Abstract
It is assumed that platelets in diseased conditions share similar properties to platelets in healthy conditions, though this has never been examined in detail for myocardial infarction (M.I.). We examined platelets from patients with ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) compared to platelets from healthy volunteers in order to evaluate for differences in platelet phenotype and function. Platelet activation was examined and post-receptor signal transduction pathways were assessed. Platelet-derived plasma biomarkers were evaluated by Receiver Operator Characteristic (ROC) analyses. Maximum platelet activation through the thromboxane receptor was greater in STEMI compared to NSTEMI but less through PAR1. Extracellular-signal related-kinase 5 (ERK5) activation which can activate platelets was increased in platelets from subjects with STEMI and especially in platelets from patients with NSTEMI. Matrix metalloproteinase 9 (MMP9) protein content and enzymatic activity were several-fold greater in platelets with M.I. compared to control. Mean plasma MMP9 concentration in patients with M.I. distinguished between STEMI and NSTEMI (AUC 75% [C.I. 60–91], p=0.006) which was superior to troponin T (AUC 66% [C.I. 48–85, p=0.08), predicting STEMI with sensitivity 80% (95% C.I. 56–94), specificity 90% (C.I. 68–99), AUC 70% (C.I. 54–86, p<0.0001), and NSTEMI with sensitivity 50% (C.I. 27–70), specificity 90% (C.I. 68–99), AUC 70% (C.I. 54–86, p=0.03). Platelets from patients with STEMI and NSTEMI show differences in platelet surface receptor activation and post-receptor signal transduction, suggesting the healthy platelet phenotype in which anti-platelet agents are often evaluated in preclinical studies is different from platelets in patients with M.I.
INTRODUCTION
More than fifty years ago, it was discovered that aspirin inhibits platelet activation by preventing platelet thromboxane synthesis and adenosine diphosphate (ADP) secretion — mechanisms which amplify platelet activation via surface receptors [1–3]. Based upon those discoveries, dual anti-platelet therapy (DAPT), consisting of aspirin and a P2Y12 receptor antagonist are the standard of care in the treatment of STEMI and NSTEMI [4–6].
Failure of platelet antagonists to have their anticipated effect in some patients has been attributed to single nucleotide polymorphisms (SNPs) in genes encoding proteins responsible for metabolism of P2Y12 antagonists [7, 8], for cyclooxygenase enzymatic activity, in aspirin transporters, and in aspirin absorption [9–11]. These studies all suggest a personalized approach to antiplatelet therapy should be considered when a patient’s treatment trajectory deviates from the expected path. Longitudinal studies acknowledge that patients with STEMI have enjoyed improved outcomes over the last two decades with advances in percutaneous intervention without much change in mortality for NSTEMI [12]. A recent study indicates patient mortality for NSTEMI has failed to improve since 2010 in spite of delivery of care consistent with established guidelines, including appropriate utilization of percutaneous revascularization procedures [13, 14].
Reasons for this difference in long-term mortality observed in patients following STEMI compared to NSTEMI may include fundamental differences in platelet biology for each condition. Whether circulating platelets in diseased conditions are phenotypically and functionally similar to platelets in healthy subjects is an under-explored area of investigation [15, 16]. We and others reported that platelet ERK5 alters the platelet phenotype in murine models of thrombotic and ischemic disease and in platelets exposed to redox stress [17, 18]. Hu et al. recently demonstrated that platelet receptor and post-receptor signaling change in diabetic platelets with consequent resistance to clopidogrel [19]. These studies suggest greater attention should be paid to changes in platelet post-receptor signal transduction pathways in diseased conditions.
Examining the true platelet phenotype in CAD is an extraordinarily difficult task given that coronary instrumentation and non-uniform blood collection techniques alter platelet function. Following M.I., patients are placed on aspirin and a P2Y12 receptor antagonist making it impossible to assess contributions of individual platelet receptor signaling pathways. In an effort to address this limitation, we isolated platelets from patients with M.I. following aspirin monotherapy, and prior to the patient undergoing coronary angiography or receiving a loading dose of a P2Y12 receptor antagonist. We carefully examined platelet receptor signaling and platelet biomarkers in M.I., compared to platelets isolated from healthy individuals. The goal was to assess the true functional contribution of platelet receptors for which oral antiplatelet medications are available. Using this information, we attempted to evaluate whether the platelet phenotype in STEMI differs from NSTEMI, offering a possible mechanistic explanation for differences in long-term outcomes.
MATERIALS AND METHODS
STUDY POPULATION AND DESIGN
This study was approved by the University of Rochester Research Subjects Review Board. The recruitment, consent, and blood collection procedures are as follows: a STEMI alert is triggered in our institution by acute ST-segment elevation in two contiguous leads of the 12-lead ECG in the ambulance or immediately upon arrival in the ED. Delayed consent was granted for patients presenting with STEMI in order to avoid interfering with the door-to-balloon time. Each subject had four plasma citrate tubes of venous blood drawn within 10 minutes of arriving in the emergency department and before P2Y12 antagonists were administered. This first sample was used for platelet isolation and patients were consented within 24 hours of blood draw following coronary angiography. The sample was discarded if the patient declined to enroll. For patients with NSTEMI, subjects were already in the ED or hospital for 1–24 hours and identified by elevated plasma cardiac troponin (plasma value greater than the 99th percentile of a healthy population with a 10% assay CV) with concomitant symptoms consistent with cardiac chest pain. Patients with NSTEMI were approached for consent prior to P2Y12 antagonists being given or coronary angiography being conducted. Each patient was treated with 325 mg aspirin in the ambulance, in the ED, or hospital at least 30 minutes prior to blood draw. We excluded any patient who could not provide consent, if the patient died, and patients with thrombocytopenia, anemia, or active hematologic malignancies. We also excluded patients who were administered a loading dose of a P2Y12 receptor antagonist in the pre-hospital setting or the ED. Healthy volunteer subjects were enrolled by answering posted documents at the University of Rochester, and were free from anti-platelet medications. We enrolled 60 subjects: 20 healthy subjects aged 20–76 and 40 patients with acute M.I. aged 29–86 (20 with STEMI, 20 with NSTEMI). Patient demographics were recorded in a confidential manner. Platelet function was studied within 1 hour of blood draw. To enhance the clinical relevance of this study, we compared the summed agonist dose-response curves only for platelet signaling pathways for which oral antagonists are available (aspirin for cyclooxygenase inhibition which decreases platelet thromboxane release, U46619 is the agonist used in this study; clopidogrel, ticagrelor, and prasugrel for P2Y12 receptor inhibition, 2-methyl-ADP [ADP] is the agonist used in this study; vorapaxar for PAR1 inhibition, Thrombin Receptor Activator Peptide 6 [TRAP] is the agonist used in this study.
