Abstract
Purpose
To determine if replacing time spent in high and low impact physical activity (PA) predicts changes in pediatric bone mineral density (BMD) and content (BMC).
Methods
We analyzed data from the longitudinal Bone Mineral Density in Childhood Study (N=2,337 with up to 7 visits). The participants were aged 5–19 years at baseline, 51.2% were female and 80.6% were non-Black. Spine, total hip, and femoral neck areal BMD (aBMD) and total body less head (TBLH) BMC Z-scores were calculated. Hours per day (h/d) spent in high and low impact PA were self-reported. Standard covariate adjusted (partition model) and time allocation sensitive isotemporal substitution modeling frameworks were applied to linear mixed models. Statistical interactions with sex, self-reported ancestry, age and bone fragility genetic scores (percentage of aBMD lowering alleles carried) were tested.
Results
In standard models, high impact PA was positively associated with bone Z-score at all four skeletal sites (e.g., TBLH-BMC Z-score: beta=0.05, P=2.0×10−22), whereas low impact PA was not associated with any of the bone Z-scores. In isotemporal substitution models, replacing 1 h/d of low-for-high impact PA was associated with higher bone Z-scores (e.g., TBLH-BMC Z-score: beta=0.06, P=2.9×10−15). Conversely, replacing 1 h/d of high-for-low impact PA was associated with lower bone Z-scores (e.g., TBLH-BMC Z-score: beta=−0.06, P=2.9×10−15). The substitution associations were similar for each sex and ancestry group, and for those with higher and lower genetic scores for bone fragility (P-interactions >0.05), but increased in strength among the older adolescents (P-age interactions <0.05).
Conclusion
Time sensitive models suggest that replacing low impact PA for high impact PA would be beneficial for the growing skeleton in the majority of children.
Keywords: Bone mineral density, children, adolescents, weight-bearing
Introduction
Osteoporosis causes fracture, morbidity and early mortality, especially for females (1). Enhancing bone accrual in early life to optimize peak bone mass is one key strategy that can help towards preventing osteoporosis (2). Promoting high impact physical activity in childhood is considered particularly effective for enhancing bone accretion (3–10), with pre and early puberty considered a particularly important period to derive the bone-related benefits of physical activity (4, 6). However, the current physical activity guidelines for American youth do not specify the proportion of time children should engage in higher impact, weight-bearing activities that are most beneficial to the developing skeleton; only that muscle and bone strengthening activities should be performed 3 days per week (11). Furthermore, genetic factors are known to play a role in bone density and osteoporosis risk (12), but it is not known if such susceptibility affects the effectiveness of physical activity as a preventive measure. In this context, established variants near genes involved in WNT signaling are of particular interest given the known role of this biological pathway in mechanosensation (13, 14).
Epidemiological studies focusing on physical activity and pediatric areal bone mineral density (aBMD) have thus far neglected to consider how time is allocated. The 60 minute per day of moderate-to-vigorous physical activity (MVPA) recommendation could be achieved by engaging in 60 minutes of low impact, non-weight bearing physical activities (e.g. swimming and biking), 60 minutes of high impact, weight bearing physical activities (e.g. tennis, basketball), or by through multiple combinations of both (e.g. 30 minutes of each). Testing if the allocation of time spent in low and high impact physical activity associates with aBMD outcomes can be achieved using isotemporal modeling (15). Indeed, this method has been applied to test statistically how substituting time spent in physical activity, sedentary behavior and sleep might affect cardiometabolic health outcomes (16, 17), but it has not yet been applied to the study of high and low impact physical activity substitution in the context of pediatric bone health. This modeling approach is highly suitable for simulating the effect of low impact physical activity being displaced by high impact physical activity in an equal time exchange.
Using data from children of European ancestry in the Bone Mineral Density in Childhood Study (BMDCS), we previously reported using standard modeling (that did not account for time allocation) that high impact, weight bearing physical activity was associated with higher aBMD Z-scores, and that the associations held even for those genetically predisposed to bone fragility (as defined by a genetic score comprised of all known bone fragility variants) (18). Low impact physical activity was not associated with bone Z-scores using standard modeling (18). In the present study, we applied isotemporal substitution modeling to predict how replacing time spent in high versus low impact physical activity associated with aBMD Z-scores. We hypothesized that replacing low-for-high and replacing high-for-low impact physical activity through statistical modeling would be associated with higher and lower bone Z-scores, respectively. We also tested if the high versus low impact physical activity replacement associations were modified by sex, self-reported ancestry and age, pubertal stage, and by genetic susceptibility to bone fragility, as defined by either an overall genetic score or WNT signaling pathway specific genetic scores.
Methods
Study Sample
The BMDCS participants were enrolled at one of five sites: Los Angeles, CA; Cincinnati, OH; Omaha, NE; Philadelphia, PA; and New York, NY (19). The participants were recruited in 2002–2003, when aged 6–18 years, and were followed-up annually over 6-years until 2008–2009(19). In 2006–2007, the study was extended to include 5 and 19 years olds, who were followed annually over 2-years until 2008–2009. Blood or saliva was collected at the final visit (2008–2009) for the purpose of extracting and genotyping DNA. As part of the DNA collection effort, the sample was expanded to include additional cross-sectional cohorts of children recruited at the Cincinnati and Omaha sites. A description of the inclusion and exclusion criteria has been provided previously (18). The parents/guardians provided written informed consent for participants <18 years, and these participants provided assent. The participants >18 years provided written informed consent. Institutional Review Boards at each study site approved the BMDCS protocol.
Bone Z-score Outcomes
Participants had their whole body, lumbar spine and proximal femur scanned by dual energy X-ray absorptiometry (DXA) annually. All scans were obtained from Hologic, Inc. densitometers (Bedford, MA: QDR4500A, QDR4500W, Delphi A and Apex models) and were centrally analyzed at the University of California, San Francisco’s DXA Core Laboratory. The scans were adjusted for machine differences and longitudinal drift. A set of phantoms was circulated to all sites and was used to cross-calibrate DXA devices. One site was selected as the reference site, and DXA values from all other sites were adjusted to align with the reference site according to the phantom measurements. Longitudinal drift in each DXA device was monitored by examination of longitudinal phantom scans and technical service reports. The CUSUM procedure in SAS was applied to identify adjustments to account for drift or changes in machine performance. We included the following aBMD and BMC bone Z-scores in our study: spine aBMD, total hip aBMD, femoral neck aBMD and TBLH-BMC. These bone Z-scores were calculated, with adjustment for height, to account for increases and sex differences in aBMD/BMC during growth, using the BMDCS reference values (19, 20). We used aBMD estimates for the three individual sites since aBMD is less size dependent since it accounts for bone area in cm2. For whole body scans, we used BMC adjusted for height Z-scores because the depth and composition of bone throughout the body is variable (e.g., ribs vs. femurs are quite different in cortical bone thickness, resulting in quite different densities) and whole body aBMD is an average of these many types of bone.
