Abstract
Background
Microscopic transsphenoidal surgery (mTSS) is a well-established method to address adenomas of the pituitary gland. Endoscopic transsphenoidal surgery (eTSS) has become a viable alternative, however. Advocates suggest that the greater illumination, panoramic visualization, and angled endoscopic views afforded by eTSS may allow for higher rates of gross total tumor resection (GTR). The aim of this meta-analysis was to determine the rate of GTR using mTSS and eTSS.
Methods
A meta-analysis of the literature was conducted using PubMed, EMBASE and Cochrane databases through September 2016 in accordance with PRISMA guidelines.
Results
Seventy case series that reported GTR rate in 8257 pituitary adenomas patients were identified. For all pituitary adenomas, eTSS (GTR=74.0%; I2=92.1%) was associated with higher GTR as compared to mTSS (GTR=66.4%; I2=84.0%) in a fixed-effect model (P-interaction<0.01). For functioning pituitary adenomas (FPAs) (n=1,170 patients), there was no significant difference in GTR rate between eTSS (GTR=75.8%; I2=63.9%) and mTSS (GTR=75.5%; I2=79.0%); (P-interaction=0.92). For non-functioning pituitary adenomas (NFPAs) (n=2,655 patients), eTSS (GTR=71.0%; I2=86.4%) was associated with higher GTR as compared to mTSS (GTR=60.7%; I2=87.5%) in a fixed-effect model (P-interaction<0.01). None of the associations were significant in a random-effect model (all P-interaction>0.05). No significant publication bias was identified for any of the outcomes.
Conclusion
Among patients who were not randomly allocated to either approach, eTSS resulted in a higher rate of GTR as compared to mTSS for all patients and for NFPA patients alone, but only in a fixed-effect model. For FPA, however, eTSS did not seem to offer a significantly higher rate of GTR. These conclusions should be stated with caution because of the nature of the included non-comparative studies.
Keywords: endoscopic transsphenoidal surgery, gross total resection, meta-analysis, microscopic transsphenoidal surgery, pituitary adenoma, transsphenoidal surgery
Introduction
The transsphenoidal approach to the sellar region was first developed for resection of sellar pathology by Schoffler in 1907 and later popularized by Cushing without the aid of lens magnification [14, 17, 60, 77]. Introduction of the operating room microscope for transsphenoidal surgery by Jules Hardy in 1960s greatly improved intra-operative visibility and surgical outcomes [13, 40, 60]. Since around the turn of the 21st century, the introduction of the endoscope have allowed for improved illumination and panoramic visualization of the anterior skull base, with many skull base centers rapidly adopting this new technology [14, 46].
Despite this, the choice between endoscopic transsphenoidal surgery (eTSS) and microscopic transsphenoidal surgery (mTSS) remains controversial in the neurosurgical community, and no head-to-head study has compared the two approaches in terms of efficacy or safety. Whereas mTSS requires either a sublabial incision or removal of the nasal septum, eTSS is most frequently performed transnasally with some disruption of the nasal anatomy [43, 57]. Perhaps as a result, some studies have showed that mTSS could also be associated with longer hospital stay postoperatively compared to eTSS [36]. On the other hand, the majority of endoscopic approaches utilize 2 dimensional endoscopic lens and are associated with a considerable learning curve [3, 12, 49, 58]. Some experts have also claimed that eTSS operations may last longer or result in higher rate of post-operative cerebrospinal fluid (CSF) leak than mTSS [66, 79]. Overall, no true consensus exists and many factors play may a role in choosing either of the modalities. Patient care could be improved by a more uniform practice and more objective comparative data.
With regard to surgical outcomes, gross total resection (GTR) remains of key importance, particularly for functioning adenomas. The presence of residual disease can necessitate adjuvant medical therapy, radiosurgery and place the patient at a greater future risk of visual decline or pituitary dysfunction. Although previous systematic reviews and meta-analyses have failed to show a significant difference in GTR for pituitary adenoma resection using either mTSS or eTSS [1, 36, 76, 81], we set out to update the estimated pooled rate of GTR after each method and to identify which patient and tumor-related factors were associated with higher rates of GTR.
Methods
Search Strategy and Paper Selection
A systematic review of the literature was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify studies reporting GTR in patients harboring pituitary adenomas in the PubMed, Embase, and Cochrane databases [65]. A search strategy was designed in consultation with a librarian, using relevant keywords for identification of articles reporting both approaches (Appendix 1).
All databases were searched on July 25, 2017 and duplicates were removed. All articles were screened for title and abstract relevance by two authors, independently, to identify articles reporting GTR for mTSS, eTSS, or both. Discrepancies in study selection were resolved by discussion and consultation with a senior author. Selected articles were subject to full-text screening. Only articles that reported GTR specifically for pituitary adenomas were included. Case reports, commentaries, abstracts, reviews, animal studies, studies with an endoscopically-assisted approach or extended approach, studies in pediatric patients (<18 years old), re-operations, and cadaveric studies were excluded. Only literature in English was reviewed.
Data Extraction
Study characteristics were extracted from the full text of selected studies including authors, publication year, country of origin, sample size, study design, and duration of study. Patient characteristics were extracted including number, sex, age, type of pituitary adenomas (non-functioning pituitary adenomas [NFPA] vs. functioning pituitary adenomas [FPA]), histological type, number of macroadenomas, number of microadenomas, surgery type, and rate of GTR.
Meta-Analysis
Data analysis was performed using Comprehensive Meta-Analysis (CMA) version 3 (Copyright 1998–2014. Biostat, Inc.). The fixed-effect model using the inverse variance method was used to obtain the overall rate and the 95% confidence intervals. The random-effects model that accounts for the within- and the between-study variances according to the method of DerSimonian and Laird was also used for comparison [25]. Pooled rate estimates of GTR together with 95% confidence intervals were used to assess the efficacy of transsphenoidal surgery among patients with any pituitary adenoma, FPA, and NFPA [25]. Heterogeneity was evaluated among studies by using Cochran’s Q test (P<0.10) and I2 percentage. An I2 value >50% was considered to be high [41]. Potential sources of heterogeneity were explored using sub-group analyses by categorical covariates: surgery type (eTSS; mTSS), tumor type (FPA vs. NFPA), continent (Asia, Australia, Europe, North America, South America), center (single vs. multiple), surgeon (single vs. multiple), male percent (high, defined as ≥ median value of 50%, vs. low <50 %), age in categories (25–35, 36–40, 41–50, 51–55, 56–60 and 61–65), study design (cohort; case series), microadenoma (low percent, defined as < median percent; high percent defined as ≥ median percent); macroadenoma (low percent; high percent), FPA type (ACTH-producing, GH-producing, and prolactinoma), and publication after 2000. It is important to note that the p-interaction resulting from the subgroup analyses should be interpreted with caution because the original studies are case series and comparing two groups of studies based on a specific covariate will not resolve all the other potential differences among the studies being compared. Meta-regression was conducted on continuous covariates including international journal impact factor and year of publication. Publication bias was assessed using funnel plots, Egger’s linear regression test, and Begg’s and Mazumdar rank correlation test. If publication bias was identified, the number of missing studies was evaluated by the trim-and-fill method. A P-value <0.05 was considered significant except where otherwise specified.
Results
Search Results
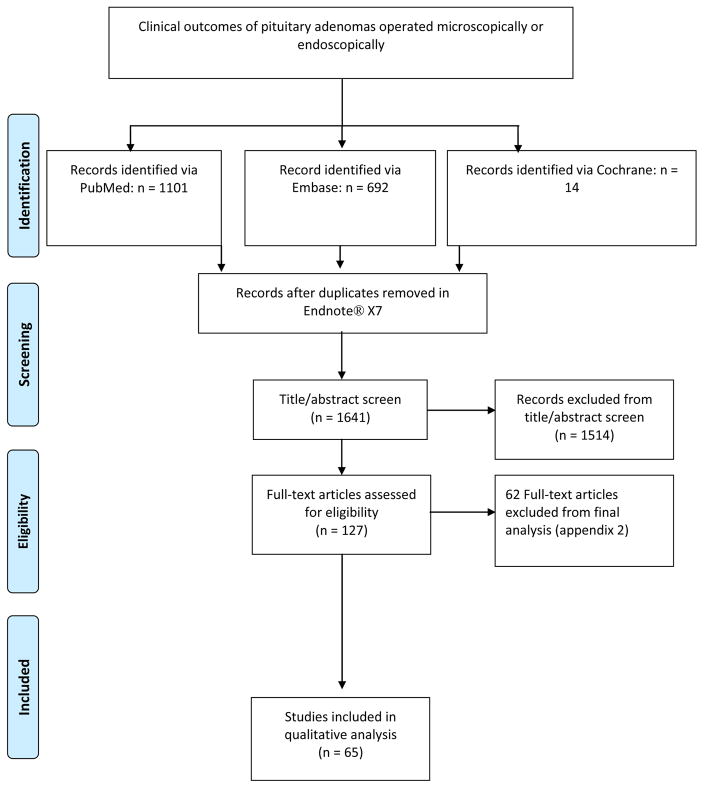
The systematic search resulted in 1641 articles after duplicates were removed. After title and abstract screening, 1514 articles were excluded, resulting in 127 articles for full text evaluation. After full-text screening, a total of 57 case series were included in the meta-analysis, with a total of 7,896 patients that had undergone surgery for a pituitary adenomas (Figure 1, Appendix 2) [4–11, 15, 16, 18–23, 27, 30–33, 35, 37–39, 42, 44, 45, 47, 48, 50–56, 59, 64, 68–71, 74, 75, 78, 80, 82–84, 86–92]. The median percentage of males was 53.0% (range: 0–72.2%). Mean age per study ranged from 31.6 to 63.5 years (median of means=50.0 years) (Table 1). The median percentage of macroadenomas was 86.25% (Table 2, Appendix 3). The median percentage of FPA was 47.28% (range: 0–100%).
Figure 1.
Flowchart: Study Selection process of the identified studies
Table 1.
Characteristics of Studies Included in the Analysis of Gross Tumor Resection (GTR)
| Study | Year of Publication | Study Design | Prospective | Country | Duration of Study | Surgical Intervention | Single Center | Single Surgeon |
|---|---|---|---|---|---|---|---|---|
| Bodhinayake et al.4 | 2014 | CS | no | USA | 2006 – 2011 | ETSS | yes | yes |
| Bokhari et al.5 | 2013 | CS | no | Australia | July 1998 – September 2010 | ETSS | yes | yes |
| Campbell et al.6 | 2010 | CS | no | USA | June 2005 – September 2009 | ETSS | yes | yes |
| Cappabianca et al.7 | 2002 | CS | no | Italy | January 1997 – July 2001 | ETSS | yes | no |
| Charalampaki et al.8 | 2009 | CS | no | Germany | January 2004 – June 2007 | ETSS | yes | NA |
| Chi F et al.9 | 2013 | Cohort study | no | China | November 2011–October 20112 | ETSS | Yes | Yes |
| Choe et al.10 | 2008 | CS | no | Korea | 2004–2007 | ETSS | yes | no |
| Choe et al.10 | 2008 | CS | no | Korea | 1997–2004 | MTSS | yes | no |
| Chone et al.11 | 2014 | CS | no | Brazil | January of 2009 – December of 2012 | ETSS | yes | NA |
| Conrad et al.15 | 2016 | Cohort study | no | USA | October 2008 – November 2009 | ETSS | yes | NS |
| Constantino et al.16 | 2016 | CS | no | Brazil | March 2010 – March 2014 | ETSS | yes | NS |
| Cusimano et al.18 | 2012 | Cohort study | No | Canada | October 1994–July 2009 | ETSS | Yes | No |
