Abstract
Inflammatory and immune signaling has been documented as a root cause of many cardiovascular pathologies. In this review, we explore the emerging role of tumor necrosis factor receptor-associated factor 6 (TRAF6)-nuclear factor kappa B (NF-κB) signaling axis in atherosclerosis, ischemic heart disease, pathologic cardiac hypertrophy or heart failure, myocarditis, and sepsis-induced cardiomyopathy. We discuss the current understanding of cardiac inflammation in heart disease, present the TRAF6 signaling axis in the heart, then summarize what is known about TRAF6 in pathophysiology of heart disease including proof-of-concept studies that identify the utility of blocking TRAF6 to attenuate cardiac dysfunction, which suggests that TRAF6 is a novel, druggable target in treating cardiovascular disease incurred by inflammatory processes.
INTRODUCTION
Cardiovascular disease is the leading global cause of death with an estimated ~17.3 million deaths per year and is expected to grow to 23.6 million by 2030.1 Underlying processes contributing to cardiovascular disease include atherosclerosis, hypertension, myocardial infarction (MI; heart attack), and cardiac hypertrophy (secondary to hypertension).1 Pharmacological intervention of hypertension in treating cardiovascular disease has not significantly changed over the past 15 years, and includes angiotensin-converting enzyme inhibitors, calcium channel blockers, and thiazide diuretics.2 Additional therapeutic options are needed to complement these therapies to improve outcomes and lessen progression to heart failure. Inflammation plays a role in mediating the pathology of Coxsackie virus (CV) disease and involves multiple signaling factors including the nuclear factor kappa B (NF-κB) pathway. The tumor necrosis factor (TNF) receptor-associated factor (TRAF6) protein is an intracellular protein widely expressed in heart (specifically cardiomyocytes), smooth muscle, skeletal muscle, and adipose tissue, as well as B-cells, endothelial cells, and macrophage.3–8 In addition, cardiac fibroblasts and cardiac endothelial cells have TRAF6, which likely have critical roles in cardiac inflammatory processes.9–11 In all cell types, TRAF6 has had a prominent role in signal transduction of NF-κB signaling. Pharmacologically blocking TRAF6 signaling (via CD40 inhibition) has been shown to reduce inflammation in peritonitis and septic shock,12 demonstrating its potential value in the clinical management of related diseases. In this review, we explore the emerging role of TRAF6 in atherosclerosis, ischemic heart disease, and pathologic cardiac hypertrophy or heart failure. Further, we offer a discussion of how TRAF6 functions in myocarditis and sepsis-induced cardiomyopathy to argue that TRAF6 is a critical factor in the development of heart failure precipitated by infection and has potential as a broad-acting therapeutic target in cardiovascular disease.
CARDIAC IMMUNITY AND INFLAMMATION
Both innate and adaptive immune responses participate in the pathogenesis of cardiovascular disease. The innate immune system protects against pathogens or tissue injury, whereas the adaptive immune system is characterized by a highly specific response mediated by B- and T-cells (see recent reviews for detail).13–15 The heart’s innate immune system is essential not only for homeostatic responses but also for tissue repair. Cardiac innate immune responses are initiated either by the detection of damage associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) or DAMPs by pattern recognition receptors acting as danger/signals/alarmins16 to detect self vs non-self.17,18 Typically, PAMPs include lipopolysaccharides, glycolipids from mycobacterium, lipopolysaccharides (LPS) from Gram-negative organism cell walls, teichoic acids of Gram-positive organism cell walls, and double-stranded viral RNA, whereas DAMPs often include damaged extracellular matrix (ECM) proteins or circulating oxidized proteins. Toll-like receptors (TLRs) are key pattern recognition receptors that are implicated in the recognition of PAMPs and DAMPs.19,20 When encountering PAMPs and DAMPs, PPRs often initiate signaling cascades to activate NF-κB, activator protein (AP-1), and interferon regulatory factor-like transcription factors to regulate the expression of target genes that encode interferons and proinflammatory cytokines in the heart.21 These proinflammatory cytokines have direct cardiodepressant effects and have been shown to directly cause heart failure in experimental models, underscoring the key role inflammation plays in cardiac pathology (as reviewed in Refs 19, 22). However, the spectrum of factors involved in mediating the intracellular signaling cascade of the inflammatory response has not been fully elucidated. Expanding our knowledge of what proteins are obligatory in generating an inflammatory response would offer greater insight on the process, in addition to uncovering specific therapeutic targets. The aim of this review is to explore the role of a particular protein, TRAF6, in mediating the inflammatory response in the heart. TRAF6 is known to participate in TLR signaling to NF-κB; however, little is understood concerning its role in the myocardium following injury.
THE TNF RECEPTOR-ASSOCIATED FACTOR UBIQUITIN LIGASE PROTEIN
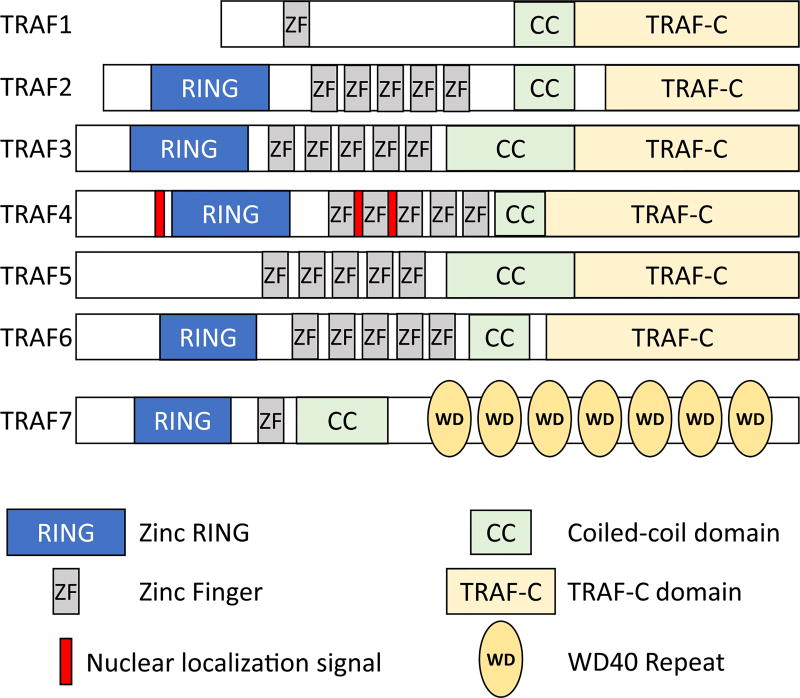
The TNF receptor-associated factor (TRAF) subfamily of proteins (consisting of 7 isoforms, TRAF1-7) functions as signaling adaptors coupling TNF-receptor superfamily members to downstream signaling events (ie, NF-κB). TRAF6 mediates signaling for a host of TNF-R superfamily receptors, including TLRs, RIG-I-like receptors, NOD-like receptors, T-cell receptors, interferon receptors, IL-1 family receptors, IL-17 family receptors, and the transforming growth factor beta (TGFβ) receptors.23 TRAF proteins contain several domains: zinc finger, coiled-coil, TRAF-C, and, with the exception of TRAF1, a RING (Really INteresting Gene) (Fig 1), where its ubiquitin ligase activity and protein docking sites reside. With their broad involvement in signal transduction, TRAF proteins serve a critical role in cellular function, particularly during pathology where many of the targeted receptors are known to be altered in various disease states.
Fig 1.
Overview of the domain structure of the 7 TRAF proteins. Based on data presented by Xie.23 TRAF, tumor necrosis factor receptor-associated factor.
Interestingly, of the TRAF isoforms, TRAF6 protein is present in most tissues including the heart, with expression specifically in cardiomyocytes. TRAF6 possesses several highly conserved binding motifs allowing it to interact with a range of targets including various membrane receptors, adaptor proteins, and intracellular kinases, making it a multifaceted mediator of signaling within the myocardium.24,25 TRAF6 is unique among the TRAF proteins as it is the only isoform involved in TLR and NF-κB signaling.26,27
Overview of TRAF6 signaling
Activation of TLRs, the induction of TRAF6, and downstream NF-κB activation plays a critical role in the pathogenesis of common cardiac diseases.28,29 As an overview, we outline TRAF6 activities in the context TLR2 and TLR4 signaling as these are the signaling pathways investigated extensively to date in the cardiovascular system. However, TRAF6 is present in an extensive number of signaling pathways from members of the TNF receptor superfamily, IL-1 family, and receptors such as CD40, Receptor Activator Of Nuclear Factor Kappa-B Ligand, and TGFβ type 1 receptors, with interactions with IRAK1/IRAK and PKCζ.24,30–33 The role of TRAF6 in cell signaling across cell types is more diverse than presented here, making it critical to understand at a cellular level once more has been published on TRAF6 in individual cell types with respect to cardiovascular disease.
