Abstract
Objective
To determine the long-term risk of cardiovascular disease and metabolic conditions in women undergoing hysterectomy with bilateral ovarian conservation compared with age-matched referent women.
Methods
Using the Rochester Epidemiology Project records-linkage system, we identified 2,094 women who underwent hysterectomy with ovarian conservation for benign indications between 1980 and 2002 in Olmsted County, Minnesota. Each woman was age-matched (±1 year) to a referent woman residing in the same county who had not undergone prior hysterectomy or any oophorectomy. These two cohorts were followed historically to identify de novo cardiovascular or metabolic diagnoses. We estimated hazard ratios and 95% confidence intervals using Cox proportional hazards models adjusted for 20 pre-existing chronic conditions and other potential confounders. We also calculated absolute risk increases and reductions from Kaplan-Meier estimates.
Results
Over a median follow-up of 21.9 years, women who underwent hysterectomy experienced increased risks of de novo hyperlipidemia (HR 1.14; 95% CI 1.05-1.25), hypertension (HR 1.13; 95% CI 1.03-1.25), obesity (HR 1.18; 95% CI 1.04-1.35), cardiac arrhythmias (HR 1.17; 95% CI 1.05-1.32), and coronary artery disease (HR 1.33; 95% CI 1.12-1.58). Women who underwent hysterectomy at age ≤35 years had a 4.6-fold increased risk of congestive heart failure and a 2.5-fold risk of coronary artery disease.
Conclusions
Even with ovarian conservation, hysterectomy is associated with an increased long-term risk of cardiovascular and metabolic conditions, especially in women who undergo hysterectomy at age ≤35 years. If these associations are causal, alternatives to hysterectomy should be considered to treat benign gynecologic conditions.
Keywords: hysterectomy, cardiovascular diseases, metabolic conditions, epidemiology, cohort study
INTRODUCTION
Over 400,000 hysterectomies with or without concurrent bilateral oophorectomy are performed each year in the U.S., most for benign disease.1,2 Studies have shown that bilateral oophorectomy increases mortality and the risk of cardiovascular disease (CVD) and other chronic diseases;3–5 thus, bilateral oophorectomy rates at the time of hysterectomy have decreased.6 On the other hand, the rates of hysterectomy with ovarian conservation are increasing, in particular for younger women.7,8 However, the possible harmful long-term outcomes of hysterectomy with ovarian conservation are understudied.
Previous studies of hysterectomy with ovarian conservation have had methodological limitations.9–14 Most studies did not control for pre-existing CVD which is increased in women undergoing hysterectomy.11,12 Some studies included women with a previous unilateral oophorectomy in the ovarian conservation group.9,10 Two recent studies have addressed some of these limitations, but had either limited data on pre-existing CVD or short-term follow-up.13,14 The aim of this study was to assess the long-term risk of CVD and metabolic conditions following hysterectomy with bilateral ovarian conservation compared to population-based referent women without prior hysterectomy or oophorectomy, after adjustment for chronic conditions present at the time of hysterectomy and for several potential confounders.
METHODS
Overall cohort study design
As part of the Mayo Clinic Study of Uterine Disease and Health (MCSUD), we studied 2,094 Olmsted County, Minnesota resident women who underwent hysterectomy with ovarian conservation for benign indications between January 1, 1980 and December 31, 2002 (23 years). Our cohort was a subset of a larger cohort previously established to study the frequency of hysterectomy, time trends, and some long-term sequelae, as reported elsewhere (Supplemental Digital Content 1).12,15–17 Women were identified using the Rochester Epidemiology Project (REP) medical records-linkage system that includes the complete inpatient and outpatient records of all medical providers in Olmsted County. Details of the REP and of the Olmsted County population have been previously published.18
As described previously, the REP electronic indices were searched for procedural codes for hysterectomy and for diagnostic codes for surgical indication.17 We included all women who underwent hysterectomy with bilateral ovarian conservation during the study period, authorized the use of their medical records for research, and were 18 years old or older on the date of hysterectomy (index date). For each woman who underwent hysterectomy, we randomly identified one woman matched by age (±1 year) who resided in Olmsted County on the index date, had not undergone a hysterectomy or oophorectomy (unilateral or bilateral) prior to the index date, and had authorized the use of her medical records for research. Approximately 97% of the women residing in Olmsted County have provided the general research authorization required by the Minnesota law for inclusion into the system.18 No other matching criteria or restrictions were used. The study was approved by the institutional review boards at Olmsted Medical Center and Mayo Clinic.
Ascertainment of conditions present at the index date
All International Classification of Diseases (ICD) codes for chronic conditions diagnosed before the index date were obtained electronically from the diagnostic indices of the REP. To align with our ongoing work on multimorbidity using the Department of Health and Human Services (DHHS) definition, we considered 18 DHHS chronic conditions as well as anxiety and obesity (total of 20 conditions listed in Supplemental Digital Content 2).19–21 To reduce the risk of false positive diagnoses, only women whose medical record contained at least two diagnostic codes separated by more than 30 days were considered to have that condition. Before 1994, a one-year separation of codes was required because finer dating of the codes was not available. Because the ICD codes were introduced in the REP in 1975, we restricted the established cohort to women who underwent hysterectomy on or after January 1, 1980 to provide a minimum of five years of diagnostic capture before the index date (1975-1979).
Cardiovascular and metabolic outcome conditions
Women in both the hysterectomy and referent cohorts were followed passively through the REP records-linkage system. The primary outcomes of the study were the following CVD and metabolic conditions: hyperlipidemia, hypertension, diabetes, obesity, cardiac arrhythmias, coronary artery disease (CAD), congestive heart failure (CHF), and stroke.20 The CVD and metabolic outcomes were obtained electronically from the REP indices, and required at least two diagnostic codes separated by more than 30 days as described above. However, to include those conditions that caused acute death, a single diagnosis found anywhere on a death certificate was also sufficient.
Statistical analyses
Each CVD and metabolic condition was evaluated separately, and women with that condition prior to hysterectomy (or index date for referent women) were excluded from the analysis in order to evaluate de novo conditions. The duration of follow-up was calculated from the index date to the date of the condition diagnosis, date of death, last contact within the REP, or the end of the study (December 31, 2015), whichever came first. Cumulative incidence curves were estimated using the Kaplan-Meier method. Cox proportional hazards models were used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) using age as the time scale with women entering the risk set at their respective index ages.
Although the hysterectomy and referent cohorts were only matched by age (±1 year) at index date during the sampling process, additional strategies were applied to limit the differences at baseline. In particular, the Kaplan-Meier curves and the Cox models were adjusted using inverse probability weights derived from a logistic regression model including 20 pre-existing chronic conditions, years of education (≤12, 13-16, >16, unknown), race (white vs. nonwhite), and age and calendar year at index date (continuous) (Supplemental Digital Content 3). Robust sandwich covariance estimates were used in the Cox models to account for women included in both cohorts (referent women with subsequent hysterectomy), and for the use of estimated weights. Absolute risks were derived from the adjusted Kaplan-Meier curves at 30 years, and differences between the two cohorts were measured using the absolute risk increase (ARI) or reduction (ARR), obtained by subtracting the two absolute risks.
