Abstract
Objective
To evaluate the frequency and clinical significance of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes.
Study Design
Amniotic fluid (AF) was retrieved from both sacs in 90 twin gestations with preterm labor and intact membranes (GA between 20+0 and 34+6 weeks). Preterm labor was defined as the presence of painful regular uterine contractions, with a frequency of at least 2 every 10 minutes, requiring hospitalization. Fluid was cultured and assayed for matrix metalloproteinase-8. Intra-amniotic inflammation was defined as an AF matrix metalloproteinase-8 concentration >23 ng/mL.
Results
The prevalence of intra-amniotic inflammation of at least 1 amniotic sac was 39% (35/90), while that of proven intra-amniotic infection for at least 1 amniotic sac was 10% (9/90). Intra-amniotic inflammation without proven microbial invasion of the amniotic cavity was found in 29% (26/90) of cases. Intra-amniotic inflammation was present in both amniotic sacs in 22 cases, in the presenting amniotic sac in 12 cases, and in the non-presenting amniotic sac in 1 case. Women with intra-amniotic inflammation observed in at least 1 amniotic sac and a negative AF culture for microorganisms had a significantly higher rate of adverse pregnancy outcome than those with a negative AF culture and without intra-amniotic inflammation (lower gestational age at birth, shorter amniocentesis-to-delivery interval, and significant neonatal morbidity). Importantly, there was no significant difference in pregnancy outcomes between women with intra-amniotic inflammation and a negative AF culture and those with a positive AF culture.
Conclusion
Intra-amniotic inflammation is present in 39% of twin pregnancies with preterm labor and intact membranes and is a risk factor for impending preterm delivery and adverse outcome, regardless of the presence or absence of bacteria detected using cultivation techniques.
Keywords: intra-amniotic infection, intra-amniotic inflammation, matrix metalloproteinase-8, preterm labor, preterm birth, twin pregnancy
Introduction
The contribution of twin gestations to preterm birth continues to increase (1). The absolute number of multiple births has increased (2). In the United States of America alone, the frequency of multiple pregnancy increased 75%—from 19.3 to 33.7 per 1000 live births—between 1980 and 2006 (3).
Twin pregnancies have a six-fold higher perinatal mortality than singleton pregnancies (4), and preterm birth is the leading cause of perinatal morbidity and death in twins (5–9). Indeed, more than half of twins are born prior to 37 completed weeks of gestation (7–9), and the rate of early preterm birth (< 32 weeks’ gestation) is 12%, which is 7-fold higher than that of singleton pregnancies (10). Specifically, Martin et al.(3) reported that while the rate of preterm birth (defined as birth <37 weeks of gestation ) in singletons was 11.1%, that of multiple gestations was 61.9%. The most recent report suggests that the twin birth rate was 33.1 per 1000 live births in 2012 (11). About one-third of preterm birth (<32 and <37 weeks’ gestation) is caused by spontaneous preterm labor in twin gestations (4, 9).
Intra-amniotic infection is a well-established cause of spontaneous preterm delivery, and occurs in approximately 25% of women with a singleton gestation who deliver a preterm neonate (12). Its presence is a risk factor for impending preterm delivery (despite tocolytic administration) and adverse neonatal outcome (4, 12–19). Intra-amniotic inflammation is more common than intra-amniotic infection and carries similar risk for adverse pregnancy and neonatal outcomes (19–21). Only a fraction of patients with proven intra-amniotic inflammation have evidence of bacteria or viruses in the amniotic cavity and this underscores the importance of intra-amniotic inflammation by itself (19, 21–24).
There is no information about the prevalence and clinical significance of intra-amniotic inflammation in twin gestations presenting with preterm labor or the contribution of proven infection to intra-amniotic inflammatory processes in twins. This study was conducted to answer these questions.
Material and Methods
Study Population
The study population consisted of women with a twin gestation who were consecutively admitted to the Seoul National University Hospital with a diagnosis of preterm labor and intact membranes (<35 weeks’ gestation) and who underwent amniocentesis of both amniotic sacs for assessment of the microbiologic status of the amniotic cavity and/or fetal lung maturity between January 1993 and December 2012 (n=90). Patients with neonates who had major congenital anomalies (n=4) or unavailable data (n=2) were excluded from the analysis of neonatal outcome (n=84) (Figure 1). Preterm labor was defined as the presence of painful regular uterine contractions, with a frequency of at least 2 every 10 minutes, requiring hospitalization and by evaluation with amniocentesis of the microbial state of the amniotic cavity. The inclusion criteria were: (1) twin gestation with live fetuses; (2) preterm labor with intact membranes; (3) gestational age at amniocentesis between 20+0/7 and 34+6/7 weeks of gestation. Rupture of membranes was excluded by performing a sterile speculum examination and by testing for pooling and nitrazine (25–28). The standard clinical practice at our institution is to offer amniocentesis to all patients admitted with a diagnosis of preterm labor (including twins). Amniotic fluid (AF) was retrieved after written informed consent was obtained from each participant. The results of AF analysis (i.e. amniotic fluid white blood cell count and culture) were provided to the attending physicians. The administration of antibiotics and tocolytic agents was left to the discretion of the physician. The Institutional Review Board of Seoul National University Hospital approved the collection and use of these samples and information for research purposes. The Seoul National University Hospital has received a Federal Wide Assurance with the Office for Human Research Protection of the Department of Health and Human Services of the United States.
Figure 1.
Patient flow diagram
Amniotic fluid studies
AF was retrieved by transabdominal amniocentesis from both sacs under ultrasound guidance. Indigo carmine was injected after fluid was retrieved from the first sac to ensure sampling of both amniotic cavities when it was difficult to distinguish sonographically from one to the other amniotic sac. The AF was transported immediately to the Department of Laboratory Medicine of our hospital using specific transport media and cultured for aerobic and anaerobic bacteria and genital mycoplasmas (Ureaplasma species and Mycoplasma hominis), as previously described (29).
Microbial invasion of the amniotic cavity (MIAC) was defined as a positive AF culture for microorganisms. Fluid not used for clinical purposes was centrifuged and stored in polypropylene tubes at −70°C. After delivery, the stored AF was analyzed for matrix metalloproteinase (MMP)-8, which was measured using a commercially available enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis, MN, USA). Each measurement was performed in duplicate. Intra- and inter-assay coefficients of variation were <10% each. MMP-8 was used because previous studies indicated that it is a sensitive and powerful predictor of intra-amniotic infection and/or inflammation (20, 21, 28, 30–32). Intra-amniotic inflammation was defined as an elevated concentration of MMP-8 (>23 ng/mL), according to the results of multiple studies (20, 21, 31, 33).
Women were divided into 3 groups according to the presence or absence of MIAC and/or intra-amniotic inflammation: 1) Group 1: patients who did not have bacteria or intra-amniotic inflammation in either sac; 2) Group 2: patients with intra-amniotic inflammation in at least one amniotic sac, but no evidence of bacteria; and 3) Group 3: patients with intra-amniotic inflammation and bacteria in at least one amniotic sac. The primary outcome measures were amniocentesis to delivery interval within 2 and 7 days, and spontaneous preterm delivery at < 34 and < 36 weeks’ gestation. The secondary outcome measures were neonatal morbidity and death.
Diagnosis of acute histologic chorioamnionitis, funisitis, clinical chorioamnionitis and neonatal morbidity
Acute histologic chorioamnionitis was defined by the presence of acute inflammatory changes in the choriodecidua and amnion, respectively; acute funisitis was diagnosed by the presence of neutrophil infiltration into umbilical vessel walls or Wharton’s jelly using criteria previously published (29, 34–36). Clinical chorioamnionitis was diagnosed when maternal temperature was elevated to 37.8°C and when ≥ 2 of the following criteria were present: uterine tenderness, foul-smelling vaginal discharge, maternal leukocytosis (>15,000 cells/mm3), maternal tachycardia (>100 beats/min), and fetal tachycardia (> 160 beats/min) as proposed by Gibbs et al (37–43). Significant neonatal morbidity was defined as the presence of any of the following conditions: respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage (grade ≥ II), proven congenital neonatal sepsis, and necrotizing enterocolitis. These conditions were diagnosed according to definitions previously described in detail (44).
