Abstract
Rationale
Although endotoxin is a recognized cause of environmental lung disease, how its relationship with respiratory outcomes varies with climate is unknown.
Objective
To examine the endotoxin predictors as well as endotoxin association with asthma, wheeze, and sensitization to inhalant allergens in various US climate regions.
Methods
We analyzed data on 6,963 participants in the National Health and Nutrition Examination Survey. Endotoxin measurements of house dust from bedroom floor and bedding were performed at the University of Iowa. Linear and logistic regression analyses were used to identify endotoxin predictors and assess endotoxin association with health outcomes.
Results
The overall median house dust endotoxin was 16.2 EU/mg; it was higher in mixed-dry/hot-dry regions (19.7 EU/mg) and lower in mixed-humid/marine areas (14.8 EU/mg). Endotoxin predictors and endotoxin association with health outcomes significantly differed across climate regions. In subarctic/very cold/cold regions, log10-endotoxin was significantly associated with higher prevalence of wheeze outcomes (OR:1.48, 95% CI:1.19–1.85 for any wheeze, OR:1.48, 95% CI:1.22–1.80 for exercise-induced wheeze, OR:1.50, 95% CI:1.13–1.98 for prescription medication for wheeze, and OR:1.95, 95% CI:1.50–2.54 for doctor/ER visit for wheeze). In hot-humid regions, log10-endotoxin was positively associated with any wheeze (OR:1.66, 95% CI:1.04–2.65) and current asthma (OR:1.56, 95% CI:1.11–2.18), but negatively with sensitization to any inhalant allergens (OR:0.83, 95% CI:0.74–0.92).
Conclusion
Endotoxin predictors and endotoxin association with asthma and wheeze differ across U.S. climate regions. Endotoxin is associated positively with wheeze or asthma in cold and hot-humid regions, but negatively with sensitization to inhalant allergens in hot-humid climates.
Keywords: Endotoxin, Asthma, Wheeze, Climate, House dust
INTRODUCTION
The incidence and severity of lung illnesses are greatly affected by environmental factors such as pathogen-associated molecular patterns (PAMPs) of which, endotoxin has arguably been the most studied (Sigsgaard & Heederik, 2011). Endotoxin is a lipopolysaccharide (LPS) from the outer membrane of Gram-negative bacteria cell wall and is ubiquitously found in our environment (Thorne & Heederik, 1999). It has been described to cause neutrophilic airway inflammation by binding to CD14 associated with TLR4 and MD2 (LY96), triggering the activation of nuclear factor κB and the stimulation of the Th1 arm of the immune system (Doreswamy & Peden, 2011). As a result, endotoxin inhalation causes neutrophilic asthma and wheeze not only in occupational settings, but also in households (Michel, Duchateau, & Sergysels, 1989; Thorne et al., 2005). There are, however, postulates that early-life exposure to low doses of endotoxin and other microbial components may protect against allergy and immunoglobulin E (IgE)-mediated asthma (von Mutius, 2016). The mechanism is not fully understood, but seems to be due to a downregulation of Th2 immune response. Yet, this Th1/Th2 paradigm does not fully explain the protective effect against asthma. Hypo-responsiveness with decreased IFN-γ, tumor necrosis factor-α, IL-10, and IL-12 has been proposed to be another likely possibility (Braun-Fahrlander et al., 2002).
Endotoxin predictors and endotoxin association with respiratory outcomes have been examined previously in the US population using the National Health and Nutrition Examination Survey (NHANES), and results suggested that endotoxin was associated with higher prevalence of wheeze irrespective of sensitization status (Thorne PS et al., 2015). Some studies have reported that climate affects the determinants of indoor pollutants such as benzene, toluene, formaldehyde, acetaldehyde, particulate matter, and nitrogen dioxide, as well as their relationship with respiratory conditions (Héroux et al., 2010; Pönkä, 1991). However, no study to date has examined whether endotoxin predictors or the association of endotoxin with respiratory outcomes differ by climate. Our study is the first to examine climatic variability in a large sample representative of the US population.
MATERIALS AND METHODS
Data source and study design
We used data from the 2005–2006 NHANES by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). It is a continuous cross-sectional survey of the US non-institutionalized civilian population selected using a complex multistage sampling design to derive a representative sample of the US population. Individuals with low-income, adolescents 12–19 years, people aged ≥ 60 years, African-Americans, and Mexican-Americans were oversampled to ensure suitable samples for these subgroups. To protect participant confidentiality, all data analysis using restricted, not publicly available variables (i.e., climate regions) was conducted at the NCHS Atlanta Research Data Center (RDC). For our study, all the 6,963 NHANES child and adult participants who were aged 1 to 150-year-old and had data on house dust endotoxin were included. NHANES protocols were approved by the Institutional Review Boards of the NCHS and CDC and informed consent was obtained from all participants (CDC, 2006).
Climate regions
As required by NCHS, 8 US climate regions were aggregated into four categories: subarctic/very cold/cold, mixed-humid/marine, hot-humid, and mixed-dry/hot-dry to avoid data suppression due to small sample cells. The definition of each of the climate region is provided by the US Department of Energy guide to determining depending on temperature and precipitation. A detailed description of the different climate regions is available at https://www1.eere.energy.gov/buildings/publications/pdfs/building_america/ba_climateguide_7_1.pdf (Baechler et al., 2010).
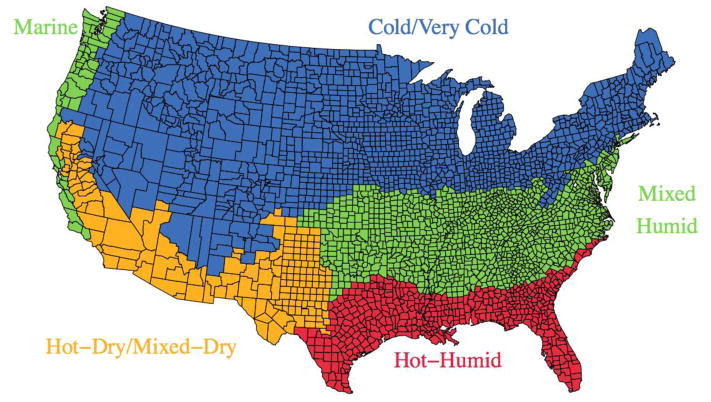
A map of the US with the climate regions by county included in the study is displayed in Figure 1.
Figure 1.
Map of the climate regions of the US based on the classification by the Department of Energy. Note that Alaska and Hawaii were not shown in the map. All of Alaska is classified as subarctic and all of Hawaii is hot-humid.
