Abstract
Objective
Implementing treat-to-target strategies require that rheumatoid arthritis (RA) patients and their rheumatologists decide on how best to escalate care when indicated. The objective of this study was to develop preference phenotypes to facilitate shared decision making at the point-of-care for patients failing methotrexate monotherapy.
Methods
We developed a conjoint analysis survey to measure RA patient preferences for triple therapy, biologics, and JAK inhibitors. The survey included seven attributes: administration, onset, bothersome side effects, serious infection, very rare side effects, amount of information, and cost. Each choice set (n=12) included three hypothetical profiles. Preference phenotypes were identified by applying latent class analysis to the conjoint data.
Results
1273 participants completed the survey. A 5-group solution was chosen based on progressively lower values of the Akaike and Bayesian information criteria. Members of the largest group (Group 3: 38.4%) were most strongly impacted by the cost of the medication. The next largest group (Group 1: 25.8%) was most strongly influenced by the risk of bothersome side effects. Members of Group 2 (11.2%) were also risk averse, but were most concerned with the risk of very rare side effects. Group 4 (6.6%) strongly preferred oral over parenteral medications. Members of Group 5 (18.0%) were most strongly and equally influenced by onset of action and the risk of serious infections.
Conclusions
RA patients' treatment preferences can be measured and represented by distinct phenotypes. Our results underscore the variability in patients' values and the importance of using a shared decision making approach to implement TTT.
Best practices for patients with rheumatoid arthritis (RA) call for patients to be treated-to-target (TTT). Adherence to this strategy requires ongoing disease activity monitoring and adjustments in treatment plans to attain, and subsequently maintain, a state of low disease activity or remission. TTT strategies are in large part possible because of the numerous effective treatment options currently available for patients with inflammatory arthritis. However, having many available options also paradoxically increases the difficulty of choosing how to adjust treatment.(1) Several studies have shown that increasing the number of options in a choice set significantly increases the difficulty of making a decision and increases the likelihood of deferral.(2, 3) Indeed, asking physicians to help patients compare and contrast triple therapy, different biologics, and JAK inhibitors, and to subsequently determine which option best fits with each patient's values and goals at the point-of-care is challenging. Consequently, patients are rarely effectively engaged in the decision making process.(4)
Decision aids have been developed for several preference sensitive decisions in order to facilitate shared decision making, and randomized controlled trials have proven them to be consistently effective in improving patients' knowledge, decreasing decisional conflict, and in some cases, improving patient participation in decision making.(5) Despite these proven benefits, however, decision aids have not been effectively integrated into clinical practice, in large part due to time constraints.(6) To address this gap, we sought to develop a decision aid which rather than asking each physician-patient dyad to consider the numerous trade-offs involved in comparing all available options, presents a set of (rigorously derived and transparent) distinct preference phenotypes and asks patients to consider which best fits with their own values and goals. Asking patients to perform a matching task is a simpler cognitive task that may be better suited to decision making at the point-of-care.
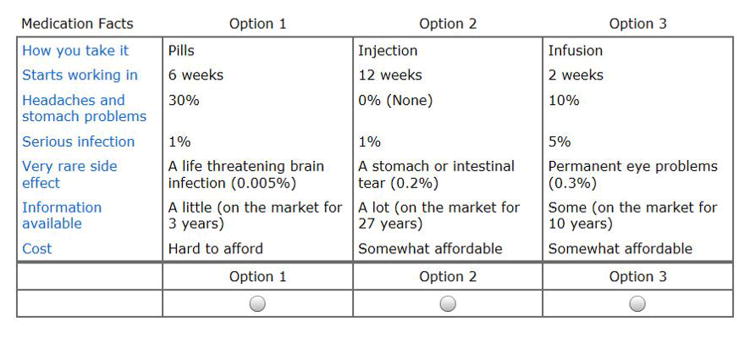
Conjoint analysis is a well-validated and widely used method to measure preferences. Originally developed to understand consumer preferences and predict market shares of innovative products, this approach is now recognized as a valuable means of assessing patient preferences for health care.(7-11) When faced with multiple alternatives, people make decisions by making trade-offs between the specific features of competing products. CA evaluates these trade-offs to determine which combination of attributes is most preferred by consumers. This approach assumes that each option is a composite of different characteristics, and that each characteristic represents one of a number of levels. Levels refer to the range of estimates for each characteristic. Respondents do not evaluate treatment alternatives directly. Rather, preferences are calculated based on how participants value differences between competing options. Answers to respondent-specific questions (see example Figure 1) allow the investigator to calculate values for specific treatment characteristics and to predict which option most closely suits each participant's individual preferences.