Statistical analysis
Proportions were compared with the use of Fisher’s exact tests. Kruskal-Wallis analysis of variance test was used for comparison of continuous variables among groups. Post hoc comparisons between groups were performed by using the Mann-Whitney U test. The McNemar test and the Wilcoxon signed rank test were used for correlated samples. The amniocentesis-to-delivery interval was compared by using the generalized Wilcoxon test for survival analysis. Cox proportional hazard modeling was used to adjust for covariates. The amniocentesis-to-delivery interval of women delivered for maternal or fetal indications was treated as a censored observation, with the censoring time equal to the amniocentesis-to-delivery interval. Generalized estimating equations were used to explore the association between the presence of intra-amniotic inflammation and neonatal outcomes, adjusting for confounding variables while accounting for the fact that twin neonates are not independent of each other. A probability value < 0.05 was considered as statistically significant. Multiple comparisons among 3 groups were adjusted using Bonferroni corrections to control for the type I error rate (results significant only when a probability value < .05/3 = .017). SPSS 22.0 for Windows (IBM, Armonk, NY, USA) was used for statistical analyses.
Results
Characteristics of study population
Amniocentesis was performed on 125 women with a twin pregnancy and preterm labor and intact membranes (Figure 1). Among them, AF was retrieved from only one sac due to technical difficulties or oligohydramnios in 27 women (2 women with a positive AF culture and 25 women with a negative culture). A total of 98 women met the inclusion criteria. AF was not available to determine MMP-8 concentrations in eight cases (one patient who had a positive AF culture and seven who had a negative culture for bacteria); therefore, these patients were excluded from further analysis because they could not be evaluated for the presence or absence of intra-amniotic inflammation.
Fifty-five women without microorganisms or intra-amniotic inflammation comprised Group 1, 26 women without microorganisms but with intra-amniotic inflammation of at least one amniotic sac comprised Group 2, and 9 women with intra-amniotic inflammation and microorganisms of at least one amniotic sac comprised Group 3.
Table 1 displays the clinical characteristics of the study population. Patients without microorganisms in either amniotic cavity but with intra-amniotic inflammation of at least one amniotic sac (Group 2) had a significantly lower gestational age at amniocentesis < 28 weeks and more advanced cervical dilatation than women without microorganisms or intra-amniotic inflammation (Group 1). There were no significant differences between women with intra-amniotic inflammation without detectable bacteria (Group 2) and those with intra-amniotic inflammation and microorganisms of at least one amniotic sac (Group 3).
Table 1.
Clinical characteristics of the study population
| Characteristics | Intra-amniotic inflammation (−)/ MIAC (−) (Group 1, n=55) |
P* | Intra-amniotic inflammation (+)/ MIAC (−) (Group 2, n=26) |
P† | Intra-amniotic inflammation (+)/ MIAC (+) (Group 3; n=9) |
P‡ |
|---|---|---|---|---|---|---|
| Maternal age, years§ | 31 (29–33) | <.001 | 34 (32–35) | NS | 32 (29–36) | NS |
| Nulliparity | 43/55 (78%) | NS | 9/26 (35%) | NS | 4/9 (44%) | NS |
| Gestational age at amniocentesis, weeks | 30.6 (28.6–32.2) | NS | 27.5 (25.1–33.3) | NS | 27.7 (23.9–33.0) | NS |
| Gestational age at amniocentesis < 28 weeks | 12/55 (22%) | .006 | 14/26 (45%) | NS | 5/9 (56%) | NS |
| Cervical dilatation, cm§ | 1.5 (0–3) | <.001 | 3.0 (1.5–4) | NS | 3.0 (1.5–5) | NS |
| Tocolytics use** | 50/53 (94%) | NS | 21/24 (88%) | NS | 5/9 (56%) | .006 |
| Corticosteroids use†† | 43/52 (83%) | NS | 14/18 (78%) | NS | 5/8 (63%) | NS |
| Clinical chorioamnionitis | 0/55 | NS | 0/26 | NS | 1/9 (11%) | NS |
Data presented as median (interquartile range) or n/N (%).
MIAC, microbial invasion of amniotic cavity; NS, not significant.
Comparison between groups 1 and 2.
Comparison between groups 2 and 3.
Comparison between groups 1 and 3.
P<.05 by Kruskal-Wallis ANOVA test.
Four women who were unavailable for information about use of tocolytics were excluded from the analysis.
Administration of antenatal corticosteroid was considered when gestational age was between 23+0 and 33+6 weeks.
Intra-amniotic inflammation
The frequency of intra-amniotic inflammation (defined as an MMP-8 concentration >23 ng/mL) of at least one amniotic sac was 39% (35/90). Intra-amniotic inflammation was present in all 9 patients (100%) with microorganisms in the amniotic fluid, and in 32% (26/81) of those without MIAC. The presenting amniotic sac had a higher rate of intra-amniotic inflammation than the non-presenting amniotic sac [presenting amniotic sac: 38% (34/90) vs. non-presenting amniotic sac: 26% (23/90); P = 0.002 using a McNemar test]. Intra-amniotic inflammation was present in both amniotic sacs in 22 cases, in the presenting amniotic sac alone in 12 cases, and in the non-presenting amniotic sac alone in one case.
The median AF MMP-8 concentration of the presenting amniotic sac was higher than the non-presenting amniotic sac [median, 3.0 ng/mL (IQR, 0.6–86.6 ng/mL) vs. median, 1.6 ng/mL (IQR, 0.4–23.6 ng/mL); P < 0.001; Wilcoxon signed rank test]. Among all patients with intra-amniotic inflammation involving at least one amniotic sac, regardless of the presence or absence of MIAC (n=35), the presenting sac had a higher median AF MMP-8 concentration than the non-presenting sac [median, 172.2 ng/mL (interquartile range, 65.1- 353.0 ng/mL) vs. median, 57.0 ng/mL (interquartile range, 13.9–237.4 ng/mL); P = 0.008; Wilcoxon signed rank test].
Microbial invasion of the amniotic cavity
The prevalence of positive amniotic fluid cultures for microorganisms for at least one amniotic sac was 10% (9/90). Microorganisms were isolated from both sacs in 6 of these 9 patients, and from the presenting amniotic sac alone in 3 cases. Ureaplasma species were the most common microorganisms isolated from the AF (from both sacs of 6 patients). Other microorganisms recovered from the AF included Mycoplasma hominis (co-isolated with Ureasplasma species in both amniotic sacs from 2 women), Group B Streptococcus (from the presenting amniotic sac in 1 patient), Streptococcus viridans (from the presenting amniotic sac in 1 patient), and Staphylococcus capitis (from the presenting amniotic sac in 1 patient).
Pregnancy outcomes of twins according to the presence or absence of intra-amniotic inflammation and/or infection
Figure 2 shows the amniocentesis-to-delivery interval in the three study groups. For 11 patients who were delivered due to maternal or fetal indications, this interval was censored. Patients with intra-amniotic inflammation without detectable bacteria (Group 2) had a significantly shorter median amniocentesis-to-delivery interval than patients without intra-amniotic inflammation and bacteria in AF (Group 1; P < 0.001); however, there was no difference in the median amniocentesis-to-delivery interval between women with intra-amniotic inflammation without detectable bacteria (Group 2) and those with intra-amniotic inflammation and microorganisms detected in at least one amniotic sac (Group 3).
Figure 2.
