Abstract
It has been a common practice to assay phosphoenolpyruvate carboxylase (PEPC) under high, nonphysiological concentrations of Mg2+ and bicarbonate. We have performed kinetic studies on the enzyme from maize (Zea mays) leaves at near physiological levels of free Mg2+ (0.4 mm) and bicarbonate (0.1 mm), and found that both the nonphosphorylated and phosphorylated enzymes exhibited a high degree of cooperativity in the binding of phosphoenolpyruvate, a much lower affinity for this substrate and for activators, and a greater affinity for malate than at high concentrations of these ions. Inhibition of the phosphorylated enzyme by malate was overcome by glycine or alanine but not by glucose-6-phosphate, either in the absence or presence of high concentrations of glycerol, a compatible solute. Alanine caused significant activation at physiological concentrations, suggesting a pivotal role for this amino acid in regulating maize leaf PEPC activity. Our results showed that the maximum enzyme activity attainable in vivo would be less than 50% of that attainable in vitro under optimum conditions. Therefore, the high levels of PEPC protein in the cytosol of C4 mesophyll cells might be an adaptation for sustaining the steady-state rate of flux through the photosynthetic CO2 assimilation pathway despite the limitations imposed by the PEPC kinetic properties and the conditions of its environment.
In the leaves of C4 plants, the initial reaction in the assimilation pathway of atmospheric CO2 is the essentially irreversible carboxylation of phosphoenolpyruvate (PEP) by phosphoenolpyruvate carboxylase (PEPC) (EC 4.1.1.31), which requires Mg2+ for its activity (Bandurski, 1955). As in the case of enzymes catalyzing reactions involving ATP and ADP, the substrate of the PEPC-catalyzed reaction is a complex between Mg2+ and substrate (Wedding et al., 1988; Rodríguez-Sotres and Muñoz-Clares, 1990; Tovar-Méndez et al., 1998). This is surprising considering that the stability constant of Mg-PEP (0.18 mm) (Wold and Ballou, 1957) is very low compared with those of MgATP or MgADP (63 and 4 mm, respectively) (Dawson et al., 1986). Because the amount of free Mg2+ estimated to be in the plant cytosol is only 0.4 mm, which is not believed to drastically change under any plausible physiological condition (Yazaki et al., 1988), the cytosolic Mg-PEP concentration is about one-tenth of the PEP concentration. We have recently proposed that the main features of the kinetics of maize (Zea mays) leaf PEPC would lead to an enzyme mostly inactive under the physiological concentrations of the substrate if the concentrations of allosteric activators are low (Tovar-Méndez et al., 1998).
The PEPC-catalyzed reaction had been regarded as a non rate-controlling step of the CO2 assimilation pathway in leaves of maize plants, because the extractable PEPC activity was in great excess of that needed for the observed flux of this photosynthetic process (Avdeva and Andreeva, 1973; Usuda, 1984). However, the importance of this step in the photosynthetic metabolism of C4 plants is underscored by the complex regulation of the activity of the C4 PEPC isoenzyme, which indicates an important role in the control of the rate of CO2 assimilation. At physiological pH, C4 PEPC is activated homotropically by its substrate, Mg-PEP (Tovar-Méndez et al., 1998), and heterotropically by phosphorylated sugars (Coombs et al., 1973; Wong and Davies, 1973) and neutral amino acids (Nishikido and Takanashi, 1973; Bandarian et al., 1992), and is inhibited by dicarboxylic acids (Huber and Edwards, 1975). The enzyme is also controlled by phosphorylation on an N-terminal Ser residue (Jiao and Chollet, 1988), which causes a decrease in affinity for the dicarboxylic acids (Jiao and Chollet, 1988; Echevarría et al., 1994) and an increase in affinity for PEP (Duff et al., 1995) or Mg-PEP (Tovar-Méndez et al., 1998). Recently, in a study of Amaranthus edulis mutants that have reduced amounts of PEPC, control coefficients of 0.26 and 0.39 were determined for the wild-type and mutant enzymes, respectively, at high light and ambient CO2 concentrations (Dever et al., 1997), implying that the PEPC protein is not in excess.
To understand the role of PEPC in varying the flux through the CO2 assimilation pathway of C4 plants under a wide range of conditions, one needs to understand the degree to which the enzyme is sensitive to changes in concentration of substrates and putative regulators in vivo. In some studies, care has been taken to simulate in vivo conditions by assaying the enzyme in the presence of the estimated cytosolic concentrations of PEP and allosteric effectors (Doncaster and Leegood, 1987; Echevarría et al., 1994; Gao and Woo, 1996) or in the presence of high concentrations of glycerol to simulate conditions of high protein concentration (Stamatakis et al., 1990). However, these studies were carried out at high, nonphysiological concentrations of Mg2+ and, consequently, substrate Mg-PEP. Moreover, the other substrate of the PEPC reaction, bicarbonate ion, has also been used at much higher concentrations than its estimated concentration (77 μm) in the cytosol of mesophyll cells under air and normal illumination conditions (Jenkins et al., 1989). Because regulation of PEPC activity by metabolite effectors (Doncaster and Leegood, 1987) or by post-translational modification is mostly exerted at subsaturating concentrations of substrate (Huber and Sugiyama, 1986; Echevarría et al., 1994), the use of high substrate concentrations would lead to erroneous estimates of in vivo PEPC activity and confusion about the role and relevance of its mechanisms of regulation.
In the present study, the kinetic features of maize leaf PEPC were investigated at concentrations of free Mg2+ and bicarbonate close to those existing in vivo in an attempt to understand how the enzyme responds to changes in the environment.
RESULTS
Effects of Mg2+ on the Kinetics of Saturation of PEPC by PEP
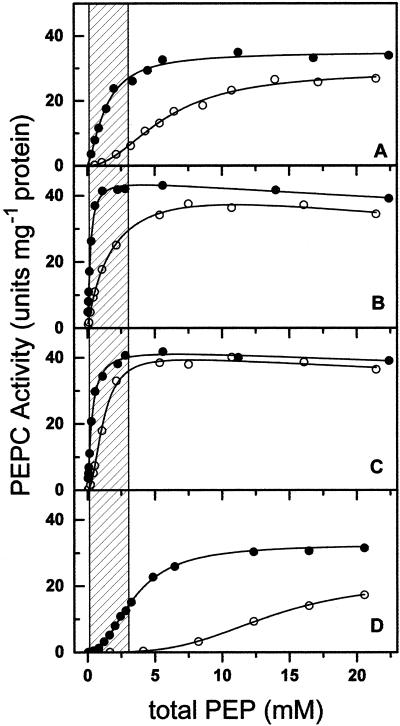
Figure 1 shows the saturation kinetics of nonphosphorylated PEPC by total PEP at 0.4 and 10 mm free Mg2+ and saturating bicarbonate (10 mm). The assays were carried out at pH 7.3, which is the reported pH of the cytosol of C4 mesophyll cells (Rajagopalan et al., 1993). As expected, since Mg-PEP is the reaction substrate, the kinetics of saturation by total PEP at 0.4 mm free Mg2+ were clearly different from those at 10 mm, both in the absence and in the presence of effectors. Vmax values at 0.4 mm free Mg2+ were very similar to those at 10 mm under all conditions tested, but the apparent S0.5 for total PEP values were around 4-fold higher at 0.4 than at 10 mm free Mg2+ (results not shown). There were important differences in the degree of cooperative binding of PEP by the enzyme, as indicated by the Hill number, particularly in the presence of 5 mm malate, at which we estimated a S0.5(total PEP) value of 13.4 mm and a n value of 3.4 ± 0.2 at the low Mg2+ concentration. Even in the absence of malate, PEPC, which exhibited a poor cooperativity at 10 mm free Mg2+ (n = 1.4 ± 0.1), became quite responsive to changes in total PEP concentrations when assayed at 0.4 mm free Mg2+ (n = 2.1 ± 0.2). Qualitatively similar results were obtained with the phosphorylated form (results not shown).