Analyses were performed for all women combined, and stratified by age at hysterectomy (≤35, 36-50, and >50 years) and by surgical indication. The inverse probability weights were derived separately within each stratum to maximize the covariate balance. We performed two sets of sensitivity analyses to 1) exclude women with any of the 20 chronic conditions prior to the index date, and 2) to censor women at the time of subsequent unilateral or bilateral oophorectomy (both women with hysterectomy and referent women) or hysterectomy (referent women). Analyses were performed using the SAS version 9.4 software package (SAS Institute, Inc., Cary, NC), and tests of statistical significance were conducted at the 2-tailed alpha level of 0.05.
RESULTS
Description of the hysterectomy and referent cohorts
Between 1980 and 2002, a total of 2,094 women underwent hysterectomy with ovarian conservation. A total of 529 women (25.3%) were age 35 or younger at the time of hysterectomy, and 271 women (12.9%) were older than 50 years. The median age at index date was 40 years (interquartile range 35 to 44). Indications for hysterectomy with ovarian conservation included uterine leiomyomas (n = 827, 39.5%), prolapse (n = 425, 20.3%), and menstrual disorders (n = 534, 25.5%; including menorrhagia and metrorrhagia). Other surgical indications comprised 14.7% (n = 308) of the cohort. Vaginal hysterectomy was performed in 1,709 women (81.6%).
The median length of follow-up was 22.5 years (interquartile range [IQR] 15.2-28.8) for women with hysterectomy, 21.3 years (IQR 13.7-28.6) for referent women, and 21.9 years (IQR 14.2-28.7) for both cohorts combined. The median density of medical contacts during follow-up was 7.3 per year (IQR 4.3-11.4) for the women with hysterectomy and 6.2 per year (IQR 3.6-9.9) for referent women (excluding contacts in the first 6 months after the index date). A total of 293 women (14.0%) died in the hysterectomy cohort and 306 (14.6%) in the referent cohort. The adjusted HR for all-cause mortality was 0.99 (95% CI 0.78-1.24; p=0.90).
Conditions present at index date and adjustments
Women undergoing hysterectomy with ovarian conservation were more likely to have pre-existing hyperlipidemia (odds ratio [OR] 1.50; 95% CI 1.11-2.02), obesity (OR 1.58; 95% CI 1.30-1.93), and a higher number of chronic conditions compared with referent women (OR 1.90; 95% CI 1.48-2.44, for having three or more of the 20 chronic conditions). Other CVD and metabolic conditions were similar between women with hysterectomy and referent women (data not shown). The two overall cohorts were not highly imbalanced on baseline characteristics before the adjustments using inverse probability weights (each standardized difference of means <25% of the SD), and the adjustments improved the balance successfully (each standardized difference of means <5% of the SD; Supplemental Digital Content 3). The range of the weights used in the overall analysis was reported in Supplemental Digital Content 3.
Overall analyses
Women who underwent hysterectomy with ovarian conservation were at higher risk of developing de novo cardiovascular and metabolic conditions compared with age-matched referent women (Table 1). We observed a significantly increased risk of hyperlipidemia (adjusted HR 1.14; 95% CI 1.05-1.25; ARI 3.8%), hypertension (adjusted HR 1.13; 95% CI 1.03-1.25; ARI 6.5%), and obesity (adjusted HR 1.18; 95% CI 1.04-1.35; ARI 4.3%) (Table 1). Moreover, the risks of cardiac arrhythmias (adjusted HR 1.17; 95% CI 1.05-1.32; ARI 5.6%) and CAD (adjusted HR 1.33; 95% CI 1.12-1.58; ARI 6.4%) were significantly increased. The risk of CHF was decreased but did not reach statistical significance. The cumulative incidence of CVD appears to diverge between women with hysterectomy and referent women five to 15 years after the index date for several conditions, and after about 20 years for CAD (Fig. 1).
TABLE 1.
Cumulative incidence of cardiovascular and metabolic conditions in women who underwent hysterectomy with ovarian conservation compared to referent women (overall analyses; all ages)
| Condition | Hysterectomy
|
Referent Women
|
Unadjusted Modelsb
|
Adjusted Modelsc
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N at Risk | Person-years | N of Events | Absolute Riska % (95% CI) | N at Risk | Person-years | N of Events | Absolute Riska % (95% CI) | Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | |
| Hyperlipidemia | 1,979 | 30,055 | 1,069 | 73.1 (70.1-76.0) | 2,015 | 29,792 | 906 | 69.3 (66.1-72.4) | 1.20 (1.10-1.31) | <0.001 | 1.14 (1.05-1.25) | 0.002 |
| Hypertension | 1,943 | 32,383 | 871 | 62.6 (59.4-65.8) | 1,963 | 31,605 | 715 | 56.1 (52.8-59.5) | 1.20 (1.09-1.32) | <0.001 | 1.13 (1.03-1.25) | 0.01 |
| Diabetes | 2,037 | 38,670 | 631 | 49.6 (46.3-53.0) | 2,056 | 36,999 | 535 | 45.9 (42.6-49.4) | 1.15 (1.03-1.28) | 0.01 | 1.09 (0.97-1.22) | 0.14 |
| Obesity | 1,815 | 32,260 | 499 | 36.0 (33.1-39.1) | 1,908 | 32,532 | 406 | 31.7 (28.9-34.8) | 1.24 (1.09-1.41) | <0.001 | 1.18 (1.04-1.35) | 0.01 |
| Cardiac arrhythmias | 2,043 | 38,831 | 620 | 45.1 (42.0-48.3) | 2,051 | 37,695 | 492 | 39.5 (36.4-42.8) | 1.24 (1.10-1.38) | <0.001 | 1.17 (1.05-1.32) | 0.006 |
| Coronary artery disease | 2,072 | 41,904 | 322 | 24.1 (21.5-27.0) | 2,078 | 40,143 | 222 | 17.7 (15.4-20.3) | 1.42 (1.20-1.67) | <0.001 | 1.33 (1.12-1.58) | 0.001 |
| Congestive heart failure | 2,089 | 44,116 | 127 | 9.5 (7.8-11.5) | 2,084 | 41,283 | 130 | 10.0 (8.3-12.0) | 0.88 (0.69-1.12) | 0.29 | 0.80 (0.62-1.02) | 0.07 |
| Stroke | 2,087 | 43,505 | 171 | 12.2 (10.3-14.5) | 2,087 | 41,080 | 145 | 11.8 (9.9-13.9) | 1.11 (0.89-1.37) | 0.37 | 1.01 (0.81-1.26) | 0.91 |
Absolute cumulative risk at 30 years after hysterectomy (or index) calculated using the Kaplan-Meier method. The estimates were adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline, years of education (≤12, 13-16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous).
