Abstract
This study aims to investigate how antioxidant enzyme activity and overall antioxidant capacity respond to short-term changes in exposure to air pollution. 201 participants were recruited before- and followed up during- and after- the 2008 Beijing Olympics. Serum levels of antioxidant enzymes including glutathione S-transferases (GST), glutathione peroxidase (GPx), glutathione reductase (GR), and total antioxidant status (TAS) were measured. We used linear mixed-effects models to compare changes in antioxidant enzymes across the three periods after adjusting for potential confounding factors. Among all participants, glutathione peroxidase (GPx) levels decreased by 12.0% when air pollution dropped by 50–60% during the Olympics and increased by 6.5% when air pollution levels rose after the Olympics. The magnitude of increase among males, smokers, and older individuals was relatively smaller compared to females, nonsmokers, and younger individuals. Among all participants, total antioxidant status (TAS) significantly decreased by 6.23% during the games and continued to decrease by 4.41% after the games. However, among females, nonsmokers, and younger participants, there was an increase in TAS response to the elevated air pollution levels. Our study observed strong responses in GPx and TAS levels to the short-term decrease and increase of air pollution levels and responses varied among subgroups.
Keywords: Air pollution, antioxidant enzymes, Beijing Olympics, glutathione peroxidase (GPx), panel study, total antioxidant status (TAS)
1. Introduction1
The impact of air pollution on human health remains a critical worldwide concern. Epidemiologic studies have consistently identified air pollution to be associated with increased morbidity and mortality (Lodovici and Bigagli, 2011; Seaton et al., 1995). Various air pollutants, including fine and ultrafine particles, ozone, nitrogen oxides, polycyclic aromatic hydrocarbons and transition metals, can act as free radical initiators generating reactive oxygen species (ROS), which directly attack cellular DNA (Lodovici and Bigagli, 2011). Oxidative stress, which results from the imbalance of reactive oxygen species (ROS) generation and antioxidant enzymes, may induce damage of tissue, lipids, proteins, and nucleic acids, and therefore, plays a critical role in environment related diseases in humans including cancer, asthma, respiratory diseases, and arteriosclerosis (Lodovici and Bigagli, 2011).
When a cell sustains oxidative stress, antioxidants present in the cell respond to quench the reactive oxygen species (ROS). Phase I and II metabolic enzymes interact with foreign and toxic compounds in the body (Delfino et al., 2011; Sies, 1997), and therefore, are important for regulating the balance between the overproduction and destruction of ROS within the cell. Glutathione peroxidase (GPx), a key front line defense phase II enzyme, is responsible for breaking down hydrogen peroxide and additional peroxides into less toxic compounds, and reduces the formation of free hydroxyl radicals (Ceballos-Picot et al., 1996). Glutathione peroxidase (GPx) requires glutathione (GSH) as a co-factor producing glutathione disulfide (GSSG) as a product. Glutathione reductase (GR) is responsible for the reduction of GSSG to glutathione (GSH), which is critical for maintaining glutathione levels and minimizing oxidative stress in cells (Carlberg and Mannervik, 1985). Glutathione S-transferases (GST), a large family of Phase II enzymes, use glutathione to detoxify xenobiotic substances to less toxic products that can be removed from the body (Sies, 1997). Among all antioxidant enzymes, GPx has been considered as the most important given its higher affinity to hydrogen peroxide compared to catalase, which also catalyzes hydrogen peroxide into water and oxygen (Baud et al., 2004; Davis and Uthus, 2003; Valko et al., 2006). Moreover, the treatment of using GPx-1 as a therapeutic target might have potential benefits for COPD patients against hydrogen peroxide and the peroxynitrite molecules from cigarette smoke-induced inflammation and emphysema (József and Filep, 2003; Vlahos and Bozinovski, 2013). In addition, it has been shown that deletion of the genes regulating glutathione enzyme activity influences the level of DNA adducts and development of cancer, specifically lung cancer (Nielsen et al., 1996).
Over the years, measures of total antioxidant status (TAS) have been developed to capture the collective effect of antioxidant defense capacity, including enzymatic and nonenzymatic systems (Fraga et al., 2014; Franco et al., 2007). Multiple antioxidant enzymes, as well as antioxidant vitamins and micronutrients, are thought to work in concert and interact with each other to maintain the balance of ROS; therefore, total antioxidant status (TAS) has been found to be a useful indication of antioxidant defense capacity (Emin et al., 2012).
To date, several published literature based on the Beijing Olympics have reported findings on inflammation and oxidative stress related biomarkers. These previous studies found responses in airway inflammation markers (fractional exhaled nitric oxide, FENO), respiratory and systemic stress markers (nitrate and nitrite in exhaled breath condensate), and DNA and lipid oxidative damage markers (8-hydroxy-2′ –deoxyguanosine (8-OHdG) and urinary malondialdehyde (MDA)) (Huang et al., 2012; Lin et al., 2015; Rich et al., 2012). However, to our knowledge, none of the studies have measured the response of antioxidant enzymes and total antioxidant status markers.
Human epidemiological studies regarding antioxidant response to air pollution remain limited. In a study among elderly subjects with coronary heart disease, GPx-1 was inversely associated with PM2.5 and PM2.5–10 (Delfino et al., 2009). Another study in a rural Indian population found women exposed to biomass smoke had higher ROS production and lower total antioxidant status (TAS) compared to liquefied petroleum gas users (Mondal et al., 2010). Given the limited human research so far, additional epidemiologic studies are needed to further understand the role of antioxidants among human populations exposed to environmental pollutants.