Figure 1.
Kinetics of saturation of nonphosphorylated maize leaf PEPC by total PEP at 10 mm bicarbonate and at 0.4 (○) or 10 mm (●) free Mg2+ in the absence (A) and presence of 10 mm Glc6P (B), 10 mm Gly (C), or 5 mm malate (D). In the concentration range of total PEP used in these experiments (0.028–22.4 mm), Mg-PEP concentrations ranged from 0.0017 to 1.36 mm at 0.4 mm free Mg2+ and from 0.0173 to 13.84 mm at 10 mm free Mg2+. Free PEP concentrations (trianionic form) ranged from 0.0236 to 18.91 mm at 0.4 mm free Mg2+ and from 0.0096 to 7.69 mm at 10 mm free Mg2+. The points are the experimental data. The lines are the result of the best fit of the experimental data to Equation 9, 10, or 11 as appropriate. The shaded area corresponds to the estimated physiological range of total PEP concentration.
Given the kinetic properties of PEPC, the differences in initial velocity between the two Mg2+ concentrations were important in the total PEP concentration range of 0.1 to 3 mm (Fig. 1, shaded area), especially in the presence of the inhibitor malate. These two concentrations of PEP are believed to be close to those existing in the cytosol of the mesophyll cells during the dark and light periods, respectively (Leegood, 1985; Stitt and Heldt, 1985; Doncaster and Leegood, 1987). It is clear that the potential in vivo PEPC activity would be greatly overestimated if assays were carried out at Mg2+ concentrations higher than the physiological ones.
Effects of Free Mg2+ on the Kinetics of Saturation of PEPC by Effectors
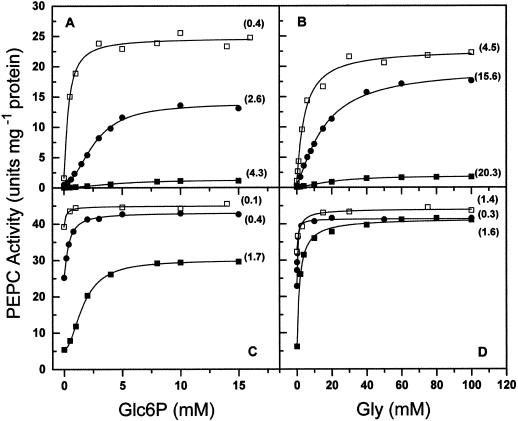
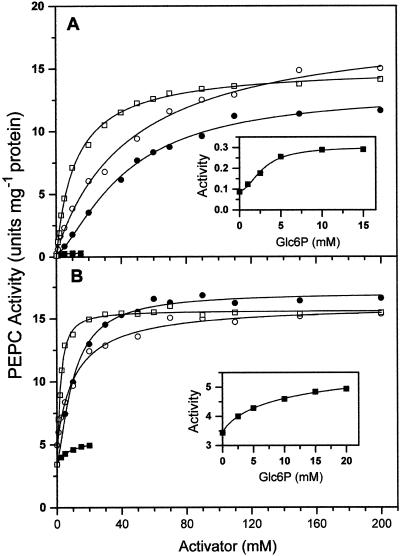
The known effectors of PEPC, activators such as Glc-6-phosphate (Glc6P) and Gly and inhibitors such as malate, exert their action mainly at low concentrations of substrate (Doncaster and Leegood, 1987). Because of that, and since the true substrate of maize leaf PEPC is the Mg-PEP complex, the evaluation of their effects should be greatly dependent on the concentration of the metal ion used in the enzyme assays, and this was found to be the case. As can be seen in Figure 2, the enzyme activities measured in the presence of Glc6P (Fig. 2, A and C) or Gly (Fig. 2, B and D) were much lower at physiological than at high free Mg2+ concentrations; this was especially true at 0.1 mm total PEP (Fig. 2, A and B), at which concentration they were very low even at saturating concentrations of any of the activators. However, when both activators were present, the activities of the enzyme measured at 0.4 mm free Mg2+ were slightly higher than those at 10 mm free Mg2+ in the absence of activators. Concentrations of Glc6P higher than 15 to 20 mm resulted in inhibition of the enzyme (results not shown), as previously reported (Mújica-Jiménez et al., 1998). No inhibition by Gly was observed even at very high concentrations.
Figure 2.
Kinetics of saturation of nonphosphorylated maize leaf PEPC by Glc6P (A and C) in the absence (●, ▪) and presence (□) of 50 mm Gly and by Gly (B and D) in the absence (▪, ●) and presence (□) of 10 mm Glc6P. The concentrations of free Mg2+ in the assays were 0.4 mm (▪, □) or 10 mm (●), and total PEP was 0.1 mm (A and B) or 3 mm (C and D). The points are the experimental data. The lines are the result of the best fit of the experimental data to Equation 12 or 13 as appropriate. Within parentheses are given the A0.5 values estimated for each data set.
The concentration of the metal ion in the assay medium also affected the A0.5 values, which measure the apparent affinity of the activators for the enzyme, and the degree of cooperativity in their binding, as assessed by the Hill number. The affinity of the enzyme for Gly was much lower than for Glc6P at 0.1 mm total PEP, but was very similar for both activators at 3 mm PEP. Thus, the ratio between the A0.5 for Glc6P values determined at 0.1 and 3 mm total PEP was 2.5, whereas the same ratio in the case of Gly was almost 13. Because of the reciprocity in the heterotropic effects, in the range of total PEP concentrations from 0.1 to 3 mm, Gly causes greater increases in the binding of this substrate to the enzyme than Glc6P when both activators are at equimolar concentrations. This was observed in the experiments shown in Figure 1, B and C. At 3 mm PEP, saturation of the enzyme with Gly was non-cooperative, whereas that of Glc6P was still cooperative. In addition, the activity measured at saturating Glc6P was much lower than that at saturating Gly. Again, similar results were found with the phosphorylated form of the enzyme (results not shown).
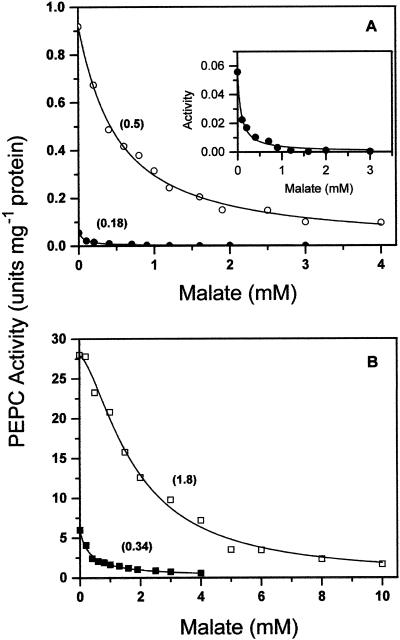
The kinetics of saturation by malate were also greatly affected by the concentration of free Mg2+ (Fig. 3). The I50 was about 3- to 5-fold lower at low compared with high free Mg2+, depending on the concentration of total PEP. Under our experimental conditions, the binding of malate to the enzyme was noncooperative, except at 3 mm total PEP and 10 mm free Mg2+, when the best fit of the data was achieved using Equation 1 (yielding a Hill number of 1.6 ± 0.1). This is an indication that the enzyme may exist in at least two states in equilibrium, one of which would be stabilized by the substrate and the other by malate. The high concentration of substrate in the latter experiment would displace the equilibrium toward the enzyme form not able to bind malate.
