of the GA due to the experimental conditions were analyzed by a multifactorial analysis of variance (ANOVA) [ i.e., species (i.e., LZR vs. OZR), and experimental condition (i.e., Control, UCMS, Ex, and UCMS+Ex)] with an interaction term (species-by-group), and a Tukey post-hoc test was performed to determine differences between groups. The effects of TEMPOL and L-NAME on the maximal dilation of the GA was examined with a repeated measures ANOVA. Clinical characteristics between the animals were compared with a One-Way ANOVA with Tukey post-hoc test, as appropriate. DHE, was examined using Kruskal Wallis test, with within group comparisons examined using the Mann-Whitney test. In all cases, p<0.05 was taken to reflect statistical significance.
Results
Animal Characteristics
The baseline characteristics of the animals used in the present study are summarized in Table 1. In comparison to LZR and OZR controls, the intervention groups had a lower body mass at the end of the intervention. Mean arterial pressure did not differ between UCMS or Ex groups. Higher fasting glucose concentrations were noted in the LZR and OZR UCMS group compared to their respective controls. Further, glucose concentrations were lower in the OZR-Ex group, but higher in the OZR-UCMS+Ex compared to OZR-controls. Markers and symptoms of stress were elevated in both LZRs and OZRs using the UCMS protocol as seen by an increase in corticosterone and coat scores in both LZR and OZR-UCMS, and UCMS+Ex groups compared to controls. Also, there was an increase in adrenal weights with UCMS in LZRs, from 16.7g to 25.1g (p=0.0001) and in OZRs, from 27.2g to 32.7g (p=0.0001) (data not shown). Adrenal weight did not significantly change in LZR- or OZR-UCMS+Ex as compared to their respective controls.
Table 1.
Baseline characteristics of animal groups
| LZR | OZR | |||||||
|---|---|---|---|---|---|---|---|---|
| Con | UCMS | Ex | UCMS+Ex | Con | UCMS | Ex | UCMS+Ex | |
| BM, g | 400 ± 36 | 361 ± 25* | 358 ± 28* | 343 ± 21* | 641 ± 54 | 585 ± 39* | 599 ± 53* | 567 ± 46* |
| MAP, mmHg | 112 ± 8 | 122 ± 13 | 120 ± 15 | 120 ± 11 | 139 ± 14 | 142 ± 16 | 140 ± 14 | 132 ± 13 |
| Glucose, mg/dl | 98 ± 15 | 124 ± 17* | 101 ± 20 | 115 ± 15 | 184 ± 29 | 230 ± 34*^ | 154 ± 29 | 219 ± 36*^ |
| Insulin, mg/dl | 1.2 ± 0.9 | 2.1 ± 0.9 | 1.4 ± 0.7 | 1.8 ± 0.9 | 6.6 ± 2.6 | 7.8 ± 3.7 | 4.6 ± 2.4 | 6.2 ± 2.4 |
| TG, mg/dl | 25 ± 7 | 54 ± 40 | 31 ± 8 | 36 ± 8 | 124 ± 19 | 114 ± 35 | 82 ± 38 | 103 ± 40 |
| TC, mg/dl | 86 ± 11 | 87 ± 12 | 95 ± 15 | 88 ± 4 | 256 ± 63 | 213 ± 42 | 181 ± 20* | 187 ± 27* |
| Corticosterone, ng/ml | 7.0 ± 0.4 | 8.8 ± 1.7* | 9.4 ± 2.9 | 10.7 ± 4.0 | 13.8 ± 1.4 | 17.3 ± 5.2* | 10.6 ± 2.9* | 15.8 ± 4.0* |
| Coat Scores, AU | 0.6 ± 0.4 | 1.5 ± 0.4* | 0.6 ± 0.5 | 1.8 ± 0.8* | 2.4 ± 0.4 | 4.8 ± 0.8* | 3.2 ± 0.6 | 4.5 ± 0.4*^ |
BM, body mass; MAP, mean arterial pressure; TG, triglycerides; TC, total cholesterol.
p<0.05 vs. control,
p<0.05 vs. Ex group. Mean ± SD. n=6–8 per group.
Effects of Experimental Conditions on GA Reactivity
Figure 1 illustrates the EDD, EID, and constriction responses of the GA for each treatment group. After 8 weeks of UCMS in LZRs, there was a significant impairment in EDD (73%, p=0.0001) compared to LZR-Controls. EDD was higher in LZR-Ex (41%, p=0.009) compared to LZR-Controls, and EDD was also higher in the LZR-UCMS+Ex (209%, p=0.0001) vs. LZR-UCMS. Importantly, now EDD in the LZR-UCMS+Ex group did not statistically differ compared to LZR-Control. In the OZR groups, UCMS did not attenuate EDD further (Fig 1A); however, in the OZR-Ex group, EDD was higher (141%, p=0.0001) compared to OZR-Controls. When Ex was combined with UCMS, the EDD was higher (174%, p=0.0001) vs. OZR-UCMS, and EDD in the OZR-UCMS+Ex group was also higher (83%, p=0.02) than OZR-Controls (Fig. 1A). Of note, there was a significant (p=0.0006) species (LZR vs. OZR) by group (Con, UCMS, Ex, UCMS+Ex) interaction for the GA EDD response.
Figure 1. The effects of UCMS and Ex on GA reactivity.