Figure 1. Example of a Choice Task.
If these were your only options, which would you choose?
Choose by clicking one of the buttons below. You can see more information by clicking on the medication facts in the left hand column.
Shared decision making is a key element of TTT because patients with the same level of disease activity have varying treatment preferences. Preference heterogeneity can be systematically examined via stratification or segmentation. Stratification separates study participants into homogeneous groups based on observed characteristics (e.g., demographics) and estimates either separate models or separate sets of coefficients for each strata. Stratification assumes that preference heterogeneity can be accurately determined a priori by observed variables; however, little empirical data support this assumption.(12, 13) In contrast, segmentation clusters respondents into groups based on unobserved/latent characteristics. Segmentation of conjoint data allows one to subdivide a large population into meaningful groups that are similar within themselves but statistically different from other groups. This approach has been successfully used to reveal varying patterns of preferences for public health interventions and drug development.(14-18)
The objective of this study was to develop a set of distinct preference phenotypes for use at the point-of-care by applying latent class analysis to preference data collected in a large group of RA patients in order to identify groups of patients whose values and preferences are similar to each other but distinct from other groups.
Methods
Participants and Recruitment Procedures
To be eligible, participants had to be at least 18 years of age (21 in Puerto Rico), speak English or Spanish, live in the U.S. or Puerto Rico, report having a diagnosis of RA made by a physician, and be taking one or more disease modifying antirheumatic drugs (DMARDs) and/or a biologic or JAK inhibitor. Those reporting being employed by pharmaceutical or insurance industries were excluded from the English language sample.
English speaking participants were recruited via email invitations to CreakyJoints (https://creakyjoints.org/) members who had previously identified themselves as having a diagnosis of RA. CreakyJoints is a large arthritis patient network of approximately 55,000 patients in all 50 states. At the time of enrollment (∼January 2016), about two-thirds of CreakyJoints members had RA. Among the CreakyJoints population who received the survey invitation and for whom demographic information was known, 90% were female, 80% were white, and the average age was 51 (SD 12). The most common conditions in the CreakyJoint community at the time of the survey were RA (67%), osteoarthritis (41%), osteoporosis (13%), psoriatic arthritis (11%) and ankylosing spondylitis (9%).
Spanish speaking participants were recruited in Spanish through a combination of Facebook ads on RA-related Facebook pages, and email invitations to Spanish-speaking RA patients through third-party respondent panel providers. Two research survey companies (Research Now and Market Cube) targeted U.S. residents of the 50 states plus Washington D.C. and Puerto Rico who had previously reported receiving a diagnosis of RA and were Spanish speakers. They also targeted Spanish speakers in the same regions with no known history of arthritis, and screened for diagnosis of RA. All CreakyJoints members and panelists agreeing to participate in the study were provided a unique survey link that allowed them to take the survey one time. Respondents recruited via Facebook were directed to a sign-up page on CreakyJoints.org and then emailed a unique link to participate in the survey. Since it was possible that an individual could sign up with multiple email addresses to receive more than one invitation/link, we de-duplicated by name and address. If someone took the survey twice, which occurred in two instances, we removed the duplicate response.