Figure 3.
Kinetics of saturation of nonphosphorylated maize leaf PEPC by malate at 0.4 mm (●, ▪) or 10 mm (○, □) free Mg2+ and 0.1 mm (A) or 3 mm (B) total PEP. The inset shows the saturation by malate at 0.4 mm free Mg2+ and 0.1 mm total PEP in a small scale. The points are the experimental data. The lines are the result of the best fit of the experimental data to Equation 14 or 15 as appropriate. In Table II are given the corresponding concentrations of free PEP and Mg-PEP. Within parentheses are given the I0.5 values estimated for each data set.
These results show that the effects of the activators are overestimated and the effects of the inhibitor underestimated if a high, nonphysiological concentration of the metal ion is used in the assays.
Effects of Activators on the Inhibition by Malate at 0.4 mm Free Mg2+
It has been reported that Glc6P effectively overcomes the inhibition by malate (Huber and Edwards, 1975; Echevarría et al., 1994). On the other hand, Gly has been found to be more effective than Glc6P in this respect (Gao and Woo, 1996). Because these studies were carried out at high concentrations of Mg2+, we were interested in examining the effects of Glc6P and Gly, alone and in combination, on the I50 for malate of the nonphosphorylated and phosphorylated PEPC forms at 0.4 mm free Mg2+. Table I shows the results of these experiments carried out at the same two fixed concentration of total PEP as above: 0.1 and 3 mm. Under all the conditions tested, Gly was much more effective in preventing inhibition by malate than was Glc6P, confirming the report of Gao and Woo (1996). The effects of 10 mm Gly were especially significant at 3 mm PEP, where the activator increased the I50 for malate more than 10-fold in both enzyme forms. At the same PEP concentration, 10 mm Glc6P caused only a 2- to 3-fold increase. When both activators were present, the I50 value was increased with respect to the value in the absence of activators 17- and 23-fold in the nonphosphorylated and phosphorylated forms, respectively. Interestingly, the ratios of the I50 values of the phosphorylated form to the I50 values of the nonphosphorylated form are 2- to 3-fold lower at low compared with high PEP concentration. Thus, the partial desensitization of the enzyme to the inhibitor malate caused by phosphorylation is increased by high PEP concentrations and by the presence of activators (i.e. under the conditions presumably prevailing during the light period of the day).
Table I.
Effect of activators on the I0.5 for malate of the nonphosphorylated and phosphorylated forms of maize leaf PEPC at pH 7.3, 0.4 mm free Mg2+, and 10 mm bicarbonate
| Enzyme Form | Total PEP | Control | Glc6P | Gly | Glc6P + Gly |
|---|---|---|---|---|---|
| mm | |||||
| Nonphosphorylated PEPC | 0.1 | 0.17 ± 0.01 | 0.23 ± 0.04 | 0.47 ± 0.05 | 1.74 ± 0.08 |
| 3.0 | 0.34 ± 0.04 | 0.77 ± 0.04 | 4.78 ± 0.23 | 5.82 ± 0.24 | |
| Phosphorylated PEPC | 0.1 | 0.37 ± 0.02 | 0.49 ± 0.03 | 0.67 ± 0.04 | 3.61 ± 0.03 |
| 3.0 | 1.53 ± 0.16 | 5.38 ± 0.16 | 17.1 ± 0.6 | 34.9 ± 2.0 |
Values ± sd are given in mm and were estimated by the best fit to Equation 6. Glc6P and Gly were given at 10 mm concentrations.
Kinetics of Saturation of PEPC by PEP, Glc6P, Gly, or Malate at 0.4 mm Free Mg2+ and 0.1 mm Bicarbonate
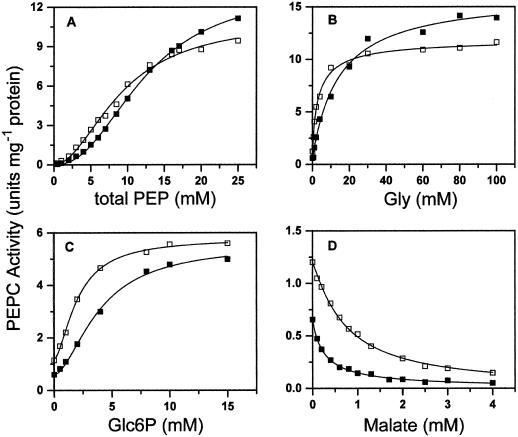
The concentration of bicarbonate in the cytosol of maize mesophyll cells has been estimated to be as low as 77 μm (Jenkins et al., 1989). This concentration is much lower than those commonly used in the assays of PEPC, which were 1 mm (Echevarría et al., 1994; Duff et al., 1995; Gao and Woo, 1996; Ogawa et al., 1997), or 10 mm (Uedan and Sugiyama, 1976; Doncaster and Leegood, 1987; McNaughton et al., 1989; Gillinta and Grover, 1995; Dong et al., 1997; Tovar-Méndez et al., 1998), concentrations that are at least 10- to 100-fold the Km for bicarbonate of the C4 PEPC (Uedan and Sugiyama, 1976; Bauwe, 1986; Janc et al., 1992; Dong et al., 1997). To see whether our conclusions might be affected qualitatively and/or quantitatively by low bicarbonate concentrations, we studied the kinetics of saturation of the nonphosphorylated and phosphorylated forms of PEPC by total PEP or by Glc6P, Gly, or malate at 0.4 mm free Mg2+ and 0.1 mm bicarbonate. Although we are aware that this concentration is still higher than the physiological one, we chose it to simplify the experiments. No exogenous bicarbonate had to be added to the cuvette, thus avoiding possible errors in estimating its concentration, and no further precautions, such as extensive degassing and isolation of the samples from air, were required. The results are shown in Figure 4, and the apparent kinetic parameters obtained by the best nonlinear fit of the experimental initial velocity data to the appropriate equations are given in Table II. Assaying the enzyme at low concentrations of bicarbonate resulted in increases in the estimated values of the S0.5 for total PEP and the A0.5 for Gly and Glc6P, and decreases in the I50 for malate values with respect to those found at a high, saturating bicarbonate concentration. These findings are in agreement with previous reports (Ogawa et al., 1997; Parvathi et al., 1998). Interestingly, the Hill numbers were not affected by lowering the concentration of bicarbonate 100-fold.
Figure 4.
Kinetics of saturation of nonphosphorylated (▪) and phosphorylated (□) maize leaf PEPC by PEP (A), Gly (B), Glc6P (C), and malate (D) at 0.4 mm free Mg2+ and 0.1 mm bicarbonate. In B to D, the total PEP concentration was 3 mm.
Table II.