Physical Activity Exposures
We used a modified version of the Slemenda questionnaire to estimate hours per day leisure time physical activity levels (21). The approach we employed has been reported previously (18). In brief, we estimated total leisure time physical activity, as well as time spent in high impact physical activities and low impact physical activities. The high impact physical activity estimate includes time spent in weight bearing physical activities with a ground reaction force greater than 2× body weight involving sprinting, turning or jumping actions (22). Whereas the low impact physical activity estimate includes non-weight-bearing physical activities or weight-bearing physical activities with a ground reaction force of 1–2× body weight (22). Note, the majority of physical activities included in the questionnaire would require at least moderate intensity aerobic effort to complete (>3 metabolic equivalents). The high and low impact physical activities are listed in Supplementary Table 1 (see Table, Supplemental Digital Content 1, physical activity impact assignment).
Genetic Scores
Extracted DNA was genome-wide genotyped for common variants, using the Illumina Infinium™ II OMNI Express plus Exome BeadChip (Illumina, San Diego) (23). We included 63common variants near known adult bone mass loci (12, 24–26). Using these variants we calculated the percentage of aBMD lowering alleles carried (27). We also restricted the list of variants to loci near genes with known involvement in the WNT signaling pathway (18 variants) (12) to calculate a WNT specific score (Supplementary Table 1). Furthermore, because some of the 18 WNT signaling pathway related variants are near genes that promote WNT signaling and others near genes that inhibit WNT signaling, we also calculated separate WNT-promotion and WNT-inhibition genetic scores (see Table, Supplemental Digital Content 2, variants used for genetic score calculations).
Covariates
We included the following covariates in our statistical models: age, self-reported population ancestry (Black or Non-Black), body mass index (BMI) Z-score, Tanner stage, and dietary calcium. BMI Z-score was calculated using U.S. standards (28). Pubertal stage was assessed by physicians or nurses with expertise in pediatric endocrinology; participants were categorized as pre-pubertal, pubertal and post-pubertal (Tanner stages I, II to IV, and V, respectively), based on testicular volume in males and breast development in females. A semi-quantitative food frequency questionnaire (Block Dietary Data Systems, Berkeley, CA) (29), which included forty-five food and beverage items, was used to estimate dietary calcium intake (mg/d).
Statistical Analysis
Linear mixed effects models, with random intercepts, were used to test for associations between time spent in high and low impact physical activity and bone Z-scores, to account for the correlation between the repeated measures. The between-subject variability was modeled as a random effect using the method of maximum likelihood (ML) estimation. Also, robust standard errors were calculated using the Huber-White approach.
For the standard, or partition, models (model M1), high and low impact physical activity were included in the same model, along with time constant covariates (Y, for each i individual) and time varying covariates (Z, for each i individual and each j time point). For the isotemporal substitution models, time spent in high impact (model M2a) and low impact (model M2b) physical activity were included in separate models, along with total physical activity (high + low impact) and the time constant and time varying covariates (15, 16).
| M1 |
| M2a |
| M2b |
with and independently
The partition model physical activity beta coefficients (model M1) are the predicted changes in bone Z-score for each additional 1 hour of high impact and low impact physical activity, adjusted for the covariates. In contrast, the isotemporal model beta coefficients are the predicted changes in bone Z-score for each additional 1 hour of high impact physical activity in place of low impact physical activity (model M2a), or for each additional 1 hour of low impact physical activity in place of high impact physical activity (model M2b), adjusted for the covariates.
Statistical interactions involving physical activity and key variables (X) were also tested using the isotemporal-modeling framework (models M3a and M3b). Specifically, statistical interactions with sex, self-reported ancestry, age and Tanner stage were tested to determine if low and high impact physical activity replacement associations with the bone Z-score outcomes were consistent for each sex and ancestry group, and across the chronological and biological age range of the sample. (Note, non-linear age interactions were tested, but there was no statistical evidence of quadratic (physical activity-age-age) or cubic age interactions (physical activity-age-age-age) and only the linear age interaction results are presented). Finally, among participants of European ancestry (defined using genetic ancestry markers) we tested for statistical interactions with genetic bone fragility scores. The analysis was restricted to these individuals because the variants comprising the score were discovered in GWAS of individuals of European ancestry. The goal was to determine if low and high impact physical activity replacements were consistent for those genetically predisposed to, or genetically protected against, bone fragility.
| M3a |
| M3b |
with and independently
All analyses were performed with both sexes combined and stratified by sex. The analytical sample we used in this study included 157 opposite sex siblings and so the older sibling was removed to meet the assumption of independent observations when both sexes were analyzed together. It is likely that siblings may have similar bone health and physical activity patterns, and we opposite sex siblings share 50% of their genetic code. We used an alpha level of 0.05 to indicate statistically significant main associations and interactions. All analyses were performed using Stata 13.0 (Stata Corp, College Station, TX).
Results
Our primary sample comprised of 2,337 participants with up to 7 annual bone Z-score estimates (Table 1). For those in the original cohort (N=1,391), with the opportunity to complete up to 7 study visits, over 50% completed all study visits and more than 86% completed 4 or more study visits (see Table, Supplemental Digital Content 3, longitudinal data patterns). For those in the extension cohort (N=454), with the opportunity to complete up to 3 study visits, 50.4% completed 2 or 3 study visits (see Table, Supplemental Digital Content 3, longitudinal data patterns). The descriptive statistics for the European ancestry sample with genetic data are provided in Supplementary Table 4 (see Table, Supplemental Digital Content 4, descriptive statistics for European ancestry participants). Just over half the sample was female (51.2% at baseline) and the majority of the sample was non-Black (80.6% at baseline). Self-reported physical activity averaged 1.60 hours per day at baseline, with high impact physical activity averaging 0.65 hours per day and low impact physical activity averaging 0.95 hours per day. Males reported more total physical activity compared to females, with the difference largely due to more time engaged in high impact physical activities (0.79 versus 0.50 hours per day at baseline). There were no major changes in the demographics of the sample over the course of the 7-year study period (Table 1), but a greater percentage of females remained at the final study visit (55.6% at visit 7) and there was a decline in the percentage of non-Black participants remaining at the final study visit (77.3% at visit 7). The average BMI Z-score of the sample declined from 0.33 at baseline to 0.28 at visit 7 (Table 1).
Table 1.