Survey Development
The survey was designed to enable patients to differentiate between triple therapy, biologics, and JAK inhibitors (i.e., options commonly considered after failing methotrexate monotherapy). An initial set of attributes and levels were developed by a rheumatologist (LF) in consultation with a patient partner (CW). The preliminary list of attributes and levels was subsequently revised based on feedback obtained during a focus group with ten RA patients. We ultimately included seven attributes: route of administration, onset of action, bothersome side effects, serious infection, very rare side effects, amount of information, and cost. Magnitude of benefit was not included as an attribute because efficacy was assumed to be equal across all options and thus not influence choice. Levels are listed in Table 1 and detailed descriptions of each level provided to participants at the beginning of the survey are included in the Appendix. We also included an instructional video which demonstrated how to complete the conjoint questions. We performed cognitive interviews with ten RA patients and revised the survey wording and instructions based on their feedback prior to study launch. After the initial English version was fielded, the survey was translated into Spanish and then back-translated into English. The survey was programmed and administered using Sawtooth Software, Inc.
Table 1. Attributes and Levels.
| Attributes | Levels |
|---|---|
| Route of administration | Pills |
| Injection | |
| Infusion | |
| Onset of action | 2 weeks |
| 6 weeks | |
| 12 weeks | |
| Bothersome side effects | 0% |
| 10% | |
| 30% | |
| Serious infection | 1% |
| 3% | |
| 5% | |
| Very rare side effects | Stomach or intestinal tear (0.2%) |
| Neurologic disease like multiple sclerosis (0.05%) | |
| Permanent eye problems (0.3%) | |
| Life threatening brain infection (0.005%) | |
| Amount of information available | A lot (on the market for 27 years) |
| Some (on the market for 10 years | |
| A little (on the market for 3 years) | |
| Cost | Easy to afford |
| Somewhat affordable | |
| Hard to afford |
We used the software's complete enumeration strategy to construct random choice sets. The complete enumeration method ensures that 1) each level is shown as few times as possible in a single task, 2) each level is shown approximately an equal number of times across the choice tasks, and 3) the level of one characteristic is chosen independently of the levels of other characteristics. Each subject answered 12 random choice sets. An example of one of the choice sets is provided in Figure 1. In addition, we included a fixed task in which the investigators defined the options in the choice set in order to gauge respondents' attention to the task. We also collected demographic and clinical characteristics. Participants were offered $25.00 after completing the survey.
Analyses
To examine the impact of each attribute on respondents' preferences we divided the range of utilities for each attribute by the sum of the ranges and multiplied by 100. Latent class analysis was used to classify subjects into mutually exclusive categories based on how they valued each medication characteristic.(19) Class solutions were replicated five times from random starting seeds. A 5-group solution was chosen based on progressively lower values of the Akaike and Bayesian information criteria.
We used Sawtooth Software's simulator to estimate preferences for four illustrative sets of treatment choices:
Triple therapy [onset of action 12 weeks, 30% risk of bothersome side effects, 1% risk of serious infection, risk of permanent eye problems (0.3%), easy to afford] versus a subcutaneous Anti-TNF biologic [onset of action 12 weeks, 10% risk of bothersome side effects, 3% risk of a serious infection, very rare risk of a neurologic disease like multiple sclerosis (0.05%), hard to afford]. Both options were described as having a lot of information available about them.
We then reran the preceding model but decreased the risk of bothersome side effects associated with triple therapy to 20%.
In a third simulation, we decreased the cost of the Anti-TNF biologic from “Hard” to “Somewhat” affordable.
Lastly, we estimated preference for an infusion [no risk of bothersome side effects, 3% risk of serious infection, risk of a life-threatening brain infection (0.005%), on the market for 10 years) versus a JAK Inhibitor (pills, 10% risk of bothersome side effects, 5% risk of serious infection, risk of stomach or intestinal tear (0.2%), on the market for 5 years].