Hazard ratios calculated using Cox proportional hazards models with age as the time scale.
Hazard ratios calculated using Cox proportional hazards models with age as the time scale and adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline, years of education (≤12, 13-16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous).
Fig. 1.
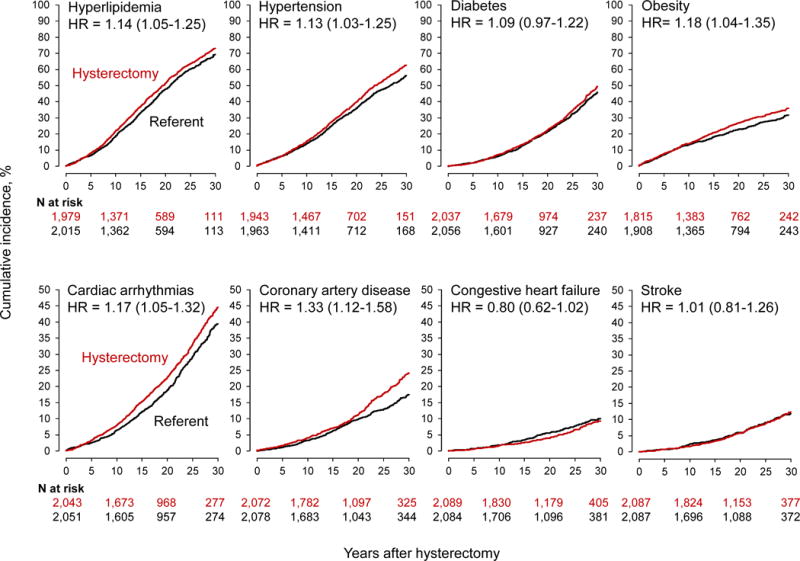
Cumulative incidence curves for cardiovascular and metabolic conditions in women who underwent hysterectomy with ovarian conservation compared with age-matched referent women (overall analyses). The curves were adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline, years of education (≤12, 13-16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). The number of women at risk varied across conditions because we excluded women with that specific condition on the index date. The hazard ratios (HRs) and corresponding 95% confidence intervals were calculated using Cox proportional hazards models with age as the time scale and adjusted using inverse probability weights. Note the different scales used for the y-axis to better show differences.
Analyses stratified by age
Women who underwent hysterectomy with ovarian conservation at age ≤35 years had significantly increased risk of several CVD and metabolic outcomes compared with referent women (Table 2). There was a 4.6-fold increase in CHF (adjusted HR 4.59; 95% CI 1.32-15.94; ARI 4.6%), a 2.5-fold increase in CAD (adjusted HR 2.49; 95% CI 1.39-4.47; ARI 6.1%), and a 1.4-fold increased risk for cardiac arrhythmias (adjusted HR 1.36; 95% CI 1.00-1.84; ARI 10.1%). In this younger age stratum, the incidence of CVD started to diverge in women with hysterectomy compared to referent women 20 to 25 years after the index date, around the time of expected natural menopause. As expected, the divergence was delayed in these younger women compared to the overall sample (Fig. 2 compared to Fig. 1).
Table 2.
Cumulative incidence of cardiovascular and metabolic conditions in strata by age at hysterectomy with ovarian conservation
| Condition | Hysterectomy
|
Referent Women
|
Unadjusted Modelsb |
Adjusted Modelsc |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N at Risk |
Person -years |
N of Events |
Absolute Riska % (95% CI) |
N at Risk |
Person -years |
N of Events |
Absolute Riska % (95% CI) |
Hazard Ratio (95% CI) |
P | Hazard Ratio (95% CI) |
P | |
| Age ≤35 years | ||||||||||||
| Hyperlipidemia | 518 | 8,995 | 211 | 57.3 (50.9-63.9) | 517 | 8,419 | 173 | 57.3 (50.8-63.9) | 1.18 (0.97-1.44) | 0.11 | 1.02 (0.82-1.26) | 0.88 |
| Hypertension | 519 | 9,736 | 160 | 44.7 (38.6-51.3) | 521 | 9,138 | 129 | 38.6 (32.5-45.3) | 1.21 (0.96-1.53) | 0.10 | 1.13 (0.89-1.44) | 0.31 |
| Diabetes | 524 | 10,502 | 124 | 38.7 (32.4-45.7) | 524 | 9,757 | 93 | 33.0 (26.8-40.1) | 1.30 (1.00-1.70) | 0.052 | 1.22 (0.92-1.62) | 0.16 |
| Obesity | 469 | 8,519 | 126 | 36.6 (30.7-43.3) | 502 | 8,423 | 106 | 33.7 (28.0-40.2) | 1.18 (0.91-1.52) | 0.21 | 1.07 (0.82-1.41) | 0.60 |
| Cardiac arrhythmias | 522 | 10,336 | 118 | 35.6 (29.8-42.3) | 526 | 9,835 | 81 | 25.5 (20.3-31.9) | 1.46 (1.10-1.93) | 0.008 | 1.36 (1.00-1.84) | 0.049 |
| Coronary artery disease | 529 | 11,174 | 40 | 13.2 (9.3-18.6) | 529 | 10,455 | 16 | 7.1 (3.9-12.7) | 2.49 (1.40-4.43) | 0.002 | 2.49 (1.39-4.47) | 0.002 |
| Congestive heart failure | 528 | 11,353 | 16 | 4.9 (2.7-8.7) | 529 | 10,556 | 3 | 0.3 (0.0-2.3) | 5.24 (1.54-17.82) | 0.008 | 4.59 (1.32-15.94) | 0.02 |
| Stroke | 527 | 11,233 | 21 | 5.0 (2.8-8.8) | 529 | 10,497 | 14 | 4.8 (2.8-8.1) | 1.51 (0.76-2.99) | 0.23 | 1.14 (0.50-2.58) | 0.75 |
| Age 36 to 50 years | ||||||||||||
| Hyperlipidemia | 1,216 | 18,212 | 724 | 78.0 (74.4-81.4) | 1,243 | 18,325 | 619 | 73.5 (69.7-77.3) | 1.19 (1.08-1.33) | <0.001 | 1.14 (1.03-1.27) | 0.01 |
| Hypertension | 1,217 | 20,199 | 570 | 65.5 (61.4-69.5) | 1,244 | 20,294 | 463 | 58.3 (54.0-62.7) | 1.26 (1.12-1.42) | <0.001 | 1.21 (1.07-1.36) | 0.002 |