Beijing is a heavily polluted city in China. This current analysis was based on a panel study conducted during the 2008 Beijing Olympics when there was a significant decline in air pollution levels and a later increase after the games (Mu et al., 2014). The current study hypothesizes that the antioxidant enzymes will respond to the drastic change of pollution levels during the three exposure periods.
2. Methods
2.1 Study design
This panel study was conducted during the 2008 Beijing Olympics and the details of the study design can be found elsewhere (Mu et al., 2014). Briefly, 201 participants, recruited from a community health service center in the Haidian district of Beijing, China, were interviewed during three visits: pre-Olympics (baseline), during-Olympics (1st follow-up), and post-Olympics (2nd follow-up). The study was approved by the Institutional Review Boards at the University at Buffalo and Peking University, China. Inclusion criteria included female and male participants ages 20 to 65 years old, of Han ethnicity, residing in the Haidian district for the past year, who did not have any prior chronic health conditions (Mu et al., 2014).
2.2 Data collection
During in-person interviews at each visit, participants completed questionnaires that collected information regarding social demographics, medical history, dietary and cooking habits, and occupational history. All the participants resided in the Beihang community of the Haidan district, which is a relatively small and closed community with an area of 0.27 km2. During the study period, particulate matter concentration was measured with a mass monitor (Met One® 531 AEROCET Particulate Profiler, Met One Instruments, Inc. Grant Pass, Oregon) located in the center of the community of Beihang. The monitor was positioned in an open space close to a main road. The monitor was placed 1.5 m above the ground and recorded PM1, PM2.5, PM7, PM10, and total suspended particulates (TSP) in duplicate, along with temperature and relative humidity, twice a day at 10:00 a.m. and 4:00 p.m. Air pollutant measures were recorded every four days over the study period. The concentration range was up to 1 mg/m3. The air pollution measurements were conducted on the dates of the blood sampling or 1–2 days before the blood sampling.
2.3 Blood collection
There were three waves of data collection among participants. The official period for the Olympic and Paralympics was from August 8, 2008 to September 17, 2008. Blood samples were collected 13–14 days before the Olympics (baseline), 29 days after the opening of the Olympics (first follow-up during the game), and 74 days after the last day of the Olympics (2nd follow-up).
The protocols were designed with oxidative stress metabolite analyses in mind to ensure the integrity and utility of the samples. All participants were asked to come for their visit without having breakfast. Participants were instructed to arrive at the center between 8–9 a.m. on the day of their interview. Prior to the physical examination, a blood sample was collected by a trained nurse under sterile and standardized conditions. All the samples were allowed to clot for 30 minutes at room temperature, separated by centrifugation and aliquoted before being stored in the freezer at −80°C. The samples were kept from light throughout the process.
2.4 Antioxidant enzymes
Glutathione S-transferases (GST), glutathione peroxidase (GPx), and glutathione reductase (GR) enzymes were analyzed using an automated enzyme kinetic methodology adapted to the Cobas Fara II automated, centrifugal analyzer (Pippenger et al., 1998). Total antioxidant status (TAS) was analyzed with the Randox TAS kit adapted to the Cobas Fara II autoanalyzer. Using the TAS kit (Randox Laboratories, Kearneysville, West Virginia), ABTS (2,2′-Azino-di-[3-ethylbenzthiazoline sulphonate]) is incubated with a peroxidase (metmyoglobin) and H2O2 to produce the radical cation ABTS+, which has a relatively stable blue-green color, and is measured at 600 nm. Antioxidants in the added sample cause suppression of this color production to a degree, which is proportional to their concentration (Miller et al., 1993).
2.5 Statistical analyses
Univariate analyses were performed to describe participant demographic information by age, sex, BMI, and smoking status among the 199 study participants (2 participants did not donate their blood samples). For continuous variables, means (SD) and their 95% confidence intervals were reported for GPx, GR, GST, and TAS. Categories were created based on their biological relevance and distribution: BMI <24 kg/m2 and ≥24 kg/m2 were based on the cut point for overweight status among Chinese and nonsmokers consisted of those who never smoked more than 100 cigarettes in their lifetime.
We used linear mixed-effects models to test the changes in the continuous antioxidant enzyme measures over the three periods to deal with the repeated measurements in this panel study. The subject ID was used to denote within-individual comparison in the models. In the analysis, period of the Olympics was used as the independent variable with time coded as 1, indicating pre-Olympics, 2 for the period during the Olympics, and 3, indicating the post-Olympics period. The correlated structure used was “unstructured”, meaning no special structure would be imposed and the correlation structure was completely determined by the data. The linear mixed-effects models presented comparisons between two periods, during- to pre, post- to during, and post- to pre-Olympics, but the present analysis only compares during- to pre- and post- to during-Olympics. We controlled for age, sex, smoking status, BMI status, and each of their interactions with the periods in the models. We used the SAS 9.4 (Cary, NC) statistical software to analyze our data.
3. Results
The temporal air quality control measures during the Beijing Olympics resulted in approximately 50–60% decline in air pollution levels compared to the pre-Olympic period, which returned to the pre-Olympic levels after the temporary measures ceased (Mu et al., 2014). The average PM10 levels were 127.8 μg/m3, 55.9 μg/m3 and 139.8 μg/m3, respectively, before, during and after the Beijing Olympics, while the average PM2.5 levels were 83.2 μg/m3, 32.7 μg/m3 and 45.7 μg/m3, respectively (Mu et al., 2014).
Mean antioxidant baseline measures are presented in Table 1 grouped by participant demographics. Mean glutathione peroxidase (GPx) was significantly higher in males, 947.70 U/L than females, 865.90 U/L (p<0.0001). Smokers had elevated GPx measures compared to nonsmokers, (930.90 U/L vs. 886.60 U/L), p=0.03. Smokers and older participants had higher mean TAS levels compared to nonsmokers and younger participants, p<0.05. However, no statistical differences were observed for GR and GST across the study subgroups of participants (Table 1).