Endotoxin measurement
Combined bed and bedroom floor dust samples were collected at each participant’s home using a Sanitaire™ Model 3683 vacuum cleaner and a Mitest™ Dust Collector (Indoor Biotechnologies, Inc., Charlottesville, VA). A 1-square yard (0.84 m2) surface on both bed and adjacent floor was independently vacuumed for two minutes. Details on the dust collection methods are available at https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/allergen_manual_06.pdf). Dust samples were analyzed for endotoxin at the University of Iowa Pulmonary Toxicology Facility using a kinetic chromogenic Limulus amebocyte lysate assay previously described and with extensive quality assurance measures. The quality assurance measures included rigorous chain of custody verification, internal and external audits, bar coding of samples, use of a single lot of assay reagents, blind repeats of NHANES dust samples (N = 665), use of a single microplate reader, and application of Westgard rules to accept or reject a run. Endotoxin concentrations were reported in Endotoxin Units (EU) per mass of sieved dust (mg). The lower limit of detection was 0.0005 EU/mg. Our updated data on dust endotoxin concentration in NHANES was released in February 2014.
Wheeze and asthma outcomes
Wheeze and asthma were assessed using a questionnaire administered to each study participant (SP) or their parent if they were a young child (less than 12-year-old). Wheeze outcomes were measured using the following questions: “In the past 12 months, {have you/has SP} had wheezing or whistling in {your/his/her} chest?” (Any wheeze), “In the past 12 months, {has your/has SP’s} chest sounded wheezy during or after exercise or physical activity?” (Exercise-induced wheeze), “In the past 12 months, {have you/has SP} taken medication prescribed by a doctor for wheezing or whistling?” (Prescription medication for wheeze), “In the past 12 months, how many times {have you/has SP} gone to the doctor’s office or the hospital emergency room for one or more of these attacks of wheezing or whistling?” (Doctor/ER visit for wheeze). The link to the NHANES wheeze questionnaire can be found at https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/RDQ_D.htm#RDQ070.
Asthma outcomes were defined using the questions: “Has a doctor or other health professional ever told {you/SP} that {you have/s/he/SP has} asthma?” (Diagnosed asthma), “{Do you/Does SP} still have asthma?” (Current asthma), “During the past 12 months, {have you/has SP} had an episode of asthma or asthma attack?” (Asthma attack in past 12 Months). Asthma medication in past 30 days was determined through self-report of prescription medications use by the participant within the one-month period prior to the survey. The link to the NHANES asthma questionnaires can be found at https://wwwn.cdc.gov/Nchs/Nhanes/2005-2006/MCQ_D.htm#MCQ010.
Sensitization to inhalant allergens
Serum IgE specific to fifteen inhalant allergens (Dermatophagoides farinae, Dermatophagoides pteronyssinus, cat, dog, cockroach, Alternaria alternata, ragweed, rye grass, bermuda grass, oak, birch, Aspergillus fumigatus, thistle, mouse, rat) was measured using the Pharmacia Diagnostics ImmunoCAP 1000 System (Kalamazoo, Michigan), now known as Thermo Scientific™ ImmunoCAP Specific IgE. Sensitization status was defined as IgE specific to any of the inhalant allergens ≥0.35 kU/L.
Covariates
Data on socio-demographics and home characteristics were collected using questionnaires. The socio-demographic characteristics considered were age, gender, race/ethnicity, and family income. The home characteristics included questions on whether the was rented or owned, the type of home (single family detached, multifamily, or trailer), when was home built, the number of years lived in the home, the presence of mildew or musty smell, carpeted surface, pets, cockroach, of a smoker, and of children in the home. Data on the room temperature was also collected during the home visits. The surveys collecting data on the outcomes and the covariates were administered approximately two weeks before the house dust samples.
Statistical analysis
In the descriptive analysis, the participants’ characteristics were compared across the climate regions using the chi-square test. For each climate region, we assessed endotoxin predictors and the endotoxin relationship with respiratory outcomes as well as sensitization to inhalant allergens. Endotoxin predictors were first determined in the overall study population using forward stepwise linear regression. The threshold for entry was set as P<0.10 and the threshold for removal was set as P>0.20. A multiple linear regression analysis was subsequently performed in each climate regions to find endotoxin predictors specific to each of them. The multiple linear regression models included all the variable found to significant in the overall sample. To investigate log10-endotoxin relation with the outcomes, multivariate logistic regression analysis was performed and odds ratios (OR) with corresponding 95% confidence intervals (CI) for effect of log10-endotoxin were reported. The logistic models were adjusted for age, gender, race/ethnicity, family income, and sensitization to any inhalant allergen (except when sensitization status was an outcome). We tested for effect modification on the association between log10-endotoxin and the outcomes by including a product term of log10-endotoxin and climate regions. All analyses were done with SAS (version 9.4; SAS Institute, Cary, NC). NHANES sample weights were used in all analyses to obtain unbiased national estimates, standard errors (SE), CIs, and P-values were developed in accordance with the complex survey design by using Taylor series linearization methods. P<0.05 were considered statistically significant (except in effect modification testing where a P<0.10 was considered as significant).
RESULTS
Characteristics of study participants
The characteristics of the study participants overall and by climate regions are described in Table 1. Compared to other regions, participants in subarctic/very cold/cold areas were more likely to be non-Hispanic White, to live in homes with a cat or a dog, but without a child, and to have room temperatures between 82 and 96 °F. Participants in mixed-humid/marine regions tended to be non-Hispanic Black and to live in homes with room temperatures between 75 and 82 °F. Participants in hot-humid regions were more likely to live in homes with cockroaches. Participants in the mixed-dry/hot-dry regions tended to be Mexican-Americans and to live in homes with a child and room temperatures between 50 and 74 °F. Higher room temperatures in subarctic/very cold/cold regions may be explained by the fact that NHANES sampled these regions in summer and these homes are less likely to be air conditioned than those in more temperate regions. The prevalence of exercise-induced wheeze was higher in subarctic/very cold/cold and hot-humid regions, while the prevalence of current asthma was higher in subarctic/very cold/cold regions. The median (IQR) endotoxin concentration in house dust in the overall population was 16.2 (8.2–34.3) EU/mg, it was higher in mixed-dry/hot-dry regions (19.7 [10.3–43.3] EU/mg) and lower in mixed-humid/marine areas (14.8 [7.2–28.9] EU/mg) (Table 1).
Table 1.