| Diabetes | 1,262 | 24,156 | 421 | 52.6 (48.4-56.8) | 1,277 | 23,406 | 363 | 49.2 (45.1-53.5) | 1.13 (0.99-1.30) | 0.07 | 1.08 (0.94-1.24) | 0.27 |
| Obesity | 1,131 | 20,225 | 330 | 36.5 (33.0-40.3) | 1,177 | 20,636 | 263 | 32.4 (28.9-36.2) | 1.28 (1.09-1.50) | 0.003 | 1.20 (1.02-1.41) | 0.03 |
| Cardiac arrhythmias | 1,263 | 24,636 | 366 | 42.9 (39.0-47.0) | 1,273 | 24,127 | 288 | 38.1 (34.2-42.2) | 1.27 (1.09-1.47) | 0.002 | 1.21 (1.04-1.41) | 0.01 |
| Coronary artery disease | 1,288 | 26,582 | 181 | 22.7 (19.5-26.4) | 1,292 | 25,664 | 124 | 16.3 (13.5-19.5) | 1.44 (1.15-1.80) | 0.001 | 1.34 (1.07-1.68) | 0.01 |
| Congestive heart failure | 1,293 | 28,066 | 41 | 5.6 (3.9-7.9) | 1,292 | 26,445 | 51 | 7.2 (5.3-9.7) | 0.76 (0.51-1.13) | 0.18 | 0.63 (0.42-0.95) | 0.03 |
| Stroke | 1,291 | 27,498 | 92 | 10.6 (8.4-13.4) | 1,294 | 26,293 | 65 | 9.5 (7.4-12.3) | 1.37 (1.00-1.87) | 0.048 | 1.22 (0.88-1.67) | 0.23 |
| Age >50 years | ||||||||||||
| Hyperlipidemia | 245 | 2,848 | 134 | 81.2 (69.4-90.6) | 255 | 3,048 | 114 | 75.9 (64.4-85.9) | 1.26 (1.01-1.57) | 0.04 | 1.19 (0.95-1.50) | 0.12 |
| Hypertension | 207 | 2,449 | 141 | 90.1 (82.9-95.1) | 198 | 2,172 | 123 | 84.5 (76.8-90.7)a | 1.00 (0.80-1.25) | 0.98 | 0.94 (0.75-1.18) | 0.62 |
| Diabetes | 251 | 4,011 | 86 | 58.7 (48.2-69.4) | 255 | 3,835 | 79 | 59.4 (48.2-70.9) | 1.01 (0.76-1.36) | 0.93 | 1.00 (0.74-1.35) | 0.98 |
| Obesity | 215 | 3,517 | 43 | 27.6 (20.1-37.0) | 229 | 3,473 | 37 | 22.1 (16.0-30.2) | 1.18 (0.76-1.83) | 0.46 | 1.17 (0.75-1.83) | 0.50 |
| Cardiac arrhythmias | 258 | 3,859 | 136 | 75.6 (67.2-83.2) | 252 | 3,734 | 123 | 78.6 (69.8-86.3) | 1.04 (0.83-1.32) | 0.71 | 1.02 (0.81-1.29) | 0.86 |
| Coronary artery disease | 255 | 4,149 | 101 | 53.9 (45.2-63.2) | 257 | 4,024 | 82 | 44.7 (36.8-53.4) | 1.16 (0.87-1.56) | 0.31 | 1.15 (0.85-1.56) | 0.35 |
| Congestive heart failure | 268 | 4,698 | 70 | 39.2 (31.3-48.2) | 263 | 4,283 | 76 | 45.4 (36.6-55.3) | 0.79 (0.57-1.09) | 0.15 | 0.84 (0.60-1.17) | 0.30 |
| Stroke | 269 | 4,773 | 58 | 36.9 (28.9-46.4) | 264 | 4,290 | 66 | 41.2 (32.4-51.3) | 0.77 (0.54-1.08) | 0.13 | 0.80 (0.56-1.14) | 0.22 |
Absolute cumulative risk at 30 years after hysterectomy (or index) calculated using the Kaplan-Meier method. For hypertension, the Kaplan-Meier estimate at 25 years after index was reported for referent women in the age >50 year stratum because this was the maximum length of follow-up available for these women. The estimates were adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline, years of education (≤12, 13-16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). These adjustments were performed separately in each stratum to maximize the balance at baseline.
Hazard ratios calculated using Cox proportional hazards models with age as the time scale.
Hazard ratios calculated using Cox proportional hazards models with age as the time scale and adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline, years of education (≤12, 13-16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). These adjustments were performed separately in each stratum to maximize the balance at baseline.
Fig. 2.
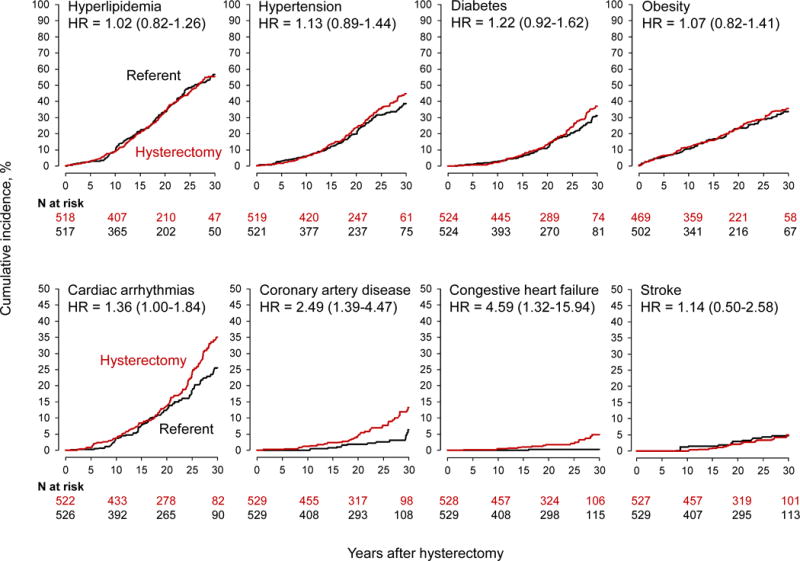
Cumulative incidence curves for cardiovascular and metabolic conditions in women who underwent hysterectomy with ovarian conservation at 35 years or younger compared with age-matched referent women (stratified analyses). The curves were adjusted using inverse probability weights derived from a logistic regression model restricted to this age stratum, and including all 20 chronic conditions present at baseline, years of education (≤12, 13-16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). The number of women at risk varied across conditions because we excluded women with that specific condition on the index date. The hazard ratios (HRs) and corresponding 95% confidence intervals were calculated using Cox proportional hazards models with age as the time scale and adjusted using inverse probability weights. Note the different scales used for the y-axis to better show differences.