| Dallapiazza et al.19 | 2014 | Cohort study | no | USA | June 2010 to January 2013 | ETSS | yes | yes |
| Dallapiazza et al.19 | 2014 | Cohort study | no | USA | June 2010 to January 2013 | MTSS | yes | yes |
| Dallapiazza et al.20 | 2015 | CS | no | USA | September 2004 – August 2008 | ETSS | yes | NS |
| Dehdashti et al.23 | 2008 | CS | No | Canada | July 2004 – March 2007 | ETSS | yes | no |
| De Paiva Neto et al.21 | 2010 | Cohort study | No | USA | July 1998–May 2008 | ETSS | Yes | Yes |
| De Witte et al.22 | 2011 | CS | no | Belgium | Februray 2007– December 2010 | ETSS | yes | yes |
| Duz et al.27 | 2008 | CS | no | Turkey | 2006–2007 | ETSS | yes | NA |
| Duz et al.27 | 2008 | CS | no | Turkey | 1996–2004 | MTSS | yes | NA |
| Fathalla et al.30 | 2015 | Cohort study | no | Canada | 2000–2013 | ETSS | yes | no |
| Fathalla et al.30 | 2015 | Cohort study | no | Canada | MTSS | yes | no | |
| Fomekong et al.31 | 2014 | CS | no | Belgium | March 2006 – October 2011 | MTSS | yes | NS |
| Frank et al.32 | 2006 | CS | no | Italy | May 1998 and December 2004 | ETSS | yes | NA |
| Gao et al.33 | 2016 | Cohort study | No | China | January 2012–November 2014 | ETSS | Yes | NA |
| Gao et al.33 | 2016 | Cohort study | No | China | January 2012–November 2014 | MTSS | Yes | NA |
| Gondim et al.35 | 2011 | CS | no | Brazil | January 1998–december 2009 | ETSS | yes | yes |
| Guo Dong et al.37 | 2016 | Cohort study | No | China | June 2010–July 2014 | ETSS | Yes | NA |
| Guo Dong et al.37 | 2016 | Cohort study | No | China | June 2010–July 2014 | MTSS | Yes | NA |
| Guvenc et al.38 | 2016 | Cohort study | no | Turkey | June 2000 – June 2014 | ETSS | yes | no |
| Guvenc et al.38 | 2016 | Cohort study | no | Turkey | June 2000 – June 2014 | ETSS | yes | no |
| Han S et al.39 | 2013 | Cohort study | No | China | May 2009–June 2012 | ETSS | Yes | Yes |
| Hofstetter et al.42 | 2011 | CS | yes | USA | February 2004–january 2010 | ETSS | yes | no |
| Jain et al.44 | 2007 | RT | yes | India | NA | ETSS | yes | no |
| Jain et al.44 | 2007 | RT | yes | India | NA | MTSS | yes | no |
| Jang JH et al.45 | 2016 | Cohort study | No | S. Korea | April 1998–December 2014 | ETSS | Yes | Yes |
| Jho, et al.47 | 2001 | CS | no | USA | 1993–1999 | ETSS | yes | NA |
| Juraschka et al.48 | 2014 | CS | no | Canada | January 2006 – June 6 | ETSS | yes | no |
| Karppinen et al.50 | 2015 | Cohort study | no | Finland | 2000 – 2011 | ETSS | yes | no |
| Karppinen et al.50 | 2015 | Cohort study | no | Finland | 2000 – 2011 | MTSS | yes | no |
| Kenan et al.51 | 2006 | Cohort studt | No | Turkey | September 1997–June 2005 | ETSS | Yes | No |
| Kumar et al.53 | 2012 | CS | no | Singapore | June 1990–May2008 | ETSS | yes | yes |
| Kuo et al.54 | 2016 | CS | no | Taiwan | 2000 – 2009 | ETSS | yes | yes |
| Kurosaki et al.55 | 2000 | Cohort study | No | Germany | January 1991–November 1999 | MTSS | Yes | NA |
| Lampropoulos et al.56 | 2013 | CS | yes | Greece | 2004–2011 | MTSS | yes | yes |
| Liu et al.60 | 2015 | CS | no | China | January 2009 – December 2012 | MTSS | yes | no |
| Messerer et al.64 | 2011 | Cohort Study | No | France | 2006–2009 | ETSS | yes | yes |
| Messerer et al.64 | 2011 | Cohort Study | No | France | 2006–2009 | MTSS | yes | yes |
| Mortini P et al.67 | 2005 | CS | No | Italy | 1990–2002 | MTSS | NA | No |
| Nakao et al.68 | 2010 | Cohort study | No | Japan | 2000–2008 | ETSS | Yes | NA |
| Nie al.69 | 2015 | CS | NA | China | January 2012 – December 2012 | ETSS | yes | NA |
| O’Malley et al.70 | 2008 | Cohort study | no | USA | 2003–2008 | ETSS | yes | yes |
| O’Malley et al.70 | 2008 | Cohort study | no | USA | 2003–2008 | MTSS | yes | yes |
| Ogawa et al.71 | 2015 | CS | no | Japan | October 2008 – January 2014 | MTSS | yes | yes |
| Pinar et al.74 | 2015 | Cohort study | No | Turkey | February 2011–December 2013 | ETSS | Yes | NA |
| Qureshi et al.75 | 2016 | CS | no | USA | 2006 – 2012 | ETSS | yes | yes |
| Sheehan et al.78 | 1999 | CS | no | USA | 1995–1997 | ETSS | yes | NA |
| Sheehan et al.78 | 1999 | CS | no | USA | 1995–1997 | MTSS | yes | NA |
| Song et al.80 | 2014 | CS | no | China | January 2007 – January 2012 | ETSS | yes | NA |
| Thomas et al.82 | 2014 | CS | yes | USA | NA | ETSS | yes | NA |
| Tosaka et al.83 | 2015 | CS | no | Japan | October 2003 – October 2009 | ETSS | yes | yes |
| Wang et al.84 | 2015 | CS | no | China | January 2007 – June 2013 | ETSS | yes | yes |
| Wongsirisuwan et al.86 | 2014 | CS | no | Thailand | January 2003 – September 2013 | ETSS | yes | yes |
| Yan et al.87 | 2015 | CS | no | China | January 2013 – June 2013 | MTSS | yes | NA |
| Yildirim et al.88 | 2016 | CS | no | Turkey | August 2009 – May 2014 | ETSS | yes | no |
| Zaidi et al.89 | 2016 | Cohort study | no | USA | October 2011–June 2014 | ETSS | yes | yes |
| Zaidi et al.89 | 2016 | Cohort study | no | USA | October 2011–June 2014 | MTSS | yes | yes |
| Zhan et al.90 | 2015 | CS | no | China | January 2008 – December 2014 | ETSS | yes | NA |
| Zhang, X et al.91 | 2008 | CS | no | China | 1998 – 2005 | ETSS | yes | NA |
| Zhou et al.92 | 2014 | CS | no | China | January 2007– July 2012 | ETSS | yes | yes |
Abbreviations: CS, Case series; RT, Randomized Trail; ETSS, Endoscopic Transsphenoidal surgery; MTSS, Microscopic Transsphenoidal Surgery; NA, Not Available; iMRI: intraoperative MRI
Table 2.
Patient Characteristics in the Selected Studies
| Study | # PA | AGE | Gender | According to Size | According to Function | Functional Pituitary Adenoma | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Mean±SD) | M | F | % Microadenoma | % Macroadenoma | % FPA | %NFPA | % GH | % ACTH | %PRL | ||
| Bodhinayake et al.4 | 64 | 46.7 (16.7) | 24 (37.5%) | 40 (62.5%) | 14.10% | 78.1% | NA | NA | NA | NA | NA |
| Bokhari et al.5 | 79 | 56.7 ± 16.3 | 35 (44%) | 44 (56%) | 8.86% | 91.14% | 50.63% | 49.37% | 47.50% | 10.00% | 40.00% |
| Campbell et al.6 | 27 | 45.7 | 14 (53.8%) | 12 (46.2%) | 14.81% | 81.48% | 100.00% | 0.00% | 85.19% | NA | 11.11% |
| Cappabianca et al.7 | 146 | 46.06 | 68 (46.57%) | 78 (53.4%) | 14.38% | 85.62% | 45.21% | 54.79% | 54.55% | 19.70% | 19.70% |
| Charalampaki et al.8 | 134 | 57 | 70 (52.23%) | 80 (59.7%) | 11.19% | 44.78% | 44.03% | 55.97% | 44.07% | 27.12% | 27.12% |
| Chi et al.9 | 80 | 50.84 (13.62) | 45 (56%) | 35 (44%) | 20% | 80% | 57% | 43% | 19.6% | 6.5% | 56.5% |
| Choe et al.10 | 12 | 47 ± 12 | 5 (41.6%) | 7 (58.33%) | 25.00% | 75% | 100.00% | 0.00% | 75.00% | 25.00% | 0.00% |
| Choe et al.10 | 11 | 48 ± 10 | 2 (18.1%) | 9 (81.8%) | 27.27% | 72.73% | 100.00% | 0.00% | 72.73% | 27.27% | 0.00% |
| Chone et al.11 | 47 | 54 | NA | NA | NA | NA | 36.17% | 63.83% | 41.18% | 29.41% | 29.41% |
| Conrad et al.15 | 40 | 56.5 (−) | 20 (50%) | 20 (50%) | 10% | 90% | 27.50% | 72.50% | NA | NA | 2 |
| Constantino et al.16 | 28 | 46 (−) | 17 (60%) | 11 (40%) | 0 | 100% | 82% | 7.10% | NA | 0 | 1 |
| Cusimano et al.18 | 29 | 50.3 (15.4) | 16 (55%) | 13 (45%) | 0 | 100% | 13.8% | 86.2% | 75% | 0 | 25% |
| Dallapiazza et al.19 | 56 | 56.2± 12.8 | 27 (48.21%) | 29 (51.78%) | NA | NA | 0% | 100% | 0 | 0 | 0 |
| Dallapiazza et al.19 | 43 | 56.7± 16.9 | 24 (55.8%) | 19 (44.1%) | NA | NA | 0% | 100% | 0 | 0 | 0 |
| Dallapiazza et al.20 | 80 | 56.6 (13) | 38 (47.5%) | 42 (52.5%) | 0 | 100% | 0% | 100% | 0 | 0 | 0 |
| Dehdashti et al.23 | 200 | 49.9 (NA) | 109 (54.5%) | 91 (45.5%) | 79% | 21% | 44.5% | 55.5% | 38.2% | 30.3% | 28.1% |
| De Paiva Neto et al.21 | 51 | 48 (16) | 63% | 37% | 0 | 100% | 23.6% | 76.4% | 25% | 8.3% | 66.7% |
| De Witte et al.22 | 81 | 50.07± 13.81 | 44 (53%) | 39 (47%) | 12.35% | 90.12% | NA | 49.38% | NA | NA | NA |
| Duz et al.27 | 28 | NA | NA | NA | NA | NA | 46.43% | 42.86% | 61.54% | 23.08% | 15.38% |
| Duz et al.27 | 40 | NA | NA | NA | NA | NA | 45.00% | 52.50% | 55.56% | 22.22% | 22.22% |
| Fathalla et al.30 | 42 | 43.2 | 21 (50) | 21 (50) | 16.7% | 83.3% | 100% | 0% | 0 | 0 | 0 |
| Fathalla et al.30 | 23 | 42.1 | 7 (30) | 16 (70) | 26.1% | 73.9% | 100% | 0% | 0 | 0 | 0 |
| Fomekong et al.31 | 73 | 50 (17) | 46 (63) | 27 (37) | 0 | 100% | NA | NA | NA | NA | NA |
| Frank et al.32 | 173 | NA | 191 (45.5%) | 227 (54.5%) | NA | NA | 128.90% | 1.12 | 39.91% | 26.01% | 32.74% |
| Gao et al.33 | 60 | 44.6 | 26 (43.3%) | 34 (56.6%) | 21.6% | 78.3% | 55% | 45% | 21.2% | 15.1% | 63.6% |
| Gao et al.33 | 45 | 48.8 | 19 (42.2%) | 26 (57.7%) | 22.2% | 77.7% | 51.1% | 48.8% | 17.4% | 13% | 69.5% |
| Gondim et al.35 | 301 | 42.44± 15.31 | 134 (44.5%) | 167 (55.5%) | 17.61% | 82.39% | 55.15% | 44.85% | 40.96% | 22.29% | 28.92% |
| Guo-Dong et al.37 | 100 | 43.4 (14) | 59% | 41% | NA | NA | 48% | 52% | 18.75% | 8.3% | 68.75% |
| Guo-Dong et al.37 | 147 | 40.4 (14.2) | 53 (36%) | 94 (64%) | NA | NA | 53.1% | 46.9% | 12.8% | 8.9% | 73.1% |
| Guvenc et al.38 | 45 | 48.3 (14.1) | 19 (42.2) | 26 (57.8) | 31.10% | 68.90% | 51.10% | 48.90% | 24.40% | 8.90% | 11.10% |
| Guvenc et al.38 | 49 | 37.0 (13.0) | 18 (36.7) | 31 (63.3) | 14.30% | 85.70% | 71.40% | 28.60% | 36.70% | 2% | 26.50% |
| Han et al.39 | 250 | 43.8 | 99 (39.6%) | 151 (60.4%) | 17.2% | 82.8% | 41.2% | 58.8% | 40.8% | 19.4% | 32% |
| Hofstetter et al.42 | 86 | 45.2±1.8 | 37 (43.0%) | 49 (57.0%) | NA | NA | 100.00% | NA | 38.37% | 20.93% | 40.70% |
| Jain et al.44 | 10 | 40.1 | NA | NA | 10.00% | NA | NA | NA | NA | NA | NA |
| Jain et al.44 | 10 | 31.6 | NA | NA | 20.00% | NA | NA | NA | NA | NA | NA |
| Jang JH et al.45 | 331 | 48.4 | 145 (43.8%) | 186 (56.2%) | 29.6% | 70.4% | 52.6% | 47.4% | 11.5% | 16.7% | 59.8% |
| Jho, et al.47 | 128 | NA | 70 (54.68) | 90 (70.3) | 23.44% | 76.56% | NA | 53.13% | NA | NA | NA |
| Juraschka et al.48 | 73 | 54.48 (14.8) | 50 (68.5) | 23 (31.5) | 0 | 100% | 11% | 89% | NA | NA | NA |
| Karppinen et al.50 | 41 | 58.5 (16) | 23 (56) | 18 (44) | 0 | 100% | 0 | 100% | 0 | 0 | 0 |
| Karppinen et al.50 | 144 | 58.4 (13) | 95 (66) | 49 (34) | NA | NA | 0 | 100% | 0 | 0 | 0 |
| Kenan et al.51 | 59 | 44.7 | NA | NA | 14% | 86% | 75.8% | 24.2% | 35.6% | 6.8% | 47.4% |
| Kumar et al.53 | 164 | 53.1 | 80 (48.7) | 91 (55.4) | 19.51% | 70.73% | NA | 38.41% | NA | NA | NA |
| Kurosaki et al.55. | 32 | 73.9 | 17 (53%) | 15 (47%) | NA | NA | 0 | 100% | 0 | 0 | 0 |
| Kuo et al.54 | 38 | 50.8 (13.1) | 24 (63.2) | 14 (36.8) | NA | NA | NA | NA | NA | NA | NA |
| Lampropoulos et al.56 | 184 | 49.77±14.23 | 99(53.8) | NA | NA | NA | 47.28% | 52.72% | 58.62% | 26.44% | 12.64% |
| Liu et al.60 | 1104 | NS | 407 (36.9) | 697 (63.1) | 11.30% | 88.70% | 60.20% | 39.80% | |||
| Messerer et al.64 | 82 | 57 | 47(57.3) | 35 (42.6) | NA | NA | NA | NA | NA | NA | NA |