Description of the study population by climate regions, NHANES 2005–2006 (N = 6,963)
| Overall | Subarctic/Very Cold/Cold | Mixed-Humid/Marine | Hot-Humid | Mixed-Dry/Hot-Dry | P | |
|---|---|---|---|---|---|---|
|
| ||||||
| Number of Participants | 6,963 | 2,236 | 2,566 | 779 | 1,382 | |
| Characteristics of participants | ||||||
| Age in years, median (Q1–Q3) | 35.3 (17.5 – 52.3) | 35.3 (17.7 – 53.5) | 36.9 (18.3 – 53.3) | 35.7 (17.3 – 49.5) | 32.3 (15.2 – 46.7) | |
|
| ||||||
| Gender, % | ||||||
| Male | 48.9 | 49.1 | 48.2 | 50.2 | 49.0 | 0.849 |
| Female | 51.1 | 50.9 | 51.8 | 49.8 | 51.0 | |
|
| ||||||
| Race/Ethnicity, % | ||||||
| Other | 10.1 | 10.4 | 8.6 | 7.8 | 14.3 | <.001 |
| Mexican American | 9.4 | 4.3 | 3.9 | 22.1 | 27.1 | |
| Non-Hispanic Black | 12.3 | 4.3 | 24.5 | 14.5 | 6.2 | |
| Non-Hispanic White | 68.1 | 81.0 | 63.0 | 55.6 | 52.4 | |
|
| ||||||
| Family Income, % | ||||||
| <$20,000 | 19.2 | 18.5 | 19.2 | 24.1 | 18.2 | 0.303 |
| ≥$20,000 | 80.8 | 81.5 | 80.8 | 75.9 | 81.8 | |
|
| ||||||
| Homes’ Characteristics | ||||||
| Home, % | ||||||
| Rented | 30.9 | 27.5 | 30.6 | 28.0 | 42.1 | 0.123 |
| Owned | 69.1 | 72.5 | 69.4 | 72.0 | 57.9 | |
|
| ||||||
| Type of home, % | ||||||
| Single family detached | 67.3 | 68.4 | 70.9 | 56.9 | 63.1 | 0.056 |
| Multifamily | 25.3 | 24.9 | 23.4 | 22.2 | 32.0 | |
| Trailer | 7.4 | 6.7 | 5.8 | 20.9 | 4.9 | |
|
| ||||||
| When was home built, % | ||||||
| (missing) | 16.4 | 12.3 | 16.9 | 19.4 | 24.3 | 0.277 |
| Before 1978 | 39.9 | 45.3 | 40.4 | 28.9 | 31.3 | |
| 1978 – present | 43.7 | 42.3 | 42.7 | 51.7 | 44.4 | |
|
| ||||||
| Years family lived in home, % | ||||||
| Up to 2 years | 37.4 | 35.2 | 35.7 | 35.4 | 48.0 | 0.122 |
| 3 to 5 years | 18.6 | 16.8 | 19.2 | 25.6 | 18.1 | |
| 6 years or more | 44.0 | 48.0 | 45.2 | 39.0 | 33.9 | |
|
| ||||||
| Mildew or musty smell in home a, % | 16.6 | 17.9 | 16.9 | 14.4 | 13.5 | 0.526 |
|
| ||||||
| Floor surface carpeted, % | 90.9 | 89.5 | 91.0 | 91.2 | 94.2 | 0.251 |
|
| ||||||
| Cat in home now, % | 26.4 | 31.6 | 25.0 | 11.8 | 24.1 | 0.002 |
|
| ||||||
| Dog in home now, % | 36.7 | 42.0 | 29.0 | 34.2 | 40.9 | 0.025 |
|
| ||||||
| Cockroaches seen in home, % | 14.8 | 3.5 | 21.0 | 46.6 | 12.0 | <.001 |
|
| ||||||
| Smoker in home, % | 20.0 | 20.5 | 23.2 | 19.1 | 12.3 | 0.072 |
|
| ||||||
| Child in the home, % | ||||||
| Child age 1–17 in home | 57.1 | 54.5 | 56.7 | 56.6 | 65.2 | 0.009 |
| No child in home | 42.9 | 45.5 | 43.3 | 43.4 | 34.8 | |
|
| ||||||
| Room Temperature (F), % | ||||||
| 82 – 96 F | 6.2 | 8.8 | 7.1 | 0.2 | 0.9 | <.001 |
| 75 – 82 F | 40.8 | 44.6 | 48.4 | 30.6 | 20.7 | |
| 67 – 74 F | 45.1 | 40.6 | 39.7 | 59.1 | 60.0 | |
| 50 – 67 F | 8.0 | 6.1 | 4.8 | 10.1 | 18.4 | |
|
| ||||||
| Respiratory/Sensitization outcomes | ||||||
| Any wheeze, % | 16.2 | 17.5 | 14.4 | 18.4 | 15.2 | 0.377 |
|
| ||||||
| Exercise-induced wheeze, % | 7.9 | 9.3 | 6.2 | 9.3 | 7.0 | 0.009 |
|
| ||||||
| Medication for wheeze, % | 9.5 | 9.7 | 8.4 | 11.5 | 10.0 | 0.504 |
|
| ||||||
| Doctor/ER visits for wheeze, % | 7.2 | 6.7 | 7.9 | 8.3 | 6.2 | 0.757 |
|
| ||||||
| Current asthma, % | 8.8 | 10.6 | 6.9 | 8.0 | 8.5 | 0.046 |
|
| ||||||
| Current asthma and any wheeze, % | 4.5 | 5.3 | 3.8 | 4.0 | 4.4 | 0.493 |
|
| ||||||
| Sensitization to inhalant allergens, % | 43.8 | 40.5 | 44.8 | 49.5 | 47.0 | 0.060 |
|
| ||||||
| Endotoxin Levels | ||||||
| Endotoxin, median (Q1–Q3) | 16.2 (8.2–34.3) | 16.2 (8.1 – 34.5) | 14.8 (7.2 – 28.9) | 19.2 (8.9 – 37.8) | 19.7 (10.3 – 43.3) | |
| Log10-Endotoxin, mean (SE) | 1.190 (0.014) | 1.195 (0.024) | 1.128 (0.027) | 1.196 (0.092) | 1.306 (0.018) | |
Abbreviations: Q, quartile; ER, emergency room; SE, standard error.
Grey shade represents characteristics significantly different across climate regions.
Endotoxin predictors by climate regions
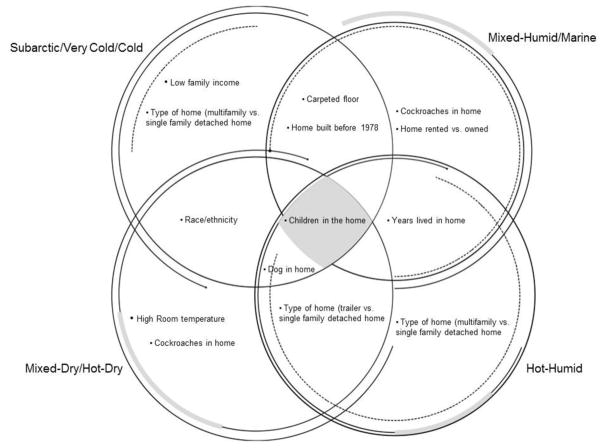
Table 2 reports endotoxin predictors in the overall population and by climate regions. In subarctic/very cold/cold regions, increased endotoxin concentration in house dust was predicted by Mexican-American race/ethnicity (compared to non-Hispanic White) (linear regression coefficient (β): 0.168, P<0.001), low family income (β: 0.218, P<0.01), home built before 1978 (β: 0.103, P<0.05), living in a home with children (β: 0.114, P<0.05) or a dog (β: 0.199, P<0.001). Predictors of decreased endotoxin in house dust included non-Hispanic Black race/ethnicity (β: −0.091, P<0.01) and living in a multi-family type of home compared to a single-family (β: −0.089, P<0.05) (Table 2).
Table 2.