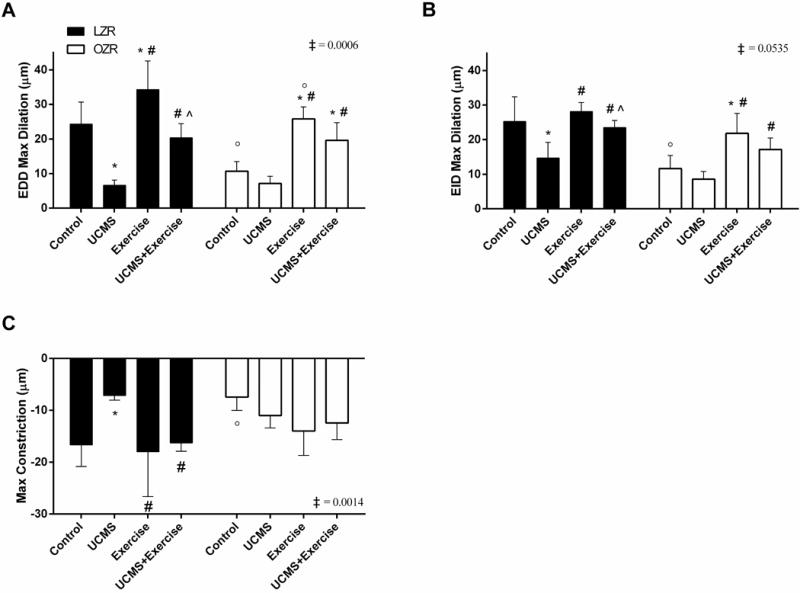
A) Assessment of endothelium-dependent dilation (EDD) to a maximal does of Ach (10−6M); B) endothelium-independent dilation (EID) to a maximal does of SNP (10−6M); and C) GA constriction to a maximal does of PE (10−7M). n = 6–8/group. Mean ± SD. *p<0.05 vs. control, #p<0.05 vs. UCMS, ^p<0.05 vs. Ex, °p<0.05 vs. matched treatment group in opposite species (i.e., LZR vs. OZR), ‡p<0.05 species (LZR vs. OZR) by group (Con, UCMS, Ex, UCMS+Ex) interaction. Please see text for additional details.
Next, we examined EID of the GA, and found that EID was lower (53%, p=0.001) in LZR-UCMS compared to LZR-Controls (Fig. 1B), but no differences were noted in EID between LZR-Ex and LZR-Control groups. Importantly, the concurrent exposure of LZR to UCMS and Ex resulted in a higher EID (56%, p=0.02) compared to LZR-UCMS, and made EID similar between LZR-UCMS+Ex and LZR-Control groups. As for the OZR groups, no significant differences in EID were noted between OZR-UCMS and OZR-Controls; however, EID was higher in the OZR-Ex (88%, p=0.003) vs. OZR-Controls (Fig 1B). Further, EID in OZR-UCMS+Ex was higher (17%, p=0.02) compared to OZR-UCMS (Fig. 1B). We also noted a close to significant (p=0.054) species-by-group interaction for the GA EID response.
The GA constriction response was evaluated by stimulating the GA with increasing doses of PE (Fig. 1C). In LZR, UCMS resulted in a smaller constriction response (57%, p=0.002) compared to LZR-Controls. The GA constriction response was similar between LZR-Ex, LZR- UCMS+Ex, and LZR-Control groups. In the OZR, the GA constriction response was similar between all groups. As such, a significant (p=0.001) species-by-group interaction was noted for the GA constriction response.
Effect of Acute TEMPOL or L-NAME on GA
To examine whether the impairment in the GA EDD was a reflection of increased oxidative stress and reduced NO bioavailability, the GA was acutely incubated with TEMPOL and L-NAME (Fig. 2). Of note, all comparisons here are compared to within the experimental group without the TEMPOL, or L-NAME incubation (i.e., LZR control-TEMPOL vs. LZR control). Acute TEMPOL incubation did not significantly impact EDD in LZR-Control, LZR+Ex, and LZR-UCMS+Ex (Fig. 2A); however, in the LZR-UCMS group, TEMPOL significantly increased EDD (81%, p=0.009), suggesting a role of oxidative stress on the impaired EDD with UCMS in LZRs. Acute TEMPOL incubation significantly increased EDD in OZR-Control (76%, p=0.01) and OZR-UCMS (149%, p=0.03) (Fig. 2B). Neither OZR-Ex nor OZR-UCMS+Ex had any improvements in EDD with acute TEMPOL incubation. A significant (p=0.0012) species-by-group interaction was noted for the EDD-TEMPOL response.
Figure 2. The effects of acute TEMPOL or L-NAME incubation on GA reactivity. The effects of.
