Abstract
Objective
Acute kidney injury (AKI) frequently complicates critical illness and is associated with high morbidity and mortality. Frailty is common in critical illness survivors, but little is known about the impact of AKI. We examined the association of AKI and frailty within a year of hospital discharge in survivors of critical illness.
Design
Secondary analysis of a prospective cohort study
Setting
Medical/surgical ICU of a US tertiary care medical center
Patients
317 participants with respiratory failure and/or shock
Interventions
None
Measurements and Main Results
AKI was determined using Kidney Disease Improving Global Outcomes stages. Clinical frailty status was determined using the Clinical Frailty Scale (CFS) at 3 and 12 months following discharge. Covariates included mean ICU sequential organ failure assessment (SOFA) score and acute physiology and chronic health evaluation II (APACHE II) score as well as baseline comorbidity (i.e., Charlson Comorbidity Index), kidney function, and CFS score. Of 317 patients, 243 (77%) had AKI and 1 in 4 patients with AKI were frail at baseline. In adjusted models, AKI stages 1, 2, and 3 were associated with higher frailty scores at 3 months (OR 1.92, 95% CI 1.14, 3.24; OR 2.40, 95% CI 1.31, 4.42; OR 4.41, 95% CI 2.20, 8.82; respectively). At 12 months, a similar association of AKI stages 1, 2, and 3 and higher CFS score was noted (OR 1.87, 95% CI 1.11, 3.14; OR 1.81, 95% CI 0.94, 3.48; OR 2.76, 95% CI 1.34, 5.66; respectively). In supplemental and sensitivity analyses, analogous patterns of association were observed.
Conclusions
AKI in survivors of critical illness predicted worse frailty status 3 and 12 months post-discharge. These findings have important implications on clinical decision making among AKI survivors and underscore the need to understand the drivers of frailty to improve patient-centered outcomes.
Keywords: frailty, critical illness, acute kidney injury, prospective cohort, clinical frailty scale, chronic kidney disease, mortality
Introduction
Acute kidney injury (AKI) affects up to half of patients admitted to the ICU (1–4). The incidence of AKI is increasing rapidly, and along with it, the population of survivors for whom optimal care has not been defined (1–4). Most post-AKI research focuses on its long-term impact on organ function. However, less attention is paid to outcomes such as quality of life and functional status (5).
Frailty is a multidimensional condition denoted by a “loss of physiologic reserve and the ability to resist stressors” (6, 7). While associated with aging, frailty is observed in younger patients with acute and chronic diseases and predicts subsequent hospitalizations, functional decline, and death (6–17). This is particularly salient to AKI survivors who experience these outcomes at markedly elevated rates; however, the mechanisms driving this relationship remain unclear.
Although AKI often occurs in patients with multiple comorbidities that may contribute to frailty, AKI may directly promote frailty even after recovery from the acute illness. We hypothesized that among critically ill patients who survived to hospital discharge, AKI would be associated with higher frailty scores at 3 and 12 months. To test this this hypothesis, we used data from the prospective BRAIN-ICU cohort of critically ill patients who were assessed at baseline, 3 months, and 12 months using the Clinical Frailty Scale (CFS) (9, 18, 19).
Materials and Methods
Study setting and design
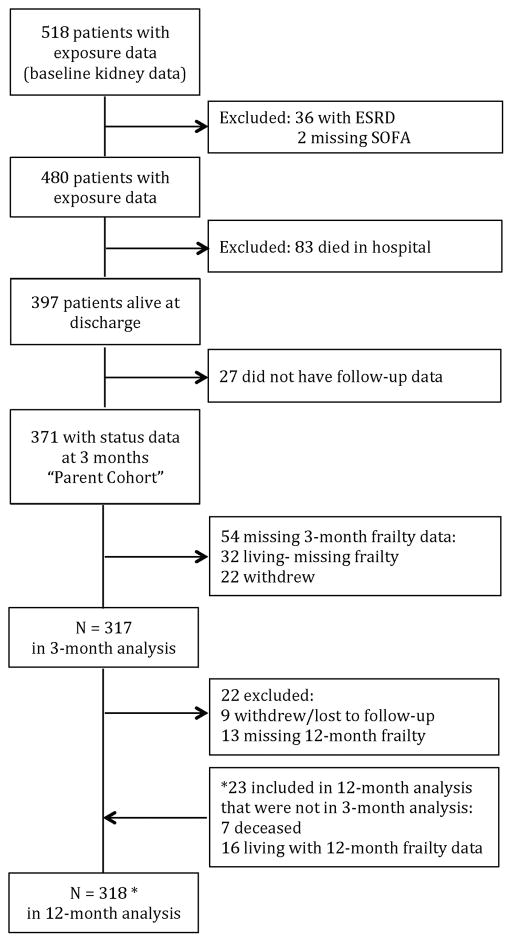
The BRAIN-ICU Study is a prospective cohort of critically ill adults (age ≥ 18 years) with acute respiratory failure and/or shock (septic or cardiogenic) enrolled between 2007 and 2010 at 5 medical centers (9, 18, 20). Follow-up occurred at 3- and 12-months post-discharge. We have previously described BRAIN-ICU enrollment in detail (9, 18, 20). Briefly, patients with recent ICU exposure (i.e., mechanical ventilation within 2 months, 5 ICU days within the prior month), an expected survival of less than 24 hours, inability to be reliably assessed for delirium (i.e., blindness, deafness, or non-English speaking), and a high likelihood of pre-existing cognitive deficits (e.g., neurodegenerative disease, anoxic brain injury, severe dementia) were excluded from the parent study. For this secondary analysis, we restricted the cohort to participants who were enrolled at Vanderbilt University Medical Center since daily serum creatinines were available for this group. Additional exclusion criteria unique to these analyses were (see Figure 1) end-stage renal disease (ESRD, i.e., baseline eGFR < 15 ml/min/1.73m2, history of kidney transplant, or receiving maintenance dialysis; n=36), missing sequential organ failure assessment (SOFA) scores (n=2), death prior to hospital discharge (n=83), or alive but absent from both the 3-month and 12-month follow-up visits (n=27). Written informed consent was obtained from all participants and/or their surrogates. The study was approved by the Vanderbilt University Medical Center IRB.