Linear regression coefficients (β) for endotoxin predictors by climate regions, NHANES 2005–2006
| Overall | Subarctic/Very Cold/Cold | Mixed-Humid/Marine | Hot-Humid | Mixed-Dry/Hot-Dry | |
|---|---|---|---|---|---|
|
| |||||
| Characteristics of participants | |||||
| Race/Ethnicity | |||||
| Other | 0.006 | 0.004 | −0.007 | 0.112 | 0.017 |
| Mexican American | 0.151*** | 0.168*** | 0.091 | 0.170 | 0.123*** |
| Non-Hispanic Black | −0.004 | −0.091** | 0.034 | 0.032 | 0.172a |
| Non-Hispanic White | Ref | Ref | Ref | Ref | Ref |
|
| |||||
| Family Income (<$20,000 vs ≥$20,000) | 0.135** | 0.218** | 0.094a | 0.059 | 0.045 |
|
| |||||
| Homes’ Characteristics | |||||
| Home (rented vs. owned) | 0.091a | −0.014 | 0.122* | −0.008 | 0.227a |
|
| |||||
| Type of home | |||||
| Single family detached | Ref | Ref | Ref | Ref | Ref |
| Multifamily | −0.085* | −0.089* | −0.108 | 0.197* | −0.163a |
| Trailer | 0.120** | 0.097a | 0.066 | 0.227** | 0.251** |
|
| |||||
| When was home built | |||||
| (missing) | 0.092a | 0.090a | 0.013 | 0.188 | 0.130 |
| Before 1978 | 0.106** | 0.103* | 0.086*** | 0.286a | 0.058 |
| 1978 – present | Ref | Ref | Ref | Ref | Ref |
|
| |||||
| Years family lived in home | |||||
| Up to 2 years | −0.018 | 0.084 | −0.073* | −0.221 | −0.025 |
| 3 to 5 years | −0.058* | 0.018 | −0.090 | −0.246* | −0.027 |
| 6 years or more | Ref | Ref | Ref | Ref | Ref |
|
| |||||
| Child age 1–17 in the home (yes vs no) | 0.124*** | 0.114* | 0.085* | 0.206*** | 0.146* |
|
| |||||
| Mildew or musty smell in home (yes vs no) | −0.005 | 0.013 | −0.062 | −0.037 | 0.035 |
|
| |||||
| Floor surface carpeted (yes vs no) | 0.217*** | 0.217*** | 0.204** | 0.096 | 0.150 |
|
| |||||
| Cat in home now (yes vs no) | 0.077*** | 0.047 | 0.056 | 0.151 | 0.145a |
|
| |||||
| Dog in home now (yes vs no) | 0.153*** | 0.199*** | 0.061 | 0.243* | 0.103* |
|
| |||||
| Cockroaches seen in home (yes vs no) | 0.111** | 0.012 | 0.190** | 0.132a | 0.145** |
|
| |||||
| Room Temperature (F) | |||||
| 82 – 96 F | 0.077 | 0.038 | 0.056 | 0.233 | 0.467*** |
| 75 – 82 F | 0.001 | −0.018 | −0.078 | 0.374 | −0.014 |
| 67 – 74 F | 0.038 | 0.003 | −0.052 | 0.184 | 0.069** |
| 50 – 67 F | Ref | Ref | Ref | Ref | Ref |
|
| |||||
| Smoker in home (yes vs no) | 0.048 | 0.059 | 0.037 | 0.040 | 0.182 |
|
| |||||
| R-squared fit statistic | 0.078 | 0.092 | 0.069 | 0.166 | 0.146 |
P < 0.05
P < 0.01
P < 0.001
0.05 ≤ P ≤ 0.10
Grey shaded cells represent predictors significant associated with log10-endotoxin.
In mixed-humid/marine regions, predictors of higher house dust endotoxin were: living in a rented home (β: 0.122, P<0.05), built before 1978 (β: 0.086, P<0.001), with a child (β: 0.085, P<0.05), with a carpeted floor (β: 0.204, P<0.01), or with cockroaches (β: 0.190, P<0.01). Lower endotoxin was associated with living in the home for ≤2 years compared to ≥6 years (β: −0.073, P<0.05) (Table 2).
In hot-humid regions, living in a multifamily (β: 0.197, P<0.05) or a trailer (β: 0.227, P<0.01) type of home, in a home with a child (β: 0.206, P<0.001) or a dog (β: 0.243, P<0.05) were associated with higher house dust endotoxin, while participants living in a home for 3–5 years tended to have less endotoxin (β: −0.246, P<0.05) (Table 2).
In mixed-dry/hot-dry regions, higher endotoxin was associated with being Mexican-American (β: 0.123, P<0.001), living in a trailer (β: 0.251, P<0.01) or in a home with a child (β: 0.146, P<0.05), with a dog (β: 0.103, P<0.05), with cockroaches (β: 0.145, P<0.01), or with room temperatures between 82 and 96 °F (β: 0.467, P<0.001) and 67 and 74 °F (β: 0.069, P<0.01) compared to temperatures between 50 and 67 °F (Table 2).
The distribution of endotoxin predictors across the different climate regions was illustrated in a Venn diagram reported in Figure 2.
Figure 2.
Venn diagram for endotoxin predictors by climate regions.
Influence of climate regions on endotoxin association with outcomes
Endotoxin and wheeze outcomes
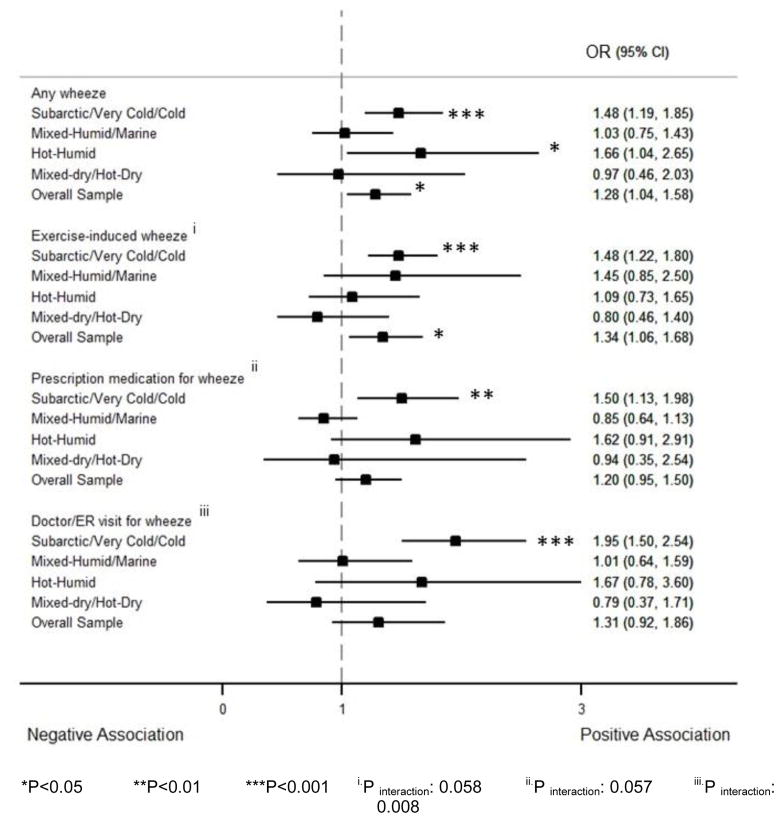
Endotoxin association with wheeze outcomes was significantly different across climate regions for exercise-induced wheeze (Pinteraction=0.058), prescription medication for wheeze (Pinteraction=0.057), and doctor/ER visit for wheeze (Pinteraction=0.008). In subarctic/very cold/cold regions, endotoxin had a strong association with any wheeze in the past 12 months (OR: 1.48, 95% CI: 1.19–1.85), exercise-induced wheeze (OR: 1.48, 95% CI: 1.22–1.80), prescription medication for wheeze (OR: 1.50, 95% CI: 1.13–1.98), and doctor/ER visit for wheeze (OR: 1.95, 95% CI: 1.50–2.54). In hot-humid regions, a log10-endotoxin increase was associated with a 66% increase in the odds of any wheeze in the past 12 months (OR: 1.66, 95% CI: 1.04–2.65). With the exception of endotoxin association with any wheeze in the past 12 months in hot-humid regions, the association between endotoxin and wheeze outcomes was still significant after adjustment for multiple comparison (i.e. they had a p-value <0.0125 which corresponds to the ratio of the 0.05 significance level over the number of wheeze outcomes (4)) (Figure 3).