Women who had hysterectomy with ovarian conservation between age 36 and 50 years had increased risks of hyperlipidemia, hypertension, obesity, cardiac arrhythmias, and CAD (Table 2). However, the risk of CHF was significantly decreased in this age group (adjusted HR 0.63; 95% CI 0.42-0.95; ARR 1.6%). Women who had hysterectomy after the age of 50 years did not have any significantly increased risk of CVD and metabolic conditions (Table 2).
Analyses stratified by indication
In women who underwent hysterectomy with ovarian conservation for uterine leiomyomas, the risks of de novo hyperlipidemia and cardiac arrhythmias were increased compared with referent women (Table 3). In women who underwent hysterectomy for menstrual disorders, the risk of CAD was significantly increased (adjusted HR 1.81; 95% CI 1.21-2.72; ARI 9.2%). The risk of hypertension was increased in both the leiomyomas and the menstrual disorders strata, but did not reach statistical significance. By contrast, in women who underwent hysterectomy for uterine prolapse, only the risk of de novo obesity was increased, whereas the risk of CHF was decreased (adjusted HR 0.59; 95% CI 0.38-0.92; ARR 4.9%; Table 3).
TABLE 3.
Cumulative incidence of cardiovascular and metabolic conditions in strata by hysterectomy indicationa
| Hysterectomy
|
Referent Women
|
Unadjusted Modelsc |
Adjusted Modelsd |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | N at Risk |
Person -years |
N of Events |
Absolute Riskb % (95% CI) |
N at Risk |
Person -years |
N of Events |
Absolute Riskb % (95% CI) |
Hazard Ratio (95% CI) |
P | Hazard Ratio (95% CI) |
P | |
| Leiomyomas | |||||||||||||
| Hyperlipidemia | 761 | 10,596 | 433 | 77.4 (72.6-82.0) | 783 | 10,773 | 358 | 72.6 (67.0-77.9) | 1.25 (1.09-1.43) | 0.001 | 1.22 (1.06-1.40) | 0.004 | |
| Hypertension | 763 | 11,899 | 355 | 63.6 (58.6-68.6) | 760 | 11,532 | 284 | 60.8 (54.8-66.8) | 1.22 (1.05-1.42) | 0.01 | 1.15 (0.99-1.34) | 0.07 | |
| Diabetes | 801 | 14,367 | 248 | 51.2 (45.7-56.9) | 810 | 13,904 | 207 | 48.6 (42.9-54.5) | 1.17 (0.98-1.39) | 0.09 | 1.14 (0.95-1.36) | 0.16 | |
| Obesity | 710 | 12,150 | 183 | 34.7 (30.0-39.9) | 745 | 12,134 | 160 | 32.2 (27.7-37.3) | 1.14 (0.93-1.41) | 0.21 | 1.09 (0.89-1.35) | 0.41 | |
| Cardiac arrhythmias | 797 | 14,330 | 250 | 48.8 (43.7-54.3) | 809 | 14,168 | 196 | 41.8 (36.6-47.4) | 1.32 (1.10-1.58) | 0.003 | 1.28 (1.07-1.54) | 0.007 | |
| Coronary artery disease | 815 | 15,621 | 116 | 24.1 (19.8-29.2) | 818 | 15,051 | 101 | 21.4 (17.3-26.3) | 1.15 (0.89-1.50) | 0.28 | 1.11 (0.85-1.45) | 0.44 | |
| Congestive heart failure | 824 | 16,616 | 47 | 9.6 (6.9-13.4) | 823 | 15,562 | 50 | 10.3 (7.5-14.0) | 0.86 (0.58-1.26) | 0.44 | 0.82 (0.55-1.21) | 0.31 | |
| Stroke | 821 | 16,314 | 67 | 13.8 (10.5-18.0) | 824 | 15,496 | 61 | 12.7 (9.6-16.8) | 1.05 (0.75-1.48) | 0.77 | 1.03 (0.73-1.46) | 0.87 | |
| Menstrual disorders | |||||||||||||
| Hyperlipidemia | 518 | 8,435 | 282 | 69.9 (64.2-75.4) | 521 | 8,337 | 240 | 68.8 (62.8-74.6) | 1.23 (1.04-1.45) | 0.02 | 1.09 (0.91-1.30) | 0.35 | |
| Hypertension | 506 | 9,238 | 214 | 58.7 (52.6-65.0) | 513 | 9,077 | 170 | 51.3 (45.1-57.7) | 1.30 (1.07-1.59) | 0.009 | 1.21 (0.98-1.49) | 0.07 | |
| Diabetes | 525 | 10,538 | 163 | 49.4 (43.1-56.1) | 529 | 10,090 | 149 | 45.1 (38.9-51.7) | 1.09 (0.88-1.36) | 0.42 | 1.06 (0.85-1.33) | 0.61 | |
| Obesity | 470 | 8,736 | 144 | 38.5 (32.9-44.7) | 486 | 8,695 | 114 | 33.6 (28.4-39.5) | 1.26 (0.99-1.61) | 0.06 | 1.14 (0.89-1.47) | 0.31 | |
| Cardiac arrhythmias | 523 | 10,564 | 146 | 37.7 (32.1-44.0) | 526 | 10,373 | 104 | 31.7 (26.3-37.8) | 1.41 (1.10-1.81) | 0.006 | 1.25 (0.96-1.62) | 0.10 | |
| Coronary artery disease | 530 | 11,345 | 75 | 21.7 (17.0-27.3) | 533 | 11,008 | 39 | 12.5 (8.7-17.8) | 1.97 (1.34-2.89) | <0.001 | 1.81 (1.21-2.72) | 0.004 | |
| Congestive heart failure | 533 | 11,806 | 28 | 7.6 (4.9-11.7) | 532 | 11,229 | 18 | 6.4 (3.8-10.8) | 1.49 (0.83-2.67) | 0.19 | 1.14 (0.62-2.11) | 0.68 | |
| Stroke | 533 | 11,684 | 32 | 8.9 (5.9-13.3) | 534 | 11,149 | 29 | 9.5 (6.6-13.5) | 1.06 (0.65-1.74) | 0.82 | 0.86 (0.50-1.49) | 0.60 | |
| Uterine prolapse | |||||||||||||
| Hyperlipidemia | 408 | 6,290 | 218 | 71.1 (63.9-78.0) | 415 | 6,189 | 177 | 67.9 (60.3-75.3) | 1.22 (1.01-1.47) | 0.04 | 1.16 (0.96-1.41) | 0.12 | |
| Hypertension | 388 | 6,264 | 193 | 69.4 (61.1-77.3) | 395 | 6,194 | 158 | 60.5 (53.2-67.9) | 1.17 (0.95-1.43) | 0.13 | 1.10 (0.89-1.35) | 0.39 | |
| Diabetes | 414 | 8,014 | 142 | 54.1 (46.6-62.0) | 412 | 7,375 | 105 | 45.3 (37.8-53.5) | 1.23 (0.97-1.57) | 0.09 | 1.14 (0.89-1.46) | 0.30 | |
| Obesity | 368 | 6,436 | 110 | 39.6 (32.9-47.0) | 393 | 6,838 | 72 | 29.2 (22.8-37.0) | 1.65 (1.23-2.22) | <0.001 | 1.59 (1.17-2.14) | 0.003 | |
| Cardiac arrhythmias | 416 | 8,011 | 135 | 48.4 (41.1-56.3) | 413 | 7,516 | 122 | 47.7 (40.4-55.5) | 0.95 (0.75-1.20) | 0.67 | 0.87 (0.68-1.11) | 0.26 | |
| Coronary artery disease | 420 | 8,717 | 81 | 27.1 (21.5-33.8) | 421 | 8,079 | 56 | 19.2 (14.6-25.0) | 1.29 (0.91-1.82) | 0.15 | 1.27 (0.89-1.81) | 0.18 | |
| Congestive heart failure | 425 | 9,176 | 35 | 11.7 (8.0-17.0) | 421 | 8,302 | 47 | 16.6 (12.3-22.2) | 0.62 (0.40-0.97) | 0.03 | 0.59 (0.38-0.92) | 0.02 | |
| Stroke | 425 | 9,084 | 45 | 14.9 (10.8-20.4) | 421 | 8,202 | 45 | 18.3 (13.6-24.4) | 0.86 (0.57-1.31) | 0.49 | 0.77 (0.50-1.18) | 0.22 | |
A total of 308 women with other indications for hysterectomy were not included in the stratified analyses.