Treatment preferences were generated using the randomized first choice model in which utilities are summed across the levels corresponding to each option and then exponentiated and rescaled so that they sum to 100. This model is based on the assumption that participants prefer the option with the highest utility (or value). The randomized first choice model accounts for the error in the point estimates of the utilities as well as the variation in each respondent's total utility for each option and has been shown to have better predictive ability than other models.(20) We examined associations between patient characteristics with group membership using ANOVA and chi-square tests for continuous and categorical variable respectively. The study was approved by the Yale Human Studies Research Program.
Results
Participants
1101 participants completed the survey in English. Of these 42 were eliminated because they completed the survey in under 10 minutes and an additional 52 people were excluded because they did not respond correctly to the attention check task. 421 participants completed the survey in Spanish. Of these 66 were eliminated because they completed the survey too quickly and an additional 89 people were excluded because they did not respond correctly to the attention check task.
The mean (SD) age of the study population (N=1273) was 50.7 (11.7). Participants' age ranged from 18 to 87 years. The majority were female (89.6%) and Caucasian (91.5%). Twenty-four percent were Hispanic. About half (51.9%) were college graduates, 46.3% reported an annual household income of $60K or greater, 50.5% were employed and 22.6% reported being on disability. All but 3.4% reported having either private or government insurance. The mean (SD) patient global score was 4.6 (2.3), and 46.3% reported having a fair or poor overall health status.
Relative Importance of Each Attribute
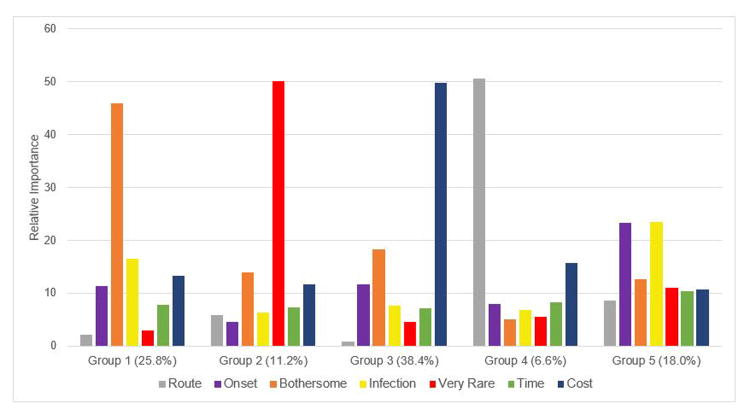
Table 2 lists the aggregate relative importance of each attribute. Given the levels included in the survey, affordability had the greatest impact on decision making followed by the probability of bothersome side effects. Variability in relative importances across clusters is illustrated in Figure 2. Members of the largest group (Group 3: 38.4%) were most strongly impacted by the cost of the medication. The next largest group (Group 1: 25.8%) was most strongly influenced by the risk of bothersome side effects. Members of Group 2 (11.2%) were also risk averse, but were most concerned with the risk of very rare side effects. Members of the smallest group (Group 4: 6.6%) strongly prioritized avoiding parenteral medications over the other medication characteristics included in the survey. Members of Group 5 (18.0%) were most strongly and equally influenced by onset of action and the risk of serious infections.
Table 2. Aggregate Relative Importances.
| Attribute | Mean (SD) Relative Importances |
|---|---|
| Cost | 24.66 (13.46) |
| Bothersome side effects | 20.73 (10.35) |
| Very rare side effects | 13.66 (9.03) |
| Onset of action | 11.50 (7.16) |
| Serious infection | 11.01 (6.68) |
| Route of administration | 10.66 (8.60) |
| Time on the market | 7.78 (4.79) |
Figure 2. Relative Importances per Cluster.