Table 1.
Antioxidant biomarkers at baseline according to demographics of the study participants, Beijing 2008 (n=199).
| N | GPx, U/L Mean (SD) |
GR, U/L Mean (SD) |
GST, U/L Mean (SD) |
TAS, mmol/L Mean (SD) |
|
|---|---|---|---|---|---|
| Sex | |||||
| Female | 114 | 865.90 (153.00) | 6.45 (1.51) | 15.7 (4.2) | 0.90 (0.23) |
| Male | 85 | 947.70 (130.10) | 6.61 (1.58) | 15.7 (2.5) | 1.17 (0.30) |
| P value | <0.0001 | 0.48 | 0.85 | <0.0001 | |
| Age | |||||
| ≤40 | 39 | 826.67 (142.64) | 6.23 (1.61) | 14.69 (3.27) | 0.95 (0.30) |
| >40–50 | 67 | 902.52 (163.97) | 6.59 (1.45) | 15.70 (4.02) | 0.98 (0.30) |
| >50 | 93 | 930.66 (128.78) | 6.59 (1.57) | 15.98 (3.27) | 1.07 (0.27) |
| P value | 0.0010 | 0.42 | 0.16 | 0.04 | |
| Education | |||||
| Primary & illiterate | 66 | 902.66 (128.0) | 6.58 (1.61) | 15.21 (3.69) | 0.98 (0.27) |
| Middle School | 105 | 902.35 (168.99) | 6.45 (1.48) | 15.82 (3.68) | 1.01 (0.31) |
| ≥High school | 27 | 889.50 (116.55) | 6.65 (1.64) | 15.93 (2.71) | 1.12 (0.25) |
| P-value | 0.92 | 0.77 | 0.50 | 0.12 | |
| Smoking | |||||
| Nonsmoker | 135 | 886.60 (159.50) | 6.52 (1.59) | 15.49 (3.92) | 0.96 (0.26) |
| Smoker | 64 | 930.90 (119.50) | 6.51 (1.44) | 15.92 (2.63) | 1.14 (0.32) |
| P value | 0.03 | 0.97 | 0.37 | <0.0001 | |
| Alcohol | |||||
| Nondrinker | 130 | 890.00 (148.00) | 6.43 (1.56) | 15.90 (3.93) | 0.96 (0.27) |
| Drinker | 67 | 923.40 (149.50) | 6.70 (1.51) | 15.14 (2.69) | 1.12 (0.31) |
| P value | 0.14 | 0.25 | 0.11 | 0.0005 | |
| BMI | |||||
| <24 | 131 | 892.59 (148.01) | 6.37 (1.54) | 15.46 (3.61) | 1.00 (0.27) |
| 24–<28 | 53 | 931.80 (152.50) | 6.80 (1.36) | 15.69 (3.54) | 1.04 (0.32) |
| ≥28 | 15 | 863.10 (134.78) | 6.77 (1.99) | 16.92 (3.01) | 1.04 (0.38) |
| P value | 0.16 | 0.18 | 0.32 | 0.72 | |
Enzyme levels were compared across the three periods and reported in Table 2. Mean GPx level was 900.81 U/L, 798.67 U/L and 857.26 U/L before, during, and after the Olympics, respectively. During the Olympics, mean GPx decreased by 11.34% (95% CI: −12.87, −9.80%) then increased again by 7.34% (95% CI: 3.40, 11.28) after the Olympics, p<0.05. After adjusting for age, sex, smoking status, BMI, and their interaction with time-points, this finding remained statistically significant. Every 10 μg/m3 change in PM2.5 was associated with a 20.23 U/L change in GPx (95% CI: 17.39, 23.08) during the Olympics and a 44.86 U/L change in GPx (95% CI: 21.48, 68.24) after the Olympics. Total antioxidant status (TAS) levels also fluctuated over the three periods. During the Olympics, TAS activity decreased significantly by 6.24% (95% CI: −8.66, −3.82%). After the Olympics, TAS activity continued to decrease by 4.61% (95% CI: −7.22, −2.01%), p=0.04 (Table 2). Every 10 μg/m3 change in PM2.5 was associated with a 0.01 mmol/L (95% CI: 0.01, 0.02) change in TAS activity during the Olympics (Table 2). Similar patterns were observed for GST and GR. However, the findings were not statistically significant in both crude and adjusted models.
Table 2.
Changes of antioxidant biomarkers before, during, and after the 2008 Olympics in Beijing.