Figure 1.
Study Enrollment and Exclusion. Analysis set: those with follow-up (frailty) data
Baseline kidney function and acute kidney injury
We estimated baseline kidney function by using the mean of all outpatient serum creatinine values from 7 to 365 days prior to the index admission (21). If the mean outpatient serum creatinine was not available, we used the lowest serum creatinine during the hospitalization as the baseline creatinine (22). To improve accuracy, we performed clinical adjudication of baseline serum creatinine in 2 settings. First, if the lowest serum creatinine during the hospitalization was ≥ 0.5mg/dl below the mean of all outpatient serum creatinines from 7 to 365 days prior to index admission. Second, for patients with a baseline serum creatinine that resulted in an eGFR < 60 ml/min/1.73m2. In these settings, 2 nephrologists (KA, ES) blinded to outcomes reviewed the electronic health record (EHR), including scanned documents, to adjudicate baseline serum creatinine by consensus. Differences in baseline creatinine values between the nephrologists were nearly always on the order of 0.1mg/dl. Notably, 21 patients’ baseline creatinine values were adjusted by clinical adjudication. We used baseline creatinine values to calculate the baseline eGFR from the CKD-EPI equation (23).
We determined AKI status (i.e., exposure of interest) using serum creatinine values collected during routine clinical care. In the vast majority of cases, patients’ serum creatinine values were available daily. We staged AKI according to Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria using the difference between baseline and peak serum creatinine (i.e., peak injury) (24). Specifically, stage 1 injury required a 50% increase from baseline creatinine or an absolute increase in baseline creatinine of ≥ 0.3mg/dl, stage 2 injury required a ≥ 100% increase from baseline serum creatinine, and stage 3 injury required a ≥ 200% increase from baseline serum creatinine or initiation of dialysis.
In addition, to examine the relationship between unresolved AKI at hospital discharge and frailty, we a priori decided to calculate KDIGO stages at hospital discharge using the serum creatinine in closest proximity to discharge (i.e., injury at discharge). To examine the potential effect of dialysis on the association between AKI and frailty, we also included receipt of dialysis at time of discharge as a separate covariate in a supplemental analysis. Further, to examine the impact of timing of AKI onset, we adjusted for peak injury occurring within 48 hours (i.e., peak creatinine within 48 hours of admission) versus beyond 48 hours in a supplemental analysis.
Covariates
In addition to baseline kidney function, we also chose to adjust for the following potential confounders, selected a priori based on biologic plausibility and prior research (9, 17, 22, 25, 26): age, gender, race, modified Charlson comorbidity index (CCI) (27), mean modified sequential organ failure assessment (SOFA) score (28), acute physiology and chronic health evaluation II (APACHE II) score (29), and baseline CFS score (described below). We modified the CCI and SOFA to remove the renal components of these indices since they were included separately in our analyses. In a supplemental analysis, we also controlled for length of study time mechanically ventilated to further adjust for potential differences in severity of illness. In the 11% of patients who were not mechanically ventilated (evenly distributed between exposure arms), the time mechanically ventilated was set to 0. We did not include length of hospital stay as a covariate because AKI has been consistently associated with longer hospital admissions, often related to monitoring of kidney function (22). All covariates were assessed at enrollment or during the enrollment hospitalization (e.g., SOFA scores) only and were not updated following discharge.
Clinical frailty assessment
Upon enrollment (i.e., within 72 hours of ICU admission), study team members trained by a geriatrician with frailty expertise, interviewed the patient and/or surrogate and reviewed the EHR to determine a pre-hospitalization CFS score (i.e., baseline CFS score). The CFS is a validated measure of clinical frailty with scores that range from 1 (very fit) to 7 (severely frail/terminally ill) (19, 30). Scores ≥ 5 indicate the presence of clinical frailty. Because every point increase in CFS score has been associated with poorer outcomes (19, 30), we analyzed the CFS as an ordinal measure (31–34).