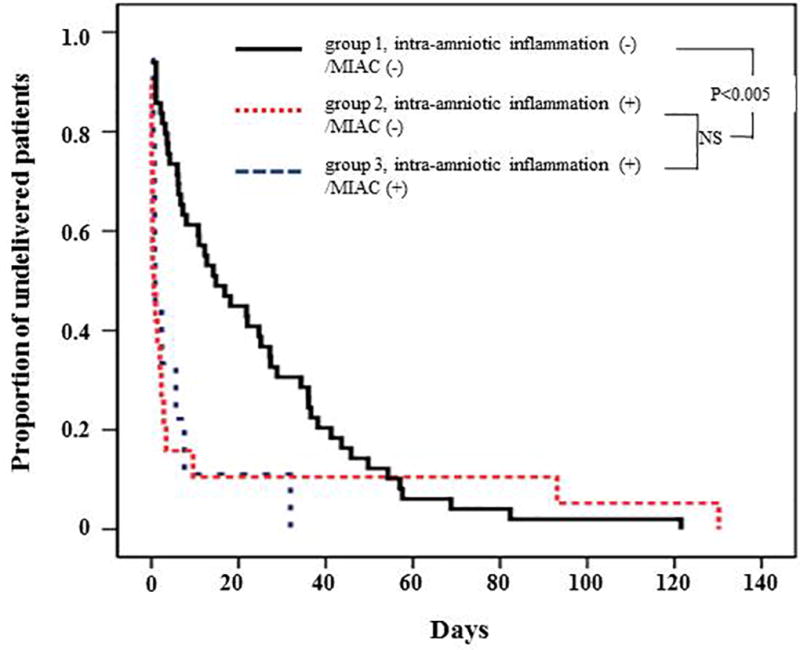
Survival analysis of the amniocentesis-to-delivery interval, according to the results of amniotic fluid (AF) culture and matrix metalloproteinase (MMP)-8 concentrations (group 1: median, 14 days [interquartile range, 4–36 days]; group 2: median, 1 day [interquartile range, 0–3 days]; group 3: median 1 day [interquartile range, 1–6 days]). Intra-amniotic inflammation was defined as an elevated amniotic fluid MMP-8 concentration (> 23 ng/mL) of at least 1 amniotic cavity, and microbial invasion of amniotic cavity (MIAC) was defined as a positive amniotic fluid culture of at least 1 amniotic cavity.
Table 2 shows the results of multivariate survival analysis. Patients with intra-amniotic inflammation in at least one amniotic sac, but without bacteria (Group 2) had a significantly shorter median amniocentesis-to-delivery interval than those without intra-amniotic inflammation or bacteria (Group 1) after adjusting for the gestational age at amniocentesis and cervical dilatation (hazard ratio, 4.14; 95% CI, 2.36–7.26; P < 0.001 by Cox proportional hazards model analysis).
Table 2.
Hazard ratios of various variables related to amniocentesis to delivery interval
| Characteristics | Unadjusted hazard ratio (95% CI) |
Adjusted hazard ratio (95% CI)* |
|---|---|---|
| Intra-amniotic inflammation and/or MIAC | ||
| Intra-amniotic inflammation (−)/ MIAC (−) (group 1) | Reference | Reference |
| Intra-amniotic inflammation (+)/ MIAC (−) (group 2) | 2.78 (1.67–4.62) | 4.14 (2.36–7.26) |
| Intra-amniotic inflammation (+)/ MIAC (+) (group 3) | 2.69 (1.25–5.80) | 4.48 (1.98–10.2) |
| Cervical dilatation | 1.61 (0.97–2.69) | 1.71 (1.01–2.89) |
| Gestational age at amniocentesis | 1.09 (1.01–1.18) | 1.17 (1.08–1.26) |
CI, confidence interval; MIAC, microbial invasion of amniotic cavity.
Adjusted for gestational age at amniocentesis, intra-amniotic inflammation, MIAC, and cervical dilatation (Cox proportional hazard model)
Table 3 compares the pregnancy outcomes of the study population after adjusting for gestational age at amniocentesis and cervical dilatation. Patients with intra-amniotic inflammation in at least one amniotic sac, but without bacteria (Group 2) had a significantly higher rate of spontaneous delivery within 1, 2 and 7 days of amniocentesis than patients without intra-amniotic inflammation or bacteria (Group 1) (adjusted odds ratio [aOR], 6.81; 95% confidence interval [CI], 2.05–22.6; P=0.002 for spontaneous delivery within 1 day: aOR, 11.2; 95% CI, 3.40–36.6; P < 0.001 for spontaneous delivery within 2 days: aOR, 19.8; 95% CI, 4.29–91.3; P < 0.001 for spontaneous delivery within 7 days: aOR, 5.23; 95% CI, 1.29–21.2, respectively).
Table 3.
Pregnancy outcomes of study population
| Characteristics | Intra-amniotic inflammation (−)/ MIAC (−) (Group 1, n=55) |
P* | Adjusted P*,§ |
Intra-amniotic inflammation (+)/ MIAC (−) (Group 2, n=26) |
P† | Intra-amniotic inflammation (+)/ MIAC (+) (Group 3; n=9) |
P‡ | Adjusted P‡,§ |
|---|---|---|---|---|---|---|---|---|
| Gestational age at delivery, weeks**,†† | 34.0 (31.4–35.1) | .004 | - | 30.2 (26.1–34.6) | NS | 28.4 (25.9–33.1) | .003 | - |
| Spontaneous delivery | ||||||||
| <24 hours | 6/54 (11%) | .001 | .002 | 12/26 (46%) | NS | 5/9 (56%) | .006 | .003 |
| <48 hours | 7/54 (13%) | <.001 | <.001 | 16/26 (62%) | NS | 5/9 (56%) | .009 | .007 |
| <7 days | 17/53 (32%) | <.001 | <.001 | 23/26 (89%) | NS | 7/9 (78%) | .021 | .017 |
| Spontaneous preterm delivery | ||||||||
| <34+0 weeks‡‡ | 23/50 (46%) | .003 | .021 | 18/21 (86%) | NS | 7/8 (88%) | NS | NS |
| <36+0 weeks | 40/49 (81%) | NS | NS | 25/26 (96%) | NS | 8/8 (100%) | NS | NS |
| Acute histologic chorioamnionitis | 12/46 (26%) | NS | NS | 11/21 (52%) | NS | 5/8 (63%) | NS | NS |
| Funisitis | 2/47 (4%) | NS | NS | 4/21 (19%) | NS | 4/8 (50%) | .003 | .003 |
Data presented as median (interquartile range) or n/N (%)
MIAC, microbial invasion of amniotic cavity; NS, not significant.
Comparison between groups 1 and 2.
Comparison between groups 2 and 3.b
Comparison between groups 1 and 3.
Adjusted for gestational age at amniocentesis and the cervical dilatation (logistic regression analysis).
Two women whose gestational age at delivery were not known were excluded.
P<.05 by Kruskal-Wallis ANOVA test.
Cases with gestational age at amniocentesis ≥ 34+0 weeks were excluded from the analysis.
Neonatal outcome of twins
Table 4 compares the neonatal outcomes among the three study groups. Neonates born to mothers who had intra-amniotic inflammation of at least one amniotic sac without MIAC (Group 2) had a significantly higher rate of adverse neonatal outcome (neonatal death and/or any significant morbidity) than those born to mothers without intra-amniotic inflammation or MIAC (Group 1), even after adjusting for gestational age at amniocentesis and cervical dilatation (P = 0.007).
Table 4.