| Antioxidants | Before Olympics | During Olympics | After Olympics | |
|---|---|---|---|---|
| GPx (U/L) | Mean (95% CI) | 900.81 (880.04, 921.58) | 798.67 (776.93, 820.42) | 857.26 (835.13, 879.39) |
| Percent change across period (95% CI) | −11.34 (−12.87, −9.80) | 7.34 (3.40, 11.28) | ||
| Change per 10 μg/m3 of PM2.5 | 20.23 (17.39, 23.08) | 44.86 (21.48, 68.24) | ||
| Crude P-value | <.0001 | 0.0002 | ||
| Adjusted P-value1 | <.0001 | 0.0002 | ||
| GR (U/L) | Mean (95% CI) | 6.52 (6.30, 6.73) | 6.45 (6.19, 6.71) | 6.37 (6.14, 6.59) |
| Percent change across period (95% CI) | −1.08 (−5.33, 3.18) | −1.30 (−5.56, 2.96) | ||
| Change per 10 μg/m3 of PM2.5 | 0.014 (−0.04, 0.07) | −0.06 (−0.28, 0.15) | ||
| Crude P-value | 0.62 | 0.55 | ||
| Adjusted P-value1 | 0.66 | 0.53 | ||
| GST (U/L) | Mean (95% CI) | 15.63 (15.13, 16.13) | 15.27 (14.74, 15.81) | 15.54 (15.00, 16.07) |
| Percent change across period (95% CI) | −2.30 (−5.15, 0.56) | 1.74 (−2.13, 5.60) | ||
| Change per 10 μg/m3 of PM2.5 | 0.07 (−0.02, 0.16) | 0.20 (−0.25, 0.65) | ||
| Crude P-value | 0.12 | 0.38 | ||
| Adjusted P-value1 | 0.13 | 0.38 | ||
| TAS (mmol/L) | Mean (95% CI) | 1.01 (0.97, 1.06) | 0.95 (0.91, 0.99) | 0.91 (0.87, 0.94) |
| Percent change across period (95% CI) | −6.24 (−8.66, −3.82) | −4.61 (−7.22, −2.01) | ||
| Change per 10 μg/m3 of PM2.5 | 0.01 (0.01, 0.02) | −0.03 (−0.07, 0.00) | ||
| Crude P-value | <0.0001 | 0.06 | ||
| Adjusted P-value1 | <0.0001 | 0.04 | ||
Adjusted for age (≤50,>50), sex, smoking status (never, ever), BMI status (<24, ≥24) and their interaction terms with time-points.
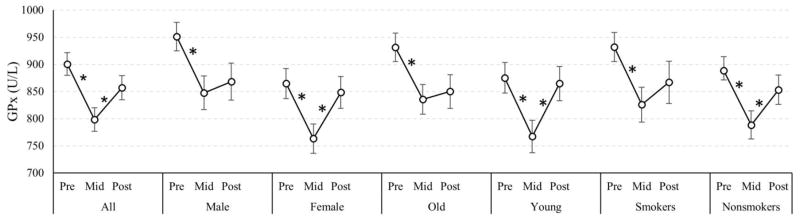
We further conducted stratified analyses to observe changes in enzyme levels across the periods by subgroups. Figure 1 shows the changes in GPx levels by subgroups of age, sex, and smoking status. In all participants, GPx levels decreased when air pollution levels declined but increased after the Olympics when air pollution levels rose. However, the responses after the Olympics differed among subgroups. The magnitude of increase among males, older participants, and smokers was smaller compared to females, younger participants, and nonsmokers. The changes in the former groups were not statistically significant (Figure 1).
Figure 1.
Glutathione Peroxidase. The levels of glutathione peroxidase (GPx) changes (U/L) over the study periods (pre, mid, post Olympics) in Beijing 2008, by age, sex and smoking status (n=199). “*” indicates statistical significance, P<0.05, and the bars above and below each mean represent the 95% confidence intervals.
Among males, GPx levels decreased from 951.35 U/L before the Olympics to 847.79 U/L during the Olympics, and the change was statistically significant (p<0.0001). However, after the Olympics GPx levels slightly increased to 868.25 U/L (95% CI: 834.16, 902.33), but the change did not reach statistical significance (Table A.1). Similar patterns were seen among smokers and older participants (Table A.2, Table A.3).
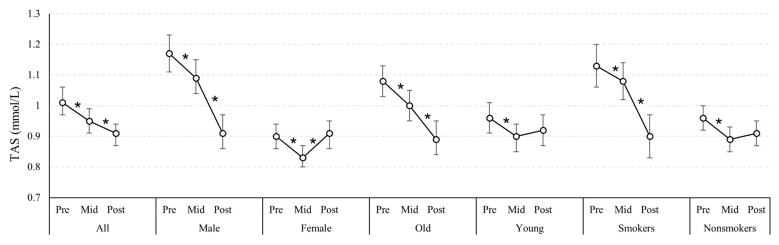
Among all the participants, the trend of TAS activity was different from GPx. TAS levels decreased during the Olympics and continued to decline when air pollution levels rose after the Olympics (Figure 2). Among younger participants, non-smokers, and females, TAS levels increased after the Olympics. However, among males, older participants, and smokers, TAS levels continued to decrease after the Olympics. The changes in TAS levels after the Olympics did not reach statistical significance among younger participants and nonsmokers (Figure 2). A significant interaction was observed between air pollution and sex, pinteraction<0.0001. Male TAS levels slightly decreased during the Olympics, from 1.17 mmol/L to 1.09 mmol/L, and kept decreasing when air pollution levels went up after the Olympics (0.91 mmol/L). However, TAS levels in females decreased during the Olympics from 0.90 mmol/L to 0.83 mmol/L, and then increased after the Olympics (0.91 mmol/L), pinteraction<0.0001 (Table A.1). A similar pattern for TAS was seen among smokers and nonsmokers (Table A.3).
Figure 2.
Total Antioxidant Status. The levels of glutathione peroxidase (TAS) changes (U/L) over the study periods (pre, mid, post Olympics) in Beijing 2008, by age, sex and smoking status (n=199). “*” indicates statistical significance, P<0.05, and the bars above and below each mean represent the 95% confidence intervals.