Apparent kinetic parametersa of the nonphosphorylated and phosphorylated forms of maize leaf PEPC at pH 7.3, 0.4 mm free Mg2+, and 0.1 mm bicarbonate
| Parameter | Nonphosphorylated | Phosphorylated |
|---|---|---|
| Saturation by total PEP | ||
| Vmax (units mg−1 protein) | 13.4 ± 0.3 | 11.0 ± 0.5 |
| S0.5 (mm) | 12.3 ± 0.3 | 9.1 ± 0.6 |
| n | 2.1 ± 0.1 | 2.0 ± 0.2 |
| Saturation by Glc6Pa | ||
| vamax (units mg−1 protein) | 5.5 ± 0.2 | 5.8 ± 0.1 |
| A0.5 (mm) | 3.9 ± 0.3 | 2.0 ± 0.1 |
| n | 1.7 ± 0.1 | 1.6 ± 0.1 |
| Saturation by Glya | ||
| vamax (units mg−1 protein) | 16.1 ± 0.5 | 11.5 ± 0.2 |
| A0.5 (mm) | 14.5 ± 1.6 | 3.1 ± 0.3 |
| n | 1.0 ± 0.1 | 0.9 ± 0.1 |
| Saturation by malatea | ||
| I50 (mm) | 0.27 ± 0.01 | 0.72 ± 0.01 |
As can be seen in Figure 4, the activity of the phosphorylated form was higher than that of the nonphosphorylated form at subsaturating concentrations of total PEP, but slightly lower at saturating and near-saturating concentrations of the substrate. The increase in the affinity of the enzyme for the substrate Mg-PEP, which was brought about by phosphorylation and observed at high, saturating concentrations of bicarbonate, was therefore also observed at subsaturating concentrations. The different effects of Glc6P and Gly on enzyme activity were also observed at low bicarbonate concentrations. Thus, at 3 mm total PEP, the binding of Glc6P was still cooperative, whereas that of Gly was not. The highest activity measured at saturating concentrations of the activators was indicative of saturation of the enzyme by this substrate concentration in the presence of Gly but not in the presence of Glc6P.
When 20 mm malate was added to the assay medium to simulate near physiological conditions, the kinetic differences between Glc6P and Gly were accentuated. Figure 5A shows the kinetics of saturation of activity catalyzed by phosphorylated PEPC by the addition of Glc6P or Gly at 3 mm total PEP, 0.4 mm free Mg2+, and 0.1 mm bicarbonate in presence of 20 mm malate. The effects on PEPC activity of Ala, the most abundant neutral amino acid in mesophyll cells (Weiner and Heldt, 1992), was also studied. Saturating the enzyme with Glc6P caused a rise of only about 3-fold in the velocity, which was still well below that measured at the same substrate concentration but in the absence of malate and Glc6P. Saturating the enzyme with Gly or Ala caused a 150-fold increase, yielding the same enzyme activity observed in the absence of malate and in the presence of saturating concentrations of the neutral amino acids. As a consequence, the maximum activity obtained under the conditions of this assay at saturating Glc6P was only about 2% of the maximum activity measured at saturating Gly or Ala. Moreover, the estimated A0.5 for Glc6P was only 1.4-fold higher than that determined in the absence of malate and otherwise identical conditions, whereas the estimated A0.5 for Gly was increased 13-fold.
Figure 5.
Kinetics of saturation of phosphorylated maize leaf PEPC by Glc6P (▪), Ala (□), or Gly (●) and by Gly in the presence of 10 mm Glc6P (○). A was in the absence and B was in the presence of 20% (v/v) glycerol. The insets show the saturation by Glc6P in a small scale. Assays were carried out in the presence of 20 mm malate at 3 mm total PEP, 0.1 mm bicarbonate, and 0.4 mm free Mg2+. The points are the experimental data. The lines are the result of the best fit of the experimental data to Equation 12 or 13 as appropriate.
These findings suggest that malate effectively prevents the binding of Gly, and vice versa. Both ligands are mutually exclusive. In contrast, Glc6P and malate appear to bind simultaneously to the enzyme, even though both are mutually competitive to a small degree. The resulting enzyme-malate-Glc6P complex seems not to bind the substrate (i.e. it behaves as an inhibited enzyme form). As shown in Figure 5B, qualitatively similar results were obtained when the experiment was performed in the presence of 20% (v/v) glycerol to simulate the low water activity level likely existing in vivo. Saturating concentrations of Gly or Ala completely overcome malate inhibition, whereas saturating Glc6P concentrations only caused small increases in the enzyme activity determined in its absence. Moreover, PEPC inhibition occurred when Glc6P was increased above 15 or 20 mm in the absence or presence of glycerol, respectively (not shown). Although the enzyme activity in the absence of activators was notably higher in the presence than in the absence of glycerol, indicating that glycerol opposed malate inhibition in some degree, it is interesting that the maximum activity reached at saturating concentrations of neutral amino acids in the absence of glycerol was very similar to that in its presence.
Whatever the mechanism of interaction between the two kinds of activators and malate, it is clear from our results that Glc6P by itself is a very inefficient activator of PEPC if malate is present. Thus, the neutral amino acids, particularly Ala, would be much better activators of the enzyme than Glc6P under the conditions prevailing during the day.
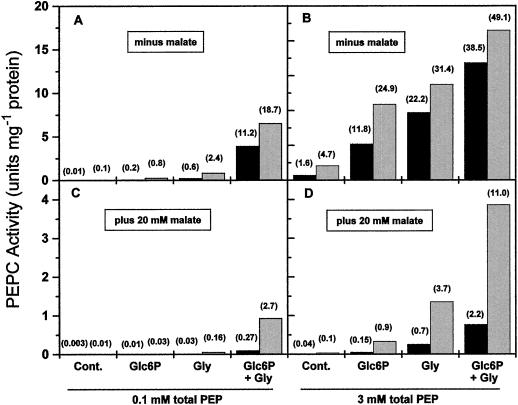
As a summary of the results described above, Figure 6 shows the combined effects of phosphorylation, activators, and PEP concentration on the activity of the phosphorylated and nonphosphorylated forms of the enzyme at concentrations of free Mg2+ and bicarbonate close to the physiological ones and in the absence and presence of 20 mm malate. At the lowest PEP concentration, the activities of both enzyme forms were negligible unless high concentrations of both activators were present, particularly in the presence of malate. It can be seen that 10 mm Gly produced higher increases in PEPC activity than 10 mm Glc6P. It is interesting that the nonphosphorylated enzyme may exhibit appreciable activity at low concentrations of total PEP if the concentration of malate is low and both activators are present. The activities of the phosphorylated form were always higher than those of the nonphosphorylated form, but the differences between the forms were small in the absence of malate. The advantages of phosphorylation were clearly seen in the presence of the inhibitor. However, phosphorylation by itself without concomitant increases in total PEP was not able to cause a significant increase in enzyme activity. Thus, at 0.1 mm total PEP and 20 mm malate, the activity of the phosphorylated enzyme when both Glc6P and Gly (10 mm each) were present was only 2.7% of the maximum activity at saturating Mg-PEP and bicarbonate. Without phosphorylation, PEPC activity was equally low at 2.2% of the maximum activity, even at high PEP and activator concentrations (Fig. 6).
Figure 6.
Effects of activators on the specific activity of nonphosphorylated (black bars) and phosphorylated (gray bars) maize leaf PEPC in the absence (A and B) and presence (C and D) of 20 mm malate. Assays were performed at 0.1 mm (A and C) or 3 mm (B and D) total PEP, 0.4 mm free Mg2+, and 0.1 mm bicarbonate in the absence or presence of 10 mm Glc6P or 10 mm Gly as indicated. The enzyme activity at each condition, as a percentage of the maximum activity achieved at saturating concentrations of Mg-PEP and bicarbonate, is given above each bar within parentheses. Cont., Control.
