Abstract
Nanotechnology offers several advantages for drug delivery. However, there is the need for addressing potential safety concerns regarding the adverse health effects of these unique materials. Some such effects may occur due to undesirable interactions between nanoparticles and the immune system, and they may include hypersensitivity reactions, immunosuppression, and immunostimulation. While strategies, models, and approaches for studying the immunological safety of various engineered nanoparticles, including metal oxides, have been covered in the current literature, little attention has been given to the interactions between iron oxide–based nanomaterials and various components of the immune system. Here we provide a comprehensive review of studies investigating the effects of iron oxides and iron-based nanoparticles on various types of immune cells, highlight current gaps in the understanding of the structure–activity relationships of these materials, and propose a framework for capturing their immunotoxicity to streamline comparative studies between various types of iron-based formulations.
Keywords: nanoparticles, immunotoxicity, immunotherapy, drug delivery, imaging
Graphical Abstract
Strategy for developing an experimental framework to assess immunotoxicity of IONPs.
IONPs have variety of effects on the immune system. Clinical translation of these formulations is often delayed or even halted due to the immunotoxicity. Here we review the literature and suggest translational considerations. The key recommendation of the review is the strategy for developing experimental framework to assess IONPs immunotoxicity. The framework can be applied to compare between different types of IONPs, as well as between brand and generic version of the same type of iron-based complex drug formulation.
1. Introduction
Nanotechnology gained much attention during the past decade due to nanoparticles’ physicochemical properties that allow investigators to cover gaps and overcome barriers in various industrial and biomedical applications [1–3]. One such barrier is related to the delivery of therapeutic and imaging agents [3]. Nanoparticles help to address this by providing the unique advantages of traceability, imaging, drug conjugation, and drug encapsulation capabilities; optimizing drug biodistribution and pharmacokinetics profiles; targeting drug delivery to the tissue of interest; and possessing antibacterial and adjuvant properties [1, 2, 4, 5]. Many different types of nanoparticles, including but not limited to liposomes, nanoemulsions, micelles, solid lipid nanoparticles, dendrimers, carbon nanotubes, fullerenes, metal colloids, and metal oxides, have been created and described in the current literature. Each type of these novel materials is characterized by a unique set of properties in addition to their class-defining size, in at least one dimension, to be within a submicron range. For example, metallic nanoparticles composed of iron oxide, cobalt ferrite, and nickel ferrite possess magnetic properties, which allow for tissue imaging and magnet-guided delivery into cells and tissues [6–10]. Colloidal gold and silver, iron oxides, and platinum nanoparticles have been engineered for use in therapeutic applications, diagnostics, and a combination thereof, known as “theranostics” [11]. In addition to their type-specific properties, each nanoparticle is unique due to a specific set of physicochemical properties (size, charge, zeta potential, surface functionality, hydrophobicity, etc.), giving specific characteristics to the formulation [12].
Despite the benefits of nanotechnology carriers, their use always requires assessment of potential safety concerns regarding the environmental, occupational, and adverse health effects. Some of these adverse effects include hypersensitivity reactions, immunosuppression, and immunostimulation, which may result from undesirable interactions between the immune system and nanoparticles [13]. Therefore, evaluation of their immunotoxicity requires special consideration. Strategies, models, and approaches for studying nanoparticle effects on the immune system have been extensively discussed elsewhere [14–16]. Earlier publications have also covered the immunotoxicity of various types of engineered nanomaterials [13, 15], reviewed the biomedical utility of metallic nanoparticles [11, 17], and even attempted to get mechanistic insights into the cytotoxicity of iron oxide nanoparticles (IONPs) as well as to identify the potential contribution of the coating to the cytotoxicity [18]. However, little attention in the current literature is given to interactions between IONPs and various components of the immune system. To cover this gap, in this review we focus on the immunotoxicity of IONPs. While these materials were previously considered to be relatively safe and biocompatible and were among the first nanoformulations to reach a clinical stage (Table 1, reviewed in [19–21]), recent studies have uncovered several toxicities that were previously unnoticed (reviewed in [22]). This observation prompted us to re-evaluate the literature about IONPs’ effects on the immune system.
Table 1. Iron-containing nonbiological complex drugs and their current status.
Formulations of iron-containing complex drugs are summarized along with the information regarding their composition, indication, FDA approval status, manufacturer, and (when applicable) reason(s) for withdrawal from market. N.A. – not applicable; Inj. – injection; IONP – iron oxide nanoparticles
| Formulation Name | Description of formulation |
Coating Material | Clinical Application | FDA status | Reason for Withdrawal |
Manufacturer | Brand/ Generic |
References |
|---|---|---|---|---|---|---|---|---|
| Feridex I.V/Ferumoxide/Endorem | IONP | Dextran | Liver imaging | Withdrawn (2008) | Hypotension, lumbar pain/leg pain, local pain, and hypersensitivity | Bayer HealthCare | Brand | [21, 50] |
| Resovist/Cliavist | IONP | Carboxydextran | Liver imaging | Withdrawn (2009) | Vasodilatation and paraesthesia | Bayer HealthCare | Brand | [29, 50] |
| Combidex/Ferumoxtran-10/Sinerem | IONP | Dextran | Prostate cancer lymph node metastases MRI | Withdrawn (2007) | Anaphylactic shock (1) and lack of improved efficacy | Advanced Magnetics, Inc./AMAG Pharma | Brand | [21, 22] |
| Feraheme/Ferumoxytol | IONP | Polyglucose sorbitol carboxymethyl ether | Iron deficiency in adult chronic kidney disease patients | Approved (2009) | N.A. | AMAG Pharma | Brand | [54, 55] |
| Lumirem/Gastromark | IONP | Silica | MRI of gastrointestinal lumen | Approved (1996) | N.A. | Guerbet | Brand | |
| Clariscan/Feruglose | Iron Dextran Inj. | Carbohydrate polyethylene glycol | MR angiography, tumor microvasculature MRI | Development discontinued | . | GE Healthcare | N.A. | [50, 51] |
| Ferrisat | . | Dextran | Iron-deficient anemia | Approved (1992) | N.A. | In France/Generic by Bayer in the US | Brand/Generic | |
| Venofer | Iron Sucrose Inj. | Sucrose complex | Iron-deficient anemia following autologous stem cell transplantation | Approved (2000) | N.A. | American Regent, Inc. | Brand | |
| DexFerrum/DexIron/Infed | Iron Dextran | Dextran | Iron-deficient anemia | Approved (1996) | N.A. | American Reagent, Inc./Allergan | Generic/Brand | |
| Ferrlecit | Sodium ferric gluconate | Sodium gluconate complex | Iron-deficient anemia | Approved (1999) | N.A. | Sanofi, Inc. | Generic/Brand | |
| Injectafter/Ferinject | Ferric carboxymaltose | Carboxymaltose | Iron-deficient anemia | Approved (2013) | N.A. | Vifor Pharma/American Regent, Inc.(Luitpold) | Generic/Brand | |
| Nanotherm | IONP | Aminosilane | Magnetic thermal ablation | Approval awaited | N.A. | MagForce AG | Brand |
2. General overview and biomedical application of iron-based nanomaterials
In its elemental form, iron can exist in two states, Fe+2 and Fe+3, which can form eight different oxides. Among these, magnetite (Fe3O4), maghemite (γ-Fe2O3), and hematite (α-Fe2O3) are the most frequently used for biomedical applications due to their magnetic properties. Some IONPs possess superparamagnetic properties, which allow them to undergo magnetization only under a magnetic field. As such, these superparamagnetic IONPs (SPIONs) are useful for magnetic resonance imaging (MRI) and are used as contrast agents for diagnostic applications [23, 24]. It is generally accepted that three main categories of SPION exist based on the particle size. They include oral (large) SPIONs with a size between 300 nm and 1.5 μm, standard SPIONs (SSPIONs) with a size between 50 and 150 nm, and ultrasmall SPIONs (USPIONs) with a size less than 50 nm [25, 26]. The magnetic property of iron oxide is attributable to the T2 spin reduction in the absorbing tissues, which enables imaging of the organs labeled with iron oxide nanoparticles [27]. Various tumors, cells, and tissues in the central nervous system can be efficiently perfused with SPIONs. Several SPION-based formulations have been approved by the United States Food and Drug Administration (FDA) for use as imaging contrast agents [28, 29] (Table 1). Due to their smaller size, SSPIONs and USPIONs can be administered via an intravenous (i.v.) route, which is useful for broader applications in MRI, whereas administration of oral SPION is limited to the gastrointestinal tract. Several efforts have been made towards cell-specific tracking using MRI. For example, a dextran-coated SPION, FeraTrack Direct®, was found to accumulate in stem cells and immune cells with minimal or nonsignificant toxicity and functional alteration [30]. This platform was suggested for MRI-based cell tracking in cancer patients.
Apart from their single-mode diagnostic utility, SPIONs also make suitable platforms for theranostic and therapeutic applications [31]. The magnetic properties of IONPs have been sought to develop therapeutics for targeted drug delivery, including various cancer treatments [32–35]. The surface of IONPs can be engineered to achieve longer circulation time and, thus, improved accumulation in tumors due to the so-called enhanced permeability and retention effect caused by the leaky nature of neovasculature [36]. SPION accumulation in tumors can be guided through the external magnetic field. Along with this tool, the addition of tumor-specific targeting moieties to the SPIONs’ surfaces can further improve their accumulation in the tumor and decrease off-target effects. Some IONPs can generate hyperthermia due to magnetic field alteration [37]. This property provides an additional benefit to cancer therapy in that, in addition to the direct elimination of cancer cells by heat, it can enable a tumor-specific immune response that eradicates secondary tumors [38]. Multifunctional IONPs containing an iron oxide core, surface coating(s), and a cytotoxic drug can also be used in combination with hyperthermia to further substantiate an antitumor response. For example, IONPs with a phospholipid–polyethylene glycol (PEG) coating and doxorubicin (DXR) demonstrated sustained drug release and increased apoptosis of cancer cells, which was further enhanced by hyperthermia [39]. Similarly, hyaluronic acid–modified IONPs with gold nanoshells provided a multifunctional capability that included both imaging and thermal ablation properties [40], while IONPs coated with a thiol-functionalized organo-silica shell demonstrated a multimodal imaging potential with fluorescent and magnetic properties [41]. Williams et al. demonstrated that FeraSpin R (a SPION formulation for preclinical MRI application)–loaded cytotoxic T lymphocytes are a potential tool for eradicating HIV-infected latent reservoirs with thermal ablation at physiologically hyperthermal temperatures without significantly altering normal T-cell function [42]. Another approach for therapeutic applications of IONPs could be gene- and biomarker-specific targeting. Janko et al. demonstrated targeted apoptosis and necrosis in tumor cells by using SPION labeled with mitoxantrone, while reducing the off-target effect on healthy peripheral blood leukocytes [43]. Alternatively, due to their effect on M1 phenotype activation in macrophages, various IONP formulations can also be explored as a possible vaccine adjuvant [44]. More recently, Couto et al. developed an IONP-based chelating chromatography method to study metalloprotein secretion from monocytes and macrophages [45]. Although it is in a very early stage, this technology was found to be more efficient and cost-effective, and it may be expanded for broader proteomics and metabolomics applications. With a similar approach, Liu et al. functionalized the SPION with HDAC5- and miRNA-specific labeling to detect the levels of HDAC5 mRNA in vivo using contrast-enhanced (MCE)–MRI [46], demonstrating a novel application in epigenetics. More recently, several studies have indicated a possible application of IONP formulations in anticancer treatments [38–40, 47–49]. A recent study using Feraheme® reported its anticancer potential via induction of pro-inflammatory macrophage polarization in tumor tissues [48].
3. Clinical experience
Following FDA approval of the first iron dextran formulation, Ferrisat, in 1992, several other products, including those containing IONPs, have gone through clinical trials (Table 1). The development of some of these formulations was halted by safety concerns. For example, Clariscan™, an MR angiography agent [50], was discontinued due to rising concerns about long-term liver toxicity [51]. Several previously approved IONP formulations have been withdrawn from clinical use due to a variety of reasons. For instance, Feridex® and Resovist®, initially approved for liver imaging in 1996, were discontinued in 2008 and 2009, respectively. In both cases, the reason for discontinuation was undesirable side effects. In the case of Feridex, they included hypotension, lumbar pain, and leg cramps [21, 50]; side effects of Resovist were vasodilatation and paraesthesia [50]. Another formulation intended for lymph node metastasis, Combidex (also known as Ferumoxtran-10 and Sinerem), was initially given conditional approval by the FDA in 2000. Several years later, in 2007, it was discontinued worldwide except for Holland [21, 22]. The main indication of current clinical formulations containing IONPs is iron-replacement therapy. In general, these formulations have a better risk–benefit outcome than iron-based imaging agents. The toxicity of iron-based complex formulations and IONPs is thought to be due to the labile iron, while hypersensitivity reactions, commonly reported for these formulations, are attributed to the coating. For example, sodium ferric gluconate complex in sucrose induced complement activation-related pseudoallergy in 3% of patients enrolled in a multicenter randomized trial [52, 53]. A similar observation was reported with IONPs coated with polyglucose sorbitol carboxymethyl ether (Feraheme), which is indicated for iron-replacement therapy in patients with chronic kidney disease. Clinical use of this formulation is often associated with severe hypersensitivity reactions. As such, the FDA has issued a cautionary warning and updated industry guidelines regarding the safety of this product [54, 55]. Patients with a prior history of allergic reactions to Feraheme or other drugs, as well as elderly patients with multiple serious medical conditions, are deemed to be at higher risk. The mechanism(s) underlying these reactions are poorly understood.
