Abstract
Purpose
To determine if testosterone supplementation is associated with retinal artery (RAO) or vein occlusions (RVO).
Methods
Retrospective matched-cohort study using data from a large national US insurance database. The testosterone cohort consisted of all male patients who filled a prescription for testosterone from 2000-2013. Five controls were matched on age (±3 years), sex, race and similar time in plan (±3 months) for every exposed patient. Exclusion occurred for <2 years in the plan, <1 eye care visit, medications known to effect androgen levels and systemic diseases associated with occlusions or increased testosterone. Cox proportional hazard regression assessed the hazard of a new diagnosis of RAO or RVO while controlling for age, race, diabetes mellitus and hypertension.
Results
35,784 incident testosterone users were compared with 178,860 matched controls. 93 (0.3%) RAOs and 50 (0.1%) RVOs were found in the testosterone cohort and contrasted with 316 (0.2%) RAOs and 232 (0.1%) RVOs in the control group. After multivariate analysis, testosterone supplementation significantly increased the hazard for RAO (HR: 1.43, 95%CI: 1.12-1.81, p=0.004), but not RVO (HR: 1.03, 95%CI: 0.74-1.42, p=0.86).
Conclusion
Although the incidence of RAO and RVO is low in users of testosterone, supplementation therapy is associated with an increased hazard of RAO, but apparently not RVO.
Keywords: Androgens, hormone-replacement therapy, retinal artery occlusion, retinal vascular occlusion, retinal vein occlusion, testosterone
Introduction
Retinal vascular occlusions, which include retinal artery occlusion (RAO) and retinal vein occlusion (RVO), are the second most common causes of blindness from retinal vascular disease.1-3 RAO and RVO can occur secondary to endogenous and exogenous thrombosis, embolization, vasculitis or vasospasm and can result in significant permanent vision loss.4-9 Both common systemic cardiovascular risk factors (i.e. hypertension, hyperlipidemia, diabetes mellitus, etc.), as well as less common hypercoaguable states (i.e. protein C or protein S deficiency, hyperhomocysteinemia, etc.)can predispose patients to develop RAO.4,5 Many of these same patients are also at risk of developing RVO due to the calcification of arterial walls impinging retinal veins, which can lead to narrowing and stasis and ultimately resulting in venous thrombosis.10,11
Another, more recently recognized cause of vascular thrombosis is the use of hormone replacement therapy. The use of oral estrogen-progestin contraceptives,8,12 clomiphene citrate13 and estrogen replacement therapies7,14 have been associated with both RAOs15-17 and RVOs.13,18-22 Multiple reports have also implicated exogenous androgenic hormone regimens in the development in thrombotic and cardiovascular events in patients with and without a previously undiagnosed thrombophilia-hypofibrinolysis.23,24
Indications for supplemental testosterone use include low libido, infertility, hair loss, prostate cancer and sarcopenia,25-27 and its use has increased dramatically worldwide in men since 2000.25,28 With this increase, a greater focus has been placed on identifying the potential risks of exogenous testosterone. To date, direct clinical evidence for a possible link to vascular disease has primarily been derived from case reports.23,24,29,30 Recently, however, a large meta-analysis found compelling evidence that 1 year of supplemental testosterone therapy significantly increased coronary artery noncalcified and total plaque volumes compared to a placebo group.31 Specific to testosterone's potential role in retinal disease, basic science studies have found androgen receptors and enzymes in endothelial cells32,33 and in retinal tissue,34-37 as well as a pro-inflammatory effect of testosterone in vascular endothelial cells.38 To date, no studies have evaluated the risk of retinal occlusive disease with exogenous testosterone. The aim of this study was to determine if supplemental testosterone use is associated with RAO or RVO.
Methods
Data Source
The Clinformatics™ Data Mart Database (OptumInsight, Eden Prarie, MN) is a de-identified administrative medical claims database containing the records of all enrollees in a large insurance network from across the United States. The data set includes demographic (age, sex, race) data, all outpatient medical claims (office visits and associated diagnoses for ocular and non-ocular medical conditions) and all outpatient pharmaceutical prescriptions filled for each participant enrolled in the insurance plan from January 1, 2000 to December 31, 2013. All individuals in the medical plan had full pharmacy coverage during their time enrolled. The University of Pennsylvania's Institutional Review Board deemed this study exempt from review due to the de-identified nature of the data.
