Abstract
Purpose
To understand trends in the incidence and mortality of two human papillomavirus (HPV)-associated cancers, cervical and oropharyngeal cancer, in Massachusetts.
Methods
From 2004–2014, the Massachusetts Cancer Registry recorded 3,996 incident cases of oropharyngeal cancer and 2,193 incident cases of cervical cancer. Mortality data were obtained from the Massachusetts Registry of Vital Records and Statistics from 2008–2014. Rates were age-standardized to the 2000 US population and trends were assessed using joinpoint regression.
Results
While the incidence rate of cervical cancer (5.46 per 100,000) decreased by 2.41% annually (p=0.004), the incidence rate of oropharyngeal cancer among males (7.85 per 100,000) increased by 2.82% annually (p=0.0002). Mortality rates for both cancers decreased from 2008–2014 but were not statistically significant (Cervical −3.73% annually, p=0.29; Oropharyngeal −1.94% annually, p=0.44).
Conclusion
The rising incidence rate of oropharyngeal cancer in men and the decreasing, but relatively high, incidence rate of cervical cancer in women highlight the need for further screening and prevention by HPV vaccination in Massachusetts.
Keywords: Cancer, cervical cancer, oropharyngeal cancer, human papillomavirus (HPV), Massachusetts, Population Surveillance
INTRODUCTION
Persistent infection with a high risk type of human papillomavirus (HPV) is known to cause cervical, oropharyngeal, vaginal, vulvar, penile, anal, and rectal cancers [1]. Cervical and oropharyngeal are the two most common types of HPV-associated cancers, representing about 71% of all HPV-associated cancers in the US [2]. Nationally, cervical cancer rates are decreasing but oropharyngeal cancer rates are increasing [3]. While there are screening tests for cervical cancer, there are no routine screening tests for oropharyngeal cancer. However, primary prevention exists in the form of vaccines for HPV. Unfortunately, HPV vaccination rates remain low in Massachusetts, with 62.0% of teen (13–17 year old) girls and 51.4% of teen boys up-to-date (2 doses if immunocompetent and started vaccination before age 15, 3 doses if not immunocompetent or started vaccination after age 15) with the vaccination series in 2016 [4].
HPV vaccines have only been FDA approved since 2006. Due to the lag time between age at vaccination (11–12 years old) and average age at diagnosis for cervical (49 years old) or oropharyngeal cancer (62 among females, 59 among males), we are not yet able to directly examine how the vaccine has impacted cancer rates [3]. However, understanding trends of the two most common HPV-associated cancers is useful for state public health officials to inform cancer prevention, including vaccination promotion and policy efforts. Trends of cervical and oropharyngeal cancer incidence and mortality in Massachusetts have not been examined and the number of cancers potentially preventable by the HPV vaccine in Massachusetts is not known.
METHODS
In 2015, the estimated population of Massachusetts was 6.79 million people, of which 51.5% were female [5]. Only 15.4% of the population was 65 years old and over, while 20.4% was under 18 years old [5]. Most of the population was non-Hispanic white (73.5%), while 8.4% was black, 11.2% was Hispanic, and 6.6% was Asian [5]. Due to legislation enacted in 2006, Massachusetts has a small percentage of people without health insurance (3.3% of those under age 65) [5].
The Massachusetts Cancer Registry (MCR) is part of the Massachusetts Department of Public Health (MDPH) and collects data on incident cancer cases in Massachusetts. The MCR has been collecting information since 1982 and the North American Association of Central Cancer Registries (NAACCR) has estimated that the MCR case ascertainment is more than 95% complete [6]. Information on new cases of cervical and oropharyngeal cancer diagnosed from 2004–2014 was obtained from the MCR. Oropharyngeal cancers included the following sites: base of tongue, tonsils, soft palate, and other parts of the oropharynx. All oropharyngeal cases were restricted to squamous cell carcinomas, and cervical cancer included squamous cell carcinomas and adenocarcinomas. The cancer death data were provided by the MDPH’s Massachusetts Registry of Vital Records and Statistics (MVRS). The MVRS has legal responsibility for collecting reports of death on Massachusetts residents. We collected information on death from cervical cancer and oropharyngeal cancer from 2008–2014. Details on ICD codes included can be found in Appendix 1.
Incidence and mortality rates were age-standardized to the 2000 US population using 18 five-year age categories. We examined incidence and mortality rates by cancer site, gender, age group, and race/ethnicity. To assess trends we used joinpoint regression to calculate the annual percent change (APC) and perform hypothesis tests [7]. For cervical cancer, we also examined stage at diagnosis to supplement incidence information because of prevalent screening. Rates and trends were considered statistically significant if p<0.05. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NY) [8].
The MCR does not include information on the HPV genotype of the cancers. To estimate the number of cases attributable to an HPV infection, we used methods previously applied and published by the CDC’s Morbidity and Mortality Weekly Report (MMWR) publication for the U.S [2]. We multiplied the percentage of cases that were HPV-positive from genotyping studies by the average annual number of cases [1–2]. The genotype prevalence data came from a national study that had data from seven cancer registries from varying times between 1993 and 2005 [1]. They did not include any states from New England. We assumed the genotype prevalence from this study would be the same as the genotype prevalence in Massachusetts. We felt comfortable making this assumption due to similar a geographic prevalence in the national study, similar rates to smaller studies done in Massachusetts, and our assessment that this is the best data available to estimate HPV prevalence (Appendix Table 2).
Appendix Table 2.