4. Immunotoxicity of iron-based nanoparticles
Interactions with various types of immune cells
Monocytes
The mononuclear phagocytic system (MPS) plays a central role in innate and adaptive immunity. Phagocytosis is the primary function of cells composing the MPS. These cells originate from a common precursor in the bone marrow; they circulate in peripheral blood, can become activated in response to various internal and external stimuli, mature, and can enter various tissues. Monocytes represent an essential component of the MPS. Along with other phagocytes, such as neutrophils and macrophages, they patrol the body for invading particulates. Monocytes are heterogeneous and vary in their expression of surface markers. They can be recruited from blood to the sites of inflammation, as well as be the source of tissue-resident macrophages and monocyte-derived dendritic cells. Early studies on IONPs focused on the intracellular magnetic labelling of monocytes [56, 57]. This utility is still under investigation [30, 58, 59]. The primary advantage of IONPs for this application was initially well-perceived among the groups studying monocyte infiltration into the brain during neurodegenerative disorders such as ischemia [60], experimental allergic encephalomyelitis [61, 62], and HIV-associated dementia [63]. However, more recent studies suggested that IONPs are not as immunologically inert as a cell-labeling agent should be. Several research groups demonstrated an increased accumulation of IONPs in U937 monocytes [64], stimulation of a Th1-biased immune response [65], and monocyte-driven endothelial cell dysfunction leading to atherosclerosis [66]. Together, these findings prompted further assessment of IONP-mediated immunotoxicity and genotoxicity analysis in various systems. Zhu et al. reported that IONPs could activate monocytes, which in turn, through production of secondary messengers, results in an increase in cytoplasmic vacuolation, mitochondrial swelling, and death of human aortic endothelial cells [66]. Importantly, this observation also suggests that a possible toxicity of IONPs leads to cardiovascular complications. Similarly, PEG-coated IONPs increased reactive oxygen species (ROS), tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL1β), and mitochondrial dysfunction in THP-1 human monocytes [67]. Another recent study reported that starch-coated IONPs alter subtle features, such as the cytoskeleton and ion channel functions, in monocytes without any apparent increase in IL1β and IL10 secretion [68]. In contrast, polyvinyl alcohol (PVA)–coated IONPs induced IL1β levels in monocytes without affecting the survival of these cells [69]. In line with these observations are the studies demonstrating that dextran-coated IONPs did not affect endothelial–monocytic cell interaction [70] and that carboxylic acid–functionalized IONPs prevented inflammatory cytokine response (IL1β, TNFα, and IL6) in monocytes treated with bacterial lipopolysaccharide (LPS) [71]. These data may suggest that particle coating contributes to both cell survival and cytokine secretion in response to IONPs or other stimuli. However, the direct comparison between these studies is not straightforward because other physicochemical properties of these particles were also different. More studies are needed to further understand structure–activity relationships and the mechanisms of the multifaceted effects of IONPs on monocytes described in the current literature. Such studies are needed to clarify the recognition of the particles by these cells.
Macrophages
Macrophages are phagocytes residing in tissues and are both phenotypically and functionally diverse. Depending on their localization, these cells were given specific names. For example, liver-resident macrophages are called Kupffer cells; those located in the central nervous system are the microglial cells; and in the lungs and bones, they are known as alveolar macrophages and osteoclasts, respectively. Their primary functions are the detection; phagocytosis; and killing of pathogens, apoptotic cells, and damaged host cells. Besides ingesting and clearing microbes and altered host cells, macrophages function as antigen-presenting cells (APCs) and thereby contribute to the effector phase of T-cell-mediated immunity. Macrophages promote the repair of damaged tissues by stimulating angiogenesis (formation of new blood vessels) and fibrosis (formation of the collagen-rich extracellular matrix). Depending on the activating stimulus, macrophages are subdivided into two subtypes that are distinct in their functional capabilities. Classically activated macrophages are efficient in killing the microbes, while alternatively activated macrophages promote tissue repair. Other subtypes of macrophages include epithelioid and giant cells.
Many studies utilized IONPs for in vivo labeling of tissue macrophages [72–76]. For example, ferumoxytol accumulated in both the infiltrating macrophages and microglial cells in the brain and performed better than a gadolinium-based MRI agent for the estimation of disease severity in an animal model of multiple sclerosis [76]. The IONPs’ uptake by macrophages correlated with clinical scores of the disease, suggesting that these nanoparticles could be used as clinical diagnostic agents for conditions associated with macrophage accumulation (e.g., inflammation, cancer immunotherapy, and autoimmune diseases). Similarly, Zhu et al. reported increased accumulation of ferric oxide nanoparticles in rat alveolar macrophages and RAW264.7 cells [64]. However, stress responses and cytotoxicity of the nanoparticles to macrophages could not be discounted. For example, microarray analysis performed in IONP-treated mouse macrophages revealed extensive reprogramming of more than 500 genes, many of which are involved in oxidative stress and inflammatory responses [77, 78]. More detailed mechanistic studies demonstrated perturbation in a variety of molecular pathways. Notably, Mulens et al. reported that both murine and human macrophage cell lines treated with polyethyleneimine (PEI)–coated IONPs showed activation of toll-like receptor 4 (TLR4)–mediated signaling and ROS production via the p38, ERK1/2, and JNK MAPK pathways [44]. This pathway further activated macrophages, as reported, by upregulating IL12, CD40, CD80, and CD86 expression, indicating M1 polarization [44]. Similarly, PVA-coated IONPs also induced IL1β levels in human monocytes, as well as the monocytes’ differentiation into monocyte-derived macrophages [69]. However, in a similar study, Venofer, Ferinject, and Ferrlecit reduced the differentiation of monocytes into M1 macrophages and myeloid dendritic cells [79], suggesting that the M1-polarized differentiation via IONPs observed in the earlier studies could be driven by the coating material. It is important to note that the IONPs used by Mulens et al. [44] and Strehl et al. [69] were not screened for endotoxins, which are common contaminants in engineered nanomaterials and activate the immune cells via TLR4 and its downstream signaling cascades. Ferucarbotran was also reported to activate RAW264.7 macrophages via an increase in oxidative stress, decrease in mitochondrial membrane potential, and increase in cell proliferation within 24 hours [80]. In contrast, magnetite and maghemite affected the cell viability of macrophages through a variety of pathways, including endoplasmic reticulum stress, mitochondrial damage, and autophagy, wherein magnetite particles were more toxic to macrophages than their maghemite counterparts [81, 82]. The induction of autophagy by bare-IONPs was correlated with activation of the ERK, but not JNK, pathway [83]. Implantation of a subcutaneous patch containing a SPION–albumin complex resulted in the accumulation of macrophages at the implantation site and local tissue inflammation [84]. Similarly, the intranasal administration of ferucarbotran in mice leads to the proliferation and activation of microglial cells [80]. However, in the latter case, no significant alteration in inflammatory cytokine secretion was detected [80].
Lymphocytes
Lymphocytes are the principal cells in both the humoral and cellular immune response. They specifically recognize and respond to antigens. Two main types of lymphocytes are the B cells, which recognize extracellular and cell-surface antigens and produce antibodies, and the T cells, which recognize antigens of intracellular microbes and either directly kill the infected cells or assist the MPS in destroying them. There are multiple subtypes of B and T lymphocytes, which differ from other cells in respective type by both expression of surface markers and function. While all lymphocytes can internalize molecules via endocytosis and pinocytosis, only B cells are phagocytic. The ability of lymphocytes to take up nanoparticles is substantially lower than that of monocytes and macrophages. Nevertheless, the internalization of nanoparticles by lymphocytes has been described in some studies. For example, an in vivo study with cross-linked iron oxide nanoparticles showed no detectable particle presence in B cells or T cells [76]. In contrast, another study reported IONP uptake by T cells via adsorptive pinocytosis or receptor-mediated endocytosis [85]. IONPs can be targeted to T cells for improved uptake and specific cell labeling. For example, IONPs derivatized with HIV Tat proteins, as well as monocrystalline IONPs, were proposed for use as MRI contrast agents to study the distribution of injected T cells [85–88].
The current understanding of IONPs’ effects on T-cell function is incomplete. Several earlier studies attempted to investigate how IONPs influence T-cell function and found no profound effects [89, 90]. In one study, mouse CD4+ T cells labeled with Feridex did not demonstrate any change in the proliferation profile or the levels of the secreted cytokines interferon gamma (IFNγ), IL4 and IL10 [89]. Likewise, uptake of IONPs by T cells was not accompanied by any measurable effects on T-cell functions [85]. More recent studies, however, demonstrated that IONPs are not immunologically inert and may affect specific molecular pathways and processes in T cells. For example, cytotoxic T cells treated with Resovist showed a slight delay in proliferation rate without any noticeable change in the cytotoxicity [90]. Using Jurkat T cells, Yan et al. demonstrated that IONPs were not cytotoxic despite the suppression of Kv1.3 channels, which is known to activate Ca+2 signaling [91]. The authors also reported an increase in NADP levels with a concomitant decrease in NADPH levels and suppression of IFNγ, IL2, and IFN response genes [91]. Collectively these data suggested a potential anti-inflammatory effect of IONPs on T cells. Another study reported that IONPs are cytotoxic to T-cell line A3 in both a size- and surface coating–dependent manner [92]. Since the interaction between T cells and APCs is critical for T-cell function, in vitro studies using purified T cells or T-cell lines are limited in the spectrum of questions they can address. In contrast, in vivo studies address this limitation by allowing researchers to investigate the full spectrum of nanoparticle effects on T cells in context with other components of the immune system.
IONPs’ effects on T cells in vivo depend on many factors, including nanoparticle physicochemical properties, dose, and the route of administration. Most importantly, IONP immunotoxicity encompasses opposite responses, including both stimulatory (T-cell-mediated delayed-type hypersensitivity [DTH]) and inhibitory (immunosuppression) reactions. Mechanistic studies, which may explain such a drastic difference in T-cell-mediated immune responses to IONPs, are missing. Below we review several descriptive studies illustrating the vast range of such effects.
The systemic administration of a low dose (5.14 mg/kg) of magnetic IONPs to mice via an i.v. route increased both the number of CD4+ and CD8+ T cells and the cytokines (IFNγ, IL10, and IL2) produced by the activated T cells [93]. Similarly, i.v. administration of polyacrylic acid (PAA)–coated IONPs increased lymphocyte infiltration in the liver [94]. In contrast, amino-PVA–coated SPIONs showed no effect on CD4+ T cells, as measured by their activation state and apoptosis [95]. Together, these reports support the earlier notion that surface coating material contributes to IONPs’ effects on the T-cell-mediated immune response. When magnetic IONPs were orally administered to mice, an increase in CD4+ and CD8+ T cells was detected at medium and high doses (600 and 1,200 mg/kg, respectively) [96]. In line with these reports, Zhu et al. demonstrated Th1 polarization and exaggeration of DTH reaction to ovalbumin (OVA) in both OVA-sensitized [97] and unchallenged [98] BALB/c mice, indicating the immunostimulatory effect of magnetic IONPs. In contrast, a series of studies by Jan et al. demonstrated the immunosuppressive effects of a carboxydextran-coated IONP, Resovist, in mice [99–101]. Notably, the attenuation of DTH parameters—such as lower IFNγ, IL6, and TNFα; reduction of T-cell infiltration; and footpad swelling at the inflammation site—were observed after animals were exposed to a single i.v. dose of Resovist [100]. In other studies from this group, Resovist resulted in a reduction in the antigen-specific T-cell antibody response and was attributable to reduced glutathione levels [99, 101]. One potential explanation for the difference in findings by Zhu et al. [97, 98] and by Jan et al. [99–101] is the route of administration. While Jan et al. used an i.v. route, Zhu et al. exposed animals to IONPs via intratracheal instillation, which activated airway exosomes in the lungs and resulted in T-cell activation, either directly or via dendritic cell (DC)–mediated antigen presentation. Other factors contributing to the discrepancy may include mouse strains and the dose of IONPs.