At 3- and 12-month follow-up, trained study team members, who were blinded to the events of the ICU course, conducted in-person CFS assessments. Because CFS scores at ICU admission were associated with mortality (9), we a priori chose to categorize death as a CFS score of 8 to reduce the effect of survivor bias in our primary analyses. In sensitivity analyses (described below), we used the 1 to 7 CFS scoring system and excluded patients who died before the respective follow-up time point.
Statistical analysis
Since CFS is an ordinal response variable (31–34), we used ordinal logistic regression to model the dependency of CFS on AKI status while adjusting for potential clinical and demographic confounders. We fit separate models for different markers of AKI, e.g., peak AKI stage and discharge AKI stage. Sociodemographic covariates of interest were age, gender, and race. Clinical covariates were baseline (i.e., pre-illness) CFS score, modified CCI score, modified mean daily SOFA score, APACHE II score, baseline creatinine, and days mechanically ventilated. We included age as a non-linear term in the models using restricted cubic splines with 3 knots. We modeled baseline creatinine both as a linear term as well as a non-linear term with restricted cubic splines. The non-linear baseline creatinine term did not change or improve representation of the data, so all of our final models relied on a linear baseline creatinine term. We also tested for an interaction between AKI status and baseline creatinine using both linear and non-linear terms for baseline creatinine. The interaction terms were uniformly non-significant and were dropped from the model for parsimony. We report the odds ratios (OR) and 95% confidence intervals (95% CI) obtained using robust standard errors from the models.
In sensitivity analyses, we repeated our main analyses while: 1) restricting the cohort to patients with a known outpatient baseline creatinine (n=177) 2) restricting the cohort to patients with a baseline CFS score ≤ 5 (i.e., mildly frail or better), and 3) restricting the cohort to survivors at 3 or 12 months, respectively (in order to understand better whether the association between AKI and frailty was driven primarily by death). Due to the smaller sample size in the analysis restricted to survivors, we combined AKI stages 2 and 3. All analyses use 95% confidence intervals. Analyses were performed using R version 3.3.0 (35).
Results
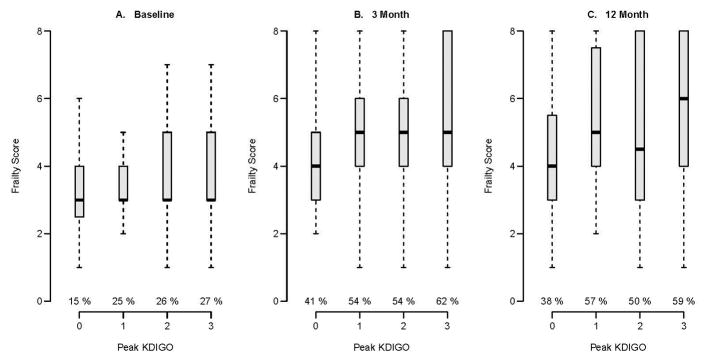
For our primary analyses, 371 patients met inclusion criteria (Parent Cohort, Figure 1). Of these, 317 and 318 were in our analytic cohorts at 3 and 12 months, respectively (Figure 1). In the analytic cohort, at baseline, participants had a median age of 57 years, 90% were white, median non-renal SOFA score at enrollment was 8, and median APACHEII score was 25. Participants had a median non-renal Charlson score of 2 and median baseline creatinine of 0.9 (Table 1). At baseline, 75 of 317 (24%) participants were clinically frail (Table 1). AKI occurred in 243 (77%) patients and was still present at discharge in 87 (27%) patients (Table 1). Approximately, 27% (65 of 243) versus 13% (10 of 74) of AKI and non-AKI patients were considered frail at baseline (i.e., CFS ≥ 5), respectively (Table 1 and Figure 2A). At 3- and 12-month follow-up, unadjusted CFS scores increased in all groups compared with baseline, and about 50% to 62% of AKI patients were considered clinically frail (Figure 2B and 2C, Supplemental Figure 1).
Table 1.