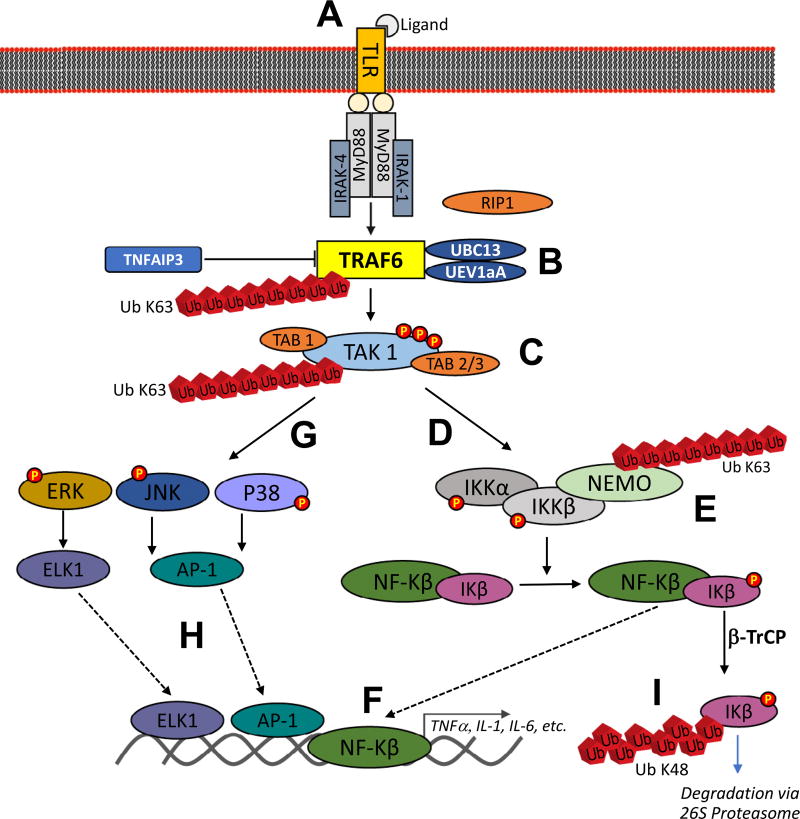
The role of TRAF6 in TLR-mediated cardiomyocyte inflammation outlines the canonical signaling targets (Fig 2). Briefly, ligand activation of TLR4 initiates MyD88 recruitment followed by binding and activation of IL-1 receptor-associated kinase1 or 4 (IRAK) through its death domain (Fig 2, A). The resulting complex activates TRAF6 (Fig 2, B), which can complex with TGFβ-activated kinase 1 (TAK1) and TAK1-binding proteins TAB1 and TAB2/3 (Fig 2, C). The TAB1/TAK1/TAB2/3 complex in conjunction with the ubiquitin conjugating enzyme Ubc13 and its variant UEV1A activates the inhibitory kappa B kinase (IKKα/IKKβ/IKKγ) complex (Fig 2, D), which in turn phosphorylates IκB. The β-TrCP ubiquitin ligase then recognizes and polyubiquitinates phosphorylated IκB (Fig 2, E), targeting it for degradation by the 26S proteasome,36–38 leading to the activation of transcription factor NF-κB (Fig 2, F). In addition, TAK1 can also lead to the activation of mitogen-activated protein kinase signaling pathways, including the extracellular signal-regulated kinase pathway, the c-Jun N-terminal kinase (JNK) pathway, and the p38 pathway (Fig 2, G). These mitogen-activated protein kinase signaling pathways are involved in activation of transcription factor AP-1 and ETS (erythroblast transformation specific domain)-like gene 1 tyrosine kinase (Fig 2, H). The activation of NF-κB and AP-1 promotes the expression of proinflammatory cytokines, such as IL-1, IL-6, and TNFα (Fig 2, I).39 TRAF6’s target specificity (eg, TLR2/4) and downstream effectors make its biological activity unique among TRAF proteins.
Fig 2.
Summary of TRAF6’s role in TLR-mediated activation of ERK, JNK, P38, and nuclear factor-κB. TRAF6 catalyzes K-63 polyubiquitination of TAK-1 with the help of Ubc13 and Uev1a. TAK-1 forms heterodimers with TAB1 and TAB2 or TAB3 for a strong interaction with polyubiquitin chains, phosphorylation and complete activation of TAK-1. The activated TAK-1 complex further phosphorylates and activates mitogen-activated protein kinases (MAPKs) [p38, c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK)] and IκB kinase (IKK)–NF-κB pathways. Original artwork based on data summarized in Ajibade et al.34; TNFAIP3 inhibition of TRAF6 by restricting its ubiquitylation based on results published by Gui et al.35 TLR, Toll-like receptor; TRAF, tumor necrosis factor receptor-associated factor.
The ubiquitin proteasome system: ubiquitin Lys48- and Lys63-linked chains
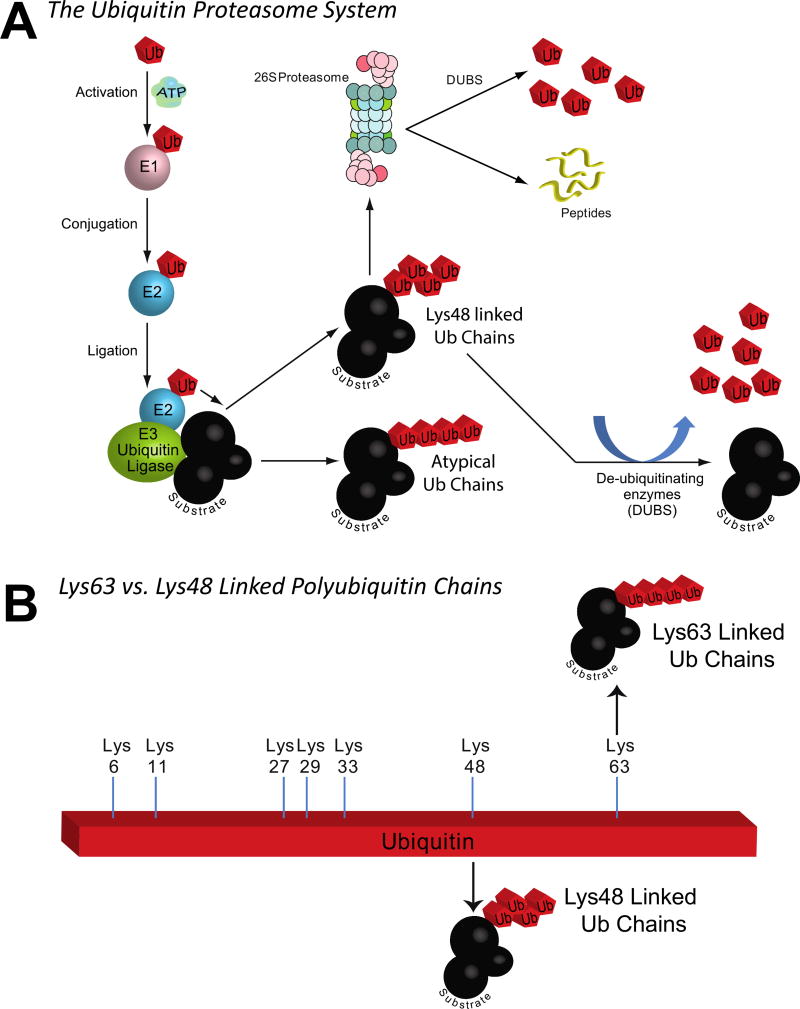
The ubiquitin ligase activity of TRAF6 is part of a broader protein quality control system present in all tissues. The ubiquitin proteasome system consists of 3 primary enzymes: E1, E2, and E3 (Fig 3, A). Ubiquitin ligases, like TRAF6, are responsible for interacting with specific substrates, which then act in concert with E1, E2, and ubiquitin to place ubiquitin chains on the substrate (Fig 3, A). The classic ubiquitination process results in protein-linked ubiquitin chains bound to each other (ubiquitin to ubiquitin) through their lysine 48 (shown in Fig 3, A, as an alternating ubiquitin chain), resulting in the protein being targeted to the 26S proteasome for degradation (Fig 3, A). However, ubiquitin ligases also catalyze Ub linkage via lysine 63 to form a linear chain (shown in Fig 3, B), which does not tag for degradation, but rather serves as a novel post-translational modification with broad effects that are just beginning to be understood. For example, Ub K63 chains can stabilize proteins (eg, the FOXO1 transcription factor) and enhance their activity.40,41 Alternatively, as in the case of TRAF6, the Lys63 polyubiquitin chains can also serve as scaffolds to link signaling protein complexes (Fig 4, A).
Fig 3.
TRAF6 as a ubiquitin ligase that mediates the addition of Lys63-linked polyubiquitin chains to substrates. A. Overview of the ubiquitin proteasome system and the functional differences between Lys48-linked polyubiquitin chains targeted substrates for 26S proteasome degradation and the Lys63-linked polyubiquitin chains that do not lead to substrate degradation. B. Overview of a single ubiquitin molecule and the 7 potential lysines that are present that could be linked to form chains, including the Lys48 and Lys63.