Neonatal outcomes of study population
| Characteristics | Intra-amniotic inflammation (−)/ MIAC (−) (Group 1, n=102) |
P* | Adjusted P*,§ |
Intra-amniotic inflammation (+)/ MIAC (−) (Group 2, n=48) |
P† | Intra-amniotic inflammation (+)/ MIAC (+) (Group 3; n=18) |
P‡ | Adjusted P‡,§ |
|---|---|---|---|---|---|---|---|---|
| Neonatal death | 5/102 (5%) | NS | NS | 6/48 (13%) | NS | 4/18 (22%) | NS | NS |
| Significant morbidity**,†† | 19/101 (19%) | .006 | .012 | 21/42 (50%) | NS | 8/15 (53%) | NS | NS |
| Respiratory distress syndrome | 13/101 (13%) | NS | NS | 5/42 (12%) | NS | 3/15 (20%) | NS | NS |
| Bronchopulmonary dysplasia | 10/97 (10%) | .002 | .015 | 18/42 (43%) | NS | 4/15 (27%) | NS | NS |
| Intraventricular hemorrhage | 8/101 (8%) | NS | NS | 2/42 (5%) | NS | 4/15 (27%) | NS | NS |
| Necrotizing enterocolitis | 3/101 (3%) | NS | NS | 4/42 (10%) | NS | 0/15 | NS | NS |
| Proven early neonatal sepsis | 1/101 (1%) | NS | NS | 1/42 (2%) | NS | 0/15 | NS | NS |
| Neonatal death and/or any significant morbidity | 21/102 (21%) | .002 | .007 | 27/48 (56%) | NS | 11/18 (61%) | .011 | NS |
Data presented as n/N (%).
MIAC, microbial invasion of amniotic cavity; NS, not significant.
Comparison between groups 1 and 2.
Comparison between groups 2 and 3.b
Comparison between groups 1 and 3.
Adjusted for gestational age at amniocentesis and the cervical dilatation (generalized estimation equation models).
Defined as the presence of any of the following conditions: proven early neonatal sepsis, respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage, and necrotizing enterocolitis.
Ten cases were excluded from the analysis because they died shortly after delivery as a result of extreme prematurity and thus could not be evaluated with respect to the presence or absence of neonatal morbidity.
Discussion
Principal findings of the study
(1) Intra-amniotic inflammation was present in 39% of twin pregnancies with preterm labor and intact membranes; (2) the diagnosis of intra-amniotic inflammation regardless of the presence or absence of microorganisms, is a risk factor for impending preterm delivery and adverse neonatal outcome; (3) intra-amniotic inflammation was more common in the presenting amniotic sac than in the non-presenting sac; (4) 63% (22/35) of twin gestations had intra-amniotic inflammation in both amniotic sacs; and (5) 67% (6/9) of twin gestations had bacteria isolated from both amniotic sacs.
Intra-amniotic inflammation in twin gestations with preterm labor
A role for intra-amniotic inflammation in the pathogenesis of preterm labor and subsequent preterm birth in singleton gestations is well-established (15, 19, 21, 28, 34, 41, 43, 45–70). However, the clinical significance of this condition in twin gestations has not been determined. Previous studies focus only on the presence of microorganisms in the amniotic cavity or MIAC (25, 71). We report herein for the first time that the prevalence of intra-amniotic inflammation for at least one amniotic sac is 39% (35/90), and bacteria could be identified in the amniotic fluid of 26% of those cases (9/35). Therefore, intra-amniotic inflammation is common in twin gestations complicated by spontaneous preterm labor. These findings are consistent with observations on singleton gestations for which the prevalence of intra-amniotic inflammation was 31% (63/206), of which 30% (19/63) were associated with the presence of bacteria in the amniotic cavity (19). Collectively, these data suggest that intra-amniotic inflammation is frequent in patients with preterm labor in both singleton and twin gestations, and that only a small percentage of those are associated with the presence of bacteria.
Although inflammation has traditionally been attributed to infection, a growing body of evidence suggests that there are non-microbial causes of inflammation, including danger signals released under conditions of cellular stress or necrosis (15, 24, 72). The precise nature of these danger signals has not been established, but alarmins have been implicated through the activation of the inflammasome family of pathways (73–76).
Concordance/discordance of intra-amniotic inflammation in twin gestation
Concordance of the presence of intra-amniotic inflammation in both amniotic sacs occurred in 63% (22/35) of cases. Of the remaining 13 cases, the inflammatory process was detectable in the presenting sac except for one case. The predominant involvement of the presenting sac suggests that the causative factor for intra-amniotic inflammation resides in the lower genital tract (i.e. bacteria ascending from the vagina or cervix), or that the danger signal responsible for inflammation affects the lower uterine pole. The observation that one patient had intra-amniotic inflammation in the non-presenting amniotic sac was unexpected. Possible explanations include difficulty in identifying the presenting sac in some cases of twin gestations or the mislabeling of a specimen.
Importance of intra-amniotic inflammation for twin gestations with preterm labor
The importance of intra-amniotic inflammation is underscored because it is a risk factor for impending preterm birth and adverse neonatal outcome in both singleton and twin gestations with preterm labor and intact membranes. Indeed, patients with intra-amniotic inflammation were more likely to deliver <24 hours, <48 hours, or <7 days than those without inflammation. Interestingly, there was no difference in the interval-to-delivery between patients with intra-amniotic inflammation with and without bacteria in the amniotic cavity. Moreover, the presence of intra-amniotic inflammation was a risk factor for adverse neonatal outcome, regardless of whether or not bacteria were detected in the amniotic fluid (see Table 4).
The subclinical nature of intra-amniotic inflammation is underscored by the fact that only 2.85% percent (one of 35 patients) with this condition met the clinical criteria for chorioamnionitis. Therefore, amniotic fluid analysis is key in assessing the presence of inflammation or infection in the amniotic cavity. A non-invasive test to detect intra-amniotic infection/inflammation is highly desirable, but despite many efforts such method is not yet available. Twin gestations represent a unique challenge because of the need to assess the inflammatory and microbial state of two amniotic sacs. The availability of rapid tests, such as those designed for the determination of MMP-8 and IL-6, allows the diagnosis of intra-amniotic inflammation (32, 59, 61,77–83).
Intra-amniotic infection in twin gestations with preterm labor
The prevalence of MIAC of at least one amniotic sac was 10% (9/90) and in all cases, involved the presenting sac. The microorganisms isolated included Ureaplasma species, Mycoplasma hominis, Streptococcus agalactiae, and Streptococcus viridans. That genital Mycoplasmas were the most common microorganisms involved is in keeping with previous reports on patients with preterm labor and a singleton gestation (27, 84–86), preterm PROM (22, 87–92), sonographic short cervix (93), and acute cervical insufficiency (20, 94, 95). The reasons for the overrepresentation of genital Mycoplasmas in intra-amniotic infection remain unknown. These microorganisms are frequently present in the lower genital tract of women (20, 96–102). Why genital Mycoplasmas ascend into the amniotic cavity rather than other commensal organisms remains an unanswered and important question. Virulence factors may explain the invasiveness of some microorganisms and not others. Indeed, we recently identified a gene in Mycoplasma hominis that was associated with infection of the amniotic cavity and preterm birth (103). Importantly, the severity of the intra-amniotic inflammatory response when infections are caused by genital mycoplasmas is more severe than that observed with other organisms (91). This has important implications for the understanding of the biology of infection in the fetus and the neonate.
The finding that 10% of patients have MIAC is in keeping with a previous study of 46 women with a twin gestation reported more than two decades ago (71). Genital Mycoplasmas were the most frequent isolates, and the presenting sac was involved in all cases, suggesting an ascending pathway for intrauterine infection. The inflammatory state of the amniotic cavity was not assessed in the prior study, and this is the novel aspect of the study reported herein.
Strengths and limitations
This is the first study to report the frequency and intensity of intra-amniotic inflammation in patients with a twin gestation. The strengths of this study are the analysis of both amniotic cavities and the relatively large sample size. A limitation of the study is that the results of the white blood cell counts and AF cultures were used in patient management. However, it would not be possible to ethically withhold information about the presence/absence of intra-amniotic infection because it may pose a risk for both the mother and her fetuses. We hope that future studies will evaluate whether knowledge of MIAC and/or intra-amniotic inflammation might better inform clinical decisions regarding use of antibiotics in selected women with preterm labor. In the current study, there was no difference in pregnancy and neonatal outcomes between patients with intra-amniotic inflammation with or without evidence of bacteria (groups 2 and 3). This might be attributed to the small sample size of groups 2 and 3 (n=26 for group 2 and n=9 for group 3). The current study used cultivation techniques, long regarded as the gold standard for the detection of bacteria. This is in keeping with the standard of medicine worldwide. Molecular microbiological techniques had been studied by our group extensively; however, they had not been developed when this study began in 1993. Moreover, the understanding of the conditions required to handle specimens in a manner that will preclude contamination when obtaining the sample at the bedside or handling the specimens in the laboratory has continued to evolve. Thus, we elected not to perform molecular microbiological studies with broad primers (e.g. 16S RNA genes etc.) because of concern with false-positive results and their interpretation. The net result is that we report a minimum estimate of the burden of the intra-amniotic infection in patients with a twin gestation and preterm labor. We used stringent criteria and excluded patients with incomplete information to focus on the question on concordance and discordance of intra-amniotic inflammation and intra-amniotic infection in twin gestation. We consider this a major strength of the study.