PLATELET FUNCTION
For each subject, blood was drawn by a medical professional into citrate plasma tubes, then centrifuged in a tabletop centrifuge at 1100 rpm for 15 mins. The platelet rich plasma (PRP) well above the buffy coat was decanted and the final platelet centrifugation step at 2600 rpm for 5 mins was conducted with a final concentration of 10 μM PGI2. The final washed platelet pellet from one human plasma citrate tube was resuspended in 1000 μL of fresh Tyrode’s solution which was diluted 1:20 in fresh Tyrode’s solution. This was aliquoted into 100 μL quadruplicate platelet samples and stimulated with 1 μL of each drug concentration. After 15 minutes, 1 μL of labeled CD62P (p-selectin) antibody was incubated in the dark for 30 minutes. This reaction was then stopped by adding 100 μL of 2% formalin to each reaction, and then quantification of platelet surface P-selectin was made possible using an Accuri Flow Cytometer (BD Biosciences) at 10K events per sample. Data was then processed through FloJo (Ashland, Oregon). Platelet surface p-selectin was quantified in quadruplicate using the geometric mean, then expressed as fold change from baseline to account for differences in experimental condition. For each concentration of drug for each patient, the sample was run in quadruplicate (baseline, four increasing doses of agonist=20 samples per agonist) for each of three agonists per patient for one patient encounter.
REAGENTS
TRAP6 (Cayman Chemicals), 2-methyl-ADP (Tocris, Bristol, UK), U46619 (Cayman Chemical), gelatin (Fisher Scientific). ERK5 antibody #3372, p-ERK5 antibody #3371, MMP9 antibody #3852, CD41 antibody #13807, CD45 antibody #13917(all from Cell Signaling Technology), actin antibody #612657 (BD Transduction Labs), TIMP1 antibody #ab38978 (Abcam), GAPDH antibody #sc-25778 (Santa Cruz Biotech.). CD62P-PE antibody Clone AK4 # 12-0628-62 (eBioscience/ThermoFisher Waltham, MA). CD41-FITC antibody #303703 (BioLegend, San Diego, CA). CD45-PE antibody #12-9459-42 (eBioscience/ThermoFisher Waltham, MA). Anti-mouse and anti-rabbit secondary antibody (GE healthcare, UK).
BIOCHEMISTRY
Platelet protein studies
Cell lysis and cell protein extraction, SDS PAGE, and Western blotting were conducted using buffers and techniques as described previously [17]. Blocking buffer was 3% BSA (Sigma Aldrich)) dissolved in Tris-buffered saline at pH 8.0 (Fisher Scientific) with 0.1% Tween-20 (TBS-T) at room temperature for 1 hour. Primary antibody was 1:1000 overnight at 4 °C in 3% BSA/TBS-T. Secondary antibody (GE Healthcare, Buckinghamshire, UK) was used in a 1:2000 titer in 5% milk/TBS-T for 1 hour at room temperature. ECL reagent used was Supersignal West Pico (Thermo Scientific) for each antibody. Final autoradiographic films (Bioblot BXR, Laboratory Product Sales, Rochester NY) were quantified by densitometry using ImageJ software (NIH).
MMP activity assay
Platelets were isolated as described above and placed in 50% vol/vol 2x non-denaturing sample buffer at the following final concentration: Tris- HCl 250 mM, 0.5% SDS, 1% glycerol, 0.05% bromophenol blue for 10 minutes without boiling and separated by SDS-PAGE (12% bis- acrylamide containing 1mg/mL final concentration gelatin within the matrix) at 125V (constant voltage, room temperature). The gel was renatured by gently rocking in 2.5% Triton-X-100 for 30 mins at room temperature, then decanting, and incubating in fresh zymogram buffer for 12 hours overnight at 37°C. The zymogram buffer was decanted, and the gel was rocked at room temperature for 4 hours in Simply Blue Safestain (Invitrogen). MMP activity was noted by clear bands in the final gel (a reverse image). Total MMP activity in each lane was quantified by densitometry using ImageJ software (NIH).
Immuno-depletion of white blood cells from PRP
PRP was obtained as above, with quality control studies taken to stay above the buffy coat in order to decrease the number of white blood cell (WBC) contaminants. We used a FITC-tagged CD41 antibody as a platelet-specific marker and a PE-tagged CD45 antibody as a WBC-specific marker, using flow cytometry for isolated platelet analysis. The final step of each PRP isolate for agonist stimulation was 100 μL in volume with a mean platelet count of 9954 ± 5 and a mean WBC count of 12 ± 1 (average WBC contamination of PRP=0.12%). For quality control, the final PRP isolate was divided into two equal volumes with one half incubated with a human anti-CD45 antibody to deplete residual WBCs using the MagniSort™ Human CD45 Depletion Kit (Invitrogen # 8804-6802-74) according to the manufacturer’s instructions. A THP1 cell line which is known to contain MMP9 and CD45 was used as a positive control for WBCs [20, 21]. We noted CD45 but not CD41 immunoreactivity in THP1. We normalized platelet MMP9 protein content to immunoreactive CD41 in CD45-depleted, CD45 (+), and control IgG non-depleted, CD45 (-), PRP. We observed a slight but not significant decrease in platelet MMP9 immunoreactivity (MMP9/CD41 ratio 1.9 ± 0.51 in depleted vs. 2.9 ± 0.86 in non-depleted PRP, P=0.36.
