Abstract
Maintaining the vitality of the dental pulp, the highly innervated and highly vascular, innermost layer of the tooth, is a critical goal of any dental procedure. Upon injury, targeting the pulp with specific therapies is challenging because it is encased in hard tissues. This project describes a method that can effectively deliver therapeutic agents to the pulp. This method relies on the use of nanoparticles that can be actively steered using magnetic forces to the pulp, traveling through naturally occurring channels in the dentin (the middle layer of the tooth). This method can reduce the inflammation of injured pulp and improve the penetration of dental adhesives into dentin. Such a delivery method would be less expensive, and both less painful and less traumatic than existing therapeutic options available for treatment of injured dental pulp. This technique would be simple and could be readily translated to clinical use.
Keywords: Magnet, nanoparticles, drug delivery, pulp, composite
Graphic Abstract
In this manuscript, technology to steer and deliver drug-laden nanoparticles to the tooth pulp is described. This technology exploits naturally occurring dentinal tubules that extend from the dentin to the pulp, and magnetic forces to actively steer iron nanoparticles deep into the tooth structure. This technology was tested on rat molar teeth and in freshly extracted human teeth and can be used to deliver drug-laden nanoparticles to the pulp, or to enhance the bond strength of commercially available resin adhesives to the tooth dentin.
Introduction
Dentinal tubules are microscopic channels that extend outwards, through dentin, from the dental pulp (soft, inner layer of the tooth) to the enamel border (hard, outer layer). In humans, these tubules are 0.3 – 2 μm in diameter and usually taper and may exhibit branching as they approach the pulp. Dentinal tubules are abundant in dentin (middle layer) and their density ranges from 10 – 30 tubules per 100 μm2 of dentin 1. Dentinal tubules serve as an important route for the delivery of nutrients to the dentin from the pulp and contain: dentinal fluids, un-mineralized collagen, processes of cells that deposit dentin (odontoblasts), sensory nerve terminals and immunoglobulins and complement proteins that assist with the defense against microorganisms 1. Dentists recognized the importance of these tubules decades ago and it is through these dentinal tubules that micromechanical retention of esthetic composite resin restorations is achieved 1, 2. It is also through these tubules that the by-products of bacterial fermentation attack the highly innervated and highly vascular, innermost layer of the tooth, the tooth pulp, and consequently, cause pulpitis and consequently dental pain 3, 4.
Maintaining pulp vitality is a goal of any procedure on the tooth and a healthy pulp is necessary for tooth nutrition, innervation, and immunocompetency 5. However, since the pulp is encased in hard tissues, targeting pulpal tissues with specific therapies is challenging. Systemic pharmacologic treatments are not effective because they do not effectively reach the pulp and topical drug delivery would be more concentrated, more beneficial and would have a more favorable side-effect profile 6.
Dentists have tried, with mixed results, passive diffusion and iontophoresis to propel charged minerals and therapeutic agents including potassium, fluoride, and bisphosphonates to reduce tooth sensitivity and treat invasive idiopathic resorption of the tooth 7–11.
Here, we describe and test an active therapeutic agent delivery method to the dental pulp. We exploit dentinal tubules and use external magnetic forces to transport drug-eluting nanoparticles through the dentinal tubules to the pulp. Unlike diffusion, which is a passive process, magnetic forces can be arranged to act in one direction; they can actively transport substantially more drug to a target than either diffusion or iontophoresis 12–14, and can be designed/constructed so as to release drugs over an extended period of time. This gentle, non-invasive and active delivery method may prove effective in introducing therapeutic agents into the pulp, along with other dental applications that involve dentinal tubules.
Methods
Nanoparticles
To deliver prednisolone to the pulp, commercially available chitosan-coated nanoparticles (300 nm, chemicell nano-screenMAG/G-Chitosan-Prednisolone, lot 1704/15, size 300nm, 25mg/ml) were used. These particles were applied to experimental cavities in rat mandibular molar teeth (1ul/per tooth).
To improve penetration of resin adhesive to the pulp, iron nanoparticles fabricated by reduction method were used. These particles were produced by reducing iron (II) in the aqueous solution of iron (II) chloride tetrahydrate by sodium borohydride with the addition of polyvinylpyrrolidone (PVP, Mw=40,000) as stabilizing ligands in a flask. The iron nanoparticles were collected by placing a magnet beneath the flask and washed with water to remove PVP. The nanoparticles were then coated with silica via the hydrolysis of tetraethyl orthosilicate (TEOS) in ethanol. A solution of methacryloxypropyltrimethoxysilate (KH570) in ethanol was then added into the reaction to introduce acrylate functional groups onto silica-coated nanoparticles. A magnet was used to collect the acrylate-functionalized magnetic nanoparticles. These nanoparticles were dispersed into a commercially available adhesive resin (Scotch Bond MultiPurpose, 3M) with the aid of a mechanical mixer (DAC 150 Speed mixer, Flacktek, Landrum, SC, USA). The control adhesive did not include any nanoparticles. For the restoration, composite resin (TPH, Caulk/Dentsply, Milford, DE) was used. All procedures involving adhesive resin and composite resin were carried out under safe yellow light.