Cohorts
We identified a cohort of exposed individuals based on filling a prescription for testosterone in any of the following forms: oral, topical gel, patch or intramuscular. Other inclusion criteria required patients be male, to have at least 2 years of continuous data in the dataset and 1 or more visits to an eye care provider prior to their first testosterone prescription (the index date). Individuals were also excluded for specific systemic diseases associated with increased risk of thrombosis, disorders associated with increased testosterone or with any medication use known to have androgen or anti-androgen effects, including patients with recorded ICD-9 codes for collagen vascular disease, coagulopathy, sickle cell disease, homocystinuria, platelet abnormalities, hyperviscosity or testicular cancer or 1 or more prescriptions for anti-androgen medications, including aromatase inhibitors: anastrozole, exemestane, toremifene citrate, fulvestrant, letrozole and testolactone. Finally, to decrease the likelihood of misdiagnosis, all patients with an ocular history of intraocular surgery (within 90 days prior to index date), diabetic retinopathy, glaucoma, optic disc drusen, Susac syndrome, retinal artery occlusion (RAO), retinal vein occlusion (RVO), Giant cell arteritis or non-arteritic anterior ischemic optic neuropathy were also excluded. (See Table, Supplemental Digital Content 1 for all ICD-9 codes used in this study.)
An unexposed cohort was also created consisting of 5 matched controls for every eligible case. The controls were matched on age (±3 years), race, sex and starting and ending eligibility time within the insurance plan (±3 months). The same index date as the matched case was assigned to the controls. Matches were also required to meet all of the same inclusion and exclusion criteria as the exposed cohort.
Outcome and Covariates of Interest
The primary outcome of interest was an incident diagnosis of RAO, RVO or a combined outcome of either RAO or RVO. Additional covariates of interest were history of diabetes mellitus (DM) (without a prior diagnosis of retinopathy) and hypertension (HTN).
Statistical Analysis
Baseline demographic data was evaluated at the time of the index date. Characteristics for the exposed and unexposed cohorts were summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. The hazard of RAO, RVO and the combined outcome were determined using Cox proportional hazard regression with censoring for end of eligibility or incidence of any the above exclusion criteria after the index date. SAS version 9.4 (SAS Institute Inc., Cary, NC) software was used for all statistical analysis.
Results
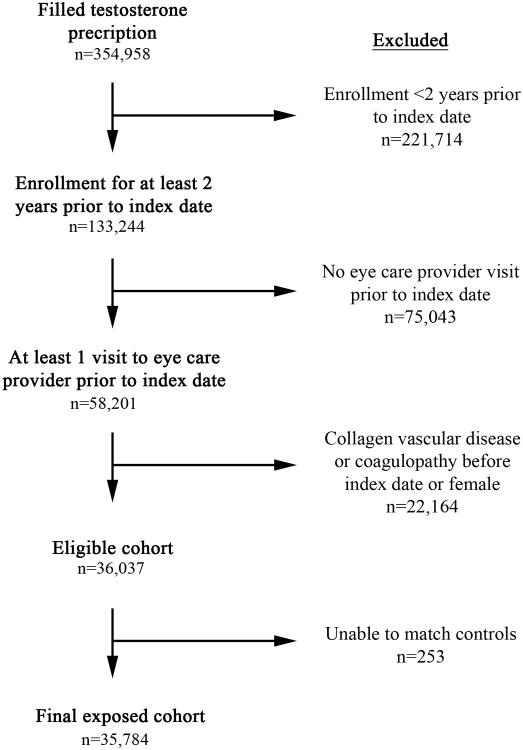
The exposed cohort consisted of 35,784 users of testosterone and 178,860 unexposed matched controls. (Figure 1) Within the testosterone cohort, 93 (0.3%) RAOs, 50 (0.1%) RVOs and 126 (0.4%) combined outcomes were found. This was compared to 316 (0.2%) RAOs, 232 (0.1%) RVOs and 532 (0.3%) combined outcomes in the unexposed cohort. The mean age of patients in the testosterone cohort was 54.9±12.0 (years±standard deviation) and in the controls was 54.7±12.1 (p=0.01).(Table 1) The racial distribution was similar for both cohorts with 34.2% white, 2.9% black, 3.5% Hispanic, 0.70% Asian and 58.7% unknown (p=1.00). The testosterone cohort had significantly more patients with diabetes mellitus (26.1% versus 13.9%, p=0.005) and hypertension (63.8% versus 45.8%, p=0.006).