Current thoughts about the mechanisms responsible for preterm labor in twins and higher order gestations
A fundamental adaptation of mammalian pregnancy is that the uterine myocytes undergo hypertrophy and hyperplasia in order to accommodate the growth of the fetus, placenta, and amniotic fluid (104, 105). Pregnant women with a multiple gestation (1, 2), polyhydramnios (often associated with congenital anomalies such as esophageal atresia) (106, 107) or certain uterine anomalies, such as a unicornuate uterus, have an increased risk for preterm delivery (108, 109). Therefore, a belief has been established in reproductive biology that there are inherent limitations to the dimensions a pregnant uterus can tolerate before mechanical signals induce labor (92). Indeed, decades ago, a pharmacologic increase in intra-amniotic pressure with the injection of hypertonic saline (110) or glucose (111) was used to induce labor/mid-trimester abortion before the availability of effective uterotonic agents, such as prostaglandins, for this indication (112). Yet, elucidation of the precise mechano-receptors and effectors of the forces responsible for the control of uterine contractility on the conditions of uterine stretch, (which should be properly referred to as biomechanical “stress”) has proven to be a challenge (113).
An increase in intra-amniotic volume induced either spontaneously in patients with congenital anomalies or by the administration of fluid within the amniotic cavity does not reliably result in an increase in intra-amniotic pressure. Myometrial relaxation in response to the increase volume load has been reported and attributed to agents such as nitric oxide. Finally, the epidemiologic relationship between the number of fetuses and the mean gestational age at birth reported in higher order multiple gestations is confounded by the increased frequency of complications of such pregnancies (e.g., preeclampsia and small for gestational age (SGA)), which may lead to medically indicated preterm delivery and may not necessarily reflect the preterm onset of labor.
The relationship between increased uterine stress (force per unit area) and parturition has been studied in human (114, 115) and non-human primates (113). In rodents (e.g. rat) with a bicornuate uterus, it is possible to generate an animal model of uterine overdistension (116). After unilateral tubal ligation to limit pregnancy to one horn, a plastic tube can be inserted in the non-gravid horn to examine the effect of increasing uterine volume. Natural experiments are also possible in some species, e.g., the tammar wallaby (a marsupial) in which only one uterine horn becomes pregnant (117). The laboratory of Steven Lye (116), as well as that of Parry and Bathgate (117), used these experimental paradigms to gain insights into the understanding of the role of mechanical signals in pregnancy and parturition. Overexpression of the chemokine monocyte chemotactic protein-1 (MCP-1), also known as CCL2 (chemokine [C-C motif] ligand 2), during the course of uterine overdistension, along with the oxytocin receptor and connexin 43, were discovered through these studies (117–120). Given the major endocrinological differences between non-human primates and rodents, Waldorf et al. has recently developed a unique animal model of uterine overdistension and preterm labor in pigtail macaques (Macaca nemestrina) (113). Two key observations from these studies are that inflation of intra-amniotically placed balloon is associated with increased uterine wall stress and that the deployment of an “inflammatory pulse” characterized by an increase in the AF concentrations of interleukin (IL)-1beta, tumor necrosis factor (TNF)-alpha, IL-6, IL-8, CCL2, prostaglandin E2, and prostaglandin F2-alpha. The investigators also demonstrated that similar changes can be induced in the myometrium of a twin gestation with early labor and in amniocytes subjected to mechanical stretch in vitro. Importantly, transcriptome analysis reveals similarities between the pattern of gene expression observed in women with polyhydramnios and twins compared to those observed in non-human primates after intra-amniotic balloon inflation (113). Collectively, these observations provide strong evidence for a relationship between an early inflammatory event after mechanical stress of the uterus and preterm labor (provided that magnitude of the stress was sufficient). The roled of different transcription factors, such as activator protein (AP)-1 and NF-κB (121), as well as other important signaling processes, including the c-Jun N-terminal kinase (JNK) and extracellular signal-regulated protein kinase (ERK), and p38 pathways (122) and caspase 3 (123) in the mechanisms responsible for uterine overdistension and perhaps preterm labor in twin gestation require further investigation (119, 124–126).
The complexity of preterm labor in twin gestation is such that this condition is not one disorder (50, 127, 128). Indeed, microbiologic studies reported in the last two decades as well as the present study have provided objective evidence of clinical heterogeneity (23, 24, 85, 90, 129–137). Intra-amniotic infection affects a subset of pregnancies with twin gestation presenting with preterm labor and such pregnancies cannot be considered equivalent to those without intra-amniotic infection. This proposition—equivalence of intra-amniotic inflammation/infection versus the absence of these conditions—would not be tenable based on what is known about the clinical presentation (15, 19, 46, 138, 139), cytokine network (140), lipidomics (including prostaglandins and other products of arachidonic acid metabolism, such as LTB-4) (141), placenta pathology (34, 142–146), and neonatal outcome (29, 135, 147–152).
To attribute most cases of preterm labor in twin gestation to uterine overdistension appears to be an oversimplification, as even in control experiments in non-human primates, the effect of uterine wall tension is variable (113). In the present study, nearly 40% of patients with preterm labor have objective evidence of intra-amniotic inflammation at the time of presentation and its mere presence is a poor prognostic sign (even in the absence of proven infection). Whether the intra-amniotic inflammatory process that we observe resembles the one observed in singleton gestation or reflects part of the spectrum of the “inflammatory pulse” reported by Waldorf et al. remains to be determined (113). It is now possible to characterize the proteome, lipidome, metabolome, and network analysis to elucidate differences and similarities between those patients who deliver preterm and those who deliver at term (63, 140, 141, 153–158). This will provide the first glance at the in vivo spontaneous “inflammatory pulse” reported by Waldorf et al. in non-human primates.
Since preterm parturition is a syndrome caused by multiple etiologies, we are open to identify other mechanisms of disease that may operate in premature labor in the complexities of placentation and biology of twin gestation. For example, discrepancies in the frequency of villitis of unknown etiology, chronic chorioamnionitis, and chronic deciduitis—lesions which had been interpreted to reflect maternal anti-fetal rejection—have been observed to be more prevalent in twin gestation (159). It would be surprising if the most common mechanism of disease in the late preterm gestation would not be operative in a subset of patients with preterm labor and twin gestation. Studies are needed to examine the placental pathology of twin gestation after preterm labor to gain insight into other pathological processes potentially responsible for preterm labor in multiple gestation.
Clinical implications
The major conclusion of this study is that the frequency of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes is similar to that of singleton gestation with preterm labor and intact membranes. Importantly, the presence of intra-amniotic inflammation is a risk factor for preterm delivery and adverse neonatal outcome, regardless of the presence or absence of bacteria detected using cultivation techniques. We envision that a rational assessment of the patient with an episode of suspected preterm labor in a twin gestation would involve systematic analysis of amniotic fluid. Recent observations that an episode of suspected preterm labor, even in the absence of preterm delivery, is a risk factor for SGA, and possibly neurodevelopmental handicap, gives further strength to the evaluation of the patients with a twin gestation (160).
Acknowledgments
This work was supported in part by the Seoul National University Bundang Hospital Research Fund (Grant No. 09-2015-003), in part, by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services, and, in part, with Federal funds under Contract No. HHSN275201300006C.