The ability of certain IONPs to activate T cells and promote T-cell-mediated immunity is explored in vaccines and immunotherapies for cancer. Certain IONPs can generate heat after exposure to an alternating magnetic field, and the resultant hyperthermia is efficient in eliminating tumors. In addition to the direct hyperthermia-mediated cytotoxicity, Bionized NanoFerrite (BNF) starch–coated IONPs can activate the immune response, leading to the generation of tumor-specific cytotoxic T cells [38]. In a local hyperthermia model for anticancer application, the IONPs increased the activation of CD8+ T cells in the draining lymph nodes and thereby provided tumor-specific resistance towards secondary tumors, including those formed due to metastasis [38]. Similarly, melanoma-targeted IONPs induced tumor-specific cytotoxic T lymphocytes (CTLs) following hyperthermia triggered by a magnetic field. The mechanism of CTL induction involved heat shock protein 72 (Hsp72) [102]. When investigated as vaccine adjuvants, the IONPs were found to be efficient in inducing both Th1 response and CTLs. For example, magnetic IONPs (Fe3O4) promoted a Th1-mediated immunity surpassing traditional adjuvant alum [103]. When assessed in an H22 hepatic tumor model, IONPs conjugated with inactivated H22 tumor cells (tumor vaccine); stimulated a CTL response; and resulted in higher IFNγ levels, lower IL4 levels, and reduction in tumor growth [103]. Several groups demonstrated IONPs’ utility as imaging agents to detect natural killer (NK) cells in the tumor microenvironment [104–108] and monitor activated platelets in cardiovascular diseases [109, 110]. However, apart from the observation that NK-cell apoptosis occurred after loading the cell with a high concentration of IONPs [105], the effect of these particles on NK cells is largely unknown.
Dendritic Cells
DCs are the most specialized APCs. They capture external antigens, process them, and transfer them to lymphatic tissue where they are presented to naïve T lymphocytes. Effector cells (mononuclear phagocytes, T cells, and other leukocytes) are needed for the effective elimination of the antigens. APCs communicate with effector cells and other host cells during various stages of immune responses via cytokines and other secondary messenger molecules. Multiple subsets of DCs have been described, and they include classical myeloid, plasmacytoid, monocyte-derived inflammatory, and follicular DCs, as well as Langerhans cells. The alteration of DC function may lead to severe alterations in both innate and adaptive immunity and, therefore, requires close attention. Below we summarize studies investigating IONPs’ effects on DCs.
Several studies reported that IONPs may inhibit DC function. IONPs inhibited antigen presentation in monocyte-derived dendritic cells (MDDCs) without affecting their phenotypic properties [111]. Moreover, this inhibition of antigen presentation via MDDCs resulted in the suppression of a variety of cytokines and chemokines from T cells [111]. Similarly, loading DCs with a low dose of IONPs led to a reduction in T-cell proliferation in response to LPS and Tetanus Toxoid [111]. Feridex treatment, only at higher concentrations (>400 μg/mL), reduced DC viability without affecting their maturation [112], whereas Endorem reduced the migratory properties of the DCs [112, 113]. Contrary to these reports, Mou et al. demonstrated no deleterious effects of IONPs on DCs, as assessed by their ability to activate T cells [114] or migrate [115]. The difference in nanoparticle coating and dose level may contribute to the difference between the studies that report the adverse effects of IONPs on DCs [111–113] and those that do not [114, 115]. This observation is consistent with other studies we reviewed earlier when we discussed IONPs’ effects on monocytes, macrophages, and lymphocytes.
Other blood cells
Unlike research on monocytes, macrophages, DCs, and T and B lymphocytes, studies investigating IONPs’ effects on other blood cells are scarce. The results of studies investigating the toxicity of IONPs to platelets are controversial. PAA-coated IONPs did not activate platelets and did not induce their aggregation, but they inhibited arachidonic acid–mediated platelet aggregation, suggesting that these IONPs are anticoagulants [116]. In contrast, USPIONs induced platelet aggregation in a concentration-dependent manner, suggesting that these particles are pro-thrombogenic [117]. The use of particles with different surface coatings and platelets from different species in these studies complicates comparison between the results. Similarly, few studies investigated IONPs’ effects on erythrocyte membranes [118, 119]. While some studies reported IONPs mediated hemagglutination without evident hemolysis [120], others suggested that IONPs can in fact damage erythrocytes [121]. Interestingly, one study recommended eryptosis, a suicidal erythrocyte death, as a better marker of IONPs’ compatibility with erythrocytes than the traditional hemolysis test [122]. In contrast to these observations, another study suggested that IONPs can protect erythrocyte membranes from photosensitizer-mediated damage [123]. Since different particles and different methods for erythrocyte analysis were used in these studies, comparing the test results to understand the structure–activity relationship does not allow us to make accurate conclusions.
Structure–Activity Relationship
It is well-established that nanoparticle physicochemical properties (size, charge, surface functionalities, hydrophobicity, etc.) determine particle interaction with the immune system [13]. Conducting structure–activity relationship studies with IONPs, however, is challenged by the fact that a change in one parameter (e.g., surface functionality) often leads to a change in another one (e.g., particle size), which complicates conclusions about the initial variable (surface functionality in our example). Another challenge to understand the immunological structure–activity relationship of IONPs is the absence of a systematic approach, as most available studies focus on short-term acute responses, often cytotoxicity in one or a few immune cell lines, which do not accurately model a potential change in the immune system’s function. Moreover, IONPs’ core material is involved in many cellular processes, and iron imbalance is known to disrupt homeostasis, which leads to a variety of disorders, including those mediated by altered function of the immune system [124–127]. The immunoregulatory properties of iron and its binding with cell-surface proteins are well-documented [128–131]. Therefore, it is plausible to assume that IONPs may also exhibit a wide range of immunotoxicities due to the release of iron ions from the nanoparticle core, the property often not tracked in the structure–activity relationship studies. With these limitations in mind, below we will summarize some structure–activity relationship studies exploring potential influences of various IONPs’ physicochemical properties on the viability and function of immune cells.
Role of size
Several studies have attempted to gain insight into the role of the particle size in the IONPs’ biocompatibility. Regrettably, immunotoxicity was not investigated in these studies. Intuitively, one may expect that IONP size contributes to particle immunotoxicity. However, a systematic approach that includes multiple immune-cell types and their functions is needed to verify this hypothesis. Investigation of genotoxic effects in the Ames test demonstrated that small (~10 nm) PEG-coated IONPs are more mutagenic than their larger (~30 nm) counterparts or PEI–coated IONPs [132]. A comparison of pharmacokinetics profile, toxicity, and gene expression in response to IONPs of different size (10, 20, 30, and 40 nm) demonstrated differences in both biodistribution and toxicity [133]. Particularly, 10-nm IONPs were primarily detected in the liver, while their 40-nm counterparts were in the spleen. The effects of the particle on tissue-resident macrophages (red pulp macrophages in the spleen and Kupffer cells in the liver) and other immune cells were not investigated. Although IONPs were found to be largely nontoxic, alterations in the levels of various genes involved in oxidative stress, iron transport, metabolic processes, and apoptosis were described and were more pronounced when small particles (10 and 20 nm) were tested.
Role of shape
While the role of the size of the IONP is usually studied using nanoparticles with a spherical shape, IONPs also exist in other shapes, which may induce different levels of cellular response. This property could be attributed in part to the increase in surface area because particles with shapes other than spherical are deemed to have a larger surface area. For example, higher levels of TNFα, ROS, necrosis, and lactate dehydrogenase (LDH) release were observed in murine macrophages treated with rod-shaped IONPs than in cells treated with spherical particles [134]. Similarly, star-shaped IONPs induced higher TNFα levels and oxidative stress as compared to their spherical counterparts [135].
Role of surface functionalities
PEG is often used for particle coating to increase hydrophilicity and prolong particle circulation time. The general trend commonly observed with PEGylated particles is improved biocompatibility [136, 137]. However, some reports suggest the opposite effect. For example, PEG-coated IONPs were found to be more genotoxic than PEI-coated particles of a similar size [132]. The immunotoxicity associated with IONPs has been widely attributed to various surface coatings, including PAA, PEI, PEG, dextran, albumin, and dendrimers [138–140]. However, the trends observed with a particular coating type are inconsistent, likely because a change in the coating was also associated with a change in one or more other physicochemical parameters, and because different models were used to study the effect of the coating. A few examples are further discussed below. Branched PEI-coated SPIONs enhanced the Th1 polarization of human DCs [141]. An intraperitoneal injection of L-DOPA–coated IONPs in mice induced local inflammation and tissue lesions [142]. In contrast, dextran-coated IONPs were immunosuppressive, as assessed by the reduction in splenic lymphocyte proliferation and the secretion of pro-inflammatory cytokines IL1β, IL4, IL6, IL10, and TNFα [143]. Silica-coated IONPs resulted in a reduction in total glutathione, catalase, and glutathione reductase, as well as an increase in lipid peroxidation and protein carbonyl formation in CHSE-214 cells [144]. Reduced viability of endothelial cells was observed with lauric acid– and lauric acid–bovine serum albumin–coated IONPs but not with dextran-coated NPs [70]. No difference in viability and expression of TNFα in a human monocyte cell line (CRL-2367) was reported when IONPs with PEI, PEG, and PAA coatings were compared [18].
Role of zeta potential
The surface charge of the IONPs is largely attributed to the coating materials. However, the net effect of the particle charge may also produce various heterogeneity in response to the IONPs. Maurizi et al. reported the effect of surface charge by comparing anionic, 2,3-dimercaptosuccinic acid (DMSA)–PEG2000–coated IONPs with neutral, PEG2000-coated counterparts [145]. The negatively charged IONPs were internalized by the murine macrophage cell line RAW264.7 to a more significant extent than particles with the neutral surface. This finding was confirmed in vivo, wherein the negatively charged IONPs demonstrated greater uptake by the resident macrophages than their neutral counterparts. Similar observations were reported with Resovist, which contains a negatively charged carboxydextran coating [145]. The higher internalization of charged particles could be attributed to the greater opsonization with serum proteins, which promotes cellular uptake [146]. Cationic, PEI-coated IONPs resulted in an increase in ROS formation in U937 monocytes [147], as well as induced higher levels of LDH and lipid peroxides when compared to slightly less cationic counterparts in which the PEI coating was partially neutralized with PEG [147]. Cationic IONPs resulted in an increase in antigen cross-presentation and, consequently, T-cell activation, whereas their negatively charged counterparts were more potent in the induction of autophagy [148]. Together, these results suggest that optimization in the surface coating can be used to improve desirable interactions between IONPs and immune cells and reduce undesirable effects.
Mechanisms of immunotoxicity
Even though both immunosuppressive and immunostimulatory responses to IONPs have been documented in vivo and in vitro, the mechanism(s) underlying these toxicities remain mostly unknown. Below we review some examples suggesting the involvement of cytokines and stress-mediated responses.
Cytokine storm
Several studies have shown induction of a variety of cytokines/chemokines in response to IONP treatment. However, the available data are controversial, and comparison between the studies is challenging due to differences in the immune-cell types, particle physicochemical properties, concentrations, doses, routes of administration, and time employed. No change in IL1β, TNFα, IL6, or IL12 secretion by macrophages, DCs, and microglial cells was detected in vitro with silica-coated INOPs or Ferumoxtran-10 [80, 149–151]. In contrast, ferumoxide resulted in a significant increase in the IL1β release from macrophages in vitro; the effect was detectable at the highest tested concentration only [149]. The elevation of IL12, TNFα, and IFNγ levels—but not IL4 levels—was observed in vitro in macrophage and immature DC cultures treated with magnetic IONPs [98]. The authors attributed the observed cytokine levels to the formation of exosomes, which activated a Th1-type immune response and inflammation [98]. This finding was confirmed in vivo, where iron oxide accumulation in the lungs was associated with elevated levels of Th1 cytokines and chemokines in the bronchoalveolar lavage [65]. The authors of this study also observed a reduction in the helper-to-cytotoxic T-cell ratio and MHC-II expression, and they suggested that the observed alterations could onset the development of the DTH reaction to the IONPs [65]. Similarly, functionalized silica-coated IONPs increased IL6, IL8, and granulocyte-macrophage colony-stimulating factor in phytohemagglutinin-activated blood cells [152]. Interestingly, despite the activation of cytokine responses to IONPs, some other cell processes are affected oppositely. For example, protamine sulfate–modified ferumoxides activated THP-1 cells, as evidenced by the activation of NF-κB with no change in TNFα secretion or CD54 and CD80 expression; at the same time, the cells were less efficient in chemotaxis towards MCP-1 [153]. Inhibition of cytokine secretion by the immune cells has also been reported. For example, carboxydextran-coated IONPs inhibited IFNγ expression in OVA-activated T cells [99]. Similarly, Resovist suppressed TNFα in rat macrophages with or without LPS stimulation [154]. On the other hand, both Resovist and USPION particles (SH U 555C) increased IL10 in mouse macrophages, which was potentiated in the presence of LPS [154]. Some nanoparticles are not pro-inflammatory per se, but can exaggerate inflammation caused by microbial products [15]. This property has so far been observed with cationic and high-aspect-ratio nanomaterials and has resulted in lysosomal dysfunction [15]. However, IONPs tend to suppress inflammatory cytokines, such as IFNγ and IL4, and inhibit the antigen-mediated antibody response [101]. Although these studies provide the evidence for the role of particle surface functionalization in the induction of cytokine response, the overall conclusion and data interpretation are not straightforward. A major deficit in the current literature is related to the studied model in that the primary focus so far was on inflammatory cells, such as monocytes and macrophages. Relatively fewer studies focused on T cells and their function. While it is plausible to expect an immunomodulatory effect of IONPs on T-cell function, more studies and better experimental models are needed.