Descriptive characteristics of the analytical sample
| Visit 1 (N=2,327) |
Visit 2 (N=1,566) |
Visit 3 (N=1,483) |
Visit 4 (N=1,241) |
Visit 5 (N=1,087) |
Visit 6 (N=945) |
Visit 7 (N=739) |
|
|---|---|---|---|---|---|---|---|
| Original Cohort (6–18y), N | 1,391 | 1,338 | 1,290 | 1,241 | 1,087 | 945 | 739 |
| Extension Cohort (5y & 19y), N | 454 | 228 | 193 | ||||
| Cross-sectional Sample (5–19y), N | 482 | ||||||
| Age, mean (SD), years | 11.4 (4.4) | 11.2 (3.5) | 12.1 (3.4) | 13.8 (3.1) | 14.7 (3.0) | 15.3 (2.7) | 15.7 (2.4) |
| BMI, mean (SD), Z-score | 0.33 (0.82) | 0.33 (0.86) | 0.33 (0.88) | 0.32 (0.90) | 0.31 (0.92) | 0.31 (0.90) | 0.28 (0.90) |
| Dietary Calcium, mean (SD), mg/d | 907.4 (537.6) | 866.6 (504.3) | 878.2 (524.7) | 840.4 (550.2) | 837.0 (568.2) | 798.9 (520.1) | 774.0 (469.3) |
| Female, N (%) | 1,191 (51.2) | 807 (51.5) | 766 (51.7) | 654 (52.7) | 580 (53.4) | 510 (54.0) | 411 (55.6) |
| Non-black, N (%) | 1,875 (80.6) | 1,187 (80.6) | 1,136 (76.6) | 959 (77.3) | 839 (77.2) | 726 (76.8) | 571 (77.3) |
| Tanner I, N (%) | 1,052 (45.2) | 720 (46.0) | 570 (38.4) | 256 (20.6) | 166 (15.3) | 103 (10.9) | 163 (22.1) |
| Tanner I–IV, N (%) | 638 (27.4) | 423 (27.0) | 392 (26.4) | 377 (30.4) | 322 (29.6) | 279 (29.5) | 131 (17.7) |
| Tanner V, N (%) | 637 (27.4) | 423 (27.0) | 521 (35.1) | 608 (49.0) | 599 (55.1) | 563 (59.6) | 445 (60.2) |
| Total Physical Activity, mean (SD), hr/d | 1.60 (1.28) | 1.53 (1.25) | 1.81 (1.26) | 1.79 (1.20) | 1.78 (1.20) | 1.82 (1.28) | 1.64 (1.22) |
| - Male | 1.78 (1.40) | 1.71 (1.34) | 1.87 (1.27) | 1.82 (1.21) | 1.93 (1.25) | 1.99 (1.25) | 1.79 (1.39) |
| - Female | 1.43 (1.13) | 1.37 (1.13) | 1.75 (1.24) | 1.76 (1.20) | 1.65 (1.14) | 1.67 (1.15) | 1.52 (1.08) |
| High Impact, mean (SD), hr/d | 0.65 (0.70) | 0.58 (0.68) | 0.92 (0.79) | 0.91 (0.77) | 0.90 (0.79) | 0.89 (0.82) | 0.81 (0.81) |
| - Male | 0.79 (0.78) | 0.73 (0.76) | 0.98 (0.81) | 0.97 (0.82) | 1.02 (0.85) | 1.02 (0.89) | 0.91 (0.90) |
| - Female | 0.50 (0.59) | 0.43 (0.56) | 0.86 (0.77) | 0.86 (0.72) | 0.80 (0.72) | 0.78 (0.74) | 0.73 (0.73) |
| Low Impact mean (SD), hr/d | 0.95 (0.90) | 0.96 (0.94) | 0.89 (0.82) | 0.88 (0.80) | 0.88 (0.75) | 0.92 (0.84) | 0.83 (0.75) |
| - Male | 0.98 (0.95) | 0.98 (0.96) | 0.89 (0.81) | 0.85 (0.78) | 0.91 (0.76) | 0.97 (0.92) | 0.87 (0.80) |
| - Female | 0.92 (0.86) | 0.94 (0.91) | 0.89 (0.82) | 0.90 (0.82) | 0.85 (0.75) | 0.88 (0.76) | 0.79 (0.71) |
| Spine aBMD, mean (SD), Z-score | −0.05 (0.93) | −0.06 (0.95) | −0.09 (0.96) | −0.09 (0.98) | −0.08 (0.99) | −0.08 (1.00) | −0.12 (1.04) |
| Total Hip aBMD, mean (SD), Z-score | 0.01 (0.98) | −0.01 (0.97) | −0.01 (0.97) | 0.01 (1.00) | 0.00 (1.00) | −0.00 (1.03) | −0.02 (1.06) |
| Femoral Neck aBMD, mean (SD), Z-score | 0.01 (0.94) | 0.00 (0.95) | 0.00 (0.96) | −0.00 (0.98) | −0.00 (0.97) | −0.01 (1.01) | −0.01 (1.03) |
| TBLH-BMC, mean (SD), Z-score | 0.08 (0.74) | 0.06 (0.73) | 0.12 (0.74) | 0.13 (0.77) | 0.10 (0.78) | 0.04 (0.81) | 0.05 (0.85) |
Partition Models
Using the partition-modeling framework, high impact physical activity was associated with higher bone Z-scores across all 4 skeletal sites (Table 2). For example, each additional hour of high impact physical activity was associated with 0.05 higher TBLH-BMC Z-score (95%CI: 0.04, 0.06; P=2.0×10−22). The high impact physical activity associations were directionally consistent among males and females, although the strength of the high impact associations were stronger among males for the femoral neck aBMD and total hip aBMD Z-scores (Table 2, sex P-interactions <0.05). In contrast, low impact physical activity was not associated with bone Z-scores (Table 2), with the exception of a negative association with female femoral neck aBMD Z-score (beta=−0.02, 95% CI: −0.04, −0.01, P=0.009; sex P-interaction=0.025). The same pattern of associations were observed for the partition models stratified by self-reported ancestry (see Table, Supplemental Digital Content 5, associations by self-reported ancestry).
Table 2.