Absolute cumulative risk at 30 years after hysterectomy (or index) calculated using the Kaplan-Meier method. The estimates were adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline, years of education (≤12, 13-16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). These adjustments were performed separately in each stratum to maximize the balance at baseline.
Hazard ratios calculated using Cox proportional hazards models with age as the time scale.
Hazard ratios calculated using Cox proportional hazards models with age as the time scale and adjusted using inverse probability weights derived from a logistic regression model including all 20 chronic conditions present at baseline, years of education (≤12, 13-16, >16, unknown), race (white vs nonwhite), and age and calendar year at baseline (continuous). These adjustments were performed separately in each stratum to maximize the balance at baseline.
Sensitivity analyses
When women with any of the 20 pre-existing chronic conditions at the index date were excluded, the risks of hyperlipidemia, obesity, cardiac arrhythmias, and CAD were increased in the hysterectomy with ovarian conservation group (n = 1,204) compared with referent women (n = 1,433; Supplemental Digital Content 4). Results stratified by age were similar to the results in the full cohort, and some of the HRs observed in the younger age stratum were particularly sizeable (2.3-fold increased risk for CAD and 3.5-fold for CHF). Hysterectomy for leiomyomas was associated with a 1.4-fold increase in cardiac arrhythmias, and hysterectomy for menstrual disorders with a 2.2-fold increase in CAD (Supplemental Digital Content 4). Sensitivity analyses censoring women at the time of subsequent oophorectomy (both women with hysterectomy and referent women) or hysterectomy (referent women) showed results similar to the primary analyses (data not shown).
DISCUSSION
This study showed HRs of de novo CVD and metabolic outcomes ranging between 1.13 and 1.33 for women who underwent hysterectomy even with conservation of both ovaries compared with referent women who did not have hysterectomy. The magnitude of the increase in CVD risk was similar after adjusting for 20 selected baseline chronic conditions, and after excluding women with previous CVD or metabolic conditions. For women age 35 years and younger at the time of hysterectomy with both ovaries conserved, the HR was 1.36 for cardiac arrhythmias, 2.49 for CAD, and 4.59 for CHF. The absolute risk increase was 10.1% for cardiac arrhythmias, 6.1% for CAD. and 4.6% for CHF over a 22 year follow-up. Fortunately, the conditions that showed a stronger association with hysterectomy were relatively rare in this younger age group (absolute risk among referent women = 0.3% for CHF and 7.1% for CAD). In addition, the number of women who developed CHF was small (n=19), and further studies are needed to replicate this finding.
Comparison with other studies
Our findings are similar to the population-based findings of Ingelsson et al., who showed an 18% increased risk of CVD after hysterectomy with ovarian conservation before age 50 years.13 Unlike their study, we were able to adjust our analyses for baseline CVD and metabolic risk factors such as hypertension and obesity, which we found to be higher among women undergoing hysterectomy. Yeh et al. also found an increase in CVD and stroke, but only for women <45 years at the time of surgery.14 However, the median follow-up time of seven years in that study may limit the comparability with our study.
Prior studies that compared women who underwent hysterectomy with bilateral oophorectomy to women who underwent hysterectomy with ovarian conservation elected to address a different question. They did not consider the risk of CVD outcomes due to hysterectomy alone.3,22 The Nurses’ Health Study (NHS) showed a 17% higher risk of coronary heart disease following hysterectomy with bilateral oophorectomy compared to women with hysterectomy and ovarian conservation.3 Our study demonstrates an additional 33% higher risk of CAD with removal of the uterus alone compared to women with no surgery. Similarly, Gierach et al. found an increasing step-wise trend in mortality from coronary heart disease for women who had no gynecological surgery (reference), hysterectomy alone (21% increase), or hysterectomy with bilateral oophorectomy (56% increase), if surgery was done at age ≤35 years.23 The choice of the referent group determines the specific question addressed by a cohort study. It may be useful in future studies to consider multiple levels of gynecological surgery compared to no gynecological surgery (e.g., removal of only the uterus, uterus and one ovary, uterus and both ovaries, only one ovary, only two ovaries, only two tubes, etc.).
Possible interpretation of the findings
As for any observational study, we cannot exclude that the observed associations could be explained by some residual bias or some yet unknown confounding variables.24 To the extent possible, we have removed or controlled the possible biases and confounders. If we hypothesize that the absolute risk differences observed are completely attributable to hysterectomy, that all confounding effects and biases have been removed, and that women are followed for 30 years, we can estimate the number needed to harm (NNH; defined as the inverse of the ARI). In women who underwent hysterectomy at age ≤35 years, the NNH was 10 for cardiac arrhythmias, 16 for CAD, and 22 for CHF (NNHs are not shown in tables).