Footnotes
Declaration of interest
All authors declare no conflicts of interest.
References
- 1.Bateni ZH, Clark SL, Sangi-Haghpeykar H, et al. Trends in the delivery route of twin pregnancies in the United States, 2006–2013. Eur J Obstet Gynecol Reprod Biol. 2016;205:120–126. doi: 10.1016/j.ejogrb.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ. Three decades of twin births in the United States, 1980–2009. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Sutton PD, et al. National vital statistics reports. 7. Vol. 57. Hyattsville, MD: National Center for Health Statistics; 2009. Births: final data for 2006. 55, no. 7. [Google Scholar]
- 4.Gardner MO, Goldenberg RL, Cliver SP, et al. The origin and outcome of preterm twin pregnancies. Obstet Gynecol. 1995;85(4):553–557. doi: 10.1016/0029-7844(94)00455-M. [DOI] [PubMed] [Google Scholar]
- 5.Sung JH, Kim SH, Kim YM, et al. Neonatal outcomes of twin pregnancies delivered at late-preterm versus term gestation based on chorionicity and indication for delivery. J Perinat Med. 2016;44(8):903–911. doi: 10.1515/jpm-2015-0401. [DOI] [PubMed] [Google Scholar]
- 6.Rao A, Sairam S, Shehata H. Obstetric complications of twin pregnancies. Best Pract Res Clin Obstet Gynaecol. 2004;18(4):557–576. doi: 10.1016/j.bpobgyn.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Kogan MD, Alexander GR, Kotelchuck M, et al. Trends in twin birth outcomes and prenatal care utilization in the United States, 1981–1997. JAMA. 2000;284(3):335–341. doi: 10.1001/jama.284.3.335. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan SP, Scardo JA, Hayes E, et al. Twins: prevalence, problems, and preterm births. Am J Obstet Gynecol. 2010;203(4):305–315. doi: 10.1016/j.ajog.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Ananth CV, Joseph KS, Demissie K, et al. Trends in twin preterm birth subtypes in the United States, 1989 through 2000: impact on perinatal mortality. Am J Obstet Gynecol. 2005;193(3 Pt 2):1076–1082. doi: 10.1016/j.ajog.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 10.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2004. Natl Vital Stat Rep. 2006;55(1):1–101. [PubMed] [Google Scholar]
- 11.Martin JA, Hamilton BE, Osterman MJK, et al. National vital statistics reports. 9. Vol. 62. Hyattsville, MD: National Center for Health Statistics; 2013. Births: final data for 2012. 62, no 9. [PubMed] [Google Scholar]
- 12.Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8(1):3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 13.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42(1):1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gravett MG, Hummel D, Eschenbach DA, et al. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986;67(2):229–237. doi: 10.1097/00006250-198602000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Espinoza J, Gonçalves LF, et al. Inflammation in preterm and term labour and delivery. Semin Fetal Neonat Med. 2006;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and Gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169(4):805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 17.Torricelli M, Voltolini C, Conti N, et al. Inflammatory and infectious risk factors are associated with the response to tocolysis in patients with preterm labor. J Matern Fetal Neonatal Med. 2011;24(1):43–46. doi: 10.3109/14767058.2010.482614. [DOI] [PubMed] [Google Scholar]
- 18.Watts DH, Krohn MA, Hillier SL, et al. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79(3):351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185(5):1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Romero R, Park CW, et al. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198(6):633.e1–633.e8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191(4):1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28(12):1394–1409. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17. doi: 10.3109/14767058.2014.954243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intraamniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–474. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazor M, Hershkovitz R, Ghezzi F, et al. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand. 1996;75(7):624–627. doi: 10.3109/00016349609054686. [DOI] [PubMed] [Google Scholar]
- 26.Yoon BH, Yang SH, Jun JK, et al. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol. 1996;87(2):231–237. doi: 10.1016/0029-7844(95)00380-0. [DOI] [PubMed] [Google Scholar]
- 27.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92(1):77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 28.Maymon E, Romero R, Chaiworapongsa T, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185(5):1149–1155. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 29.Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172(3):960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 30.Angus SR, Segel SY, Hsu CD, et al. Amniotic fluid matrix metalloproteinase-8 indicates intra-amniotic infection. Am J Obstet Gynecol. 2001;185(5):1232–1238. doi: 10.1067/mob.2001.118654. [DOI] [PubMed] [Google Scholar]
- 31.Park JS, Romero R, Yoon BH, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185(5):1156–1161. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 32.Chaiyasit N, Romero R, Chaemsaithong P, et al. Clinical chorioamnionitis at term VIII: A rapid MMP-8 test for the identification of intra-amniotic inflammation. J Perinat Med. 2017;45(5):539–550. doi: 10.1515/jpm-2016-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Romero R, Kim SM, et al. A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM. J Matern Fetal Neonatal Med. 2016;29(17):2727–2737. doi: 10.3109/14767058.2015.1103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim CJ, Romero R, Chaemsaithong P, et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–S52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J, Park JW, Kim BJ, et al. Funisitis is more common in cervical insufficiency than in preterm labor and preterm premature rupture of membranes. J Perinat Med. 2016;44(5):523–529. doi: 10.1515/jpm-2015-0123. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term VI: Acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med. 2016;44(1):33–51. doi: 10.1515/jpm-2015-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibbs RS. Diagnosis of intra-amniotic infection. Semin Perinatol. 1977;1(1):71–77. [PubMed] [Google Scholar]
- 38.Gibbs RS, Blanco JD, St Clair PJ, et al. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145(1):1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol. 1980;136(6):709–713. doi: 10.1016/0002-9378(80)90445-7. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: Microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43(1):19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016;44(1):5–22. doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazaki-Tovi S, Vaisbuch E. Clinical chorioamnionitis - an ongoing obstetrical conundrum. J Perinat Med. 2016;44(1):1–4. doi: 10.1515/jpm-2015-0366. [DOI] [PubMed] [Google Scholar]
- 43.Oh KJ, Kim SM, Hong JS, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol. 2017;216(6):604.e1–604.e11. doi: 10.1016/j.ajog.2017.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182(3):675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 45.Casey ML, Cox SM, Beutler B, et al. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J Clin Invest. 1989;83(2):430–436. doi: 10.1172/JCI113901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez R, Ghezzi F, Romero R, et al. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22(2):281–342. [PubMed] [Google Scholar]
- 47.Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol. 2001;6(1):153–163. doi: 10.1902/annals.2001.6.1.153. [DOI] [PubMed] [Google Scholar]
- 48.Abrahams VM, Bole-Aldo P, Kim YM, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173(7):4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 49.Vogel I, Thorsen P, Curry A, et al. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):516–525. doi: 10.1111/j.0001-6349.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 50.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLOS One. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friel LA, Romero R, Edwin S, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35(5):385–393. doi: 10.1515/JPM.2007.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21(8):529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamill N, Romero R, Gotsch F, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36(3):217–227. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holst RM, Laurini R, Jacobsson B, et al. Expression of cytokines and chemokines in cervical and amniotic fluid: relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med. 2007;20(12):885–893. doi: 10.1080/14767050701752601. [DOI] [PubMed] [Google Scholar]
- 56.Kusanovic JP, Romero R, Mazaki-Tovi S, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21(12):902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazaki-Tovi S, Romero R, Kusanovic JP, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008;36(6):485–496. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nhan-Chang CL, Romero R, Kusanovic JP, et al. A role for CXCL13 (BCA-1) in pregnancy and intraamniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21(11):763–775. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J, Oh KJ, Yang HJ, et al. The importance of intraamniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med. 2009;22(10):917–923. doi: 10.1080/14767050902994705. [DOI] [PubMed] [Google Scholar]