| Messerer et al.64 | 82 | 56.5 | 51 (62.19) | 31 (37.8) | NA | NA | NA | NA | NA | NA | NA |
| Mortini P et al.67 | 361 | 51.9 ±0.7 | NA | NA | NA | NA | NA | 100% | 0 | 0 | 0 |
| Nakao et al.68 | 43 | 55 | 23 (53.4%) | 20 (46.6%) | 0 | 100% | 0 | 100% | 0 | 0 | 0 |
| Nie al.69 | 52 | 46.8 | 24 (46.2) | 28 (53.8) | 13.50% | 86.50% | 67.30% | 32.70% | 0 | ||
| O’Malley et al.70 | 21 | 47.9 | 15 (60%) | 10(40%) | 12% | 88% | NA | NA | NA | NA | NA |
| O’Malley et al.70 | 22 | 50.8 | 16 (64%) | 9 (36%) | 8% | 92% | NA | NA | NA | NA | NA |
| Ogawa et al.71 | 23 | 63.5 (13.7) | 12 (52.2) | 11 (47.8) | NA | NA | 95.70% | 4.30% | NA | NA | NA |
| Pinar et al.74 | 32 | 48.6 | 14 (43.8%) | 18 (56.2%) | 37.5% | 62.5% | 68.8% | 31.2% | 36.4% | 22.7% | 27.3% |
| Qureshi et al.75 | 78 | 52.2 (18.1) | 43 (55.1) | 35 (44.9) | 3.80% | 96.20% | NA | NA | NA | NA | NA |
| Sheehan et al.78 | 26 | 59.2 ± 15.1 | 18 (69.2) | 8 (30.7) | 0.00% | 100% | 0.00% | 100% | NA | NA | NA |
| Sheehan et al.78 | 44 | 57.8 ± 14.9 | 31 (70.45) | 13 (29.5) | 0.00% | 100% | 0.00% | 100% | NA | NA | NA |
| Song et al.80 | 22 | NA | 12 (40.0) | 18 (60.0) | NA | NA | 100% | 0 | 0 | ||
| Thomas et al.82 | 50 | NA | 21 (42.0) | 29 (58.0) | 18.00% | 82% | 24.00% | 76.00% | 4 | ||
| Tosaka et al.83 | 30 | 51.5 | 14 (46.7) | 16 (53.3) | 0 | 100% | NA | NA | NA | NA | NA |
| Wang et al.84 | 1166 | 40.3 (15.25) | 517 (44.3) | 649 (55.7) | 21.00% | 79% | 50.50% | 49.50% | 68 | ||
| Wongsirisuwan et al.86 | 38 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Yan et al.87 | 99 | NA | 0 (0.0) | 99 (100.0) | 68.70% | 31.3% | 100.00% | 0 | 0 | 0 | |
| Yildirim et al.88 | 160 | 48.5 | 88 (55.0) | 72 (45.0) | 0 | 100% | 0 | 100% | 0 | 0 | 0 |
| Zaidi et al.89 | 55 | 55.9 ± 13.8 | 35 (63.6) | 20(36.4) | NA | NA | 0.00% | 100% | NA | NA | NA |
| Zaidi et al.89 | 80 | 59.1 ± 14.6 | 50 (62.5) | 30 (37.5) | NA | NA | 0.00% | 100% | NA | NA | NA |
| Zhan et al.90 | 313 | 60.1 | 188 (60.0) | 125 (39.9) | 16.90% | 83.10% | 0 | 100% | 0 | 0 | 0 |
| Zhang, X et al.91 | 78 | 45.1 | 36 (46.2%) | 42 (53.8%) | 14.10% | 85.90% | 44.87% | 55.13% | 25.71% | 11.43% | 48.57% |
| Zhou et al.92 | 133 | 38.3 | 96 (72.2) | 37 (27.8) | 12.80% | 87.20% | 100% | 0 | 0 | 0 | 0 |
Abbreviations: PA, pituitary adenoma; NA, not available; FPA, functional pituitary adenoma; NFPA, nonfunctional pituitary adenoma; GH, growth hormone; PRL, prolactin
Pituitary Adenomas
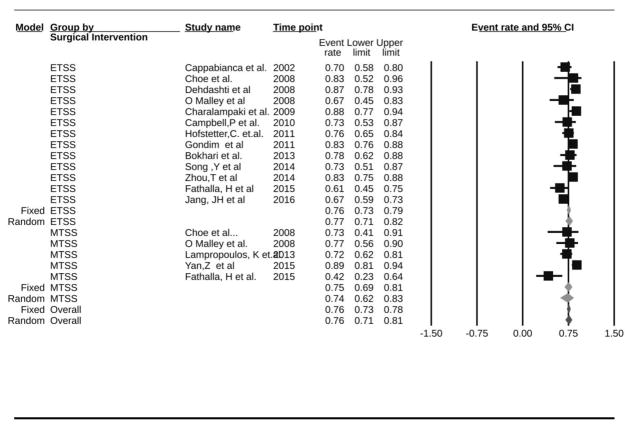
GTR was available for n=8257 patients (Table 3). Using the fixed-effect model, the pooled rate of GTR among all studies was 71.0% (95%CI: 69.9–72.1%, I2=91.2%; P-heterogeneity<0.01 under the fixed-effect model (Table 4) [4–11, 15, 16, 18–23, 27, 30–33, 35, 37–39, 42, 44, 45, 47, 48, 50–56, 59, 64, 68, 70, 71, 74, 75, 78, 80, 82–84, 86–92]. When eTSS and mTSS were compared, GTR rate was significantly higher in eTSS (n=50 studies, GTR=74.0%, 95%CI: 72.6–75.3%, I2=92.1%; P-heterogeneity<0.01) than in mTSS (n=20 studies, GTR=66.4%, 95%CI: 64.5–68.2%, I2=84.0%; P-heterogeneity<0.01) (Figure 2). This difference was significant in a fixed-effect model (P-interaction<0.01), but not in a random-effect models (P-interaction=0.40). To further assess the considerable heterogeneity in GTR observed in the pituitary adenomas overall, functioning pituitary adenomas (FPA) and non-functioning pituitary adenomas (NFPA) were assessed separately.
Table 3.
Result of Gross Tumor Resection In Pituitary adenoma
| Study (Year of Publication) | Surgical Intervention | PA (n) | Total Cases of GTR (n,(%)) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PA | Macroadenoma | Microadenoma | NFPA | FPA | GH | ACTH | PRL | |||
| Bodhinayake et al.4 | ETSS | 64 | 44/64 (68.7) | NR | NR | NR | NR | NR | NR | NR |
| Bokhari et al.5 | ETSS | 79 | 50/79 (63.2) | 43/72 (59.7) | 7/7 (100) | 19/39 (48.7) | 31/40 (77.5) | 14/19 (73.68) | 4/4 (100) | 12/16 (75) |
| Campbell et al.6 | ETSS | 26 | 19/26 (73.1) | NR | NR | NR | 19/26 (73.1) | 19/26 (73.1) | ||
| Cappabianca et al.7 | ETSS | 146 | 91/146 (62.3) | 73/125 (58.4) | 18/25 (85.7) | 45/80 (56.2) | 46/66 (69.7) | 23/36 (63.88) | 10/13 (76.9) | 10/13 (76.9) |
| Charalampaki et al.8 | ETSS | 134 | 126/134 (94.0) | NR | NR | 74/75 (98.7) | 52/59 (88.1) | 23/26 (88.4) | 14/16 (87.5) | 15/16 (93.75) |
| Chi et al.9 | ETSS | 80 | 51/80 (63.75) | NR | NR | NR | NR | NR | NR | NR |
| Choe et al.10 | ETSS | 12 | 10/12 (83.3) | NR | NR | NR | 10/12 (83.3) | 8/9 (88.8) | 2/3 (66.6) | NR |
| Choe et al.10 | MTSS | 11 | 8/11 (72.7) | NR | NR | NR | 8/11 (72.7) | 5/8 (62.5) | 3/3 (100) | NR |
| Chone et al.11 | ETSS | 47 | 45/47 (95.7) | NR | NR | 28/30 (93.3) | NR | NR | NR | NR |
| Conrad et al.15 | ETSS | 40 | 34/40 (85) | NR | NR | NR | NR | NR | NR | NR |
| Constantino et al.16 | ETSS | 28 | 4/28 (14.3) | 4/28 (14.3) | NR | NR | NR | NR | NR | NR |
| Cusimano et al.18 | ETSS | 29 | 23/29 (79.3) | NR | NR | NR | NR | NR | NR | NR |
| Dallapiazza et al.19 | ETSS | 56 | 54/56 (96.4) | NR | NR | 54/56 (96.4) | NR | NR | NR | NR |
| Dallapiazza et al.19 | MTSS | 43 | 40/43 (93.02) | NR | NR | 40 (93.02) | NR | NR | NR | NR |
| Dallapiazza et al.20 | ETSS | 80 | 57/80 (71) | 57/80 (71) | NR | 57/80 (71) | NR | NR | NR | NR |
| Dehdashti et al.23 | ETSS | 200 | 176/200 (88) | 158/200 (79) | 42/200 (21) | 98/111 (88) | 75/86 (87.2) | 29/34 (85) | 23/27 (85) | 23/25 (92) |
| De Paiva Neto et al.21 | ETSS | 51 | 21/51 (41.2) | NR | NR | NR | NR | NR | NR | NR |
| De Witte et al.22 | ETSS | 81 | 24/81 (29.6) | NR | NR | NR | NR | NR | NR | NR |
| Duz et al.27 | ETSS | 28 | 15/28 (53.5) | NR | NR | NR | NR | NR | NR | NR |
| Duz et al.27 | MTSS | 40 | 20/40 (50) | NR | NR | NR | NR | NR | NR | NR |
| Fathalla et al.30 | ETSS | 41 | 25/41 (61) | NR | NR | NR | 25/41 (61) | 25/41 (61) | NR | NR |
| Fathalla et al.30 | MTSS | 19 | 8/19 (42) | NR | NR | NR | 8/19 (42) | 8/19 (42) | NR | NR |
| Fomekong et al.31 | MTSS | 73 | 47/73 (65) | 47/73 (65) | NR | NR | NR | NR | NR | NR |
| Frank et al.32 | ETSS | 173 | 106/173 (62.2) | 105/172 (61.1) | 1/1 (100) | 106/173 (62.2) | NR | NR | NR | NR |
| Gao et al.33 | ETSS | 60 | 49/60 (81.7) | 37/47 (78.7) | 12/13 (92.3) | NR | NR | NR | NR | NR |
| Gao et al.33 | MTSS | 45 | 28/45 (62.2) | 19/35 (54.3) | 9/10 (90) | NR | NR | NR | NR | NR |
| Gondim et al.35 | ETSS | 301 | 233/301 (77.4) | NR | NR | 96/135 (71.1) | 137/166 (82.5) | 58/68 (85.3) | 27/37 (72.9) | 41/48 (85.4) |
| Guo-Dong et al.37 | ETSS | 100 | 60/100 (60) | NR | NR | NR | NR | NR | NR | NR |
| Guo-Dong et al.37 | MTSS | 147 | 85/147 (57.8) | NR | NR | NR | NR | NR | NR | NR |
| Guvenc et al.38 | ETSS | 45 | 31/45 (68.9) | 20/31 (65) | 11/14 (78.6) | NR | NR | NR | NR | NR |
| Guvenc et al.38 | MTSS | 49 | 38/49 (77.6) | 32/42 (76.2) | 6/7 (85.7) | NR | NR | NR | NR | NR |
| Han S et al.39 | ETSS | 250 | 216/250 (86.4) | NR | NR | NR | NR | NR | NR | NR |
| Hofstetter et al.42 | ETSS | 86 | 65/86 (78.6) | NR | NR | NR | 65/86 (75.5) | 26/33 (78.8) | 13/18 (72.2) | 26/35 (74.3) |
| Jain et al.44 | ETSS | 10 | 5/10 (50) | NR | NR | NR | NR | NR | NR | NR |
| Jain et al.44 | MTSS | 10 | 5/10 (50) | NR | NR | NR | NR | NR | NR | NR |
| Jang JH et al.45 | ETSS | 331 | 229/331 (69.2) | NR | NR | 98/157 (62.4) | 18/20 (90) | 25/29 (86.2) | 73/104 (68.3) | |
| Jho, et al.47 | ETSS | 68 | 53/68 (77.9) | NR | NR | 53/68 (77.9) | NR | NR | NR | NR |
| Juraschka et al.48 | ETSS | 73 | 16/73 (24.2) | 16/73 (24.2) | NR | NR | NR | NR | NR | NR |
| Karppinen et al.50 | ETSS | 41 | 23/41 (56) | 23/41 (56) | NR | NR | NR | NR | NR | NR |
| Karppinen et al.50 | MTSS | 144 | 64/144 (45) | NR | NR | NR | NR | NR | NR | NR |
| Kenan et al.51 | ETSS | 59 | 38/59 (64.4) | 38/59 (64.4) | NR | NR | NR | NR | NR | NR |
| Kumar et al.53 | ETSS | 66 | 56/66 (84.8) | NR | NR | 56/66 (84.8) | NR | NR | NR | NR |
| Kurosaki et al.55 | MTSS | 32 | 24/32 (75) | NR | NR | NR | NR | NR | NR | NR |
| Kuo et al.54 | ETSS | 38 | 8/38 (21.1) | NR | NR | NR | NR | NR | NR | NR |
| Lampropoulos et al.56 | MTSS | 184 | 124/184 (67.4) | NR | NR | 61/97 (62.9) | 63/87 (72.4) | 36/51 (70.6) | 17/23 (73.9) | 8/11 (72.7) |
| Liu et al.59 | MTSS | 1104 | 781/1104 (70.7) | NR | NR | NR | NR | NR | NR | NR |
| Messerer et al.64 | ETSS | 82 | 61/82 (74) | NR | NR | 61/82 (74) | NR | NR | NR | NR |
| Messerer et al.64 | MTSS | 82 | 41/82 (50) | NR | NR | 41/82 (50) | NR | NR | NR | NR |
| Mortini P et al.67 | MTSS | 361 | 234 (64.8) | NR | NR | 234 (64.8) | NR | NR | NR | NR |
| Nakao et al.68 | ETSS | 43 | 20/43 (47) | NR | NR | NR | NR | NR | NR | NR |
| Nie al.69 | ETSS | 52 | 46/52 (88.5) | NR | NR | NR | NR | NR | NR | NR |
| O’Malley et al.70 | ETSS | 21 | 14/21 (66.6) | NR | NR | NR | NR | NR | NR | NR |
| O’Malley et al.70 | MTSS | 22 | 17/22 (77.2) | NR | NR | NR | NR | NR | NR | NR |
| Ogawa et al.71 | MTSS | 23 | 20/23 (87.0) | NR | NR | NR | NR | NR | NR | NR |
| Pinar et al.74 | ETSS | 32 | 18/32 (56.2) | 9/20 (45) | 9/12 (75) | NR | NR | NR | NR | NR |
| Qureshi et al.75 | ETSS | 78 | 73/78 (93.6) | NR | NR | NR | NR | NR | NR | NR |
| Sheehan et al.78 | ETSS | 16 | 7/16 (43.8) | NR | NR | 7/16 (43.8) | NR | NR | NR | NR |
| Sheehan et al.78 | MTSS | 36 | 15/36 (41.7) | NR | NR | 15/36 (41.7) | NR | NR | NR | NR |
| Song et al.80 | ETSS | 22 | 16/22 (72.7) | NR | NR | NR | 16/22 (72.7) | NR | NR | NR |
| Thomas et al.82 | ETSS | 50 | 37/50 (74.0) | NR | NR | NR | NR | NR | NR | NR |
| Tosaka et al.83 | ETSS | 30 | 15/30 (50.0) | 15/30 (50.0) | NR | NR | NR | NR | NR | NR |
| Wang et al.84 | ETSS | 1166 | 1069/1166 (91.7) | 829/921 (90.0) | 240/245 (98.0) | NR | NR | NR | NR | NR |
| Wongsirisuwan et al.86 | ETSS | 38 | 28/38 (73.3) | NR | NR | NR | NR | NR | NR | NR |
| Yan et al.87 | MTSS | 99 | 88/99 (88.9) | NR | NR | NR | 88/99 (88.9) | NR | NR | 88/99 (88.9) |
| Yildirim et al.88 | ETSS | 160 | 144/160 (90.0%) | 144/160 (90.0%) | NR | 144/160 (90.0) | NR | NR | NR | NR |
| Zaidi et al.89 | ETSS | 55 | 43/55 (78.2) | NR | NR | 43/55 (78.2) | NR | NR | NR | NR |
| Zaidi et al.89 | MTSS | 80 | 65/80 (81.3) | NR | NR | 65/80 (81.3) | NR | NR | NR | NR |
| Zhan et al.90 | ETSS | 313 | 239/313 (76.4) | NR | NR | 239/313 (76.4) | NR | NR | NR | NR |
| Zhang X et al.91 | ETSS | 78 | 62/78 (79.4) | NR | NR | NR | NR | NR | NR | NR |
| Zhou et al.92 | ETSS | 133 | 110/133 (82.7) | NR | NR | NR | 110/133 (82.7) | 110/133 (82.7) | NR | NR |
Abbreviations: GTR, gross total resection, PA, pituitary adenoma; NR, not reported; FPA, functional pituitary adenoma; NFPA, nonfunctional pituitary adenoma; GH, growth hormone; PRL, prolactin
Table 4.