4. Discussion
The current panel study observed strong antioxidant responses, especially in GPx and TAS, to the drastic changes in air pollution levels over the three study periods. Among all participants, we observed that GPx activity declined when the air quality improved during the games then increased in response to higher air pollution levels after the Olympics. Overall, the GPx responses among subgroups were similar. However, males, older participants, and smokers had a smaller magnitude of increase in GPx levels after the Olympics compared to females, younger participants, and nonsmokers. Moreover, compared to the consistent drop of TAS levels during the games among all participants, TAS levels increased in response to the elevated air pollution levels after the Olympics among females, younger participants, and nonsmokers. However, it continued to decrease among males, older participants, and smokers after the Olympics. The current study did not find statistically significant responses in GR and GST to the changes in air pollution.
4.1 Glutathione peroxidase (GPx)
The antioxidant defense system is responsible for maintaining the balance of generated ROS. GPx is the major selenoenzyme antioxidant that is responsible for reducing lipid and hydrogen peroxides generated during periods of oxidative stress (Espinoza et al., 2008). Animal studies have shown that mice with knockout of GPx-1 do not survive long after exposure to paraquat or hydrogen peroxide. The study indicated that neurons in mice lacking GPx were more susceptible to H2O2-mediated toxicity compared to the wild-type (de Haan et al., 1998). These results support the notion that GPx is needed to deal with oxidative stress and eliminate ROS when exposed to high levels of air pollution.
In the current study, we observed males, older participants, and smokers had a relatively smaller magnitude of increase in GPx levels in response to the elevated air pollution levels after the games compared to females, younger participants, and nonsmokers. Findings from the literature have been diverse. A longitudinal study among older participants with coronary artery disease in Los Angeles, California, (≥65 years old), reported that PM2.5 and PM2.5–10 were inversely associated with GPx-1 (Delfino et al., 2009). The results are different with our findings among older healthy individuals (>50years old) who showed a weak however positive response to air pollution levels. The weaker response may be explained by chronic erosion of GPx activity in blood cells due to accumulated oxidative stress over time with aging and heavy environmental exposure (Delfino et al., 2009; Espinoza et al., 2008). However, healthier and younger individuals may have better antioxidant capabilities. This notion was supported in the same longitudinal study where a positive association between particulate matter and GPx was found among a healthy subgroup (Delfino et al., 2009).
We suspect that compared to healthy individuals, smokers and elderly individuals might have damaged antioxidant enzyme activity due to long-term oxidative stress and aging. This hypothesis was supported by previous experimental and human studies (Adiga, 2008; Andersen et al., 1997; Bentley et al., 2008; Metta et al., 2015; Van Eeden and Hogg, 2000). One study showed that chronic smoking exposure causes phenotypic changes in polymorphonuclear leukocytes leading to leukocytosis and neutrophilia (Van Eeden and Hogg, 2000). Moreover, chronic cigarette smoking might cause depletion of the antioxidant enzyme response in cells (Bikkad et al., 2014; Metta et al., 2015). Studies have also reported decreased activities of antioxidant enzymes including GPx, superoxide dismutase (SOD), and catalase in erythrocytes as a function of old age (Adiga, 2008; Andersen et al., 1997). Thus, smokers and elderly might have a blunted antioxidant response to environmental threats. In addition, those differed responses can be observed in a short time period. Delfino et al. (2009), found that compared to elderly individuals, a healthier subgroup of individuals presented a positive response in antioxidant activity to air pollution exposure over the previous 3–5 days.
The association between GPx activity and smoking has been inconsistent across epidemiological studies. Some studies have found lower GPx activity in smokers compared to nonsmokers (Bentley et al., 2008; Kluchova et al., 2007; Yildiz et al., 2002) while others have found GPx activity is higher among smokers (Brown et al., 1996). In our study, the blunted response in GPx in males (Fig. 1) may also be explained by the much higher proportion of current smokers in males, (54.12%) compared to that in females (4.35%). Among smokers, we also observed that GPx levels were higher than nonsmokers at baseline; however, their response to the increased air pollution levels after the Olympics was not as strong as that among nonsmokers. It has been hypothesized that the modification of the selenoenzymes inhibits the ability of GPx and other antioxidant enzymes, including Cn, Zn-SOD, to reduce increased formation of oxidative species (Staimer et al., 2012). Cigarette smoke and environmental pollutants contain toxic electrophilic carbonyls that can deactivate the nucleophilic active sites of selenocysteine enzymes like GPx-1 through covalent bonding, therefore preventing GPx from its antioxidant abilities (Staimer et al., 2012). This proposed mechanism lends support to our findings among smokers in the subgroup analysis that showed a relatively weaker GPx response to the increased air pollution levels after the Olympics compared to nonsmokers. It is also possible that smoking might mask some of the effect from the decrease in air pollution among smokers. However, we cannot make the conclusion based on our data. This needs to be investigated in further future experimental and human studies.
Evidence from animal and human studies have shown that environmental stressors can contribute to the depletion of antioxidant defense; therefore, a potential use of GPx-1 as a therapeutic target in vivo has been proposed (Vlahos and Bozinovski, 2013). A previous study reported the use of a GPx mimetic, Ebselen, which helped to resolve inflammation as a result of cigarette smoking in patients with COPD (Vlahos and Bozinovski, 2013). This would especially be important among smokers or elderly individuals who have a damaged GPx response.
4.2 Total antioxidant status (TAS)
Total antioxidant status is a marker of total antioxidant capacity and it provides an overall measure for the cumulative action of all antioxidants in the blood as opposed to the summing of all the measures of antioxidants, even ones that remain undiscovered (Bakhtiari et al., 2015; Ghiselli et al., 2000). In the current study, we found that when air pollution levels increased after the Olympics, males, smokers, and older participants had continued decreased activity of TAS, while females, nonsmokers, and younger participants had an increased response in TAS. The response seen in females, nonsmokers, and younger participants may be explained by the oxidant-antioxidant balance, also known as maintaining redox homeostasis after exposure to air pollution that generates increased ROS (Dröge, 2002).