Figure 3.
Forest plot reporting the adjusted OR and CI for the association between log10-endotoxin and wheeze outcomes in past 12 months by climate regions. The analysis was adjusted for age, gender, race/ethnicity, family income, and sensitization to inhalant allergens. The figure shows that endotoxin was associated with higher prevalence of wheeze outcomes in subarctic/very cold/cold regions. In hot-humid regions, endotoxin was associated with a higher prevalence of any wheeze. The association of endotoxin with wheeze outcomes was significantly different across climate regions for exercise-induced wheeze, prescription medication for wheeze, and doctor/ER visit for wheeze.
Endotoxin and asthma outcomes
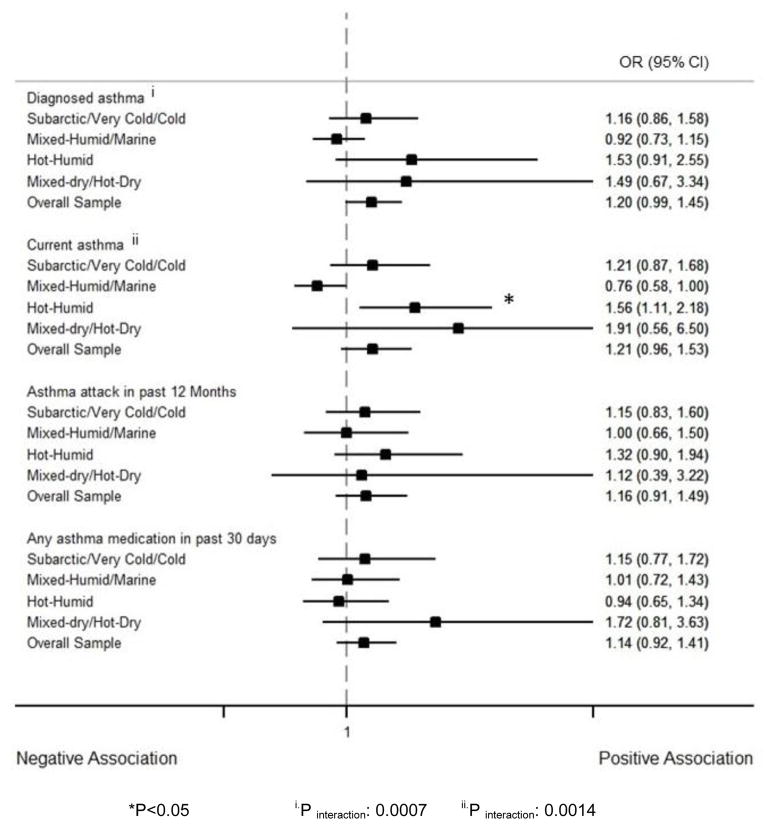
Endotoxin association with asthma outcomes significantly changed across climate regions for asthma diagnosis (Pinteraction=0.0007) and current asthma (Pinteraction=0.0014). In hot-humid regions, log10-endotoxin was associated with a 56% increase in the odds of current asthma (OR: 1.56, 95% CI: 1.11–2.18). However, in mixed-humid/marine climates endotoxin had a marginally significant negative association with current asthma (OR: 0.76, 95% CI: 0.58–1.00). The association between endotoxin and current asthma in hot-humid regions was still significant after adjustment for multiple comparison (p-value <0.0125) (Figure 4).
Figure 4.
Forest plot reporting the adjusted OR and CI for the association between log10-endotoxin and asthma outcomes by climate regions. The analysis was adjusted for age, gender, race/ethnicity, family income, and sensitization to inhalant allergens. The figure shows that endotoxin was associated with a higher prevalence of current asthma in hot-humid regions. The association of endotoxin with asthma outcomes was different across climate regions for asthma diagnosis and current asthma.
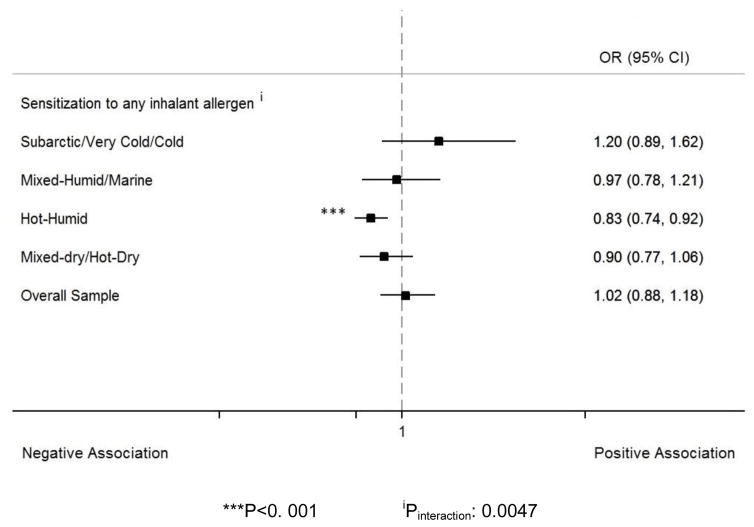
Endotoxin and sensitization to inhalant allergens
The association of endotoxin with sensitization to inhalant allergens was significantly different by climate regions (Pinteraction=0.043). In hot-humid climate regions, endotoxin was negatively associated with sensitization to any inhalant allergen (OR: 0.83, 95% CI: 0.74–0.92), while in the other regions, no significant relationship was found (Figure 5).
Figure 5.
Forest plot reporting the adjusted OR and CI for the association between log10-endotoxin and sensitization to any allergen by climate regions. The analysis was adjusted for age, gender, race/ethnicity, and family income. Endotoxin was negatively associated with sensitization to inhalant allergens in hot-humid regions. Endotoxin association with sensitization to inhalant allergens was significantly different across climate regions.