DISCUSSION
Effect of Mg2+ and PEP in the Response of the Enzyme to Its Allosteric Effectors
The kinetics of PEPC at 0.4 mm free Mg2+ are quite different from the kinetics at high free Mg2+ (Figs. 1–3). The experimental data shown in Figure 1A are fully consistent with the kinetic model we have recently proposed for maize leaf PEPC (Tovar-Méndez et al., 1998), and they give a very good fit to this model when free PEP or Mg-PEP are considered as the variable substrate, yielding identical Vmax and S0.5 for Mg-PEP values regardless of the metal ion concentration (results not shown). Thus, the results in this paper support the role of Mg2+ ions in the kinetics of the enzyme as part of the Mg-PEP complex.
As part of the substrate, Mg2+ indirectly affects the binding of the allosteric ligands to the enzyme. The same reasoning applies to PEP. We attempted to determine whether, in addition, free Mg2+ or free PEP could directly modulate the response of the enzyme to its effectors by comparing the results obtained at 10 mm free Mg2+ with those obtained at 0.4 mm free Mg2+.
For nonessential activators, the relationship between the A0.5 value and the normalized substrate concentration ([S]/Ks) is
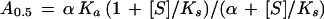 |
1 |
where α is the interaction factor that describes the influence that the binding of the substrate has on the binding of the activator and vice versa, and Ka is the activation constant (i.e. the dissociation constant of the activator from the complex enzyme-activator). When there is cooperative binding of the substrate and activator, Equation 1 becomes:
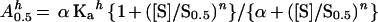 |
2 |
When bicarbonate is saturating, the reaction catalyzed by PEPC may be considered a single substrate reaction in which the normalized substrate concentration ([S]/S0.5) is related to the initial velocity (vo) by Equation 3, which was derived from the Hill equation (Eq. 10):
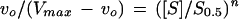 |
3 |
Thus, combining Equations 2 and 3, it is possible to relate the A0.5 value with the degree of saturation of the enzyme by the substrate, as indicated by the ratio of the initial velocity in the absence of inhibitor to the corresponding maximum velocity (vo/Vmax):
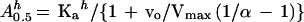 |
4 |
Assuming that Glc6P and Gly behave as nonessential activators able to bind to the free enzyme and to the enzyme-substrate complex, and taking into account the degree of saturation of the enzyme by Mg-PEP (measured as vo/Vmax under each condition) and the A0.5 obtained at the two concentrations of free Mg2+, it is thus possible to estimate theoretical values for Ka for a given value of α using Equation 4.
Similarly, the I50 value for a competitive inhibitor is an apparent kinetic parameter related to the dissociation constant of the inhibitor-enzyme complex (Ki) and to the normalized concentration of the substrate by the following expression (Segel, 1975):
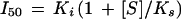 |
5 |
or when there is cooperative binding of the substrate and inhibitor:
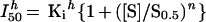 |
6 |
Combining Equations 3 and 6, we obtain:
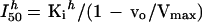 |
7 |
Thus, assuming that malate behaves as a competitive inhibitor with respect to Mg-PEP, we can estimate theoretical Ki values using Equation 7 and the experimentally determined I50 values. Therefore, for a given Mg-PEP concentration, the Ka and Ki values estimated by Equations 4 and 7, respectively, should be independent of the concentration of Mg2+ and PEP if these ligands affect the binding of the allosteric effectors only through the formation of the enzyme/Mg-PEP complex. This was found for malate and Glc6P within experimental error, but for Gly we found important differences between the theoretical Ka values estimated at low and high free Mg2+ and also between those estimated at low and high total PEP for any given value of α (not shown). Therefore, the binding of Gly appears not only to be dependent on the steady-state level of the enzyme/Mg-PEP complex, but also on the level of the enzyme/Mg or enzyme/PEP complex. This conclusion is consistent with our previous finding that Gly greatly increases the binding of free Mg2+ and free PEP to the active site of the enzyme (Tovar-Méndez et al., 1998), and might explain why the A0.5 for Gly changed to a much greater extent than the A0.5 for Glc6P when Mg or PEP were varied in a given concentration range (Fig. 2). Although Gly promotes the binding of free Mg2+ to the active site more than that of free PEP, we have also shown that free PEP activates the enzyme by binding to the Glc6P allosteric site (Tovar-Méndez et al., 1998). In fact, Gly increases the affinity of the allosteric site for free PEP. Therefore, it is expected that the free PEP bound to the allosteric site would have a positive effect on the binding of Gly, as has been found for phosphomycin, another ligand of this allosteric site (Mújica-Jiménez et al., 1998).
Effect of Bicarbonate in the Response of the Enzyme to PEP and the Allosteric Effectors
We observed a decrease in Vmax and an increase in S0.5 for total PEP when the concentration of bicarbonate was reduced from 10 to 0.1 mm at 0.4 mm free Mg2+. Kinetic studies of maize leaf PEPC carried out at pH 7.8 indicated that the addition of PEP and bicarbonate to PEPC is random, but the reaction pathway in which bicarbonate adds after PEP is preferred (Janc et al., 1992). In such a mechanism, Vmax is a function of the concentration of bicarbonate (Segel, 1975):
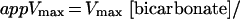 |
8 |
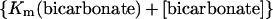 |
Therefore, the 2-fold decrease observed in the Vmax value when the concentration of bicarbonate was lowered is accounted for by the kinetic mechanism assuming that the Km(bicarbonate) at pH 7.3 is 0.1 mm (Dong et al., 1997). The decrease in the apparent affinity of the enzyme for total PEP could also be explained by the kinetic mechanism if the value of the dissociation constant of Mg-PEP from the free enzyme is higher than the value of its dissociation constant from the enzyme/Mg-PEP/bicarbonate complex in a random mechanism, or if the value of the dissociation constant of Mg-PEP from the free enzyme (Kia) is higher than Km(Mg-PEP) in a steady-state ordered mechanism (Segel, 1975). However, in a previous study, Janc et al. (1992) found that Kia is considerably lower than Km(PEP). These discrepancies may arise because total PEP and total Mg2+ was used in those experiments and the data were analyzed assuming that PEPC has three substrates, Mg2+, PEP, and bicarbonate.
Assuming that the allosteric transition takes place upon formation of the enzyme/Mg-PEP complex, the observed effects that decreasing the concentration of bicarbonate had on the affinity of the enzyme for its allosteric regulators could be explained if the steady-state levels of the enzyme/Mg-PEP complex were lower at low compared with high bicarbonate concentrations. This would imply some degree of synergism in the binding of both substrates, which is consistent with the observed effects of bicarbonate on the apparent affinity for total PEP at a fixed free Mg2+ concentration. The finding that changing the concentration of bicarbonate 100-fold did not affect the degree of cooperativity observed in the kinetics of saturation by total PEP rules out a cooperative binding of bicarbonate under our experimental conditions, which is in agreement with previous results (Uedan and Sugiyama, 1976; Bauwe, 1986; Janc et al., 1992; Dong et al., 1997). However, Parvathi et al. (1998) observed cooperativity in the binding of bicarbonate to the enzyme and postulated a bicarbonate-induced conformational change to explain the effects of this ion on the sensitivity of the enzyme to its allosteric effectors.