Animal anesthesia and preparation
All experiments involving animals were performed under a University of Maryland Baltimore approved IACUC protocol and in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Adult male and female Long Evans Rats (n=81; Charles River Laboratories) were used. Animals were anesthetized using ketamine/xylazine (100/10 mg/kg, I.P.) or isoflurane (5% for induction and 1–3% for maintenance). Anesthetized animals were placed on a surgical table on a regulated heating blanket in the supine position. A small horizontal metal bar secured to the surgical table was placed on top of the mandibular incisors and used to stabilize the mandible. A stereotaxic incisor holder was attached to the maxillary incisors and used to open the mouth. A microscope was used to visualize the teeth during preparation. A high speed dental hand piece was used to prepare rat mandibular molars. Irrigation with cold, sterile isotonic saline was applied every 5 seconds during preparation to avoid excessive heat and suction was applied at the same time to prevent saline from penetrating the animal’s airway. A round carbide bur was used (0.4 mm in diameter). The cavity preparation extended 0.25 mm into dentin. The cavities were left exposed for two weeks before nanoparticle treatment.
Nanoparticle application in rats
Animals were anesthetized using ketamine/xylazine (100/10 mg/kg, I.P.) or isoflurane (5% for induction and 1–3% for maintenance). The teeth were washed and cleaned thoroughly from debris. A magnet (maximum internal field:1.4T, surface field: 0.54T; BX0X0X0-N52; K&J Magnetics, Inc.) was placed under the mandible and prednisolone-eluting nanoparticles (1 μL, >180 million particles) were applied to the cavity. After 30 minutes, the magnet was removed and the cavity was washed using isotonic saline and suction. The cavity was then etched using 37% phosphoric acid, and primer and bonding agent (Scotch Bond Multipurpose, 3M) were applied and light-cured. Composite resin was applied and light cured and the restoration was finished and polished to ensure that it conforms to the tooth contours and that it is not sharp or rough.
Extraction of specimens
For qRT-PCR, the animals were deeply anesthetized with Ketamine/Xylazine and sacrificed. The mandibular teeth were immediately extracted and flash frozen using liquid nitrogen. The teeth were stored in −80 °C freezer until time of use. For histology, the animals were euthanized with sodium pentobarbital (100 mg/kg). The rats were perfused transcardially with buffered saline followed by 4% buffered paraformaldehyde. The mandibular jaw, containing the teeth was removed, decalcified, embedded in paraffin and sectioned.
qRT- PCR analysis
Primers for all SYBR assays were designed using Primer 3 50. Also see http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi). Melting curve analysis was performed to ensure single-product amplification for all primer pairs. TaqMan® assays were obtained from Applied Biosystems. RT-PCR was performed on the ABI 7900HT Fast Real Time PCR System (Applied Biosystems) using assays specific for each gene of interest. For SYBR assays, each reaction well contained 5mL of Power SYBR Green PCR Master Mix (Applied Biosystems, Cat#4367659), cDNA equivalent to 20ng of total RNA and 400nM each of forward and reverse amplification primers in a reaction volume of 10mL. For TaqMan® assays, each reaction well contained 5mL of Gene Expression Master Mix (Applied Biosystems, Cat#4370074), cDNA equivalent to 20ng of total RNA and 0.5mL of 20X concentrated TaqMan® assay in a reaction volume of 10mL. Cycling conditions were as follows: 95°C for 10 minutes for polymerase activation, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Data analysis was performed using Sequence Detection System software from Applied Biosystems, version 2.4. The experimental Ct (cycle threshold) was calibrated against the endogenous control product GAPDH. Samples were analyzed for relative gene expression by the ΔΔCt method 51. Two-way Analysis of Variance (ANOVA) followed by Dunnet’s multiple comparison test was used for statistical analysis. A p<0.05 was considered significant.
Histological analysis
Mandibular jaws containing the teeth were decalcified, embedded in paraffin and sectioned sagittaly (7 μm thick) for histological evaluation. The sections were mounted on slides, deparaffinized and cleared using xylene. Filtered 0.1 % hematoxylin was used to stain the sections for 10 minutes then rinsed in distilled water. The sections were then stained with eosin and dehydrated using alcohol. Mounting medium was then applied and the slides were coverslipped before examination under the microscope. A calibrated Oral & Maxillofacial Pathology investigator evaluated all the slides for the following criteria: Pulp inflammation (range of scores 0–3, lower number indicates less inflammation), pulp vascularity (scores: 0–9, lower score indicates normal vascularity), the width of periodontal ligament (0–3, lower score indicates normal ligament with no fibrosis), and the presence of periapical inflammation (0–7, lower score indicates a normal periapical area). Five sections from each animal were evaluated. The sum of scores was calculated for each animal, and then averaged across animals in each group. The average cumulative score was compared across the groups using the Kruskal-Wallis test, a P <0.05 was considered significant.
Tooth Bonding
Human teeth were mounted in a PVC plastic tube. The occlusal one-third of the tooth crown was removed to expose the mid-coronal dentin using a low speed saw (IsoMet Low Speed Saw, Buehler Ltd., Illinois, USA). The distance from the occlusal surface of the dentin, to the bottom of the PVC tube was 25 mm. The dentin surface was polished with 600-grit SiC paper. The teeth were etched with 37% phosphoric acid gel for 15 s, and rinsed with distilled water. The primer was applied (Adper Scotchbond Multi-Purpose Primer, 3M ESPE) and the solvent was removed with a stream of air for 5 s. The adhesive (Adper Scotchbond Multi-Purpose Adhesive, 3M ESPE) was then applied and a magnet (Neodymium N52, described above) was placed immediately below the tooth for a period of 3 minutes before light-curing for 10 s (Optilux VCL 401, Demetron Kerr, Danbury, CT). Composite resin was applied and light-cured for 60 s. In control experiments, control adhesive was applied without nanoparticles or magnetic pull. One-way ANOVA followed by Dunnett’s multiple comparison test was used to compare between the groups. A P<0.05 was considered significant.