Figure 1.
Flowchart demonstrating the patients excluded for each criteria and the final exposed cohort in the study.
Table 1. Baseline characteristics.
| Characteristic | Factor | Exposed (n=35,769) | Unexposed (n=178,845) | p-value* |
|---|---|---|---|---|
| Age (years) | Mean (±SD) | 54.9 (±12.0) | 54.7 (±12.1) | 0.01 |
| Race | White | 12,221 (34.2%) | 61,105 (34.2%) | 1.00 |
| Black | 1,024 (2.9%) | 5,120 (2.9%) | ||
| Hispanic | 1,267 (3.5%) | 6,335 (3.5%) | ||
| Asian | 247 (0.7%) | 1,235 (0.7%) | ||
| Unknown | 21,010 (58.7%) | 105,050 (58.7%) | ||
| Diabetes mellitus | No | 26,444 (73.9%) | 153,993 (86.1%) | 0.005 |
| Yes | 9,325 (26.1%) | 24852 (13.9%) | ||
| Hypertension | No | 12,950 (36.2%) | 96,917 (54.2%) | 0.006 |
| Yes | 22,819 (63.8%) | 81,928 (45.8%) |
p-value <0.05 is significant
SD = standard deviation
Univariate analysis (Table 2) revealed that increasing age, DM and HTN were each associated with an increasing hazard for each of the outcomes (p<0.01 for all comparisons). Race was not significantly associated with the development of any of the outcomes (p>0.12 for all comparisons). Testosterone supplementation was associated with a significantly increased HR for RAO (HR: 1.52, 95% CI: 1.21-1.93, p <0.001) and the combined outcome (HR: 1.23, 95% CI: 1.01-1.48, p=0.04), but was not associated with RVOs (HR: 1.11, 95% CI: 0.81-1.53, p=0.51).
Table 2.
Univariate analysis for each outcome.
| Total Patients | RAO | RVO | RAO/RVO combined | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Hazard Ratio (95% CI) | p-value* | n (%) | Hazard Ratio (95% CI) | p-value* | n (%) | Hazard Ratio (95% CI) | p-value* | |||
| Age | 214,644 | 409 (0.2%) | 1.07 (1.06-1.08) | <0.001 | 282 (0.1%) | 1.07 (1.06-1.08) | <0.001 | 658 (0.3%) | 1.07 (1.06-1.08) | <0.001 | |
| Race | White | 73,338 | 146 (0.2%) | -- | 0.12 | 119 (0.2%) | -- | 0.66 | 253 (0.3%) | -- | 0.46 |
| Black, Hispanic, Asianτ | 15,232 | 35 (0.2%) | 1.21 (0.78-1.88) | 26 (0.2%) | 1.10 (0.71-1.68) | 55 (0.4%) | 1.09 (0.81-1.48) | ||||
| Unknown | 126,074 | 228 (0.2%) | 1.28 (1.01-1.61) | 137 (0.1%) | 0.91 (0.69-1.21) | 350 (0.3%) | 1.11 (0.94-1.32) | ||||
| DM | No | 180,463 | 327 (0.2%) | -- | 0.01 | 217 (0.1%) | -- | <0.001 | 517 (0.3%) | -- | <0.001 |
| Yes | 34,181 | 82 (0.2%) | 1.42 (1.09-1.86) | 65 (0.2%) | 1.67 (1.26, 2.23) | 141 (0.4%) | 1.53 (1.27, 1.86) | ||||
| HTN | No | 109,873 | 125 (0.1%) | -- | <0.001 | 87 (0.1%) | -- | <0.001 | 205 (0.2%) | -- | <0.001 |
| Yes | 104,771 | 284 (0.3%) | 2.58 (2.06-3.24) | 195 (0.2%) | 2.49 (1.88, 3.31) | 453 (0.4%) | 2.48 (2.10, 2.94) | ||||
| Testosterone | No | 178,860 | 316 (0.2%) | -- | <0.001 | 232 (0.1%) | -- | 0.51 | 532 (0.3%) | -- | 0.04 |
| Yes | 35,784 | 93 (0.3%) | 1.52 (1.21-1.93) | 50 (0.1%) | 1.11 (0.81, 1.53) | 126 (0.4%) | 1.23 (1.01-1.48) | ||||
p-value <0.05 considered significant
combined groups due to low individual numbers
-- comparator group
DM = diabetes mellitus
HTN = hypertension
RAO = retinal artery occlusion
RVO = retinal vein occlusion
CI = confidence interval
After controlling for all covariates of interest, multivariate analysis (Table 3) showed that testosterone supplementation was associated with an increased hazard of RAO (HR: 1.43, 95% CI: 1.12-1.81, p=0.004), but not RVO (HR: 1.03, 95% CI: 0.74-1.42, p=0.86), or the combined RAO/RVO outcome (HR: 1.14, 95% CI: 0.94-1.38, p=0.18). Increasing age and hypertension also continued to be associated with an increased hazard of each of the three outcomes (p<0.007 for all comparisons).