IMMUNOASSAY
Enzyme-linked immunosorbent assays (ELISAs) were conducted according to the manufacturer’s instructions using the sandwich ELISA technique. The ELISAs for plasma MMP9 (cat #DY911) and TIMP1 (cat# DY970-05) were purchased from R&D Systems (Minneapolis, MN). The ELISA for plasma thromboxane B2 (cat# 501020) was purchased from Cayman Chemicals.
STATISTICAL ANALYSIS
Data were analyzed by using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA). Dichotomous clinical variables are presented as frequencies. Continuous clinical variables are presented as mean with standard error of the mean (SEM) unless otherwise stated. Differences between groups were assessed with ANOVA for repeated measurements. Where pairwise comparisons between the baseline and experimental condition could be made, a t-test was used. Plasma biomarker data was evaluated by ROC curve analysis with performance of the biomarker for predicting the clinical condition reported as Area Under Curve (AUC), sensitivity, and specificity with 95% confidence interval.
RESULTS
Baseline Population
The general characteristics of the subjects in this investigation are shown in Table 1. Demographic data, clinical variables, and cardiovascular co-morbidities were similar between patients with STEMI and NSTEMI with the notable exception that patients with STEMI had a greater peak cardiac troponin plasma concentration.
Table 1. Patient Demographics.
40 subjects with M.I. were studied (20 STEMI, 20 NSTEMI).
| STEMI (n=20) | NSTEMI(n=20) | |
|---|---|---|
| Sex(M/F) | 14/6 | 13/7 |
| Age (years±sem) | 67 ±3 | 67 ± 3 |
| Race (C/B/A) | 13/6/1 | 18/2/0 |
| Diabetes (n) | 10 | 6 |
| Dyslipidemia (n) | 12 | 13 |
| Hypertension (n) | 16 | 17 |
| Tobacco Use (n) (Current/Former/No) | 4/7/9 | 2/4/6 |
| CKD (No/1/2/3/4) | 0/6/9/4/1 | 6/1/7/6/0 |
| Regular Aspirin Use (n) | 9 | 8 |
| Regular P2Y12 antagonist Use (n) | 3 | 1 |
| Anticoagulant Use (n) | 2 | 1 |
| Morphine administration in ED (n) | 6 | 3 |
| Platelet Count (mean × 103 ± sem) | 219 ± 18 | 210 ± 14 |
| First LVEF after PCI (%) | 47 ± 4.9 | 51 ± 4 |
| First cTnT (ng/mL ± sem) | 0.67 ± 0.45 | 0.46 ± 0.17 |
| Peak cTnT (ng/mL, mean ± sem) | 1.87 ±0.7 | 0.69 ± 0.2* |
| [LDL] (mg/dL, mean ± sem ) | 110 ± 10 | 99 ± 10 |
| [HDL] (mg/dL, mean ± sem ) | 47 ± 3 | 44 ± 3 |
| Statin Use (n) | 11 | 11 |
Patient demographics are noted. The first venous blood sample was used for most analyses.
p=0.03, otherwise no significant difference in continuous variables between groups.
For race: C=Caucasian, B=black, A=Asian. CKD=chronic kidney disease. LVEF=left ventricular ejection fraction. cTnT=cardiac troponin T. LDL=low density lipoprotein. HDL=high density lipoprotein.
Platelet Function at the time of STEMI is different from NSTEMI
Using a well-validated method of flow cytometry to detect platelet surface p-selectin expression as an index of platelet activation [22], we observed a wide range of individual platelet responses to surface receptor agonists for patients presenting with STEMI and NSTEMI in spite treatment with 325 mg aspirin at least 30 minutes before blood draw. For comparison, healthy volunteer agonist dose-responses for platelet surface receptors are shown on the same graphs (Online Fig. I). We found no difference in P2Y12 receptor signaling at any agonist concentration between platelets from patients with STEMI compared to NSTEMI (Fig. 1A). The platelet thromboxane receptor signaling pathway was more activated in patients presenting with STEMI (Fig. 1B) while the platelet PAR1 signaling pathway was more activated in patients presenting with NSTEMI (Fig. 1C). Using a transient thromboxane metabolite (thromboxane B2, TxB2) as an indirect plasma marker of cyclooxygenase (COX) activity, patients with STEMI and NSTEMI showed a similar plasma concentration of TxB2, suggesting factors that may alter aspirin absorption such as morphine and changes in autonomic activity at the time of M.I. were similar between groups (Online Fig. II).
Fig. 1. Platelet reactivity in patients with STEMI is different from NSTEMI.
All patients were given 325 mg aspirin at least 30 minutes prior to blood draw. Platelets were isolated and examined basally (0) or after stimulation with agonists for (A) the P2Y12 receptor (ADP), (B) the thromboxane receptor (U46619), and (C) Protease-Activated Receptor 1 (PAR1) for 15 mins and activation assessed by FACS by P-selectin expression, Mean Fluorescence Intensity (MFI) ± SEM, all performed in quadruplicate in each group, n=12–17. * p< 0.05 and **P<0.01 between STEMI and NSTEMI.