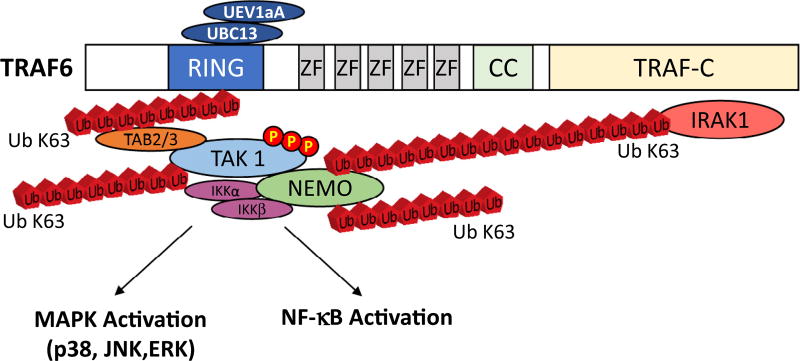
Fig 4.
The ubiquitin ligase TRAF6 is activated by extracellular stimuli, conjugating Lys63-linked polyubiquitin chains to transduce signal, linking NEMO, TAK1, IRAK, and TRAF6 itself. IRAK, IL-1 receptor-associated kinase; TRAF, tumor necrosis factor receptor-associated factor.
TRAF6 activity in NF-κB signaling
In contrast to the classic, phosphorylation-centric regulation of signal transduction, TRAF6 mediates signal transduction through its ubiquitin ligase activity, which acts to create ubiquitin chains that scaffold or molecular bridge between TNF superfamily receptors and TAK1 (Fig 2, B). The TRAF6 protein has 4 distinct domains, including an N-terminal RING finger domain, followed by 4 zinc finger (ZF) domains, a coiled-coil domain, and a TRAF-C domain.42 In conjunction with the E2 enzyme (UBC13-UEV1A) required for ubiquitin chain formation (described more in the next paragraph), the TRAF6 RING domain houses the E3 (ubiquitin ligase), facilitating its own site-specific ubiquitination with Lys-63-linked polyubiquitin chains.42 This unique K63 modification does not tag substrates for degradation but rather acts as a scaffold that activates IKK and stress kinase pathways (Fig 2). Counteracting this formation of TRAF6 ubiquitin chains, the TNF alpha-induced protein 3 (TNFAIP3, aka A20) is a cytoplasmic protein that inhibits the interaction of TRAF6 with E2 enzymes, blocking ubiquitination,43 to inhibit TRAF6’s scaffold formation (ie, Ub chain formation) and downstream signaling.44,45 Of the 4 ZF domains, the C-terminal domains 2–4 are dispensable for activating IKK, p38, and JNK via IL-1 or LPS; however, the first ZF domain and the RING domain are essential.42 The activation of IKK then leads to the upregulation of NF-κB and downstream genes, including TNFα, IL-1, IL-6, which all act as direct cardiodepressants, as described above. The critical role of TRAF6 in NF-κB signaling is central in the pathophysiology of common cardiovascular diseases and is, therefore, an important subject of the studies aimed to discover the molecular targets underlying pathologic phenomena.
TRAF6 IN CARDIAC DEVELOPMENT
Work on the TRAF family has shown various effects during development in knockout mice. TRAF6−/− deletion does lead to increased mortality; mice fail to thrive and die prematurely. TRAF6−/− mice develop severe osteopetrosis (bone thickening), spleen enlargement, and modest hepato- and cardiomegaly.46,47 Although the primary developmental effect of TRAF6 deletion has been suggested to involve noncardiac tissues, the role of TRAF6 in signaling under normal physiological conditions may be minimal. The more likely situation for TRAF6’s role in cardiovascular homeostasis is in the context of injury.
TRAF6 AND ATHEROSCLEROSIS
There is variable evidence on the role of TRAF6 in the pathogenesis of atherosclerosis. In initial studies, ApoE−/− mice fed a high-fat diet for 12 weeks and treated with statins or aerobic exercise (vs. no treatment) demonstrated reductions in angiotensin II and endothelin 1 levels along with reduced vascular TLR4 levels.48 This paralleled altered microRNA expression; miR-146a and miR-126 increased and miR155 decreased with decreased TRAF6 expression (via increased miR146a expression blocking Traf6 mRNA).49–51 Although these studies suggest a link between treatment, TLR4, and TRAF6 expression with atherosclerosis severity,48 evidence of a concrete role for TRAF6 in atherogenesis has not been established. In LDLR−/−/TRAF6+/− mice reconstituted with TRAF6-deficient fetal liver cells and on a high cholesterol diet for 18 weeks, TRAF6-deficient resident cells did not affect atherosclerosis lesion size in aortic roots and abdominal aortas.52 In patients with stable coronary heart disease, acute coronary heart disease, and those without heart disease, the TRAF6 total blood RNA expression did not change.52 Together, these studies suggest that TRAF6 is not required in endogenous inflammatory cells in mice during the development of atherosclerosis and does not correlate with human disease severity.52 However, animal models of atherosclerosis do not track well with human disease (eg, ApoE−/− mice), and only a minority of human plaques trigger vascular events (not seen in animals).
Interestingly, TRAF6 does appear to play a role in the formation of neointima in after balloon injury model of rabbit carotid disease. In this model, rabbit carotid arteries are injured, allowed 28 days of recovery followed by a stent placement, and given either a dominant-negative TRAF6 via electroporation and followed for neointimal formation.53 Rabbits with the dominant-negative TRAF6 had smaller in-stent lesions than did controls, with evidence that the dominant-negative TRAF6 suppressed macrophage infiltration, activation of proteases, and proteoglycan accumulation in the in-stent intima.53 These findings suggest a role of TRAF6 in cell replication, inflammatory cell infiltration, protease activation, and ECM accumulation in in-stent lesion development.53 Subsequent studies have identified that the statin rosuvastatin attenuates CD40L-induced downregulation of ECM production (eg, negative MMP9 activity) in human aortic smooth muscle cells via TRAF6-JNK-NF-κB signaling pathway.54 These data taken together support a primary role for TRAF6 in the pathogenesis of atherosclerosis.
TRAF6 IN ISCHEMIA/REPERFUSION INJURY AND MYOCARDIAL INFARCTION
Given the role of innate inflammation (ie, NF-κB signaling) in cardiac ischemia/reperfusion (I/R) injury and MI, it is not surprising that TRAF6 would be a primary player. Recent studies have demonstrated that the TLR4 receptor is a critical activator of NF-κB in cardiac I/R injury.55 In these studies, TLR4−/− mice were challenged with 45 minutes of cardiac ischemia followed by 4 hours of reperfusion.56 Infarct size (normalized to the area at risk) was reduced in TLR4−/− mice by 51.2% compared with wild type.56 Similarly, cardiomyocyte apoptosis was decreased in TLR4−/− compared with wild type, broadly demonstrating the role of TLR4 in cardiac I/R injury.
TLR4-TRAF6 signaling in cardiac I/R injury, the bromodomain (BRD) and extraterminal (BET) family of proteins have been implicated in the pathology of MI. After acute MI, BRD2 and BRD4 mRNA and protein levels increased with JQ1 treatment.57 Moreover, JQ1 decreased myocardial damage compared with shams, as indicated by circulating lactate dehydrogenase (LDH) and creatine kinase MB isoenzyme (CK-MB).4 Although acute MI increased TLR4, TRAF6, and NF-κB protein levels, JQ1 treatment significantly attenuated expression.4 Additionally, work on another Ub ligase, Pellino1, offers additional evidence for TRAF6 action in the post-MI inflammation response. MI induced greater TRAF6 ubiquitination.58 Together, these findings demonstrate how inhibiting BET family proteins suppresses clinically relevant manifestations of the disease, in part, by inhibiting TLR4/TRAF6/NF-κB expression, which suggests TRAF6 is a novel target for treating acute MI.
In addition to TRAF6’s regulation of TLR4-mediated NF-κB signaling in a PI3K/Akt-dependent mechanism, TRAF6 gene expression itself is modulated during cardiac I/R injury. The small noncoding micro-RNA miR-146b inhibits TRAF6 mRNA expression, thus miRNA-146b knockdown (using antagomir) enhances TRAF6 expression.59 Additionally, knockdown of miRNA-146b enhanced TRAF6 and NF-κB activation, promoted FOXP3 protein, and anti-apoptotic gene expression, while downregulating pro-apoptotic genes.59 Similarly, miRNA-125b protects against myocardial I/R injury by targeting TRAF6 mRNA expression.60 In these studies, mice transfected with lentivirus-expressing miR-125b (LmiR-125b) were challenged to 45 minutes of ischemia followed by 4 hours up to 7 days of reperfusion.60 Increased expression of miR-125b significantly decreased I/R-induced infarct size by 60% and prevented I/R-induced systolic dysfunction (by echocardiography).60 Using miR-125b transgenic mice, a similar amelioration of myocardial I/R injury was observed through inhibition of caspase-3/7 and caspase-8 activity, with suppression of p53 and Bak1 in the myocardium.60 Transfection with LmiR-125R suppressed TRAF6 expression and prevented I/R-induced NF-κB activation.60 TRAF6 activity can be inhibited by the microRNAs 146b and 125b, with specific evidence that miR-125b can inhibit TRAF6 and significantly attenuate cardiac I/R injury in vivo.