Grey: Route of Administration
Purple: Onset of Action
Orange: Bothersome side effects
Yellow: Risk of serious infection
Red: Risk of serious, but rare side effect
Green: Time available on the market
Dark Blue: Cost (Affordability)
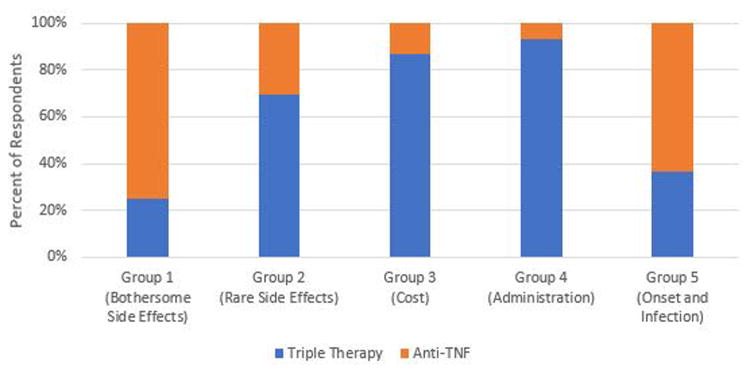
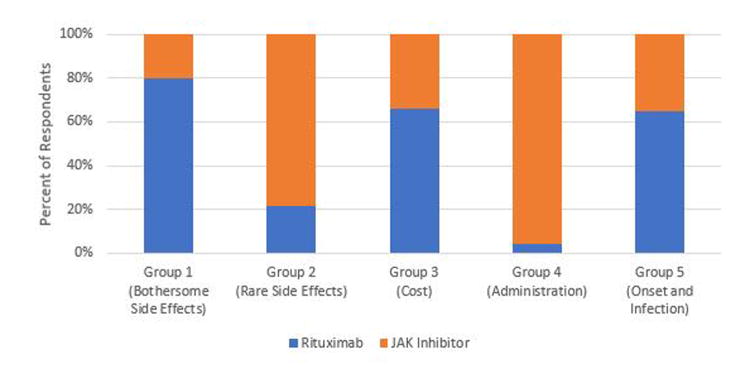
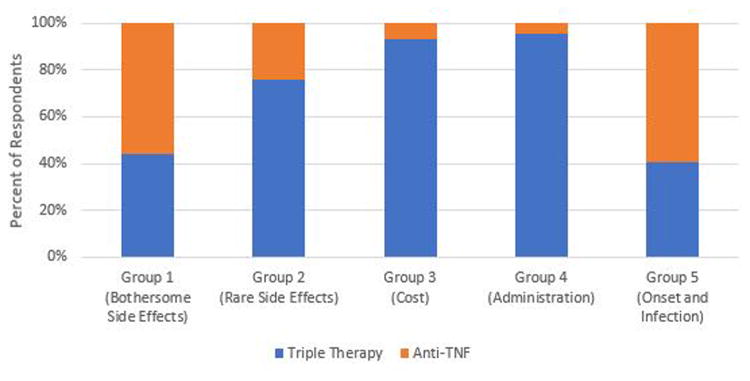
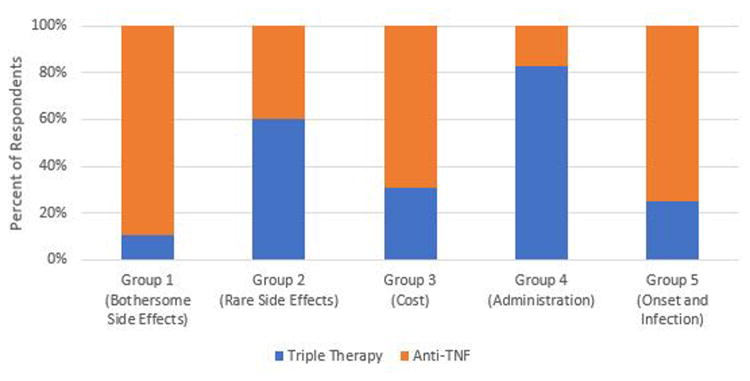
Illustrative examples of how the five preference phenotypes are related to treatment preference are provided in Figures 3 through 6. Preference for triple therapy versus a subcutaneous Anti-TNF biologic are described in Figure 3. Triple therapy is preferred by the majority of participants in Groups 2, 3, and 4. In contrast, those prioritizing avoiding bothersome side effects (Group 1) and rapid onset of action (Group 5) prefer the subcutaneous Anti-TNF. Biologics remain the preferred option for Groups 1 and 5 even when the risk of bothersome side effects associated with triple therapy is decreased (Figure 4). If, however, the cost of Anti-TNFs is assumed to be “Somewhat” instead of “Hard to afford”, biologics become the preferred option in members of Group 3 (who are most concerned with cost) (Figure 5). The impact of varying patient values on treatment preference is further illustrated in Figure 6 which describes preferences for rituximab versus a JAK Inhibitor. Members of Group 4 who prioritize route of administration (specifically strongly preferring oral versus parenteral medications) and those of Group 2 who are most concerned with the risk of very rare side effects (specifically are much less worried about the risk of an intestinal tear compared to the remote risk of progressive multifocal leukoencephalopathy) strongly prefer a JAK Inhibitor, whereas the remainder would choose rituximab.
Figure 3. Preferences for Triple Therapy vs Subcutanteous Anti-TNF.
Blue: Preference for Triple therapy
Orange: Preference for Anti-TNF
Figure 6. Preferences for Rituximab vs JAK Inhibitor.