Details on genotyping study used to estimate HPV prevalence for the cancers and related studies from Massachusetts
| Paper (Year) | Study Type and Number of Cases | Geographic Area | Years Incident Cases Collected | Prevalence HPV + Cases | Findings/Use/Limitations | |
|---|---|---|---|---|---|---|
| Cervical | Oropharyngeal | |||||
| Genotyping Data Nationally | ||||||
|
| ||||||
| Sarayia et al (2015) [1] | Cross-sectional study of select US cancer registries, 777 cervical cases, 588 oropharyngeal cases | Los Angeles, Hawaii, Iowa, Kentucky, Florida, Louisiana, Michigan | 1993–2005 1 registry 1993–1999 1 registry 2000–2004 1 registry 1994–2004 4 registries 2004–2005 |
90.6% | 70.1% | Used for HPV + prevalence in our estimates |
|
| ||||||
| Steinau et al (2014) [appendix source 2] | Cross-sectional study of select US cancer registries, 557 oropharyngeal cases | Los Angeles, Hawaii, Iowa, Kentucky, Florida, Louisiana, Michigan | 1995–2005 1 registry 1995–1999 1 registry 2000–2004 1 registry 1994–2004 4 registries 2004–2005 |
NA | 72.4% | High-risk HPV prevalence by registry: Los Angeles = 17 cases (85.0%) Hawaii = 33 cases (84.6%) Iowa = 4 cases (30.7%) Kentucky = 74 cases (63.8%) Florida = 101 cases (72.1%) Louisiana = 75 cases (78.9%) Michigan = 92 cases (68.6%) |
|
| ||||||
| Genotyping Data in Massachusetts | ||||||
|
| ||||||
| Wright et al (2013) [appendix source 3] | Chart review, Brigham and Women’s Hospital, 80 cervical cases | Boston for treatment | 2005–2011 | 96.3% | NA | To compare MA prevalence to national prevalence |
|
| ||||||
| Addison et al (2017) [19] | Case series from Massachusetts General Hospital, 235 oropharynx cases | Boston for treatment | 2002–2012 | NA | 64.7% | To compare MA prevalence to national prevalence Eligible patients had to be undergoing radiation |
|
| ||||||
| Lorch et al (2015) [20] | Chart review, Dana Farber Cancer Institute, 500 oropharyngeal cases | Boston for treatment | 2001–2011 | NA | 43% HPV + 44% unknown status | To compare MA prevalence to national prevalence Eligible patients had to be stage III or IV |
| Paper (Year) | Study Type and Number of Cases | Geographic Area | Years Incident Cases Collected | Prevalence HPV + Cases | Findings/Use/Limitations | |
|---|---|---|---|---|---|---|
| Cervical | Oropharyngeal | |||||
| Genotyping Data in Massachusetts | ||||||
|
| ||||||
| Nelson et al (2017) [21] | Population-based greater Boston area, 486 pharyngeal cases | Greater Boston area | 1999–2003 and 2006–2011 | NA | 60.7% | To compare MA prevalence to national prevalence |
|
| ||||||
| Nichols et al (2010) [22] | Case series from Partners Healthcare System, 68 oropharynx cases | Massachusetts | 1996–2006 | NA | 78% HPV 16 | To compare MA prevalence to national prevalence Eligible patients had to be undergoing chemoradiation |
|
| ||||||
| Ringstrom et al (2002) [23] | Case series from Dana-Farber, 29 oropharynx cases | Boston for treatment | 1994–1998 | NA | 52% HPV 16 oropharynx 64% HPV 16 tonsil |
To compare MA prevalence to national prevalence |
Saraiya M, Unger ER, Thompson TD, et al (2015) US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. JNCI Journal of the National Cancer Institute 107(6):djv086. doi:10.1093/jnci/djv086.
Steinau M, Saraiya M, Goodman MT, et al (2014). Human Papillomavirus Prevalence in Oropharyngeal Cancer before Vaccine Introduction, United States. Emerging Infectious Diseases. 20(5):822–828.
Wright AA, Howitt BE, Myers AP, et al (2013). Oncogenic mutations in cervical cancer: genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer. 119(21):3776–83.
Addison D, Seidelmann SB, Jangua SA, et al (2017). Human Papillomavirus Status and the Risk of Cerebrovascular Events Following Radiation Therapy for Head and Neck Cancer. J Am Heart Assoc. 6(9):e006453.
Lorch JH, Hanna GJ, Posner MR, et al (2015). Human Papillomavirus and Induction Chemotherapy versus Concurrent Chemoradiotherapy in Locally Advanced Oropahyrygneal Cancer: The Dana Farber Experience. Head & Neck. 38(S1):E1618–E1624.
Nelson HH, Pawlita M, Michaud DS, et al (2017). Immune Response to HPV16 E6 and E7 Proteins and Patient Outcomes in Head and Neck Cancer. JAMA Oncology. 3(2):178–185.
Nichols AC, Finkelstein DM, Faquin WC, et al (2010). Bcl2 and Human Papilloma Virus 16 as Predictors of Outcome following Concurrent Chemoradiation for Advanced Oropharyngeal Cancer. Clin Can Res. 16(7):2138–2146.
Ringstrom E, Peters E, Hasegawa M, et al (2002). Human Papillomavirus Type 16 and Squamous Cell Carcinoma of the head and Neck. Clin Cancer Res. 8(10):3187–3192.
About 30% of oropharyngeal cancers in the US are not related to HPV [2]. Risk factors for oropharyngeal cancer include smoking, alcohol, and the number of sexual and oral sex partners. Since we do not have information on the HPV status of the tumors in the MCR, we assessed trends in risk factors for oropharyngeal cancer to help us determine whether the potential burden of disease of these cancers in Massachusetts might be associated with HPV. To do this we used the Behavioral Risk Factor Surveillance System (BRFSS) Survey, which collects self-reported information on current cigarette smoking, heavy drinking, and binge drinking among adults in Massachusetts and the US from 1990–2010 using a random sample of land and cellular telephone numbers [9]. The number of sexual partners was collected from the Massachusetts BRFSS from 2000–2010. Per capita ethanol consumption data was collected from the National Institute on Alcohol Abuse and Alcoholism from 1977–2010 [10]. It is important that teens are vaccinated for HPV before they begin any sexual activity. Additionally, we see that most HPV infections in the US occur when people are in their 20s, so understanding teen sexual behaviors is important for prevention efforts [11]. Data on teen sexual behaviors including sexual intercourse with 4 or more partners and sexual intercourse before the age of 13 was collected by the Youth Risk Behavioral Surveillance System (YRBSS) for Massachusetts and the US sample from 1993–2015 [12]. The YRBSS surveys are state- and nationally-representative surveys of high school youth, grades 9–12, that assess health risk behaviors [12]. We again used joinpoint regression to calculate the APC for trends in these risk factors over time.