DISCUSSION
In this study representative of the US population, we report that endotoxin predictors and endotoxin association with respiratory outcomes, as well as sensitization to inhalant allergens significantly vary with climate regions. Endotoxin is associated with higher prevalence of wheeze outcomes in subarctic/very cold/cold areas, while in hot-humid regions, it is associated with higher prevalence of wheeze and current asthma, but lower prevalence of sensitization to inhalant allergens. This is a surprising finding given that prior studies conducted in a single climate region have often presumed a national representativeness (Bischof et al., 2002; Gehring et al., 2004; Wickens et al., 2003). Therefore, the present study is the first to examine how endotoxin predictors and endotoxin association with respiratory outcomes differ with climate regions.
Despite differences in endotoxin predictors, the presence of children in homes was consistently associated with endotoxin across climate regions and has frequently been reported to be a predictor of endotoxin (Jacobs et al., 2014; Thorne, Cohn, Mav, Arbes, & Zeldin, 2009; Waser et al., 2004). Although we found housing type to be a predictor of endotoxin in all climate regions except in mixed-humid/marine areas, previous studies have produced conflicting results. In a nationally representative US study, Thorne et al. found single-housing compared to multi-housing units only associated with kitchen floor dust endotoxin (Thorne et al., 2009). In Europe, Bischof et al. found the type of home to be a predictor of living room floor endotoxin (Bischof et al., 2002), whereas Gehring et al. did not find it to be a determinant of endotoxin in bedding (Gehring et al., 2004). It has been proposed that pets could increase house endotoxin levels because their gastrointestinal tracts may be colonized primarily with Gram-negative bacteria (Waser et al., 2004), which is consistent with our findings that the presence of dogs in homes was a predictor of endotoxin in all the climate regions except in mixed-humid/marine areas (where they may spend more time outside in than in cold and hot climates). Furthermore, dogs may be more likely than cats to carry Gram-negative bacteria and endotoxin on their fur. Carpets were predictive of higher endotoxin in subarctic/very cold/cold and mixed-humid/marine regions where they are more likely to retain moisture, which itself was associated with higher endotoxin levels in New Zealand (Wickens et al., 2003) but not in the US (Thorne et al., 2009). Likewise, race/ethnicity differentially affected house endotoxin; Mexican-Americans lived in houses with higher endotoxin than non-Hispanic Whites in cold temperatures. The exact reason of this is unclear to us. We hypothesize that since low income was a contributing factor of higher endotoxin in cold regions, residual confounding might explain our findings for Hispanics who are known to have the lowest socioeconomic status in the US (Braveman, Cubbin, Egerter, Williams, & Pamuk, 2010). Endotoxin was also higher in Mexican-Americans’ homes in the mixed-dry/hot-dry regions, possibly for the reason that they could disproportionately be living in the Southern US, where the climate is more favorable to microbial growth, compared to non-Hispanic Whites (Thorne et al., 2015). Although cockroach carcasses contain endotoxin, it is not clear why in hot-humid regions, where the presence of cockroaches in homes was more prevalent, cockroaches were only marginally significant predictors of endotoxin concentration. Also, cockroaches were not associated with endotoxin in colder regions where they are rare (Kutintara & Parrott, 2003). It is unclear why the number of years lived in the home, living in a home built before 1978, or living in a rented home predicted endotoxin in some regions but not in others.
Consistent with our finding of a stronger endotoxin association with wheeze outcomes in subarctic/very cold/cold climates, cold weather has been shown to enhance the effect of indoor pollutants on respiratory conditions even at lower concentrations (Pönkä, 1991). In cold temperatures, low air humidity and home heating increases the indoor air dryness. When inhaled, dry air is associated with increased production of neutrophils, eosinophils and leukotrienes causing inflammation and hyper-reactivity in small airways (Makra et al., 2008; Millqvist, 1999). It has been suggested that exercising in cold weather might lead to airway remodeling as well as bronchoconstriction due to hyperpnoea resulting from the airway fluid hyperosmolarity (D’Amato et al., 2015). To possibly explain the aggravating effect of cold climate on wheeze associated with exposure to pollutants, cold air has been reported to impair the lung mucociliary clearance of contaminants (Clary-Meinesz, Cosson, Huitorel, & Blaive, 1992). It remains unclear whether the effect of cold weather on endotoxin association with wheeze is truly causal or the result of other determinants. For instance, space heaters, furnaces, or gas stoves used in some climate regions increase humidity, contributing to dust mite allergen, oxidant gases, and other indoor air pollutants related to wheeze (Arif, Delclos, Lee, Tortolero, & Whitehead, 2003). Houses with poor ventilation and increased humidity are known to be associated with wheeze and also tend to have higher endotoxin (Mi, Norbäck, Tao, Mi, & Ferm, 2006; Wickens et al., 2003). Although endotoxin had strong positive associations with wheeze outcomes in subarctic/very cold/cold regions, it was not significantly associated with any of the asthma outcomes in these areas.
In hot-humid regions, we found endotoxin positively associated with any wheeze and current asthma, but negatively with sensitization to inhalant allergens. In contrast, in mixed-humid/marine regions, endotoxin had a marginally significant negative association with current asthma (P=0.05). The reason for the changing directionality in endotoxin relationship with respiratory outcomes by climate regions is unclear. Some studies have suggested that LPS types may be different in certain climatic conditions and have different effects. Some bacteria may adapt to climate by undergoing structural and functional changes that produce different LPS (Kumar, Jagannadham, & Ray, 2002, Knirel et al., 2005). Several studies have reported that different endotoxin types may have different associations with asthma and wheeze depending on their chemical structure (Zhao et al., 2008, Norbäck et al., 2014). With regard to the number of acyl chains attached to the lipid A, some authors have speculated that penta-acylated endotoxin could be protective against allergic sensitization and IgE-mediated asthma, whereas the hexa-acylated form would increase the risk (Brix, Eriksen, Larsen, & Bisgaard, 2014; Norbäck et al., 2014). The distinct effect of penta- and hexa-acylated endotoxin on inflammation and cytokine profiles has also been confirmed by animal studies in which a higher inflammatory response was associated with hexa-acylated endotoxin structure (Hađina et al., 2005). In mixed-humid/marine regions, the presence of cockroaches in the home was a strong predictor of higher endotoxin and cockroaches are typically associated with high humidity and poor ventilation, nevertheless there was a marginally significant inverse relationship between endotoxin and current asthma (Munir et al., 1994; Thorne et al., 2009). Interestingly, one study found a negative association between exposure to cockroaches and recurrent wheeze in urban children and that cockroaches were associated with beneficial dust bacteria such as Bacteroidetes or Prevotellaceae which contain a penta-acylated type of LPS (Larsen et al., 2015; Lynch et al., 2014). Another logical explanation of the different endotoxin effects across climates might be related to endotoxin predictors. Congruent with different associations of endotoxin with sensitization to allergens in different climates, it is known that different climate regions have different allergens (Peat et al., 1996) and that endotoxin has different relationships with different allergens depending on age of exposure (Min & Min, 2015).