Blue: Preference for Rituximab
Orange: Preference for JAK Inhibitor
Figure 4. Preferences for Lower Risk Triple Therapy vs Subcutaneous Anti-TNF.
Blue: Preference for Triple therapy
Orange: Preference for Anti-TNF
Figure 5. Preferences for Triple Therapy vs Less Costly Subcutaneous Anti-TNF.
Blue: Preference for Triple therapy
Orange: Preference for Anti-TNF
Associations between Group Membership and Participant Characteristics (Table 3)
Table 3. Patient Characteristics by Group.
| Characteristic | Group 1 (n=329) n (%) |
Group 2 (n=142) n (%) |
Group 3 (n=489) n (%) |
Group 4 (n=84) n (%) |
Group 5 (n=229) n (%) |
P Value |
|---|---|---|---|---|---|---|
| Female (n=1140) |
281 (85) | 129 (91) | 451 (92) | 80 (95) | 199 (87) | 0.006 |
| Caucasian (n=1165) |
307 (93) | 130 (92) | 455 (93) | 68 (81) | 205 (90) | 0.003 |
| Hispanic (n=311) |
86 (26) | 49 (35) | 74 (15) | 36 (43) | 66 (29) | <.0001 |
| Private Insurance (n=873) |
245 (75) | 86 (61) | 328 (67) | 57 (68) | 157 (69) | 0.04 |
| Patient Global (Mean, SD)* |
4.6 (2.3) | 4.4 (2.3) | 4.8 (2.1) | 4.0 (2.2) | 4.6 (2.4) | 0.02 |
Tukey's procedure demonstrated that the only paired comparison reaching statistical significance (p<0.05) was between Groups 3 and 4.
We found no significant differences in age, education, employment status, income, overall self-reported health status or current biologic use across groups (data not shown). Caucasians were less likely than non-Caucasians (6% versus 15%) while Hispanic subjects were more likely than non-Hispanics (12% versus 5%) to belong to Group 4 (prioritized oral over parenteral treatment). Female participants were more likely than males to belong to Group 3 (40% versus 29%) which prioritized cost, and less likely to belong to Group 1 (25% versus 36%) which prioritized avoiding bothersome side effects. The patient global score was significantly higher in Group 3 (prioritizing cost) than in Group 4 (prioritized oral treatment).