Scanning Electron Microscopy
The resin bonded discs were polished to a high gloss with 600-grit emery paper and 0.05μm alumina under a stream of water. The sections were demineralized in hydrochloric acid (HCl) solution (6mol/L) for 10 s, and deproteinated in 1% sodium hypochlorite (NaOCl) solution for 10 min. The sections were sputter-coated with gold and examined in a Hitachi SEM model S-2500 using an accelerating voltage of 15.0 kV. The micromorphology at the dentin-restoration interface was randomly visualized under SEM for each specimen. To quantify resin tag length, a blinded investigator measured the length of each resin tag from the edge of the hybrid layer to the tip of the resin tag. An average resin tag length was calculated for each specimen from three randomly acquired SEM images of the dentin/tooth interface. The overall average resin tag length was calculated for each group (n=5 teeth/group). To quantify resin tag density, the number of resin tags present relative to the width of field of view was calculated and averaged for each specimen. The average density was then averaged for each group. A Student “t” test was used to compare between the groups. A P<0.05 was considered significant.
Results
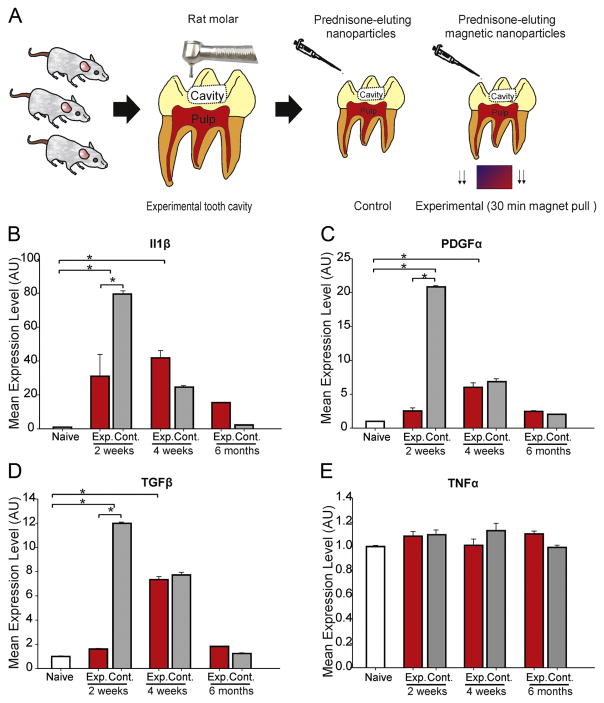
To experimentally inflame the pulp, we prepared moderate-size experimental cavities localized to the dentin of rat mandibular molars. The cavities were left untreated for 2 weeks before undergoing treatment using prednisolone-eluting magnetic nanoparticles that were actively guided to the tooth pulp using strong external magnetic forces (Figure 1A). In control experiments, untreated (naïve) teeth and teeth treated with the same nanoparticles, without magnetic pull, were used. The teeth were harvested at 3 different survival periods (2 weeks, 4 weeks and 6 months) to assess changes in expression of 4 different cytokines in the dental pulp.
Figure 1.
The effect of prednisolone eluting nanoparticles on the expression of inflammatory cytokines and growth factors in the rat tooth pulp. (A) Experimental design: Experimental cavities were prepared in rat mandibular molar teeth and cavities were left exposed for 2 weeks. After two weeks, the cavities on one side of the mandible were treated with prednisolone-eluting nanoparticles and a magnet was placed under the jaw for 30 minutes. On the other side, nanoparticles were placed in the cavity for 30 minutes, but without magnetic pull. The teeth were then cleaned and restored with composite resin. After a survival period of 2 weeks, 4 weeks and 6 months, qRT-PCR was performed to study changes in the levels of (B) Il1β, (C) PDGFα, (D) TGFβ and (E) TNFα. * denotes significant statistical difference. Error bars indicate standard deviations.
The expression levels of interleukin 1 beta (Il1β) was significantly different between the control and experimental groups (F=13.83, P<0.001, ANOVA, Figure 1B). Il1β was significantly elevated following cavity preparation and nanoparticle treatment 2 and 4 weeks after treatment in the experimental group, before approaching baseline values at the 6-month time point. Il1β levels were significantly reduced when prednisolone-eluting nanoparticles were actively pulled to the pulp compared to controls (no magnetic pull) 2 weeks after treatment (Figure 1B).
The expression levels of platelet-derived growth factor alpha (PDGFα) (Figure 1C) and transforming growth factor beta (TGFβ) (Figure 1D) were also significantly different between the groups (PDGFα: F=7.48, P<0.001, TGFβ: F=11.52, P<0.001, ANOVA). Both of these cytokines followed a similar pattern to that of Il1β, with both being significantly elevated compared to untreated teeth, and their expression being significantly less in treated teeth at 2 weeks, when magnetic pull was applied, compared to controls. The levels of tumor necrosis factor (TNFα) were not influenced by cavity preparation or nanoparticle application (F=1.197, P=0.31, ANOVA, Figure 1E).