There is growing evidence that hysterectomy with ovarian conservation increases the risk of future CVD, but the mechanisms remain unclear. To the best of our knowledge, the uterus does not produce any recognized endocrine factors that could directly impact the cardiovascular system. Therefore, the effects are probably mediated by the effects on the ovaries. One theory is that the loss of collateral blood flow to the ovaries caused by hysterectomy results in decreased ovarian reserve and its sequelae. Alternatively, the uterus itself could have a paracrine or endocrine effect on the ovaries.25 There is evidence that ovarian dysfunction is at least part of the mechanism because the symptoms of ovarian insufficiency may occur up to four years earlier in women who had hysterectomy with ovarian conservation.26 Also, Trabuco et al. demonstrated a significant reduction in antimüllerian hormone, a key marker of ovarian reserve, one year after hysterectomy with ovarian conservation.27
The risk of future CVD may be even higher if both patients and physicians assume that the ovarian function is sufficient following hysterectomy, and that hormone therapy is not required. Two studies have shown that women who had undergone hysterectomy with ovarian conservation were equally or less likely to be using hormone therapy than women with bilateral oophorectomy or women without hysterectomy.28,29 We did not have complete information concerning the use of hormonal therapy after the index date. This information was not available electronically in the REP until 1998. However, for a subsample of 792 women who underwent hysterectomy in 1998-2002, we were able to study hormone use. The overall hormone use following hysterectomy was infrequent; less than a quarter of women who underwent hysterectomy (22.8%) used estrogen alone or in combination with a progestogen compared with 12.6% of referent women (data not shown in tables). Therefore, the use of hormonal therapy was relatively low in Olmsted County even before the publication of the results from the Women’s Health Initiative clinical trials.30,31
The reasons why hysterectomy was associated with a significantly increased HR of CHF in the age ≤35 years stratum and with a significantly reduced HR of CHF in the 36 to 50 years stratum remain unclear. We can hypothesize that women in the 36 to 50 years stratum who were at high risk of CHF (e.g., long history of CAD or uncontrolled hypertension) were excluded from the surgery (confounding by pre-existing high-risk conditions).
Strengths and limitations
Our study design overcomes several of the limitations of prior studies. First, women were followed continuously both before and after the hysterectomy or index date. Therefore, there was no time gap between the hysterectomy and recruitment into the study (left censoring was minimized by design). Second, because the data collection was historical, women did not need to provide a study-specific informed consent but only a general research authorization (as per Minnesota legal requirements), thus minimizing non-participation (approximately 97% participation of women in the REP).18 Third, because women had been included in the REP for a median of 20.8 years (IQR 11.2-30.1) preceding the index date, this study better captures the CVD and metabolic conditions present at baseline.9,32 Fourth, all women had both ovaries conserved at baseline contrary to studies which included women with prior unilateral oophorectomy in the referent group.9,10,33–35
Fifth, we did not rely on the recall or the self-report of hysterectomy and oophorectomy. In a prior study of self-reported surgical data, 11% of women were misclassified as to their oophorectomy status.9 Finally, with more than 20 years of follow-up through the REP, we could detect more CVD events compared to two well-designed population-based studies of hysterectomy with ovarian conservation in which the follow-up was limited to ten or fewer years.13,14 As shown in Fig. 1 and 2, the length of follow-up is critical because hysterectomy occurs in relatively younger women, but CVD risk increases with age. The curves for CVD outcomes started to diverge only 10, 15, or 20 years after the hysterectomy.
Our study also has limitations. First, because the CVD and metabolic outcomes were detected through a passive follow-up system, we cannot exclude some difference in detection across the hysterectomy and referent cohorts (surveillance bias). However, the length of follow-up, the density of medical contacts, and the all-cause mortality were similar in the two cohorts. Second, in using the REP indexes to detect CVD and metabolic conditions before or after the index date, we may have missed some conditions that were not diagnosed. In addition, electronic indices may misclassify CVD or metabolic outcomes due to incorrect coding during routine medical care; however, missing data and misclassified diagnoses should not be differential in the hysterectomy and referent cohorts. In addition, to address misclassified diagnoses, we required two diagnostic codes to confirm non-fatal CVD and metabolic conditions. Third, despite the median follow-up of 22 years for both cohorts combined, our cohorts are relatively young to study mortality. To date, a total of 293 women with hysterectomy and 306 referent women have died. We plan to continue to follow the two cohorts for future analyses of mortality effects.
Fourth, our electronic indices did not include lifestyle variables such as physical activity or smoking and income level which may be associated with hysterectomy. We were able to partly adjust for socioeconomic status by including years of education and race in our models; however, we cannot exclude some residual confounding effects. Fifth, because we tested a number of associations, and some of them may not be independent, some of the findings may represent type 1 errors. Therefore, our results will need replication in independent samples. Sixth, Olmsted County is predominantly comprised of white women of European descent, similar to the population of the Upper Midwest of the United States; however, hysterectomy rates vary by region, and there is no one area that represents the entire country.36 Finally, approximately 82% of the hysterectomies in our study were vaginal. This percent may be different in other parts of the country. However, there is no evidence that the type of surgery would influence the outcomes considered.
CONCLUSIONS
Hysterectomy with ovarian conservation is associated with a significantly increased risk of several CVD and metabolic conditions, even after adjusting for CVD and metabolic conditions diagnosed before hysterectomy, and for several additional possible confounders. If these associations are causal, they have both scientific and clinical implications. From a research perspective, we hypothesize that the increased risk could be mediated at least in part through impaired ovarian function secondary to the surgery. Therefore, further studies are needed to clarify the direct effects of hysterectomy on ovarian function and subsequent clinical outcomes. From a clinical perspective, uterine-preserving treatments for heavy menstrual bleeding and leiomyomas should be considered.37 In addition, for women who need to undergo a hysterectomy, hormonal treatment should be offered or clearly considered.
Supplementary Material
Supplemental Digital Content 1. Figure with flow chart of the derivation of the study cohorts from previously defined broader cohorts. pdf
Supplemental Digital Content 2. Table with list of the 20 chronic conditions used in the study and corresponding ICD-9 codes. pdf
Supplemental Digital Content 3. Figure showing the balance of characteristics at baseline obtained using inverse probability weights (IPW). pdf
Supplemental Digital Content 4. Table that provides the cumulative incidence of cardiovascular and metabolic conditions among women who did not have any of the 20 chronic conditions at baseline, overall and in strata by age at hysterectomy and by indication. pdf
Acknowledgments
The authors thank Robin Adams for formatting the manuscript and Cathy Schleck for assistance with the creation of the cohort.
Funding/support: This study was supported by the Office of Research on Women’s Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Building Interdisciplinary Research Careers in Women’s Health (BIRCWH, K12 HD065987-2), the National Institute on Aging (R01 AG034676 and P50 AG044170), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD060503). WAR was partly supported by other grants from the National Institutes of Health (R01 AG052425, U01 AG006786, and P01 AG004875).
Footnotes
Financial disclosure/conflicts of interest: EAS receives funding from Bayer.
Presented at the Society for Gynecologic Investigation Annual Meeting, Florence, Italy, March 2014.