Physical activity and bone Z-score associations from partition and isotemporal substitution models
| Standard Partition Model | Isotemporal Substitution Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Site | N | Obs | Physical Activitya |
Beta (95% CI) | P-value | P-Interaction (Sex) |
Beta (95% CI) | P-value | P-Interaction (Sex) |
| Both | FN | 2337 | 9388 | Low | −0.01 (−0.02,0.01) | 0.345 | 0.025 | −0.06 (−0.08,−0.04) | 4.35E-09 | 0.601 |
| Female | FN | 1265 | 5366 | Low | −0.02 (−0.04,−0.01) | 0.009 | −0.06 (−0.08,−0.03) | 2.08E-05 | ||
| Male | FN | 1229 | 4999 | Low | 0.00 (−0.02,0.02) | 0.986 | −0.07 (−0.09,−0.04) | 1.09E-06 | ||
| Both | FN | 2337 | 9388 | High | 0.05 (0.04,0.07) | 6.66E-14 | 0.011 | 0.06 (0.04,0.08) | 4.35E-09 | 0.601 |
| Female | FN | 1265 | 5366 | High | 0.03 (0.02,0.05) | 7.55E-05 | 0.06 (0.03,0.08) | 2.08E-05 | ||
| Male | FN | 1229 | 4999 | High | 0.07 (0.05,0.08) | 2.92E-12 | 0.07 (0.04,0.09) | 1.09E-06 | ||
| Both | TH | 2337 | 9388 | Low | 0.00 (−0.01,0.01) | 0.876 | 0.287 | −0.05 (−0.07,−0.03) | 1.86E-08 | 0.216 |
| Female | TH | 1265 | 5366 | Low | −0.01 (−0.02,0.00) | 0.128 | −0.05 (−0.07,−0.02) | 3.65E-05 | ||
| Male | TH | 1229 | 4999 | Low | 0.00 (−0.02,0.01) | 0.709 | −0.06 (−0.09,−0.04) | 6.56E-08 | ||
| Both | TH | 2337 | 9388 | High | 0.05 (0.04,0.06) | 7.11E-14 | 0.021 | 0.05 (0.03,0.07) | 1.86E-08 | 0.216 |
| Female | TH | 1265 | 5366 | High | 0.04 (0.02,0.05) | 5.75E-06 | 0.05 (0.02,0.07) | 3.65E-05 | ||
| Male | TH | 1229 | 4999 | High | 0.06 (0.04,0.08) | 4.23E-12 | 0.06 (0.04,0.09) | 6.56E-08 | ||
| Both | SP | 2334 | 9381 | Low | 0.00 (−0.01,0.01) | 0.953 | 0.362 | −0.04 (−0.06,−0.02) | 2.27E-05 | 0.329 |
| Female | SP | 1263 | 5346 | Low | −0.01 (−0.02,0.01) | 0.311 | −0.03 (−0.06,−0.01) | 0.005 | ||
| Male | SP | 1228 | 5012 | Low | 0.00 (−0.02,0.01) | 0.735 | −0.05 (−0.08,−0.03) | 1.64E-05 | ||
| Both | SP | 2334 | 9381 | High | 0.04 (0.03,0.05) | 4.76E-09 | 0.051 | 0.04 (0.02,0.06) | 2.27E-05 | 0.329 |
| Female | SP | 1263 | 5346 | High | 0.03 (0.01,0.05) | 3.15E-03 | 0.03 (0.01,0.06) | 0.005 | ||
| Male | SP | 1228 | 5012 | High | 0.05 (0.03,0.07) | 4.19E-09 | 0.05 (0.03,0.08) | 1.64E-05 | ||
| Both | TB | 2334 | 9224 | Low | −0.01 (−0.02,0.00) | 0.122 | 0.557 | −0.06 (−0.07,−0.04) | 2.89E-15 | 0.359 |
| Female | TB | 1265 | 5259 | Low | −0.01 (−0.03,0.00) | 0.065 | −0.05 (−0.07,−0.03) | 5.45E-08 | ||
| Male | TB | 1226 | 4930 | Low | −0.01 (−0.02,0.00) | 0.194 | −0.07 (−0.08,−0.05) | 4.32E-11 | ||
| Both | TB | 2334 | 9224 | High | 0.05 (0.04,0.06) | 2.00E-22 | 0.092 | 0.06 (0.04,0.07) | 2.89E-15 | 0.359 |
| Female | TB | 1265 | 5259 | High | 0.04 (0.03,0.05) | 2.58E-10 | 0.05 (0.03,0.07) | 5.45E-08 | ||
| Male | TB | 1226 | 4930 | High | 0.06 (0.04,0.07) | 3.11E-15 | 0.07 (0.05,0.08) | 4.32E-11 | ||
All models adjusted for age, race, Tanner stage, BMI Z-score, and dietary calcium. When both sexes were combined, sex was included as a covariate and the older of the opposite sex siblings was dropped.
Abbreviations: FN, femoral neck aBMD; TH, total hip aBMD; SP, spine aBMD; TB, total body less head BMC.
Low refers to time spent in low impact physical activity; the partition model beta coefficients are the change in bone Z-score for each additional 1 hour of low impact physical activity, adjusted for the covariates (including high impact physical activity); the isotemporal model beta coefficients are the change in bone Z-score for each additional 1 hour of low impact physical activity in place of high impact physical activity. aHigh refers to time spent in high impact physical activity; the partition model beta coefficients are the change in bone Z-score for each additional 1 hour of high impact physical activity, adjusted for the covariates (including low impact physical activity); the isotemporal model beta coefficients are the change in bone Z-score for each additional 1 hour of high impact physical activity in place of low impact physical activity.
Isotemporal Models
Using the isotemporal-modeling framework, replacing low-for-high impact physical activity was associated with higher bone Z-scores across all 4 sites (Table 2 and Figure 1). For example, replacing1 hour per day of low-for-high impact physical activity was associated with 0.06 higher TBLH-BMC Z-score (95%CI: 0.04, 0.07; P=2.9×10−15). Replacing high-for-low impact physical activity was associated with lower bone Z-scores across all 4 sites in a direct reciprocal manner (Table 2 and Figure 1). Therefore, for TBLH-BMC this translates to an overall +0.12 Z-score difference for a 1-hour low-for-high physical activity replacement and a −0.12 Z-score difference for a 1-hour high-for-low physical activity replacement.
Figure 1.