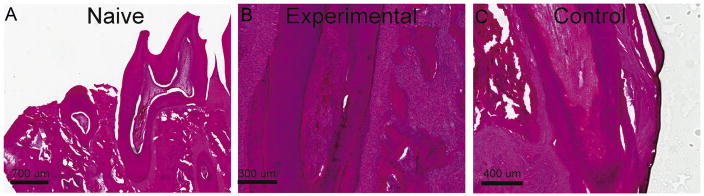
We further evaluated the anti-inflammatory effects of magnetic delivery of therapeutic nanoparticles using histological evaluation of teeth that were pre-prepared with experimental cavities as described above. Magnetic delivery of prednisolone-eluting nanoparticles was most effective in reducing cytokine levels 2 weeks after treatment, and therefore, we focused our histological analysis on that time point. We evaluated the tooth pulp for the presence of inflammation, and changes in vascularity. We also evaluated the periodontal ligament for signs of widening, and the periapical area for signs of inflammation. In Figure 2, representative examples of sagittal sections through molar teeth obtained from naïve, experimental and control animals are shown. Tissues from animals in which prednisolone-eluting nanoparticles were actively steered to the pulp exhibited scores that were not significantly different from those of tissues from untreated animals (P=0.004, Kruskal-Wallis test, Table 1). In control animals, when no magnetic pull was performed, pulp and periodontal tissues scored significantly higher for signs of inflammation and tissue damage when compared to tissues obtained from untreated animals (Table 1). Taken together, these findings indicate that active steering of drug-eluting nanoparticles is necessary for the anti-inflammatory effects observed, further suggesting that iron nanoparticles, guided through dentinal tubules using magnetic forces, can serve as an effective drug delivery system to the pulp.
Figure 2.
The effect of prednisolone eluting nanoparticles on the histology of the rat tooth pulp. (A) Sagittal section of a naïve untreated decalcified rat mandibular molar showing the dentin, viable healthy pulp, and alveolus. (B) An example of rat tooth viable healthy pulp and surrounding root of a rat tooth treated with prednisolone-eluting nanoparticles and magnetic forces. (C) An example of rat tooth hyperemic pulp and surrounding structures of a rat tooth treated with prednisolone-eluting nanoparticles, without magnetic pull.
Table 1.
Histological evaluation of harvested teeth
| Group | Pulp Inflammation (score range: 0–3) | Pulpal Vascularity (0–9) | Periodontal Ligament Widening (0–3) | Periapical Area Inflammation (0–7) | Average Total Score (Sum) |
|---|---|---|---|---|---|
| Naïve | 0 | 0 | 0 | 0 | 0a |
| Experimental | 1.4 | 1.2 | 1.4 | 1 | 5ab |
| Control | 3.6 | 2.2 | 2.6 | 1.4 | 9.8b |
Values modified with the same superscript letter are not significantly different.
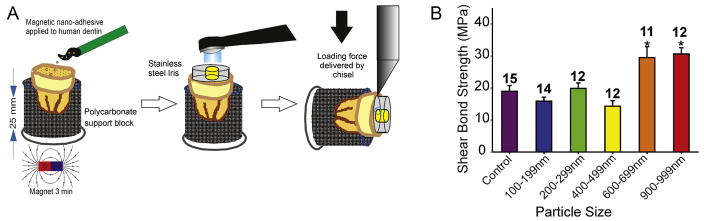
In addition to serving as vessels to deliver nutrients to and from the pulp 15, dentinal tubules play a significant role in facilitating the retention of composite resin restorations (esthetic fillings) that are commonly used to restore dental cavities 1, 2. Dentinal tubules allow for the formation of resin tags that assist in mechanically anchoring these restorations to dentin 16, 17. Therefore, we tested if our delivery system can be used to actively steer resin adhesives into dentinal tubules, and if such active guidance enhances the initial shear bond strength of composite resin to dentin. We used freshly extracted human third molar teeth, and a commercially available adhesive resin (Adper Scotch Bond, Multi-Purpose Adhesive, 3M) dispersed with acrylate-functionalized magnetic iron nanoparticles of various sizes (100–1000 nm, 2% w:v). We used this nanoparticle-doped adhesive, in combination with brief (3 min) external magnetic force application, to bond composite resin to prepared teeth (Figure 3A). We discovered that shear bond strength of composite resin to dentin is significantly increased when the diameter of nanoparticles used was ≥ 600 nm (F=11.399, P<0.001, Figure 3B). These findings indicate that actively guiding nanoparticle-doped adhesive into dentin enhances the initial bond strength of composite resin restorations to dentin and suggests that this active delivery method increases the probability of resin tag formation, improving the penetration of resins into dentin.
Figure 3.
The effect of iron nanoparticles on the shear bond strength of composite resin to dentin. (A) Experimental design: Human molar teeth were sectioned to expose the dentin. Etch (15 s) then rinse, prime, and bond was performed using a nanoparticle-doped adhesive. Magnetic pull for 3 minutes was performed when nanoparticle-doped adhesive was applied. The adhesive resin was then cured and composite resin applied and cured. (B) The shear bond strength of composite resin to dentin was significantly increased by incorporating nanoparticles in resin adhesive. This increase was dependent on particle size. * denotes significant statistical difference. Error bars indicate standard deviations.