Discussion
In this study, we found that RA patients' treatment preferences can be measured and represented by distinct phenotypes. Our results underscore the variability in patients' values and the importance of using a shared decision making approach to implement TTT. Presenting patients with a range of phenotypes can facilitate shared decision making by 1) emphasizing that there is no single best option for patients with RA who continue to have moderate to high disease activity despite adequate trials of methotrexate, and 2) assisting them to clarify their concerns and preferences. For example, patients identifying with Group 1 are most concerned with bothersome side effects. They were much less concerned about the route of administration and the risk of very rare complications. Thus, how best to escalate care for these patients should focus on options which differ in the probability, and type, of bothersome side effects. In contrast, patients identifying with Group 2 are most concerned with very rare side effects, and for them sufficient time to differentiate between rare black box warnings as well underscoring the low probability of these events would best address patients' specific information needs. As previously reported (16, 21), we found that a small group of patients are reluctant to consider parenteral treatment. For some patients identifying with Group 4 a specific educational session with a nurse regarding parenteral therapy may be helpful prior to making a treatment decision whereas for others focusing on triple therapy or a JAK Inhibitor would be most appropriate. Patients with concerns matching those of Group 5 are those most concerned with onset of action and the risk of serious infections. An efficient personalized approach to shared decision making for these patients could focus on the how triple therapy, biologics and JAK Inhibitors differ across these two characteristics.
Despite being a mostly insured population, cost had the strongest influence on treatment preferences, with Group 3 being the largest cluster. Concerns over deductibles and expectations related to future cost increases are pervasive among RA patients. The importance attributed to cost highlights the need for rheumatologists to present comparative cost data to patients when discussing therapeutic alternatives. Unfortunately, out-of-pocket expenses differ from patient to patient (even among those with the same insurance plans) and obtaining these estimates at the point-of-care is generally not feasible.
While we used robust methods to measure preferences and elicited input from a large RA population (including representation of Spanish-speaking patients) there are also important limitations to this study. Patients recruited through a research panel and an online arthritis community do not represent a population-based sample. Moreover, diagnosis was ascertained based on self-report of RA and current use of a DMARD and/or biologic, and was not confirmed by medical record or claims data. In addition, our sample included few African American RA patients. The attributes and levels included in the study were chosen to reflect a broad range of medication characteristics; still, the results can only be generalized to those included in the survey. The impact of cost, for example would not be expected to be relevant to patients whose co-pays are affordable and do not differ across options. In addition, cost is likely to be of much less importance to patients outside of the U.S. with effective and stable drug coverage.
In summary, we developed distinct RA preference phenotypes by applying latent class analyses to conjoint data generated by a large number of English and Spanish speaking RA patients. Preferences examined in this study include those available to RA patients who have failed methotrexate monotherapy and are eligible for escalation to triple therapy, biologics or JAK Inhibitors. Future research will examine the feasibility of implementing a decision aid incorporating these phenotypes at the point-of-care.
Supplementary Material
Acknowledgments
The authors would like to thank all the patients who took the time to participate in this research project. We would also like to thank Seth Ginsberg and David Curtis for their continuous support.
Research reported in this publication was supported by the Rheumatology Research Foundation Innovative Research Grant program and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR060231-06 (Fraenkel). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The study was approved by the Yale Human Studies Research Program.
Raw deidentified will be shared once resulting manuscripts from this grant have been published.
Footnotes
The authors do not have any conflicts of interest related to the content of this manuscript.
None of the authors have any competing interests to disclose and all authors meet criteria for authorship.