Baseline characteristics
| Characteristic | Non-AKI (N = 74) | AKI (N=243) |
|---|---|---|
| Age (years) | 56 (43, 66) | 57 (47, 67) |
| Female | 37 (50) | 113 (47) |
| Race | ||
| White | 68 (92) | 217 (89) |
| African-American | 6 (8) | 26 (11) |
| Education (years) | 12.0 (12.0, 14.0) | 12.0 (12.0, 14.0) |
| BMI | 27 (24, 32) | 30 (25, 36) |
| Mean SOFA* | 6.1 (5.0, 7.3) | 7.0 (5.7, 8.5) |
| APACHE II at ICU admission | 22 (17, 26) | 26 (19, 31) |
| ICU LOS (days) | 2.0 (1.2, 3.9) | 5.9 (2.8, 11.9) |
| LOS (days) | 7 (5, 12) | 13 (9, 22) |
| Mechanically ventilated | 65 (88) | 219 (90) |
| Days mechanically ventilated | 0.9 (0.2, 2.0) | 2.9 (0.9, 7.9) |
| CCI* | 2 (0, 3) | 2 (1, 3) |
| Baseline serum creatinine (mg/dl) | 0.90 (0.71, 1.12) | 0.95 (0.74, 1.25) |
| Baseline eGFR (ml/min/1.73m2) | 88 (64, 105) | 79 (55, 101) |
| AKI KDIGO stage (peak) | ||
| 0 | 74 (100) | 0 (0) |
| 1 | NA | 104 (43) |
| 2 | NA | 70 (29) |
| 3 | NA | 69 (28) |
| AKI KDIGO stage (discharge) | ||
| 0 | 74 (100) | 156 (64) |
| 1 | NA | 50 (21) |
| 2 | NA | 7 (3) |
| 3 | NA | 30 (12) |
| Baseline CFS score | ||
| 1: Very fit | 3 (4) | 13 (5) |
| 2: Well | 15 (20) | 37 (15) |
| 3: Well, with comorbidities | 33 (45) | 82 (34) |
| 4: Apparently vulnerable | 13 (18) | 46 (19) |
| 5: Mildly frail | 7 (9) | 29 (12) |
| 6: Moderately frail | 3 (4) | 29 (12) |
| 7: Severely frail | 0 (0) | 7 (3) |
Continuous variables are presented as medians (and interquartile ranges). Categorical variables are expressed as frequencies (and percentages).
SOFA and CCI were modified to remove their renal components
BMI body mass index, SOFA sequential organ failure assessment, APACHE II acute physiology and chronic health evaluation II, ICU intensive care unit, LOS length of stay, CCI Charlson Comorbidity Index, eGFR estimated glomerular filtration rate, AKI acute kidney injury, KDIGO Kidney Disease Improving Global Outcomes, CFS clinical frailty scale
Figure 2.
Clinical Frailty Scale scores by acute kidney injury status using stage based on peak injury
In this BRAIN-ICU cohort, only 1 in 6 patients without AKI and 1 in 4 with AKI were frail at baseline. At both 3- and 12-month evaluations, over 1 in 2 AKI patients were frail.
For each KDIGO stage, the percentage of Clinical Frailty Scale scores ≥ 5 (i.e., frail) is given below the boxplots
Association between Peak AKI and Frailty
In fully adjusted models, relative to no AKI, peak AKI stage 1, 2, and 3 were associated with higher CFS scores at 3 months (OR 1.92, 95% CI 1.14, 3.24; OR 2.40, 95% CI 1.31, 4.42; OR 4.41, 95% CI 2.20, 8.82) respectively; Table 2). Similarly, peak AKI was generally associated with higher CFS scores at 12 months (AKI stage 1: OR 1.87, 95% CI 1.11, 3.14; AKI stage 2: OR 1.81, 95% CI 0.94, 3.48; AKI stage 3: OR 2.76, 95% CI 1.34, 5.66; Table 2). The fully adjusted probabilities of having a CFS score that increased from 4 (apparently vulnerable) or less to 5 or greater (frail) based on peak AKI stage are shown in Figure 3. There was no significant interaction between AKI stage and baseline creatinine in any of the peak AKI and frailty analyses.
Table 2.
Adjusted Association of Peak Acute Kidney Injury Stage and Clinical Frailty Scale Scores at 3- and 12-months
| Variable | 3-month CFS Model | 12-month CFS model |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| AKI stage 1 | 1.92 (1.14, 3.24) | 1.87 (1.11, 3.14) |
| AKI stage 2 | 2.40 (1.31, 4.42) | 1.81 (0.94, 3.48) |
| AKI stage 3 | 4.41 (2.20, 8.82) | 2.76 (1.34, 5.66) |
| Baseline creatinine* | 0.83 (0.52, 1.34) | 1.07 (0.63, 1.80) |
| Age* | -- | -- |
| Female | 0.89 (0.59, 1.32) | 1.07 (0.72, 1.61) |
| Black | 1.34 (0.68, 2.65) | 0.98 (0.45, 2.12) |
| Charlson Score† | 1.12 (0.99, 1.26) | 1.11 (0.98, 1.26) |
| SOFA Score† | 0.90 (0.82, 1.00) | 0.89 (0.80, 1.00) |
| APACHE | 0.99 (0.96, 1.02) | 1.01 (0.98, 1.03) |
| Baseline CFS=2 | 2.95 (0.66, 13.23) | 1.55 (0.53, 4.54) |
| Baseline CFS=3 | 4.11 (0.90, 18.84) | 1.84 (0.62, 5.46) |
| Baseline CFS=4 | 6.70 (1.32, 34.10) | 3.06 (0.94, 10.00) |
| Baseline CFS=5 | 7.56 (1.43, 40.01) | 4.75 (1.49, 15.17) |
| Baseline CFS=6 | 19.17 (3.67, 100.24) | 4.67 (1.41, 15.48) |
| Baseline CFS=7 | 22.48 (2.39, 211.11) | 8.89 (0.71, 111.17) |
Baseline creatinine was centered. Age was modeled nonlinearly using restricted cubic splines with 3 knots; individual ORs are not generalizable.