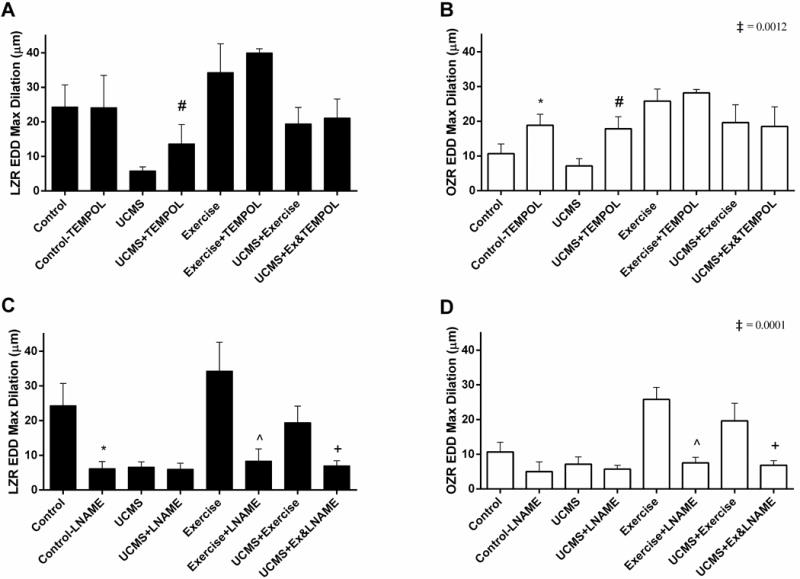
acute TEMPOL incubation on EDD reactivity in LZR (A) and OZR (B). The effects of acute L-NAME incubation on EDD reactivity in LZR (C) and OZR (D). n = 6–8/group. Mean ± SD. *p<0.01 vs. control, #p<0.01 vs. UCMS, ^p<0.05 vs. exercise, +p<0.05 within group comparison for change in TEMPOL or L-NAME. ‡p<0.05 species (LZR vs. OZR) by group (Con, UCMS, Ex, UCMS+Ex) interaction. See text for additional details.
As with TEMPOL, we also incubated the GA with L-NAME to examine the role of NO. Acute L-NAME incubation severely blunted the EDD in LZR-Control (75%, p=0.0001), LZR-Ex (76%, p=0.0001), and LZR-UCMS+Ex (64%, p=0.0001) groups but had minimal effect on EDD in the LZR-UCMS group (9%, p=0.99) (Fig. 2C). In the OZR groups, acute L-NAME incubation only significantly blunted EDD in OZR-Ex (71%, p=0.0001) and OZR-UCMS+Ex (65%, p=0.0001), with no significant effects in the OZR-Controls, and OZR-UCMS groups (Fig. 2D). We also noted a significant (p=0.0001) species by group interaction for the EDD-L-NAME response.
Effects of Experimental Conditions on GA Remodeling
Following assessment of vessel reactivity in our experimental groups, we then examined, under passive conditions, the GA remodeling. There were minimal changes in ID, OD, and WT in the LZR-UCMS vs. LZR-Control groups (Table 2); however, an increase in the ß-slope of the GA stress-strain relationship (p=0.05) was noted in LZR-UCMS vs. LZR-Control. In contrast, no differences were noted between LZR-Ex, LZR-UCMS+Ex, or LZR-Control groups for ID, OD, WT, or ß-slope, suggesting that Ex limited the increased GA stiffness noted with UCMS alone in LZRs. As for the effects of the experimental conditions in OZR, we noted a significantly lower ID and OD in OZR-UCMS vs. OZR-Controls (p=0.01), and as such WT did not differ between OZR-UCMS vs. OZR-Controls (Table 2). Further, an increase in the ß-slope of the GA stress-strain relationship (p=0.01) was noted in the OZR-UCMS vs. OZR-Controls (Table 2). No significant differences were noted in ID, OD, WT, and the ß-slope of the stress-strain relationship between OZR-Ex and OZR-Control groups. However, when Ex was combined with UCMS, the ID and OD were reduced (16%-18%, p<0.05) and the ß-slope was increased (80%, p=0.001) in OZR-UCMS+Ex vs. OZR-Controls, as such the effects of UCMS+Ex on GA remodeling was similar to that noted with UCMS alone in OZRs (Table 2). No significant species-by-group interactions were identified for GA remodeling.
Table 2.
Gracilis arteriole remodeling due to MetS and UCMS in the GA.
| LZR | OZR | |||||||
|---|---|---|---|---|---|---|---|---|
| Con | UCMS | Ex | UCMS+Ex | Con | UCMS | Ex | UCMS+Ex | |
| ID (μm) | 210 ± 8 | 204 ± 50 | 194 ± 11 | 192 ± 15 | 184 ± 31 | 151 ± 5* | 189 ± 31 | 168 ± 24* |
| OD (μm) | 306 ± 4 | 294 ± 38 | 294 ± 24 | 292 ± 28 | 273 ± 28 | 230 ± 5* | 281 ± 28 | 258 ± 31*# |
| WT (μm) | 48 ± 2 | 45 ± 11 | 50 ± 6 | 50 ± 6 | 45 ± 3 | 40 ± 2 | 46 ± 2 | 45 ± 6 |
| Wall:Lumen Ratio | 0.5 ± 0.04 | 0.5 ± 0.19 | 0.5 ± 0.04 | 0.5 ± 0.03 | 0.5 ± 0.11 | 0.5 ± 0.03 | 0.5 ± 0.11 | 0.5 ± 0.09 |
| β-Slope Coefficient | 4.5 ± 0.9 | 6.0 ± 0.9* | 3.5 ± 0.7 | 4.3 ± 1.0 | 4.5 ± 1.3 | 8.1 ± 3.4* | 4.6 ± 1.2 | 8.7 ± 5.0* |
ID, inner GA diameter; OD, outer GA diameter; WT, wall thickness. Mean ± SD. n = 6–8/group.
p<0.01 vs. control,
p<0.01 vs. UCMS.