RESULTS
Incidence rates by gender
From 2004 to 2014, 2,193 cases of cervical cancer and 3,996 cases of oropharyngeal cancer were diagnosed in Massachusetts (Table 1). The incidence rate of cervical cancer (5.46 cases per 100,000 females) was higher than the incidence rate of oropharyngeal cancer among males and females together (4.68 cases per 100,000). However, oropharyngeal is much more common in males and the incidence rate of oropharyngeal cancer among males is higher than the incidence rate of cervical cancer (7.85 vs 5.46 per 100,000 males and females respectively).
Table 1.
Number and age-adjusted incidence (2004–2014) and mortality (2008–2014) ratesa of cervical and oropharyngeal cancers in Massachusetts by sex, age, and race/ethnicity
| Characteristic | Cervical Cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| No. Cases | Incidence Rate | Annual Percent Change | No. Deaths | Mortality Rate | Annual Percent Change | |||
| % | p value | % | p value | |||||
| Total | 2,193 | 5.46 | −2.41 | 0.004 | 374 | 1.29 | −3.73 | 0.294 |
|
| ||||||||
| Age Group (Years) | ||||||||
| 20–29 | 105 | 2.12 | −6.40 | 0.073 | - | - | - | |
| 30–39 | 418 | 8.71 | −3.93 | 0.027 | 16 | 0.54 | 17.36 | 0.145 |
| 40–49 | 545 | 9.76 | −0.15 | 0.899 | 69 | 1.99 | −10.93 | 0.211 |
| 50–59 | 453 | 8.74 | −3.84 | 0.0002 | 94 | 2.75 | 08-10:30.61 10-14:-6.11 |
0.026 0.034 |
| 60–69 | 332 | 9.34 | 0.14 | 0.916 | 89 | 3.63 | −11.93 | 0.025 |
| 70–79 | 188 | 8.24 | −2.53 | 0.126 | 51 | 3.53 | 3.11 | 0.511 |
| ≥80 | 145 | 7.15 | −4.01 | 0.084 | 49 | 3.77 | −4.78 | 0.634 |
|
| ||||||||
| Race/Ethnicity | ||||||||
| White, Non-Hispanic | 1,642 | 5.04 | −2.54 | 0.020 | 305 | 1.25 | −4.65 | 0.112 |
| Black, Non-Hispanic | 192 | 8.72 | −4.74 | 0.038 | 34 | 2.28 | −5.12 | 0.669 |
| Asian | 116 | 6.62 | 3.73 | 0.261 | 15 | 1.34 | 5.04 | 0.624 |
| Hispanic | 195 | 8.07 | −6.36 | 0.010 | 20 | 1.41 | 4.07 | 0.868 |
| Characteristic | Oropharyngeal Cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| No. Cases | Incidence Rate | Annual Percent Change | No. Deaths | Mortality Rate | Annual Percent Change | |||
| % | p value | % | p value | |||||
| Total | 3,996 | 4.68 | 3.01 | 0.0006 | 820 | 1.46 | −1.94 | 0.439 |
|
| ||||||||
| Sex | ||||||||
| Male | 3,127 | 7.85 | 2.82 | 0.0002 | 586 | 2.35 | −3.01 | 0.289 |
| Female | 869 | 1.91 | 04-12: 6.62 12-14:-13.73 |
0.0007 0.132 |
234 | 0.73 | 0.78 | 0.872 |
|
| ||||||||
| Age Group (Years) | ||||||||
| 20–29 | - | - | - | - | - | - | ||
| 30–39 | 48 | 0.51 | 1.81 | 0.701 | - | - | - | |
| 40–49 | 489 | 4.46 | 0.52 | 0.729 | 35 | 0.51 | −2.44 | 0.836 |
| 50–59 | 1,378 | 13.70 | 1.37 | 0.144 | 177 | 2.72 | −0.36 | 0.939 |
| 60–69 | 1,208 | 18.00 | 04-11: 9.85 11-14: -8.78 |
0.004 0.175 |
240 | 5.38 | −3.79 | 0.197 |
| 70–79 | 622 | 15.41 | 6.06 | 0.001 | 195 | 7.54 | −4.23 | 0.449 |
| ≥80 | 243 | 7.90 | 3.91 | 0.127 | 166 | 8.42 | 2.13 | 0.771 |
|
| ||||||||
| Race/Ethnicity | ||||||||
| White, Non-Hispanic | 3,672 | 4.97 | 3.43 | 0.0005 | 733 | 1.50 | −1.78 | 0.499 |
| Black, Non-Hispanic | 127 | 3.15 | −0.61 | 0.823 | 46 | 1.83 | −12.53 | 0.260 |
| Asian | 44 | 1.59 | 11.24 | 0.069 | 15 | 0.91 | 2.09 | 0.796 |
| Hispanic | 133 | 3.63 | −0.67 | 0.883 | 23 | 1.09 | 20.68 | 0.313 |
Per 100,000 person-years, standardized to the 2000 U.S. standard population, numbers may not add up due to unknown demographic, cells with fewer than 10 people not shown, multiple annual percent change values are shown when the regression indicated a statistically significant breakpoint
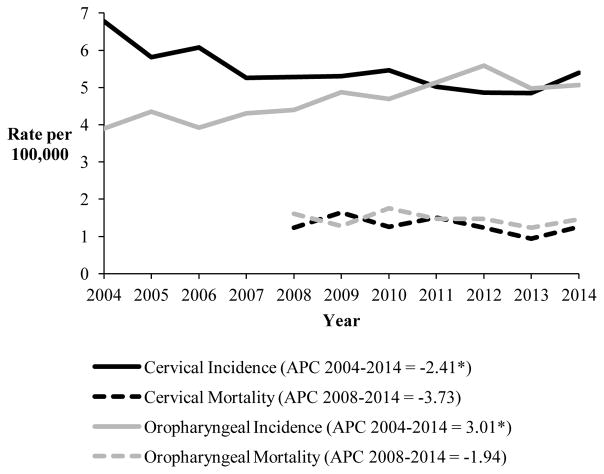
In Massachusetts, the incidence rate of cervical cancer has decreased by 2.41% annually (p=0.004), while the incidence rate of oropharyngeal cancer among males and females has increased by 3.01% annually from 2004 to 2014 (p=0.0006) (Figure 1). Among males, there was a 2.82% annual increase in the incidence rate of oropharyngeal cancer from 2004 to 2014 (p=0.0002). Females had a statistically significant increase in the rate of oropharyngeal cancer from 2004 to 2012 by 6.62% annually (p=0.0007), but then had a 13.73% decrease from 2012 to 2014 which was not statistically significant (p=0.13).