References
- 1.Schwartz B. The paradox of choice: Why more is less. Revised. New York: Harper Collins; 2016. [Google Scholar]
- 2.Iyengar SS, Lepper MR. When choice is demotivating: Can one desire too much of a good thing? J Person Soc Psychol. 2000;79:995–1006. doi: 10.1037//0022-3514.79.6.995. [DOI] [PubMed] [Google Scholar]
- 3.Shah AM, Wolford G. Buying behavior as a function of parametric variation of number of choices. Psychol Sci. 2007;18:369–70. doi: 10.1111/j.1467-9280.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- 4.Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368:6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]
- 5.Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Of Systematic Reviews. 2017;4 doi: 10.1002/14651858.CD001431.pub5. Cd001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravel K, Legare F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: A systematic review of health professionals' perceptions. Implementation Sci. 2006;1:16. doi: 10.1186/1748-5908-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauber BA, Fairchild AO, Johnson FR. Quantifying benefit-risk preferences for medical interventions: An overview of a growing empirical literature. Appl Health Econ Health Policy. 2013;11:319–29. doi: 10.1007/s40258-013-0028-y. [DOI] [PubMed] [Google Scholar]
- 8.Bridges JFP. Stated preference methods in health care evaluation: an emerging methodological paradigm in health economics. Appl Health Econ Health Policy. 2003;2:213–24. [PubMed] [Google Scholar]
- 9.Bridges JFP, Onukwugha E, Johnson FR, Hauber AB. Patient preference methods - A patient-centered evaluation paradigm. ISPOR Connections. 2007;13:4–7. [Google Scholar]
- 10.Fraenkel L, Bogardus ST, Wittink DR. Understanding patient preferences for the treatment of lupus nephritis with adaptive conjoint analysis. Med Care. 2001;39:1203–16. doi: 10.1097/00005650-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–3. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iragüen P, de Dios Ortúzar J. Willingness-to-pay for reducing fatal accident risk in urban areas: An Internet-based Web page stated preference survey. Accident Anal Prev. 2004;36:513–24. doi: 10.1016/S0001-4575(03)00057-5. [DOI] [PubMed] [Google Scholar]
- 13.Morey E, Greer Rossmann K. Using stated-preference questions to investigate variations in willingness to pay for preserving marble monuments: Classic heterogeneity, random parameters, and mixture models. J Cult Econ. 2003;27:215–29. [Google Scholar]
- 14.Cunningham CE, Bruce BS, Snowdon AW, et al. Modeling improvements in booster seat use: a discrete choice conjoint experiment. Accident Anal Prev. 2011;43:1999–2009. doi: 10.1016/j.aap.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Deal K. Segmenting patients and physicians using preferences from discrete choice experiments. Patient. 2014;7:5–21. doi: 10.1007/s40271-013-0037-9. [DOI] [PubMed] [Google Scholar]
- 16.Fraenkel L, Suter L, Cunningham CE, Hawker G. Understanding preferences for disease modifying drugs in osteoarthritis. Arthritis Care Res (Hoboken) 2014;66:1186–92. doi: 10.1002/acr.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waschbusch DA, Cunningham CE, Pelham WE, et al. A discrete choice conjoint experiment to evaluate parent preferences for treatment of young, medication naive children with ADHD. J Clin Child Adolesc Psychol. 2011;40:546–61. doi: 10.1080/15374416.2011.581617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraenkel L, Lim J, Garcia-Tsao G, Reyna V, Monto A, Bridges JF. Variation in treatment priorities for chronic hepatitis C: A latent class analysis. Patient. 2016;9:241–9. doi: 10.1007/s40271-015-0147-7. [DOI] [PubMed] [Google Scholar]
- 19.CBC Latent Class Technical Paper. Sequim, WA: Sawtooth Software; [cited 2017 August 8]. Available from: https://www.sawtoothsoftware.com/support/technical-papers/sawtooth-software-products/cbc-latent-class-technical-paper-2004. [Google Scholar]
- 20.Huber J, Orme B, Miller R. The value of choice simulators In: Gustafsson A, Herrmann AF, editors Conjoint measurement. Fourth. New York: Springer; 2007. pp. 347–62. [Google Scholar]
- 21.Constantinescu F, Goucher S, Weinstein A, Smith W, Fraenkel L. Arthritis Care Res. Vol. 61. Hoboken: 2009. Understanding why rheumatoid arthritis patient treatment preferences differ by race; pp. 413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.