Because TRAF6 is found in cardiomyocytes,61 fibroblasts,9,11 neutrophils,62 endothelial cells,7 and macrophage,8 the role of TRAF6 in I/R can further be identified at the level of the cell. The protective role of decreasing TRAF6 in cardiomyocytes is clear from both in vitro and in vivo studies using the miR-125b.60 When TRAF6 expression is reduced by miR125b in the H9C2 cardiomyocyte cell line and challenged with hypoxia/reoxygenation (2 hours/24 hours), a clear cardioprotection is observed (significant reduction in LDH release).60 When mice are treated with the LmiR-125b or when miR-125b transgenic mice are challenged with I/R injury, significant reductions in cardiomyocyte apoptosis are observed.60 Neutrophils feature prominently in the myocardial I/R injury, releasing oxygen free radicals, cytokines, and other proinflammatory mediators that activate other neutrophils and the coronary vascular endothelium.63 Decreasing TRAF6 (by increasing miR125b) blocked neutrophil infiltration in the myocardium,60 resulting from either decreased myocyte signaling to neutrophils, decreased responses of neutrophils, or reduced activation of coronary endothelial cells (possibly via reduced TRAF6). Transfection of macrophages with miR-125b mimics the hypoxia/reoxygenation attenuation in wild-type mice, suggesting that miR-125b may be protective by its regulation of TRAF6 in macrophages.64 Lastly, the TGFβ1/TRAF6 signaling pathway has been implicated in angiotensin II-induced atrial fibroblast proliferation,11 making TRAF6 blockage post-MI potentially protective against mal-adaptive remodeling. Together, these findings suggest that blocking TRAF6 in cardiomyocytes, neutrophils, endothelial cells, macrophage, and fibroblasts may be protective against cardiac I/R injury at the level of most of the cell types involved in vivo.
TRAF6 IN CARDIAC HYPERTROPHY
At the level of the whole organ, cardiac hypertrophy manifests as a thickening of the interventricular wall or septum.65 At the cellular level, it involves the enlargement of cardiomyocytes accompanied by structural and organizational changes in sarcomere along with increased protein synthesis.66 Physiological hypertrophy is induced (eg, exercise) to enhance cardiac pump function and efficiency. In contrast, pressure overload-induced pathologic hypertrophy (eg, high blood pressure) promotes cardiac function and growth in the short term; however, over time the risk of developing heart failure increases significantly.67 In patients, differentiating these 2 types of cardiac responses is challenging, but easily done in experimental models by molecular analyses. What we know about TRAF6 as a player in pathologic hypertrophy stems from experimental models of disease, where signaling pathways have begun to be delineated in vivo.
One of several key small molecules mediating pathologic hypertrophy is angiotensin II, resulting from the activation of the renin-angiotensin-aldosterone system.68 In human dilated cardiomyopathy hearts, TRAF6 protein is increased >2 fold compared with normal heart controls.69 Moreover, pressure overload-induced cardiac hypertrophy in mice similarly increased TRAF6 protein expression at weeks 2–8.69 Inducing cardiomyocyte hypertrophy in neonatal rat cardiomyocytes with angiotensin II or phenylephrine similarly elevated TRAF6 protein at 48 hours.69 These studies also identified that reactive oxygen species production regulates TRAF6 expression during the development of cardiac hypertrophy using H2O2 and reactive oxygen species scavengers.69
Both cardiomyocyte-specific (α-myosin heavy chain promoter driven) TRAF6 transgenic (Tg) and TRAF6−/−mouse models have been used to delineate TRAF6’s role in pressure overload-induced cardiac hypertrophy.69 In response to pressure overload-induced hypertrophy by aortic banding, TRAF6 Tg mice exhibited markedly exaggerated heart wt/tibia length ratios.69 Histologic analysis revealed increased cardiomyocyte hypertrophy and fibrosis in the interstitial and perivascular space compared with non-Tg controls and elevated markers of pathologic hypertrophy (Anp, Bnp, β-myosin heavy chain, collagen I, collagen II, and Ctgf mRNA).69 In contrast, inducible cardiac-specific TRAF6−/− mice subjected to aortic banding exhibited attenuated cardiac enlargement and cardiac dysfunction compared with controls, including decreased heart weight/tibia length ratios, ameliorated cardiac dysfunction, and less interstitial and perivascular fibrosis, along with markers of pathologic hypertrophy.69
TRAF6’s regulation of pathologic cardiac hypertrophy in response to aortic banding appeared to be through the TAK1-JNK1/2-p38 signaling axis. JNK1/2 and p38 are upregulated in response to various stimuli, such as pressure overload. Analysis of TRAF6’s impact on signaling revealed effects on JNK1/2, p38, and upstream TAK1.69 Mechanistically, inhibiting TAK1 abolished TRAF6’s pro-hypertrophic effects, further implicating it in cardiac hypertrophy development in response to pressure overload. TRAF6 directly binds TAK1, which results in ubiquitination (K63-linked chains). Other studies have found that deleting TRAF6’s ring domain (with ubiquitin ligase activity) inhibits both K63 ubiquitination and P38 and JNK activation.70 Overall, TRAF6 plays a critical role in cardiac hypertrophy and may prove to be a therapeutic target through approaches to disrupt the interaction between TRAF6 and TAK1. However, given the importance of TRAF6 throughout the body, organ-specific inhibition would likely be necessary.
TRAF6 IN CARDIAC REMODELING AND HEART FAILURE
The cardiac ECM provides mechanical support, in addition to providing a molecular signal transmission platform in the healthy heart. Cardiac remodeling during the development of heart failure secondary to sustained pressure-overload (hypertension-induced) cardiac hypertrophy, post-MI, etc. results in dynamic changes in the ECM that drive both the inflammatory process and the tissue repair.71 Early in the remodeling process, matrix fragments are released, activating a proinflammatory signaling process (as recently reviewed).71 Formation of provisional matrix to facilitate leukocyte infiltration and activation of myofibroblasts results in the deposition of ECM proteins.71 Pressure and volume overload, secondary to heart failure, also induces profound alterations in the ECM composition, regulated by proteases in the heart that are critical in cardiac repair and remodeling. Several lines of evidence suggest that TRAF6 may regulate these changes in cardiac ECM during remodeling, as outlined below.
Although several lines of evidence indicate that TRAF6 supports vascular NF-κB signaling, evidence for TRAF6’s role in heart failure is indirect. In a model of mammary tumorigenesis, tumor-induced cardiac fibrosis and myofibrillar disorganization paralleled increased Smad-3, NF-κB, and TRAF6.72 Activation of cardiac Akt/mTOR occurs in rat hearts challenged with tumors.72 Exercise training prevented activation of NF-κB and TRAF6, demonstrating the role of these signaling pathways via tumor-derived cytokines in mediating heart failure as part of the phenotype seen in cancer cachexia.72 The progression of heart failure is largely attributable in the loss of cardiac myocytes via cell death, with prolonged NF-κB implicated in promoting heart failure.73 Given NF-κB’s critical role in heart failure progression and regulation of cardiac remodeling,73 TRAF6 is an emerging factor of interest, given its role in cardiac NF-κB signaling.