- 60.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16(1):56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 61.Park CW, Lee SM, Park JS, et al. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36(6):497–502. doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cruciani L, Romero R, Vaisbuch E, et al. PENTRAXIN 3 in amniotic fluid: a novel association with intra-amniotic infection and inflammation. J Perinat Med. 2010;38(2):161–171. doi: 10.1515/JPM.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero R, Mazaki-Tovi S, Vaisbuch E, et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med. 2010;23(12):1344–1359. doi: 10.3109/14767058.2010.482618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soto E, Romero R, Richani K, et al. Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J Matern Fetal Neonatal Med. 2009;22(11):983–992. doi: 10.3109/14767050902994747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–125.e15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 66.Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40(4):329–343. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiefer DG, Peltier MR, Keeler SM, et al. Efficacy of midtrimester short cervix interventions is conditional on intraamniotic inflammation. Am J Obstet Gynecol. 2016;214(2):276.e1–276.e6. doi: 10.1016/j.ajog.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Madan I, Romero R, Kusanovic JP, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38(3):275–279. doi: 10.1515/JPM.2010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manuck TA, Esplin MS, Biggio J, et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol. 2015;212(4):487.e1–487.e11. doi: 10.1016/j.ajog.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim SM, Romero R, Lee J, et al. About one-half of early spontaneous preterm deliveries can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. J Matern Fetal Neonatal Med. 2016;29(15):2414–2422. doi: 10.3109/14767058.2015.1094049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romero R, Shamma F, Avila C, et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol. 1990;163(3):757–761. doi: 10.1016/0002-9378(90)91063-i. [DOI] [PubMed] [Google Scholar]
- 72.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24(12):1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21(9):605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plazyo O, Romero R, Unkel R, et al. HMGB1 induces an inflammatory response in the chorioamniotic membranes that is partially mediated by the inflammasome. Biol Reprod. 2016;95(6):130. doi: 10.1095/biolreprod.116.144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gomez-Lopez N, Romero R, Xu Y, et al. A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci. 2017;24(6):934–953. doi: 10.1177/1933719116675058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romero R, Xu Y, Plazyo O, et al. A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol. 2016 doi: 10.1111/aji.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197(3):292.e1–292.e5. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 78.Nien JK, Yoon BH, Espinoza J, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol. 2006;195(4):1025–1030. doi: 10.1016/j.ajog.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 79.Chaemsaithong P, Romero R, Docheva N, et al. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2017:1–17. doi: 10.1080/14767058.2017.1281904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J Matern Fetal Neonatal Med. 2015;28(13):1510–1519. doi: 10.3109/14767058.2014.961417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29(3):360–367. doi: 10.3109/14767058.2015.1006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med. 2016;29(3):349–359. doi: 10.3109/14767058.2015.1006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kacerovsky M, Musilova I, Stepan M, et al. Detection of intraamniotic inflammation in fresh and processed amniotic fluid samples with the interleukin-6 point of care test. Am J Obstet Gynecol. 2015;213(3):435–436. doi: 10.1016/j.ajog.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 84.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189(4):919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 85.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161(3):817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 86.Kim SM, Romero R, Lee J, et al. Gastric fluid versus amniotic fluid analysis for the identification of intraamniotic infection due to ureaplasma species. J Matern Fetal Neonatal Med. 2016;29(16):2579–2587. doi: 10.3109/14767058.2015.1098614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gauthier DW, Meyer WJ. Comparison of Gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol. 1992;167(4 Pt 1):1092–1095. doi: 10.1016/s0002-9378(12)80044-5. [DOI] [PubMed] [Google Scholar]
- 88.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and Gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169(4):839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 89.Averbuch B, Mazor M, Shoham-Vardi I, et al. Intrauterine infection in women with preterm premature rupture of membranes: maternal and neonatal characteristics. Eur J Obstet Gynecol Reprod Biol. 1995;62(1):25–29. doi: 10.1016/0301-2115(95)02176-8. [DOI] [PubMed] [Google Scholar]
- 90.Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179(5):1254–1260. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 91.Oh KJ, Lee KA, Sohn YK, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203(3):211.e1–211.e8. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82(5):423–431. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 93.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34(1):13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oh KJ, Lee SE, Jung H, et al. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38(3):261–268. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Romero R, Gonzalez R, Sepulveda W, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167(4 Pt 1):1086–1091. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 96.Redelinghuys MJ, Ehlers MM, Dreyer AW, et al. Antimicrobial susceptibility patterns of ureaplasma species and Mycoplasma hominis in pregnant women. BMC Infect Dis. 2014;14:171. doi: 10.1186/1471-2334-14-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marovt M, Keše D, Kotar T, et al. Ureaplasma parvum and Ureaplasma urealyticum detected with the same frequency among women with and without symptoms of urogenital tract infection. Eur J Clin Microbiol Infect Dis. 2015;34(6):1237–1245. doi: 10.1007/s10096-015-2351-8. [DOI] [PubMed] [Google Scholar]
- 98.Chaban B, Links MG, Jayaprakash TP, et al. Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome. 2014;2:23. doi: 10.1186/2049-2618-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis. 2013;26(3):231–240. doi: 10.1097/QCO.0b013e328360db58. [DOI] [PubMed] [Google Scholar]
- 100.Donders GGG, Ruban K, Bellen G, et al. Mycoplasma/Ureaplasma infection in pregnancy: To screen or not to screen. J Perinat Med. 2017;45(5):505–515. doi: 10.1515/jpm-2016-0111. [DOI] [PubMed] [Google Scholar]
- 101.Kwak DW, Cho HY, Kwon JY, et al. Usefulness of maternal serum C-reactive protein with vaginal Ureaplasma urealyticum as a marker for prediction of imminent preterm delivery and chorioamnionitis in patients with preterm labor or preterm premature rupture of membranes. J Perinat Med. 2015;43(4):409–415. doi: 10.1515/jpm-2014-0142. [DOI] [PubMed] [Google Scholar]
- 102.Musilova I, Pliskova L, Kutova R, et al. Ureaplasma species and Mycoplasma hominis in cervical fluid of pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2016;29(1):1–7. doi: 10.3109/14767058.2014.984606. [DOI] [PubMed] [Google Scholar]
- 103.Allen-Daniels MJ, Serrano MG, Pflugner LP, et al. Identification of a gene in Mycoplasma hominis associated with preterm birth and microbial burden in intraamniotic infection. Am J Obstet Gynecol. 2015;212(6):779.e1–779.e13. doi: 10.1016/j.ajog.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reynolds SR. The relation of hydrostatic conditions in the uterus to the size and shape of the conceptus during pregnancy; a concept of uterine accommodation. Anat Rec. 1946;95:283–296. doi: 10.1002/ar.1090950303. [DOI] [PubMed] [Google Scholar]
- 105.Reynolds SR. Uterine accommodation of the products of conception; physiologic considerations. Am J Obstet Gynecol. 1947;53(6):901–913. doi: 10.1016/s0002-9378(16)39767-8. [DOI] [PubMed] [Google Scholar]
- 106.Houben CH, Curry JI. Current status of prenatal diagnosis, operative management and outcome of esophageal atresia/tracheo-esophageal fistula. Prenat Diagn. 2008;28(7):667–675. doi: 10.1002/pd.1938. [DOI] [PubMed] [Google Scholar]
- 107.Odibo IN, Newville TM, Ounpraseuth ST, et al. Idiopathic polyhydramnios: persistence across gestation and impact on pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol. 2016;199:175–178. doi: 10.1016/j.ejogrb.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 108.Hiersch L, Yeoshoua E, Miremberg H, et al. The association between Mullerian anomalies and short-term pregnancy outcome. J Matern Fetal Neonatal Med. 2016;29(16):2573–2578. doi: 10.3109/14767058.2015.1098613. [DOI] [PubMed] [Google Scholar]