Post-receptor signaling in platelets differs in patients with STEMI compared to NSTEMI
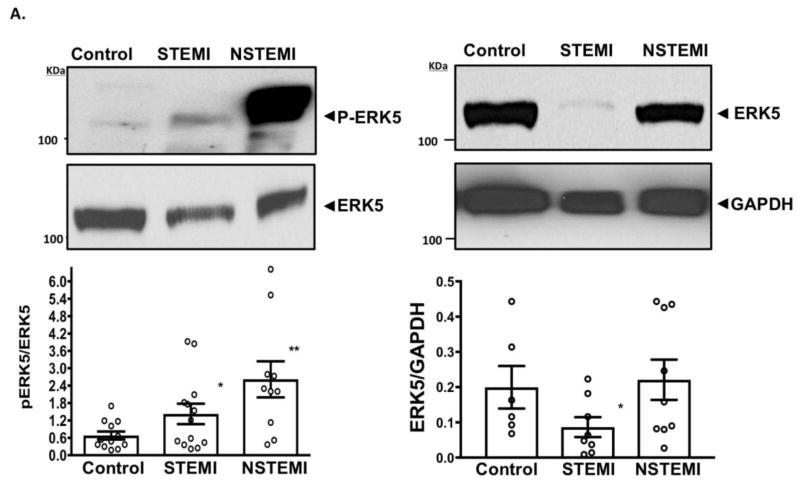
Consistent with a prior report in mice that platelet ERK5, a redox-activated kinase, promotes dysregulated platelet activity in a murine model of myocardial infarction[17], we observed that ERK5 activation occurs in human platelets at the time of M.I. Using a phospo-specific antibody which detects the ERK5 activation motif, a mild increase (2.2-fold over baseline) in platelet ERK5 activation was noted in patients presenting with STEMI and a marked increase (4.4-fold over baseline) in NSTEMI. We also observed a concomitant 2.2-fold decrease in platelet ERK5 protein expression in patients presenting at the time of STEMI compared to NSTEMI, likely explaining the difference in activation between groups (Fig. 2A). Using an inhibitor of ERK5, we could dose-dependently inhibit agonist-induced platelet activation in healthy platelets which was slightly more efficacious in platelets taken from patients with NSTEMI (Fig. 2B).
Fig. 2.
Fig. 2A. Platelet ERK5 Activation in STEMI and NSTEMI: All patients were given 325 mg aspirin at least 30 minutes prior to blood draw. Platelets were isolated from healthy volunteer subjects (control) or from patients with STEMI or NSTEMI. ERK5 activation was assessed by Western blotting using a phospho-specific antibody (P-ERK5). A pan-ERK5 antibody was used to detect total ERK5 and the ratio (P-ERK5/ERK5) was used to report ERK5 activity as mean ± SEM,. *p<0.05 control vs. STEMI, **p=0.01 control vs NSTEMI, n=10–13. GAPDH was used as a loading control for normalizing ERK5 expression, * P<0.05 vs.* control, n=6–9. The molecular weight is indicated in kiloDaltons (kDa).
Fig. 2B. ERK5 Inhibition Prevents Platelet Activation: All patients were given 325 mg aspirin at least 30 minutes prior to blood draw. Platelets were isolated from healthy volunteer subjects (control, white) or from patients with NSTEMI (black) and incubated with BIX 02189 (0–100 μM, 30 mins) prior to platelet stimulation with TRAP6 (10μM, 15 mins). Platelet activation was assessed by FACS by P-selectin expression, Mean Fluorescence Intensity (MFI) ± SEM, all performed in quadruplicate in each group, n=4. * p< 0.05 between NSTEMI/TRAP6 and NSTEMI/TRAP6 + BIX 02189 and **P<0.05 control/TRAP6 and control/TRAP6 + BIX 02189.
Platelets are enriched in matrix metalloproteinases (MMPs) [23]. MMPs may be important for platelet signaling and tissue remodeling at remote sites, including myocardial infarct extension and myocardial rupture [17, 24]. MMP9 is enriched in the coronary sinus of patients following M.I. [25, 26] and has been implicated in deleterious cardiac remodeling in murine models of M.I. [17, 27] while platelet-mediated MMP2 release was reported to occur irrespective of aspirin treatment [28]. Supporting previous studies, we observed that healthy human platelets express only a negligible quantity of MMP9 protein [29, 30] though MMP9 protein content in platelets persisted after accounting for the possibility of occasional white blood cell contaminants in PRP (Online Fig. III). We observed a 3-fold upregulation in MMP9 protein expression in platelets from patients with STEMI and NSTEMI (Fig. 3A) as further evidence of a different platelet phenotype compared to healthy conditions. Interestingly, MMP9 activity by zymography was 9-fold greater in STEMI but only 3-fold greater in NSTEMI compared to control platelets (Fig. 3B). We simultaneously observed the reciprocal trend of less tissue inhibitor of MMP protein 1 (TIMP1) protein expression in platelets from patients with STEMI and NSTEMI compared to control (0.46 ± 0.16, 0.47 ± 0.1, and 0.97 ± 0.17, respectively) (Fig. 3C). Finally, we detected MMP9 activity in more plasma samples from patients with STEMI than NSTEMI which corresponded with our observation in platelets (Fig. 4A–B).
Fig. 3. Platelet Biomarker Evaluation in STEMI and NSTEMI.
All patients were given 325 mg aspirin at least 30 minutes prior to blood draw. Platelets were isolated from healthy volunteer subjects (control) or from patients with STEMI or NSTEMI. (A) MMP9 protein content was assessed by Western blotting. (B) MMP activity was assessed by gel zymography. (C )TIMP1 protein expression was assessed by Western blotting. GAPDH is a loading controls. Protein expression or MMP activity are represented was mean ± SEM (n=5–19). *p<0.05 vs control, ** p<0.01 vs control. The molecular weight is indicated in kiloDaltons (kDa). MMP=matrix metalloproteinase. TIMP=tissue inhibitor of MMP.
Fig. 4. Plasma MMP Activity in STEMI and NSTEMI.
All patients were given 325 mg aspirin at least 30 minutes prior to blood draw. Plasma was isolated and (A) MMP activity was assessed by gel zymography and (B) displayed graphically as % active MMP9 detected in plasma (n=15 samples for STEMI, n=9 samples for NSTEMI). (C) Plasma, platelets, or coronary thrombus were examined by zymography in the same patient with an acute inferior STEMI attributed to a right coronary artery (RCA) aspirated thrombus. MMP9 appears to be the most active gelatinase in these samples.