Figure 1.
Trends in cervical and oropharyngeal cancer incidence (2004–2014) and mortality rates (2008–2014) in Massachusetts
Rates are age-adjusted to the 2000 US standard population. The annual percent change (APC) values for the trends in incidence and mortality rates are presented in the legend. Statistically significant trend (p<0.05) in the APC are denoted with an *.
Incidence rates by age group
The average age at diagnosis for cervical cancer was 52.0 years old (standard deviation = 15.9, median=50, mode=45). For cervical cancer, incidence rates were the highest among females in their 40s (9.76 per 100,000) and 60s (9.34 per 100,000) (Table 1). Females of most ages experienced annual decreases in the incidence rate of cervical cancer, with the only statistically significant decreases occurring among females in their 30s (−3.93% APC, p=0.027) and 50s (−3.84% APC, p=0.0002). The average age at diagnosis for oropharyngeal cancer was 61.1 years old (standard deviation = 11.1, median=60, mode = 59). Oropharyngeal cancer had the highest incidence rate among people in their 60s (18.00 per 100,000) and 70s (15.41 per 100,000). Most age groups had annual increases in the incidence of oropharyngeal cancer, with the only statistically significant increases observed among people in their 70s (6.06% APC, p=0.0001) from 2004–2014 and people in their 60s from 2004–2011 (9.85% APC, p=0.004).
Incidence rates by race/ethnicity
Non-Hispanic white females had lower incidence rates of cervical cancer than non-Hispanic black, Hispanic, and Asian females (5.04 vs 8.72, 8.07, and 6.62 per 100,000, respectively) (Table 1). For oropharyngeal cancer, non-Hispanic white people had higher incidence rates than non-Hispanic black, Hispanic, and Asian people (4.97 vs 3.15, 3.63, and 1.59 per 100,000, respectively). When we further examined stage at diagnosis for cervical cancer, Hispanic females were more likely to be diagnosed at stage I or II than all other race/ethnicities (Hispanic = 93.9%, Asian = 86.2%, non-Hispanic white = 83.4%, non-Hispanic black = 79.2%) (Appendix Table 3).
Appendix Table 3.
Cervical cancer stage at diagnosis by age and race/ethnicity in Massachusetts, 2004–2014
| Stage I Number (%) |
Stage II Number (%) |
Stage III Number (%) |
Stage IV Number (%) |
|
|---|---|---|---|---|
| Total | 1,075 (50.1) | 729 (34.0) | 284 (13.2) | 57 (2.7) |
|
| ||||
| Age Group (Years) | ||||
| 20–29 | 76 (72.4) | 23 (21.9) | - | - |
| 30–39 | 295 (70.6) | 100 (23.9) | 16 (3.8) | - |
| 40–49 | 328 (60.2) | 157 (28.8) | 49 (9.0) | 11 (2.0) |
| 50–59 | 198 (43.7) | 174 (38.4) | 72 (15.9) | - |
| 60–69 | 112 (33.7) | 143 (43.1) | 71 (21.4) | - |
| 70–79 | 54 (28.7) | 78 (41.5) | 43 (22.9) | 13 (6.9) |
| ≥80 | 35 (24.1) | 63 (43.5) | 34 (23.5) | 13 (9.0) |
|
| ||||
| Race/Ethnicity | ||||
| White, Non-Hispanic | 817 (49.8) | 552 (33.6) | 232 (14.1) | 41 (2.5) |
| Black, Non-Hispanic | 86 (44.8) | 66 (34.4) | 30 (15.6) | 10 (5.2) |
| Asian | 62 (53.5.1) | 38 (32.8.6) | 13 (11.2) | - |
| Hispanic | 110 (56.4) | 73 (37.4) | - | - |
Cells with counts under 10 people are not shown, SEER summary stages are presented, percentages are the percent of total cases with that stage for the subgroup provided in each row, numbers may not add up due rounding and missing race/ethnicity values
Trends in incidence rates for cervical and oropharyngeal cancer varied by race. Non-Hispanic white, non-Hispanic black, and Hispanic females had statistically significant annual decreases in the incidence rate of cervical cancer (−2.54% p=0.020, −4.74% p=0.038, and −6.36% p=0.010, respectively). However, the incidence rate of cervical cancer for Asian females increased by 3.73% annually, but this increase was not statistically significant (p=0.26). For oropharyngeal cancer, the incidence rate for non-Hispanic white people had statistically significant annual increases (3.43%, p=0.0005) and Asian people had a non-statistically significant annual increase (11.24%, p=0.069). Non-Hispanic black and Hispanic people had non-statistically significant annual decreases (−0.61% p=0.82, and −0.67% p=0.88, respectively).