Interaction term: ID = 0.884, OD = 0.456, WT = 0.991, WLR 0.98
NO and ROS Levels
The improvement in EDD with acute TEMPOL or Ex suggested that the endothelial dysfunction in MetS with and without UCMS was in part, mediated by changes in ROS and NO levels. We therefore, examined NO levels in the aorta as determined by DAF-FM diacetate assay. NO bioavailability was lower in LZR-UCMS (68%, p=0.0001) compared to LZR-Controls, but higher in LZR-Ex (15%, p=0.001) vs. LZR-Control, and NO bioavailability was higher (25%, p=0.0001) in the LZR-UCMS+Ex vs. LZR-UCMS group. As for the OZR groups, NO bioavailability was similar between OZR-UCMS and OZR-Controls; however, NO bioavailability was higher (146%, p=0.0001) in OZR-Ex vs. OZR-Controls, and slightly higher (14%, p=0.057) in OZR-UCMS+Ex vs. OZR-UCMS group (Fig. 3A).
Figure 3. The effects of UCMS and Ex on NO and ROS levels.
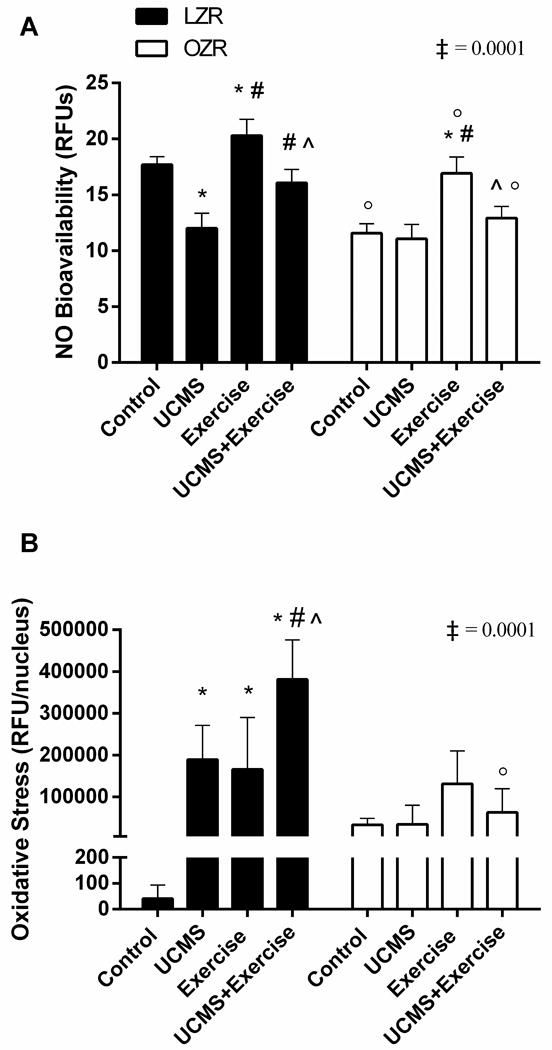
A) NO levels in the aorta as determined by DAF-FM diacetate assay; and B) DHE staining indicating ROS levels in the aortas of each group. Mean ± SD. *p<0.05 vs. control, #p<0.05 vs. UCMS, ^p<0.05 vs. exercise, °p<0.05 vs. matched treatment group in opposite species. ‡p<0.05 species (LZR vs. OZR) by group (Con, UCMS, Ex, UCMS+Ex) interaction. See text for additional details.
Figure 3B illustrates ROS levels in the femoral artery. Levels of ROS were higher in LZR-UCMS (99%, p=0.0001), LZR-Ex (99%, p=0.003), and LZR-UCMS+Ex (100%, p=0.0001) vs. LZR-Controls. Further, the ROS levels were higher in LZR-UCMS+Ex vs. LZR-Ex (130%, p=0.0001), and LZR-UCMS (101%, p=0.04). In the OZR groups, as with NO bioavailability, ROS levels did not differ between OZR-UCMS and OZR-Controls. No differences in ROS levels were noted between the OZR-UCMS+Ex vs. OZR-Controls, or OZR-UCMS groups.
Discussion
The present study is the first to look at the comorbidity between MetS undergoing chronic stress and the effects of Ex on limiting the peripheral vascular effects associated MetS and UCMS. Our results suggest that: 1) exposure to UCMS in LZR resulted in vascular pathologies similar to that evidence in OZR-Controls without the major changes in body mass, glucose or lipid profiles; 2) exposure to UCMS in OZR did not further affect EDD; and 3) Ex combined with UCMS improved peripheral microvascular dysfunction associated with UCMS.
The Impact of UCMS on the GA
Chronic psychosocial stress has been proposed as a risk factor for the development of the MetS (35,36). Thus, we were interested in understanding to what extent exposing a healthy rat (LZR) to the UCMS protocol would result in a MetS phenotype. The UCMS protocol resulted in some mild changes in fasting glucose, with a slight, but non-significant increase in triglycerides and MAP, and an actual reduction in BM in the LZR. Thus, one cannot say that the LZR now resembles a MetS phenotype based on the classification of MetS (i.e., BM, blood pressure, glucose, cholesterol); however, the LZR established substantial peripheral microvascular dysfunction that would represent the vasculopathies noted in an OZR. Indeed, the UCMS protocol severely blunted EDD and EID in LZR-Control. We have shown that UCMS significantly decreased NO and increased ROS production, which is the most likely cause of the impairment of EDD in LZRs. These results were also supported by the fact that acute incubation with the ROS scavenger, TEMPOL, improved EDD in LZR-UCMS, likely allowing NO levels to rise. Further, we also noted an increased production of ROS and a corresponding decrease in NO-bioavailability in the LZR-UCMS group. The UCMS-induced impairment of EID found in the present study is contrary to a similar study using UCMS in mice (37), but is in concurrence with a study using chronic social isolation in prairie voles (38). Factors that may have contributed to the smooth muscle damage include dysfunction of certain secondary messengers and their receptors, and increased cyclooxygenase signaling. One such secondary messenger is soluble guanylyl cyclase, which can be inhibited by increased levels of ROS. Inhibition of cyclic adenosine monophosphate expression by overproduction of cortisol could also influence smooth muscle function (39–41). Furthermore, an increase in thromboxane production can lead to smooth muscle cell dysfunction, thus creating chronic vasoconstriction (42,43). However, further research is needed to address the potential reasons for the impaired EID with UCMS.