Platelet-derived biomarkers in the plasma predict M.I
Examining plasma, isolated platelets, and aspirated intracoronary thrombus at the time of coronary intervention in a patient with an inferior STEMI, we observed multiple enzymatically-active gelatinases, though MMP9 activity appeared to be most prominent in coronary thrombus, aligning with the same band in platelets and plasma (Fig. 4C).
We observed a stepwise increase in MMP9 concentration in the plasma of patients with NSTEMI and STEMI, respectively, compared to normal subjects. We also observed a reciprocal decrease in plasma TIMP1 expression in the same subjects (Fig. 5). Using the first blood sample drawn from the patient on arrival, plasma MMP9 predicted STEMI with 90% specificity and 80% sensitivity and NSTEMI with 90% specificity and 50% sensitivity (Fig. 6A). Moreover, plasma MMP9 could distinguish between patients with STEMI and NSTEMI with 85% specificity and 60% sensitivity which was superior to cardiac troponin T from the same sample (Fig 6B). Thus, MMP9, serving as an early platelet-derived-biomarker, could be useful in distinguishing M.I. from other causes of chest pain in an undifferentiated patient presenting with a non-acute ECG, and plasma cardiac biomarkers that are not yet released or less than the assay detection limit.
Fig. 5. Plasma Biomarker Evaluation in STEMI and NSTEMI.
Blood was taken from patients immediately upon presentation with STEMI or NSTEMI and compared to healthy volunteer subjects. Plasma was isolated and biomarker concentration was evaluated by ELISA. Patients with STEMI or NSTEMI were given 325 mg aspirin at least 30 minutes prior to blood draw. Data are represented as mean ± SEM (n=20 each group) for (A) Plasma MMP9, * p<0.05 vs. control ** p<0.01 vs. control and p=0.025 STEMI vs. NSTEMI. (B) Plasma TIMP1, p=NS between all groups. (C) Plasma MMP9/TIMP1 ratio * p<0.05 vs. control p<0.001 vs. control and p=0.015 STEMI vs. NSTEMI. The mean plasma concentration of each marker is shown below each graph. ELISA= Enzyme-Linked Immunosorbant Assay.
Fig 6. Plasma Biomarker Evaluation in STEMI and NSTEMI by ROC Analysis.
Blood was taken from patients immediately upon presentation with STEMI or NSTEMI and compared to healthy volunteer subjects. Patients with STEMI or NSTEMI were given 325 mg aspirin at least 30 minutes prior to blood draw. ROC analysis was used to determine the performance of (A) plasma MMP9 from the first blood sample obtained from the patient. * p<0.0001 predicting STEMI. § p=0.03 predicting NSTEMI and (B) Plasma MMP9 and cTnT in distinguishing between STEMI and NSTEMI, *p=0.006 for MMP9 between groups and ¶ p=0.08 for cTnT between groups. AUC=area under curve. cTnT=cardiac troponin T.
DISCUSSION
To our knowledge, this the first investigation of platelet receptor function and post-receptor signaling comparing patients presenting with STEMI to NSTEMI immediately upon arrival following only aspirin monotherapy and prior to percutaneous instrumentation. Platelets from patients presenting with STEMI were found to be different from patients with NSTEMI, with residual platelet activation observed following aspirin treatment in both groups, but with different platelet receptor agonist sensitivities and with different post-receptor signaling properties. Platelets from patients with STEMI and NSTEMI were also found to be phenotypically different from platelets in healthy volunteers with STEMI platelets favoring the thromboxane receptor signaling pathway and NSTEMI platelets favoring the PAR1 pathway. The P2Y12 agonist dose-response curve in platelets from patients with STEMI and NSTEMI was virtually indistinguishable between groups at all concentrations of agonist used. Previous investigations reported plasma microribonucleic acid (miRNA) and platelet ribonucleic acid (RNA) profiles are different in patients presenting with STEMI and NSTEMI [31, 32]. Our study in platelets supports and extends these observations. A phenotypically different platelet population in STEMI compared to NSTEMI may be a hitherto unexplored possibility for the different patient outcomes observed since the recommended anti-platelet therapy for either is the same.
A significant advantage of our study design was the provision of delayed patient consent for blood draw and therefore obtaining the blood before loading with a P2Y12 receptor antagonist, and before coronary angiography or instrumentation. These factors all contribute significantly to experimental variability with respect to platelet function and biomarker evaluation, they limit interpretation of clinical data, and may contaminate clinical trial design with antiplatelet medications [33–35]. Since we challenged platelets with surface receptor agonists only for clinically-relevant signaling pathways for which oral antagonists are available, we gained insight into which platelet signaling pathways may be important in STEMI and NSTEMI. This opportunity may have been lost had we relied on indirect aggregometry and examination of basal (unstimulated) platelet activation.
The biological processes involved in platelet activation and thrombosis in STEMI and NSTEMI may be different—a phenomenon which was proposed previously. With STEMI, arterial thrombosis manifests as a flow-limiting occlusion of an epicardial coronary artery due to plaque rupture, erosion, and infrequently a calcified nodule [36]. Atheroembolism, conversely, is thought to be the signature of platelet activation in NSTEMI and may be a recurrent phenomenon. Recurrent myocardial hypoperfusion events in NSTEMI were previously linked to adverse long-term patient outcomes [37]. By inference, NSTEMI may be a disorder of platelet function while STEMI may be a disorder with an equal contribution of the vasculature and platelets. Hence, it stands to reason that uniformly treating patients with aspirin and a P2Y12 receptor antagonist following STEMI and NSTEMI with the assumption that they are the same biological process might yield different long-term outcomes as reported [15].