Results of Gross tumor resection rate and 95% Confidence Interval in the Following case (Combine Subgroups Using Fixed- and random-effect model)
| Operative modality | Number Of studies | Fixed-effect model | Random-effect model | Heterogeneity | P-interaction | P-value for Begg’s test | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| GTR rate (%) | 95%-CI | GTR rate (%) | 95%-CI | I2 value (%) | P-heterogeneity | Fixed effect model | Random effect model | |||
| ETSS PA Overall | 50 | 74.0 | 72.6–75.3 | 71.2 | 66.2–75.8 | 92.1 | <0.01 | <0.01 | 0.44 | 0.33 |
| MTSS PA Overall | 19 | 66.6 | 64.6–68.6 | 67.5 | 58.5–75.4 | 84.8 | <0.01 | |||
| ETSS FPA | 13 | 75.8 | 72.8–78.5 | 76.7 | 70.5–81.8 | 63.9 | <0.01 | 0.92 | 0.67 | 0.91 |
| MTSS FPA | 5 | 75.5 | 69.1–80.9 | 74.1 | 62.2–83.2 | 79.0 | <0.01 | |||
| ETSS NFPA | 19 | 71.0 | 68.7–73.3 | 74.1 | 67.5–79.7 | 86.3 | <0.01 | <0.01 | 0.127 | 0.08 |
| MTSS NFPA | 8 | 60.7 | 57.3–64.0 | 64.6 | 53.1–74.7 | 87.4 | <0.01 | |||
Abbreviations: GTR, gross total resection, PA, pituitary adenoma; NR, not reported; FPA, functional pituitary adenoma; NFPA, nonfunctional pituitary adenoma; ETSS, Endoscopic Transsphenoidal surgery; MTSS, Microscopic Transsphenoidal Surgery
Figure 2.
Subgroup Analysis by The type of TSS, Forest Plot of Gross tumor resection rate and 95 % CI for Patient with PA who had transsphenoidal Surgery
Functioning Pituitary Adenomas
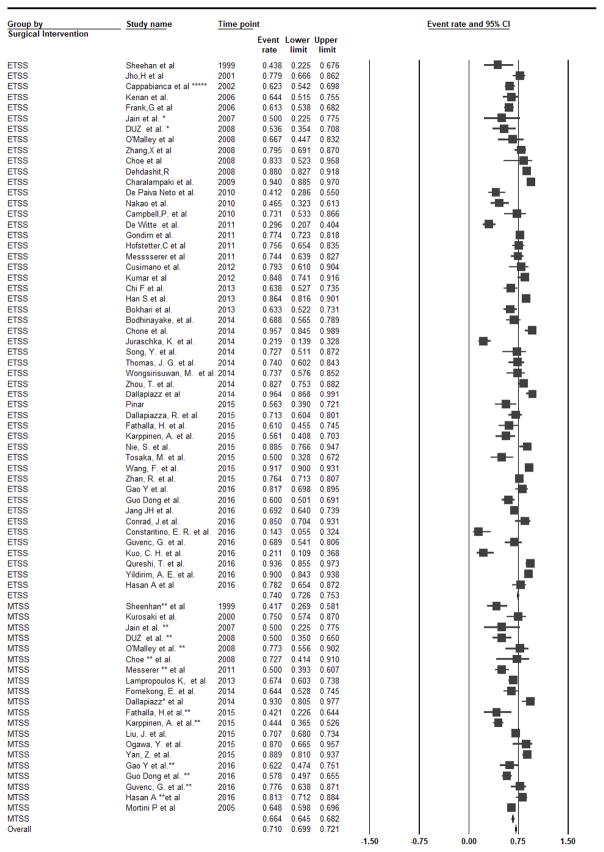
Eighteen studies reported GTR rate among FPAs (n=1170) [5–10, 18, 21, 23, 30, 33, 35, 37, 39, 42, 45, 51, 55, 56, 74, 80, 87, 92]. Using the fixed-effect model, the overall GTR rate was 75.7% (95%CI: 73.1–78.2%, I2=67.5%, p-heterogeneity<0.01). In a subgroup analysis for eTSS vs. mTSS, GTR rate was not significantly different comparing eTSS (GTR=75.8%, 13 studies) and mTSS (GTR=75.5%, 5 studies) using both the fixed- (P-interaction=0.92) and the random-effect models (P-interaction=0.67, Figure 3).
Figure 3.
Subgroup Analysis by The type of TSS, Forest Plot of Gross tumor resection rate and 95 % CI for Patient with Functional PA who had Transsphenoidal Surgery
All of the 13 studies reporting GTR after eTSS were published after 2000 and only 3 studies reported GH-producing to be the type of FPA. Using the fixed-effect model, significant sources of heterogeneity were identified for microadenoma percent (P=0.04; high percent: 67.6%, 3 studies, which had a lower GTR than studies with low percent microadenoma: 80.1%, 2 studies), number of centers (P=0.01; single center: 74.9%, multiple centers: 87.2%), age (P=0.01; 36–40 years: 82.7%, 41–45 years: 70.5%, 46–50 years: 71.5%, 56–60 years: 83.2%), and study design (P<0.01; cohort: 66.7%; case series: 78.3%). Non-significant interactions were identified for continent, country, male percent, and number of surgeons (all P>0.05). No significant sources of heterogeneity were identified using the random-effect model (not shown). Meta-regression on journal impact factor and year of publication were not significant in both random- and fixed-effect models (P >0.05 for all).
All of the five studies reporting GTR after mTSS were case series, conducted in a single center and published after 2000. Using the fixed-effect model, significant interactions were identified for age category (p=0.03; category 51–55: 77.3%, 1 study, which had a higher GTR than each of 46–50: 72.4%, 2 studies; 41–45: 42.1%, 1 study), type of FPA (p<0.01; 1 study with prolactinoma patients had a higher GTR rate of 88.9% than one study with GH-producing: 42.1%), in addition to continent (p<0.01; GTR in Asia: 86.7%, 2 studies, which was higher than in Europe: 72.4%, 1 study; and North America: 59.5%, 2 studies). Using the random-effect model, however, sources of heterogeneity could be identified for age category: p<0.01; and types of FPA: P<0.01. Other variables such as continent, male percent, single surgeon, and microadenoma percentage were not a significant source of heterogeneity. Meta-regression on journal impact factor was significant in a fixed-effect model (slope=−0.74: 95%-CI: −1.47; −0.01, P=0.046) which suggested that a lower GTR percent was associated with a higher journal impact factor, but this association was not significant in a random-effect model (P=0.38). Meta-regression on year of publication was not significant in both models (P>0.05 for both).
Non-Functioning Pituitary Adenomas
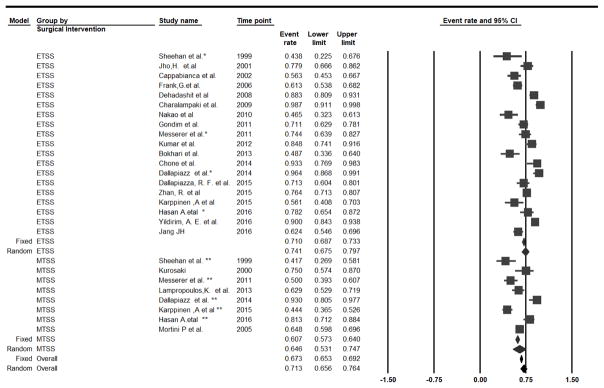
Twenty-seven studies reported GTR for NFPA (n=2,655) [5, 7, 8, 11, 19, 20, 23, 32, 35, 45, 53, 56, 64, 78, 88–90]. Under the fixed-effect model, the overall GTR rate for NFPA was 67.3% (95%CI: 65.3–69.2%, I2=87.7%, p-heterogeneity<0.01). In a subgroup analysis for eTSS vs. mTSS, GTR rate was significantly higher in eTSS (GTR=71.0%, 19 studies) than in mTSS (GTR=60.7%, 8 studies) (P-interaction<0.01), although this difference was not significant in the random-effect model (P-interaction= 0.13, Figure 4).
Figure 4.
Subgroup Analysis by The type of TSS, Forest Plot of Gross tumor resection rate and 95 % CI for Patient with Non-Functional PA who had Transsphenoidal Surgery
Among the 19 studies reporting GTR after eTSS, they were all conducted in a single center. Using the fixed-effect model, significant interactions were identified for the following variables: continent: P <0.01 (GTR in North America: 78.2%, 6 studies, which was higher than in Europe: 68.4%, 6 studies; Asia: 70.5%, 4 studies; South America: 73.3%, 2 studies; and Australia: 48.7%, 1 study); age category: P <0.01 (age category 46–50: 74.5%, 2 studies, which had a higher GTR than each of 51–55: 46.5%, 1 study, and 56–60: 73.7%, 7 studies); publication after 2000 (P=0.02; before 2000: 43.8%, 1 study, vs. after 2000 71.3%, 18 studies); study design (P<0.01; cohort: 58.5%, 3 studies; case series: 73.6%; 16 studies). Non-significant interaction was identified for microadenoma percent (P=0.41) and male percentage (P=0.66). Using the random-effect model, only study design was identified as a significant source of heterogeneity (P<0.01). Other variables such as number of surgeons were not available in many studies and were therefore not used for stratification. Meta-regression on year of publication was significant in a fixed-effect model (P<0.01, beta: 0.03) suggesting an increased GTR with later publication year, but not in a random-effect model (P=0.15). Meta-regression on journal impact factor was not significant in a random-effect model (P=0.20), yet it was significant in the fixed-effect models (beta: −0.13; P<0.01) suggesting that studies published in a higher impact factor journal tended to report a lower GTR than studies published in lower impact factor journals.
Among the eight single center studies reporting GTR after mTSS, significant interactions were identified with the following variables using the fixed-effect model: continent (P<0.01; GTR in North America 71.8%, 3 studies; GTR in Europe 59.0%, 5 studies), age (P=0.013; age category 71–75: 75%, 1 study, which had a higher GTR than 56–50: 54.4%, 5 studies), study design: (P<0.01; cohort: GTR=49.2%, 2 studies; case series: GTR=63.5%, 6 studies), and publication before 2000 (P=0.019; before 2000: 41.7%, 1 study, after 2000: 61.6%, 7 studies). However, using the random-effect model, no significant sources of heterogeneity could be identified for the following variables: continent (P-interaction=0.15), age category (P=0.96), publication before 2000 (P=0.141), and study design (P=0.524). While 7 studies did not report the microadenoma percentage, only 1 study indicated it had a higher macroadenoma percentage; 6 other studies reported a higher male percentage. Meta-regression on journal impact factor was significant with the fixed-effect model (slope=0.13, 95% CI: 0.008–0.25, p=0.04) indicating a direct association between a higher journal impact factor and a higher GTR rate, but this association was not significant in a random-effect model (p=0.79). Meta-regression on study year was not significant in random- (P=0.42) or fixed-effect (P=0.41) models.