Previous studies by our group and others have examined the effects of MetS and UCMS on microvascular function separately (5,6,11,37,44,45) but to what extent the development of vascular pathologies occurs with the progression of MetS undergoing UCMS remained unclear. The condition of MetS already has significant microvascular dysfunction, therefore, it was important to examine the vascular pathologies during comorbidity. The UCMS protocol did not have a significant impact on EDD or EID in the OZR. The lack of UCMS effect on EDD in OZR may be due to the fact OZR-Controls demonstrate elevated ROS levels, and thus diminished bioavailable NO, unlike LZR-Control. Our group has previously shown in the GA that NO levels in OZR-Control are substantially lower than LZR-Control, as well as in other models of disease states such as hypertension, high-fructose diet, and high-salt diet (9). Therefore, the severity of impairment in OZRs may make them less susceptible to the effects of UCMS than in LZRs. TEMPOL increased EDD in OZR-Control and OZR-UCMS, suggesting that ROS scavenging can recover the reduction in NO caused by UCMS and MetS. These data were confirmed by the effect of acute incubation with L-NAME, which blunted dilation response in the Ex and UCMS+Ex groups.
There are various other mechanisms that could be affecting the microvasculature during the comorbidity between UCMS and MetS. For example, with chronic stress and depression there is an increase in cortisol/corticosterone levels as we have seen here in our LZR- and OZR-UCMS groups. Chronically elevated cortisol results in overproduction of angiotensin II (Ang II), which causes vasoconstriction. Ang II has also been shown to cause an increase in endothelim-1, thromboxane, and ROS through activation of AT1 type receptors (46). Thus, the increase in circulating Ang II in vivo causes changes in the smooth muscle and endothelium, possible contributing to the impairment in EDD seen in vitro in LZR- and OZR-UCMS groups.
Peripheral microvascular wall dimensions were not affected by UCMS in LZRs but the stress-strain relationship was shifted to the left, suggesting an increase in stiffness. This response could reflect changes in the collagen deposition and elastin fractionation in the LZR GA (47,48). In contrast, UCMS significantly decreased both inner and outer diameters in OZRs as compared to their controls, indicating eutrophic inward remodeling. Eutrophic inward remodeling could be caused by repositioning of vascular smooth muscle cells to adapt to the chronic circumferential stress (49). OZR-UCMS also had a significant leftward shift in the stress-strain relationship, implying a stiffening of the vessels. Physical inactivity, a by-product of depression and a cause of MetS, produces oxidative stress and inflammation (50). Therefore, Ex is the logical intervention to augment the deleterious effects of MetS and depression.
Exercise Can Improve Aspects of the Comorbidity of MetS and UCMS
Exercise increased LZR EDD but Ex had a much greater effect on OZR EDD response overall. Exercise significantly enhanced NO bioavailability in LZRs and OZRs, which could explain why there was a greater improvement in dilation in LZR- and OZR-Ex. An earlier study found NO production and arginine conversion (indicating eNOS activity) were both increased with Ex (20). Following Ex, EDD was unaffected by the ROS levels as supported by the lack of TEMPOL effect in both LZR- and OZR-Ex groups. The specific cause of the elevation of ROS levels is unclear given Ex has been shown to have antioxidant effects itself (25,26). However, corticosterone levels were elevated in the Ex and UCMS+Ex groups vs. the controls which may, in part, reflect the slight stress induced by use of the forced treadmill Ex protocol. It has been shown that glucocorticoids can increase ROS directly, including superoxide, hydrogen peroxide, and peroxynitrite (12). Given that DHE can interact with these oxidants, it could be speculated the higher ROS levels seen in LZR-Ex and UCMS+Ex are a byproduct of increased corticosterone. The fact that in Ex and UCMS+Ex EDD was improved despite elevated corticosterone levels, suggests EDD augmentation was likely mediated from non-corticosterone pathways. L-NAME blunted maximum dilation back down to LZR- and OZR-Control+LNAME values, suggesting the Ex augmentation of EDD may be solely through a NO-dependent pathway. The expression and function of eNOS is upregulated after Ex training due to increased shear stress (23,51), thus could explain why EDD in LZR- and OZR-Ex was improved in the skeletal muscle arterioles. Ex did not change EID response in LZRs or OZRs as compared to their respective controls, which was supported by a previous study from our group also showing no change in EID in LZRs and OZRs (20).
Ex did not result in GA structure remodeling in either the LZRs or OZRs, but Ex was able to prevent the level of impairment in EDD seen in LZR- and OZR-UCMS. Perhaps the increase in NO bioavailability in the UCMS+Ex groups, relative to the UCMS groups, limited the GA structure remodeling. LZR- and OZR-UCMS+Ex did not show an increase in EDD of the GA when incubated with TEMPOL as compared to their respective Control+TEMPOL values, which would suggest that TEMPOL did not have an additive effect when paired with exercise. EID was also increased with exercise in LZR- and OZR-UCMS+Ex. Furthermore, in the LZR, the combination of Ex with UCMS prevented the leftward shift (and increase GA stiffness) in the stress strain relationship as evident in the LZR-UCMS group, suggesting a global improvement in microvascular function (i.e., EDD, EID with reduced arterial stiffness). However, the combination of Ex with UCMS in OZR was unable to prevent the increase in GA arterial stiffness. Perhaps here the comorbidity of MetS and UCMS was too much of stressful stimulus for 8 weeks of Ex to prevent. Further, research is needed to identify whether longer periods of Ex or a combination of Ex with ‘destiffening’ agents (i.e., ALT-711) could be used to prevent the increase in arterial stiffness with UCMS in the OZR.