The Charlson Comorbidity Index and SOFA score were modified to remove the chronic kidney disease and acute kidney injury terms, respectively.
CFS Clinical Frailty Scale, AKI acute kidney injury, SOFA Sequential Organ Failure Assessment, APACHE Acute Physiology and Chronic Health Evaluation
Figure 3.
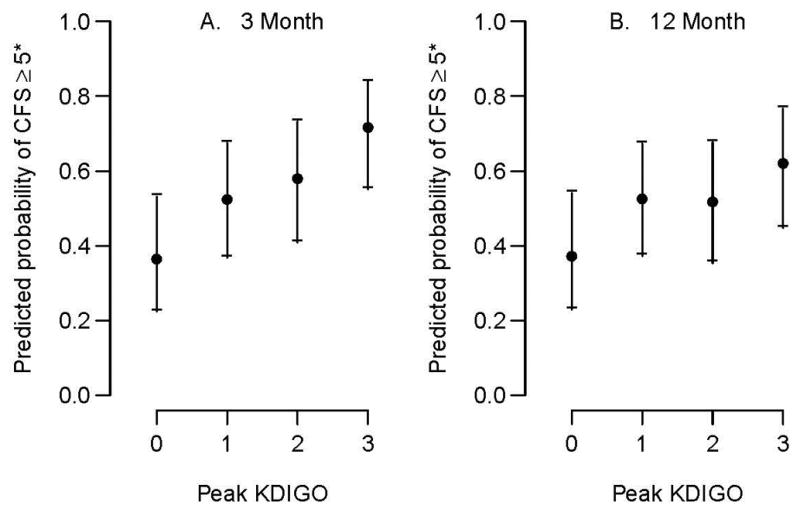
Estimated probability of an increase in Clinical Frailty Scale score from 4 (apparently vulnerable) or less to 5 or greater (frail) at 3 and 12 months by acute kidney injury status based on peak creatinine.
*Specifically, the presented probabilities are for white males, age 57 years, baseline creatinine = 1, Apache = 24, non-renal Charlson = 2, average non-renal SOFA = 7, and baseline Clinical Frailty Scale score = 4 (apparently vulnerable)
Supplemental Analyses
In fully adjusted models, AKI stage 2 and 3 at discharge were associated with higher CFS scores at 3 months (Supplemental Table 1). At 12 months, AKI stage 3 at discharge remained associated with higher CFS scores, while AKI stage 1 and 2 at discharge trended towards an association (Supplemental Table 1).
When adjusting for dialysis status at discharge, the findings were similar; AKI was associated with higher CFS scores at 3 and 12 months (Supplemental Table 2). In addition, when adjusting for timing of AKI onset as a covariate (peak creatinine ≤ 48 hours vs. > 48 hours), the findings were similar; AKI was associated with higher CFS scores at 3 and 12 months (Supplemental Table 3). Notably, timing of AKI (>48 hours) was also associated with frailty status; however, controlling for this did not abrogate the association between AKI and frailty, which remained robust (Supplemental Table 3). When adjusting for length of study time mechanically ventilated, the results were similar with AKI stage 1 and 3 associated with higher CFS scores at 3 and 12 months (Supplemental Table 4).
Sensitivity Analyses
In the sensitivity analysis restricted to patients with a known outpatient baseline creatinine (Supplemental Table 5), the findings were modestly attenuated and the confidence intervals widened. AKI stage 1 was no longer significantly associated with frailty scores at 3 or 12 months (OR 1.71, 95% CI 0.86, 3.38; OR 1.65, 95% CI 0.85, 3.22, respectively) although the direction of the association and point estimate of the effect remained similar. AKI stage 2 was not associated with higher CFS scores (3 months: OR 2.02, 95% CI 0.84, 4.88; 12 month: OR 1.16, 95% CI 0.44, 3.10); however, AKI stage 3 was associated with higher CFS scores (3 months: OR 5.77, 95% CI 2.33, 14.30; 12 months: OR 3.54, 95% CI 1.43, 8.75).
In analyses restricted to patients with baseline CFS score ≤ 5 (i.e., mildly frail), the main results were unchanged; AKI stages were associated with higher CFS scores at 3 and 12 months (Supplemental Table 6). In the sensitivity analysis restricted to survivors at 3 or 12 months, respectively, AKI stage 1 was not associated with CFS score at 3 months but bordered on an association with higher CFS scores at 12 months (3 months: OR 1.59, 95% CI 0.88, 2.88; 12 months: OR 1.85, 95% CI 0.97, 3.53; respectively). Conversely, AKI stage 2+ was associated with higher CFS scores at 3 months only (3 month: OR 2.11, 95% CI 1.14, 3.91; 12 month: OR 1.13, 95% CI 0.58, 2.19; Supplemental Table 7).