Publication Bias
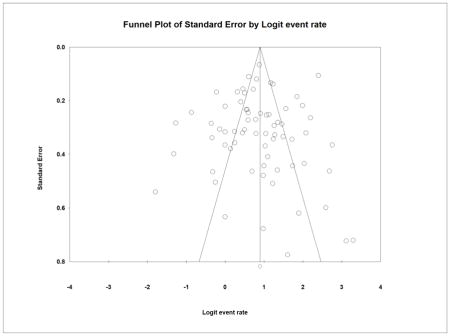
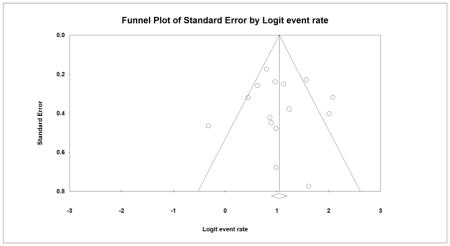
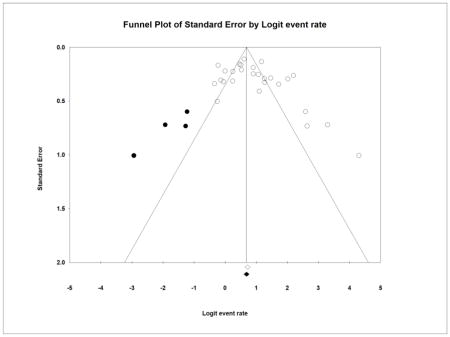
A symmetrical inverted funnel plot suggested the absence of publication bias in the GTR analysis for pituitary adenomas (Appendix 4). Furthermore, no significant publication bias was identified using Begg’s (P=0.29) and Egger’s test (P=0.52). In the analysis for FPA, a symmetrical funnel plot suggested the absence of publication bias (Appendix 5), which was also confirmed by Begg’ (P=0.91) and Egger’s Tests (P=0.82). In the analysis for NFPA, a slightly asymmetrical inverted funnel plot suggested the presence of publication bias where smaller studies showing a lower GTR rate could have been unpublished (Appendix 6); however, Begg’s (P=0.11) and Egger’s test (P=0.07) indicated no publication bias. After imputing 4 studies to the left of the pooled estimate using the trim and fill method, the new pooled GTR rate slightly decreased from 67.3% to 66.5% under the fixed effect model.
Discussion
This meta-analysis indicates that among patients who are not randomly allocated to either approach, eTSS results in a higher rate of GTR compared to mTSS, for all pituitary adenomas and for NFPA in fixed-effect models. For all FPAs, however, eTSS does not offer a significantly higher rate of GTR in both models. Despite these significant associations, the great heterogeneity among studies reporting both approaches could not be corrected by meta-regression, indicating that the results should be interpreted with caution.
Despite detailed meta-regression by both study and patient-level characteristics, the heterogeneity between studies of both modalities could not be alleviated. Due to the relatively low quality of evidence of the included studies, which mostly consisted of retrospective case series, this heterogeneity is not surprising. Some of the reasons for the great heterogeneity may include the learning curve associated with endoscopic resection, with more and less experienced surgeons reporting significantly different rate of GTR.
One recent survey among neurosurgeons found a significant correlation between the number of pituitary adenomas resections performed and post-operative complication rates (p<0.05) [12]. For GTR specifically, one study found a significant relation when comparing the first 40 patients with the last 40 patients in their case series (52.5% vs. 75.0%, p=0.036), while another study only found a nonsignificant trend towards higher rate of GTR with growing experience [5, 9]. However, another study comparing an inexperienced neurosurgeon performing eTSS to an experienced neurosurgeon performing mTSS showed no significant difference in GTR (p=0.67), suggesting learning curve may not always compromise GTR [89]. In a multivariate model, however, the same study showed that larger pituitary adenomas were associated with a lower extent of resection [89]. In this meta-analysis, only a difference in percentage of macroadenomas was identified as a source of heterogeneity for mTSS NFPA resection, and difference in microadenoma percentage as a source of heterogeneity for eTSS FPA resection. The lack of a significant difference in GTR for eTSS and mTSS may be explained by longer experience with microscopic resection mTSS, despite improved visibility with eTSS.
One other meta-analysis also reported a significant difference in GTR between eTSS and mTSS, but heterogeneity was not described (79% vs. 65% respectively, p<0.01) [24]. Similarly, one study examining 15 cohort studies also reported a higher rate of GTR for eTSS (OR = 1.86, 95%-CI: 1.36–2.54) [34]. Another study found similar results for pituitary adenomas invading the cavernous sinus (47% vs. 21% respectively, p<0.01) [26]. Three other systematic reviews have suggested no significant differences in GTR between the two modalities [76, 79, 81].
Although it remains unclear which of the two treatment modalities, eTSS or mTSS, is superior for GTR, other factors may also play a key role in outcomes for patients with pituitary adenomas, and this meta-analysis cannot fully address these concerns. For example, eTSS may be associated with shorter length of stay and lower costs [24, 36, 73, 76]. Other experts have suggested, however, that eTSS, which generally requires longer operative times, may adversely affect both patient and financial outcomes [66]. Furthermore, one meta-analysis found an association between eTSS and vascular complications when compared to mTSS (1.58% vs. 0.50%, p<0.01) [1]. Proposed reasons for this difference include more aggressive surgical excision in patients undergoing eTSS, perhaps due to the superior visualization permitted by this modality. Other patient-related factors that may alter the choice may be quality of life and visual improvement after surgery, which have not been compared between the modalities [62, 85]. Also, a meta-analysis showed that eTSS was associated with more post-operative visual improvement [24]. Remission of hypersecretion of FPA may also form an indication for either of the modalities, although one meta-analysis showed a non-significant difference [36, 67]. This is particularly relevant as for most FPAs the main goal of the surgery is to achieve hormonal recovery instead of GTR [29, 67]. This is why hormonal recovery could be viewed as a far superior outcome to GTR for FPA patients. Nevertheless, GTR is suggested to be predictive of hormonal recovery [42]. However, it remains to be elucidated what the exact contribution of GTR is to post-operative hormonal recovery rate, as many other factors also contribute to this outcome (e.g., dopamine-antagonists for prolactinoma) [72]. Finally, recurrence, progression free and overall survival, which were also not directly compared, could further aid decision making. Recently, an analysis of nearly 6000 operations demonstrated that eTSS was associated with higher rates of complications, longer postoperative hospital stays, and increased costs when compared to mTSS. It is important to remember that these economic factors may also play a role in decisions regarding methodological choice, beyond just patient- and prognosis-related variables [2].
Strengths of this meta-analysis include the systematic search strategy and fully updated reference list. This is the largest meta-analysis conducted to date on this topic, and the second to identify significant difference in GTR between the two modalities [24]. Additionally, this meta-analysis reported and attempted to address heterogeneity via subgroup analysis by numerous study and patient-level characteristics.
Limitations of this meta-analysis include the high heterogeneity identified among the studies for both eTSS and mTSS. Additionally, odds ratios or relative risks could not be calculated due to the study design of the included studies as the vast majority was retrospective case series. Furthermore, due to inconsistent reporting among the studies included, meta-regression by Knosp score, Hardy-Wilson tumor grading, or asymmetric suprasellar extension was not possible [26]. Furthermore, using both fixed- and random-effect models may help determine the true difference in GTR, but a random-effect model is often not significant when a fixed-effect model is. As with any meta-analysis, its strength is determined only by the strength of the studies included within it. The literature on this topic mostly consists of retrospective case series of varying size; thus, pooled analysis is limited in showing causality. Furthermore, it was not possible to incorporate surgeon experience, which may also influence GTR rate [5, 9]. Surgical outcomes after giant pituitary adenomas resection could not be compared separately as only outcomes after ETSS were reported in five studies and after ETSS, MTSS, and craniotomy in one study [16, 18, 21, 48, 54, 68]. The latter suggests that ETSS results in significantly higher GTR rate among giant pituitary adenomas [18]. This study also examined only GTR and not the many other factors that determine selection of surgical modality. GTR is an important but limited marker for surgical success, especially when resecting FPAs, for which hormonal recovery determines surgical success, and when stereotactic radiosurgery (SRS) is available [28, 67]. This limits the implications of this meta-analysis for FPA patients.
As the technology for eTSS continues to advance, it is likely that eTSS will continue to displace mTSS as the primary approach for sellar lesions, regardless of whether carefully collected evidence indicates superiority. The gold standard for comparison between the two modalities would of course be a prospective, randomized, controlled trial comparing eTSS to mTSS for a large number of patients, as suggested by the IDEAL (Idea, Development, Exploration, Assessment, Long-term Follow-up, Improving the Quality of Research in Surgery) Framework [63]. The IDEAL criteria require careful introduction accompanied by prospective evaluation for initial patients. This should than be followed by a randomized controlled trial to show true benefit [63]. There are many reasons why such a study is unlikely to occur, including surgeon preference and difficulties with patient enrollment. In light of these difficulties and the unlikelihood of such high-quality data, meta-analyses of currently existing studies represent the highest quality data available. Further studies may be improved by focusing on smaller subsets of these reports with the aim to reduce heterogeneity and identify more granular differences in the two approaches. Furthermore, a focus on evaluation of relevant outcomes to patients, such as hormonal recovery for FPA, visual recovery, and quality of life, is of vital importance. Also, alternative trial design may aid finding methodologically just ways of comparing these surgical modalities [61, 63].
Conclusion
The pooled GTR rate in all pituitary adenoma patients undergoing eTSS (74.0%) was significantly higher than the GTR rate in patients undergoing mTSS (66.6%). For NFPA, eTSS resulted in a significantly higher GTR rate (71.0%) than mTSS (60.7%) in a fixed-effect model. However, none of these differences were significant in random-effect models. A direct comparison between the two modalities was impossible, however, due to the high heterogeneity among studies.
Supplementary Material
Acknowledgments
Funding: No funding was acquired for this study.
Appendices
Appendix (1): Search Strategy
| Pubmed |
| (“Pituitary Neoplasms”[Mesh] OR “Pituitary Neoplasms/surgery”[Mesh] OR Pituitary Neoplasms[TW] OR Pituitary Neoplasm[TW] OR Neoplasm, Pituitary[TW] OR Neoplasms, Pituitary[TW] OR Pituitary Tumors[TW] OR Pituitary Tumor [TW] OR Tumor, Pituitary[TW] OR Tumors, Pituitary[TW] OR Adenoma, Pituitary[TW] OR Adenomas, Pituitary[TW] OR Pituitary adenoma [TW] OR Pituitary Adenomas[TW] OR Macroadenoma [TW] OR Microadenoma [TW] OR Pituitary Microadenoma [TW]) And (“Neuroendoscopy”[Mesh] OR “Neuroendoscopes”[mesh] OR Neuroendoscopy [TW] OR Neuroendoscope*[TW] OR “Neuroendoscopy/adverse effects”[Mesh] OR “Neuroendoscopy/methods”[MAJOR] OR “Neuroendoscopes”[MAJOR] OR trans-sphenoidal[tw] OR Transsphenoidal [tw] OR Transnasal endoscopic resection [TW] OR TER [TW] OR Transsphenoidal pituitary surgery[TW] OR Transsphenoidal endoscopic surgery [TW] OR endoscopic transsphenoidal surgery [TW] OR endoscopic endonasal transsphenoidal [TW] OR nasal endoscopic transsphenoidal surgery [TW] OR ETSS [tw] OR trans-nasal [TW] OR Transsphenoidal microscopic surgery [TW] OR microscopic transsphenoidal surgery [TW] OR MTS [TW]OR microscopic endonasal transsphenoidal [TW] OR MTSS [TW] OR micro-surger*[TW] OR microsurger* [TW] OR “Cerebral Revascularization”[Mesh] OR “Microsurgery”[Mesh:NoExp]) AND (“neoplasm, residual”[MeSH] OR residual neoplasm [Text Word] OR residual tumor [tw] OR residual cancer [tw] OR GROSS TUMOR RESECTION [TW] OR “Length of Stay”[Mesh] OR length of stay [tw] OR “perioperative period”[MeSH Terms] OR perioperative period [Text Word] OR “postoperative complications”[MeSH] OR postoperative complication*[Text Word] OR complication rate[tw]) |
| Embase |
| exp hypophysis tumor/su [Surgery] OR Pituitary Neoplasms.ti,ab,tw. OR Pituitary Tumors.ti,ab,tw.OR Pituitary adenoma.ti,ab,tw. OR exp hypophysis adenoma/OR Pituitary Microadenoma.ti,ab,tw. OR Pituitary Adenomas.ti,ab,tw. OR exp microadenoma/su [Surgery] OR microadenoma.ti,ab,tw. OR exp adenoma/su [Surgery] OR Macroadenoma.ti,ab,tw. AND Exp neuroendoscopy/OR neuroendoscopy.ti,ab,tw. OR Neuroendoscopic surgery.tw OR neuroendoscopic procedure.tw. OR neuroendoscope.tw. OR neuroendoscopes.tw OR neurological Procedure.tw. OR exp transsphenoidal surgery/OR transsphenoidal surgery.ti,ab,tw. OR Transsphenoid surgery.tw. OR transsphenoidal treatment.tw. OR Transsphenoidal microscopic surgery.ti,ab,tw. OR Transsphenoidal endoscopic surgery.ti,ab,tw. OR Microscopic transsphenoidal surgery.ti,ab,tw. OR Endoscopic Transsphenoidal surgery.ti,ab,tw. OR Microscopic endonasal transsphenoidal.ti,ab,tw. OR Endoscopic endonasal transsphenoidal.ti,ab,tw. OR Nasal endoscopic transsphenoidal surgery.ti,ab,tw. OR endoscopic neurosurgery.ti,ab,tw. AND Gross tumor resection.ti,ab,tw OR exp perioperative period/OR Peri-operative period.ti,ab,tw OR Complication Rate.ti,ab,tw OR postoperative complications.ti,ab,tw OR exp Postoperative Complication/OR Length of Stay.ti,ab,tw. OR exp Length of stay. |