Limitations
The present study is not without limitations. We realize the limitation associated with measuring tissue superoxide levels by fluorescence-based assessment of hydroethidine oxidation. While our excitation/emission gating does detect signals produced by oxidation products that may originate from reactions independent of superoxides, the spectral overlap does not exclude considerable contributions from 2-hydroxyethidium, the superoxide-specific oxidation product of hydroethidine (52). The possible explanation for the higher ROS levels in LZR-Ex and LZR-UCMS+Ex is the generation of hydrogen peroxide by Ex. Hydrogen peroxide has been shown to be a vasodilator and produced during Ex (53). Further, it was interesting to note that corticosterone levels were elevated in the Ex and UCMS+Ex groups vs. the controls, which could also have contributed to more hydrogen peroxide production, thus increasing fluorescence intensity in the Ex and UCMS+Ex groups. Future research should use HPLC to look for superoxide and 2-OH-E+ levels to obtain a more quantitative indicator of these products.
Another limitation that may, in part, affect the translational relevance of our study was that we deployed a forced treadmill running protocol as opposed to voluntary wheel running, which in itself can cause stress. However, given the sedentary nature of the OZR, we wanted to ensure that all rats were exposed to a similar Ex stimulus. In addition, given the size of the OZRs (by 12–15 weeks), it would be difficult for them to fit into a running wheel. Thus, although voluntary Ex would be more translationally relevant, unfortunately, in the OZR rats forced treadmill exercise was the most feasible option, and we believe the benefits of forced Ex outweighed the stress the rats might undergo.
Conclusions
The data presented here shows that the chronic exposure to stressful conditions in healthy rats leads to substantial vasculopathies similar to that in an OZR. Further, the comorbidity between chronic stress and MetS, does not exacerbate the effects of one another on skeletal muscle arteriole EDD response. It does, however, lead to the development of other vasculopathy adaptations in relation to constriction response. We have found that Ex can improve these pathological maladaptations and that the NO pathway has the potential to be a therapeutic target in clinical settings.
Acknowledgments
This work was supported by the American Heart Association grants IRG 14330015 and pre-doctoral fellowship AHA 14PRE 20380386, and the National Institute of General Medical Sciences of the National Institutes of Health under award numbers U54GM104942 and 5P20GM109098.
The authors would like to thank Chris Pitzer, Stuart Clayton, Colton Allen, and all other undergraduate interns who helped with the exercise and depression protocol. Thank you to Christa Lilly for statistical consultation. We are grateful to the WVU Animal Facility husbandry and veterinary staff for their continued assistance.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest. The results of the present study do not constitute endorsement by the American College of Sports Medicine and are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA [Internet] 2014;311(8):806–14. doi: 10.1001/jama.2014.732. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24570244%5Cnhttp://jama.jamanetwork.com/article.aspx?articleid=1832542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 3.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009;13:1–7. [PubMed] [Google Scholar]
- 4.Falkner B, Cossrow NDFH. Prevalence of metabolic syndrome and obesity-associated hypertension in the racial ethnic minorities of the United States. Vol 16 Current Hypertension Reports. 2014;16(7):449. doi: 10.1007/s11906-014-0449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maksimovic M, Vlajinac H, Radak D, Marinkovic J, Jorga J. Relationship between peripheral arterial disease and metabolic syndrome. Angiology [Internet] 2009;60:546–53. doi: 10.1177/0003319708325445. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19147526. [DOI] [PubMed] [Google Scholar]
- 6.Frisbee JC, Delp MD. Vascular function in the metabolic syndrome and the effects on skeletal muscle perfusion: lessons from the obese Zucker rat. Essays Biochem [Internet] 2006;42:145–61. doi: 10.1042/bse0420145. Available from: http://www.ncbi.nlm.nih.gov/sites/entrez?db=PubMed&cmd=retrieve&dopt=AbstractPlus&list_uids=17144886%5Cnpapers2://publication/doi/10.1042/bse0420145. [DOI] [PubMed] [Google Scholar]
- 7.Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R307–16. doi: 10.1152/ajpregu.00114.2005. [DOI] [PubMed] [Google Scholar]
- 8.Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15(6):318–30. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Frisbee JC, Butcher JT, Frisbee SJ, Olfert IM, Chantler PD, Tabone LE, et al. Increased peripheral vascular disease risk progressively constrains perfusion adaptability in the skeletal muscle microcirculation. Am J Physiol - Hear Circ Physiol [Internet] 2016;310(4):H488–504. doi: 10.1152/ajpheart.00790.2015. Available from: http://ajpheart.physiology.org/lookup/doi/10.1152/ajpheart.00790.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratt AG, Norris ER, Kaufmann M. Peripheral vascular disease and depression. J Vasc Nurs [Internet] 2005;23(4):123-7-9. doi: 10.1016/j.jvn.2005.09.004. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16326330. [DOI] [PubMed] [Google Scholar]