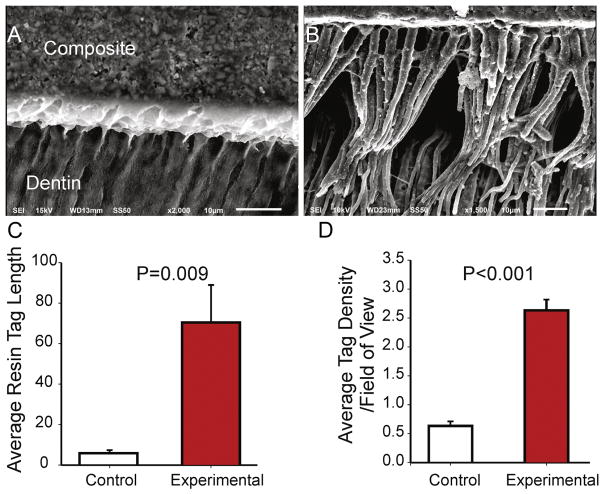
To determine if magnetic pull does in fact improve the penetration of resin into dentin, we obtained scanning electron microscopy (SEM) images from cross sections of (1) teeth restored with the nanoparticle-doped adhesive and magnetic pull (900 nm, 2%, n=5 teeth, 3 images/tooth), and (2) teeth restored with control adhesive (no nanoparticles, n=5 teeth). Representative images are shown in Figure 4A–B. Teeth restored using the nanoparticle-doped adhesive and magnetic pull displayed an extensive network of resin tags penetrating dentin, both vertically and horizontally, compared to control (Figure 4AB). The average length of resin tag and the number of resin tags per field of view were also significantly greater in teeth restored with the nanoparticle-doped adhesive compared to controls (Figure 4C–D). These findings further emphasize the utility of iron nanoparticles and magnetic forces as a delivery system in dental applications.
Figure 4.
Examples of scanning electron microscopy images of the resin-dentin interface obtained from (A) Control specimen and (B) Experimental specimen where a nanoparticle-doped adhesive resin was used. (C) Quantification of average resin tag length in control and experimental specimens (n=5/group). (D) Quantification of average resin tag density per field of view. Error bars indicate standard deviations.
Discussion
This project describes a non-invasive delivery method that exploits naturally occurring dentinal tubules, and uses magnetic forces, to actively steer therapeutic agents to the dental pulp. We demonstrate that such methods can reduce the expression of inflammatory cytokines and reduce the inflammation of injured pulp. The results also indicate that this delivery method can be used to improve the penetration of dental adhesives into dentin, which enhances the shear bond strength of composites to dentin.
Nanoparticles are used routinely in dentistry. They are an integral component of esthetic fillings, porcelains, cements, and mineralized bone scaffolds. To target pulpal tissues, we used nanoparticles that are commercially available Chemicell (Berlin, Germany). These particles consist of an iron oxide core coated with polysaccharides (starch or chitosan) to confer biocompatibility. Chitosan-coated nanoparticles were used in this project because chitosan-coated nanoparticles may be more beneficial than starch-coated nanoparticles. Particles coated with chitosan can ionically bind greater drug loads due to their high zeta potential (+23 mV). These particles can, in turn, be loaded with drugs such as steroids (e.g.: prednisolone). These particles have been tested and determined to be biocompatible and non-toxic in both preclinical models 18, 19 and in human clinical trials 20, 21, where they were shown to be biocompatible in quantities much greater than the amounts used in this project, as such, the particles themselves are likely to have minimal effect on pulpal biology. These particles have also been used with great success to treat inner and middle ear diseases 12, 14, 22, 23, and this delivery method may be instrumental in treating pulpal conditions that are refractory to current treatments, such as invasive cervical resorption of the dental pulp 24.
Properties that regulate the penetration of materials across dentinal tubules have been subjected to considerable study 25. Thus, particle size will significantly influence the amount of drugs that can be delivered to the pulp. The most effective particle size will depend on both the applied magnetic forces and the resistance to motion within the tubules, including resistance due to pulpal pressure 26. For a single particle, the magnetic force varies with particle volume (with the cube of particle diameter). If the particles are too small, they may not experience sufficient magnetic force; if they are too large, they may aggregate rendering them difficult to move through the narrow tubules. Only one size of nanoparticles (300 nm) was used to deliver nanoparticles to the pulp, and the force was applied for a period of 30 minutes. However, additional experiments are needed to assess the effect of particle size, and delivery time, on the efficiency of drug delivery to the pulp.
It is important to note that rat molar teeth are anatomically and developmentally similar to human teeth, albeit smaller in size 27; and moreover, rat molar teeth exhibit dentinal tubules that are within the range of diameters reported for human dentinal tubules 28; and the histologically observed healing of dental pulp tissue after injury in rats is comparable with the healing process described in humans and other mammals 29. Nonetheless, the results of this study are the first step in exploring the benefits of delivery of therapeutic agents locally to the dental pulp. The results generated from this study will be used to design future preclinical and clinical trials and test the utility of drug-laden nanoparticles for the treatment of dental disease.
This technology can also be used to improve bond strength of composite resins to dentin. A major concern with contemporary adhesive resins is their limited ability to infiltrate and penetrate demineralized dentinal collagen fibrils exposed by acid-etching or self-etch adhesives. This is due to inability of the adhesive to replace and remove loosely bound water around collagen fibrils 30; and due to the presence of highly hydrated negatively charged proteoglycans in the interfibrillar spaces preventing the adhesive from penetrating deep into the dentin hybrid layer resulting in a weaker adhesive/dentin interface31. This incomplete infiltration renders exposed collagen fibrils susceptible to enzymatic attack by metalloproteinases, which will degrade the composite bond to dentin and reduce its durability 32–35. Therefore, an adhesive resin which improved penetration, as demonstrated in this study, could be used to provide for a successful, durable restoration 33, 36.