Our study had limitations. Due to the cross-sectional design of the study, temporality between endotoxin exposure and asthma or wheeze cannot be evaluated and causality cannot be established. House dust was only sampled once, but dust endotoxin and allergens from mattress and bedroom floor have been proposed to be representative of long time exposures (Heinrich et al., 2003). Asthma and wheeze outcomes were self-reported and could not be verified. The NHANES did not collect data on the seasonal variation of endotoxin. It only reported the 6-month period (November–April versus May–October) of the participant’s examination which was not significantly associated with endotoxin levels. We also did not have data on occupational exposure to endotoxin. Endotoxin was quantified using the Limulus amebocyte lysate assay which does not allow one to distinguish between penta- and hexa-acylated endotoxins (Brix et al., 2014) and no data on the chemical structure of the LPS was available. Nonetheless, our study has major strengths. It is the first to investigate endotoxin predictors and endotoxin association with respiratory outcomes and with sensitization to inhalant allergens across different climate regions. It includes a large sample representative of the US child and adult population from the NHANES, which is the largest cohort study inclusive of endotoxin exposure to date. Endotoxin predictors were selected from a broad range of variables which were tested for significance. Sensitization to inhalant allergens was determined from a panel of fifteen common allergens. House dust endotoxin was measured using extensive quality assurance, and in order to conduct this study, we were granted access to non-public data on US climate regions.
In conclusion, the predictors of house dust endotoxin as well as endotoxin association with asthma, wheeze, and sensitization status differ with climate regions in the US. In Subarctic/Very Cold/Cold regions, endotoxin is mainly associated with higher prevalence of wheeze. In hot-humid climate, endotoxin is positively associated with wheeze and asthma, but negatively with sensitization to inhalant allergens. Future research should examine the potential mediators of these variations, including the different endotoxin structures using appropriate techniques to test the hypotheses generated by these findings.
HIGHLIGHTS.
Endotoxin predictors and association with lung conditions differ with macroclimate.
In cold regions, endotoxin is associated with higher prevalence of wheeze.
In hot-humid regions, endotoxin is positively associated with wheeze and asthma.
Endotoxin had an inverse relationship with sensitization in hot-humid climates.
Acknowledgments
Sample extraction and endotoxin analysis work at the University of Iowa was funded by CDC/NCHS (200-2010-34238 NCE1). Data analysis was funded through a grant to the University of Iowa, Environmental Health Sciences Research Center (NIH P30 ES005605), the University of Iowa Center for Health Effects of Environmental Contamination (CHEEC), and through a contract to Social & Scientific Systems, Inc. (HHSN273201600002). This work was also funded, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (NIH Z01 ES025041). The authors acknowledge Dr. Nervana Metwali for leading the analysis of the NHANES dust samples for endotoxin.
Footnotes
Author Contributions: Angelico Mendy assisted with development of the research questions and data analysis and was principal author of this manuscript. Peter S. Thorne directed the endotoxin analysis of the NHANES samples, contributed to the study design, collaborated on development of the research questions and edited the manuscript. Darryl C. Zeldin and Päivi Salo contributed to the conception, hypothesis delineation and design of this component of the NHANES study. Jesse Wilkerson and Richard D. Cohn contributed to the statistical analysis and interpretation of the data. All authors provided edits and comments on the manuscript.
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arif AA, Delclos GL, Lee ES, Tortolero SR, Whitehead LW. Prevalence and risk factors of asthma and wheezing among US adults: An analysis of the NHANES III data. The European Respiratory Journal. 2003;21(5):827–833. doi: 10.1183/09031936.03.00054103a. [DOI] [PubMed] [Google Scholar]
- Baechler MC, Williamson JL, Gilbride TL, Cole PC, Hefty MG, Love PM. High-performance home technologies: Guide to determining climate regions by county. Pacific Northwest National Laboratory; 2010. [Google Scholar]
- Bischof W, Koch A, Gehring U, Fahlbusch B, Wichmann H, Heinrich J. Predictors of high endotoxin concentrations in the settled dust of german homes. Indoor Air. 2002;12(1):2–9. doi: 10.1034/j.1600-0668.2002.120102.x. [DOI] [PubMed] [Google Scholar]
- Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L … Allergy and Endotoxin Study Team. Environmental exposure to endotoxin and its relation to asthma in school-age children. The New England Journal of Medicine. 2002;347(12):869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. American Journal of Public Health. 2010;100(S1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix S, Eriksen C, Larsen JM, Bisgaard H. Metagenomic heterogeneity explains dual immune effects of endotoxins. Journal of Allergy and Clinical Immunology. 2014;135(1):277–280. doi: 10.1016/j.jaci.2014.09.036. DOI: http://dx.doi.org/10.1016/j.jaci.2014.09.036. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) Analytic and reporting guidelines: The national health and nutrition examination survey (NHANES) baltimore, MD: Hyattsville; 2006. [Google Scholar]
- Clary-Meinesz C, Cosson J, Huitorel P, Blaive B. Temperature effect on the ciliary beat frequency of human nasal and tracheal ciliated cells. Biology of the Cell. 1992;76:335–338. doi: 10.1016/0248-4900(92)90436-5. https://doi.org/10.1016/0248-4900(92)90436-5. [DOI] [PubMed] [Google Scholar]
- D’Amato G, Holgate ST, Pawankar R, Ledford DK, Cecchi L, Al-Ahmad M, … Baena-Cagnani CE. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the world allergy organization. World Allergy Organization Journal. 2015;8(1):25. doi: 10.1186/s40413-015-0073-0. https://doi.org/10.1186/s40413-015-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doreswamy V, Peden DB. Modulation of asthma by endotoxin. Clinical & Experimental Allergy. 2011;41(1):9–19. doi: 10.1111/j.1365-2222.2010.03628.x. [DOI] [PubMed] [Google Scholar]
- Gehring U, Bischof W, Borte M, Herbarth O, Wichmann H, Heinrich J. Levels and predictors of endotoxin in mattress dust samples from east and west German homes. Indoor Air. 2004;14(4):284–292. doi: 10.1111/j.1600-0668.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- Hađina S, Weiss JP, McCray PB, Jr, Kulhankova K, Thorne PS. MD-2–dependent pulmonary immune responses to inhaled lipooligosaccharides: Effect of acylation state. American Journal of Respiratory Cell and Molecular Biology. 2008;38(6):647–654. doi: 10.1165/rcmb.2007-0418OC. https://doi.org/10.1165/rcmb.2007-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich J, Hölscher B, Douwes J, Richter K, Koch A, Bischof W, … Wichmann H. Reproducibility of allergen, endotoxin and fungi measurements in the indoor environment. Journal of Exposure Science and Environmental Epidemiology. 2003;13(2):152–160. doi: 10.1038/sj.jea.7500267. [DOI] [PubMed] [Google Scholar]