Our data indicate no acute differences in signaling through the P2Y12 (ADP) receptor for STEMI and NSTEMI platelets, but preference for PAR1 (TRAP6) signaling in NSTEMI, and preference for the thromboxane signaling pathway (U46619) in STEMI. This observation implies aspirin with or without PAR1 blockade may be beneficial for NSTEMI. The Thrombin-Receptor Antagonist Vorapaxar in Acute Coronary Syndromes (TRACER) study showed a nonsignificant relative reduction of 8% in the primary end point but the study employed triple anti-platelet therapy of aspirin, clopidogrel, and vorapaxar with a significant trend toward bleeding [38]. While it remains unclear whether a trial of aspirin plus a PAR1 antagonist or a PAR1 antagonist alone would change long-term patient outcomes in NSTEMI, a recent study by Wong et al. using a PAR4 antagonist elegantly demonstrated marked platelet inhibition without being blighted by the specter of bleeding diatheses common to PAR1 antagonists [39]. Together, these observations emphasize the importance of returning to the fundamentals of platelet biology and understanding the nuances of platelet signaling to improve patient outcomes.
Thromboxane receptor and PAR1receptor signaling but not P2Y12 receptor signaling was different in STEMI and NSTEMI platelets in the current investigation. A downstream commonality for PAR1 and thromboxane receptor signaling is stimulating the Gαq G-protein to activate the MAPK family member ERK5 in the platelet which was previously demonstrated in healthy human and murine platelets[17]. Our study supports and complements the literature by showing the platelet PAR1 and thromboxane receptors and downstream ERK5 signaling distinguish STEMI from NSTEMI platelets, but P2Y12 receptor signaling, which was reported not to be a strong ERK5 activator in platelets, does not.[17]. ERK5 has been demonstrated to activate platelets, particularly in environments of redox stress which is typical for M.I. [17, 18, 40]. These observations propose ERK5 as a potential pharmacologic target of dysregulated platelet activity in thrombotic disease, especially in patients with ongoing ischemic disease as is the case with NSTEMI. ERK5 tends to drive cell cycle progression in nucleated cells [41, 42], and so the presence in the anucleate platelet suggests a carryover remnant from the precursor nucleated megakaryocyte. By inference, ischemic cardiac disease may ‘reprogram’ bone marrow megakarycoytes to generate dysfunctional platelets with enhanced thrombotic potential as was suggested in peripheral artery disease, diabetes, and atrial fibrillation [19, 43, 44].
Platelets from patients with STEMI and NSTEMI, unlike platelets from healthy subjects, synthesize, and likely secrete large quantities of activated MMP9. Platelet MMP activity was previously demonstrated to affect platelet activation and aggregation, while post-translational MMP9 modifications including s-nitrosylation and phosphorylation alter MMP9 enzymatic activity and MMP stability [45–47]. MMPs were also reported to propagate coronary plaque rupture, tissue remodeling, and myocardial infarct expansion [17, 27, 48, 49]. The difference in MMP9 activity in platelets from STEMI compared to NSTEMI in spite of similar quantities of MMP9 protein and a similar decrease in platelet TIMP1 (inhibitor) content, further suggest different biochemical properties of platelets from each patient population. Platelets are activated prior to coronary thrombosis and subsequent myocardial necrosis. Platelet-derived biomarkers therefore may be useful in early risk stratification [50]. This notion aligns with a previous study which showed coronary artery MMP9 concentration at the site of plaque rupture was an independent predictor of STEMI [26]. MMP9 synthesis and activation—at least in part—may be one of the pathophysiological consequences of ‘aspirin resistance’ or dysregulated platelet activity in the post-M.I. environment.
Our investigation does have some limitations. The population size, while heterogeneous, was relatively small, and will need to be validated in a larger cohort. A small number of patients were taking anti-platelet agents prior to arrival with M.I. and we could not determine the true adherence to these agents. This could present as a source of interference in the platelet function studies. Patients with STEMI in our study were immediately and objectively diagnosed by ECG in the prehospital setting or the ED and the first blood sample was used for platelet function studies. Patients with NSTEMI—as would be expected by relying on a positive cardiac biomarker for the diagnosis—were identified 1–24 hours after presentation and sometimes only after serial blood draws. A difference in the time to diagnose NSTEMI compared to STEMI may theoretically affect platelet function. The contribution of platelets and additional cells such as monocytes and neutrophils to the plasma MMP9 pool measured in our patients as predictive of STEMI and NSTEMI is unclear and will need further investigation.
This translational study demonstrated that receptors and post-receptor signaling properties as well as platelet reactivity are different in platelets from patients presenting acutely with STEMI and NSTEMI. The platelet proteome could be exploited to discover biomarkers of prognostic value for M.I. given that platelet activation precedes coronary thrombosis and myocardial necrosis.
Supplementary Material
AT A GLANCE COMMENTARY.
Background
A poorer long-term mortality for patients with NSTEMI compared to STEMI is observed in spite of anti-platelet inhibitor prescription. Unpredictable behavior of platelet inhibitors administered in some patients with MI was previously attributed to differences in absorption and metabolism of antiplatelet drugs.
Translational Significance
This study shows that the platelet phenotype may be fundamentally different in patients with NSTEMI compared to STEMI, with differences in agonist sensitivity and the ability to synthesize and secrete biomarkers. Platelet ERK5 appears to be a central mediator of dysregulated platelet activity during MI in humans which supports prior observations in murine models.
Acknowledgments
Conflict of interest: All authors have read the journal’s policy on authorship agreement and disclosures of potential conflicts of interest and have none to declare. The authors have read the journal’s authorship agreement and the manuscript has been reviewed and approved by all named authors. The manuscript was written and edited only by the named authors. This study was supported by the following grants: NIH grants 5T32HL066988-1, HL120200, and K08HL128856 as well as a University of Rochester Department of Medicine Pilot Grant to SJC, National Institutes of Health 5R01HL124018 and AHA 13EIA14250023 to CNM. We would like to thank Dr. John Gassler for critical input on study design which ultimately made the observations possible.