| Cochrane |
Cochrane Central Register of Controlled Trials (CCTR)
Cochrane database for SR
Cochrane Methodology Register
|
Appendix (2): Studies excluded in the Analysis of Gross Tumor Resection (GTR)
| Author | Year of Publica tion |
Countr y |
Study Design |
Retrospe ctive |
Prospec tive |
# Of Grou ps |
Diagnosis | Surgical Interventi on |
Reason |
|---|---|---|---|---|---|---|---|---|---|
| Abosch, A | 1998 | USA | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION |
| Atkinson et al. | 2008 | USA | Not clear | Yes | No | 2 | Cushing Disease | STT/CTS | COMPARING MTSS VS COMBINED (IT DIDN’T MEET OUR CRTIERIA) |
| Bao X | 2016 | China | CS | Yes | No | 1 | PA | ETSS | NO AVAILABLE INFORMATION ABOUT GTR |
| Barzaghi, L. | 2007 | Italy | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION |
| Berker, M. | 2012 | Turkey | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION |
| Berkman S. | 2014 | Germany | CS | Yes | No | 1 | PA | MTSS | NO AVAILABLE INFORMATION ABOUT GTR |
| Black, P. M. | 1987 | USA | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION |
| Cappabianca, P. | 1999 | Italy | CS | Yes | No | 2 | PA | ETSS/MTSS | Overlapping cohorts |
| Cappabianca, P. | 2002 | Italy | CS | Yes | No | 1 | PA, Craiopharyingiomas, chordoma |
ETSS | Overlapping cohorts |
| Casler | 2005 | USA | CS | Yes | No | 2 | PA | ETSS/MTSS | IT REPORTED GTR IN ETSS AND MTSS BUT THEY WERE USED TWO OR COMBINED TECHNIQUE FOR 10 PATIENT AND RESULT REOPORTED IN GENERAL (I DON’T KNOW THE RESULT FOR THEM) |
| Chacko, A. G. | 1997 | Vellore | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION |
| Chen, L.; | 2011 | China | CS | No | Yes | 1 | PA | EATSS | It was EATSS doesn’t meet our criteria |
| Cheng, R. -X; | 2011 | China | CS | Yes | No | 2 | PA | ETSS/MTSS | GTR INFORMATION NA / THEY MENTION REMISSION RATE HERE INFORM OF GTR BUT IT IS DEFINED IN THE STUDY AS HORMONAL CONTROL |
| Cho D.Y | 2002 | China | Randomized | NA | NA | 2 | Prolactinomas | ETSS/MTSS | NO AVAILABLE INFORMATION GTR |
| Cho, D. Y.; | 2002 | China | CS | No | No | 2 | PA | ETSS/MTSS | Didn’t design study to report GTR INFORMATION |
| Chung T.G. | 2015 | U.S.A. | CS | Yes | No | NS | PA | NS | NO AVAILABLE INFORMATION ABOUT GTR |
| Comtois, R. | 1991 | Canada | CS | Yes | No | 1 | PA | ETSS | Study published before 1992 |
| Ciric, I. | 1983 | USA | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION |
| D’Haens, J. | 2009 | Belgium | CS | Yes | No | 2 | PA | ETSS/MTSS | No GTR INFORMATION, THEY REPORTED REMISSION RATE IN FPA NOT GTR AS OUR DEFINATION in the Meta-analysis |
| Dehdashti, A. | 2007 | Canada | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION |
| Esposito, V. | 2004 | Italy | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION |
| Fatemi, N. | 2008 | USA | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION |
| Gendeh, B. S. | 2006 | Kualalumpur | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION |
| Graham S.M., | 2009 | USA | CS | Yes | No | 2 | PA | ETSS/ Open Pituitary surgery | NO AVAILABLE INFORMATION GTR |
| Gondim, J.A. | 2014 | Brazil | CS | Yes | No | 1 | PA | ETSS | Overlapping cohorts |
| Gondim, J.A. | 2015 | Brazil | CS | Yes | No | 1 | PA | ETSS | Overlapping cohorts |
| Gondim, J.A. | 2010 | Brazil | CS | Yes | No | 1 | PA | ETSS | Overlapping cohorts |
| Halvorsen, H. | 2014 | Norway | Cohort | Yes | No | 2 | PA | ETSS/MTSS | Didn’t design study to report GTR INFORMATION |
| Higgins, T.S. | 2008 | United states | Cohort | Yes | No | 1 | Sellar pathology | MTSS | Not specific for PA |
| Ho, K. H. | 1987 | Singapore | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION |
| Kawamata, T. | 2002 | Japan | CS | No | Yes | 2 | PA | EATSS/MTSS | First of all the procedure is EATSS and didn’t design study to report GTR INFORMAT ION / need to check with Hasan |
| Kim, M. | 2009 | Korea | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION, only Reported remission rate in Acromegaly |
| Koren et al. | 1999 | Israel | CS | Yes | No | 2 | Pituitary Tumors | ETSS/MTSS | NO AVIALBLE INFORMATION ABOUT GTR |
| Lu, Y. -J | 2009 | Taiwan | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION |
| Minet, W. | 2008 | Ontario | Cohort | Yes | No | 2 | Sella-based tumour | EATSS/ETSS | Didn’t design study to report GTR INFORMATION / two group EATSS AND PETSS / NEED TO CHECK? |
| Nasseri, S. | 2001 | USA | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION |
| Neal et al. | 2007 | USA | Not clear | Yes | No | 3 | PA | ETSS/MTSS | NO AVAILABLE INFORMATION ABOUT GTR |
| Patel K.S. | 2014 | U.S.A. | CS | No | Yes | 1 | PA | NS | NO AVAILABLE INFORMATION ABOUT GTR |
| Pinar E. | 2015 | Turkey | CS | Yes | No | 1 | PA | ETSS | NO AVAILABLE INFORMATION ABOUT GTR |
| Prevedello, D. M. | 2008 | USA | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION, IT report Remission rate in Cushing |
| Razak, A. A. | 2013 | UK | Cohort | No | Yes | 2 | PA | ETSS/MTSS | Didn’t design study to report GTR INFORMATION, IT report Remission rate in FPA |
| Rudnik, A. | 2007 | Poland | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION, |
| Sand, M. S. | 2011 | Malaysia | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION, |
| Santos Rde, P. | 2007 | Brazil | CS | Yes | No | 1 | Sellar tumors | ETSS | Didn’t design study to report GTR INFORMATION, |
| Sarkar S. | 2016 | India | CS | Yes | No | 1 | PA | ETSS | NO AVAILABLE INFORMATION ABOUT GTR |
| Semple, P. L | 1999 | USA | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION, |
| Senior, B. A. | 2008 | USA | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION, |
| Shah S. & Har-El G. | 2001 | USA | CS | Yes | No | NO AVAILABLE INFORMATION GTR | |||
| Shah, S.; Har-El, G. | 2001 | USA | Cohort | Yes | No | 2 | PA | ETSS/MTSS | Didn’t design study to report GTR INFORMATION, |
| Shimon, I | 2001 | Israel | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION, only Remission rate in Acromegally |
| Shimon, I. | 2002 | Israel | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION, only Remission rate in Cushing |
| Shou, X. -F; | 2005 | China | CS | Yes | No | 1 | PA | MTSS | GTR reported but they did more than one type of TSS and result in general we don’t know I how many patient reach to GTR in MTSS or ETSSl |
| Smith, S. J. | 2010 | UK | Cohort | Yes | No | 2 | Skull base | ETSS/MTSS | Didn’t design study to report GTR INFORMATION, |
| Tindal, G.T. | 1978 | United states | CS | Yes | No | 1 | PA | ETSS | Study before 1992 |
| Turner, H. E. | 1999 | UK | CS | Yes | No | 1 | Microprolactinomas | MTSS | Didn’t design study to report GTR INFORMATION, only Remission rate in microprolactinoma |
| Uren, B. | 2007 | Australia | CS | Yes | No | 1 | Pituitary Tumors | ETSS | Didn’t design study to report GTR INFORMATION, only Remission rate |
| Van Bunderen, C. | 2013 | The Netherland | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION, only Remission rate |
| Wagenmakers, M. | 2013 | The Netherland | CS | Yes | Yes | 1 | PA | ETSS | Didn’t design study to report GTR INFORMAT ION, only Remission rate |
| Wagenmakers, M. | 2011 | The Netherland | CS | Yes | No | 1 | PA | ETSS | Didn’t design study to report GTR INFORMATION, only Remission rate |
| White et al. | 2004 | USA | Case control | Yes | No | 2 | PA | ETSS/MTSS | NO AVAILABLE INFORMATION ABOUT GTR |
| Yamada S. | 2014 | Japan | CS | Yes | No | 2 | PA | ETSS/MTSS | NO AVAILABLE INFORMATION ABOUT GTR |
| Yamada, S.; | 1997 | Japan | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION, only Remission rate |
| Yan Z. | 2015 | China | CS | Yes | No | 1 | PA | NS | NO AVAILABLE INFORMATION ABOUT GTR |
| Zaidi, H.A. | 2016 | United states | CS | Yes | No | 1 | PA | ETSS | Patients were operated using iMRI |
| Zhang, H. W. | 2008 | China | CS | Yes | No | 1 | PA | MTSS | Didn’t design study to report GTR INFORMATION, only Remission rate |
| Zhao, B. | 2010 | China | CS | Yes | No | 1 | PA | Extended TSS | Doesn’t meet Criteria because they used Two ETSS/AND in some group MTSS (it called Extended Approach) |
CS, Case series; RT, Randomized Trail; ETSS, Endoscopic Transsphenoidal surgery; MTSS, Microscopic Transsphenoidal Surgery; EATSS, Endoscope-Assisted Endonasal Trans-sphenoidal microsurgery; PA, Pituitary Adenomas; GTR, Gross Tumor Resection; NS, Not specified; NA, Not Available; iMRI, intraoperative Magnetic Resonance Imaging
Appendix (3): Analysis of Gross Tumor Resection (GTR) In Pituitary Adenoma Patients according to Tumor Characteristic in the selected studies
| Study | Year of Publication |
Number of groups |
Surgical Intervention |
Result of GTR reported for | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PA | Macroaden oma |
Microade noma |
NF PA |
FP A |
G H |
AC TH |
P R L |
||||
| Bodhinayake et al.4 | 2014 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Bokhari et al.5 | 2013 | 1 | ETSS | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Campbell et al.6 | 2010 | 1 | ETSS | ✖ | ✖ | ✖ | ✖ | ✔ | ✔ | ✖ | ✖ |
| Cappabianca et al.7 | 2002 | 1 | ETSS | ✔ | ✔ | ✔ | ✖ | ✔ | ✔ | ✔ | ✔ |
| Charalampaki et al.8 | 2009 | 1 | ETSS | ✔ | ✖ | ✖ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Chi et al.9 | 2013 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Choe et al.10 | 2008 | 2 | ETSS | ✖ | ✖ | ✖ | ✖ | ✔ | ✔ | ✔ | ✖ |
| MTSS | ✖ | ✖ | ✖ | ✖ | ✔ | ✔ | ✔ | ✖ | |||
| Chone et al.11 | 2014 | 1 | ETSS | ✔ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| Conrad et al.15 | 2016 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Constantino et al.16 | 2016 | 1 | ETSS | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Cusimano et al.18 | 2012 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Dallapiazza et al.19 | 2014 | 2 | ETSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ | |||
| Dallapiazza et al.20 | 2015 | 1 | ETSS | ✔ | ✔ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| Dehdashti et al.23 | 2008 | 1 | ETSS | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| De Paiva Neto et al.21 | 2010 | 1 | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | |
| De Witte et al.22 | 2011 | 1 | ETSS | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Duz et al.27 | 2008 | 2 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | |||
| Fathalla et al.30 | 2015 | 2 | ETSS | ✔ | ✖ | ✖ | ✖ | ✔ | ✔ | ✖ | ✖ |
| MTSS | ✔ | ✖ | ✖ | ✖ | ✔ | ✔ | ✖ | ✖ | |||
| Fomekong et al.31 | 2014 | 1 | MTSS | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Frank et al.32 | 2006 | 1 | ETSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| Gao et al.33 | 2016 | 2 | ETSS | ✔ | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✔ | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | |||
| Gondim et al.35 | 2011 | 1 | ETSS | ✔ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| Guo-Dong et al.37 | 2016 | 2 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | |||
| Guvenc et al.38 | 2016 | 2 | ETSS | ✔ | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✔ | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | |||
| Han et al.39 | 2013 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Hofstetter et al.42 | 2011 | 1 | ETSS | ✖ | ✔ | ✔ | ✖ | ✔ | ✔ | ✔ | ✔ |
| Jain et al.44 | 2007 | 2 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | |||
| Jang et al.45 | 2016 | 1 | ETSS | ✔ | ✖ | ✖ | ✔ | ✖ | ✔ | ✔ | ✔ |
| Jho et al.47 | 2001 | 1 | ETSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| Juraschka et al.48 | 2014 | 1 | ETSS | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Karppinen et al.50 | 2015 | 2 | ETSS | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | |||
| Kenan et al.51 | 2006 | 1 | ETSS | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Kuo et al.54 | 2016 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Kumar et al.53 | 2012 | 1 | ETSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| Kurosaki et al.55 | 2000 | 1 | MTSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Lampropoulos et al.56 | 2013 | 1 | MTSS | ✖ | ✖ | ✖ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Liu et al.59 | 2015 | 1 | MTSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Messerer et al.64 | 2011 | 2 | ETSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ | |||
| Mortini et al.67 | 2005 | 1 | MTSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| Nakao et al.68 | 2011 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Nie al.69 | 2015 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Ogawa et al.71 | 2015 | 1 | MTSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| O’Malley et al.70 | 2008 | 2 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | |||