TRAF6 IN MYOCARDITIS
Myocarditis is a major cause of heart failure in young people, which can progress to a chronic form leading to dilated cardiomyopathy and congestive heart failure. The pathogenesis of viral myocarditis is not completely understood despite extensive research over several decades and there is no effective treatment currently available against viral myocarditis. Coxsackie virus B3 (CVB3) is a common cause of viral myocarditis in humans and is used in genetically vulnerable mouse strains to recapitulate clinical pathology.74,75 CVB3 can permanently damage myocardium, and experimental studies demonstrate that activation of inflammatory pathways is principally involved in the myocyte damage in myocarditis.76,77 Elevated levels of circulating proinflammatory cytokines including TNFα, IL-6, and IL-1β have been detected in patients with myocarditis. Modulation of inflammatory response is, therefore, a suitable therapeutic strategy for the management of viral myocarditis.78
Recent studies have revealed that Myd88-mediated TLR signaling exacerbates CVB3-induced myocarditis by altering TRAF6 activity.79 Mice inoculated with CVB3 intraperitoneally exhibited increased expression of TNFa, IL-1b, IL-6, and MCP-1 that persisted during the progression of CVB3-induced myocarditis and positively correlated with disease severity.35 Injection of an adenovirus expressing TNFAIP3 significantly reduced cytokine production and severity of myocarditis, including an attenuation of body weight loss, improved survival, decreased serum CK, CK-MB, and cTnI levels, and a reduced histologic myocarditis score based on cellular infiltrates.35 The mechanism by which CVB3-regulated inflammation occurs is thought to be because of its regulation of TRAF6 ubiquitination. Evidence for this came from studies where TNFAIP3 expression was increased, resulting in significantly inhibited NF-κB signaling.79 Because TNFAIP3 acts to prevent TRAF6 ubiquitination (described above, Fig 2), these studies demonstrate a role of TRAF6 in CVB3-induced myocarditis.35 The anti-inflammatory effects of the Chinese medicinal Shenqi Fuzheng injection derived from herbs have been tested in the CVB3 acute myocarditis mouse model.80 During myocarditis progression, TRAF6 mRNA and protein levels were persistently increased, paralleling increases in CK, CK-MB, LDH, and aspartate aminotransferase.80 Injection of Shenqi Fuzheng injection reduced both CVB3-induced TRAF6 production while attenuating the severity of myocarditis.80
TRAF6 IN SEPSIS-RELATED CARDIOMYOPATHY
Sepsis is caused by a bacterial infection that leads to systemic inflammation characterized by hypotension, ischemia, multiple organ failure, and death. Life-threatening sepsis resulting from a body’s own response to infection can lead to end-stage organ damage and cardiac dysfunction and is a common serious medical emergency. While end-stage damage is caused by indirect inflammatory effects resulting in increased vascular permeability, cardiac dysfunction can occur directly through activation of TLRs and cytokine receptors in cardiomyocytes. In addition to bacterial interaction with TLRs, other circulating mediators have been found in septic patients, including circulating plasma histones. Extracellular histones act as endogenous DAMPs, interacting with TLR2 and TLR4 on various cell types, including cardiomyocytes,81,82 which can induce sepsis symptoms in the absence of bacteria-inducing ischemia, liver damage, and hemorrhagic shock.83–86 An important feature of sepsis is cardiac dysfunction, which is attributed to increased inflammation and loss of ATP because of suppression of glucose and fatty acid oxidation.87 Innate immune response and inflammation are the potent mediators of sepsis-related cardiac dysfunction.88,89 In Gram-negative bacterial sepsis, LPS, a bacterial endotoxin, is thought to be chiefly involved in myocardial depression and multi-organ failure during sepsis.90 Innate immune response induced by LPS is mediated by TLR-4 receptors that recognize LPS and trigger the recruitment of adaptors including MyD88, TRAF6, and IL-1 receptor-associated kinases.87
One of the first links between sepsis-induced cardiomyopathy and TRAF6 involved investigating the link between TLR signaling and Akt2. When wild-type mice were challenged with LPS (4 mg/kg, 4 hours), significant reductions in systolic function occurred, in conjunction with increased apoptosis, upregulated caspase 3/12, increased ubiquitin and TRAF6, along with a decrease in mitochondrial membrane potential.91 In contrast, LPS-challenged Akt2−/− mice had significantly attenuated or mitigated LPS-induced changes in cardiac function, apoptosis, ubiquitination, but not TRAF6 expression, compared with wild-type mice.91 Although LPS facilitated Akt, GSK3b, and p38 phosphorylation in wild-type mice, p38 activation was ablated in Akt2−/−mice.91 The use of a TRAF6 inhibitory peptide or RNA silencing of TRAF6 significantly attenuated LPS-induced Akt2 ubiquitination, cardiac contractile anomalies, and apoptosis, suggesting that TRAF6 plays a pivotal role in LPS-induced cardiac dysfunction via Akt2 ubiquitination.91
In the prior section on myocardial I/R injury, we reviewed how the 2 micro RNAs (miRNA-125b and miRNA-146a) are protective by inhibiting TRAF6 expression and attenuating NF-κB signaling. Similarly, these miRNAs have been reported to protect against sepsis-induced cardiomyopathy as well. In the first set of studies, miRNA-125b was found to be a significant inhibitor of polymicrobial sepsis-induced cardiomyopathy by targeting TRAF6.92 In these studies, mice transfected with lentivirus expressing miR-125b (or control lentivirus) and challenged with cecal ligation and puncture (CLP)-induced sepsis. Six hours after CLP, echocardiographic studies identified the LmiRNA-125b treated mice had significant attenuation of CLP-induced cardiac dysfunction (fractional shortening %, ejection fraction %) and improved survival.92 Cardiac transfection of LmiRNA suppressed ICAM-1 and VCAM-1 expression, while attenuating the accumulation of macrophages and neutrophils in the myocardium.92 Transfection of LmiRNA also resulted in decreased serum levels of IL-1b, TNFα, TRAF6-mediated NF-κB activation.92 Additionally, transfection of cultured endothelial cells with miR-125b mimics attenuated LPS-induced ICAM-1 and VCAM-1 expression by suppressing TRAF6 and NF-κB activation in vivo.92 Together, these studies identified that miRNA-125b expression inhibited CLP-induced TRAF6 and NF-κB activation to protect against sepsis-induced cardiomyopathy.
The role of the microRNA-146a in sepsis has been studied in sepsis-induced cardiomyopathy. A lentivirus-expressing miR-146a (LmiR-146a) or scramble control was delivered into the myocardium via the right carotid artery, and after 7 days, were challenged with CLP for 6 hours and then assessed for echocardiographic changes.61 LmiR-146a attenuated sepsis-induced cardiac dysfunction, suppressed sepsis-induced NF-κB activity, IRAK, and TRAF6 expression in the myocardium, while also suppressing sepsis-induced cytokines in both plasma and peritoneal fluid.61 LmiR-146a also inhibited sepsis-induced infiltration of neutrophils and macrophages in the heart.61 In vitro studies also identified that LmiR-146a reduced LPS-induced IκB alpha phosphorylation and inflammatory cytokines production in H9C2 cardiomyocyte and in J774 macrophage cell lines.61 Overall, these studies indicated that miR-146a–inhibited TRAF6 (and IRAK) in cardiomyocytes decreased sepsis-induced neutrophil and macrophage infiltration into the myocardium to protect against sepsis-induced cardiomyopathy in vivo. With TRAF6 present in cardiomyocytes,61 macrophage,8 and endothelial cells,7 TRAF6 inhibition in multiple cell types may be responsible for the microRNA-146a protection in sepsis-induced cardiomyopathy.
GENETIC VARIANTS OF TRAF6 IN CARDIOVASCULAR DISEASES
A recent study investigated 816 patients with ischemic stroke (IS) and compared them with 816 controls to identify associations between 2 TRAF6 SNPs (rs5030411 and rs5030416) and the susceptibility to IS. The IS cases were recruited from inpatients at the First Affiliated Hospital of Guangxi University of Chinese Medicine (July 2009 to July 2014). The diagnosis was determined by at least 2 neurologists according to the criteria from the Fourth National Academic Conference on Cerebral Vascular Disease.93 The control group consisted of healthy individuals matched for gender and age, randomly recruited from those undergoing a physical examination and orthopedic patients without serious illness or neurologic disease (free of autoimmune diseases and history of cardio-cerebrovascular disease).93 These studies identified that in the Chinese Han population, the TRAF6 gene variant rs5030416 was associated with increased risk of IS.93 No significant differences in total cholesterol, triglycerides, high density lipoprotein, LDL, systolic blood pressure, or diastolic blood pressure were identified in the case group compared with age- and gender-matched controls.93 Coagulation studies were not reported for correlation. The rs5030411 TRAF6 was not strongly associated with susceptibility to IS but was associated with increased total cholesterol levels in study subjects.93 Given TRAF6’s role in NF-κB signaling and the association of toll-like receptors in the activation of inflammatory cascades in cerebral ischemia,94,95 it is likely that the TRAF6 gene variant rs5030416 associated with an increased risk of ischemia may be because of a gain of function, although this has not been studied directly. Consistent with this idea, the downregulation of TLRs through preconditioning has been shown to reduce inflammatory injury after cerebral ischemia.96 No other associations of TRAF6 genetic variants with other cardiovascular diseases have been reported to our knowledge, although further studies will offer additional insight into how the genetics of TRAF6 incur susceptibility to cardiac risk in patients.
THERAPEUTIC CONSIDERATIONS
Direct TRAF6 inhibitors
Therapies to inhibit TRAF6 are being developed and used in preclinical trials of cardiovascular disease. In atherosclerosis, blocking the interaction between CD40 and TRAF6 is a promising strategy in mouse models,12,97 as recently reviewed.98 Among small molecule inhibitors of CD40-TRAF6 interaction, at least 7 compounds have been identified that diminish NF-κB activation and lower IL-6 and IL-1β expression in a dose-dependent manner in RAW cells and in CD40-stimulated bone marrow-derived macrophages, respectively.12 These compounds directly bind the TRAF6 C-domain as demonstrated with surface plasmon resonance experiments. Compounds 6877002 and 6860766 reduced the atherosclerosis in the aortic arch by 47% and 66.8%, respectively. The number of plaque monocytes/macrophages was also significantly decreased with a reduced recruitment of monocytes to the endothelium and diminished adhesion of neutrophils. Importantly, treatment with these compounds abolished CD40-induced expression of IL-6, IL-10, IL-12, TNFα, and IL-1β.97 Furthermore, these inhibitory compounds have potential action against polymicrobial sepsis and peritonitis in mouse models, significantly increasing survival rates in sepsis.12
Indirect TRAF6 inhibitors
Studies demonstrating the effects of blocking TRAF6 directly or anti-TRAF6 treatment in cardiac hypertrophy, MI, I/R injury, myocarditis, and sepsis-induced cardiomyopathy have not been reported. Drugs directly targeting or blocking TRAF6 activity have not been tested yet for their effectiveness in cardiovascular disease. However, based on its crucial role in cardiovascular pathophysiology, TRAF6 can be considered as a potential therapeutic target in future therapeutic approaches. JQ1 is a well-known inhibitor of BRD proteins that play a central role in gene regulation during the pathogenesis of heart failure. JQ1 is a novel thienotriazolo-1,4-diazepine, possessing an appended, bulky t-butyl ester functional group created to prevent the interaction between the BRD4 fusion oncoprotein and chromatin, prompting differentiation and anti-proliferative properties in squamous carcinoma in humans.99 The BRD4 protein associates with chromatin and interacts with transcriptions factors (eg, GATA1100) and linked to alterations in downstream NF-κB activity in human immunodeficiency virus-associated kidney disease.101 In acute MI, inhibition of BET proteins by JQ1 led to the suppression the protein levels of TLR4, TRAF6, and NF-κB in rats.4 Small molecules structurally similar to JQ1 are in clinical trials for a variety of cancers, including as mono-and combination therapy in advanced myeloma (NCT03068351), multiple myeloma (NCT02157636), relapsed or refractory hematologic malignancies (NCT02543879), progressive lymphoma (NCT01949883), myelodysplastic syndrome (NCT02158858), carcinoma (NCT01587703), prostate cancer (NCT02711956), among others (https://goo.gl/nlcesa).