Discussion
In this study, we demonstrated that more than half of the survivors of critical illness who experienced AKI were frail 3 and 12 months after hospital discharge. Further, AKI was associated with worse clinical frailty scores in survivors after adjusting for illness severity. This relationship was robust for severe AKI (KDIGO stages 3), and appeared stronger if injury persisted to hospital discharge.
Our data suggest that AKI may play a role in the link between kidney disease and frailty. While our analyses cannot establish causality, potential renal-specific contributions to frailty include the well-recognized complications of volume overload (36), which may limit mobility and physical exertion; anemia (37), which can contribute to fatigue and decreased physical activity; and potential cognitive deficits(20, 38) and medication toxicities (39). Moreover, AKI has been consistently associated with longer hospital stays(22), which may worsen muscle deconditioning (40). AKI is also associated with subclinical pathophysiologic changes including inflammation, immune compromise, and metabolic derangements (36, 41, 42). These effects may be especially evident in patients with more severe AKI or in those who do not recover from their AKI. Future studies are needed to clarify whether the intersection of AKI and frailty results from the kidneys acting as a sensitive and easily assessed barometer of clinical status and/or the deleterious effects of AKI on health.
The American Society of Nephrology has identified the transition of care following AKI as an opportunity to improve long-term outcomes of the disease.(43) This has become especially important in light of reports highlighting a rapid growth in the incidence of AKI.(44) To our knowledge, this is the first study to examine the effect of AKI on frailty. For critical care providers, our findings highlight that critical illness survivors with AKI, especially those with severe or persistent injury, are at higher risk for clinical frailty for a prolonged duration. Interventions such as physical rehabilitation (including home-based therapy), nutritional evaluation and supplementation (and home-based meal assistance if necessary), and psychosocial support (including psychiatric evaluation and efforts to increase social engagement) may improve or mitigate the development of frailty.(45) Hence, close attention to these factors is necessary, especially during transitions of care. Further, our findings may inform patient and family discussions regarding the increased likelihood for a prolonged rehabilitation and for experiencing important patient-centered manifestations of clinical frailty, such as fatigue, slowing, and decrements in independence.
In patients with CKD, frailty correlates with quality of life and is an important prognostic marker (10, 11, 46–49). In addition, frail incident ESRD patients are more than three times as likely to die within 1 year of initiating dialysis. Hence, frailty is an important consideration that informs dialysis-decision-making in this population.(46, 48–50) Because patients who survive severe AKI are at the highest risk for developing advanced stages of CKD and ESRD, the high prevalence of frailty in this population suggests that routine assessments be considered.(51) Future studies will need to determine the impact of frailty on recovery from AKI and downstream outcomes and whether interventions that can decrease frailty can improve clinical and patient-centered outcomes. Because survivors of severe AKI often suffer marked declines in quality of life,(25) additional information to guide patient and clinician decision making is essential.(51)
Although we observed an association between frailty and AKI in survivors of critical illness, this relationship was attenuated in our 12-month analysis that excluded death. Patients with the most severe AKI die at markedly elevated rates, which may limit longitudinal assessments in some patients leaving relatively healthier survivors (Supplemental Table 8). We attempted to address these issues by including death as the most severe frailty score, under the assumption that decedents had worsened frailty status shortly prior to death. However, some decedents may have died from sudden events that were not preceded by frailty. Reassuringly, 85% of patients with AKI 2+ who died between 3 and 12 months, were clinically frail at their 3 month assessment (data not shown). Moreover, our sensitivity analyses continued to support an association between AKI 2+ and frailty at 3-month follow-up after excluding patients who died.
Our study has several notable strengths: we systematically measured CFS at baseline and longitudinally in a critically ill cohort, follow-up rates were high given the cohort disease burden, and creatinine data were available to provide granular phenotyping of AKI. Our study also has several important limitations. First, this was a single-center study that requires confirmation, and our findings may not be generalizable to the extremely healthy or the severely chronically ill, as few patients had baseline CFS scores in this range. Second, the observational design of this study is unable to determine whether the association between AKI and frailty was causative; we did not have data to explore postulated mediators of the AKI—frailty association further and residual confounding may be present. Third, while the CFS is pragmatic, validated, and well-suited for use in critically ill patients, it is subjective and may not optimally differentiate patients across the spectrum of frailty. However, referent frailty instruments often include performance based measures that are quite difficult to obtain in patients with acute critical illness and provider-based clinical frailty assessments appear to perform comparably to more objective measures.(52, 53) Fourth, we did not have an outpatient assessment of baseline kidney function in all patients. However, our sensitivity analyses continued to demonstrate an association between severe AKI and frailty score. Fifth, the relatively few patients in the non-AKI exposure arm, which is expected in an ICU study requiring acute respiratory failure or shock, is a potential challenge for generalizing the results of non-AKI subjects. Sixth, we did not adjudicate patients’ AKI etiology. However, because the cohort consisted of ICU patients with a high severity of illness and shock, we anticipate that the predominant etiology would be ischemic acute tubular necrosis, consistent with prior ICU studies.(54) Finally, we did not have systematic, longitudinal follow-up of renal function to allow us to examine how long-term kidney function trajectories (e.g., ESRD) relate to frailty.