Mortality rates by gender
From 2008 to 2014 there were 374 deaths from cervical cancer and 820 deaths from oropharyngeal cancer, of which 71% occurred in males (Table 1). The mortality rate from oropharyngeal cancer among males (2.35 deaths per 100,000 males) was higher than the mortality rate from cervical cancer (1.29 deaths per 100,000 females) and oropharyngeal cancer among females (0.73 deaths per 100,000 females) (Figure 1). None of the trends in mortality from 2008 to 2014 in Massachusetts for cervical or oropharyngeal cancer were statistically significant. The annual mortality rate from cervical cancer decreased by 3.73% (p=0.29) and the annual mortality rate from oropharyngeal cancer decreased by 1.94% (p=0.44).
Mortality rates by age group
For both cervical and oropharyngeal cancers, the mortality rate increased with age. Women aged 80 and above had an age-specific mortality rate of 3.77 deaths per 100,000 for cervical cancer (Table 1). For oropharyngeal cancer, people aged 80 and above had an age-specific mortality rate of 8.42 deaths per 100,000. Women in their 50s had a statistically significant increase in the mortality rate of cervical cancer from 2008 to 2010 (30.61%, p=0.026) followed by a statistically significant decrease in mortality from 2010 to 2014 (−6.11%, p=0.034). Women in their 60s also experienced a statistically significant decrease in the mortality rate of cervical cancer from 2008 to 2014 (−11.93%, p=0.025). There were no statistically significant trends in the mortality rate of oropharyngeal cancer by age group.
Mortality rates by race/ethnicity
For both cervical and oropharyngeal cancers, non-Hispanic black people had a higher mortality rate than non-Hispanic white people (cervical: 2.28 vs 1.25 per 100,000, respectively; oropharyngeal: 1.83 vs 1.50 per 100,000, respectively) (Table 1). Non-Hispanic white and black people had non-statistically significant decreases in cervical (−4.65% p=0.11, and −5.12% p=0.67, respectively) and oropharyngeal cancer mortality (−1.78% p=0.50, and −12.53% p=0.26, respectively), while Asian and Hispanic people had non-statistically significant increases in cervical (5.04% p=0.62, and 4.07% p=0.87, respectively) and oropharyngeal cancer mortality (2.09% p=0.80, and 20.68% p=0.31, respectively). However, these numbers were small and data should be interpreted with caution.
Number attributable to HPV
In Massachusetts, 180 cases (91%) of cervical cancer and 250 cases (70%) of oropharyngeal cancers were estimated to be attributable to infection with any type of HPV each year (Table 2). Of those cases estimated to be attributable to any type of HPV, 130 cases of cervical cancer ( 72%), and 220 cases of oropharyngeal cancer (88%) are estimated to be attributable to the two high risk types of HPV, HPV 16 and 18, which are targeted by all available HPV vaccines. Of those cases estimated to be attributable to any type of HPV, 160 cases of cervical cancer (89%) and 240 cases of oropharyngeal cancer (96%) are estimated to be attributable to HPV 16, 18, 31, 33, 45, 52, and 58, which are targeted by the new 9-valent vaccine.
Table 2.
Estimated annual average number of cancers attributable to HPV for cervical and oropharyngeal cancer in Massachusetts from 2004 to 2014
| Cancer | Average Annual Number | Estimated attributable to any HPV type Number (%) | Estimated attributable to HPV 16/18 Number (%) | Estimated attributable to HPV 16/18/31/33/45/52/58 Number (%) |
|---|---|---|---|---|
|
| ||||
| Cervical | 199 | 180 (90.6) | 130 (66.2) | 160 (80.9) |
| Oropharyngeal | 363 | 250 (70.1) | 220 (60.2) | 240 (65.9) |
| Males | 284 | 210 (72.4) | 180 (63.4) | 190 (67.8) |
| Females | 79 | 50 (63.3) | 40 (50.8) | 50 (60.3) |
Number estimated to be attributable to HPV is rounded to the nearest 10, percentages from source [1], average annual number is total from 2004–2014 divided by 11
Oropharyngeal cancer risk factors
There are many risk factors for oropharyngeal cancer including tobacco, alcohol, and HPV-associated risk factors such as the number of sexual partners. In Massachusetts, 14% of adults reported current cigarette smoking in 2010. The percentage of adults smoking decreased from 1990 to 2006 by 1.88% annually and then decreased from 2006 to 2010 by 5.56% annually (p<0.0001 and p<0.0001, respectively) (Appendix 2a). Massachusetts had a per capita annual ethanol consumption of 2.50 gallons in 2010 (Appendix 2b). The consumption decreased from 1977 to about 1992 and then remained more constant. The prevalence of heavy drinking was 7.2% in 2015, with 17.7% reporting binge drinking. Among Massachusetts adults ages 18 to 64 who reported being sexually active in 2010, 92.7% reported having 1 partner, 5.4% reported having 2–3 partners, and 1.9% reported 4+ partners. From 2000–2010, there was a 5.32% decrease in the percentage of adults having sex with 2–3 partners (p=0.015) and a 2.21% decrease in the percentage of adults having 4+ partners (p=0.38). For teen sexual behaviors in Massachusetts, there was a statistically significant decrease in the percentage of high school students ever having sex with four or more partners from 1993–2003 (−3.3% annually, p=0.009) and 2009–2013 (−8.2% annually, p=0.02) (Appendix 3a), and a statistically significant decrease in the percentage of high school students having sex before age 13 from 1993–2001 (−6.4% annually, p=0.008) and 2007–2015 (−9.2% annually, p=0.005) (Appendix 4b).
DISCUSSION
In Massachusetts, the incidence rates of the two most common HPV-associated cancers are moving in opposite directions. The incidence rate of oropharyngeal cancer has been increasing while the incidence rate of cervical cancer has been decreasing. Recent reports on oropharyngeal cancer in the US project that the prevalence of oropharyngeal cancer will pass the prevalence of cervical cancer by 2020 [13]. In Massachusetts this has already happened; the number of cases of oropharyngeal cancer is greater than the number of cases of cervical cancer (363 vs 199 on average each year) and the incidence rate of oropharyngeal cancer among males is already higher than the incidence rate of cervical cancer among females (7.85 vs 5.46 per 100,000). Reasons for the early crossing of incidence rates for cervical and oropharyngeal cancer should be explored further.