Table 3.
Multivariate analysis for each outcome.
| RAO | RVO | RAO/RVO combined | |||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value* | Hazard Ratio (95% CI) | p-value* | Hazard Ratio (95% CI) | p-value* | ||
| Age | 1.06 (1.05-1.07) | <0.001 | 1.07 (1.06-1.08) | <0.001 | 1.07 (1.06-1.08) | <0.001 | |
| Race | White | -- | 0.01 | -- | 0.64 | -- | 0.045 |
| Black, Hispanic, Asianτ | 1.36 (0.88-2.11) | 1.22 (0.80-1.88) | 1.22 (0.91-1.65) | ||||
| Unknown | 1.41 (1.12-1.78) | 1.02 (0.77-1.35) | 1.23 (1.04-1.346) | ||||
| DM | Yes | 0.91 (0.69-1.20) | 0.50 | 1.11 (0.82-1.49) | 0.50 | 1.01 (0.83-1.23) | 0.90 |
| HTN | Yes | 1.64 (1.29-2.09) | <0.001 | 1.53 (1.12-2.10) | 0.007 | 1.57 (1.31-1.88) | <0.001 |
| Testosterone | Yes | 1.43 (1.12-1.81) | 0.004 | 1.03 (0.74-1.42) | 0.86 | 1.14 (0.94-1.38) | 0.18 |
p-value <0.05 considered significant
combined groups due to low individual numbers
-- comparator group
DM = diabetes mellitus
HTN = hypertension
RAO = retinal artery occlusion
RVO = retinal vein occlusion
CI = confidence interval
Discussion
Although the overall incidence of RAO in patients using supplemental testosterone in this study was low (0.3%), this use demonstrated a 43% increased hazard of developing an RAO. We did not find an association with RVOs. Despite this low incidence, the increasing use of testosterone may still lead to significant ramifications for the health of its users given the impact thrombotic events can have on a patient's well being.
The exact mechanisms underlying increased risk of vascular occlusion with testosterone use are unknown, but likely involve multiple factors. Many arterial thrombotic diseases are believed to occur secondary to platelet activation.39 Rupture of an atherosclerotic plaque results in recruitment of platelets. This propagates a cyclical process of thrombus formation by platelet binding and release of platelet factors resulting in further platelet recruitment, adhesion and activation.24,39 In a recent study in men given supplemental testosterone therapy for 1 year, a significantly greater increase in both coronary artery noncalcified and total plaque volume was seen compared to the placebo group as measured by coronary computed tomographic angiography.31 This effect on arterial plaque volume in coronary arteries may also occur elsewhere in the body, increasing risk for plaque rupture and distal arterial occlusion, including RAO.