References
- 1.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110:1091–1095. doi: 10.1097/01.AOG.0000285997.38553.4b. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122:233–241. doi: 10.1097/AOG.0b013e318299a6cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mytton J, Evison F, Chilton PJ, Lilford RJ. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novetsky AP, Boyd LR, Curtin JP. Trends in bilateral oophorectomy at the time of hysterectomy for benign disease. Obstet Gynecol. 2011;118:1280–1286. doi: 10.1097/AOG.0b013e318236fe61. [DOI] [PubMed] [Google Scholar]
- 7.Asante A, Whiteman MK, Kulkarni A, Cox S, Marchbanks PA, Jamieson DJ. Elective oophorectomy in the United States: trends and in-hospital complications, 1998-2006. Obstet Gynecol. 2010;116:1088–1095. doi: 10.1097/AOG.0b013e3181f5ec9d. [DOI] [PubMed] [Google Scholar]
- 8.Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121:717–726. doi: 10.1097/AOG.0b013e3182887a47. [DOI] [PubMed] [Google Scholar]
- 9.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. Hysterectomy, oophorectomy, and heart disease risk factors in older women. Am J Public Health. 1997;87:676–680. doi: 10.2105/ajph.87.4.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falkeborn M, Schairer C, Naessen T, Persson I. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol. 2000;53:832–837. doi: 10.1016/s0895-4356(00)00187-6. [DOI] [PubMed] [Google Scholar]
- 11.Matthews KA, Gibson CJ, El Khoudary SR, Thurston RC. Changes in cardiovascular risk factors by hysterectomy status with and without oophorectomy: Study of Women’s Health Across the Nation. J Am Coll Cardiol. 2013;62:191–200. doi: 10.1016/j.jacc.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laughlin-Tommaso SK, Khan Z, Weaver AL, Schleck CD, Rocca WA, Stewart EA. Cardiovascular risk factors and diseases in women undergoing hysterectomy with ovarian conservation. Menopause. 2016;23:121–128. doi: 10.1097/GME.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32:745–750. doi: 10.1093/eurheartj/ehq477. [DOI] [PubMed] [Google Scholar]
- 14.Yeh JS, Cheng HM, Hsu PF, et al. Hysterectomy in young women associates with higher risk of stroke: a nationwide cohort study. Int J Cardiol. 2013;168:2616–2621. doi: 10.1016/j.ijcard.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 15.Blandon RE, Bharucha AE, Melton LJ, 3rd, et al. Incidence of pelvic floor repair after hysterectomy: a population-based cohort study. Am J Obstet Gynecol. 2007;197:664.e661–667. doi: 10.1016/j.ajog.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melton LJ, 3rd, Achenbach SJ, Gebhart JB, Babalola EO, Atkinson EJ, Bharucha AE. Influence of hysterectomy on long-term fracture risk. Fertil Steril. 2007;88:156–162. doi: 10.1016/j.fertnstert.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babalola EO, Bharucha AE, Schleck CD, Gebhart JB, Zinsmeister AR, Melton LJ., 3rd Decreasing utilization of hysterectomy: a population-based study in Olmsted County, Minnesota, 1965-2002. Am J Obstet Gynecol. 2007;196:214.e211–217. doi: 10.1016/j.ajog.2006.10.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca WA, Gazzuola-Rocca L, Smith CY, et al. Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc. 2016;91:1577–1589. doi: 10.1016/j.mayocp.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen JW, Cohen SB, Banthin JS. The medical expenditure panel survey: a national information resource to support healthcare cost research and inform policy and practice. Med Care. 2009;47:S44–50. doi: 10.1097/MLR.0b013e3181a23e3a. [DOI] [PubMed] [Google Scholar]
- 22.Jacoby VL, Grady D, Wactawski-Wende J, et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study. Arch Intern Med. 2011;171:760–768. doi: 10.1001/archinternmed.2011.121. [DOI] [PubMed] [Google Scholar]
- 23.Gierach GL, Pfeiffer RM, Patel DA, et al. Long-term overall and disease-specific mortality associated with benign gynecologic surgery performed at different ages. Menopause. 2014;21:592–601. doi: 10.1097/GME.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120:920–927. doi: 10.1097/AOG.0b013e31826af61a. [DOI] [PubMed] [Google Scholar]
- 25.Stewart EA. Gonadotropins and the uterus: is there a gonad-independent pathway? J Soc Gynecol Investig. 2001;8:319–326. [PubMed] [Google Scholar]
- 26.Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. Bjog. 2005;112:956–962. doi: 10.1111/j.1471-0528.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 27.Trabuco EC, Moorman PG, Algeciras-Schimnich A, Weaver AL, Cliby WA. Association of ovary-sparing hysterectomy with ovarian reserve. Obstet Gynecol. 2016;127:819–827. doi: 10.1097/AOG.0000000000001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeigler-Johnson CM, Holmes JL, Lassila HC, Sutton-Tyrrell K, Kuller LH. Subclinical atherosclerosis in relation to hysterectomy status in black women. Stroke. 1998;29:759–764. doi: 10.1161/01.str.29.4.759. [DOI] [PubMed] [Google Scholar]
- 29.Gavin KM, Jankowski C, Kohrt WM, Stauffer BL, Seals DR, Moreau KL. Hysterectomy is associated with large artery stiffening in estrogen-deficient postmenopausal women. Menopause. 2012;19:1000–1007. doi: 10.1097/gme.0b013e31825040f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 31.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 32.Ritterband AB, Jaffe IA, Densen PM, Magagna JF, Reed E. Gonadal function and the development of coronary heart disease. Circulation. 1963;27:237–251. doi: 10.1161/01.cir.27.2.237. [DOI] [PubMed] [Google Scholar]
- 33.Palmer JR, Rosenberg L, Shapiro S. Reproductive factors and risk of myocardial infarction. Am J Epidemiol. 1992;136:408–416. doi: 10.1093/oxfordjournals.aje.a116513. [DOI] [PubMed] [Google Scholar]
- 34.Luoto R, Kaprio J, Reunanen A, Rutanen EM. Cardiovascular morbidity in relation to ovarian function after hysterectomy. Obstet Gynecol. 1995;85:515–522. doi: 10.1016/0029-7844(94)00456-N. [DOI] [PubMed] [Google Scholar]
- 35.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 36.Borah BJ, Laughlin-Tommaso SK, Myers ER, Yao X, Stewart EA. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67–77. doi: 10.1097/AOG.0000000000001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laughlin SK, Stewart EA. Uterine leiomyomas: individualizing the approach to a heterogeneous condition. Obstet Gynecol. 2011;117:396–403. doi: 10.1097/AOG.0b013e31820780e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Figure with flow chart of the derivation of the study cohorts from previously defined broader cohorts. pdf
Supplemental Digital Content 2. Table with list of the 20 chronic conditions used in the study and corresponding ICD-9 codes. pdf
Supplemental Digital Content 3. Figure showing the balance of characteristics at baseline obtained using inverse probability weights (IPW). pdf
Supplemental Digital Content 4. Table that provides the cumulative incidence of cardiovascular and metabolic conditions among women who did not have any of the 20 chronic conditions at baseline, overall and in strata by age at hysterectomy and by indication. pdf