Oxidative stress
Oxidative stress has been reported in the current literature as the primary mechanism of IONPs’ cytotoxicity to immune cells. Under the normal homeostatic conditions, cells utilize iron in various biochemical processes, including ATP generation in the mitochondria, where iron serves as an electron exchanger during the electron transfer chain [155, 156]. Naturally, dysregulation of iron levels affects the mitochondrial homeostasis, leading to oxidative stress. ROS generated in response to IONP treatment activates Fenton or Haber–Weiss reactions, potentiating the damage [157]. The increase in oxidative stress depends on the tissue specificity and concentration of the IONPs [158]. For example, the exposure of hematite nanoparticles to inflammatory airways and lung-draining lymph nodes leads to cell death via excessive generation of ROS [159]. Further, in a comparative study using a nonclinical model, iron dextran was shown to produce oxidative stress, but iron sucrose and ferumoxytol were not [160]. Several other studies have demonstrated increased oxidative stress due to a variety of IONP formulations with different coatings. Regardless of the subcellular source of ROS, the oxidative stress induced by the IONPs primarily modulates the antioxidant enzymes, such as glutathione reductase, catalase, and superoxide dismutase, which consecutively increase lipid peroxidation, protein carbonyl formation, and free radical formation [22, 161]. For example, erythrocytes exposed to IONPs showed an increase in total ROS production, which could be reversed with the use of an antioxidant, N-acetyl cysteine [122]. Similarly, in a murine macrophage cell line and primary lymphocytes, IONPs increased ROS production, leading to endoplasmic reticulum stress and DNA damage, respectively, in addition to alteration in several redox gene expressions [162, 163]. The alteration in the redox status can further lead to perturbations in normal cell cycle progression [164]. Besides the cells from the immune compartment, several other tissues, such as the lungs, liver, skin, kidney, and brain, are also affected by the IONP-mediated ROS [165–169]. Taken together, an increase in oxidative stress triggered by the IONPs in immune cells raises concerns of system-wide toxicity. While excessive ROS production is toxic to the healthy cells, this attribute of IONPs could be a beneficial property for anticancer treatment. Recently, Huang et al. demonstrated the improved efficacy of the anticancer agent β-lapachone in the presence of IONPs via synergistic interaction to increase ROS and thereby increase tumor cell death [170]. However, a more careful approach is required while formulating the IONPs for anticancer therapeutics to avoid stress to other healthy compartments.
Other mechanisms
Apart from inflammation and oxidative stress, a handful of studies have attempted to elucidate IONP-mediated cytotoxicity via alternative mechanisms, such as endoplasmic reticulum stress and autophagy. Particularly, increased autophagy involving ERK pathway activation is observed in cells of the immune system, such as dendritic cells [148], macrophages [81], and total blood cells [171]. Also, studies from endothelial cells further explain the role of oxidative stress, mitochondrial damage, lysosomal activation, and endoplasmic reticulum stress [172–175], which may also contribute to IONPs’ toxicity in immune cells. Unlike conventional apoptosis, ferroptosis is a non-apoptotic cell death due to the defective antioxidant defense of the cells, primarily in response to iron [176]. The degradation of the iron-storage protein ferritin plays a major role in cross-talk between autophagy and ferroptosis [177]. Therefore, the role of ferroptosis in a variety of physiological responses requires further attention.
6. Translational considerations for improving immunological compatibility of iron nanoparticles
To inform the design of safe iron-based nanomaterials, a better understanding of the immunotoxicity of IONPs with different surface properties, size, hydrophobicity, and release of iron ions is needed. Systematic studies, which evaluate not only the cytotoxicity of IONPs on the immune cells but also include a broad spectrum of both structural and functional changes in various types of the immune cells under physiologically relevant conditions, are needed. Current literature contains evidence for both the immunosuppressive and immunostimulatory properties of IONPs. However, it is unclear what nanoparticle physicochemical parameter(s), route of administration, and dose are critical and what, if any, difference exists between different cell types. In addition to the conventional cytotoxicity response as assessed by cytokine secretion, oxidative stress, mitochondrial damage, and apoptotic signals, subtle features such as cytoskeletal structures and proteins such as actin and tubulin need to be assessed since changes in the cell structure may affect overall function. Similarly, various ion channels are present in immune cells and play vital roles in the maintenance of ionic concentrations in the cells. Therefore, the effect of IONPs on ion channels needs further evaluation in the assessment of IONP-mediated toxicity. Moreover, both immunosuppression and DTH were described for the same type of nanoparticle, for example, Feraheme (Dobrovolskaia M.A., unpublished observation). Collectively, these data suggest that an effect on the function of lymphocytes is highly probable; however, no comprehensive study is yet available. It is also essential to distinguish toxicities specific to the iron core and iron ion release from those triggered by the coating materials.
To assist the safe design of IONP and development of relevant generic formulations many traditional studies are used. The traditional approach could further benefit from an experimental framework that evaluates the critical mechanistic components of IONPs’ effects target immune cells (Figure 1). In such framework, one IONP formulation can be selected as a reference. Next, pre-clinical and/or clinical data available to this formulation are reviewed to identify toxicity typically observed with the formulation. Next, a mechanistic study is performed to identify molecular and cellular mechanisms of the observed immunotoxicity. The mechanistic study serves to identify functional endpoints representative of the immunotoxicity of the given IONP formulation. Next, several assays addressing such functional endpoints are grouped into a framework. Finally, the framework is used for comparison between brand and generic formulations of IONPs and assist bio(nano)similarity of these products (Figure 1). If proven, that the observed immunotoxicity is not unique to one particular IONP formulation and broadly applies to other IONPs, the framework could also be used to assess multiple IONP formulations.
Figure 1. Strategy for developing an experimental framework to assess the immunotoxicity of IONPs.
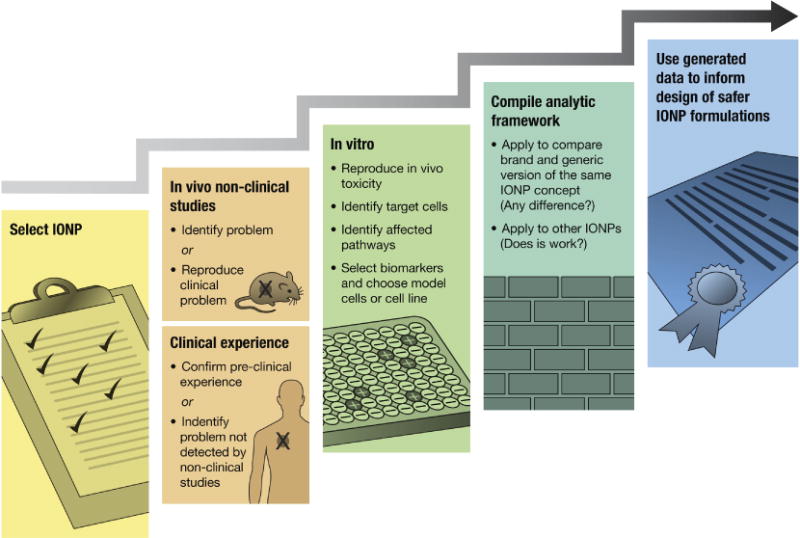
This strategy starts with focusing on a specific formulation (e.g., Feraheme). The clinical experience is examined either to identify the immunological problem that was not detected during preclinical studies or to confirm the findings of preclinical studies. In vivo nonclinical data is used to identify the problem. However, in the cases when standard toxicology studies fail to detect the immunotoxicity and it is later experienced in the clinical setting, additional functional in vivo nonclinical studies are used to reproduce the immunotoxicity relevant to the patient population. In vitro experiments are then conducted to examine the toxicity; understand the molecular and cellular mechanism(s); and identify target cells and affected pathways to select biomarkers. This can be monitored in the future batches of the same material, or to compare the brand product with a generic counterpart. Additional in vitro work can be done to select the appropriate model (e.g., to decide whether to proceed with primary cells or use a particular cell line). This information is used to compile a network of bioassays that accurately, consistently, and reproducibly identify selected biomarkers in relevant cell models. The framework is applied for comparing brand and generic formulations, and it can also be implemented for other particles from the same family. The immunotoxicity may be unique to the given formulation; in such a case, the framework can only be used for comparison between batches or between the brand and generic versions of the particular nanomaterial. If, however, the immunotoxicity is shared by an entire class of iron oxides, the framework becomes applicable to screen for a broader range of IONPs. The information generated in this research should be used to design safer IONPs.
7. Summary and future directions
In summary, despite extensive efforts to produce clinically safe iron oxide nanoformulations, there is a lack of more standardized guidelines for their evaluation. As discussed in the current review, a considerable body of literature on the toxicity of IONPs suggests that IONPs induce immune dysfunction. However, with the conventional approach, most studies focus on monocytes and macrophages, which serve as a primary line of defense in the immune system. Except for a handful of reports, there is a severe lack of mechanistic studies, which could deepen understanding of how the IONPs affect the long-term immune response and different types of immune cells. Moreover, additional in vivo models are required to more rigorously study the immune reactions in response to the IONP formulations. Differences in immune responses between various animal models (e.g. Th1-biased vs Th2-biased animals) have to be considered. Due to the genetic diversity between individuals, the sensitivity to the nanomaterials could also vary from patient to patient. Therefore, future studies require more careful assessment of patient-specific responses. A functional assay to predict how IONPs would interact with the immune system of the patient over a more extended period could provide a more accurate compatibility check. To design safer formulations, more studies need to focus on intracellular pathways that may be responsible for iron-mediated immunotoxicity. The translational considerations discussed above may assist in achieving this goal.
Highlights.
This manuscript provides review of the literature regarding immunotoxicity of iron oxide nanoparticles (IONPs). A considerable body of literature suggests that IONP induce immune dysfunction. However, most available studies focus on monocytes and macrophages and use cytotoxicity as the main end point of evaluation. The effects of these materials on the other types of immune cells and the mechanisms of toxicity are incompletely understood.
The available literature contains evidence for both immunosuppressive and immunostimulatory properties of IONPs. However, neither nanoparticle physicochemical parameter(s), route of administration, dose, nor any other critical parameter(s) for determining these toxicities are well-established.
Therefore, a better understanding of the immunotoxicity of IONPs with different surface properties, size, hydrophobicity, and release of iron ions is needed. It is also essential to distinguish between toxicities specific to the iron core, the released iron ions, and the coating materials.
Systematic studies, which evaluate not only the cytotoxicity of IONPs on the immune cells but also include a broad spectrum of both structural and functional changes in various types of immune cells under physiologically relevant conditions, are also required to fill the current gap.
To inform the safe design of IONPs and other iron-based nanomedicines, we propose an experimental framework, which could assist with evaluation of the critical mechanistic components of IONPs’ effects on the immune system. Such a framework could be used to compare brand and generic formulations of IONPs and assist with bio(nano)similarity evaluation of these products.