Epidemiological studies regarding TAS and air pollution remain limited. One previous study in India reported increased ROS generation from exposure to biomass fuels but a depletion in TAS in women exposed to chronic biomass smoke (Mondal et al., 2010). In our current study, the subgroups of young, female and nonsmokers are relatively healthy, and therefore might have a more robust antioxidant defense mechanism to boost TAS levels and maintain the oxidative and anti-oxidative balance. However, other groups might have fairly damaged antioxidant defense systems due to aging or long-term smoking exposure (Nadeem et al., 2003). This finding is supported by other observational studies. A cross-sectional study found lower TAS levels among elderly who lived in Mexico City, since the inhabitants in this city had continuous high air pollution exposure for at least the past 10 years (Sánchez-Rodríguez et al., 2004). The researchers suggested that this was due to the antioxidant system becoming increasing overwhelmed by the over generation of free radicals, from high levels of air pollution (Sánchez-Rodríguez et al., 2004). Furthermore, the results for TAS levels among smokers follows the self-defense adaptation mechanism proposed in other studies. Cigarette smoking may constantly increase generation of ROS and lower antioxidant enzymatic activities. Therefore, smokers may have already depleted antioxidant abilities. Exposure to high air pollution levels again may further exacerbate the depletion of the antioxidant defense among smokers (Zalata et al., 2007). As seen in our study, elderly and smokers do not have the same capability as younger participants and nonsmokers to respond to the changes in air pollution because of their damaged antioxidant defense system.
To our knowledge, there are a limited number of human studies regarding glutathione reductase (GR) and glutathione-s-transferase (GST) activity in response to environmental exposures. One study found that participants exposed to environmental tobacco smoke in their workplace had a nonsignificant increase in GR activity by 4% (Howard et al., 1998). Another study in animal rats exposed to cigarette smoke for 12 weeks, found a significant decrease in antioxidant enzymes including SOD, catalase activity, and GPx and GR (Anbarasi et al., 2006). While we did not find a statistically significant relationship for GR and GST response to the changes in air pollution, GR activity showed a consistent drop across the three periods, while GST activity decreased during the Olympics but increased after the Olympics. In addition, our study only investigated the short-term response of these enzymes. GST and GR might be less sensitive and need a longer time to show significant responses to environmental pollutants. However, we cannot make any conclusion based on our results. The reason for the weaker response of GR and GST in our study remains unknown and needs to be explored further in future studies.
The current panel study has some limitations. The study did not have individual exposure measurements of air pollution, which would introduce misclassification of air pollution exposure. We only measured particulate matter in this study. However, air pollution is a complex mixture of various pollutants, which may have an interactive effect on antioxidant markers. Moreover, ambient temperature and humidity were not adjusted in the models since we only had one measure at each time point. Residual confounding may be a concern because of the categorization of smokers as “yes or no”, and smoking may not have been completely controlled for in the analyses. TAS assays may not be able to perfectly measure all antioxidant constituents (Sies, 2007) because potentially unknown molecules, unidentified pathways, or interactions that may contribute to total antioxidant status (Rubio et al., 2016). While we recognize its limitations, TAS can still be a useful tool for estimating redox status in vivo and in epidemiological studies with good sensitivity and specificity (Romieu et al., 2008), given that measuring each individual molecule is not always feasible (Ghiselli et al., 2000; Serafini and Del Rio, 2004). Like our present findings, a couple of epidemiological studies have also observed associations between traffic-related air pollution (Jiang et al., 2016), biomass fuels (Mondal et al., 2010), and response in TAS. In addition, among other oxidative stress related biomarkers measured in the parent study including biomarkers of DNA damage and lipid damage (MDA, 8-OHdG, and thiobarbituric acid reactive substances (TBARS)), TAS and GPx showed the strongest responses to the changes in air pollution levels. What we have found in this current study contributes to better understanding the role of antioxidant biomarkers in air pollution related biological responses. Lastly, besides glutathione enzymes, there are other antioxidant enzymes including thioredoxin reductase, catalase or hydrogen peroxide activity that may play a role in air pollution related antioxidant response, and need to be studied in the future.
Our current study also has many major strengths. First, we had repeated measurements of antioxidant enzymes and air pollution during a short time period, which allowed us to study the effect of short-term changes in particulate matter, from high to low levels during the Olympics and to high levels again after the Olympics. Second, the comparison within individuals allows for minimized confounding that may result from selecting a comparison that may not necessarily be comparable. Third, to our knowledge limited epidemiological studies exist regarding circulating antioxidant measures and their relationship with air pollution. Additionally, although several studies were conducted during the Beijing Olympics, we were the only study that measured antioxidant enzymes and total antioxidant status (Huang et al., 2012; Lin et al., 2015; Rich et al., 2012). Lastly, our study includes four measures of antioxidant defense biomarkers including TAS, which encapsulates the comprehensive effect of the antioxidant defense system in deactivating ROS within cells.
5. Conclusions
In conclusion, in this panel study, we observed significant responses in GPx and TAS enzymatic activity to the drastic changes in both directions of air pollution levels and those responses varied among subgroups. In response to increased air pollution levels after the Olympics, females, nonsmokers, and younger participants showed increased TAS activity, while males, smokers, and older participants showed a continuous decrease in TAS activity. The results among the subgroups are consistent with the hypothesis that high air pollution exposure generates ROS and may deplete antioxidants. Therefore, individuals with damaged antioxidant defense systems do not have the same capability to respond to threats from environmental pollutants, ultimately leading to oxidative damage. The antioxidant defense mechanism is critical in maintaining the balance between the overproduction and destruction of ROS. The potential role of antioxidant enzymes as a therapeutic target should be explored further, especially for individuals with damaged antioxidant defense systems.