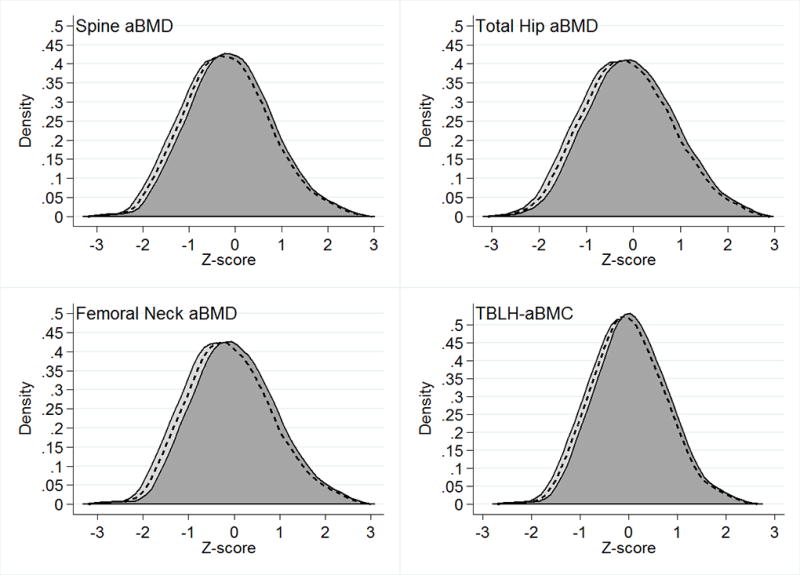
Bone Z-score frequency distributions by weight bearing physical activity composition. The dashed lines represent bone Z-scores for a population spending 1 hour per day in total physical activity with equal time in high and low impact physical activity. The light gray distributions (shifted to the left) represent bone Z-scores for a population spending 1 hour per day in low impact physical activity and no time in high impact physical activity. The dark gray distributions (shifted to the right) represent bone Z-scores for a population spending no time in low impact physical activity and 1 hour per day in high impact physical activity.
The low-for-high and high-for-low impact physical activity substitutions were directionally consistent and of similar strength for males and females (Table 2, sex P-interaction >0.05). Similarly, there was no evidence of statistical interactions between low-for-high and high-for-low impact physical activity substitutions and self-reported ancestry (see Table, Supplemental Digital Content 5, associations by self-reported ancestry), with the exception for a stronger substitution association observed for non-Black participants for femoral neck aBMD (see Table, Supplemental Digital Content 5, associations by self-reported ancestry).
In contrast, the low-for-high and high-for-low impact physical activity substitution associations with bone Z-scores were consistently modified by age (P-age interactions <0.05). Specifically, significant associations were first observed between ages 8 to 11 (depending on the skeletal site) and increased in strength towards age 19 (Figure 2). In sex-stratified analyses, the age interactions remained statistically significant among males, but not females [see Figure, Supplemental Digital Content 6, High and low impact physical activity substitutions and associations with bone Z-scores by age among (A) males and (B) females]. The total numbers of observations at age are given in Supplementary Figure 2 (see Figure, Supplemental Digital Content 7, observations by age).
Figure 2.
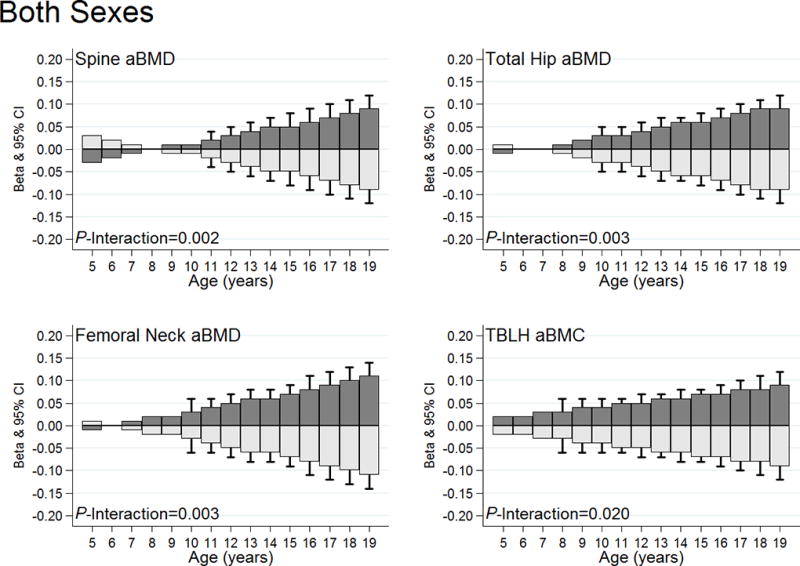
High and low impact physical activity substitutions and associations with bone Z-scores by age. The dark gray bars illustrate substitution associations for low-for-high impact physical activity. Light gray bars illustrate substitution associations for high-for-low impact physical activity. Standard error bars are included for the age-specific beta coefficients that are statistically significant (P<0.05). All models adjusted for age, sex, Tanner stage, BMI Z-score, and dietary calcium. The sex-specific graphs can be viewed in Supplementary Figure 1 [see Figure, Supplemental Digital Content 6, High and low impact physical activity substitutions and associations with bone Z-scores by age among (A) males and (B) females]. The numbers of observations provided at each age are provided in Supplementary Figure 2 (see Figure, Supplemental Digital Content 7, The total observations by 1-year age groups for both sexes, males and females).
We additionally tested for Tanner stage interactions. In this analysis we observed that the low-for-high and high-for-low impact physical activity substitution associations with spine aBMD, femoral neck aBMD and TBLH-BMC Z-scores were modified by biological age (P-Tanner interactions <0.05). Specifically the substitution associations were observed among pre- and post-pubertal children (Figure 3). Similar findings were observed when we stratified by sex [see Figure, Supplemental Digital Content 8, High and low impact physical activity substitutions and associations with bone Z-scores by Tanner stage categories among (A) males and (B) females].
Figure 3.
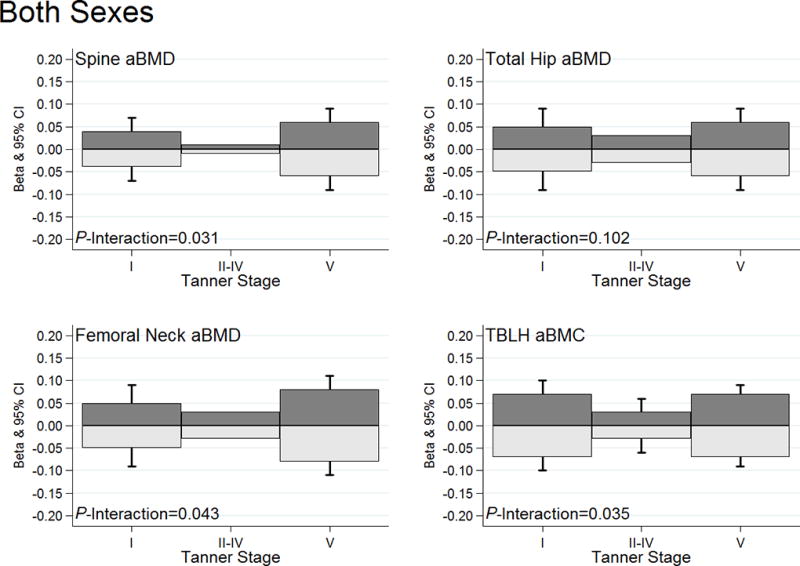
High and low impact physical activity substitutions and associations with bone Z-scores by Tanner stage categories. The dark gray bars illustrate substitution associations for low-for-high impact physical activity. Light gray bars illustrate substitution associations for high-for-low impact physical activity. Standard error bars are included for the age-specific beta coefficients that are statistically significant (P<0.05). All models adjusted for age, sex, Tanner stage, BMI Z-score, and dietary calcium. The sex-specific graphs can be viewed in Supplementary Figure 3 [see Figure, Supplemental Digital Content 8, High and low impact physical activity substitutions and associations with bone Z-scores by Tanner stage categories among (A) males and (B) females].