Abbreviations
- STEMI
ST Segment Myocardial Infarction
- NSTEMI
Non-ST Segment Myocardial Infarction (NSTEMI)
- MI
Myocardial infarction
- MMP9
matrix metalloproteinase 9
- ERK5
Extracellular-signal-regulated kinase 5
- ROC
Receiver Operator Characteristic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Born GV, Cross MJ. The Aggregation of Blood Platelets. J Physiol. 1963;168:178–95. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien JR. Effects of salicylates on human platelets. Lancet. 1968;1:779–83. doi: 10.1016/s0140-6736(68)92228-9. [DOI] [PubMed] [Google Scholar]
- 3.Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest. 1968;47:2169–80. doi: 10.1172/JCI105903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of Dual Antiplatelet Therapy: A Systematic Review for the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1116–39. doi: 10.1016/j.jacc.2016.03.512. [DOI] [PubMed] [Google Scholar]
- 5.Damman P, van ‘t Hof AW, Ten Berg JM, Jukema JW, Appelman Y, Liem AH, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: comments from the Dutch ACS working group. Neth Heart J. 2017;25:181–5. doi: 10.1007/s12471-016-0939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 7.Carlquist JF, Knight S, Horne BD, Huntinghouse JA, Rollo JS, Muhlestein JB, et al. Cardiovascular risk among patients on clopidogrel anti-platelet therapy after placement of drug-eluting stents is modified by genetic variants in both the CYP2C19 and ABCB1 genes. Thromb Haemost. 2013;109:744–54. doi: 10.1160/TH12-05-0336. [DOI] [PubMed] [Google Scholar]
- 8.Viviani Anselmi C, Briguori C, Roncarati R, Papa L, Visconti G, Focaccio A, et al. Routine assessment of on-clopidogrel platelet reactivity and gene polymorphisms in predicting clinical outcome following drug-eluting stent implantation in patients with stable coronary artery disease. JACC Cardiovasc Interv. 2013;6:1166–75. doi: 10.1016/j.jcin.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Becker DM, Segal J, Vaidya D, Yyanek LR, Herrera-Galeano JE, Bray PF, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA. 2006;295:1420–7. doi: 10.1001/jama.295.12.1420. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Zhao K, Ma N, Sun S, Miao Z, Zhao Z. Association of ABCB1 promoter methylation with aspirin exposure, platelet function, and clinical outcomes in Chinese intracranial artery stenosis patients. Eur J Clin Pharmacol. 2017 doi: 10.1007/s00228-017-2298-z. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL, Grosser T, Dong JF, Logan D, Jeske W, Angiolillo DJ, et al. Enteric Coating and Aspirin Nonresponsiveness in Patients With Type 2 Diabetes Mellitus. J Am Coll Cardiol. 2017;69:603–12. doi: 10.1016/j.jacc.2016.11.050. [DOI] [PubMed] [Google Scholar]
- 12.McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–7. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Jr, Granger CB, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 14.Puymirat E, Simon T, Cayla G, Cottin Y, Elbaz M, Coste P, et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-elevation Myocardial Infarction) 1995 to 2015. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.030798. [DOI] [PubMed] [Google Scholar]
- 15.Chan MY, Sun JL, Newby LK, Shaw LK, Lin M, Peterson ED, et al. Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation. 2009;119:3110–7. doi: 10.1161/CIRCULATIONAHA.108.799981. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson NS, Panzer-Knodle SG, Haas NF, Taite BB, Szalony JA, Page JD, et al. Assessment of platelet function assays. Am Heart J. 1998;135:S170–8. doi: 10.1016/s0002-8703(98)70245-5. [DOI] [PubMed] [Google Scholar]
- 17.Cameron SJ, Ture SK, Mickelsen D, Chakrabarti E, Modjeski KL, McNitt S, et al. Platelet Extracellular Regulated Protein Kinase 5 Is a Redox Switch and Triggers Maladaptive Platelet Responses and Myocardial Infarct Expansion. Circulation. 2015;132:47–58. doi: 10.1161/CIRCULATIONAHA.115.015656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang M, Cooley BC, Li W, Chen Y, Vasquez-Vivar J, Scoggins NO, et al. Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions. Blood. 2017;129:2917–27. doi: 10.1182/blood-2016-11-750133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu L, Chang L, Zhang Y, Zhai L, Zhang S, Qi Z, et al. Platelets Express Activated P2Y12 Receptor in Patients with Diabetes. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.026995. [DOI] [PubMed] [Google Scholar]
- 20.Vandooren J, Knoops S, Aldinucci Buzzo JL, Boon L, Martens E, Opdenakker G, et al. Differential inhibition of activity, activation and gene expression of MMP-9 in THP-1 cells by azithromycin and minocycline versus bortezomib: A comparative study. PLoS One. 2017;12:e0174853. doi: 10.1371/journal.pone.0174853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favier B, Espinosa E, Tabiasco J, Dos Santos C, Bonneville M, Valitutti S, et al. Uncoupling between immunological synapse formation and functional outcome in human gamma delta T lymphocytes. J Immunol. 2003;171:5027–33. doi: 10.4049/jimmunol.171.10.5027. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz G, Rothe G, Ruf A, Barlage S, Tschope D, Clemetson KJ, et al. European Working Group on Clinical Cell Analysis: Consensus protocol for the flow cytometric characterisation of platelet function. Thromb Haemost. 1998;79:885–96. [PubMed] [Google Scholar]
- 23.Seizer P, May AE. Platelets and matrix metalloproteinases. Thromb Haemost. 2013;110:903–9. doi: 10.1160/TH13-02-0113. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;115:599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higo S, Uematsu M, Yamagishi M, Ishibashi-Ueda H, Awata M, Morozumi T, et al. Elevation of plasma matrix metalloproteinase-9 in the culprit coronary artery in patients with acute myocardial infarction: clinical evidence from distal protection. Circ J. 2005;69:1180–5. doi: 10.1253/circj.69.1180. [DOI] [PubMed] [Google Scholar]