Acknowledgments
The study was supported in whole or in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sangtani A, Nag OK, Field LD, Breger JC, Delehanty JB. Multifunctional nanoparticle composites: progress in the use of soft and hard nanoparticles for drug delivery and imaging, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2017 doi: 10.1002/wnan.1466. [DOI] [PubMed] [Google Scholar]
- 2.Medina-Reyes EI, Garcia-Viacobo D, Carrero-Martinez FA, Chirino YI. Applications and Risks of Nanomaterials Used in Regenerative Medicine, Delivery Systems, Theranostics, and Therapy. Critical reviews in therapeutic drug carrier systems. 2017;34:35–61. doi: 10.1615/CritRevTherDrugCarrierSyst.2017016983. [DOI] [PubMed] [Google Scholar]
- 3.Anchordoquy TJ, Barenholz Y, Boraschi D, Chorny M, Decuzzi P, Dobrovolskaia MA, Farhangrazi ZS, Farrell D, Gabizon A, Ghandehari H, Godin B, La-Beck NM, Ljubimova J, Moghimi SM, Pagliaro L, Park JH, Peer D, Ruoslahti E, Serkova NJ, Simberg D. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS nano. 2017;11:12–18. doi: 10.1021/acsnano.6b08244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du J, Zhang YS, Hobson D, Hydbring P. Nanoparticles for immune system targeting. Drug discovery today. 2017 doi: 10.1016/j.drudis.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. International journal of nanomedicine. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelakeris M. Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochim Biophys Acta. 2017;1861:1642–1651. doi: 10.1016/j.bbagen.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Pol SV, Pol VG, Frydman A, Churilov GN, Gedanken A. Fabrication and magnetic properties of Ni nanospheres encapsulated in a fullerene-like carbon. J Phys Chem B. 2005;109:9495–9498. doi: 10.1021/jp050692j. [DOI] [PubMed] [Google Scholar]
- 8.Pouponneau P, Soulez G, Beaudoin G, Leroux JC, Martel S. MR imaging of therapeutic magnetic microcarriers guided by magnetic resonance navigation for targeted liver chemoembolization. Cardiovasc Intervent Radiol. 2014;37:784–790. doi: 10.1007/s00270-013-0770-4. [DOI] [PubMed] [Google Scholar]
- 9.Tu C, Louie AY. Nanoformulations for molecular MRI, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2012;4:448–457. doi: 10.1002/wnan.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puntes VF, Krishnan KM, Alivisatos AP. Colloidal nanocrystal shape and size control: the case of cobalt. Science. 2001;291:2115–2117. doi: 10.1126/science.1057553. [DOI] [PubMed] [Google Scholar]
- 11.Chapman S, Dobrovolskaia M, Farahani K, Goodwin A, Joshi A, Lee H, Meade T, Pomper M, Ptak K, Rao J, Singh R, Sridhar S, Stern S, Wang A, Weaver JB, Woloschak G, Yang L. Nanoparticles for cancer imaging: The good, the bad, and the promise. Nano today. 2013;8:454–460. doi: 10.1016/j.nantod.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 13.Dobrovolskaia MA, Shurin M, Shvedova AA. Current understanding of interactions between nanoparticles and the immune system. Toxicology and applied pharmacology. 2016;299:78–89. doi: 10.1016/j.taap.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekkers S, Oomen AG, Bleeker EA, Vandebriel RJ, Micheletti C, Cabellos J, Janer G, Fuentes N, Vazquez-Campos S, Borges T, Silva MJ, Prina-Mello A, Movia D, Nesslany F, Ribeiro AR, Leite PE, Groenewold M, Cassee FR, Sips AJ, Dijkzeul A, van Teunenbroek T, Wijnhoven SW. Towards a nanospecific approach for risk assessment. Regulatory toxicology and pharmacology: RTP. 2016;80:46–59. doi: 10.1016/j.yrtph.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Dobrovolskaia MA. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: Challenges, considerations and strategy. Journal of controlled release: official journal of the Controlled Release Society. 2015;220:571–583. doi: 10.1016/j.jconrel.2015.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedata P, Petrarca C, Garzillo EM, Di Gioacchino M. Immunotoxicological impact of occupational and environmental nanoparticles exposure: The influence of physical, chemical, and combined characteristics of the particles. International journal of immunopathology and pharmacology. 2016;29:343–353. doi: 10.1177/0394632015608933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soh JH, Gao Z. Complex-Shaped Metal Nanoparticles. Wiley-VCH Verlag GmbH & Co KGaA; 2012. Metal Nanoparticles in Biomedical Applications; pp. 477–519. [Google Scholar]
- 18.Xu Y, Sherwood JA, Lackey KH, Qin Y, Bao Y. The responses of immune cells to iron oxide nanoparticles. J Appl Toxicol. 2016;36:543–553. doi: 10.1002/jat.3282. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 20.Anselmo AC, Mitragotri S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015;17:1041–1054. doi: 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioengineering & Translational Medicine. 2016;1:10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh N, Jenkins GJ, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION) Nano Rev. 2010;1 doi: 10.3402/nano.v1i0.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin MM, Kim DK, El Haj AJ, Dobson J. Development of superparamagnetic iron oxide nanoparticles (SPIONS) for translation to clinical applications. IEEE Trans Nanobioscience. 2008;7:298–305. doi: 10.1109/TNB.2008.2011864. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63:24–46. doi: 10.1016/j.addr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Elias A, Tsourkas A. Imaging circulating cells and lymphoid tissues with iron oxide nanoparticles. Hematology Am Soc Hematol Educ Program. 2009:720–726. doi: 10.1182/asheducation-2009.1.720. [DOI] [PubMed] [Google Scholar]
- 26.Shan L. Molecular Imaging and Contrast Agent Database (MICAD) Bethesda (MD): 2004. Superparamagnetic iron oxide nanoparticles (SPION) stabilized by alginate. [PubMed] [Google Scholar]
- 27.Nakamura H, Ito N, Kotake F, Mizokami Y, Matsuoka T. Tumor-detecting capacity and clinical usefulness of SPIO-MRI in patients with hepatocellular carcinoma. J Gastroenterol. 2000;35:849–855. doi: 10.1007/s005350070022. [DOI] [PubMed] [Google Scholar]
- 28.Leung K. Molecular Imaging and Contrast Agent Database (MICAD) Bethesda (MD): 2004. Ferumoxides. [Google Scholar]
- 29.Reimer P, Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur Radiol. 2003;13:1266–1276. doi: 10.1007/s00330-002-1721-7. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Lewis B, Steiner MS, Bissa UV, Dose C, Frank JA. Superparamagnetic iron oxide nanoparticles for direct labeling of stem cells and in vivo MRI tracking. Contrast Media Mol Imaging. 2016;11:55–64. doi: 10.1002/cmmi.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TH, Lee S, Chen X. Nanotheranostics for personalized medicine. Expert Rev Mol Diagn. 2013;13:257–269. doi: 10.1586/erm.13.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Sotomayor R, Ahualli S, Viota JL, Rudzka K, Delgado AV. Iron/Magnetite Nanoparticles as Magnetic Delivery Systems for Antitumor Drugs. J Nanosci Nanotechnol. 2015;15:3507–3514. doi: 10.1166/jnn.2015.9856. [DOI] [PubMed] [Google Scholar]
- 33.Gunduz U, Keskin T, Tansik G, Mutlu P, Yalcin S, Unsoy G, Yakar A, Khodadust R, Gunduz G. Idarubicin-loaded folic acid conjugated magnetic nanoparticles as a targetable drug delivery system for breast cancer. Biomed Pharmacother. 2014;68:729–736. doi: 10.1016/j.biopha.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Prabha G, Raj V. Preparation and characterization of chitosan-Polyethylene glycol-polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. J Biomed Mater Res B Appl Biomater. 2016;104:808–816. doi: 10.1002/jbm.b.33637. [DOI] [PubMed] [Google Scholar]
- 35.Rodzinski A, Guduru R, Liang P, Hadjikhani A, Stewart T, Stimphil E, Runowicz C, Cote R, Altman N, Datar R, Khizroev S. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci Rep. 2016;6:20867. doi: 10.1038/srep20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Chatterjee DK, Diagaradjane P, Krishnan S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther Deliv. 2011;2:1001–1014. doi: 10.4155/tde.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toraya-Brown S, Sheen MR, Zhang P, Chen L, Baird JR, Demidenko E, Turk MJ, Hoopes PJ, Conejo-Garcia JR, Fiering S. Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine. 2014;10:1273–1285. doi: 10.1016/j.nano.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinto CA, Mohindra P, Tong S, Bao G. Multifunctional superparamagnetic iron oxide nanoparticles for combined chemotherapy and hyperthermia cancer treatment. Nanoscale. 2015;7:12728–12736. doi: 10.1039/c5nr02718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Hu Y, Yang J, Wei P, Sun W, Shen M, Zhang G, Shi X. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials. 2015;38:10–21. doi: 10.1016/j.biomaterials.2014.10.065. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura M, Hayashi K, Kubo H, Kanadani T, Harada M, Yogo T. Relaxometric property of organosilica nanoparticles internally functionalized with iron oxide and fluorescent dye for multimodal imaging. J Colloid Interface Sci. 2017;492:127–135. doi: 10.1016/j.jcis.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Williams JP, Southern P, Lissina A, Christian HC, Sewell AK, Phillips R, Pankhurst Q, Frater J. Application of magnetic field hyperthermia and superparamagnetic iron oxide nanoparticles to HIV-1-specific T-cell cytotoxicity. International journal of nanomedicine. 2013;8:2543–2554. doi: 10.2147/IJN.S44013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janko C, Durr S, Munoz LE, Lyer S, Chaurio R, Tietze R, Lohneysen S, Schorn C, Herrmann M, Alexiou C. Magnetic drug targeting reduces the chemotherapeutic burden on circulating leukocytes. Int J Mol Sci. 2013;14:7341–7355. doi: 10.3390/ijms14047341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulens-Arias V, Rojas JM, Perez-Yague S, Morales MP, Barber DF. Polyethylenimine-coated SPIONs trigger macrophage activation through TLR-4 signaling and ROS production and modulate podosome dynamics. Biomaterials. 2015;52:494–506. doi: 10.1016/j.biomaterials.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 45.Couto C, Neves B, Ferreira R, Daniel-da-Silva AL, Vitorino R. Proteomic studies with a novel nano-magnetic chelating system to capture metalloproteins and its application in the preliminary study of monocyte and macrophage sub-secretome. Talanta. 2016;158:110–117. doi: 10.1016/j.talanta.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 46.Liu PK, Liu CH. Epigenetics of amphetamine-induced sensitization: HDAC5 expression and microRNA in neural remodeling. J Biomed Sci. 2016;23:90. doi: 10.1186/s12929-016-0294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Askari S, Salehi R, Zarghami N, Akbarzadeh A, Rahmati-Yamchi M. The anticancer effects of biodegradable nanomagnetic dual natural components on the leptin gene expression in lung cancer. Artif Cells Nanomed Biotechnol. 2016;44:1753–1763. doi: 10.3109/21691401.2015.1101000. [DOI] [PubMed] [Google Scholar]
- 48.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, Daldrup-Link HE. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shevtsov MA, Nikolaev BP, Yakovleva LY, Parr MA, Marchenko YY, Eliseev I, Yudenko A, Dobrodumov AV, Zlobina O, Zhakhov A, Ischenko AM, Pitkin E, Multhoff G. 70-kDa heat shock protein coated magnetic nanocarriers as a nanovaccine for induction of anti-tumor immune response in experimental glioma. Journal of controlled release: official journal of the Controlled Release Society. 2015;220:329–340. doi: 10.1016/j.jconrel.2015.10.051. [DOI] [PubMed] [Google Scholar]
- 50.Wang YX. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant Imaging Med Surg. 2011;1:35–40. doi: 10.3978/j.issn.2223-4292.2011.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stirrat CG, Vesey AT, McBride OMB, Robson JMJ, Alam SR, Wallace WA, Semple SI, Henriksen PA, Newby DE. Ultra-small superparamagnetic particles of iron oxide in magnetic resonance imaging of cardiovascular disease. Journal of Vascular Diagnostics and Interventions. 2014;2:99–112. [Google Scholar]
- 52.Wysowski DK, Swartz L, Borders-Hemphill BV, Goulding MR, Dormitzer C. Use of parenteral iron products and serious anaphylactic-type reactions. Am J Hematol. 2010;85:650–654. doi: 10.1002/ajh.21794. [DOI] [PubMed] [Google Scholar]
- 53.Coyne DW, Adkinson NF, Nissenson AR, Fishbane S, Agarwal R, Eschbach JW, Michael B, Folkert V, Batlle D, Trout JR, Dahl N, Myirski P, Strobos J, Warnock DG, Ferlecit I. Sodium ferric gluconate complex in hemodialysis patients. II. Adverse reactions in iron dextran-sensitive and dextran-tolerant patients. Kidney Int. 2003;63:217–224. doi: 10.1046/j.1523-1755.2003.00703.x. [DOI] [PubMed] [Google Scholar]
- 54.Drug Label. 2015 in, FDA.gov.