Cervical cancer screening may have caused the decreasing incidence rate of cervical cancer in Massachusetts and the US. Massachusetts has a lower rate of cervical cancer incidence and mortality than the US (incidence: 5.2 vs 7.4 per 100,000 for 2008–2012; mortality: 1.3 vs 2.3 per 100,000 for 2009–2013) [2,14]. While Massachusetts saw a 2.4% decrease in the incidence rate and a 3.7% decrease in the mortality rate of cervical cancer from 2004–2014, nationally there was 12.7% decrease in the incidence rate of cervical cancer from 1990–2006 followed by a 0.4% decrease from 2006–2014, and a 0.7% decrease in mortality from 2004 to 2014 [15]. Screening for cervical cancer, using cytology with or without HPV cotesting, is widely available but there are no routine screening tests for oropharyngeal cancer. Massachusetts has a small percentage of the population without health insurance as health insurance has been mandatory since 2006. In 2014, 88.0% of females ages 21–65 reported having a Pap smear in the last 3 years in Massachusetts compared to the US median of 82.6% [9]. In addition to the higher screening prevalence, vastly fewer females in Massachusetts were diagnosed at a distant stage when compared to the US (3% in Massachusetts vs 14% nationally) and the mortality rate from cervical cancer was lower in Massachusetts than the US [14]. If anything, the high screening rate for cervical cancer in Massachusetts should result in a higher incidence of early stage cancers if screening tests are catching slow growing non-symptomatic cases. However, cervical cancer screening also detects pre-cancerous lesions which can be removed before they become cancerous. The lower incidence, coupled with relatively lower mortality suggests that the true incidence of cervical cancer among women in Massachusetts is lower than that of the general US population.
However, due to the prevalence of screening tests and more recently the HPV vaccine, cervical cancer should be almost completely preventable. Further research is needed to understand why the rate of cervical cancer is still so high in Massachusetts. This may be due to problems accessing care after positive screening tests and a lack of education about screening tests and vaccination, particularly among black women who have the greatest disparities in incidence and mortality. However, it is of note that the rates for black women have been decreasing in the US from 1990 to 2014 [16]. Also of note, there appears to be an increasing incidence and mortality for cervical cancer among Asian women. This trend requires further monitoring as future data becomes available and additional research to understand the reason for this increase.
The increase in oropharyngeal cancers nationally is thought to be due to a rise in HPV-positive cancers while HPV-negative cancers have been decreasing [17]. HPV-negative oropharyngeal cancers are associated with heavy tobacco and alcohol use [17]. Massachusetts had a higher incidence rate of oropharyngeal cancer than that in the US (5.0 vs 4.5 per 100,000 for 2008–2012) [2]. The incidence rate of oropharyngeal cancer in Massachusetts increased by 2.8% annually from 2004–2014, while the incidence rate of oropharyngeal cancer in the US increased by 3.1% annually from 1999–2014 [15]. Massachusetts had a slightly higher per capita ethanol consumption than the US (2.50 vs 2.26 gallons in 2010), as well as a higher prevalence of heavy drinking (7.2% vs 5.9%) and binge drinking (17.7% vs 16.3%) in 2015 [9–10]. However, Massachusetts had a lower prevalence of current smoking than the US with 14.0% current smokers in Massachusetts compared to 17.5% of the US in 2010 [9]. Yet, looking at the trends of smoking and alcohol consumption over time (Appendix 2), these behaviors have been decreasing in both the US and Massachusetts which makes it unlikely that they account for the recent increases in oropharyngeal cancer incidence. The higher alcohol intake in Massachusetts may explain why Massachusetts has a higher incidence rate of oropharyngeal cancer than the US, but information on the HPV status of the cancers is needed.
The HPV-positive oropharyngeal cancers tend to be diagnosed in younger males who are non-smokers and have a better prognosis than HPV-negative cancers [17]. In Massachusetts, there was an increasing incidence rate for all age groups, with statistically significant increases for people in their 70s from 2004–2014 and 60s from 2004–2011. Unlike the trends at the national level, there was no statistically significant increase in middle-aged males in Massachusetts, potentially reflecting more HPV-negative cases in Massachusetts. However, the somewhat decreasing mortality rate is in line with the better prognosis of the HPV-positive cases. Risk factors for the HPV-positive oropharyngeal cancers include an increased number of sexual or oral sexual partners [17]. Unfortunately, long-term data on oral sex in Massachusetts are unavailable. Nationally, more males and females engaged in oral sex in the 2009 National Survey of Sexual Health and Behavior study than the 1992 National Health and Social Life Survey [18]. The 2009 National Survey of Sexual Health and Behavior study found that most adults have engaged in oral sex, with over half of women and men age 18 to 49 receiving oral sex in the past year [18]. The increase in the number of oral sex partners may be why there is an increase in the rates of oropharyngeal cancer; however, further research is needed to understand the risk factors for HPV-positive oropharyngeal cancers.
The rise in the incidence rate of oropharyngeal cancer, especially among males, and the declines in traditional risk factors, highlights the need to improve HPV vaccination coverage. While we are not yet able to see the impact of HPV vaccination on cancer incidence rates, understanding these trends sends a strong message about the need for prevention. It is estimated that of the cases that are thought to be caused by HPV, up to 89% of cervical cancers and 96% of oropharyngeal cancers may be caused by a strain of HPV currently covered by the vaccine. Massachusetts has a higher vaccination rate than the US average but improvements are needed. In 2016, 78% of girls and 66% of boys in Massachusetts had at least one dose compared with 65% of girls and 56% of boys nationally [4]. However, the percentage completing the vaccine series is even lower, especially among boys. The recommendation of the Advisory Committee on Immunization Practices for a two dose HPV vaccine series, for adolescents who do not have certain immunocompromising conditions and begin vaccination before age 15, will provide an opportunity to improve immunization coverage and achieve protection against vaccine-preventable HPV-related cancers [19].