Conclusions
In conclusion, the majority of survivors of critical illness complicated by AKI are clinically frail 3 and 12 months after discharge, and AKI is independently associated with frailty. Further research is needed to understand potential mechanistic links and the effect of frailty on outcomes in patients who develop AKI during critical illness. Because frailty is associated with functional decline in the critically ill, interventions to maintain independent function among patients who survive AKI are needed. As the US population ages and the incidence of AKI and AKI risk factors increase, such information will be integral to engaging patients and families in informed discussions of treatment options and anticipated outcomes.
Supplementary Material
Acknowledgments
Source of Funding
This work was supported by National Institutes of Health grants K23DK090304 (KA), the Vanderbilt Center for Kidney Disease (KA, EDS), the ASSESS-AKI Study 5U01 DK082192-08 (EDS), and the Veterans Affairs Health Services Research and Development IIR 13-073 (EDS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Reprints will not be ordered/requested
Conflicts of Interest
The authors have no conflicts to declare.
Copyright form disclosure: Drs. Abdel-Kader, Girard, Brummel, Blume, Ely, Ikizler, and Pandharipande received support for article research from the National Institutes of Health (NIH). Dr. Girard’s institution received funding from the NIH. Dr. Ely’s institution received funding from the NIH and from VA funding, and he received funding from Orion, Abbott, and Pfizer. Dr. Bell’s institution received funding from a NIH-National Institute of Aging K23 career development award. Dr. Archer’s institution received funding from PCORI and the Department of Defense; she received funding from Pacira Pharmaceuticals; and she received other support from NIDILRR, American Physical Therapy Association, and Brown University. Dr. Ikizler’s institution received funding from NIDDK. Dr. Siew received other support from Vanderbilt Center for Kidney Disease (VCKD), and he disclosed work for hire.
References
- 1.Cerda J, Lameire N, Eggers P, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):881–886. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- 2.Pisoni R, Wille KM, Tolwani AJ. The epidemiology of severe acute kidney injury: from BEST to PICARD, in acute kidney injury: new concepts. Nephron Clin Pract. 2008;109(4):c188–191. doi: 10.1159/000142927. [DOI] [PubMed] [Google Scholar]
- 3.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 4.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nature reviews Nephrology. 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 5.Villeneuve PM, Clark EG, Sikora L, et al. Health-related quality-of-life among survivors of acute kidney injury in the intensive care unit: a systematic review. Intensive care medicine. 2016;42(2):137–146. doi: 10.1007/s00134-015-4151-0. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K, Fox RA, Stolee P, et al. Frailty in elderly people: an evolving concept. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 1994;150(4):489–495. [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 9.Brummel NE, Bell SP, Girard TD, et al. Frailty and Subsequent Disability and Mortality Among Patients With Critical Illness. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelm-Leen ER, Hall YN, MKT, et al. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122(7):664–671. e662. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afilalo J. Frailty in Patients with Cardiovascular Disease: Why, When, and How to Measure. Current cardiovascular risk reports. 2011;5(5):467–472. doi: 10.1007/s12170-011-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flint K. Which came first, the frailty or the heart disease?: exploring the vicious cycle. J Am Coll Cardiol. 2015;65(10):984–986. doi: 10.1016/j.jacc.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Young P, Shah J, Zhang C, et al. Frailty in Postmenopausal African American and Hispanic HIV-Infected Women. The Journal of frailty & aging. 2016;5(4):242–246. doi: 10.14283/jfa.2016.104. [DOI] [PubMed] [Google Scholar]
- 15.Jha SR, Hannu MK, Chang S, et al. The Prevalence and Prognostic Significance of Frailty in Patients With Advanced Heart Failure Referred for Heart Transplantation. Transplantation. 2016;100(2):429–436. doi: 10.1097/TP.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 16.Bouillon K, Kivimaki M, Hamer M, et al. Diabetes risk factors, diabetes risk algorithms, and the prediction of future frailty: the Whitehall II prospective cohort study. Journal of the American Medical Directors Association. 2013;14(11):851e851–856. doi: 10.1016/j.jamda.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Critical care medicine. 2015;43(5):973–982. doi: 10.1097/CCM.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 18.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfaadhel TA, Soroka SD, Kiberd BA, et al. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol. 2015;10(5):832–840. doi: 10.2215/CJN.07760814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siew ED, Fissell WH, Tripp CM, et al. Acute Kidney Injury as a Risk Factor for Delirium and Coma during Critical Illness. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201603-0476OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7(5):712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87(1):62–73. doi: 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 25.Johansen KL, Smith MW, Unruh ML, et al. Predictors of health utility among 60-day survivors of acute kidney injury in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol. 2010;5(8):1366–1372. doi: 10.2215/CJN.02570310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh J, Aggett J, Goodland A, et al. Frailty and comorbidity are independent predictors of outcome in patients referred for pre-dialysis education. Clin Kidney J. 2016;9(2):324–329. doi: 10.1093/ckj/sfv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive care medicine. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 29.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 30.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basic D, Shanley C. Frailty in an older inpatient population: using the clinical frailty scale to predict patient outcomes. Journal of aging and health. 2015;27(4):670–685. doi: 10.1177/0898264314558202. [DOI] [PubMed] [Google Scholar]
- 32.McIsaac DI, Taljaard M, Bryson GL, et al. Comparative assessment of two frailty instruments for risk-stratification in elderly surgical patients: study protocol for a prospective cohort study. BMC Anesthesiol. 2016;16(1):111. doi: 10.1186/s12871-016-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero-Ortuno R, Wallis S, Biram R, et al. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: An observational study. European journal of internal medicine. 2016;35:24–34. doi: 10.1016/j.ejim.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 34.Shimura T, Yamamoto M, Kano S, et al. Impact of the Clinical Frailty Scale on Outcomes After Transcatheter Aortic Valve Replacement. Circulation. 2017;135(21):2013–2024. doi: 10.1161/CIRCULATIONAHA.116.025630. [DOI] [PubMed] [Google Scholar]
- 35.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2013. [Google Scholar]
- 36.Butcher BW, Liu KD. Fluid overload in AKI: epiphenomenon or putative effect on mortality? Current opinion in critical care. 2012;18(6):593–598. doi: 10.1097/MCC.0b013e32835a1c44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi JS, Kim YA, Kang YU, et al. Clinical impact of hospital-acquired anemia in association with acute kidney injury and chronic kidney disease in patients with acute myocardial infarction. PloS one. 2013;8(9):e75583. doi: 10.1371/journal.pone.0075583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Go AS, Parikh CR, Ikizler TA, et al. The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: design and methods. BMC Nephrol. 2010;11:22. doi: 10.1186/1471-2369-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox ZL, McCoy AB, Matheny ME, et al. Adverse drug events during AKI and its recovery. Clin J Am Soc Nephrol. 2013;8(7):1070–1078. doi: 10.2215/CJN.11921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prowle JR, Kolic I, Purdell-Lewis J, et al. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014;9(6):1015–1023. doi: 10.2215/CJN.11141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing research reviews. 2011;10(1):104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Rabb H, Griffin MD, McKay DB, et al. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J Am Soc Nephrol. 2016;27(2):371–379. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein SL, Jaber BL, Faubel S, et al. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol. 2013;8(3):476–483. doi: 10.2215/CJN.12101112. [DOI] [PubMed] [Google Scholar]
- 44.Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37–42. doi: 10.1681/ASN.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cameron ID, Fairhall N, Langron C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. Journal of the American Society of Nephrology. 2007;18(11):2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez Villarreal I, Ortega O, Hinostroza J, et al. Geriatric assessment for therapeutic decision-making regarding renal replacement in elderly patients with advanced chronic kidney disease. Nephron Clin Pract. 2014;128(1–2):73–78. doi: 10.1159/000363624. [DOI] [PubMed] [Google Scholar]
- 48.Kurella Tamura M, Winkelmayer WC. Treated and untreated kidney failure in older adults: what’s the right balance? JAMA. 2012;307(23):2545–2546. doi: 10.1001/jama.2012.6667. [DOI] [PubMed] [Google Scholar]
- 49.Faller B, Beuscart JB, Frimat L, et al. Competing-risk analysis of death and dialysis initiation among elderly (>/=80 years) newly referred to nephrologists: a French prospective study. BMC Nephrol. 2013;14(1):103. doi: 10.1186/1471-2369-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemmelgarn BR, James MT, Manns BJ, et al. Rates of treated and untreated kidney failure in older vs younger adults. JAMA. 2012;307(23):2507–2515. doi: 10.1001/jama.2012.6455. [DOI] [PubMed] [Google Scholar]
- 51.Williams AW. Older Adults with CKD and Acute Kidney Failure: Do We Know Enough for Critical Shared Decision Making? Journal of the American Society of Nephrology. 2013 doi: 10.1681/ASN.2013090981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Loon IN, Goto NA, Boereboom FTJ, et al. Frailty Screening Tools for Elderly Patients Incident to Dialysis. Clin J Am Soc Nephrol. 2017;12(9):1480–1488. doi: 10.2215/CJN.11801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theou O, Brothers TD, Mitnitski A, et al. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61(9):1537–1551. doi: 10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- 54.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.