- 55.FDA Drug Safety Communication. FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol) FDA Drug Safety Communication; 2015. FDA.gov. [Google Scholar]
- 56.Sipe JC, Filippi M, Martino G, Furlan R, Rocca MA, Rovaris M, Bergami A, Zyroff J, Scotti G, Comi G. Method for intracellular magnetic labeling of human mononuclear cells using approved iron contrast agents. Magn Reson Imaging. 1999;17:1521–1523. doi: 10.1016/s0730-725x(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 57.Dousset V, Ballarino L, Delalande C, Coussemacq M, Canioni P, Petry KG, Caille JM. Comparison of ultrasmall particles of iron oxide (USPIO)-enhanced T2-weighted, conventional T2-weighted, and gadolinium-enhanced T1-weighted MR images in rats with experimental autoimmune encephalomyelitis. AJNR Am J Neuroradiol. 1999;20:223–227. [PMC free article] [PubMed] [Google Scholar]
- 58.Bietenbeck M, Florian A, Sechtem U, Yilmaz A. The diagnostic value of iron oxide nanoparticles for imaging of myocardial inflammation–quo vadis? J Cardiovasc Magn Reson. 2015;17:54. doi: 10.1186/s12968-015-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Virel A, Rehnmark A, Oradd G, Olmedo-Diaz S, Faergemann E, Stromberg I. Magnetic resonance imaging as a tool to image neuroinflammation in a rat model of Parkinson’s disease–phagocyte influx to the brain is promoted by bilberry-enriched diet. Eur J Neurosci. 2015;42:2761–2771. doi: 10.1111/ejn.13044. [DOI] [PubMed] [Google Scholar]
- 60.Rausch M, Baumann D, Neubacher U, Rudin M. In-vivo visualization of phagocytotic cells in rat brains after transient ischemia by USPIO. NMR Biomed. 2002;15:278–283. doi: 10.1002/nbm.770. [DOI] [PubMed] [Google Scholar]
- 61.Floris S, Blezer EL, Schreibelt G, Dopp E, van der Pol SM, Schadee-Eestermans IL, Nicolay K, Dijkstra CD, de Vries HE. Blood-brain barrier permeability and monocyte infiltration in experimental allergic encephalomyelitis: a quantitative MRI study. Brain. 2004;127:616–627. doi: 10.1093/brain/awh068. [DOI] [PubMed] [Google Scholar]
- 62.Rausch M, Hiestand P, Baumann D, Cannet C, Rudin M. MRI-based monitoring of inflammation and tissue damage in acute and chronic relapsing EAE. Magn Reson Med. 2003;50:309–314. doi: 10.1002/mrm.10541. [DOI] [PubMed] [Google Scholar]
- 63.Zelivyanskaya ML, Nelson JA, Poluektova L, Uberti M, Mellon M, Gendelman HE, Boska MD. Tracking superparamagnetic iron oxide labeled monocytes in brain by high-field magnetic resonance imaging. J Neurosci Res. 2003;73:284–295. doi: 10.1002/jnr.10693. [DOI] [PubMed] [Google Scholar]
- 64.Zhu MT, Feng WY, Wang Y, Wang B, Wang M, Ouyang H, Zhao YL, Chai ZF. Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicol Sci. 2009;107:342–351. doi: 10.1093/toxsci/kfn245. [DOI] [PubMed] [Google Scholar]
- 65.Park EJ, Oh SY, Lee SJ, Lee K, Kim Y, Lee BS, Kim JS. Chronic pulmonary accumulation of iron oxide nanoparticles induced Th1-type immune response stimulating the function of antigen-presenting cells. Environ Res. 2015;143:138–147. doi: 10.1016/j.envres.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 66.Zhu MT, Wang B, Wang Y, Yuan L, Wang HJ, Wang M, Ouyang H, Chai ZF, Feng WY, Zhao YL. Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis. Toxicol Lett. 2011;203:162–171. doi: 10.1016/j.toxlet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 67.Escamilla-Rivera V, Uribe-Ramirez M, Gonzalez-Pozos S, Lozano O, Lucas S, De Vizcaya-Ruiz A. Protein corona acts as a protective shield against Fe3O4-PEG inflammation and ROS-induced toxicity in human macrophages. Toxicol Lett. 2016;240:172–184. doi: 10.1016/j.toxlet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 68.Gonnissen D, Qu Y, Langer K, Ozturk C, Zhao Y, Chen C, Seebohm G, Dufer M, Fuchs H, Galla HJ, Riehemann K. Comparison of cellular effects of starch-coated SPIONs and poly(lactic-co-glycolic acid) matrix nanoparticles on human monocytes. International journal of nanomedicine. 2016;11:5221–5236. doi: 10.2147/IJN.S106540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strehl C, Gaber T, Maurizi L, Hahne M, Rauch R, Hoff P, Haupl T, Hofmann-Amtenbrink M, Poole AR, Hofmann H, Buttgereit F. Effects of PVA coated nanoparticles on human immune cells. International journal of nanomedicine. 2015;10:3429–3445. doi: 10.2147/IJN.S75936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matuszak J, Dorfler P, Zaloga J, Unterweger H, Lyer S, Dietel B, Alexiou C, Cicha I. Shell matters: Magnetic targeting of SPIONs and in vitro effects on endothelial and monocytic cell function. Clin Hemorheol Microcirc. 2015;61:259–277. doi: 10.3233/CH-151998. [DOI] [PubMed] [Google Scholar]
- 71.Grosse S, Stenvik J, Nilsen AM. Iron oxide nanoparticles modulate lipopolysaccharide-induced inflammatory responses in primary human monocytes. International journal of nanomedicine. 2016;11:4625–4642. doi: 10.2147/IJN.S113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corot C, Petry KG, Trivedi R, Saleh A, Jonkmanns C, Le Bas JF, Blezer E, Rausch M, Brochet B, Foster-Gareau P, Baleriaux D, Gaillard S, Dousset V. Macrophage imaging in central nervous system and in carotid atherosclerotic plaque using ultrasmall superparamagnetic iron oxide in magnetic resonance imaging. Invest Radiol. 2004;39:619–625. doi: 10.1097/01.rli.0000135980.08491.33. [DOI] [PubMed] [Google Scholar]
- 73.Korchinski DJ, Taha M, Yang R, Nathoo N, Dunn JF. Iron Oxide as an MRI Contrast Agent for Cell Tracking. Magn Reson Insights. 2015;8:15–29. doi: 10.4137/MRI.S23557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Periyathambi P, Sastry TP, Anandasadagopan SK, Manickavasagam K. Macrophages mediated diagnosis of rheumatoid arthritis using fibrin based magnetic nanoparticles as MRI contrast agents. Biochim Biophys Acta. 2017;1861:2992–3001. doi: 10.1016/j.bbagen.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 75.Venneti S, Lopresti BJ, Wiley CA. Molecular imaging of microglia/macrophages in the brain. Glia. 2013;61:10–23. doi: 10.1002/glia.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirschbaum K, Sonner JK, Zeller MW, Deumelandt K, Bode J, Sharma R, Kruwel T, Fischer M, Hoffmann A, Silva MCostada, Muckenthaler MU, Wick W, Tews B, Chen JW, Heiland S, Bendszus M, Platten M, Breckwoldt MO. In vivo nanoparticle imaging of innate immune cells can serve as a marker of disease severity in a model of multiple sclerosis. Proc Natl Acad Sci USA. 2016;113:13227–13232. doi: 10.1073/pnas.1609397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kodali V, Littke MH, Tilton SC, Teeguarden JG, Shi L, Frevert CW, Wang W, Pounds JG, Thrall BD. Dysregulation of macrophage activation profiles by engineered nanoparticles. ACS nano. 2013;7:6997–7010. doi: 10.1021/nn402145t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, Chen Z, Gu N, Wang J. Effects of DMSA-coated Fe3O4 magnetic nanoparticles on global gene expression of mouse macrophage RAW264.7 cells. Toxicol Lett. 2011;205:130–139. doi: 10.1016/j.toxlet.2011.05.1031. [DOI] [PubMed] [Google Scholar]
- 79.Fell LH, Seiler-Mussler S, Sellier AB, Rotter B, Winter P, Sester M, Fliser D, Heine GH, Zawada AM. Impact of individual intravenous iron preparations on the differentiation of monocytes towards macrophages and dendritic cells. Nephrol Dial Transplant. 2016;31:1835–1845. doi: 10.1093/ndt/gfw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang CY, Tai MF, Lin CP, Lu CW, Wang JL, Hsiao JK, Liu HM. Mechanism of cellular uptake and impact of ferucarbotran on macrophage physiology. PLoS One. 2011;6:e25524. doi: 10.1371/journal.pone.0025524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park EJ, Choi DH, Kim Y, Lee EW, Song J, Cho MH, Kim JH, Kim SW. Magnetic iron oxide nanoparticles induce autophagy preceding apoptosis through mitochondrial damage and ER stress in RAW264.7 cells. Toxicol In Vitro. 2014;28:1402–1412. doi: 10.1016/j.tiv.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 82.Park EJ, Umh HN, Choi DH, Cho MH, Choi W, Kim SW, Kim Y, Kim JH. Magnetite- and maghemite-induced different toxicity in murine alveolar macrophage cells. Arch Toxicol. 2014;88:1607–1618. doi: 10.1007/s00204-014-1210-1. [DOI] [PubMed] [Google Scholar]
- 83.Park EJ, Umh HN, Kim SW, Cho MH, Kim JH, Kim Y. ERK pathway is activated in bare-FeNPs-induced autophagy. Arch Toxicol. 2014;88:323–336. doi: 10.1007/s00204-013-1134-1. [DOI] [PubMed] [Google Scholar]
- 84.Schlachter EK, Widmer HR, Bregy A, Lonnfors-Weitzel T, Vajtai I, Corazza N, Bernau VJ, Weitzel T, Mordasini P, Slotboom J, Herrmann G, Bogni S, Hofmann H, Frenz M, Reinert M. Metabolic pathway and distribution of superparamagnetic iron oxide nanoparticles: in vivo study. International journal of nanomedicine. 2011;6:1793–1800. doi: 10.2147/IJN.S23638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sundstrom JB, Mao H, Santoianni R, Villinger F, Little DM, Huynh TT, Mayne AE, Hao E, Ansari AA. Magnetic resonance imaging of activated proliferating rhesus macaque T cells labeled with superparamagnetic monocrystalline iron oxide nanoparticles. J Acquir Immune Defic Syndr. 2004;35:9–21. doi: 10.1097/00126334-200401010-00002. [DOI] [PubMed] [Google Scholar]
- 86.Dodd CH, Hsu HC, Chu WJ, Yang P, Zhang HG, Mountz JD, Jr, Zinn K, Forder J, Josephson L, Weissleder R, Mountz JM, Mountz JD. Normal T-cell response and in vivo magnetic resonance imaging of T cells loaded with HIV transactivator-peptide-derived superparamagnetic nanoparticles. J Immunol Methods. 2001;256:89–105. doi: 10.1016/s0022-1759(01)00433-1. [DOI] [PubMed] [Google Scholar]
- 87.Hu DE, Kettunen MI, Brindle KM. Monitoring T-lymphocyte trafficking in tumors undergoing immune rejection. Magn Reson Med. 2005;54:1473–1479. doi: 10.1002/mrm.20717. [DOI] [PubMed] [Google Scholar]
- 88.Garden OA, Reynolds PR, Yates J, Larkman DJ, Marelli-Berg FM, Haskard DO, Edwards AD, George AJ. A rapid method for labelling CD4+ T cells with ultrasmall paramagnetic iron oxide nanoparticles for magnetic resonance imaging that preserves proliferative, regulatory and migratory behaviour in vitro. J Immunol Methods. 2006;314:123–133. doi: 10.1016/j.jim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 89.Anderson SA, Shukaliak-Quandt J, Jordan EK, Arbab AS, Martin R, McFarland H, Frank JA. Magnetic resonance imaging of labeled T-cells in a mouse model of multiple sclerosis. Ann Neurol. 2004;55:654–659. doi: 10.1002/ana.20066. [DOI] [PubMed] [Google Scholar]
- 90.Beer AJ, Holzapfel K, Neudorfer J, Piontek G, Settles M, Kronig H, Peschel C, Schlegel J, Rummeny EJ, Bernhard H. Visualization of antigen-specific human cytotoxic T lymphocytes labeled with superparamagnetic iron-oxide particles. Eur Radiol. 2008;18:1087–1095. doi: 10.1007/s00330-008-0874-4. [DOI] [PubMed] [Google Scholar]
- 91.Yan L, Liu X, Liu WX, Tan XQ, Xiong F, Gu N, Hao W, Gao X, Cao JM. Fe2O3 nanoparticles suppress Kv1.3 channels via affecting the redox activity of Kvbeta2 subunit in Jurkat T cells. Nanotechnology. 2015;26:505103. doi: 10.1088/0957-4484/26/50/505103. [DOI] [PubMed] [Google Scholar]