In addition, better vaccination coverage is needed as teens in Massachusetts continue to be sexually active. In Massachusetts, the percentage of high school students reporting having sex did not change from 2003 to 2013 [20]. While the percentage of students having sex with four or more partners and the percentage of students having sex before age 13 decreased from 1993 to 2015, the need for HPV vaccination before the start of sexual activity and sexual education regarding HPV and other sexually transmitted infections remains important (Appendix 3) [12].
This study contains some limitations that should be considered when interpreting the findings. Data on cancer incidence may be under-reported in areas of Massachusetts close to neighboring states. However, the MCR has a reciprocal reporting agreement with 41 states, which should help reduce the problem of under-reporting. When data are analyzed by cancer site and another characteristic small numbers reduce precision; as a result, differences in sub group rates may be due to chance. The MCR does not contain information on whether HPV DNA is present in cancer tissues so the proportion attributable to HPV is assumed to be the same as the results of a genotyping study [1]. While the assumptions made to apply these data were stated in the methods and Appendix Table 2, we can see that our study has fewer non-Hispanic black cases, more male cases, and more cases diagnosed at an older age (age 70+) than the genotype paper [1]. Non-Hispanic blacks tend to have a lower prevalence of HPV than non-Hispanic whites, so this would lead our estimates to be too low [1]. People diagnosed at an older age tend to have a lower HPV prevalence so this would lead our estimate to be too high [1]. Many studies of oropharyngeal cancer in Massachusetts show lower prevalence of HPV positive cases than the national studies [21–25]. However, these studies tend to be smaller, may represent people treated in Boston rather than people who live in Massachusetts, and tend to not be population-based. If the HPV positive prevalence in Massachusetts is truly lower than the national study then our numbers attributable are over-estimates. Lastly, we did not adjust for hysterectomy prevalence when calculating rates of cervical cancer. From the data available in the Massachusetts BRFSS, we were not able to get the prevalence of hysterectomies by age and race for 2004–2014. Additionally there was a change in BRFSS methodology in 2011 that precluded combining data from before and after 2011. A different study using MCR data and modeling hysterectomy prevalence shows that adjusting for hysterectomies increases the rate of cervical cancer [26]. Thus cervical cancer rates presented in this analysis are likely underestimates of the corrected rates, due to a population denominator that is too large since women with hysterectomies have not been removed, and depending on the trends in hysterectomy by race/ethnicity may underestimate the level of disparity in cervical cancer incidence and mortality.
Massachusetts has a strong public health program working on HPV vaccination and HPV-associated cancers. The MDPH Comprehensive Cancer Control and Prevention Network has developed a plan which includes evidence-based strategies for both decreasing the number of HPV-associated cancers and improving HPV immunization coverage in both males and females [27]. In addition, the Cervical Cancer Working Group, Oral HPV Prevention Task Force, and other MDPH program partners have an integrated multidisciplinary approach to education, immunization, screening and care, and are dedicated to the reduction of HPV-associated cancer in Massachusetts [28]. Historical reports of cancers in Massachusetts can be found on the Massachusetts Cancer Registry website through the statewide and special reports [29].
In Massachusetts, while the incidence rate of oropharyngeal cancer has been rising among males and is higher than the rate in the US, the incidence rate of cervical cancer has been decreasing among females and is lower than the rate in the US. These findings are useful to state public health departments to support the promotion of HPV vaccination among both girls and boys. It also supports patient and provider efforts to promote cancer prevention, especially for HPV-associated cancers.
Supplementary Material
Appendix Figure 2: The current cigarette smoking prevalence is shown for Massachusetts males, females, and the entire population of Massachusetts from 1990 to 2010 in comparison to the median prevalence in the United States from 1996 to 2010 in Figure 2a. The per capita ethanol consumption for Massachusetts and the United States from 1977 to 2010 is shown in Figure 2b. The annual percent change values for the trends are presented in the legend. For figures 2a and 2b, statistically significant trends (p<0.05) in the APC are denoted with an *.
Appendix Figure 3: The prevalence of high school students having sex with 4 or more persons in Massachusetts and the United States is shown in Figure 3a. The prevalence of high school students having sex before age 13 in Massachusetts and the United States is shown in Figure 3b. The annual percent change values for the trends are presented in the legend. For figures 3a and 3b, statistically significant trends (p<0.05) in the APC are denoted with an *.
Acknowledgments
Funding: R25 CA 98566-10, T32 CA 009001-40, T32 ES 007069, U58 DP000821-05
We would like to thank Annie MacMillan and Richard Knowlton at the Massachusetts Cancer Registry and Susan Lett from the Division of Epidemiology and Immunization at the Massachusetts Department of Public Health for their help with this project. This journal article was supported by the Grant or Cooperative Agreement Number, NU58D006271, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. We would also like to acknowledge funding from the grants R25 CA 98566-10, T32 CA 009001-40, and T32 ES 007069.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Saraiya M, Unger ER, Thompson TD, et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. JNCI Journal of the National Cancer Institute. 2015;107(6):djv086. doi: 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viens LJ, Henley SJ, Watson M, et al. Human Papillomavirus–Associated — Cancers United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661–666. doi: 10.15585/mmwr.mm6526a1. doi: http://dx.doi.org/10.15585/mmwr.mm6526a1. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HPV, Cancer [online] [accessed Mar 22, 2017];2016 URL: https://www.cdc.gov/cancer/hpv/statistics/cases.htm.
- 4.U.S. Department of Health and Human Services (DHHS) The 2016 National Immunization Survey - Teen. Hyattsville, MD: Centers for Disease Control and Prevention; 2017. National Center for Health Statistics. [Google Scholar]
- 5.United States Census Bureau. QuickFacts Massachusetts [online] [accessed Mar 29, 2017];2016 URL: https://www.census.gov/quickfacts/table/PST045216/25.