Finally, we observed no statistical evidence that the effect of replacing low-for-high or high-for-low impact physical activity on bone Z-scores differed by the overall genetic susceptibility score or by the WNT signaling specific genetic scores, restricted to participants of European ancestry (Figure 4, genetic score P-interactions >0.05). This was also the case in sex-stratified analyses for males and females [see Figure, Supplemental Digital Content 9, High and low impact physical activity substitution by genetic risk score tertiles for (A) males and (B) females].
Figure 4.
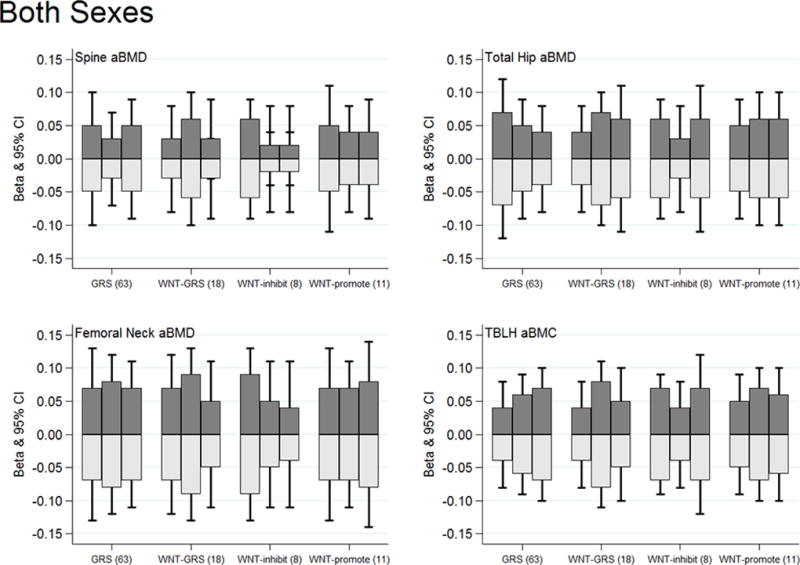
High and low impact physical activity substitutions associations with bone Z-scores by genetic risk score tertiles for both sexes combined. The dark gray bars illustrate substitution associations for low-for-high impact physical activity. Light gray bars illustrate substitution associations for high-for-low impact physical activity. No statistical evidence of any physical activity substitution by genetic score interactions (P-interactions >0.05). All models adjusted for age, sex, Tanner stage, BMI Z-score, and dietary calcium. The sex-specific graphs can be viewed in Supplementary Figure 4 [see Figure, Supplemental Digital Content 9, High and low impact physical activity substitution by genetic risk score tertiles for (A) males and (B) females].
Discussion
We investigated associations between high and low impact physical activity in childhood and aBMD Z-scores at 3 skeletal sites and TBLH-BMC Z-scores. We applied standard (partition) and isotemporal substitution modeling frameworks. As previously reported, the standard models revealed that high impact physical activity was associated with higher bone Z-scores and that low impact physical activity was not associated with bone Z-scores. However, by accounting for time allocation the isotemporal substitution models revealed the adverse effects of low impact physical, in place of high impact physical activity, on bone density outcomes. Importantly, we demonstrated that benefits of high impact physical activity, in place of low impact physical activity, were consistent for each sex and reported ancestry group, and for children genetically predisposed to bone fragility. These findings underscore the importance of accumulating at least 60 minutes of moderate to vigorous intensity physical activity (MVPA) per day, of which a large proportion is dedicated to high impact physical activity, in order to aid pediatric bone accretion.
The current physical activity guidelines for American youth state that children and adolescents should aim to accumulate at least 60 minutes of MVPA on most days of the week, and perform muscle and bone strengthening activities as part of their daily physical activity on at least 3 days per week (11). The guidelines were based on multiple observational and experimental studies, including studies showing the benefits of weight-bearing physical activity on pediatric bone density phenotypes (4, 6). Not all studies agree that this threshold is optimal for pediatric bone health (30), but our data generally support the guidelines. However, we suggest a refinement such that the proportion of MVPA dedicated to high and low impact activity may need to be directly recommended in the guideline to help optimize bone health. By using a self-reported physical activity measurement approach the participants likely overestimated their hours per day of physical activity, making it challenging to quantify an exact refinement. In relative terms the average proportion of total physical activity dedicated to high impact physical activity was 45% in our sample; therefore, a refinement to recommend at least 50% of daily MVPA (i.e. 30 minutes daily) to be dedicated towards high impact physical activity could be considered with respect to bone accretion in childhood. Replication of our findings in an independent sample is needed, including using raw accelerometry output, in gravitational units, to estimate time spent in high and low impact physical activity (7, 30–32).
It is especially important to optimize bone accretion in early life among females, who are more likely than males to be diagnosed with osteoporosis in later life (1). It is noteworthy that we did not observe evidence of statistical interactions between sex and physical activity with respect to the bone Z-score outcomes using the isotemporal substitution framework. It is also noteworthy that the proportion of total physical activity dedicated to high impact activities was lower in females, compared to the males, in our study. Therefore, females are equally responsive to the bone-related benefits of high impact physical activity in early life compared to males, but the lower amount of high impact physical activity in which they engage is a concern. Public health approaches that specifically target increases in high impact physical activity among females may be beneficial for bone health.
In addition to testing for sex differences, we also tested if any of the physical activity substitution predictions with bone Z-scores were modified by population ancestry group. Overall, we did not observe extensive evidence of any such modification, with only a stronger substitution association observed for non-black participants for femoral neck aBMD. In contrast, the benefits of replacing low-for-high and the detriments of replacing high-for-low physical activity were modified by chronological and biological age. Linear age interaction models first revealed associations from ages 8 to 11 (depending on the skeletal site) that gained in strength towards age 19. This age modification persisted in males, but not females, in sex-stratified analyses (although the age-specific beta coefficients were stronger among older females for spine, total hip and femoral neck aBMD). However, the Tanner stage interactions models revealed associations specifically among the pre- and post-pubertal children.