Possible Physiological Role of Allosteric Regulation
Despite the low intracellular concentration of free Mg2+, all potential activity of the enzyme could be realized if the levels of total PEP were high enough to produce saturating concentrations of Mg-PEP. However, considering that the S0.5(total PEP) under near physiological conditions is at least 10 mm, the levels of total PEP required for saturation of the enzyme would probably not be attainable in vivo. By increasing the affinity of the enzyme for Mg-PEP, any of the allosteric activators would allow saturation at much lower total PEP concentrations, particularly if the in vivo water activity resembles that in the presence of 20% (v/v) glycerol. Because we assayed a tetrameric PEPC, the observed effects of the allosteric activators or glycerol on PEPC activity are due to activator- or glycerol-induced conformational changes that did not involve changes in the aggregation state of the enzyme, which is in agreement with the finding of homotropic and heterotropic effects with the tetrameric form of maize leaf PEPC (Tovar-Méndez et al., 1995; Mújica-Jiménez et al., 1998). With respect to glycerol, it is known that co-solutes that alter water activity can affect the affinities of some proteins for their ligands without affecting their state of aggregation (Colombo et al., 1992; Rand et al., 1993).
The two allosteric sites are by no means redundant. Aside from connecting the CO2 assimilation pathway with the metabolic pathways of phosphorylated sugars and neutral amino acids, the ligand-bound allosteric sites affect the binding of the substrate Mg-PEP and inhibitor malate in quite different ways. These kinetic differences acquire special relevance under conditions close to those prevailing under illumination (Fig. 5), when the degree of activation of the enzyme brought about by Glc6P is much lower than that brought about by neutral amino acids. It is important to point out that the magnitude of the effects of both kind of activators on malate inhibition cannot be fully appreciated by measuring increases in I50 for malate caused by a fixed concentration of a given activator (Echevarría et al., 1994; Gao and Woo, 1996). To evaluate the full potential of the activators in the presence of the inhibitor, it is necessary to determine the maximum enzyme activity achieved at the saturating concentration of the activator and at the fixed physiological concentration of the inhibitor.
Among the phosphorylated sugars that bind to the allosteric Glc6P site, Glc6P is the strongest activator (Doncaster and Leegood, 1987; Bandarian et al., 1992; Mújica-Jiménez et al., 1998; A. Tovar-Méndez and R.A. Muñoz-Clares, unpublished results). Because of this, it is expected that saturation of the site by another phosphorylated sugar will not result in higher PEPC activity than that determined at saturating Glc6P. The Glc6P site could be important during the night or at the onset of illumination before the buildup of malate during the first hour after illumination (Rodríguez-Sotres et al., 1987). Once the levels of malate are high, saturation of the Glc6P site would give only a marginal advantage. It is interesting that increasing the concentrations of Glc6P above 20 mm did not result in further increases in PEPC activity. In fact, inhibition results. However, it is not likely that inhibition would occur in vivo, because concentrations of triose-P and Glc6P estimated to exist under conditions of illumination do not exceed this level (Stitt and Heldt, 1985).
Our results indicate that the allosteric site for neutral amino acids is crucial for achieving appreciable levels of PEPC activity under near-physiological conditions. Unlike phosphorylated sugars, the neutral amino acids do not inhibit PEPC at high concentrations. Therefore, they will produce further increases in activity even if their levels are increased above 100 mm. Under conditions of illumination and ambient CO2, the concentration in mesophyll cytosol of two ligands of this site, Gly and Ser, are low (about 2 mm; Weiner and Heldt, 1992), but the concentrations of Ala are sufficiently high (30 to 40 mm; Leegood, 1985; Weiner and Heldt, 1992) to increase PEPC activity significantly, even in the presence of high concentrations of malate (Fig. 5). Thus, Ala may be the principal ligand of this allosteric site under normal conditions. However, PEPC activation by Gly may be important at low CO2, when the concentration of Gly reaches levels similar to those of Ala at high CO2, but the Ala concentration decreases dramatically (Leegood and von Caemmerer, 1994). In this way, Gly may help to increase the flux through the C4 pathway under photorespiratory conditions.
We estimated that the limiting PEPC activity attainable in vivo if saturation by Mg-PEP takes place would be less than 50% of the maximum activity attainable in vitro under optimum conditions of bicarbonate concentration. Laisk and Edwards (1997), on the basis of very different experiments, proposed that the activity of C4 PEPC during steady-state conditions of photosynthesis is 25% of the maximum enzyme capacity. The high level of PEPC protein in the cytosol of C4 mesophyll cells (Uedan and Sugiyama, 1976; Hague and Sims, 1980) might be an adaptation for sustaining the steady-state rate of flux through the photosynthetic CO2 assimilation pathway despite the limitations imposed by the PEPC kinetic properties and the conditions of its environment.
MATERIALS AND METHODS
Chemicals and Biochemicals
PEP (monocyclohexylammonium salt), NADH (disodium salt), Glc6P, malate, Gly, Ala, porcine heart malic dehydrogenase, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were purchased from Sigma Chemical (St. Louis). EDTA (disodium salt) was from Merck KGaA (Darmstadt, Germany). All other chemicals of analytical grade were from standard suppliers.
Enzyme Purification and Assay
The purification procedure of the nontruncated and nonphosphorylated, night form of PEPC from maize (Zea mays L. cv Chalqueño) leaves, and the storage conditions of the pure enzyme were as described elsewhere (Tovar-Méndez et al., 1997). PEPC activity was measured spectrophotometrically with a coupled enzyme assay using malate dehydrogenase, and following the oxidation of NADH at 340 nm with a spectrophotometer (DU-7500, Beckman Instruments, Fullerton, CA) equipped with a kinetics software package (Tovar-Méndez et al., 1998). The specific activity of the enzyme preparation used, determined in a standard assay in the presence of 5 mm total PEP and 10 mm total Mg2+ at pH 7.3 and 30°C, was 33 μmol min−1 mg−1 protein. The enzyme preparation was fully phosphorylated in vitro by the method described by Duff et al. (1995). The phosphorylation status of PEPC was assessed as described in Tovar-Méndez et al. (1998).
Kinetic Studies
Steady-state initial velocity studies were performed at 30°C in a final volume of 0.5 mL of 100 mm HEPES-KOH buffer (pH 7.3), 1 mm EDTA, 0.2 mm NADH, and 8 units mL−1 malate dehydrogenase, with the concentrations of NaHCO3, total PEP, free Mg2+, malate, Glc6P, Gly, Ala, or glycerol stated in each experiment. The amounts of total Mg2+ (as MgCl2) and PEP used to give the desired concentrations of the free species were calculated using the procedure and dissociation constants of the Mg-PEP, Mg-Glc6P, and Mg-Gly complexes described by Tovar-Méndez et al. (1998). The dissociation constants used for Mg-malate and Mg-Ala were 28.2 and 10 mm, respectively (Dawson et al., 1986). No exogenous bicarbonate was added to the assays, in which the concentration of bicarbonate was 0.1 mm. Theoretical calculations (Segel, 1976) assuming a partial pressure of CO2 of 300 μbar gave a concentration of bicarbonate of 107 μm in aqueous solutions at pH 7.3 in equilibrium with air. We confirmed this theoretical value by end point determinations following a modification of the method described by Bauwe (1986). We determined the amount of NADH produced in an coupled assay at 30°C using pure maize leaf PEPC and saturating concentrations of PEP and Mg2+ without adding exogenous bicarbonate.