SUMMARY: TRAF6 IN CARDIOVASCULAR PATHOLOGY
TRAF6, as a key component in TLR signaling, is invariably involved in a range of cardiovascular disorders (summarized in Table I). In addition to TLR signaling, TRAF6 plays a detrimental role in the cardiovascular system including the CD40-mediated immune response and critically contributes to the progression of atherosclerosis. Similarly, in ischemia and MI, it interacts with receptor interacting protein and BET proteins, which were reported to be involved in the pathogenesis of I/R and MI, respectively. Ang II-mediated cardiac hypertrophy also includes TRAF6 as a crucial component of the signaling cascade. The cardioprotective role of TNFAIP3 in myocarditis was based on its inhibition of TRAF6. TRAF6 also appears to be a key mediator of sepsis-related cardiomyopathy through Akt signaling, which aggravates LPS-induced cardiac injury. The prominent role of TRAF6 in the modulation of inflammatory and immune signaling and the pathophysiology of cardiovascular diseases in the proof-of-concept studies presented here demonstrates that therapeutic strategies targeting TRAF6 may be a valuable avenue of development for treating cardiovascular diseases. In the future, we will need to more thoroughly understand the role of TRAF6 in individual tissues and cell types (eg, circulating lymphocytes, neutrophils, endothelial cells, macrophage), to first determine the necessity of directing TRAF6 inhibition as an effective therapy for cardiovascular disease. At the same time, we will need to better understand the need for specific targeting, including understanding the detrimental effects of blocking TRAF6 in tissues outside of the cardiovascular system and the potential unintended effects of chronic TRAF6 inhibition at the organismal level.
Table I.
Summary of the role of TRAF6 in common human cardiovascular diseases and animal models
| Disease process | TRAF6 expression in disease |
Model or organism and mechanism involved |
Effects or evidence that TRAF6 is involved |
|---|---|---|---|
| Cardiac hypertrophy | Increased TRAF6 expression reported69 | Human, mouse and rat hearts. | TRAF6 overexpression was associated with cardiac hypertrophy and TRAF6 knock down ameliorated cardiac hypertrophy (Evidence: Mouse). Increased TRAF6 protein was found in hypertrophic human hearts (Evidence: Human).69 |
| Samples from failing human hearts, transgenic and knock out mouse models of TRAF6 and Ang II stimulated rat cardiomyocytes were used. | |||
| The main signaling pathway found to be involved was TAK1,JNK1/2, and p38. | |||
| Cardiac ischemia/reperfusion injury | TRAF6 expression increased in I/R injury102 | Mouse and rat. | TRAF6 levels were increased during ischemia/reperfusion injury (Evidence: Rat).102 |
| Ischemia/reperfusion was induced by coronary artery ligation in mice and rats.Adult mouse cardiomyocytes were subjected to 2-hour hypoxia followed by 24 hours of reoxygenation. | miR-125b was found to decrease the levels of TRAF6 thereby inhibiting NF-κB activation and prevented against myocardial ischemia/reperfusion injury (Evidence: Mouse).60 | ||
| TRAF6 mediates inflammatory and apoptotic signaling as a copartner of receptor interacting protein (RIP1) and through NF-κB activation. TLR4 signaling is involved. | |||
| Myocardial infarction | N.D. | Rats. | BET protein inhibitors suppress acute myocardial infarction and this effect of BET inhibitors was in turn mediated by the inhibition of TLR4/TRAF6/NF-κB (Evidence: Rat).4 |
| Acute myocardial infarction was induced by coronary artery ligation. | |||
| BET (bromodomain and extraterminal) proteins signaling is involved, which is partly mediated by TLR4/TRAF6/NF-κB. | |||
| Atherosclerosis | N.D. | Mouse model of atherosclerosis. | TRAF6 promotes the atherosclerosis in endothelial cells and TRAF6 deficiency abrogated atherosclerosis in endothelial cells (Evidence: Mouse).103,104 |
| Apoe−/−, CD 40−/−, and LDLR−/− mice were used. | |||
| Src/ERK1/2, IKK/NF-κB, modified lipid induced activation of NF-κB and TLR signaling was involved. | |||
| Myocarditis | TRAF6 levels were markedly increased in CVB3 induced myocarditis.80 | Mice intraperitoneally inoculated with Coxsackie virus B3 (CVB3) for the induction of myocarditis. | TRAF6 inhibition by TNFα-induced protein 3 (TNFAIP3) alleviated myocarditis (Evidence: Mouse).35 |
| Myd88- and TRIF-mediated NF-κB activation was found to be involved. | |||
| Sepsis-related cardiomyopathy | N.D. | Mouse model of sepsis. | TRAF6 knockdown in mouse cardiomyocytes attenuates LPS-induced cardiac dysfunction (Mouse). miR-146a (which targets/inhibits TRAF6) similarly attenuates polymicrobial sepsis-induced cardiac dysfunction (Evidence: Mouse).61,91 |
| Mice administered with 4 mg/kg Escherichia coli O55:B5 LPS. | |||
| TRAF6 knockdown in H9c2 rat heart cell line. | |||
| Akt signaling was involved. |
Abbreviations: N.D., not determined or reported in the literature to date; TRAF, tumor necrosis factor receptor-associated factor.
Acknowledgments
This work was supported by the National Institutes of Health (R01HL104129 to M.S.W.), the Leducq Foundation Transatlantic Networks of Excellence (11CVD04) (to M.S.W.), and the International Research Support Initiative Program (IRSIP) by HEC Pakistan (to M.A.).
Abbreviations
- A20
aka TNFα induced protein 3/TNFAIP3
- AP-1
activator protein-1
- CVB3
Coxsackie virus b3 strain
- DAMPs
damage associated molecular patterns
- IKK
inhibitor of κB kinase
- IRAK1/2/4
IL-1 receptor-associated kinase 1/2/4
- JNK
cJun N-terminal kinase
- LPS
lipopolysaccharide
- PAMPs
pathogen-associated molecular patterns
- RAAS
renin-angiotensin-aldosterone system
- TAB1/2
TAK1-binding proteins
- TAK1
TGFβ-activated kinase
- TLR
Toll-like receptor
- TNFAIP3
TNFα-induced protein 3
- TRAF1/2/3/4/5/6
TNF receptor-associated factor 1/2/3/4/5/6
Footnotes
The authors confirm they have no conflict of interest to declare as per the journal’s guidelines.
The authors declare they have read the authorship agreement.