- 11.d’Audiffret AC, Frisbee SJ, Stapleton PA, Goodwill AG, Isingrini E, Frisbee JC. Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol. 2010;108(5):1041–51. doi: 10.1152/japplphysiol.01440.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golbidi S, Frisbee JC, Laher I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am J Physiol - Hear Circ Physiol [Internet] 2015;308(12):H1476–98. doi: 10.1152/ajpheart.00859.2014. Available from: http://ajpheart.physiology.org/lookup/doi/10.1152/ajpheart.00859.2014. [DOI] [PubMed] [Google Scholar]
- 13.Brostow DP, Petrik ML, Starosta AJ, Waldo SW. Depression in patients with peripheral arterial disease: A systematic review. Eur J Cardiovasc Nurs [Internet] 2017 doi: 10.1177/1474515116687222. [cited 2017 Jul 10];16(3):147451511668722. Available from: https://doi.org/10.1177/1474515116687222. [DOI] [PubMed]
- 14.Smolderen KGE, Aquarius AE, De Vries J, Smith ORF, Hamming JF, Denollet J, et al. Depressive symptoms in peripheral arterial disease: A follow-up study on prevalence, stability, and risk factors. J Affect Disord [Internet] 2008 doi: 10.1016/j.jad.2007.12.238. [cited 2017 Jul 10];110:27–35 Available from: www.elsevier.com/locate/jad. [DOI] [PubMed]
- 15.Wong SYS, Woo J, Hong AWL, Leung JCS, Leung PC. Clinically relevant depressive symptoms and peripheral arterial disease in elderly men and women. Results from a large cohort study in Southern China. doi: 10.1016/j.jpsychores.2007.06.016. [cited 2017 Jul 10]; Available from: http://ac.els-cdn.com/S0022399907002528/1-s2.0-S0022399907002528-main.pdf?_tid=715d6850-659d-11e7-bc66-00000aab0f01&acdnat=1499711435_226b53fcef62a45ec6e902b8596ad84c. [DOI] [PMC free article] [PubMed]
- 16.Chrapko WE, Jurasz P, Radomski MW, Lara N, Archer SL, Le Mellédo J-M. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry [Internet] 2004;56(2):129–34. doi: 10.1016/j.biopsych.2004.03.003. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15231445. [DOI] [PubMed] [Google Scholar]
- 17.Dhir A, Kulkarni SK. Nitric oxide and major depression. Nitric Oxide [Internet] 2011;24(3):125–31. doi: 10.1016/j.niox.2011.02.002. Available from: http://dx.doi.org/10.1016/j.niox.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Isingrini E, Surget A, Belzung C, Freslon J-L, Frisbee J, O’Donnell J, et al. Altered aortic vascular reactivity in the unpredictable chronic mild stress model of depression in mice: UCMS causes relaxation impairment to ACh. Physiol Behav [Internet] 2011;103(5):540–6. doi: 10.1016/j.physbeh.2011.04.002. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21504753%5Cnhttp://dx.doi.org/10.1016/j.physbeh.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Donley DA, Fournier SB, Reger BL, DeVallance E, Bonner DE, Olfert IM, et al. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol [Internet] 2014 Apr;116:1396–404. doi: 10.1152/japplphysiol.00151.2014. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24744384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2006;291(5):H2483–92. doi: 10.1152/ajpheart.00566.2006. [DOI] [PubMed] [Google Scholar]
- 21.Bowles DK, Woodman CR, Laughlin MH. Coronary smooth muscle and endothelial adaptations to exercise training. Exerc Sport Sci Rev. 2000;28(2):57–62. [PubMed] [Google Scholar]
- 22.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol [Internet] 2004;561(Pt 1):1–25. doi: 10.1113/jphysiol.2004.068197. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1665322&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingwell BA. Nitric oxide-mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. Faseb J [Internet] 2000;14(12):1685–96. doi: 10.1096/fj.99-0896rev. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10973917. [DOI] [PubMed] [Google Scholar]
- 24.Walther C, Gielen S, Hambrecht R. The effect of exercise training on endothelial function in cardiovascular disease in humans. Exerc Sport Sci Rev. 2004;32(4):129–34. doi: 10.1097/00003677-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Molecular and Cellular Biochemistry. 2003;253:307–12. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44(2):126–31. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aleixandre de Artiñano A, Miguel Castro M. Experimental rat models to study the metabolic syndrome. Br J Nutr. 2009;102(9):1246–53. doi: 10.1017/S0007114509990729. [DOI] [PubMed] [Google Scholar]
- 29.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev. 1992;16(4):525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 30.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) [Internet] 1997;134(4):319–29. doi: 10.1007/s002130050456. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9452163. [DOI] [PubMed] [Google Scholar]
- 31.Vollmayr B, Henn FA. Stress models of depression. Clinical Neuroscience Research. 2003;3:245–51. [Google Scholar]
- 32.Willner P. Neurobiology of Stress The chronic mild stress (CMS) model of depression : History, evaluation and usage. Neurobiol Stress [Internet] 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. Available from: http://dx.doi.org/10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frisbee JC, Brooks SD, Stanley SC, d’Audiffret AC. An Unpredictable Chronic Mild Stress Protocol for Instigating Depressive Symptoms, Behavioral Changes and Negative Health Outcomes in Rodents. J Vis Exp [Internet] 2015;(106):1–8. doi: 10.3791/53109. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26650668. [DOI] [PMC free article] [PubMed]