Supplementary Material
Highlights.
Antioxidant enzyme activities were measured pre-, mid-, and post- 2008 Beijing Olympics.
GPx and TAS activity showed significant responses to changes in air quality.
Smokers and elderly had a blunted antioxidant response to altered air quality.
Acknowledgments
Source of funding:
This work was supported in part by the National Institute of Environmental Health Sciences grant awarded to Dr. Lina Mu (Grant number: R01ES018846, R21ES026429) and in part by Department of Epidemiology and Environmental Health, UB School of Public Health and Health Professions.
Footnotes
GST, glutathione S-transferases; GPx, glutathione peroxidase; GR, glutathione reductase; TAS, total antioxidant status; ROS, reactive oxygen species; GSSG, glutathione disulfide, PM, particulate matter.
The authors declare they have no actual or potential competing conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adiga U. Total Antioxidant Activity in old age. Biomedical Research. 2008:19. [Google Scholar]
- Anbarasi K, Vani G, Balakrishna K, Devi CS. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sciences. 2006;78:1378–1384. doi: 10.1016/j.lfs.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Andersen HR, Nielsen JB, Nielsen F, Grandjean P. Antioxidative enzyme activities in human erythrocytes. Clinical chemistry. 1997;43:562–568. [PubMed] [Google Scholar]
- Bakhtiari S, Azimi S, Mehdipour M, Amini S, Elmi Z, Namazi Z. Effect of cigarette smoke on salivary total antioxidant capacity. Journal of dental research, dental clinics, dental prospects. 2015;9:281. doi: 10.15171/joddd.2015.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud O, Greene AE, Li J, Wang H, Volpe JJ, Rosenberg PA. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. Journal of Neuroscience. 2004;24:1531–1540. doi: 10.1523/JNEUROSCI.3989-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley AR, Emrani P, Cassano PA. Genetic variation and gene expression in antioxidant-related enzymes and risk of chronic obstructive pulmonary disease: a systematic review. Thorax. 2008 doi: 10.1136/thx.2007.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikkad MD, Ghuge SH, Somwanshi SD, Ingle SB. Evaluation of Lipid Peroxide and Antioxidants in Smokers. International Journal of Basic and Applied Medical Sciences. 2014;4:1–6. [Google Scholar]
- Brown K, Morrice P, Arthur J, Duthie G. Effects of vitamin E supplementation on erythrocyte antioxidant defence mechanisms of smoking and non-smoking men. Clinical Science. 1996;91:107–111. doi: 10.1042/cs0910107. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. [59] Glutathione reductase. Methods in enzymology. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Ceballos-Picot I, Merad-Boudia M, Nicole A, Thevenin M, Hellier G, Legrain S, Berr C. Peripheral antioxidant enzyme activities and selenium in elderly subjects and in dementia of Alzheimer’s type—place of the extracellular glutathione peroxidase. Free Radical Biology and Medicine. 1996;20:579–587. doi: 10.1016/0891-5849(95)02058-6. [DOI] [PubMed] [Google Scholar]
- Davis CD, Uthus EO. Dietary folate and selenium affect dimethylhydrazine-induced aberrant crypt formation, global DNA methylation and one-carbon metabolism in rats. The Journal of nutrition. 2003;133:2907–2914. doi: 10.1093/jn/133.9.2907. [DOI] [PubMed] [Google Scholar]
- de Haan JB, Bladier C, Griffiths P, Kelner M, O’Shea RD, Cheung NS, Bronson R, Silvestro MJ, Wild S, Zheng SS. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. Journal of Biological Chemistry. 1998;273:22528–22536. doi: 10.1074/jbc.273.35.22528. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, Kleinman MT, Vaziri ND, Longhurst J, Sioutas C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environmental health perspectives. 2009;117:1232. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Vaziri ND. Air pollution and circulating biomarkers of oxidative stress. Air Quality, Atmosphere & Health. 2011;4:37–52. doi: 10.1007/s11869-010-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiological reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Emin O, Hasan A, Aysegul D, Rusen D. 5 Total Antioxidant Status and Oxidative Stress and Their Relationship to Total IgE Levels and Eosinophil Counts in Children With Allergic Rhinitis. Journal of Investigational Allergology and Clinical Immunology. 2012;22:188. [PubMed] [Google Scholar]
- Espinoza SE, Guo H, Fedarko N, DeZern A, Fried LP, Xue QL, Leng S, Beamer B, Walston JD. Glutathione peroxidase enzyme activity in aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63:505–509. doi: 10.1093/gerona/63.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga CG, Oteiza PI, Galleano M. In vitro measurements and interpretation of total antioxidant capacity. Biochimica et Biophysica Acta (BBA)-General Subjects. 2014;1840:931–934. doi: 10.1016/j.bbagen.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Franco MC, Kawamoto EM, Gorjão R, Rastelli VM, Curi R, Scavone C, Sawaya AL, Fortes ZB, Sesso R. Biomarkers of oxidative stress and antioxidant status in children born small for gestational age: evidence of lipid peroxidation. Pediatric Research. 2007;62:204–208. doi: 10.1203/PDR.0b013e3180986d04. [DOI] [PubMed] [Google Scholar]
- Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radical Biology and Medicine. 2000;29:1106–1114. doi: 10.1016/s0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Howard DJ, Ota RB, Briggs LA, Hampton M, Pritsos CA. Environmental tobacco smoke in the workplace induces oxidative stress in employees, including increased production of 8-hydroxy-2′-deoxyguanosine. Cancer Epidemiology and Prevention Biomarkers. 1998;7:141–146. [PubMed] [Google Scholar]