Reviews and meta-analyses of pediatric RCTs on exercise and bone outcomes have led to a consensus that physical activity positively effects bone accretion specifically during the pre-pubertal years (4, 6). This consensus is drawn from experimental studies of which few were designed to test for puberty differences in exercise response. We reported consistent evidence of the benefits of high impact physical activity on bone Z-score outcomes among pre- and post-pubertal children as defined by chronological and biological age. However, our chronological and biological age interaction observation were inconsistent with respect to the peri-pubertal years. Overall, the beneficial effects of high impact physical activity were observed in childhood, and into early adulthood when a large proportion of bone is accrued before peak bone mass is achieved (33). The lack of a statistical significant association in the peri-pubertal group could be due to bone gains in this particular period being driven by growth. That is, the benefits of high impact physical activity are harder to detect when growth is the primary driver of bone accrual.
As with all complex diseases and traits, osteoporosis and aBMD are partly heritable (12). We have shown that bone fragility loci discovered in adults operate in the pediatric setting (27, 34). We have also shown, using standard (partition) modeling that physical activity was beneficial for aBMD regardless of the overall genetic risk score for bone fragility (18). However, in our previous study we did observe borderline evidence of a physical activity interaction with a variant near a gene involved in WNT signaling (18). This pathway has a number of roles in skeletal development, but its function within osteocytes appears primarily be to translate mechanical loads into chemical signals for bone (re)modeling (14). Therefore, WNT signaling related variants that associate with aBMD could alter mechanosensitivity and thereby the musculoskeletal response to physical activity. In the present study we extended on our previous work by using isotemporal substitution modeling and including WNT-specific genetic scores. In line with our previous observations, we draw a similar conclusion: high impact physical activity is beneficial to the developing skeleton regardless of genetic susceptibility to bone fragility. This conclusion is based on current knowledge of the genetics of bone fragility and could be adapted as new genetic discoveries are made.
Our study has several limitations. We used an observational study design and did not experimentally test the physical activity substitutions. Our physical activity questionnaire allowed for estimation of high and low impact physical activity, but relied on participants’ recall. It is possible that younger children have less ability to recall their physical activities, and this should be considered when interpreting the age/Tanner interaction results. Also, our physical activity measurement approach assumes all participants complete an activity to the same ability; again age-related differences in motor skills, strength/lean mass and aerobic capacity should be considered when interpreting the age/Tanner interaction results; older children are perhaps more able to optimally load their skeleton when engaging in high impact physical activity. Furthermore, we asked participants to primarily recall time spent in activities that require at least moderate intensity effort; we therefore do not know how replacing sedentary behavior to engage in low impact physical could affect bone Z-scores; or if replacing high impact physical activity to engage in sedentary behavior is more detrimental than replacing to engage in low impact physical activity. We adjusted for BMI in our models, but we did not specifically adjust for lean mass. It would be warranted to test how low and high impact physical activities contribute to lean mass, and if any gain in lean mass translate into an osteogenic effect (35). Our genetic findings can only be generalized to U. S. children of European descent, and while we had good cohort retention not all participants remained in the study that further limits generalizability. Finally, DXA estimated aBMD does not provide volumetric density or an estimate of trabecular and cortical bone density, or macro and micro structural properties, and follow-up studies using other imaging methodologies are needed.
Conclusions
Isotemporal substitution modeling predictions confirmed the beneficial effects of high impact physical activity on pediatric bone density, but highlighted that spending time in low-impact physical activities at the expense of high-impact physical activities may exert a detrimental effect on bone density. Importantly, recommending physical activity for bone health, especially high impact physical activity, is applicable to children genetically predisposed to bone fragility, as defined by an overall genetic score and WNT signaling specific scores.
Supplementary Material
SDC 1—Physical activities reported and impact group designation
SDC 2—GWAS implicated bone variants used to calculate genetic risk scores
SDC 3—Longitudinal data patterns for the original cohort (up to 7 study visits) and the extension cohort (up to 3 study visits)
SDC 4—Descriptive characteristics of the European ancestry analytical sample with genetic data
SDC 5—Physical activity and bone Z-score associations from partition and isotemporal substitution models by self-reported race
SDC 6—High and low impact physical activity substitutions and associations with bone Z-scores by age among (A) males and (B) females.
SDC 7—The total observations by 1-year age groups for both sexes, males and females.
SDC 8—High and low impact physical activity substitutions and associations with bone Z-scores by Tanner stage categories among (A) males and (B) females.
SDC 9—High and low impact physical activity substitution by genetic risk score tertiles for (A) males and (B) females.
Acknowledgments
We appreciate the dedication of the study participants and their families, and the support of Dr. Karen Winer, Scientific Director of the Bone Mineral Density in Childhood Study. The study was supported by funding from the National Institutes of Health (NIH), grant numbers: R01HD58886 and UL1TR000077. The study was also supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) contracts: N01-HD-1-3228, -3329, -3330, -3331, -3332, -3333. Jonathan Mitchell was support by NIH grant K01HL123612. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose. The results of the present study do not constitute endorsement by ACSM.