In addition to thrombus formation, testosterone may also directly affect platelets. Androgen receptors have been found on mammalian platelets40 and may regulate the expression of thromboxane A2 in platelets.41 It is through this mechanism that testosterone may cause a similar effect to that described in patients receiving supplemental estrogen and progesterone. One study in women receiving hormone replacement therapy for 12 weeks reported a significant increase in the amount of circulating activated platelets.30 Furthermore, dihydrotestosterone in endothlelial cells has been found to induce vascular cell adhesion molecule-1 expression, which increased monocyte binding to the endothelium and acute coronary events.32 Despite this circumstantial evidence, the exact role for how hormone replacement therapy is related to venous thrombosis is still unclear, as platelets play a significant role in thrombosis and hemostasis in conditions of high shear stress, which generally does not occur in the venous circulation.42
We did not find an association between supplemental testosterone use in men and RVOs. In one case series of women that developed vascular thrombosis, including RVO, while on testosterone therapy, all three women were found to have gene mutations predisposing them to thrombophilia and/or hypofibrinolysis.23 It is possible that there is a differential effect of testosterone between the sexes, which was not evaluated in our study. Additionally, we excluded patients with known predisposing conditions to thrombophilia. It is possible that testosterone may still pose additional risks in these patients. Further studies are needed to better understand testosterone use in patients with thrombophilia or hypofibrinolysis to better counsel them when considering supplemental testosterone use.
This study had several strengths. First, the matched-cohort study design allows for both a reduction in selection bias, as well as for studying the temporal sequence of exposure and outcome. Next, we were able to access data on a large sample of insured individuals throughout the United States, which allowed for a better powered study. Finally, as we obtained the medication usage from the pharmacy records, instead of patient self-reports, recall bias was eliminated.
Our study also has several limitations that need to be considered. First, due to the nature of the claims data we could not access chart level data to verify diagnoses. Lacking this access may have lead to some patients being erroneously included or excluded as RAO or RVO. However, this would only be problematic if there was a difference in the misclassification between the exposed and unexposed cohorts and it is unlikely that physicians would be influenced for or against diagnosing RAO or RVO due to a testosterone prescription. Next, given the study design we were not able to control for non-prescription or over-the-counter testosterone supplements that may have been used by other patients in either cohort. Since unexposed patients would in actuality be exposed, this would bias towards the null, suggesting our estimate of the true association is low. Finally, since all patients included in the study had health insurance, the results may not be generalizable to the greater population, especially to patients that do not have insurance or are enrolled in other insurance systems.
Lastly, it should be noted that as with any observational study, confounding is of the utmost concern. We attempted to reduce this by excluding all patients with any history of diseases that would predispose someone to forming a retinal emboli or vein occlusion, as well as all patients who had a prescription for a medication that may impact systemic androgen levels. Despite these efforts, residual confounding or unmeasured confounding may exist within our results. For example, we were unable to control for plasma fibrinogen or lipoprotein levels which may influence any specific person's likelihood for an emboli or clot. Similarly, although we were able to control for hypertension as a diagnosis, we were unable to control for specific systolic blood pressure levels, limiting our ability to discern between hypertension that is well or poorly controlled.
In this study, we evaluated the occurrence of RAO and RVO in men using supplemental testosterone therapy and found a significantly increased risk of developing RAO in those patients. As patients using testosterone supplements are at greater risk of retinal vascular occlusions, this diagnosis should encourage prompt referral for evaluation of other cardiovascular risk factors. Furthermore, education of patients of risk associated with supplemental testosterone therapy prior to initiation can promote early intervention and guide management if an adverse event occurs.
Supplementary Material
Summary Statement.
This is a study that evaluated for an association between testosterone supplementation and retinal vascular occlusions, which found a significantly increased hazard for developing retinal arterial occlusion with exogenous testosterone use.
Acknowledgments
Funding: National Institutes of Health K23 Award (1K23EY025729 - 01) and University of Pennsylvania Core Grant for Vision Research (2P30EYEY001583). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. Funding from each of the above sources was received in the form of block research grants to the Scheie Eye Institute. None of the organizations had any role in the design or conduction of the study.