Assays were initiated by the addition of 10 to 15 μg of PEPC to cuvettes with 10 mm bicarbonate and 50 to 100 μg to cuvettes with 0.1 mm bicarbonate. The progress of the reaction was followed during the first 30 s. To avoid cold inactivation (Kleczkowski and Edwards, 1990), the enzyme was kept at room temperature throughout the experiments, which were started at least 3 h after thawing the frozen enzyme preparation. We used an enzyme preparation in which PEPC is tetrameric, as assessed by exclusion chromatography and as indicated by the lack of hysteresis in assays in which the reaction was allowed to proceed for several minutes. PEPC cannot significantly dissociate during the assay procedure in any of the conditions tested, given that the half-time for dissociation in the incubation mixture in the absence of substrates is very long (30 min in the presence of 20 mm malate and 190 min in its absence) (A. Tovar-Méndez and R.A. Muñoz-Clares, unpublished results). Each point shown in the figures is the average of duplicate determinations. Initial velocities are expressed in μmoles of product formed per minute. We display the results of the kinetics of saturation of the enzyme by its substrate PEP by considering total PEP as the variable substrate instead of free PEP or Mg-PEP to facilitate the evaluation of the effects of the different conditions tested in the physiological range of concentration of this metabolite.
Data Analysis
PEPC kinetic data were analyzed by nonlinear regression calculations using a commercial computing program formulated with the algorithm of Marquardt (1963). Kinetic data, which were dependent upon varied concentration of substrate, were fitted to the Michaelis-Menten equation (Eq. 9) for hyperbolic kinetics, to the Hill equation (Eq. 10) for sigmoidal kinetics, or to the substrate inhibition equation (Eq. 11),
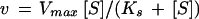 |
9 |
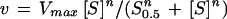 |
10 |
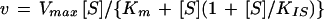 |
11 |
where v is the experimentally determined initial velocity, Vmax the maximum velocity, [S] is the concentration of the variable substrate, Ks and S0.5 are the concentrations of substrate that give half-maximum velocity, KIS the inhibition constant for the substrate, and n is the Hill number.
In the experiments in which the concentration of the activator was varied at a constant concentration of substrates, Equations 12 or 13 were used to fit the data to hyperbolic or sigmoidal saturation curves, respectively.
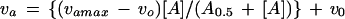 |
12 |
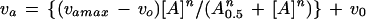 |
13 |
where va and vo are the initial velocities in the presence and absence of activator, respectively, vamax is the highest velocity obtained at saturating activator concentrations, [A] is the activator concentration, and A0.5 is the concentration of activator that gives half-maximum activation at fixed concentrations of substrates.
When the concentration of inhibitor was varied at a constant concentration of substrates, the experimental data were fitted to Equation 14 or 15 for data conforming to hyperbolic or to sigmoidal binding of the inhibitor, respectively:
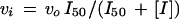 |
14 |
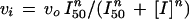 |
15 |
where vi and vo are the initial velocities in the presence and absence of inhibitor, respectively, I50 is the concentration of inhibitor that gives half-maximum inhibition, and [I] is the inhibitor concentration.
The points in the figures are the experimentally determined values, whereas the curves are calculated from fits of these data by the appropriate equation. The best fits were determined by the relative fit error, error of the constants, and absence of significant correlation between the residuals, and other relevant variables such as observed velocities, substrate concentration, and data number.
ACKNOWLEDGMENT
We thank David Maciel-Zaragoza for carrying out preliminary experiments.
Footnotes
This work was supported by the Dirección General de Apoyo al Personal Académico de Universidad Nacional Autónoma de México (grant no. DGAPA–IN 211694). A.T.M. was the recipient of a Dirección General de Apoyo al Personal Académico de Universidad Nacional Autónoma de México scholarship.
LITERATURE CITED
- Avdeva TA, Andreeva TF. Nitrogen nutrition and activities of CO2-fixing enzymes and glyceraldehyde phosphate dehydrogenase in broad bean and maize. Photosynthetica. 1973;7:140–145. [Google Scholar]
- Bandarian V, Phoehner J, Grover SD. Metabolite activation of Crassulacean acid metabolism and C4 phosphoenolpyruvate carboxylase. Plant Physiol. 1992;100:1411–1416. doi: 10.1104/pp.100.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski RS. Further studies on the enzymatic synthesis of oxalacetate from phosphorylenolpyruvate and carbon dioxide. J Biol Chem. 1955;217:137–150. [PubMed] [Google Scholar]
- Bauwe H. An efficient method for the determination of Km values for HCO3− of phosphoenolpyruvate carboxylase. Planta. 1986;169:356–360. doi: 10.1007/BF00392131. [DOI] [PubMed] [Google Scholar]
- Colombo MF, Rau DC, Parsegian VA. Protein solvation in allosteric regulation: a water effect on hemoglobin. Science. 1992;256:655–659. doi: 10.1126/science.1585178. [DOI] [PubMed] [Google Scholar]
- Coombs J, Baldry CW, Bucke C. The C-4 pathway in Pennisetum purpureum: the allosteric nature of PEP carboxylase. Planta. 1973;110:95–107. doi: 10.1007/BF00384832. [DOI] [PubMed] [Google Scholar]
- Dawson RMC, Elliot DC, Elliot WH, Jones KM. Data for Biochemical Research. Ed 3. Oxford: Clarendon Press; 1986. [Google Scholar]
- Dever LV, Bariley KJ, Leegood RC, Lea PJ. Control of photosynthesis in Amaranthus edulis mutants with reduced amounts of PEP carboxylase. Aust J Plant Physiol. 1997;24:469–476. [Google Scholar]
- Doncaster HD, Leegood RC. Regulation of phosphoenolpyruvate carboxylase activity in maize leaves. Plant Physiol. 1987;84:82–87. doi: 10.1104/pp.84.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong LY, Ueno Y, Hata S, Izui K. Effects of site-directed mutagenesis of conserved Lys606 residue on catalytic and regulatory functions of maize C4-form phosphoenolpyruvate carboxylase. Plant Cell Physiol. 1997;38:1340–1345. doi: 10.1093/oxfordjournals.pcp.a029127. [DOI] [PubMed] [Google Scholar]
- Duff SMG, Andreo CS, Pactquit V, Lepiniec L, Sarath G, Condon SA, Vidal P, Chollet R. An engineered change in the l-malate sensitivity of a site-directed mutant of sorghum phosphoenolpyruvate carboxylase: the effect of sequencial mutagenesis and S-carboxymethylation at position 8. Eur J Biochem. 1995;228:92–95. [Google Scholar]
- Echevarría C, Pacquit V, Bakrim N, Osuna L, Delgado B, Arrio-Dupont M, Vidal J. The effect of pH on the covalent and metabolic control of C4 phosphoenolpyruvate carboxylase from sorghum leaf. Arch Biochem Biophys. 1994;315:425–430. doi: 10.1006/abbi.1994.1520. [DOI] [PubMed] [Google Scholar]
- Gao Y, Woo KC. Regulation of phosphoenolpyruvate carboxylase in Zea mays by protein phosphorylation and metabolites and their roles in photosynthesis. Aust J Plant Physiol. 1996;23:25–32. [Google Scholar]
- Gillinta J, Grover SD. Kinetic interactions of glycine with substrates and effectors of phosphoenolpyruvate carboxylase from maize leaves. Photosynth Res. 1995;45:121–126. doi: 10.1007/BF00032583. [DOI] [PubMed] [Google Scholar]