Incorporation of fillers into resin materials can produce adhesives with better mechanical properties, generating a stronger hybrid layer that is more resistant to degradation and water sorption, as shown in several studies 37, 38. Niobium pentoxide, colloidal silica, hydroxyapatite, ytterbium trifluoride, tantalum oxide, glass and zirconia are among the filler particles that have been tested 39–45. However, these studies yielded mixed results. Some studies reported improved mechanical properties and bond strength when filled adhesives were used 40, 41, 43, 46, while other studies showed no change, or deterioration in mechanical properties or bond strength 47–49. However, none of these studies actively pulled the fillers to penetrate dentin.
Furthermore, previous studies of nanofilled adhesives show that nanoparticles were mostly unable to penetrate the interfibrillar spaces due primarily to a difference in diffusion rate between monomers and particles 37. The reasoning for this possibly includes the presence of non-collagenous proteins and proteoglycans in the interfibrillar spaces which may exert steric hindrance for nanoparticle infiltration 43. The use of active force to steer the nanoparticles may assist in overcoming these limitations, however, future studies are needed to determine the ability of this method to penetrate interfibrillar spaces within dentin.
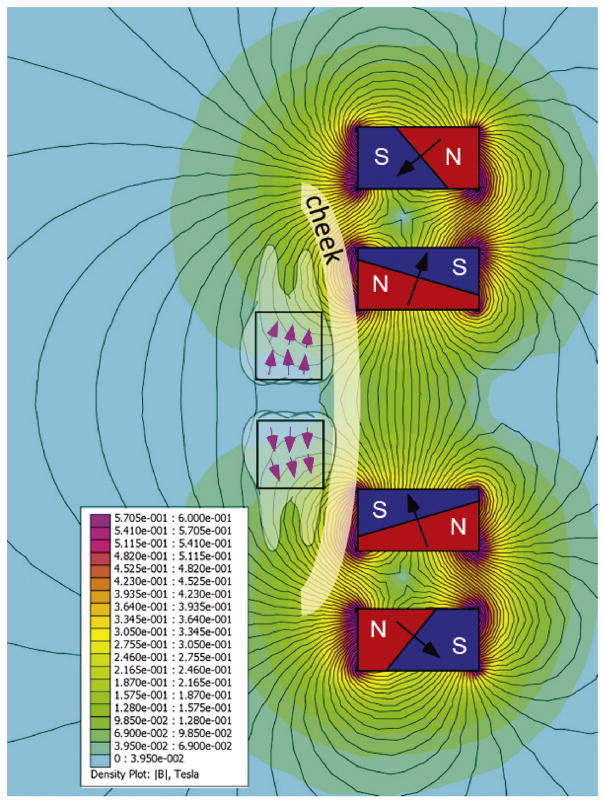
In our in vitro experiments, we are applied a single magnet under the teeth to align the magnetic force with dentinal tubule direction. In clinical situations, this is possible for mandibular teeth where the magnet can be placed under the chin. Such an approach may be challenging for maxillary teeth. However, an array of extraoral magnets can be designed (e.g. Figure 5) to deliver strong perpendicular forces to the teeth, regardless of their position in the arch.
Figure 5.
Custom made array made of 4 Neodynium N52 magnets (N=north pole of the magnet, S=south) for application of nanoparticles to maxillary teeth. To design this array, “Finite Element Method Magnetics” was used to compute the magnetic field and Matlab was used for optimization. Optimization was performed along a line 1.5 cm away from the face of the array. This configuration allows the pulling of nanoparticles apically in maxillary teeth, and mandibular teeth if necessary. Black lines represent magnetic fields.
The active delivery method we describe relies on iron nanoparticles and magnetic forces. This method exploits naturally occurring dentinal tubules, and can be used to deliver medications to reduce pulpal inflammation, and to enhance bond strength of composite resin to dentin. Such a delivery method is economical, less invasive and pain-free. It can allow for intervention at an earlier stage of the disease, i.e. before the pulp dies. The technique would be simple and could be readily translated to clinical use.