Footnotes
Conflicts Of Interest: None
Financial Disclosures: None
Portions of this data will be presented at the 2017 International Conference on Pharmacoepidemiology Annual Meeting in Montreal, Canada in August, 2017
References
- 1.Cheung N, Klein R, Wang JJ, et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: the multiethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. 2008;49(10):4297–4302. doi: 10.1167/iovs.08-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1094–1101. doi: 10.1016/j.ophtha.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 4.Brown GC, Magargal LE, Shields JA, Goldberg RE, Walsh PN. Retinal arterial obstruction in children and young adults. Ophthalmology. 1981;88:18–25. doi: 10.1016/s0161-6420(81)35080-5. [DOI] [PubMed] [Google Scholar]
- 5.Brown GC, Magargal LE. Central retinal artery obstruction and visual acuity. Ophthalmology. 1982;89:14–19. doi: 10.1016/s0161-6420(82)34853-8. [DOI] [PubMed] [Google Scholar]
- 6.Karjalainen K. Occlusion of the central retinal artery and retinal branch arterioles: a clinical, tonographic and fluorescein angiographie study of 175 patients. Acta Ophthalmol (Copenh) 1971;109(1):l–95. [PubMed] [Google Scholar]
- 7.1Jick H, Derby LE, Myers MW, Vasilakis C, Newton KM. Risk of hospital admission for idiopathic venous thromboembolism among users of postmenopausal oestrogens. Lancet. 1996;348:981–983. doi: 10.1016/S0140-6736(96)07114-0. [DOI] [PubMed] [Google Scholar]
- 8.Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1995;346:1575–1582. [PubMed] [Google Scholar]
- 9.Lidegaard O. Thrombotic diseases in young women and the influence of oral contraceptives. Am J Obstet Gynecol. 1998;179:S62–S67. doi: 10.1053/ob.1998.v179.a91674. [DOI] [PubMed] [Google Scholar]
- 10.Rath EZ, Frank RN, Shin DH, Kim C. Risk factors for retinal vein occlusions. A case-control study. Ophthalmology. 1992;99:509–514. doi: 10.1016/s0161-6420(92)31940-2. [DOI] [PubMed] [Google Scholar]
- 11.Elman MJ, Bhatt AK, Quinlan PM, Enger C. The risk for systemic vascular diseases and mortality in patients with central retinal vein occlusion. Ophthalmology. 1990;97:1543–1548. doi: 10.1016/s0161-6420(90)32379-5. [DOI] [PubMed] [Google Scholar]
- 12.Petitti DB. Clinical practice. Combination estrogen-progestin oral contraceptives. N Engl J Med. 2003;349(15):1443–1450. doi: 10.1056/NEJMcp030751. [DOI] [PubMed] [Google Scholar]
- 13.Viola MI, Meyer D, Kruger T. Association between clomiphene citrate and visual disturbances with special emphasis on central retinal vein occlusion: a review. Gynecol Obstet Invest. 2011;71(2):73–76. doi: 10.1159/000319497. [DOI] [PubMed] [Google Scholar]
- 14.Glueck CJ, Wang P, Fontaine RN, Sieve-Smith L, Lang JE. Estrogen replacement therapy, thrombophilia, and atherothrombosis. Metabolism. 2002;51(6):724–732. doi: 10.1053/meta.2002.32729. [DOI] [PubMed] [Google Scholar]
- 15.Vastag O, Tornóczky J. Arterial occlusion in the ocular fundus induced by oral contraceptives. Orv Hetil. 1984;125(51):3121–3125. Hungarian. [PubMed] [Google Scholar]
- 16.Paufique L, Lequin M. Thrombosis of the retinal artery and oral contraceptives. Bull Soc Ophtalmol Fr. 1968;68(4):512–515. French. [PubMed] [Google Scholar]
- 17.Blade J, Darleguy P, Chanteau Y. Early thrombosis of the central retinal artery and oral contraceptives. Bull Soc Ophtalmol Fr. 1971;71(1):48–49. French. [PubMed] [Google Scholar]
- 18.Murray DC, Christopoulou D, Hero M. Combined central retinal vein occlusion and cilioretinal artery occlusion in a patient on hormone replacement therapy. Br J Ophthalmol. 2000;84(5):549–550. doi: 10.1136/bjo.84.5.546e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer H. A contribution about serious ophthalmic complications with oral contraceptives. Klin Monbl Augenheilkd. 1979;175(5):677–680. German. [PubMed] [Google Scholar]
- 20.Jaworek K. Case of central retinal vein thrombosis in a young woman treated with progesterone. Klin Oczna. 1976;46(1):75–78. Polish. [PubMed] [Google Scholar]
- 21.Leong KC, Tan PL. Central retinal vein thrombosis in a woman on contraceptive pills. Singapore Med J. 1974;15(2):156–157. [PubMed] [Google Scholar]