- 6.North American Association of Central Cancer Registries (NAACCR) [accessed June 7, 2017];Certified Registries [online] 2016 https://www.naaccr.org/certified-registries/
- 7.Joinpoint Regression Program, Version 4.4.0.0. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute; Jan, 2017. [Google Scholar]
- 8.SAS 9.3. Cary, NC, USA: SAS Institute Inc; [Google Scholar]
- 9.Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. [accessed May 10, 2017];BRFSS Prevalence & Trends Data [online] 2015 URL: https://www.cdc.gov/brfss/brfssprevalence/
- 10.National Institute on Alcohol Abuse and Alcoholism. [Accessed May 17, 2017];Surveillance Report #102 Apparent Per Capita Alcohol Consumption: National, State, and Regional Trends, 1977–2013. URL: https://pubs.niaaa.nih.gov/publications/surveillance102/tab4-3_13.htm.
- 11.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV Infection Among Females in the United States. JAMA. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. [Accessed on May 17, 2017];Youth Risk Behavior Survey Questionnaire. 1993–2015 Available at: www.cdc.gov/yrbs.
- 13.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human Papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SEER Cancer Stat Facts: Cervix Uteri Cancer. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/statfacts/html/cervix.html. [Google Scholar]
- 15.Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2014. National Cancer Institute; Bethesda MD: 2016. https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site April 2017. [Google Scholar]
- 16.Centers for Disease Control and Prevention. [accessed January 15, 2018];Gynecologic Cancers: Cervical Cancer Rates by Race Ethnicity [online] 2017 URL: https://www.cdc.gov/cancer/cervical/statistics/race.htm.
- 17.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50(5):380–386. doi: 10.1016/j.oraloncology.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbenick D, Reece M, Schick V, et al. Sexual Behavior in the United States: Results from a National Probability Sample of Men and Women Ages 14–94. J Sex Med. 2010;7(suppl5):255–265. doi: 10.1111/j.1743-6109.2010.02012.x. [DOI] [PubMed] [Google Scholar]
- 19.Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination – Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mort Wkly Rep. 2016;65:1405–1408. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 20.Massachusetts Department of Public Health. Health Survey Program. [Accessed May 17, 2017];Data Brief: Massachusetts Adolescent Health: Sexual Health, Experiences, and Behaviors. 2016 Spring ; URL: http://www.mass.gov/eohhs/docs/dph/behavioral-risk/mass-adole-health-sexual-health.pdf.
- 21.Addison D, Seidelmann SB, Jangua SA, et al. Human Papillomavirus Status and the Risk of Cerebrovascular Events Following Radiation Therapy for Head and Neck Cancer. J Am Heart Assoc. 2017;6(9):e006453. doi: 10.1161/JAHA.117.006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorch JH, Hanna GJ, Posner MR, et al. Human Papillomavirus and Induction Chemotherapy versus Concurrent Chemoradiotherapy in Locally Advanced Oropahyrygneal Cancer: The Dana Farber Experience. Head & Neck. 2015;38(S1):E1618–E1624. doi: 10.1002/hed.24289. [DOI] [PubMed] [Google Scholar]
- 23.Nelson HH, Pawlita M, Michaud DS, et al. Immune Response to HPV16 E6 and E7 Proteins and Patient Outcomes in Head and Neck Cancer. JAMA Oncology. 2017;3(2):178–185. doi: 10.1001/jamaoncol.2016.4500. [DOI] [PubMed] [Google Scholar]
- 24.Nichols AC, Finkelstein DM, Faquin WC, et al. Bcl2 and Human Papilloma Virus 16 as Predictors of Outcome following Concurrent Chemoradiation for Advanced Oropharyngeal Cancer. Clin Can Res. 2010;16(7):2138–2146. doi: 10.1158/1078-0432.CCR-09-3185. [DOI] [PubMed] [Google Scholar]
- 25.Ringstrom E, Peters E, Hasegawa M, et al. Human Papillomavirus Type 16 and Squamous Cell Carcinoma of the head and Neck. Clin Cancer Res. 2002;8(10):3187–3192. [PubMed] [Google Scholar]
- 26.Stang A, Hawk H, Knowlton RK, et al. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995 to 2010. Annals of Epidemiology. 2014;24(11):849–854. doi: 10.1016/j.annepidem.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Massachusetts Department of Public Health. [Accessed July 19, 2017];Massachusetts Comprehensive Cancer Prevention and Control Network. http://www.mass.gov/eohhs/gov/departments/dph/programs/community-health/cancer-prev-and-control/program-structure.html.
- 28.Wagner R, Villa A. Oral Human Papillomavirus Infections and the Role of the Dental Professional. Journal of the Massachusetts Dental Society. 2107;65(4):12–15. [PubMed] [Google Scholar]
- 29.Massachusetts Cancer Registry, Massachusetts Department of Public Health. [Accessed January 12, 2018]; www.mass.gov/dph/mcr.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 2: The current cigarette smoking prevalence is shown for Massachusetts males, females, and the entire population of Massachusetts from 1990 to 2010 in comparison to the median prevalence in the United States from 1996 to 2010 in Figure 2a. The per capita ethanol consumption for Massachusetts and the United States from 1977 to 2010 is shown in Figure 2b. The annual percent change values for the trends are presented in the legend. For figures 2a and 2b, statistically significant trends (p<0.05) in the APC are denoted with an *.
Appendix Figure 3: The prevalence of high school students having sex with 4 or more persons in Massachusetts and the United States is shown in Figure 3a. The prevalence of high school students having sex before age 13 in Massachusetts and the United States is shown in Figure 3b. The annual percent change values for the trends are presented in the legend. For figures 3a and 3b, statistically significant trends (p<0.05) in the APC are denoted with an *.