List of Abbreviations
- Il1β
Interleukin 1 beta
- PDGFα
Platelet-derived growth factor alpha
- SEM
Scanning electron microscopy
- TGFβ
Transforming growth factor beta
- TNF α
Tumor necrosis factor
Footnotes
Disclosure: The authors declare no commercial affiliations that might pose a potential, perceived or real conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mjor IA, Sveen OB, Heyeraas KJ. Pulp-dentin biology in restorative dentistry. Part 1: Normal structure and physiology. Quintessence Int. 2001;32(6):427–446. [PubMed] [Google Scholar]
- 2.Mjor IA, Nordahl I. The density and branching of dentinal tubules in human teeth. Arch Oral Biol. 1996;41(5):401–412. doi: 10.1016/0003-9969(96)00008-8. [DOI] [PubMed] [Google Scholar]
- 3.Love RM, Jenkinson HF. Invasion of dentinal tubules by oral bacteria. Crit Rev Oral Biol Med. 2002;13(2):171–183. doi: 10.1177/154411130201300207. [DOI] [PubMed] [Google Scholar]
- 4.Zero DT, Zandona AF, Vail MM, Spolnik KJ. Dental caries and pulpal disease. Dent Clin North Am. 2011;55(1):29–46. doi: 10.1016/j.cden.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Zanini M, Meyer E, Simon S. Pulp inflammation diagnosis from clinical to inflammatory mediators: A systematic review. J Endod. 2017;43(7):1033–1051. doi: 10.1016/j.joen.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Lacevic A, Vranic E, Zulic I. Endodontic-periodontal locally delivered antibiotics. Bosn J Basic Med Sci. 2004;4(1):73–78. doi: 10.17305/bjbms.2004.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aparna S, Setty S, Thakur S. Comparative efficacy of two treatment modalities for dentinal hypersensitivity: A clinical trial. Indian J Dent Res. 2010;21(4):544–548. doi: 10.4103/0970-9290.74213. [DOI] [PubMed] [Google Scholar]
- 8.Gillam DG, Newman HN. Iontophoresis in the treatment of cervical dentinal sensitivity--a review. J West Soc Periodontol Periodontal Abstr. 1990;38(4):129–133. [PubMed] [Google Scholar]
- 9.Ikeda H, Suda H. Facilitatory effect of ac-iontophoresis of lidocaine hydrochloride on the permeability of human enamel and dentine in extracted teeth. Arch Oral Biol. 2013;58(4):341–347. doi: 10.1016/j.archoralbio.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Ikeda H, Suda H. Determination of the functional space for fluid movement in the rat dentinal tubules using fluorescent microsphere. Arch Oral Biol. 2013 doi: 10.1016/j.archoralbio.2013.01.007. In press. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi M, Takeda M, Matsumoto S. Somatostatin inhibits tooth-pulp-evoked rat cervical dorsal horn neuronal activity. Exp Brain Res. 2008;184(4):617–622. doi: 10.1007/s00221-007-1261-0. [DOI] [PubMed] [Google Scholar]
- 12.Komanee A, Lee R, Nacev A, Probst R, Sarwar A, Rutel I, Depireux DA, Dormer K, Shapiro B. Putting Therapeutic Nanoparticles Where They Need To Go By Magnet Systems Design and Control. Magnetic Drug Targeting. 2012 [Google Scholar]
- 13.Sarwar A, Nemirovski A, Shapiro B. Optimal halbach permanent magnet designs for maximally pulling and pushing nanoparticles. J Magn Magn Mater. 2012;324(5):742–754. doi: 10.1016/j.jmmm.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni S, Ramaswamy B, Horton E, Gangapuram S, Nacev A, Depireux D, Shimoji M, Shapiro B. Quantifying the motion of magnetic particles in excised tissue: Effect of particle properties and applied magnetic field. J Magn Magn Mater. 2015:393243–252. doi: 10.1016/j.jmmm.2015.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magloire H, Maurin JC, Couble ML, Shibukawa Y, Tsumura M, Thivichon-Prince B, Bleicher F. Topical review. Dental pain and odontoblasts: Facts and hypotheses. J Orofac Pain. 2010;24(4):335–349. [PubMed] [Google Scholar]
- 16.Yap UJ, Stokes AN, Pearson GJ. Concepts of adhesion--a review. N Z Dent J. 1994;90(401):91–97. [PubMed] [Google Scholar]
- 17.Perdigao J, Frankenberger R, Rosa BT, Breschi L. New trends in dentin/enamel adhesion. Am J Dent. 2000;13(Spec):25D–30D. [PubMed] [Google Scholar]
- 18.Hellstern D, Schulze K, Schopf B, Petri-Fink A, Steitz B, Kamau S, Hilbe M, Koch-Schneidemann S, Vaughan L, Hottiger M, Hofmann M, Hofmann H, Von Rechenberg B. Systemic distribution and elimination of plain and with cy3. 5 functionalized poly(vinyl alcohol) coated superparamagnetic maghemite nanoparticles after intraarticular injection in sheep in vivo. J Nanosci Nanotechnol. 2006;6(9–10):3261–3268. doi: 10.1166/jnn.2006.482. [DOI] [PubMed] [Google Scholar]
- 19.Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm. 2008;5(2):316–327. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 20.Lubbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K, Matthias M, Dorken B, Herrmann F, Gurtler R, Hohenberger P, Haas N, Sohr R, Sander B, Lemke AJ, Ohlendorf D, Huhnt W, Huhn D. Clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996;56(20):4686–4693. [PubMed] [Google Scholar]
- 21.Lubbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res. 2001;95(2):200–206. doi: 10.1006/jsre.2000.6030. [DOI] [PubMed] [Google Scholar]
- 22.Sarwar A, Lee R, Depireux DA, Shapiro B. Magnetic injection of nanoparticles into rat inner ears at a human head working distance. IEEE Transactions on Magnetics. 2013:49440–452. [Google Scholar]
- 23.Nacev A, Probst R, Komanee A, Lee R, Depireux DA, Emmert-Buck M, Shapiro B. Towards control of magnetic fluids in patients: Directing therapeutic nanoparticles to disease locations (tutorial) IEEE Control System Magazine. 2012:3232. [Google Scholar]