- Huang W, Wang G, Lu SE, Kipen H, Wang Y, Hu M, Lin W, Rich D, Ohman-Strickland P, Diehl SR. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. American journal of respiratory and critical care medicine. 2012;186:1150–1159. doi: 10.1164/rccm.201205-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Bo L, Gong C, Du X, Kan H, Xie Y, Song W, Zhao J. Traffic-related air pollution is associated with cardio-metabolic biomarkers in general residents. International archives of occupational and environmental health. 2016;89:911–921. doi: 10.1007/s00420-016-1129-3. [DOI] [PubMed] [Google Scholar]
- József L, Filep JG. Selenium-containing compounds attenuate peroxynitrite-mediated NF-κB and AP-1 activation and interleukin-8 gene and protein expression in human leukocytes. Free Radical Biology and Medicine. 2003;35:1018–1027. doi: 10.1016/s0891-5849(03)00439-8. [DOI] [PubMed] [Google Scholar]
- Kluchova Z, Petrasova D, Joppa P, Dorkova Z, Tkacova R. The association between oxidative stress and obstructive lung impairment in patients with COPD. Physiological Research. 2007;56:51. doi: 10.33549/physiolres.930884. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhu T, Xue T, Peng W, Brunekreef B, Gehring U, Huang W, Hu M, Zhang Y, Tang X. Association between changes in exposure to air pollution and biomarkers of oxidative stress in children before and during the Beijing Olympics. American journal of epidemiology. 2015;181:575–583. doi: 10.1093/aje/kwu327. [DOI] [PubMed] [Google Scholar]
- Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. Journal of toxicology. 2011 doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metta S, Basalingappa DR, Uppala S, Mitta G. Erythrocyte antioxidant defenses against cigarette smoking in ischemic heart disease. Journal of clinical and diagnostic research: JCDR. 2015;9:BC08. doi: 10.7860/JCDR/2015/12237.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical science. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- Mondal NK, Mukherjee B, Das D, Ray MR. Micronucleus formation, DNA damage and repair in premenopausal women chronically exposed to high level of indoor air pollution from biomass fuel use in rural India. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2010;697:47–54. doi: 10.1016/j.mrgentox.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Mu L, Deng F, Tian L, Li Y, Swanson M, Ying J, Browne RW, Rittenhouse-Olson K, Zhang JJ, Zhang ZF. Peak expiratory flow, breath rate and blood pressure in adults with changes in particulate matter air pollution during the Beijing Olympics: a panel study. Environmental research. 2014;133:4–11. doi: 10.1016/j.envres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. Journal of Allergy and Clinical Immunology. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- Nielsen PS, Okkels H, Sigsgaard T, Kyrtopoulos S, Autrup H. Exposure to urban and rural air pollution: DNA and protein adducts and effect of glutathione-S-transferase genotype on adduct levels. International archives of occupational and environmental health. 1996;68:170–176. doi: 10.1007/BF00381627. [DOI] [PubMed] [Google Scholar]
- Pippenger C, Browne RW, Armstrong D. Regulatory antioxidant enzymes. Free radical and antioxidant protocols. 1998:299–313. doi: 10.1385/0-89603-472-0:299. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. Jama. 2012;307:2068–2078. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. European Respiratory Journal. 2008;31:179–197. doi: 10.1183/09031936.00128106. [DOI] [PubMed] [Google Scholar]
- Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: an update. BMC veterinary research. 2016;12:166. doi: 10.1186/s12917-016-0792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rodríguez MA, Retana-Ugalde R, Ruiz-Ramos M, Mendoza-Núñez VM. Antioxidant capacity in relationship to serum lipid peroxides levels in healthy elderly of Mexico City. Acta bioquím clín latinoam. 2004;38:193–198. [Google Scholar]
- Seaton A, Godden D, MacNee W, Donaldson K. Particulate air pollution and acute health effects. The lancet. 1995;345:176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: is the total antioxidant capacity the right tool? Redox report. 2004;9:145–152. doi: 10.1179/135100004225004814. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: oxidants and antioxidants. Experimental physiology. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Sies H. Total antioxidant capacity: appraisal of a concept. The Journal of nutrition. 2007;137:1493–1495. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- Staimer N, Nguyen TB, Nizkorodov SA, Delfino RJ. Glutathione peroxidase inhibitory assay for electrophilic pollutants in diesel exhaust and tobacco smoke. Analytical and bioanalytical chemistry. 2012;403:431–441. doi: 10.1007/s00216-012-5823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological interactions. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Van Eeden S, Hogg J. The response of human bone marrow to chronic cigarette smoking. European Respiratory Journal. 2000;15:915–921. doi: 10.1034/j.1399-3003.2000.15e18.x. [DOI] [PubMed] [Google Scholar]
- Vlahos R, Bozinovski S. Glutathione peroxidase-1 as a novel therapeutic target for COPD. Redox Report. 2013;18:142–149. doi: 10.1179/1351000213Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz L, Kayaoğlu N, Aksoy H. The changes of superoxide dismutase, catalase and glutathione peroxidase activities in erythrocytes of active and passive smokers. Clinical chemistry and laboratory medicine. 2002;40:612–615. doi: 10.1515/CCLM.2002.106. [DOI] [PubMed] [Google Scholar]
- Zalata A, Yahia S, El-Bakary A, Elsheikha HM. Increased DNA damage in children caused by passive smoking as assessed by comet assay and oxidative stress. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2007;629:140–147. doi: 10.1016/j.mrgentox.2007.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.