References
- 1.Writing Group Members. Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 3.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–6. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 4.Dong LH, Li L, Song Y, et al. TRAF6-mediated SM22alpha K21 ubiquitination promotes G6PD activation and NADPH production, contributing to GSH homeostasis and VSMC survival in vitro and in vivo. Circ Res. 2015;117:684–94. doi: 10.1161/CIRCRESAHA.115.306233. [DOI] [PubMed] [Google Scholar]
- 5.Navani S. Manual evaluation of tissue microarrays in a high-throughput research project: the contribution of Indian surgical pathology to the Human Protein Atlas (HPA) project. Proteomics. 2016;16:1266–70. doi: 10.1002/pmic.201500409. [DOI] [PubMed] [Google Scholar]
- 6.Iwata S, Yamaoka K, Niiro H, et al. Increased Syk phosphorylation leads to overexpression of TRAF6 in peripheral B cells of patients with systemic lupus erythematosus. Lupus. 2015;24:695–704. doi: 10.1177/0961203314560424. [DOI] [PubMed] [Google Scholar]
- 7.Su ZF, Sun ZW, Zhang Y, Wang S, Yu QG, Wu ZB. Regulatory effects of miR-146a/b on the function of endothelial progenitor cells in acute ischemic stroke in mice. Kaohsiung J Med Sci. 2017;33:369–78. doi: 10.1016/j.kjms.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi R, Sakamoto A, Yamamoto T, et al. Di-(2-ethylhexyl) phthalate suppresses IL-12p40 production by GM-CSF-dependent macrophages via the PPARalpha/TNFAIP3/TRAF6 axis after lipopolysaccharide stimulation. Hum Exp Toxicol. 2017 doi: 10.1177/0960327117714038. 960327117714038. [DOI] [PubMed] [Google Scholar]
- 9.Chen XQ, Liu X, Wang QX, et al. Pioglitazone inhibits angiotensin II-induced atrial fibroblasts proliferation via NF-kappaB/TGF-beta1/TRIF/TRAF6 pathway. Exp Cell Res. 2015;330:43–55. doi: 10.1016/j.yexcr.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Luong le A, Fragiadaki M, Smith J, et al. Cezanne regulates inflammatory responses to hypoxia in endothelial cells by targeting TRAF6 for deubiquitination. Circ Res. 2013;112:1583–91. doi: 10.1161/CIRCRESAHA.111.300119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu J, Liu X, Wang QX, et al. Angiotensin II increases CTGF expression via MAPKs/TGF-beta1/TRAF6 pathway in atrial fibroblasts. Exp Cell Res. 2012;318:2105–15. doi: 10.1016/j.yexcr.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Zarzycka B, Seijkens T, Nabuurs SB, et al. Discovery of small molecule CD40-TRAF6 inhibitors. J Chem Inf Model. 2015;55:294–307. doi: 10.1021/ci500631e. [DOI] [PubMed] [Google Scholar]
- 13.Knowlton AA. Paying for the tolls: the high cost of the innate immune system for the cardiac myocyte. Adv Exp Med Biol. 2017;1003:17–34. doi: 10.1007/978-3-319-57613-8_2. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Knowlton AA. Innate immunity and cardiomyocytes in ischemic heart disease. Life Sci. 2014;100:1–8. doi: 10.1016/j.lfs.2014.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Trujillo L, Vazquez-Garza E, Castillo EC, Garcia-Rivas G, Torre-Amione G. Role of adaptive immunity in the development and progression of heart failure: new evidence. Arch Med Res. 2017;48:1–11. doi: 10.1016/j.arcmed.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126–48. doi: 10.1111/imr.12574. [DOI] [PubMed] [Google Scholar]
- 17.Rivera Vargas T, Apetoh L. Danger signals: chemotherapy enhancers? Immunol Rev. 2017;280:175–93. doi: 10.1111/imr.12581. [DOI] [PubMed] [Google Scholar]
- 18.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 19.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–68. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–29. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–45. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–98. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 23.Xie P. TRAF molecules in cell signaling and in human diseases. J Mol Signal. 2013;8:7. doi: 10.1186/1750-2187-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mu Y, Sundar R, Thakur N, et al. TRAF6 ubiquitinates TGFbeta type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun. 2011;2:330. doi: 10.1038/ncomms1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebban-Benin H, Pescatore A, Fusco F, et al. Identification of TRAF6-dependent NEMO polyubiquitination sites through analysis of a new NEMO mutation causing incontinentia pigmenti. Hum Mol Genet. 2007;16:2805–15. doi: 10.1093/hmg/ddm237. [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Arron JR. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. Bioessays. 2003;25:1096–105. doi: 10.1002/bies.10352. [DOI] [PubMed] [Google Scholar]
- 27.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higashikuni Y, Tanaka K, Kato M, et al. Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through interleukin-1beta upregulation via nuclear factor kappaB activation. J Am Heart Assoc. 2013;2:e000267. doi: 10.1161/JAHA.113.000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha T, Li Y, Hua F, et al. Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc Res. 2005;68:224–34. doi: 10.1016/j.cardiores.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 30.Song Y, Du ZW, Yang QW, et al. Association of genes variants in RANKL/RANK/OPG signaling pathway with the development of osteonecrosis of the femoral head in Chinese population. Int J Med Sci. 2017;14:690–7. doi: 10.7150/ijms.19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aarts S, Seijkens TTP, Kusters PJH, et al. Inhibition of CD40-TRAF6 interactions by the small molecule inhibitor 6877002 reduces neuroinflammation. J Neuroinflammation. 2017;14:105. doi: 10.1186/s12974-017-0875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Gao X, Wang J, et al. Hypoxia-induced microRNA-146a represses Bcl-2 through Traf6/IRAK1 but not Smad4 to promote chondrocyte autophagy. Biol Chem. 2017;398:499–507. doi: 10.1515/hsz-2016-0211. [DOI] [PubMed] [Google Scholar]
- 33.Feng Y, Longmore GD. The LIM protein Ajuba influences interleukin-1-induced NF-kappaB activation by affecting the assembly and activity of the protein kinase Czeta/p62/TRAF6 signaling complex. Mol Cell Biol. 2005;25:4010–22. doi: 10.1128/MCB.25.10.4010-4022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ajibade AA, Wang HY, Wang RF. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 2013;34:307–16. doi: 10.1016/j.it.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Gui J, Yue Y, Chen R, Xu W, Xiong S. A20 (TNFAIP3) alleviates CVB3-induced myocarditis via inhibiting NF-kappaB signaling. PLoS ONE. 2012;7:e46515. doi: 10.1371/journal.pone.0046515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanarek N, Ben-Neriah Y. Regulation of NF-kappaB by ubiquitination and degradation of the IkappaBs. Immunol Rev. 2012;246:77–94. doi: 10.1111/j.1600-065X.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 37.Kanarek N, London N, Schueler-Furman O, Ben-Neriah Y. Ubiquitination and degradation of the inhibitors of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000166. doi: 10.1101/cshperspect.a000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaron A, Hatzubai A, Davis M, et al. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature. 1998;396:590–4. doi: 10.1038/25159. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Lv J, Jiang S, et al. The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death Dis. 2016;7:e2234. doi: 10.1038/cddis.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li HH, Willis MS, Lockyer P, et al. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest. 2007;117:3211–23. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schisler JC, Willis MS, Patterson C. You spin me round: MaFBx/Atrogin-1 feeds forward on FOXO transcription factors (like a record) Cell Cycle. 2008;7:440–3. doi: 10.4161/cc.7.4.5451. [DOI] [PubMed] [Google Scholar]
- 42.Lamothe B, Campos AD, Webster WK, Gopinathan A, Hur L, Darnay BG. The RING domain and first zinc finger of TRAF6 coordinate signaling by interleukin-1, lipopolysaccharide, and RANKL. J Biol Chem. 2008;283:24871–80. doi: 10.1074/jbc.M802749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng Z, Zhao T, Zhou K, et al. A20 ameliorates intracerebral hemorrhage-induced inflammatory injury by regulating TRAF6 polyubiquitination. J Immunol. 2017;198:820–31. doi: 10.4049/jimmunol.1600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coornaert B, Carpentier I, Beyaert R. A20: central gatekeeper in inflammation and immunity. J Biol Chem. 2009;284:8217–21. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383–91. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Lomaga MA, Yeh WC, Sarosi I, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–24. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strickson S, Emmerich CH, Goh ETH, et al. Roles of the TRAF6 and Pellino E3 ligases in MyD88 and RANKL signaling. Proc Natl Acad Sci USA. 2017;114:E3481–9. doi: 10.1073/pnas.1702367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu XD, Zeng K, Liu WL, et al. Effect of aerobic exercise on miRNA-TLR4 signaling in atherosclerosis. Int J Sports Med. 2014;35:344–50. doi: 10.1055/s-0033-1349075. [DOI] [PubMed] [Google Scholar]
- 49.Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Combination of statin plus renin angiotensin system inhibition for the prevention or the treatment of atherosclerotic cardiovascular disease. Curr Pharm Des. 2014;20:6299–305. doi: 10.2174/1381612820666140620115756. [DOI] [PubMed] [Google Scholar]
- 50.Wu D, Cerutti C, Lopez-Ramirez MA, et al. Brain endothelial miR-146a negatively modulates T-cell adhesion through repressing multiple targets to inhibit NF-kappaB activation. J Cereb Blood Flow Metab. 2015;35:412–23. doi: 10.1038/jcbfm.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi Y, Satoh M, Minami Y, Tabuchi T, Itoh T, Nakamura M. Expression of miR-146a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: effect of renin-angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin Sci. 2010;119:395–405. doi: 10.1042/CS20100003. [DOI] [PubMed] [Google Scholar]