References
- 1.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6. doi: 10.1002/jbmr.2269. Epub 2014/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janz KF, Letuchy EM, Burns TL, Eichenberger Gilmore JM, Torner JC, Levy SM. Objectively measured physical activity trajectories predict adolescent bone strength: Iowa Bone Development Study. Br J Sports Med. 2014;48(13):1032–6. doi: 10.1136/bjsports-2014-093574. Epub 2014/05/20. bjsports-2014-093574 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behringer M, Gruetzner S, McCourt M, Mester J. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J Bone Miner Res. 2014;29(2):467–78. doi: 10.1002/jbmr.2036. [DOI] [PubMed] [Google Scholar]
- 5.Duckham RL, Rantalainen T, Ducher G, Hill B, Telford RD, Telford RM, et al. Effects of Habitual Physical Activity and Fitness on Tibial Cortical Bone Mass, Structure and Mass Distribution in Pre-pubertal Boys and Girls: The Look Study. Calcif Tissue Int. 2016;99(1):56–65. doi: 10.1007/s00223-016-0128-4. [DOI] [PubMed] [Google Scholar]
- 6.Specker B, Thiex NW, Sudhagoni RG. Does Exercise Influence Pediatric Bone? A Systematic Review. Clin Orthop Relat Res. 2015;473(11):3658–72. doi: 10.1007/s11999-015-4467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deere K, Sayers A, Rittweger J, Tobias JH. Habitual levels of high, but not moderate or low, impact activity are positively related to hip BMD and geometry: results from a population-based study of adolescents. J Bone Miner Res. 2012;27(9):1887–95. doi: 10.1002/jbmr.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabel L, Macdonald HM, Nettlefold L, McKay HA. Physical Activity, Sedentary Time, and Bone Strength From Childhood to Early Adulthood: A Mixed Longitudinal HR-pQCT study. J Bone Miner Res. 2017 doi: 10.1002/jbmr.3115. [DOI] [PubMed] [Google Scholar]
- 9.Golden NH, Abrams SA. Optimizing bone health in children and adolescents. Pediatrics. 2014;134(4):e1229–43. doi: 10.1542/peds.2014-2173. Epub 2014/10/01. peds.2014-2173 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Lofgren B, Dencker M, Nilsson JA, Karlsson MK. A 4-year exercise program in children increases bone mass without increasing fracture risk. Pediatrics. 2012;129(6):e1468–76. doi: 10.1542/peds.2011-2274. [DOI] [PubMed] [Google Scholar]
- 11.Services UDoHaH, editor. Services UDoHaH. 2008 Physical Activity Guidelines for Americans. 2008. [Google Scholar]
- 12.Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. Epub 2012/04/17. doi: ng.2249 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morse A, McDonald M, Kelly N, Melville K, Schindeler A, Kramer I, et al. Mechanical Load Increases in Bone Formation via a Sclerostin-Independent Pathway. J Bone Miner Res. 2014;29(11):2456–67. doi: 10.1002/jbmr.2278. Epub 2014/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature medicine. 2013;19(2):179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 15.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–27. doi: 10.1093/aje/kwp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang WY, Wong SH, He G, Salmon JO. Isotemporal Substitution Analysis for Sedentary Behavior and Body Mass Index. Med Sci Sports Exerc. 2016;48(11):2135–41. doi: 10.1249/MSS.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 17.Buman MP, Winkler EA, Kurka JM, Hekler EB, Baldwin CM, Owen N, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol. 2014;179(3):323–34. doi: 10.1093/aje/kwt292. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell JA, Chesi A, Elci O, McCormack SE, Roy SM, Kalkwarf HJ, et al. Physical Activity Benefits the Skeleton of Children Genetically Predisposed to Lower Bone Density in Adulthood. J Bone Miner Res. 2016;31(8):1504–12. doi: 10.1002/jbmr.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. The Journal of clinical endocrinology and metabolism. 2011;96(10):3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265–73. doi: 10.1210/jc.2009-2057. Epub 2010/01/28. doi: jc.2009-2057 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CC., Jr Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6(11):1227–33. doi: 10.1002/jbmr.5650061113. Epub 1991/11/11. [DOI] [PubMed] [Google Scholar]
- 22.Groothausen J, Siemer H, Kemper H, Twisk J, Welten D. Influence of Peak Strain on Lumbar Bone Mineral Density: An Analysis of 15-Year Physical Activity in Young Males and Females. Pediatric Exercise Science. 1997;9:159–73. [Google Scholar]
- 23.Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448(7153):591–4. doi: 10.1038/nature06010. Epub 2007/07/17. doi: nature06010 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371(9623):1505–12. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, et al. Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008;358(22):2355–65. doi: 10.1056/NEJMoa0801197. [DOI] [PubMed] [Google Scholar]
- 26.Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199–206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell JA, Chesi A, Elci O, McCormack SE, Roy SM, Kalkwarf HJ, et al. Genetic Risk Scores Implicated in Adult Bone Fragility Associate With Pediatric Bone Density. J Bone Miner Res. 2016;31(4):789–95. doi: 10.1002/jbmr.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000(314):1–27. Epub 2001/02/24. [PubMed] [Google Scholar]
- 29.Ollberding NJ, Gilsanz V, Lappe JM, Oberfield SE, Shepherd JA, Winer KK, et al. Reproducibility and intermethod reliability of a calcium food frequency questionnaire for use in Hispanic, non-Hispanic Black, and non-Hispanic White youth. J Acad Nutr Diet. 2015;115(4):519–27 e2. doi: 10.1016/j.jand.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gracia-Marco L, Moreno LA, Ortega FB, Leon F, Sioen I, Kafatos A, et al. Levels of physical activity that predict optimal bone mass in adolescents: the HELENA study. Am J Prev Med. 2011;40(6):599–607. doi: 10.1016/j.amepre.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Deere KC, Hannam K, Coulson J, Ireland A, McPhee JS, Moss C, et al. Quantifying Habitual Levels of Physical Activity According to Impact in Older People: Accelerometry Protocol for the VIBE Study. J Aging Phys Act. 2016;24(2):290–5. doi: 10.1123/japa.2015-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobias JH, Gould V, Brunton L, Deere K, Rittweger J, Lipperts M, et al. Physical Activity and Bone: May the Force be with You. Front Endocrinol (Lausanne) 2014;5:20. doi: 10.3389/fendo.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormack SE, Cousminer DL, Chesi A, Mitchell JA, Roy SM, Kalkwarf HJ, et al. Association Between Linear Growth and Bone Accrual in a Diverse Cohort of Children and Adolescents. JAMA Pediatr. 2017;171(9):e171769. doi: 10.1001/jamapediatrics.2017.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell JA, Chesi A, McCormack SE, Roy SM, Cousminer DL, Kalkwarf HJ, et al. Rare EN1 Variants and Pediatric Bone Mass. J Bone Miner Res. 2016;31(8):1513–7. doi: 10.1002/jbmr.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlachopoulos D, Ubago-Guisado E, Barker AR, Metcalf BS, Fatouros IG, Avloniti A, et al. Determinants of Bone Outcomes in Adolescent Athletes at Baseline: The PRO-BONE Study. Med Sci Sports Exerc. 2017;49(7):1389–96. doi: 10.1249/MSS.0000000000001233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1—Physical activities reported and impact group designation
SDC 2—GWAS implicated bone variants used to calculate genetic risk scores
SDC 3—Longitudinal data patterns for the original cohort (up to 7 study visits) and the extension cohort (up to 3 study visits)
SDC 4—Descriptive characteristics of the European ancestry analytical sample with genetic data
SDC 5—Physical activity and bone Z-score associations from partition and isotemporal substitution models by self-reported race
SDC 6—High and low impact physical activity substitutions and associations with bone Z-scores by age among (A) males and (B) females.
SDC 7—The total observations by 1-year age groups for both sexes, males and females.
SDC 8—High and low impact physical activity substitutions and associations with bone Z-scores by Tanner stage categories among (A) males and (B) females.
SDC 9—High and low impact physical activity substitution by genetic risk score tertiles for (A) males and (B) females.