- 24.Kitchens JA, Schwartz SA, Schindler WG, Hargreaves KM. Iontophoresis significantly increases the trans-dentinal delivery of osteoprotegerin, alendronate, and calcitonin. J Endod. 2007;33(10):1208–1211. doi: 10.1016/j.joen.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Pashley DH, Livingston MJ. Effect of molecular size on permeability coefficients in human dentine. Arch Oral Biol. 1978;23(5):391–395. doi: 10.1016/0003-9969(78)90098-5. [DOI] [PubMed] [Google Scholar]
- 26.Heyeraas KJ, Berggreen E. Interstitial fluid pressure in normal and inflamed pulp. Crit Rev Oral Biol Med. 1999;10(3):328–336. doi: 10.1177/10454411990100030501. [DOI] [PubMed] [Google Scholar]
- 27.Dammaschke T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab Anim. 2010;44(1):1–6. doi: 10.1258/la.2009.008120. [DOI] [PubMed] [Google Scholar]
- 28.Forssell-Ahlberg K, Brannstrom M, Edwall L. The diameter and number of dentinal tubules in rat, cat, dog and monkey. A comparative scanning electron microscopic study. Acta Odontol Scand. 1975;33(5):243–250. doi: 10.3109/00016357509004629. [DOI] [PubMed] [Google Scholar]
- 29.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: A review of current status and a call for action. J Endod. 2007;33(4):377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Kim YK, Mai S, Mazzoni A, Liu Y, Tezvergil-Mutluay A, Takahashi K, Zhang K, Pashley DH, Tay FR. Biomimetic remineralization as a progressive dehydration mechanism of collagen matrices--implications in the aging of resin-dentin bonds. Acta Biomater. 2010;6(9):3729–3739. doi: 10.1016/j.actbio.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott JE, Thomlinson AM. The structure of interfibrillar proteoglycan bridges (shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J Anat. 1998;192(Pt 3):391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83(3):216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 33.Pashley DH, Agee KA, Wataha JC, Rueggeberg F, Ceballos L, Itou K, Yoshiyama M, Carvalho RM, Tay FR. Viscoelastic properties of demineralized dentin matrix. Dent Mater. 2003;19(8):700–706. doi: 10.1016/s0109-5641(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Tjaderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90(8):953–968. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto M. A review--micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. J Biomed Mater Res B Appl Biomater. 2010;92(1):268–280. doi: 10.1002/jbm.b.31535. [DOI] [PubMed] [Google Scholar]
- 36.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: Aging and stability of the bonded interface. Dent Mater. 2008;24(1):90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Tay FR, Moulding KM, Pashley DH. Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent. 1999;1(2):103–117. [PubMed] [Google Scholar]
- 38.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22(3):211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Leitune VC, Collares FM, Takimi A, De Lima GB, Petzhold CL, Bergmann CP, Samuel SM. Niobium pentoxide as a novel filler for dental adhesive resin. J Dent. 2013;41(2):106–113. doi: 10.1016/j.jdent.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Sadat-Shojai M, Atai M, Nodehi A, Khanlar LN. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent Mater. 2010;26(5):471–482. doi: 10.1016/j.dental.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Conde MC, Zanchi CH, Rodrigues-Junior SA, Carreno NL, Ogliari FA, Piva E. Nanofiller loading level: Influence on selected properties of an adhesive resin. J Dent. 2009;37(5):331–335. doi: 10.1016/j.jdent.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Schulz H, Schimmoeller B, Pratsinis SE, Salz U, Bock T. Radiopaque dental adhesives: Dispersion of flame-made ta2o5/sio2 nanoparticles in methacrylic matrices. J Dent. 2008;36(8):579–587. doi: 10.1016/j.jdent.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Lohbauer U, Wagner A, Belli R, Stoetzel C, Hilpert A, Kurland HD, Grabow J, Muller FA. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010;6(12):4539–4546. doi: 10.1016/j.actbio.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Collares FM, Leitune VC, Rostirolla FV, Trommer RM, Bergmann CP, Samuel SM. Nanostructured hydroxyapatite as filler for methacrylate-based root canal sealers. Int Endod J. 2012;45(1):63–67. doi: 10.1111/j.1365-2591.2011.01948.x. [DOI] [PubMed] [Google Scholar]
- 45.Collares FM, Klein M, Santos PD, Portella FF, Ogliari F, Leitune VC, Samuel SM. Influence of radiopaque fillers on physicochemical properties of a model epoxy resin-based root canal sealer. J Appl Oral Sci. 2013;21(6):533–539. doi: 10.1590/1679-775720130334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyazaki M, Ando S, Hinoura K, Onose H, Moore BK. Influence of filler addition to bonding agents on shear bond strength to bovine dentin. Dent Mater. 1995;11(4):234–238. doi: 10.1016/0109-5641(95)80055-7. [DOI] [PubMed] [Google Scholar]
- 47.Kim JS, Cho BH, Lee IB, Um CM, Lim BS, Oh MH, Chang CG, Son HH. Effect of the hydrophilic nanofiller loading on the mechanical properties and the microtensile bond strength of an ethanol-based one-bottle dentin adhesive. J Biomed Mater Res B Appl Biomater. 2005;72(2):284–291. doi: 10.1002/jbm.b.30153. [DOI] [PubMed] [Google Scholar]
- 48.Lee YK, Pinzon LM, O’keefe KL, Powers JM. Effect of filler addition on the bonding parameters of dentin bonding adhesives bonded to human dentin. Am J Dent. 2006;19(1):23–27. [PubMed] [Google Scholar]
- 49.Nunes MF, Swift EJ, Perdigao J. Effects of adhesive composition on microtensile bond strength to human dentin. Am J Dent. 2001;14(6):340–343. [PubMed] [Google Scholar]
- 50.Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001;29(17):E88–8. doi: 10.1093/nar/29.17.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfaffl MW. A new mathematical model for relative quantification in real-time rt-pcr. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]