- 34.Yalcin I, Aksu F, Belzung C. Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol. 2005;514(2–3):165–74. doi: 10.1016/j.ejphar.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. Bmj [Internet] 2006;332(7540):521–4. doi: 10.1136/bmj.38693.435301.80. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16428252%5Cnhttp://pubmedcentralcanada.ca/picrender.cgi?accid=PMC1388129&blobtype=pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergmann N, Gyntelberg F, Faber J. The appraisal of chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr Connect [Internet] 2014;3(2):R55–80. doi: 10.1530/EC-14-0031. Available from: http://www.endocrineconnections.com/cgi/doi/10.1530/EC-14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanley SC, Brooks SD, Butcher JT, d’Audiffret AC, Frisbee SJ, Frisbee JC. Protective effect of sex on chronic stress- and depressive behavior-induced vascular dysfunction in BALB/cJ mice. J Appl Physiol [Internet] 2014;117(9):959–70. doi: 10.1152/japplphysiol.00537.2014. Available from: http://jap.physiology.org/cgi/doi/10.1152/japplphysiol.00537.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peuler JD, Scotti M-AL, Phelps LE, McNeal N, Grippo AJ. Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiol Behav [Internet] 2012 Jun 25; doi: 10.1016/j.physbeh.2012.03.019. [cited 2017 Jul 8];106:(4)476–84 Available from: http://www.ncbi.nlm.nih.gov/pubmed/22469565. [DOI] [PMC free article] [PubMed]
- 39.BRÜNE B, SCHMIDT K-U, ULLRICH V. Activation of soluble guanylate cyclase by carbon monoxide and inhibition by superoxide anion. Eur J Biochem. 1990;192(3):683–8. doi: 10.1111/j.1432-1033.1990.tb19276.x. [DOI] [PubMed] [Google Scholar]
- 40.Weber M, Lauer N, Mülsch A, Kojda G. The effect of peroxynitrite on the catalytic activity of soluble guanylyl cyclase. Free Radic Biol Med. 2001;31(11):1360–7. doi: 10.1016/s0891-5849(01)00706-7. [DOI] [PubMed] [Google Scholar]
- 41.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, et al. Cysteine Redox Sensor in PKGIa Enables Oxidant-Induced Activation. Science (80-) [Internet] 2007;317(5843):1393–7. doi: 10.1126/science.1144318. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 42.Sparks MA, Makhanova NA, Griffiths RC, Snouwaert JN, Koller BH, Coffman TM. Thromboxane receptors in smooth muscle promote hypertension, vascular remodeling, and sudden death. Hypertension. 2013;61(1):166–73. doi: 10.1161/HYPERTENSIONAHA.112.193250. [DOI] [PubMed] [Google Scholar]
- 43.Dorn GW, 2nd, Becker MW. Thromboxane A2 stimulated signal transduction in vascular smooth muscle. J Pharmacol Exp Ther. 1993;265(1):447–56. [PubMed] [Google Scholar]
- 44.Brooks SD, DeVallance E, d’Audiffret AC, Frisbee SJ, Tabone LE, Shrader CD, et al. Metabolic syndrome impairs reactivity and wall mechanics of cerebral resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol [Internet] 2015 Dec 1; doi: 10.1152/ajpheart.00691.2015. [cited 2015 Dec 11];309:(11)H1846–59. Available from: http://ajpheart.physiology.org/content/309/11/H1846.full-text.pdf+html. [DOI] [PMC free article] [PubMed]
- 45.Isingrini E, Surget A, Belzung C, Freslon JL, Frisbee J, O’Donnell J, et al. Altered aortic vascular reactivity in the unpredictable chronic mild stress model of depression in mice. UCMS causes relaxation impairment to ACh. Physiol Behav [Internet] 2011;103(5):540–6. doi: 10.1016/j.physbeh.2011.04.002. Available from: http://dx.doi.org/10.1016/j.physbeh.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Carey RM. The intrarenal renin-angiotensin system in hypertension. Adv Chronic Kidney Dis. 2015;22(3):204–10. doi: 10.1053/j.ackd.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Cattell MA, Anderson JC, Hasleton PS. Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin Chim Acta. 1996;245(1):73–84. doi: 10.1016/0009-8981(95)06174-6. [DOI] [PubMed] [Google Scholar]
- 48.Venkataraman L, Ramamurthi A. Induced Elastic Matrix Deposition Within Three-Dimensional Collagen Scaffolds. Tissue Eng Part A [Internet] 2011;17(21–22):2879–89. doi: 10.1089/ten.tea.2010.0749. Available from: http://www.liebertonline.com/doi/abs/10.1089/ten.tea.2010.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castorena-Gonzalez JA, Staiculescu MC, Foote C, Martinez-Lemus LA. Mechanisms of the inward remodeling process in resistance vessels: Is the actin cytoskeleton involved? Microcirculation. 2014;21(3):219–29. doi: 10.1111/micc.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ann het Rot M, Collins KA, Fitterling HL. Physical exercise and depression. Mt Sinai J Med. 2009;76(2):204–14. doi: 10.1002/msj.20094. [DOI] [PubMed] [Google Scholar]
- 51.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587(Pt 15):3885–97. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radical Biology and Medicine. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson AT, Franklin NC, Norkeviciute E, Bian JT, Babana JC, Szczurek MR, et al. Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. J Hypertens [Internet] 2016;34(7):1309–16. doi: 10.1097/HJH.0000000000000946. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27137176%5Cnhttp://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004872-201607000-00012. [DOI] [PubMed] [Google Scholar]