| Pinar et al.74 | 2015 | 1 | ETSS | ✔ | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Qureshi et al.75 | 2016 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Sheehan et al.78 | 1999 | 2 | ETSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ | |||
| Song et al.80 | 2014 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ |
| Thomas et al.82 | 2014 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Tosaka et al.83 | 2015 | 2 | ETSS | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Wang et al.84 | 2015 | 1 | ETSS | ✔ | ✔ | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Wongsirisuwan et al.86 | 2014 | 2 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Yan et al.87 | 2015 | 1 | MTSS | ✔ | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✔ |
| Yildirim et al.88 | 2016 | 1 | ETSS | ✔ | ✔ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| Zaidi et al.89 | 2016 | 2 | ETSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| MTSS | ✖ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ | |||
| Zhan et al.90 | 2015 | 1 | ETSS | ✔ | ✖ | ✖ | ✔ | ✖ | ✖ | ✖ | ✖ |
| Zhang X et al.91 | 2008 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ | ✖ |
| Zhou et al.92 | 2014 | 1 | ETSS | ✔ | ✖ | ✖ | ✖ | ✔ | ✔ | ✖ | ✖ |
ETSS, Endoscopic Transsphenoidal surgery; MTSS, Microscopic Transsphenoidal Surgery; PA, Pituitary Adenomas; GTR, Gross Tumor Resection; NA, No Available; FPA, Functional Pituitary Adenoma; NFPA, Non- Functional Pituitary Adenoma ACTH, Cushing Disease; PRL, Prolactinoma; GH, Growth Hormone Hypersecretion
Appendix 4: Funnel plot for publication bias for overall pituitary adenoma
Appendix 5: Funnel plot for publication bias for functional pituitary adenoma
Appendix 6: Funnel plot for publication bias for non-functional pituitary adenoma
Footnotes
Presentation at conference: Contents of this manuscript were presented at the North American Skull Base Society meeting, New Orleans, March 3–5, 2017
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84:843–849. doi: 10.1136/jnnp-2012-303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asemota AO, Ishii M, Brem H, Gallia GL. Comparison of Complications, Trends, and Costs in Endoscopic vs Microscopic Pituitary Surgery: Analysis From a US Health Claims Database. Neurosurgery. 2017;81:458–472. doi: 10.1093/neuros/nyx350. [DOI] [PubMed] [Google Scholar]
- 3.Barker FG, 2nd, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996–2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 2003;88:4709–4719. doi: 10.1210/jc.2003-030461. [DOI] [PubMed] [Google Scholar]
- 4.Bodhinayake I, Ottenhausen M, Mooney MA, Kesavabhotla K, Christos P, Schwarz JT, Boockvar JA. Results and risk factors for recurrence following endoscopic endonasal transsphenoidal surgery for pituitary adenoma. Clinical neurology and neurosurgery. 2014;119:75–79. doi: 10.1016/j.clineuro.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Bokhari AR, Davies MA, Diamond T. Endoscopic transsphenoidal pituitary surgery: a single surgeon experience and the learning curve. British journal of neurosurgery. 2013;27:44–49. doi: 10.3109/02688697.2012.709554. [DOI] [PubMed] [Google Scholar]
- 6.Campbell PG, Kenning E, Andrews DW, Yadla S, Rosen M, Evans JJ. Outcomes after a purely endoscopic transsphenoidal resection of growth hormone-secreting pituitary adenomas. Neurosurgical focus. 2010;29:E5. doi: 10.3171/2010.7.FOCUS10153. [DOI] [PubMed] [Google Scholar]
- 7.Cappabianca P, Cavallo LM, Colao A, De Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. Journal of neurosurgery. 2002;97:293–298. doi: 10.3171/jns.2002.97.2.0293. [DOI] [PubMed] [Google Scholar]
- 8.Charalampaki P, Ayyad A, Kockro RA, Perneczky A. Surgical complications after endoscopic transsphenoidal pituitary surgery. Journal of Clinical Neuroscience. 2009;16:786–789. doi: 10.1016/j.jocn.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Chi F, Wang Y, Lin Y, Ge J, Qiu Y, Guo L. A learning curve of endoscopic transsphenoidal surgery for pituitary adenoma. The Journal of craniofacial surgery. 2013;24:2064–2067. doi: 10.1097/SCS.0b013e3182a24328. [DOI] [PubMed] [Google Scholar]
- 10.Choe JH, Lee KS, Jeun SS, Cho JH, Hong YK. Endocrine outcome of endoscopic endonasal transsphenoidal surgery in functioning pituitary adenomas. J Korean Neurosurg Soc. 2008;44:151–155. doi: 10.3340/jkns.2008.44.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chone CT, Sampaio MH, Sakano E, Paschoal JR, Garnes HM, Queiroz L, Vargas AA, Fernandes YB, Honorato DC, Fabbro MD, Guizoni H, Tedeschi H. Endoscopic endonasal transsphenoidal resection of pituitary adenomas: preliminary evaluation of consecutive cases. Brazilian journal of otorhinolaryngology. 2014;80:146–151. doi: 10.5935/1808-8694.20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–236. doi: 10.1097/00006123-199702000-00001. discussion 236–227. [DOI] [PubMed] [Google Scholar]
- 13.Ciric I, Rosenblatt S, Zhao JC. Transsphenoidal microsurgery. Neurosurgery. 2002;51:161–169. doi: 10.1097/00006123-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Gadol AA, Liu JK, Laws ER., Jr Cushing’s first case of transsphenoidal surgery: the launch of the pituitary surgery era. J Neurosurg. 2005;103:570–574. doi: 10.3171/jns.2005.103.3.0570. [DOI] [PubMed] [Google Scholar]
- 15.Conrad J, Ayyad A, Wuster C, Omran W, Weber MM, Konerding MA, Muller-Forell W, Giese A, Oertel J. Binostril versus mononostril approaches in endoscopic transsphenoidal pituitary surgery: clinical evaluation and cadaver study. J Neurosurg. 2016;125:334–345. doi: 10.3171/2015.6.JNS142637. [DOI] [PubMed] [Google Scholar]
- 16.Constantino ER, Leal R, Ferreira CC, Acioly MA, Landeiro JA. Surgical outcomes of the endoscopic endonasal transsphenoidal approach for large and giant pituitary adenomas: Institutional experience with special attention to approach-related complications. Arquivos de Neuro-Psiquiatria. 2016;74:388–395. doi: 10.1590/0004-282X20160042. [DOI] [PubMed] [Google Scholar]
- 17.Cushing H. The Pituitary Body and its Disorders: Clinical Status Produced by Disorders of the Hypophysis Cerebri. Philadelphia: JB Lippincott; 1912. [Google Scholar]
- 18.Cusimano MD, Kan P, Nassiri F, Anderson J, Goguen J, Vanek I, Smyth HS, Fenton R, Muller PJ, Kovacs K. Outcomes of surgically treated giant pituitary tumours. Can J Neurol Sci. 2012;39:446–457. doi: 10.1017/s0317167100013950. [DOI] [PubMed] [Google Scholar]
- 19.Dallapiazza R, Bond AE, Grober Y, Louis RG, Payne SC, Oldfield EH, Jane JA., Jr Retrospective analysis of a concurrent series of microscopic versus endoscopic transsphenoidal surgeries for Knosp Grades 0–2 nonfunctioning pituitary macroadenomas at a single institution. Journal of neurosurgery. 2014;121:511–517. doi: 10.3171/2014.6.JNS131321. [DOI] [PubMed] [Google Scholar]
- 20.Dallapiazza RF, Grober Y, Starke RM, Laws ER, Jr, Jane JA., Jr Long-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas. Neurosurgery. 2015;76:42–52. doi: 10.1227/NEU.0000000000000563. discussion 52–43. [DOI] [PubMed] [Google Scholar]
- 21.De Paiva Neto MA, Vandergrift A, Fatemi N, Gorgulho AA, Desalles AA, Cohan P, Wang C, Swerdloff R, Kelly DF. Endonasal transsphenoidal surgery and multimodality treatment for giant pituitary adenomas. Clinical Endocrinology. 2010;72:512–519. doi: 10.1111/j.1365-2265.2009.03665.x. [DOI] [PubMed] [Google Scholar]
- 22.De Witte O, Carlot S, Devuyst F, Choufani G, Hassid S. Minimally invasive endoscopic unilateral transsphenoidal surgery for pituitary adenomas. B-ENT. 2011;7:27–32. [PubMed] [Google Scholar]
- 23.Dehdashti AR, Ganna A, Karabatsou K, Gentili F. Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery. 2008;62:1006–1015. doi: 10.1227/01.neu.0000325862.83961.12. discussion 1015–1007. [DOI] [PubMed] [Google Scholar]
- 24.DeKlotz TR, Chia SH, Lu W, Makambi KH, Aulisi E, Deeb Z. Meta-analysis of endoscopic versus sublabial pituitary surgery. Laryngoscope. 2012;122:511–518. doi: 10.1002/lary.22479. [DOI] [PubMed] [Google Scholar]
- 25.Dersimonian R, Laird N. Meta-analysis in clinical trials. Contemporary Clinical Trials. 1985;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Dhandapani S, Singh H, Negm HM, Cohen S, Anand VK, Schwartz TH. Cavernous Sinus Invasion in Pituitary Adenomas: Systematic Review and Pooled Data Meta-Analysis of Radiologic Criteria and Comparison of Endoscopic and Microscopic Surgery. World Neurosurg. 2016;96:36–46. doi: 10.1016/j.wneu.2016.08.088. [DOI] [PubMed] [Google Scholar]
- 27.Duz B, Harman F, Secer HI, Bolu E, Gonul E. Transsphenoidal approaches to the pituitary: a progression in experience in a single centre. Acta Neurochirurgica. 2008;150:1133–1138. doi: 10.1007/s00701-008-0135-y. discussion 1138–1139. [DOI] [PubMed] [Google Scholar]
- 28.Erridge SC, Conkey DS, Stockton D, Strachan MW, Statham PF, Whittle IR, Grant R, Kerr GR, Gregor A. Radiotherapy for pituitary adenomas: long-term efficacy and toxicity. Radiother Oncol. 2009;93:597–601. doi: 10.1016/j.radonc.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Fatemi N, Dusick JR, Mattozo C, McArthur DL, Cohan P, Boscardin J, Wang C, Swerdloff RS, Kelly DF. Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery. 2008;63:709–718. doi: 10.1227/01.NEU.0000325725.77132.90. discussion 718–709. [DOI] [PubMed] [Google Scholar]
- 30.Fathalla H, Cusimano MD, Di Ieva A, Lee J, Alsharif O, Goguen J, Zhang S, Smyth H. Endoscopic versus microscopic approach for surgical treatment of acromegaly. Neurosurgical review. 2015;38:541–548. doi: 10.1007/s10143-015-0613-7. discussion 548–549. [DOI] [PubMed] [Google Scholar]
- 31.Fomekong E, Duprez T, Docquier MA, Ntsambi G, Maiter D, Raftopoulos C. Intraoperative 3T MRI for pituitary macroadenoma resection: Initial experience in 73 consecutive patients. Clinical neurology and neurosurgery. 2014;126:143–149. doi: 10.1016/j.clineuro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Frank G, Pasquini E, Farneti G, Mazzatenta D, Sciarretta V, Grasso V, Fustini MF. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology. 2006;83:240–248. doi: 10.1159/000095534. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Zheng H, Xu S, Zheng Y, Wang Y, Jiang J, Zhong C. Endoscopic Versus Microscopic Approach in Pituitary Surgery. The Journal of craniofacial surgery. 2016;27:e157–e159. doi: 10.1097/SCS.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Zhong C, Wang Y, Xu S, Guo Y, Dai C, Zheng Y, Wang Y, Luo Q, Jiang J. Endoscopic versus microscopic transsphenoidal pituitary adenoma surgery: a meta-analysis. World J Surg Oncol. 2014;12:94. doi: 10.1186/1477-7819-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gondim JA, Almeida JP, Albuquerque LA, Schops M, Gomes E, Ferraz T, Sobreira W, Kretzmann MT. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary. 2011;14:174–183. doi: 10.1007/s11102-010-0280-1. [DOI] [PubMed] [Google Scholar]
- 36.Goudakos JK, Markou KD, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery: a systematic review and meta-analysis. Clin Otolaryngol. 2011;36:212–220. doi: 10.1111/j.1749-4486.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 37.Guo-Dong H, Tao J, Ji-Hu Y, Wen-Jian Z, Xie-Jun Z, Jian G, Zhen L, Tai-Peng J, Jian-Jun D, Yong-Zhong G, Wenlan L, Wei-Ping L. Endoscopic Versus Microscopic Transsphenoidal Surgery for Pituitary Tumors. The Journal of craniofacial surgery. 2016;27:e648–e655. doi: 10.1097/SCS.0000000000003000. [DOI] [PubMed] [Google Scholar]
- 38.Guvenc G, Kizmazoglu C, Pinar E, Imre A, Kaya I, Bezircioglu H, Yuceer N. Outcomes and Complications of Endoscopic Versus Microscopic Transsphenoidal Surgery in Pituitary Adenoma. The Journal of craniofacial surgery. 2016;27:1015–1020. doi: 10.1097/SCS.0000000000002684. [DOI] [PubMed] [Google Scholar]
- 39.Han S, Ding X, Tie X, Liu Y, Xia J, Yan A, Wu A. Endoscopic endonasal trans-sphenoidal approach for pituitary adenomas: Is one nostril enough? Acta Neurochirurgica. 2013;155:1601–1609. doi: 10.1007/s00701-013-1788-8. [DOI] [PubMed] [Google Scholar]
- 40.Hardy J. Surgery of the pituitary gland, using the trans-sphenoidal approach. Comparative study of 2 technical methods. Union Med Canada. 1967:96. (Fr) [PubMed] [Google Scholar]
- 41.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofstetter CP, Shin BJ, Mubita L, Huang C, Anand VK, Boockvar JA, Schwartz TH. Endoscopic endonasal transsphenoidal surgery for functional pituitary adenomas. Neurosurgical focus. 2011;30:E10. doi: 10.3171/2011.1.FOCUS10317. [DOI] [PubMed] [Google Scholar]
- 43.Isolan GR, de Aguiar PH, Laws ER, Strapasson AC, Piltcher O. The implications of microsurgical anatomy for surgical approaches to the sellar region. Pituitary. 2009;12:360–367. doi: 10.1007/s11102-009-0167-1. [DOI] [PubMed] [Google Scholar]
- 44.Jain AK, Gupta AK, Pathak A, Bhansali A, Bapuraj JR. Excision of pituitary adenomas: Randomized comparison of surgical modalities. British journal of neurosurgery. 2007;21:328–331. doi: 10.1080/02688690701395447. [DOI] [PubMed] [Google Scholar]