- 92.Ying E, Hwang HM. In vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytes. Sci Total Environ. 2010;408:4475–4481. doi: 10.1016/j.scitotenv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 93.Chen BA, Jin N, Wang J, Ding J, Gao C, Cheng J, Xia G, Gao F, Zhou Y, Chen Y, Zhou G, Li X, Zhang Y, Tang M, Wang X. The effect of magnetic nanoparticles of Fe(3)O(4) on immune function in normal ICR mice. International journal of nanomedicine. 2010;5:593–599. doi: 10.2147/ijn.s12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Couto D, Freitas M, Costa VM, Chiste RC, Almeida A, Lopez-Quintela MA, Rivas J, Freitas P, Silva P, Carvalho F, Fernandes E. Biodistribution of polyacrylic acid-coated iron oxide nanoparticles is associated with proinflammatory activation and liver toxicity. J Appl Toxicol. 2016;36:1321–1331. doi: 10.1002/jat.3323. [DOI] [PubMed] [Google Scholar]
- 95.Strehl C, Schellmann S, Maurizi L, Hofmann-Amtenbrink M, Haupl T, Hofmann H, Buttgereit F, Gaber T. Effects of PVA-coated nanoparticles on human T helper cell activity. Toxicol Lett. 2016;245:52–58. doi: 10.1016/j.toxlet.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Chen B, Jin N, Xia G, Chen Y, Zhou Y, Cai X, Ding J, Li X, Wang X. The changes of T lymphocytes and cytokines in ICR mice fed with Fe3O4 magnetic nanoparticles. International journal of nanomedicine. 2011;6:605–610. doi: 10.2147/IJN.S16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu M, Li Y, Shi J, Feng W, Nie G, Zhao Y. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small. 2012;8:404–412. doi: 10.1002/smll.201101708. [DOI] [PubMed] [Google Scholar]
- 98.Zhu M, Tian X, Song X, Li Y, Tian Y, Zhao Y, Nie G. Nanoparticle-induced exosomes target antigen-presenting cells to initiate Th1-type immune activation. Small. 2012;8:2841–2848. doi: 10.1002/smll.201200381. [DOI] [PubMed] [Google Scholar]
- 99.Shen CC, Liang HJ, Wang CC, Liao MH, Jan TR. A role of cellular glutathione in the differential effects of iron oxide nanoparticles on antigen-specific T cell cytokine expression. International journal of nanomedicine. 2011;6:2791–2798. doi: 10.2147/IJN.S25588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen CC, Liang HJ, Wang CC, Liao MH, Jan TR. Iron oxide nanoparticles suppressed T helper 1 cell-mediated immunity in a murine model of delayed-type hypersensitivity. International journal of nanomedicine. 2012;7:2729–2737. doi: 10.2147/IJN.S31054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shen CC, Wang CC, Liao MH, Jan TR. A single exposure to iron oxide nanoparticles attenuates antigen-specific antibody production and T-cell reactivity in ovalbumin-sensitized BALB/c mice. International journal of nanomedicine. 2011;6:1229–1235. doi: 10.2147/IJN.S21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sato A, Tamura Y, Sato N, Yamashita T, Takada T, Sato M, Osai Y, Okura M, Ono I, Ito A, Honda H, Wakamatsu K, Ito S, Jimbow K. Melanoma-targeted chemo-thermo-immuno (CTI)-therapy using N-propionyl-4-S-cysteaminylphenol-magnetite nanoparticles elicits CTL response via heat shock protein-peptide complex release. Cancer Sci. 2010;101:1939–1946. doi: 10.1111/j.1349-7006.2010.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu H, Zhanl D, Du Y. The immunotherapeutic effect of Fe3O4 nanoparticles as adjuvants on mice H22 live cancer. J Nanosci Nanotechnol. 2010;10:514–519. doi: 10.1166/jnn.2010.1591. [DOI] [PubMed] [Google Scholar]
- 104.Daldrup-Link HE, Meier R, Rudelius M, Piontek G, Piert M, Metz S, Settles M, Uherek C, Wels W, Schlegel J, Rummeny EJ. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol. 2005;15:4–13. doi: 10.1007/s00330-004-2526-7. [DOI] [PubMed] [Google Scholar]
- 105.Jang ES, Shin JH, Ren G, Park MJ, Cheng K, Chen X, Wu JC, Sunwoo JB, Cheng Z. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials. 2012;33:5584–5592. doi: 10.1016/j.biomaterials.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li K, Gordon AC, Zheng L, Li W, Guo Y, Sun J, Zhang G, Han G, Larson AC, Zhang Z. Clinically applicable magnetic-labeling of natural killer cells for MRI of transcatheter delivery to liver tumors: preclinical validation for clinical translation. Nanomedicine (Lond) 2015;10:1761–1774. doi: 10.2217/nnm.15.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mallett CL, McFadden C, Chen Y, Foster PJ. Migration of iron-labeled KHYG-1 natural killer cells to subcutaneous tumors in nude mice, as detected by magnetic resonance imaging. Cytotherapy. 2012;14:743–751. doi: 10.3109/14653249.2012.667874. [DOI] [PubMed] [Google Scholar]
- 108.Sheu AY, Zhang Z, Omary RA, Larson AC. MRI-monitored transcatheter intra-arterial delivery of SPIO-labeled natural killer cells to hepatocellular carcinoma: preclinical studies in a rodent model. Invest Radiol. 2013;48:492–499. doi: 10.1097/RLI.0b013e31827994e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bachelet-Violette L, Silva AKA, Maire M, Michel A, Brinza O, Ou P, Ollivier V, Nicoletti A, Wilhelm C, Letourneur D, Menager C, Chaubet F. Strong and specific interaction of ultra small superparamagnetic iron oxide nanoparticles and human activated platelets mediated by fucoidan coating. RSC Advances. 2014;4:4864–4871. [Google Scholar]
- 110.Ta HT, Li Z, Hagemeyer CE, Cowin G, Zhang S, Palasubramaniam J, Alt K, Wang X, Peter K, Whittaker AK. Molecular imaging of activated platelets via antibody-targeted ultra-small iron oxide nanoparticles displaying unique dual MRI contrast. Biomaterials. 2017;134:31–42. doi: 10.1016/j.biomaterials.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 111.Blank F, Gerber P, Rothen-Rutishauser B, Sakulkhu U, Salaklang J, De Peyer K, Gehr P, Nicod LP, Hofmann H, Geiser T, Petri-Fink A, Von Garnier C. Biomedical nanoparticles modulate specific CD4+ T cell stimulation by inhibition of antigen processing in dendritic cells. Nanotoxicology. 2011;5:606–621. doi: 10.3109/17435390.2010.541293. [DOI] [PubMed] [Google Scholar]
- 112.Dekaban GA, Snir J, Shrum B, de Chickera S, Willert C, Merrill M, Said EA, Sekaly RP, Foster PJ, O’Connell PJ. Semiquantitation of mouse dendritic cell migration in vivo using cellular MRI. J Immunother. 2009;32:240–251. doi: 10.1097/CJI.0b013e318197b2a0. [DOI] [PubMed] [Google Scholar]
- 113.Verdijk P, Scheenen TW, Lesterhuis WJ, Gambarota G, Veltien AA, Walczak P, Scharenborg NM, Bulte JW, Punt CJ, Heerschap A, Figdor CG, de Vries IJ. Sensitivity of magnetic resonance imaging of dendritic cells for in vivo tracking of cellular cancer vaccines. Int J Cancer. 2007;120:978–984. doi: 10.1002/ijc.22385. [DOI] [PubMed] [Google Scholar]
- 114.Mou Y, Chen B, Zhang Y, Hou Y, Xie H, Xia G, Tang M, Huang X, Ni Y, Hu Q. Influence of synthetic superparamagnetic iron oxide on dendritic cells. International journal of nanomedicine. 2011;6:1779–1786. doi: 10.2147/IJN.S23240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mou Y, Hou Y, Chen B, Hua Z, Zhang Y, Xie H, Xia G, Wang Z, Huang X, Han W, Ni Y, Hu Q. In vivo migration of dendritic cells labeled with synthetic superparamagnetic iron oxide. International journal of nanomedicine. 2011;6:2633–2640. doi: 10.2147/IJN.S24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giraldo-Villegas MM, Urquijo JP, Arnache-Olmos O, Rojas M. In vitro effect of polyacrylic acid-coated iron oxide nanoparticles, a tool to track monocytes in vivo, on LPS-induced mononuclear phagocyte activation and platelet aggregation. Frontiers in Immunology [Google Scholar]
- 117.Nemmar A, Beegam S, Yuvaraju P, Yasin J, Tariq S, Attoub S, Ali BH. Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Particle and Fibre Toxicology. 2016;13:22. doi: 10.1186/s12989-016-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rao L, Bu LL, Xu JH, Cai B, Yu GT, Yu X, He Z, Huang Q, Li A, Guo SS, Zhang WF, Liu W, Sun ZJ, Wang H, Wang TH, Zhao XZ. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small. 2015;11:6225–6236. doi: 10.1002/smll.201502388. [DOI] [PubMed] [Google Scholar]
- 119.Rao L, Xu JH, Cai B, Liu H, Li M, Jia Y, Xiao L, Guo SS, Liu W, Zhao XZ. Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake. Nanotechnology. 2016;27:085106. doi: 10.1088/0957-4484/27/8/085106. [DOI] [PubMed] [Google Scholar]
- 120.Loeb SK, V NAP, Valiyaveettil S. Investigating the Toxicity of Iron(III) Oxide Nanoparticles, Zinc(II) Oxide Nanorods and Multi-Walled Carbon Nanotubes on Red Blood Cells; 15th National Undergraduate Research Opportunities (NUROP) Congress; 2010; Singapore: National University of Singapore; 2010. [Google Scholar]
- 121.Creangă DE, Culea M, Nădejde C, Oancea S, Curecheriu L, Racuciu M. Magnetic nanoparticle effects on the red blood cells. Journal of Physics: Conference Series. 2009;170:012019. [Google Scholar]
- 122.Ran Q, Xiang Y, Liu Y, Xiang L, Li F, Deng X, Xiao Y, Chen L, Chen L, Li Z. Eryptosis Indices as a Novel Predictive Parameter for Biocompatibility of Fe3O4 Magnetic Nanoparticles on Erythrocytes. Sci Rep. 2015;5:16209. doi: 10.1038/srep16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al Akhras MH, Al Khalili DJ. The Influence of Iron Oxide Nanoparticles on the Red Blood Cells Photohemolysis Sensitized with Photofrin: Temperature Effect. Jordan Journal of Biological Sciences. 2015;8:55–59. [Google Scholar]
- 124.Bandyopadhyay S, Rogers JT. Alzheimer’s disease therapeutics targeted to the control of amyloid precursor protein translation: maintenance of brain iron homeostasis. Biochem Pharmacol. 2014;88:486–494. doi: 10.1016/j.bcp.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tao Y, Wang Y, Rogers JT, Wang F. Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42:679–690. doi: 10.3233/JAD-140396. [DOI] [PubMed] [Google Scholar]
- 126.Kew MC. Hepatic iron overload and hepatocellular carcinoma. Cancer Lett. 2009;286:38–43. doi: 10.1016/j.canlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 127.Porto G, De Sousa M. Iron overload and immunity. World J Gastroenterol. 2007;13:4707–4715. doi: 10.3748/wjg.v13.i35.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Sousa M. Immune cell functions in iron overload. Clin Exp Immunol. 1989;75:1–6. [PMC free article] [PubMed] [Google Scholar]
- 129.De Sousa M. T lymphocytes and iron overload: novel correlations of possible significance to the biology of the immunological system. Mem Inst Oswaldo Cruz. 1992;87(Suppl 5):23–29. doi: 10.1590/s0074-02761992000900003. [DOI] [PubMed] [Google Scholar]
- 130.Good MF, Powell LW, Halliday JW. Iron status and cellular immune competence. Blood Rev. 1988;2:43–49. doi: 10.1016/0268-960x(88)90007-0. [DOI] [PubMed] [Google Scholar]
- 131.Walker EM, Jr, Walker SM. Effects of iron overload on the immune system. Ann Clin Lab Sci. 2000;30:354–365. [PubMed] [Google Scholar]
- 132.Liu Y, Xia Q, Liu Y, Zhang S, Cheng F, Zhong Z, Wang L, Li H, Xiao K. Genotoxicity assessment of magnetic iron oxide nanoparticles with different particle sizes and surface coatings. Nanotechnology. 2014;25:425101. doi: 10.1088/0957-4484/25/42/425101. [DOI] [PubMed] [Google Scholar]
- 133.Yang L, Kuang H, Zhang W, Aguilar ZP, Xiong Y, Lai W, Xu H, Wei H. Size dependent biodistribution and toxicokinetics of iron oxide magnetic nanoparticles in mice. Nanoscale. 2015;7:625–636. doi: 10.1039/c4nr05061d. [DOI] [PubMed] [Google Scholar]