- 52.Stachon P, Missiou A, Walter C, et al. Tumor necrosis factor receptor associated factor 6 is not required for atherogenesis in mice and does not associate with atherosclerosis in humans. PLoS ONE. 2010;5:e11589. doi: 10.1371/journal.pone.0011589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyahara T, Koyama H, Miyata T, et al. Inflammatory responses involving tumor necrosis factor receptor-associated factor 6 contribute to in-stent lesion formation in a stent implantation model of rabbit carotid artery. J Vasc Surg. 2006;43:592–600. doi: 10.1016/j.jvs.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Wang XL, Zhou YL, Sun W, Li L. Rosuvastatin attenuates CD40L–induced downregulation of extracellular matrix production in human aortic smooth muscle cells via TRAF6-JNK-NF-kappaB pathway. PLoS ONE. 2016;11:e0153919. doi: 10.1371/journal.pone.0153919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hua F, Ha T, Ma J, et al. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol. 2007;178:7317–24. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 56.Vilahur G, Badimon L. Ischemia/reperfusion activates myocardial innate immune response: the key role of the toll-like receptor. Front Physiol. 2014;5:496. doi: 10.3389/fphys.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Y, Huang J, Song K. BET protein inhibition mitigates acute myocardial infarction damage in rats via the TLR4/TRAF6/NF-kappaB pathway. Exp Ther Med. 2015;10:2319–24. doi: 10.3892/etm.2015.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu W, Hu Y, Li J, et al. Silencing of Pellino1 improves post-infarct cardiac dysfunction and attenuates left ventricular remodelling in mice. Cardiovasc Res. 2014;102:46–55. doi: 10.1093/cvr/cvu007. [DOI] [PubMed] [Google Scholar]
- 59.Lu Y, Hippen KL, Lemire AL, et al. miR-146b antagomir-treated human Tregs acquire increased GVHD inhibitory potency. Blood. 2016;128:1424–35. doi: 10.1182/blood-2016-05-714535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Ha T, Zou J, et al. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102:385–95. doi: 10.1093/cvr/cvu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao M, Wang X, Zhang X, et al. Attenuation of cardiac dysfunction in polymicrobial sepsis by MicroRNA-146a is mediated via targeting of IRAK1 and TRAF6 expression. J Immunol. 2015;195:672–82. doi: 10.4049/jimmunol.1403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen G, Ye X, Zhang J, et al. Limb remote ischemic postconditioning reduces ischemia-reperfusion injury by inhibiting NADPH oxidase activation and MyD88-TRAF6-P38MAP-kinase pathway of neutrophils. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43:860–78. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 64.Ren D, Wang X, Ha T, et al. SR-A deficiency reduces myocardial ischemia/reperfusion injury; involvement of increased microRNA-125b expression in macrophages. Biochim Biophys Acta. 2013;1832:336–46. doi: 10.1016/j.bbadis.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang F, Gardner DG. Mechanical strain activates BNP gene transcription through a p38/NF-kappaB-dependent mechanism. J Clin Invest. 1999;104:1603–12. doi: 10.1172/JCI7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–9. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 67.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 68.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 69.Ji YX, Zhang P, Zhang XJ, et al. The ubiquitin E3 ligase TRAF6 exacerbates pathological cardiac hypertrophy via TAK1-dependent signalling. Nat Commun. 2016;7:11267. doi: 10.1038/ncomms11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao Q, Wang Y, Huang L, Wang F, Chen S. TNF receptor-associated factor 6 (TRAF6) mediates the angiotensin-induced non-canonical TGF-beta pathway activation of c-kit(+) cardiac stem cells. Am J Transl Res. 2015;7:2233–43. [PMC free article] [PubMed] [Google Scholar]
- 71.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest. 2017;127:1600–12. doi: 10.1172/JCI87491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Padrao AI, Moreira-Goncalves D, Oliveira PA, et al. Endurance training prevents TWEAK but not myostatin-mediated cardiac remodelling in cancer cachexia. Arch Biochem Biophys. 2015;567:13–21. doi: 10.1016/j.abb.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 73.Kouskoukis CE. Stump the experts. Extra-mammary Paget’s disease in the perianal region. J Dermatol Surg Oncol. 1990;16:352–82. doi: 10.1111/j.1524-4725.1990.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 74.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–55. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 75.Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 2007;41:118–22. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leipner C, Grun K, Borchers M, Stelzner A. The outcome of coxsackievirus B3-(CVB3-) induced myocarditis is influenced by the cellular immune status. Herz. 2000;25:245–8. doi: 10.1007/s000590050014. [DOI] [PubMed] [Google Scholar]
- 77.Calabrese F, Thiene G. Myocarditis and inflammatory cardiomyopathy: microbiological and molecular biological aspects. Cardiovasc Res. 2003;60:11–25. doi: 10.1016/s0008-6363(03)00475-9. [DOI] [PubMed] [Google Scholar]
- 78.Schultz JC, Hilliard AA, Cooper LT, Jr, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84:1001–9. doi: 10.1016/S0025-6196(11)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng Y, Chao W. Toll-like receptors and myocardial inflammation. Int J Inflam. 2011;2011:170352. doi: 10.4061/2011/170352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen J, Wang Z, Wu T. Shenqi fuzheng injection improves cvb3-induced myocarditis via inhibiting traf6 expression. Cell Mol Biol. 2013;59(Suppl):OL1826–34. [PubMed] [Google Scholar]
- 81.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–31. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006;72:384–93. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ostrowski SR, Berg RM, Windelov NA, et al. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: a prospective study. J Crit Care. 2013;28:586–96. doi: 10.1016/j.jcrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Abrams ST, Zhang N, Manson J, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–9. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalbitz M, Grailer JJ, Fattahi F, et al. Role of extracellular histones in the cardiomyopathy of sepsis. FASEB J. 2015;29:2185–93. doi: 10.1096/fj.14-268730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep. 2015;12:130–40. doi: 10.1007/s11897-014-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediators Inflamm. 2013;2013:165974. doi: 10.1155/2013/165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7:163–83. doi: 10.2174/157340311798220494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fernandes CJ, Jr, de Assuncao MS. Myocardial dysfunction in sepsis: a large, unsolved puzzle. Crit Care Res Pract. 2012;2012:896430. doi: 10.1155/2012/896430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, Xu X, Ceylan-Isik AF, et al. Ablation of Akt2 protects against lipopolysaccharide-induced cardiac dysfunction: role of Akt ubiquitination E3 ligase TRAF6. J Mol Cell Cardiol. 2014;74:76–87. doi: 10.1016/j.yjmcc.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma H, Wang X, Ha T, et al. MicroRNA-125b prevents cardiac dysfunction in polymicrobial sepsis by targeting TRAF6-mediated nuclear factor kappaB activation and p53-mediated apoptotic signaling. J Infect Dis. 2016;214:1773–83. doi: 10.1093/infdis/jiw449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Su L, Chen Z, Yan Y, et al. Association between TRAF6 gene polymorphisms and susceptibility of ischemic stroke in Southern Chinese Han population. J Mol Neurosci. 2015;57:386–92. doi: 10.1007/s12031-015-0580-z. [DOI] [PubMed] [Google Scholar]
- 94.Li M, Liu J, Bi Y, Chen J, Zhao L. Potential medications or compounds acting on toll-like receptors in cerebral ischemia. Curr Neuropharmacol. 2017 doi: 10.2174/1570159X15666170601125139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang PF, Xiong XY, Chen J, Wang YC, Duan W, Yang QW. Function and mechanism of toll-like receptors in cerebral ischemic tolerance: from preconditioning to treatment. J Neuroinflammation. 2015;12:80. doi: 10.1186/s12974-015-0301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang YC, Lin S, Yang QW. Toll-like receptors in cerebral ischemic inflammatory injury. J Neuroinflammation. 2011;8:134. doi: 10.1186/1742-2094-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seijkens T, Zarzycka B, Soehnlein O, et al. Abstract 14: small molecule inhibitors of the CD40-TRAF6 interaction reduce atherosclerosis by inducing hypo-inflammatory myeloid cells. Arterioscler Thromb Vasc Biol. 2013;33(Suppl 1):A14. [Google Scholar]
- 98.Back M, Hansson GK. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol. 2015;12:199–211. doi: 10.1038/nrcardio.2015.5. [DOI] [PubMed] [Google Scholar]
- 99.Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lamonica JM, Deng W, Kadauke S, et al. Bromodomain protein Brd3 associates with acetylated GATA1 to promote its chromatin occupancy at erythroid target genes. Proc Natl Acad Sci USA. 2011;108:E159–68. doi: 10.1073/pnas.1102140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang G, Liu R, Zhong Y, et al. Down-regulation of NF-kappaB transcriptional activity in HIV-associated kidney disease by BRD4 inhibition. J Biol Chem. 2012;287:28840–51. doi: 10.1074/jbc.M112.359505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu SZ, He XM, Zhang X, Zeng FC, Wang F, Zhou XY. Ischemic preconditioning-induced SOCS-1 protects rat intestinal ischemia reperfusion injury via degradation of TRAF6. Dig Dis Sci. 2017;62:105–14. doi: 10.1007/s10620-016-4277-0. [DOI] [PubMed] [Google Scholar]
- 103.Lutgens E, Lievens D, Beckers L, et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010;207:391–404. doi: 10.1084/jem.20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Polykratis A, van Loo G, Xanthoulea S, Hellmich M, Pasparakis M. Conditional targeting of tumor necrosis factor receptor-associated factor 6 reveals opposing functions of Toll-like receptor signaling in endothelial and myeloid cells in a mouse model of atherosclerosis. Circulation. 2012;126:1739–51. doi: 10.1161/CIRCULATIONAHA.112.100339. [DOI] [PubMed] [Google Scholar]