- 109.Fox NS, Roman AS, Stern EM, et al. Type of congenital uterine anomaly and adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2014;27(9):949–953. doi: 10.3109/14767058.2013.847082. [DOI] [PubMed] [Google Scholar]
- 110.Ballard CA, Quilligan EJ. Mid-trimester abortion with intraamniotic saline and intravenous oxytocin. Obstet Gynecol. 1973;41(3):447–450. [PubMed] [Google Scholar]
- 111.Brosset A. The induction of therapeutic abortion by means of a hypertonic glucose solution injected into the amniotic sac. Acta Obstet Gynecol Scand. 1958;37(4):519–525. doi: 10.3109/00016345809160060. [DOI] [PubMed] [Google Scholar]
- 112.Roth-Brandel U, Bygdeman M, Wiqvist N, et al. Prostaglandins for induction of therapeutic abortion. Lancet. 1970;1(7639):190–191. doi: 10.1016/s0140-6736(70)90427-7. [DOI] [PubMed] [Google Scholar]
- 113.Adams Waldorf KM, Singh N, Mohan AR, et al. Uterine overdistention induces preterm labor mediated by inflammation: observations in pregnant women and nonhuman primates. Am J Obstet Gynecol. 2015;213(6):830.e1–830.e19. doi: 10.1016/j.ajog.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fisk NM, Ronderos-Dumit D, Tannirandorn Y, et al. Normal amniotic pressure throughout gestation. Br J Obstet Gynaecol. 1992;99(1):18–22. doi: 10.1111/j.1471-0528.1992.tb14385.x. [DOI] [PubMed] [Google Scholar]
- 115.Deyer TW, Ashton-Miller JA, Van Baren PM, et al. Myometrial contractile strain at uteroplacental separation during parturition. Am J Obstet Gynecol. 2000;183(1):156–159. doi: 10.1067/mob.2000.105819. [DOI] [PubMed] [Google Scholar]
- 116.Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138(12):5398–5407. doi: 10.1210/endo.138.12.5624. [DOI] [PubMed] [Google Scholar]
- 117.Parry LJ, Bathgate RA. The role of oxytocin and regulation of uterine oxytocin receptors in pregnant marsupials. Exp Physiol. 2000;85:91S–99S. doi: 10.1111/j.1469-445x.2000.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 118.Hua R, Pease JE, Sooranna SR, et al. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-jB activation. Endocrinology. 2012;153(1):481–491. doi: 10.1210/en.2011-1506. [DOI] [PubMed] [Google Scholar]
- 119.Shynlova O, Lee YH, Srikhajon K, et al. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 120.Lee YH, Shynlova O, Lye SJ. Stretch-induced human myometrial cytokines enhance immune cell recruitment via endothelial activation. Cell Mol Immunol. 2015;12(2):231–242. doi: 10.1038/cmi.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.MacIntyre DA, Lee YS, Migale R, et al. Activator protein 1 is a key terminal mediator of inflammation-induced preterm labor in mice. FASEB J. 2014;28(5):2358–2368. doi: 10.1096/fj.13-247783. [DOI] [PubMed] [Google Scholar]
- 122.Oldenhof AD, Shynlova OP, Liu M, et al. Mitogen-activated protein kinases mediate stretch-induced cfos mRNA expression in myometrial smooth muscle cells. Am J Physiol Cell Physiol. 2002;283(5):C1530–C1539. doi: 10.1152/ajpcell.00607.2001. [DOI] [PubMed] [Google Scholar]
- 123.Kyathanahalli C, Organ K, Moreci RS, et al. Uterine endoplasmic reticulum stress-unfolded protein response regulation of gestational length is caspase-3 and −7-dependent. Proc Natl Acad Sci USA. 2015;112(45):14090–14095. doi: 10.1073/pnas.1518309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeyasuria P, Wetzel J, Bradley M, et al. Progesterone-regulated caspase 3 action in the mouse may play a role in uterine quiescence during pregnancy through fragmentation of uterine myocyte contractile proteins. Biol Reprod. 2009;80(5):928–934. doi: 10.1095/biolreprod.108.070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lei K, Georgiou EX, Chen L, et al. Progesterone and the repression of myometrial inflammation: the roles of MKP-1 and the AP-1 system. Mol Endocrinol. 2015;29(10):1454–1467. doi: 10.1210/me.2015-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mendelson CR, Montalbano AP, Gao L. Fetal-to-maternal signaling in the timing of birth. J Steroid Biochem Mol Biol. 2017;170:19–27. doi: 10.1016/j.jsbmb.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gotsch F, Gotsch F, Romero R, et al. The preterm parturition syndrome and its implications for understanding the biology, risk assessment, diagnosis, treatment and prevention of preterm birth. J Matern Fetal Neonatal Med. 2009;22(Suppl2):5–23. doi: 10.1080/14767050902860690. [DOI] [PubMed] [Google Scholar]
- 129.Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12(4):262–279. [PubMed] [Google Scholar]
- 130.Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163(3):968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 131.Romero R, Avila C, Santhanam U, et al. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85(5):1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165(4 Pt 1):821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 133.Gomez R, Romero R, Ghezzi F, et al. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 134.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179(1):186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 135.Yoon BH, Romero R, Kim KS, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181(4):773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 136.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynaecol. 2007;50(3):652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 137.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71(4):330–358. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gomez R, Romero R, Edwin SS, et al. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11(1):135–176. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 139.Romero R, Espinoza J, Gonçalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213(6):836.e1–836.e18. doi: 10.1016/j.ajog.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maddipati KR, Romero R, Chaiworapongsa T, et al. Lipidomic analysis of patients with microbial invasion of the amniotic cavity reveals up-regulation of leukotriene B4. FASEB J. 2016;30(10):3296–3307. doi: 10.1096/fj.201600583R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim CJ, Yoon BH, Romero R, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol. 2001;185(2):496–500. doi: 10.1067/mob.2001.116689. [DOI] [PubMed] [Google Scholar]
- 143.Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11(1):18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 144.Park CW, Moon KC, Park JS, et al. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications. Placenta. 2009;30(1):56–61. doi: 10.1016/j.placenta.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23(7):1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim CJ, Romero R, Chaemsaithong P, et al. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213(4Suppl):S53–S69. doi: 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174(5):1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 148.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177(1):19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 149.Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183(5):1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 150.Korzeniewski SJ, Romero R, Cortez J, et al. A “multihit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J Perinat Med. 2014;42(6):731–743. doi: 10.1515/jpm-2014-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sorokin Y, Romero R, Mele L, et al. Umbilical cord serum interleukin-6, C-reactive protein, and myeloperoxidase concentrations at birth and association with neonatal morbidities and long-term neurodevelopmental outcomes. Am J Perinatol. 2014;31(8):717–726. doi: 10.1055/s-0033-1359723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim SM, Romero R, Park JW, et al. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med. 2015;28(13):1500–1509. doi: 10.3109/14767058.2014.961009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab. 2002;87(6):2431–2434. doi: 10.1210/jcem.87.6.8689. [DOI] [PubMed] [Google Scholar]
- 154.Bujold E, Romero R, Kusanovic JP, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern Fetal Neonatal Med. 2008;21(10):697–713. doi: 10.1080/14767050802053289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Romero R, Espinoza J, Rogers WT, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med. 2008;21(6):367–388. doi: 10.1080/14767050802045848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Romero R, Kusanovic JP, Gotsch F, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/ inflammation. J Matern Fetal Neonatal Med. 2010;23(4):261–280. doi: 10.3109/14767050903067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oggé G, Romero R, Lee DC, et al. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. J Pathol. 2011;223(4):553–565. doi: 10.1002/path.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee J, Romero R, Chaiworapongsa T, et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am J Reprod Immunol. 2013;70(4):265–284. doi: 10.1111/aji.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bang H, Bae GE, Park HY, et al. Chronic placental inflammation in twin pregnancies. J Pathol Transl Med. 2015;49(6):489–496. doi: 10.4132/jptm.2015.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Paules C, Pueyo V, Martí E, et al. Threatened preterm labor is a risk factor for impaired cognitive development in early childhood. Am J Obstet Gynecol. 2017;216(2):157.e1–157.e7. doi: 10.1016/j.ajog.2016.10.022. [DOI] [PubMed] [Google Scholar]