- Hague DR, Sims TL. Evidence for light-stimulated synthesis of phosphoenolpyruvate carboxylase in leaves of maize. Plant Physiol. 1980;66:505–509. doi: 10.1104/pp.66.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Edwards GE. Inhibition of phosphoenolpyruvate carboxylase from C4 plants by malate and aspartate. Can J Bot. 1975;53:1925–1933. [Google Scholar]
- Huber SC, Sugiyama T. Changes in sensitivity to effectors of maize leaf phosphoenolpyruvate carboxylase during light/dark transitions. Plant Physiol. 1986;81:674–677. doi: 10.1104/pp.81.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janc JW, O'Leary MH, Cleland WW. A kinetic investigation of phosphoenolpyruvate carboxylase from Zea mays. Biochemistry. 1992;31:6421–6426. doi: 10.1021/bi00143a009. [DOI] [PubMed] [Google Scholar]
- Jenkins CLD, Furbank RT, Hatch MD. Mechanism of C4 photosynthesis. Plant Physiol. 1989;91:1372–1381. doi: 10.1104/pp.91.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Chollet R. Light/dark regulation of maize leaf phosphoenolpyruvate carboxylase by in vivo phosphorylation. Arch Biochem Biophys. 1988;261:409–417. doi: 10.1016/0003-9861(88)90357-8. [DOI] [PubMed] [Google Scholar]
- Kleczkowski LA, Edwards GE. Hysteresis and reversible cold inactivation of maize phosphoenolpyruvate carboxylase. Z Naturforsch. 1990;45c:42–46. [Google Scholar]
- Laisk A, Edwards GE. CO2 and temperature-dependent induction in C4 photosynthesis: an approach to the hierarchy of rate-limiting processes. Aust J Plant Physiol. 1997;24:505–516. [Google Scholar]
- Leegood RC. The intercellular compartmentation of metabolites. Planta. 1985;164:163–171. doi: 10.1007/BF00396078. [DOI] [PubMed] [Google Scholar]
- Leegood RC, von Caemmerer S. Regulation of photosynthetic carbon assimilation in leaves of C3-C4 intermediate species of Moricandia and Flaveria. Planta. 1994;192:232–238. [Google Scholar]
- Marquardt DW. An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math. 1963;11:431–441. [Google Scholar]
- McNaughton GAL, Fewson CA, Wilkins MB, Nimmo HG. Purification, oligomerization state and malate sensitivity of maize leaf phosphoenolpyruvate carboxylase. Biochem J. 1989;261:349–355. doi: 10.1042/bj2610349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mújica-Jiménez C, Castellanos-Martínez A, Muñoz-Clares RA. Studies of the allosteric properties of maize leaf phosphoenolpyruvate carboxylase with the phosphoenolpyruvate analog phosphomycin as activator. Biochim Biophys Acta. 1998;1386:132–144. doi: 10.1016/s0167-4838(98)00093-4. [DOI] [PubMed] [Google Scholar]
- Nishikido T, Takanashi H. Glycine activation of PEP carboxylase from monocotyledonous C4 plants. Biochem Biophys Res Commun. 1973;53:126–133. doi: 10.1016/0006-291x(73)91410-1. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Kai T, Yabuta N, Izui K. Phosphoenolpyruvate carboxylase of maize leaves: an improved method for purification and reduction of the inhibitory effect of malate by ethylene glycol and bicarbonate. Plant Cell Physiol. 1997;38:76–80. [Google Scholar]
- Parvathi K, Bhagwat AS, Raghavendra AS. Modulation by bicarbonate of catalytic and regulatory properties of C4 phosphoenolpyruvate carboxylase from Amaranthus hypochondriacus: desensitization to malate and glucose 6-phosphate and sensitization to Mg2+ Plant Cell Physiol. 1998;39:1294–1298. [Google Scholar]
- Rajagopalan AV, Tirumala Devi M, Raghavendra AS. Patterns of phosphoenolpyruvate carboxylase activity and cytosolic pH during light activation and dark deactivation in C3 and C4 plants. Photosynth Res. 1993;38:51–60. doi: 10.1007/BF00015061. [DOI] [PubMed] [Google Scholar]
- Rand RP, Fuller NL, Butko P, Francis G, Nicholls P. Measured changes in protein solvation with substrate binding and turnover. Biochemistry. 1993;32:5925–5929. doi: 10.1021/bi00074a001. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Sotres R, López-Pozos R, Muñoz-Clares RA. Further studies of the short-term regulation of maize leaf phosphoenolpyruvate carboxylase by light. J Plant Physiol. 1987;129:191–199. [Google Scholar]
- Rodríguez-Sotres R, Muñoz-Clares RA. Kinetic evidence of the existence of a regulatory phosphoenolpyruvate binding site in maize leaf phosphoenolpyruvate carboxylase. Arch Biochem Biophys. 1990;276:180–190. doi: 10.1016/0003-9861(90)90025-t. [DOI] [PubMed] [Google Scholar]
- Segel IH. Enzyme Kinetics. New York: Wiley Interscience; 1975. [Google Scholar]
- Segel IH. Biochemical Calculations. Ed 2. New York: John Wiley & Sons; 1976. [Google Scholar]
- Stamatakis K, Skaliora I, Gavalas NA, Manetas Y. Re-evaluation of the effects of glucose 6-phosphate and malate on the catalytic properties of phosphoenolpyruvate carboxylase from Cynodon lactylon under physiological assay conditions. Aust J Plant Physiol. 1990;17:407–411. [Google Scholar]
- Stitt M, Heldt HW. Generation and maintenance of concentration gradients between the mesophyll and bundle sheath in maize leaves. Biochim Biophys Acta. 1985;808:400–414. [Google Scholar]
- Tovar-Méndez A, Mújica-Jiménez C, Muñoz-Clares RA. Desensitization to glucose 6-phosphate of phosphoenolpyruvate carboxylase from maize leaves by pyridoxal 5′-phosphate. Biochim Biophys Acta. 1997;1337:207–216. doi: 10.1016/s0167-4838(96)00166-5. [DOI] [PubMed] [Google Scholar]
- Tovar-Méndez A, Rodríguez-Sotres R, López-Valentín D, Muñoz-Clares RA. Re-examination of the roles of PEP and Mg2+ in the reaction catalysed by the phosphorylated and non-phosphorylated forms of phosphoenolpyruvate carboxylase from leaves of Zea mays: effects of the activators glucose 6-phosphate and glycine. Biochem J. 1998;332:633–642. doi: 10.1042/bj3320633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Méndez A, Yampara-Iquise H, Mújica-Jiménez C, Muñoz-Clares RA. Binding of ligands to the glucose-6-phosphate allosteric site in maize-leaf phosphoenolpyruvate carboxylase. In: Mathis P, editor. Photosynthesis: From Light to Biosphere. Vol. 5. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 155–158. [Google Scholar]
- Uedan K, Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976;57:906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H. Variations in the photosynthesis rate and activity of photosynthetic enzymes in maize leaf tissue of different ages. Plant Cell Physiol. 1984;25:1297–1301. [Google Scholar]
- Wedding RT, Rustin P, Meyer CR, Black MK. Kinetic studies of the form of substrate bound by phosphoenolpyruvate carboxylase. Plant Physiol. 1988;88:976–979. doi: 10.1104/pp.88.4.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H, Heldt HW. Inter- and intracellular distribution of amino acids and other metabolites in maize (Zea mays) leaves. Planta. 1992;187:242–246. doi: 10.1007/BF00201946. [DOI] [PubMed] [Google Scholar]
- Wold F, Ballou CE. Studies on the enzyme enolase. J Biol Chem. 1957;227:301–312. [PubMed] [Google Scholar]
- Wong KF, Davies DD. Regulation of phosphoenolpyruvate carboxylase of Zea mays by metabolites. Biochem J. 1973;131:451–458. doi: 10.1042/bj1310451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki Y, Asukagawa N, Ishikawa Y, Ohta E, Sakata M. Estimation of cytoplasmic free Mg2+ levels and phosphorylation potentials in mung bean root tips by in vivo31P NMR spectroscopy. Plant Cell Physiol. 1988;29:919–924. [Google Scholar]