- 26.Nishiguchi T, Tanaka A, Taruya A, Emori H, Ozaki Y, Orii M, et al. Local Matrix Metalloproteinase 9 Level Determines Early Clinical Presentation of ST-Segment-Elevation Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2016;36:2460–7. doi: 10.1161/ATVBAHA.116.308099. [DOI] [PubMed] [Google Scholar]
- 27.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–8. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcinelli E, Giannini S, Boschetti E, Gresele P. Platelets release active matrix metalloproteinase-2 in vivo in humans at a site of vascular injury: lack of inhibition by aspirin. Br J Haematol. 2007;138:221–30. doi: 10.1111/j.1365-2141.2007.06632.x. [DOI] [PubMed] [Google Scholar]
- 29.Mastenbroek TG, Feijge MA, Kremers RM, van den Bosch MT, Swieringa F, De Groef L, et al. Platelet-Associated Matrix Metalloproteinases Regulate Thrombus Formation and Exert Local Collagenolytic Activity. Arterioscler Thromb Vasc Biol. 2015;35:2554–61. doi: 10.1161/ATVBAHA.115.306153. [DOI] [PubMed] [Google Scholar]
- 30.Sheu JR, Fong TH, Liu CM, Shen MY, Chen TL, Chang Y, et al. Expression of matrix metalloproteinase-9 in human platelets: regulation of platelet activation in in vitro and in vivo studies. Br J Pharmacol. 2004;143:193–201. doi: 10.1038/sj.bjp.0705917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward JA, Esa N, Pidikiti R, Freedman JE, Keaney JF, Tanriverdi K, et al. Circulating Cell and Plasma microRNA Profiles Differ between Non-ST-Segment and ST-Segment-Elevation Myocardial Infarction. Fam Med Med Sci Res. 2013;2:108. doi: 10.4172/2327-4972.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eicher JD, Wakabayashi Y, Vitseva O, Esa N, Yang Y, Zhu J, et al. Characterization of the platelet transcriptome by RNA sequencing in patients with acute myocardial infarction. Platelets. 2016;27:230–9. doi: 10.3109/09537104.2015.1083543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinhubl SR. The illusion of “optimal” platelet inhibition. JACC Cardiovasc Interv. 2012;5:278–80. doi: 10.1016/j.jcin.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Bhatt DL. Aspirin resistance: more than just a laboratory curiosity. J Am Coll Cardiol. 2004;43:1127–9. doi: 10.1016/j.jacc.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Chen WH, Lee PY, Ng W, Tse HF, Lau CP. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol. 2004;43:1122–6. doi: 10.1016/j.jacc.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 36.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–75. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 37.Kuhl JT, Linde JJ, Kober L, Kelbaek H, Kofoed KF. The Transmural Extent and Severity of Myocardial Hypoperfusion Predicts Long-Term Outcome in NSTEMI: An MDCT Study. JACC Cardiovasc Imaging. 2015;8:684–94. doi: 10.1016/j.jcmg.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33. doi: 10.1056/NEJMoa1109719. [DOI] [PubMed] [Google Scholar]
- 39.Wong PC, Seiffert D, Bird JE, Watson CA, Bostwick JS, Giancarli M, et al. Blockade of protease-activated receptor-4 (PAR4) provides robust antithrombotic activity with low bleeding. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aaf5294. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Z, Gao W, Fan X, Chen X, Mei H, Liu J, et al. Extracellular Signal-regulated Kinase 5 Associates with Casein Kinase II to Regulate GPIb-IX-mediated Platelet Activation via the PTEN/PI3K/Akt Pathway. J Thromb Haemost. 2017 doi: 10.1111/jth.13755. [DOI] [PubMed] [Google Scholar]
- 41.Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–6. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 42.Inesta-Vaquera FA, Campbell DG, Tournier C, Gomez N, Lizcano JM, Cuenda A. Alternative ERK5 regulation by phosphorylation during the cell cycle. Cell Signal. 2010;22:1829–37. doi: 10.1016/j.cellsig.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Wisman PP, Teraa M, de Borst GJ, Verhaar MC, Roest M, Moll FL. Baseline Platelet Activation and Reactivity in Patients with Critical Limb Ischemia. PLoS One. 2015;10:e0131356. doi: 10.1371/journal.pone.0131356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wysokinski WE, Tafur A, Ammash N, Asirvatham SJ, Wu Y, Gosk-Bierska I, et al. Impact of atrial fibrillation on platelet gene expression. Eur J Haematol. 2017;98:615–21. doi: 10.1111/ejh.12879. [DOI] [PubMed] [Google Scholar]
- 45.Sawicki G, Salas E, Murat J, Miszta-Lane H, Radomski MW. Release of gelatinase A during platelet activation mediates aggregation. Nature. 1997;386:616–9. doi: 10.1038/386616a0. [DOI] [PubMed] [Google Scholar]
- 46.Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, Ballermann BJ, et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–95. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy SM, Bove PF, Matthews DE, Akaike T, van der Vliet A. Nitric oxide regulation of MMP-9 activation and its relationship to modifications of the cysteine switch. Biochemistry. 2008;47:5832–40. doi: 10.1021/bi702496v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmermann WH. New Biologics for the Modulation of Post-Infarct Remodeling: Matricryptins. J Am Coll Cardiol. 2015;66:1375–7. doi: 10.1016/j.jacc.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 49.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–38. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferroni P, Riondino S, Vazzana N, Santoro N, Guadagni F, Davi G. Biomarkers of platelet activation in acute coronary syndromes. Thromb Haemost. 2012;108:1109–23. doi: 10.1160/TH12-08-0550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.