- Héroux M, Clark N, Ryswyk KV, Mallick R, Gilbert NL, Harrison I, … Guay M. Predictors of indoor air concentrations in smoking and non-smoking residences. International Journal of Environmental Research and Public Health. 2010;7(8):3080–3099. doi: 10.3390/ijerph7083080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Krop E, Borras-Santos A, Zock J, Taubel M, Hyvarinnen A, … Heederik D. Endotoxin levels in settled airborne dust in european schools: The HITEA school study. Indoor Air. 2014;24(2):148–157. doi: 10.1111/ina.12064. [DOI] [PubMed] [Google Scholar]
- Knirel YA, Lindner B, Vinogradov E, Shaikhutdinova RZ, Sof’ya NS, Kocharova NA, … Anisimov AP. Cold temperature-induced modifications to the composition and structure of the lipopolysaccharide of yersinia pestis. Carbohydrate Research. 2005;340(9):1625–1630. doi: 10.1016/j.carres.2005.04.007. https://doi.org/10.1016/j.carres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kumar GS, Jagannadham MV, Ray MK. Low-temperature-induced changes in composition and fluidity of lipopolysaccharides in the antarctic psychrotrophic bacterium pseudomonas syringae. Journal of Bacteriology. 2002;184(23):6746–6749. doi: 10.1128/JB.184.23.6746-6749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutintara B, Parrott KR. Home environments and allergen avoidance practices in a hot, humid climate. Housing and Society. 2003;30(1):69–85. http://dx.doi.org/10.1080/08882746.2003.11430484. [Google Scholar]
- Larsen JM, Musavian HS, Butt TM, Ingvorsen C, Thysen AH, Brix S. Chronic obstructive pulmonary disease and asthma-associated proteobacteria, but not commensal prevotella spp., promote toll-like receptor 2-independent lung inflammation and pathology. Immunology. 2015;144(2):333–342. doi: 10.1111/imm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, … Matsui E. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. Journal of Allergy and Clinical Immunology. 2014;134(3):593–601. doi: 10.1016/j.jaci.2014.04.018. https://doi.org/10.1016/j.jaci.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makra L, Tombácz S, Bálint B, Sümeghy Z, Sánta T, Hirsch T. Influences of meteorological parameters and biological and chemical air pollutants on the incidence of asthma and rhinitis. Climate Research. 2008;37(1):99–119. doi: 10.3354/cr00752. [DOI] [Google Scholar]
- Mi Y, Norbäck D, Tao J, Mi Y, Ferm M. Current asthma and respiratory symptoms among pupils in shanghai, china: Influence of building ventilation, nitrogen dioxide, ozone, and formaldehyde in classrooms. Indoor Air. 2006;16(6):454–464. doi: 10.1111/j.1600-0668.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- Michel O, Duchateau J, Sergysels R. Effect of inhaled endotoxin on bronchial reactivity in asthmatic and normal subjects. J Appl Physiol. 1989;66(3):1059–1064. doi: 10.1152/jappl.1989.66.3.1059. [DOI] [PubMed] [Google Scholar]
- Millqvist E. Effect of nasal air temperature on lung function. Allergy. 1999;54(s57):106–111. doi: 10.1111/j.1398-9995.1999.tb04411.x. [DOI] [PubMed] [Google Scholar]
- Min K, Min J. Exposure to household endotoxin and total and allergen-specific IgE in the US population. Environmental Pollution. 2015;199:148–154. doi: 10.1016/j.envpol.2014.12.012. https://doi.org/10.1016/j.envpol.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Munir A, Björkstén B, Einarsson R, Schou C, Ekstrand-Tobin A, Warner A, Kjellman N. Cat (fel d I), dog (can f I), and cockroach allergens in homes of asthmatic children from three climatic zones in sweden. Allergy. 1994;49(7):508–516. doi: 10.1111/j.1398-9995.1994.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Norbäck D, Markowicz P, Cai G, Hashim Z, Ali F, Zheng Y, … Hashim JH. Endotoxin, ergosterol, fungal DNA and allergens in dust from schools in johor bahru, malaysia-associations with asthma and respiratory infections in pupils. PloS One. 2014;9(2):e88303. doi: 10.1371/journal.pone.0088303. https://doi.org/10.1371/journal.pone.0088303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, Woolcock AJ. House dust mite allergens. A major risk factor for childhood asthma in Australia. American Journal of Respiratory and Critical Care Medicine. 1996;153(1):141–146. doi: 10.1164/ajrccm.153.1.8542107. https://doi.org/10.1164/ajrccm.153.1.8542107. [DOI] [PubMed] [Google Scholar]
- Pönkä A. Asthma and low-level air pollution in Helsinki. Archives of Environmental Health. 1991;46(5):262–270. doi: 10.1080/00039896.1991.9934386. http://dx.doi.org/10.1080/00039896.1991.9934386. [DOI] [PubMed] [Google Scholar]
- Sigsgaard T, Heederik D. Occupational asthma. Springer Science & Business Media; 2011. [Google Scholar]
- Teghanemt A, Zhang D, Levis EN, Weiss JP, Gioannini TL. Molecular basis of reduced potency of underacylated endotoxins. Journal of Immunology. 2005;175(7):4669–4676. doi: 10.4049/jimmunol.175.7.4669. DOI: https://doi.org/10.4049/jimmunol.175.7.4669. [DOI] [PubMed] [Google Scholar]
- Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, Rose KM, Zeldin DC. Endotoxin exposure: Predictors and prevalence of associated asthma outcomes in the US. American journal of respiratory and critical care medicine. 2015;192(11):1287–1297. doi: 10.1164/rccm.201502-0251OC. https://doi.org/10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS, Cohn RD, Mav D, Arbes SJ, Zeldin DC. Predictors of endotoxin levels in US housing. Environmental Health Perspectives. 2009;117(5):763. doi: 10.1289/ehp.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS, Heederik D. Indoor Bioaerosols–Sources and characteristics. Organic Indoor Air Pollutants: Occurrence-Measurement-Evaluation. 1999:275–288. [Google Scholar]
- Thorne PS, Kulhánková K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: The national survey of endotoxin in United States housing. American Journal of Respiratory and Critical Care Medicine. 2005;172(11):1371–1377. doi: 10.1164/rccm.200505-758OC. https://doi.org/10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mutius E. The microbial environment and its influence on asthma prevention in early life. Journal of Allergy and Clinical Immunology. 2016;137(3):680–689. doi: 10.1016/j.jaci.2015.12.1301. https://doi.org/10.1016/j.jaci.2015.12.1301. [DOI] [PubMed] [Google Scholar]
- Waser M, Schierl R, Maisch S, Carr D, Riedler J, Eder W, … Braunfahrländer C. Determinants of endotoxin levels in living environments of farmers’ children and their peers from rural areas. Clinical & Experimental Allergy. 2004;34(3):389–397. doi: 10.1111/j.1365-2222.2004.01873.x. [DOI] [PubMed] [Google Scholar]
- Wickens K, Douwes J, Siebers R, Fitzharris P, Wouters I, Doekes G, … Crane J. Determinants of endotoxin levels in carpets in New Zealand homes. Indoor Air. 2003;13(2):128–135. doi: 10.1034/j.1600-0668.2003.00187.x. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Sebastian A, Larsson L, Wang Z, Zhang Z, Norback D. Asthmatic symptoms among pupils in relation to microbial dust exposure in schools in Taiyuan, China. Pediatric Allergy and Immunology. 2008;19(5):455–465. doi: 10.1111/j.1399-3038.2007.00664.x. [DOI] [PubMed] [Google Scholar]