- 45.Jang JH, Kim KH, Lee YM, Kim JS, Kim YZ. Surgical Results of Pure Endoscopic Endonasal Transsphenoidal Surgery for 331 Pituitary Adenomas: A 15-Year Experience from a Single Institution. World Neurosurgery. 2016;96:545–555. doi: 10.1016/j.wneu.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 46.Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, Wayoff M. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102:198–202. doi: 10.1288/00005537-199202000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Jho HD. Endoscopic transsphenoidal surgery. Journal of neuro-oncology. 2001;54:187–195. doi: 10.1023/a:1012969719503. [DOI] [PubMed] [Google Scholar]
- 48.Juraschka K, Khan OH, Godoy BL, Monsalves E, Kilian A, Krischek B, Ghare A, Vescan A, Gentili F, Zadeh G. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: Institutional experience and predictors of extent of resection - Clinical article. Journal of neurosurgery. 2014;121:75–83. doi: 10.3171/2014.3.JNS131679. [DOI] [PubMed] [Google Scholar]
- 49.Kari E, Oyesiku NM, Dadashev V, Wise SK. Comparison of traditional 2-dimensional endoscopic pituitary surgery with new 3-dimensional endoscopic technology: intraoperative and early postoperative factors. Int Forum Allergy Rhinol. 2012;2:2–8. doi: 10.1002/alr.20036. [DOI] [PubMed] [Google Scholar]
- 50.Karppinen A, Kivipelto L, Vehkavaara S, Ritvonen E, Tikkanen E, Kivisaari R, Hernesniemi J, Setala K, Schalin-Jantti C, Niemela M. Transition From Microscopic to Endoscopic Transsphenoidal Surgery for Nonfunctional Pituitary Adenomas. World Neurosurg. 2015;84:48–57. doi: 10.1016/j.wneu.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 51.Kenan K, Ihsan A, Dilek O, Burak C, Gurkan K, Savas C. The learning curve in endoscopic pituitary surgery and our experience. Neurosurgical review. 2006;29:298–305. doi: 10.1007/s10143-006-0033-9. [DOI] [PubMed] [Google Scholar]
- 52.Koc K, Anik I, Ozdamar D, Cabuk B, Keskin G, Ceylan S. The learning curve in endoscopic pituitary surgery and our experience. Neurosurgical review. 2006;29:298–305. doi: 10.1007/s10143-006-0033-9. discussion 305. [DOI] [PubMed] [Google Scholar]
- 53.Kumar S, Darr A, Hobbs CG, Carlin WV. Endoscopic, endonasal, trans-sphenoidal hypophysectomy: retrospective analysis of 171 procedures. The Journal of laryngology and otology. 2012;126:1033–1040. doi: 10.1017/S0022215112001223. [DOI] [PubMed] [Google Scholar]
- 54.Kuo CH, Yen YS, Wu JC, Chang PY, Chang HK, Tu TH, Huang WC, Cheng H. Primary Endoscopic Transnasal Transsphenoidal Surgery for Giant Pituitary Adenoma. World Neurosurgery. 2016;91:121–128. doi: 10.1016/j.wneu.2016.03.092. [DOI] [PubMed] [Google Scholar]
- 55.Kurosaki M, Ludecke DK, Flitsch J, Saeger W. Surgical treatment of clinically nonsecreting pituitary adenomas in elderly patients. Neurosurgery. 2000;47:843–848. doi: 10.1097/00006123-200010000-00009. discussion 848–849. [DOI] [PubMed] [Google Scholar]
- 56.Lampropoulos KI, Samonis G, Nomikos P. Factors influencing the outcome of microsurgical transsphenoidal surgery for pituitary adenomas: A study on 184 patients. Hormones. 2013;12:254–264. doi: 10.14310/horm.2002.1409. [DOI] [PubMed] [Google Scholar]
- 57.Laws ER, Jr, Barkhoudarian G. The transition from microscopic to endoscopic transsphenoidal surgery: the experience at Brigham and Women’s Hospital. World Neurosurg. 2014;82:S152–154. doi: 10.1016/j.wneu.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 58.Leach P, Abou-Zeid AH, Kearney T, Davis J, Trainer PJ, Gnanalingham KK. Endoscopic transsphenoidal pituitary surgery: evidence of an operative learning curve. Neurosurgery. 2010;67:1205–1212. doi: 10.1227/NEU.0b013e3181ef25c5. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Li C, Xiao Q, Gan C, Chen X, Sun W, Li X, Xu Y, Chen J, Shu K, Lei T. Comparison of pituitary adenomas in elderly and younger adults: Clinical characteristics, surgical outcomes, and prognosis. Journal of the American Geriatrics Society. 2015;63:1924–1930. doi: 10.1111/jgs.13590. [DOI] [PubMed] [Google Scholar]
- 60.Liu JK, Das K, Weiss MH, Laws ER, Jr, Couldwell WT. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95:1083–1096. doi: 10.3171/jns.2001.95.6.1083. [DOI] [PubMed] [Google Scholar]
- 61.Mansouri A, Shin S, Cooper B, Srivastava A, Bhandari M, Kondziolka D. Randomized controlled trials and neuro-oncology: should alternative designs be considered? Journal of neuro-oncology. 2015;124:345–356. doi: 10.1007/s11060-015-1870-6. [DOI] [PubMed] [Google Scholar]
- 62.McCoul ED, Bedrosian JC, Akselrod O, Anand VK, Schwartz TH. Preservation of multidimensional quality of life after endoscopic pituitary adenoma resection. J Neurosurg. 2015;123:813–820. doi: 10.3171/2014.11.JNS14559. [DOI] [PubMed] [Google Scholar]
- 63.McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, Nicholl J, Balliol C, Aronson JK, Barkun JS, Blazeby JM, Boutron IC, Campbell WB, Clavien PA, Cook JA, Ergina PL, Feldman LS, Flum DR, Maddern GJ, Nicholl J, Reeves BC, Seiler CM, Strasberg SM, Meakins JL, Ashby D, Black N, Bunker J, Burton M, Campbell M, Chalkidou K, Chalmers I, de Leval M, Deeks J, Ergina PL, Grant A, Gray M, Greenhalgh R, Jenicek M, Kehoe S, Lilford R, Littlejohns P, Loke Y, Madhock R, McPherson K, Meakins J, Rothwell P, Summerskill B, Taggart D, Tekkis P, Thompson M, Treasure T, Trohler U, Vandenbroucke J. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 64.Messerer M, De Battista JC, Raverot G, Kassis S, Dubourg J, Lapras V, Trouillas J, Perrin G, Jouanneau E. Evidence of improved surgical outcome following endoscopy for nonfunctioning pituitary adenoma removal. Neurosurg Focus. 2011;30:E11. doi: 10.3171/2011.1.FOCUS10308. [DOI] [PubMed] [Google Scholar]
- 65.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Mortini P. Cons: endoscopic endonasal transsphenoidal pituitary surgery is not superior to microscopic transsphenoidal surgery for pituitary adenomas. Endocrine. 2014;47:415–420. doi: 10.1007/s12020-014-0365-0. [DOI] [PubMed] [Google Scholar]
- 67.Mortini P, Losa M, Barzaghi R, Boari N, Giovanelli M. Results of Transsphenoidal Surgery in a Large Series of Patients with Pituitary Adenoma. Neurosurgery. 2005;56:1222–1233. doi: 10.1227/01.neu.0000159647.64275.9d. [DOI] [PubMed] [Google Scholar]
- 68.Nakao N, Itakura T. Surgical outcome of the endoscopic endonasal approach for non-functioning giant pituitary adenoma. J Clin Neurosci. 2011;18:71–75. doi: 10.1016/j.jocn.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 69.Nie S, Li K, Huang Y, Zhao J, Gao X, Sun J. Endoscopic endonasal transsphenoidal surgery for treating pituitary adenoma via a sub-septum mucosa approach. International Journal of Clinical and Experimental Medicine. 2015;8:5137–5143. [PMC free article] [PubMed] [Google Scholar]
- 70.O’Malley BW, Jr, Grady MS, Gabel BC, Cohen MA, Heuer GG, Pisapia J, Bohman LE, Leibowitz JM. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus. 2008;25:E10. doi: 10.3171/FOC.2008.25.12.E10. [DOI] [PubMed] [Google Scholar]
- 71.Ogawa Y, Tominaga T. Sellar and parasellar tumor removal without discontinuing antithrombotic therapy. J Neurosurg. 2015;123:794–798. doi: 10.3171/2014.9.JNS141088. [DOI] [PubMed] [Google Scholar]
- 72.Oh MC, Aghi MK. Dopamine agonist-resistant prolactinomas. J Neurosurg. 2011;114:1369–1379. doi: 10.3171/2010.11.JNS101369. [DOI] [PubMed] [Google Scholar]
- 73.Oosmanally N, Paul JE, Zanation AM, Ewend MG, Senior BA, Ebert CS., Jr Comparative analysis of cost of endoscopic endonasal minimally invasive and sublabial-transseptal approaches to the pituitary. Int Forum Allergy Rhinol. 2011;1:242–249. doi: 10.1002/alr.20048. [DOI] [PubMed] [Google Scholar]
- 74.Pinar E, Yuceer N, Imre A, Guvenc G, Gundogan O. Endoscopic endonasal transsphenoidal surgery for pituitary adenomas. The Journal of craniofacial surgery. 2015;26:201–205. doi: 10.1097/SCS.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 75.Qureshi T, Chaus F, Fogg L, Dasgupta M, Straus D, Byrne RW. Learning curve for the transsphenoidal endoscopic endonasal approach to pituitary tumors. Br J Neurosurg. 2016:1–6. doi: 10.1080/02688697.2016.1199786. [DOI] [PubMed] [Google Scholar]
- 76.Rotenberg B, Tam S, Ryu WH, Duggal N. Microscopic versus endoscopic pituitary surgery: a systematic review. Laryngoscope. 2010;120:1292–1297. doi: 10.1002/lary.20949. [DOI] [PubMed] [Google Scholar]
- 77.Schloffer H. Erfolgreiche Operation eines Hypophysentumors auf nasalem wege. Wien Klin Wochenschr. 1907;20:621–624. [Google Scholar]
- 78.Sheehan MT, Atkinson JL, Kasperbauer JL, Erickson BJ, Nippoldt TB. Preliminary comparison of the endoscopic transnasal vs the sublabial transseptal approach for clinically nonfunctioning pituitary macroadenomas. Mayo Clin Proc. 1999;74:661–670. doi: 10.4065/74.7.661. [DOI] [PubMed] [Google Scholar]
- 79.Singh H, Essayed WI, Cohen-Gadol A, Zada G, Schwartz TH. Resection of pituitary tumors: endoscopic versus microscopic. Journal of neuro-oncology. 2016 doi: 10.1007/s11060-016-2124-y. [DOI] [PubMed] [Google Scholar]
- 80.Song Y, Li H, Liu H, Li W, Zhang X, Guo L, Tan G. Endoscopic endonasal transsphenoidal approach for sellar tumors beyond the sellar turcica. Acta Oto-Laryngologica. 2014;134:326–330. doi: 10.3109/00016489.2013.857785. [DOI] [PubMed] [Google Scholar]
- 81.Strychowsky J, Nayan S, Reddy K, Farrokhyar F, Sommer D. Purely endoscopic transsphenoidal surgery versus traditional microsurgery for resection of pituitary adenomas: systematic review. J Otolaryngol Head Neck Surg. 2011;40:175–185. [PubMed] [Google Scholar]
- 82.Thomas JG, Gadgil N, Samson SL, Takashima M, Yoshor D. Prospective Trial of a Short Hospital Stay Protocol after Endoscopic Endonasal Pituitary Adenoma Surgery. World Neurosurgery. 2014;81:576–583. doi: 10.1016/j.wneu.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 83.Tosaka M, Nagaki T, Honda F, Takahashi K, Yoshimoto Y. Multi-slice computed tomography-assisted endoscopic transsphenoidal surgery for pituitary macroadenoma: A comparison with conventional microscopic transsphenoidal surgery. Neurological research. 2015;37:951–958. doi: 10.1179/1743132815Y.0000000078. [DOI] [PubMed] [Google Scholar]
- 84.Wang F, Zhou T, Wei S, Meng X, Zhang J, Hou Y, Sun G. Endoscopic endonasal transsphenoidal surgery of 1,166 pituitary adenomas. Surgical Endoscopy and Other Interventional Techniques. 2015;29:1270–1280. doi: 10.1007/s00464-014-3815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolf A, Coros A, Bierer J, Goncalves S, Cooper P, Van Uum S, Lee DH, Proulx A, Nicolle D, Fraser JA, Rotenberg BW, Duggal N. Quantitative evaluation of vision-related and health-related quality of life after endoscopic transsphenoidal surgery for pituitary adenoma. J Neurosurg. 2016:1–8. doi: 10.3171/2016.7.JNS16200. [DOI] [PubMed] [Google Scholar]
- 86.Wongsirisuwan M, Karnchanapandh K. Comparative outcomes of keyhole supraorbital approach (KSA) and endonasal endoscopic transsphenoidal approach (EETA) in pituitary surgery. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2014;97:386–392. [PubMed] [Google Scholar]
- 87.Yan Z, Wang Y, Shou X, Su J, Lang L. Effect of transsphenoidal surgery and standard care on fertility related indicators of patients with prolactinomas during child-bearing period. International Journal of Clinical and Experimental Medicine. 2015;8:21557–21564. [PMC free article] [PubMed] [Google Scholar]
- 88.Yildirim AE, Sahinoglu M, Ekici I, Cagil E, Karaoglu D, Celik H, Nacar OA, Belen AD. Nonfunctioning Pituitary Adenomas Are Really Clinically Nonfunctioning? Clinical and Endocrinological Symptoms and Outcomes with Endoscopic Endonasal Treatment. World Neurosurgery. 2016;85:185–192. doi: 10.1016/j.wneu.2015.08.073. [DOI] [PubMed] [Google Scholar]
- 89.Zaidi HA, Awad AW, Bohl MA, Chapple K, Knecht L, Jahnke H, White WL, Little AS. Comparison of outcomes between a less experienced surgeon using a fully endoscopic technique and a very experienced surgeon using a microscopic transsphenoidal technique for pituitary adenoma. Journal of neurosurgery. 2016;124:596–604. doi: 10.3171/2015.4.JNS15102. [DOI] [PubMed] [Google Scholar]
- 90.Zhan R, Ma Z, Wang D, Li X. Pure endoscopic endonasal transsphenoidal approach for nonfunctioning pituitary adenomas in the elderly: Surgical outcomes and complications in 158 patients. World Neurosurgery. 2015;84:1572–1578. doi: 10.1016/j.wneu.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X, Fei Z, Zhang W, Zhang JN, Liu WP, Fu LA, Cao WD, Jiang XF, Song SJ. Endoscopic endonasal transsphenoidal surgery for invasive pituitary adenoma. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2008;15:241–245. doi: 10.1016/j.jocn.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 92.Zhou T, Wang F, Meng X, Ba J, Wei S, Xu B. Outcome of endoscopic transsphenoidal surgery in combination with somatostatin analogues in patients with growth hormone producing pituitary adenoma. Journal of Korean Neurosurgical Society. 2014;56:405–409. doi: 10.3340/jkns.2014.56.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.