- 22.Güven D, Sayinalp N, Kalayci D, Dündar S, Hasiripi H. Risk factors in central retinal vein occlusion and activated protein C resistance. Eur J Ophthalmol. 1999;9(1):43–48. doi: 10.1177/112067219900900107. [DOI] [PubMed] [Google Scholar]
- 23.Glueck CJ, Bowe D, Valdez A, Wang P. Thrombosis in three postmenopausal women receiving testosterone therapy for low libido. Womens Health (Lond) 2013;9(4):405–410. doi: 10.2217/whe.13.31. [DOI] [PubMed] [Google Scholar]
- 24.Glueck CJ, Wang P. Testosterone therapy, thrombosis, thrombophilia, cardiovascular events. Metabolism. 2014;63(8):989–994. doi: 10.1016/j.metabol.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548–551. doi: 10.5694/mja13.10111. [DOI] [PubMed] [Google Scholar]
- 26.Dorff TB, Vogelzang NJ. Use of testosterone replacement therapy in patients with prostate cancer. Curr Urol Rep. 2011;12(3):223–228. doi: 10.1007/s11934-011-0176-2. [DOI] [PubMed] [Google Scholar]
- 27.Morley JE. Scientific overview of hormone treatment used for rejuvenation. Fertil Steril. 2013;99:1807–1813. doi: 10.1016/j.fertnstert.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Medm. 2013;173(15):1465–1466. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greven CM, Slusher MM, Weaver RG. Retinal arterial occlusions in young adults. Am J Ophthalmol. 1995;120:776–783. doi: 10.1016/s0002-9394(14)72731-x. [DOI] [PubMed] [Google Scholar]
- 30.Thijs A, van Baal WM, van der Mooren MJ, et al. Effects of hormone replacement therapy on blood platelets. Eur J Clin Invest. 2002;32(8):613–618. doi: 10.1046/j.1365-2362.2002.01039.x. [DOI] [PubMed] [Google Scholar]
- 31.Budoff MJ, Ellenberg SS, Lewis CE, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708–716. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Death AK, McGrath KC, Sader MA, et al. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway. Endocrinology. 2004;145(4):1889–1897. doi: 10.1210/en.2003-0789. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Wang LY, Jiang TY, et al. Effects of testosterone and 17-betaestradiol on TNF-alpha-induced E-selectin and VCAM-1 expression in endothelial cells. Analysis of the underlying receptor pathways. Life Sci. 2002;71(1):15–29. doi: 10.1016/s0024-3205(02)01567-9. [DOI] [PubMed] [Google Scholar]
- 34.Gelinas D, Callard GV. Immunocytochemical and biochemical evidence for aromatase in neurons of the retina, optic tectum and retinotectal pathways in goldfish. J Neuroendocrinol. 1993;5:635–641. doi: 10.1111/j.1365-2826.1993.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 35.Nagle CA, Cardinali DP, de Laborde NP, Rosner JM. Sex-dependent changes in rat retinal hydroxyindole-o-methyl transferase. Endocrinology. 1974;94:294–297. doi: 10.1210/endo-94-1-294. [DOI] [PubMed] [Google Scholar]
- 36.Lanthier A, Patwardhan VV. In vitro steroid metabolism by rat retina. Brain Research. 1988;463:403–406. doi: 10.1016/0006-8993(88)90419-2. [DOI] [PubMed] [Google Scholar]
- 37.Rocha EM, Wickham LA, Silverira LA, et al. Identification of androgen receptor protein and 5a-reductase mRNA in human ocular tissues. Br J Ophthalmol. 2000;84:76–84. doi: 10.1136/bjo.84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Annibalini G, Agostini D, Calcabrini C, et al. Effects of sex hormones on inflammatory reseponse in male and female vascular endothelial cells. J Endocrinol Invest. 2014;37:861–869. doi: 10.1007/s40618-014-0118-1. [DOI] [PubMed] [Google Scholar]
- 39.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451(7181):914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91:2742–2747. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 41.Ruige JB, Ouwens M, Kaufman JM. Beneficial and adverse effects of testosterone on the cardiovascular system in men. Endocrin Metab. 2013;98:4300–4310. doi: 10.1210/jc.2013-1970. [DOI] [PubMed] [Google Scholar]
- 42.Frojmovic MM. Platelet aggregation in flow: differential roles for adhesive receptors and ligands. Am Heart J. 1998;135:S119–S131. doi: 10.1016/s0002-8703(98)70240-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.