- 134.Lee JH, Ju JE, Kim BI, Pak PJ, Choi EK, Lee HS, Chung N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ Toxicol Chem. 2014;33:2759–2766. doi: 10.1002/etc.2735. [DOI] [PubMed] [Google Scholar]
- 135.Xia W, Song HM, Wei Q, Wei A. Differential response of macrophages to core-shell Fe3O4@Au nanoparticles and nanostars. Nanoscale. 2012;4:7143–7148. doi: 10.1039/c2nr32070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 137.van Vlerken LE, Vyas TK, Amiji MM. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res. 2007;24:1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- 138.Soenen SJ, De Cuyper M. Assessing iron oxide nanoparticle toxicity in vitro: current status and future prospects. Nanomedicine (Lond) 2010;5:1261–1275. doi: 10.2217/nnm.10.106. [DOI] [PubMed] [Google Scholar]
- 139.Hong SC, Lee JH, Lee J, Kim HY, Park JY, Cho J, Lee J, Han DW. Subtle cytotoxicity and genotoxicity differences in superparamagnetic iron oxide nanoparticles coated with various functional groups. International journal of nanomedicine. 2011;6:3219–3231. doi: 10.2147/IJN.S26355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fulop T, Nemes R, Meszaros T, Urbanics R, Kok RJ, Jackman JA, Cho N, Storm G, Szebeni J. Complement activation in vitro and reactogenicity of low-molecular weight dextran-coated SPIONs in the pig CARPA model: Correlation with physicochemical features and clinical information. Journal of controlled release: official journal of the Controlled Release Society. 2017;270:268–274. doi: 10.1016/j.jconrel.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 141.Hoang MD, Lee HJ, Lee HJ, Jung SH, Choi NR, Vo MC, Nguyen-Pham TN, Kim HJ, Park IK, Lee JJ. Branched Polyethylenimine-Superparamagnetic Iron Oxide Nanoparticles (bPEI-SPIONs) Improve the Immunogenicity of Tumor Antigens and Enhance Th1 Polarization of Dendritic Cells. J Immunol Res. 2015;2015:706379. doi: 10.1155/2015/706379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Comanescu MV, Mocanu MA, Anghelache L, Marinescu B, Dumitrache F, Badoi AD, Manda G. Toxicity of L-DOPA coated iron oxide nanoparticles in intraperitoneal delivery setting - preliminary preclinical study. Rom J Morphol Embryol. 2015;56:691–696. [PubMed] [Google Scholar]
- 143.Easo SL, Mohanan PV. In vitro hematological and in vivo immunotoxicity assessment of dextran stabilized iron oxide nanoparticles. Colloids Surf B Biointerfaces. 2015;134:122–130. doi: 10.1016/j.colsurfb.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 144.Srikanth K, Trindade T, Duarte AC, Pereira E. Cytotoxicity and oxidative stress responses of silica-coated iron oxide nanoparticles in CHSE-214 cells. Environ Sci Pollut Res Int. 2016 doi: 10.1007/s11356-016-7870-z. [DOI] [PubMed] [Google Scholar]
- 145.Maurizi L, Papa AL, Dumont L, Bouyer F, Walker P, Vandroux D, Millot N. Influence of Surface Charge and Polymer Coating on Internalization and Biodistribution of Polyethylene Glycol-Modified Iron Oxide Nanoparticles. J Biomed Nanotechnol. 2015;11:126–136. doi: 10.1166/jbn.2015.1996. [DOI] [PubMed] [Google Scholar]
- 146.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoskins C, Cuschieri A, Wang L. The cytotoxicity of polycationic iron oxide nanoparticles: common endpoint assays and alternative approaches for improved understanding of cellular response mechanism. J Nanobiotechnology. 2012;10:15. doi: 10.1186/1477-3155-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mou Y, Xing Y, Ren H, Cui Z, Zhang Y, Yu G, Urba WJ, Hu Q, Hu H. The Effect of Superparamagnetic Iron Oxide Nanoparticle Surface Charge on Antigen Cross-Presentation. Nanoscale Res Lett. 2017;12:52. doi: 10.1186/s11671-017-1828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004;39:56–63. doi: 10.1097/01.rli.0000101027.57021.28. [DOI] [PubMed] [Google Scholar]
- 150.Muller K, Skepper JN, Posfai M, Trivedi R, Howarth S, Corot C, Lancelot E, Thompson PW, Brown AP, Gillard JH. Effect of ultrasmall superparamagnetic iron oxide nanoparticles (Ferumoxtran-10) on human monocyte-macrophages in vitro. Biomaterials. 2007;28:1629–1642. doi: 10.1016/j.biomaterials.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 151.Kunzmann A, Andersson B, Vogt C, Feliu N, Ye F, Gabrielsson S, Toprak MS, Buerki-Thurnherr T, Laurent S, Vahter M, Krug H, Muhammed M, Scheynius A, Fadeel B. Efficient internalization of silica-coated iron oxide nanoparticles of different sizes by primary human macrophages and dendritic cells. Toxicology and applied pharmacology. 2011;253:81–93. doi: 10.1016/j.taap.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 152.Zasonska BA, Liskova A, Kuricova M, Tulinska J, Pop-Georgievski O, Ciampor F, Vavra I, Dusinska M, Ilavska S, Horvathova M, Horak D. Functionalized porous silica&maghemite core-shell nanoparticles for applications in medicine: design, synthesis, and immunotoxicity. Croat Med J. 2016;57:165–178. doi: 10.3325/cmj.2016.57.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Janic B, Iskander AS, Rad AM, Soltanian-Zadeh H, Arbab AS. Effects of ferumoxides-protamine sulfate labeling on immunomodulatory characteristics of macrophage-like THP-1 cells. PLoS One. 2008;3:e2499. doi: 10.1371/journal.pone.0002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Siglienti I, Bendszus M, Kleinschnitz C, Stoll G. Cytokine profile of iron-laden macrophages: implications for cellular magnetic resonance imaging. J Neuroimmunol. 2006;173:166–173. doi: 10.1016/j.jneuroim.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 155.Cecchini G. Function and structure of complex II of the respiratory chain. Annu Rev Biochem. 2003;72:77–109. doi: 10.1146/annurev.biochem.72.121801.161700. [DOI] [PubMed] [Google Scholar]
- 156.Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, Uzarska MA, Webert H, Wilbrecht C, Muhlenhoff U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta. 2012;1823:1491–1508. doi: 10.1016/j.bbamcr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 157.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 158.Naqvi S, Samim M, Abdin M, Ahmed FJ, Maitra A, Prashant C, Dinda AK. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. International journal of nanomedicine. 2010;5:983–989. doi: 10.2147/IJN.S13244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 159.Gustafsson A, Bergstrom U, Agren L, Osterlund L, Sandstrom T, Bucht A. Differential cellular responses in healthy mice and in mice with established airway inflammation when exposed to hematite nanoparticles. Toxicology and applied pharmacology. 2015;288:1–11. doi: 10.1016/j.taap.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 160.Toblli JE, Cao G, Oliveri L, Angerosa M. Assessment of the extent of oxidative stress induced by intravenous ferumoxytol, ferric carboxymaltose, iron sucrose and iron dextran in a nonclinical model. Arzneimittelforschung. 2011;61:399–410. doi: 10.1055/s-0031-1296218. [DOI] [PubMed] [Google Scholar]
- 161.Al Faraj A, Shaik AP, Shaik AS. Effect of surface coating on the biocompatibility and in vivo MRI detection of iron oxide nanoparticles after intrapulmonary administration. Nanotoxicology. 2015;9:825–834. doi: 10.3109/17435390.2014.980450. [DOI] [PubMed] [Google Scholar]
- 162.Duan J, Kodali VK, Gaffrey MJ, Guo J, Chu RK, Camp DG, Smith RD, Thrall BD, Qian WJ. Quantitative Profiling of Protein S-Glutathionylation Reveals Redox-Dependent Regulation of Macrophage Function during Nanoparticle-Induced Oxidative Stress. ACS nano. 2016;10:524–538. doi: 10.1021/acsnano.5b05524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rajiv S, Jerobin J, Saranya V, Nainawat M, Sharma A, Makwana P, Gayathri C, Bharath L, Singh M, Kumar M, Mukherjee A, Chandrasekaran N. Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Hum Exp Toxicol. 2016;35:170–183. doi: 10.1177/0960327115579208. [DOI] [PubMed] [Google Scholar]
- 164.Periasamy VS, Athinarayanan J, Alhazmi M, Alatiah KA, Alshatwi AA. Fe3 O4 nanoparticle redox system modulation via cell-cycle progression and gene expression in human mesenchymal stem cells. Environ Toxicol. 2016;31:901–912. doi: 10.1002/tox.22098. [DOI] [PubMed] [Google Scholar]
- 165.Konczol M, Weiss A, Stangenberg E, Gminski R, Garcia-Kaufer M, Giere R, Merfort I, Mersch-Sundermann V. Cell-cycle changes and oxidative stress response to magnetite in A549 human lung cells. Chem Res Toxicol. 2013;26:693–702. doi: 10.1021/tx300503q. [DOI] [PubMed] [Google Scholar]
- 166.Ahamed M, Alhadlaq HA, Alam J, Khan MA, Ali D, Alarafi S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr Pharm Des. 2013;19:6681–6690. doi: 10.2174/1381612811319370011. [DOI] [PubMed] [Google Scholar]
- 167.Baratli Y, Charles AL, Wolff V, Ben Tahar L, Smiri L, Bouitbir J, Zoll J, Piquard F, Tebourbi O, Sakly M, Abdelmelek H, Geny B. Impact of iron oxide nanoparticles on brain, heart, lung, liver and kidneys mitochondrial respiratory chain complexes activities and coupling. Toxicol In Vitro. 2013;27:2142–2148. doi: 10.1016/j.tiv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 168.Petters C, Thiel K, Dringen R. Lysosomal iron liberation is responsible for the vulnerability of brain microglial cells to iron oxide nanoparticles: comparison with neurons and astrocytes. Nanotoxicology. 2016;10:332–342. doi: 10.3109/17435390.2015.1071445. [DOI] [PubMed] [Google Scholar]
- 169.Wu J, Ding T, Sun J. Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology. 2013;34:243–253. doi: 10.1016/j.neuro.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 170.Huang G, Chen H, Dong Y, Luo X, Yu H, Moore Z, Bey EA, Boothman DA, Gao J. Superparamagnetic iron oxide nanoparticles: amplifying ROS stress to improve anticancer drug efficacy. Theranostics. 2013;3:116–126. doi: 10.7150/thno.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shi M, Cheng L, Zhang Z, Liu Z, Mao X. Ferroferric oxide nanoparticles induce prosurvival autophagy in human blood cells by modulating the Beclin 1/Bcl-2/VPS34 complex. International journal of nanomedicine. 2015;10:207–216. doi: 10.2147/IJN.S72598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang X, Zhang H, Liang X, Zhang J, Tao W, Zhu X, Chang D, Zeng X, Liu G, Mei L. Iron Oxide Nanoparticles Induce Autophagosome Accumulation through Multiple Mechanisms: Lysosome Impairment, Mitochondrial Damage, and ER Stress. Mol Pharm. 2016;13:2578–2587. doi: 10.1021/acs.molpharmaceut.6b00405. [DOI] [PubMed] [Google Scholar]
- 173.Halamoda Kenzaoui B, Chapuis Bernasconi C, Guney-Ayra S, Juillerat-Jeanneret L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem J. 2012;441:813–821. doi: 10.1042/BJ20111252. [DOI] [PubMed] [Google Scholar]
- 174.Khan MI, Mohammad A, Patil G, Naqvi SA, Chauhan LK, Ahmad I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33:1477–1488. doi: 10.1016/j.biomaterials.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 175.Yokoyama T, Tam J, Kuroda S, Scott AW, Aaron J, Larson T, Shanker M, Correa AM, Kondo S, Roth JA, Sokolov K, Ramesh R. EGFR-targeted hybrid plasmonic magnetic nanoparticles synergistically induce autophagy and apoptosis in non-small cell lung cancer cells. PLoS One. 2011;6:e25507. doi: 10.1371/journal.pone.0025507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]


