Abstract
Parental stress exposures are implicated in the risk for offspring neurodevelopmental and neuropsychiatric disorders, prompting critical examination of preconception and prenatal periods as vulnerable to environmental insults such as stress. Evidence from human studies and animal models demonstrates the influence that both maternal and paternal stress exposures have in changing the course of offspring brain development. Mechanistic examination of modes of intergenerational transmission of exposure during pregnancy has pointed to alterations in placental signaling, including changes in inflammatory, nutrient-sensing, and epigenetic pathways. Transmission of preconception paternal stress exposure is associated with changes in epigenetic marks in sperm, with a primary focus on the reprogramming of DNA methylation, histone post-translational modifications, and small non-coding RNAs. In this review, we discuss evidence supporting the important contribution of intergenerational parental stress in offspring neurodevelopment and disease risk, and the currently known epigenetic mechanisms underlying this transmission.
Keywords: Stress, Intergenerational transmission, PTSD, placenta, sperm, epigenetics
Introduction
Early life stress is a well-established risk factor for neuropsychiatric disorders across the lifespan. ‘Stress’ encompasses various environmental challenges that disrupt organismal homeostasis and result in physiological and/or behavioral responses (1). In humans, stressors can include metabolic challenges (e.g. famine), immune challenges (e.g. illness), and perceived psychological threats (e.g. social/emotional distress). In preclinical animal studies, stress can be imparted using psychological and/or physical challenges, including immobilization, social defeat, and isolation (2). During pregnancy, maternal stress alters the maternal milieu, which can directly or indirectly impact fetal development (3–4). Because the impact of stress is transmitted from parent to offspring, the term intergenerational transmission has been applied (5). However, preconception stress in either parent can impact germ cells, thus influencing development in one or more generations, resulting in transgenerational effects (6–7). As the most recent studies focusing on germ cell epigenetic transmission have been largely examined in paternal models, we discuss these studies in detail regarding stress programming of sperm as causal in offspring phenotypes.
Understanding the mechanisms by which parental stress exposure is ultimately communicated to the developing offspring brain is critical for elucidating the etiology of mental health disorders. Epigenetic control of gene expression, including DNA methylation, histone post-translational modifications (PTMs), and non-coding RNAs, evolved to regulate and establish cell- and tissue-specific gene expression programs and to control normal cellular functions (8–9). Stress experienced during critical developmental windows when these epigenetic patterns are generated can result in reprogramming of cellular epigenomes, leading to long-term changes in patterns of gene expression and cellular function. More specifically, stress exposure can lead to such epigenetic alterations in sperm and oocytes, resulting in transmission of altered marks to the zygote (7). Following conception, stress exposure can also directly alter epigenetic programming of the fetus by disrupting the function of extra-embryonic tissues, including the placenta, to promote alterations in key developmental signals throughout gestation. Thus, parental stress exposures during the preconception and prenatal windows can have lasting consequences on offspring development and, subsequently, adult outcomes.
In both human studies and animal models, intergenerational transmission of stress exposures have been associated with endophenotypes of stress-related neuropsychiatric disease in adult offspring, including disruption of the hypothalamus-pituitary-adrenal (HPA) stress axis. The HPA stress axis is important for glucocorticoid production in response to physiological and psychological challenges, and its dysregulation is an underlying feature of most neuropsychiatric disorders (10–11). Thus, many studies have focused on understanding developmental programming of the HPA stress axis as a readout of intergenerational transmission of stress exposure. In this review, we discuss the impact of maternal and paternal stress exposure on offspring neurodevelopmental outcomes, with a focus on offspring programming of the HPA stress axis. Moreover, we focus our discussion on the epigenetic mechanisms by which intergenerational transmission of stress exposure may be signaled to developing offspring.
Maternal Mechanisms of Intergenerational Stress Transmission
Stress during pregnancy is associated with an increased risk for autism spectrum disorders (ASD), schizophrenia, affective disorders, and attention deficit hyperactivity disorder (ADHD) in offspring, largely related to the specific stage of pregnancy in which stress experience occurred (4). For instance, epidemiological and clinical studies report that early pregnancy, when epigenomic patterning is established, has the greatest impact on offspring brain development (12–16). Risk and outcome of stress exposure is also related to fetal sex, such that males whose mothers experience psychological stress during the first and second trimesters show an increased risk for schizophrenia and ASD (13–15), whereas female offspring exposed to high levels of cortisol during early pregnancy are at higher risk for affective disorders (16). Moreover, late gestation may be a sensitive period wherein stress exposure can lead to long-term alterations in cognitive function and risk for ADHD, particularly in females (17–19). Although fetal development differs between species, rodent models of maternal stress exposure are valuable for elucidating the proximate mechanisms on programming offspring stress sensitivity. Our lab and others have shown that stress during early pregnancy has the greatest long-term impact on the offspring HPA stress axis, cognitive, and metabolic function, particularly in male offspring (20–25).
Numerous biological mechanisms converge to impart sex-specific alterations on offspring neurodevelopment following maternal stress, including effects on the maternal milieu, the placenta, and the developing fetus. While these factors have been explored for their contributions to offspring brain development, they are so tightly intertwined that alterations in one environment typically produce changes in the others. For instance, the placenta is a key source of corticotropin-releasing factor (CRF) (26), which feeds back to both the fetal and maternal pituitary (27–28). Placental CRF is critical for regulating the fetal HPA axis, and for proper production of glucocorticoids and androgens from the fetal adrenal gland (27; 29). These steroids are necessary for organ maturation (30). Maternal stress enhances placental CRF production and signaling, which in turn modifies fetal HPA feedback and development (31). Rodent studies using treatment with the synthetic glucocorticoid, dexamethasone, during pregnancy demonstrate that potentiation of the maternal HPA axis reduces HPA axis sensitivity in adult offspring by attenuating the expression of glucocorticoid (GR) and mineralocorticoid receptors (MR) in the hippocampus, and enhancing anxiety-like behaviors and stress responsivity (32–33).
Similar to maternal stress, maternal infection during pregnancy is associated with an increased risk for ASD and schizophrenia, suggesting that these insults may have overlapping mechanisms that promote long-term changes in development (34–39). Interestingly, rodent models of maternal immune activation produce offspring phenotypes similar to those observed with maternal stress (40). Both maternal stress and infection promote an inflammatory state by increasing cytokine production during pregnancy, weakening the maternal immune system (41). In mice, signaling of the pro-inflammatory cytokine, interleukin-6 (IL-6) is enhanced by both of these maternal insults, and inhibiting IL-6 signaling rescues the impact of maternal infection on offspring development (42–43). Prenatal stress exposure increases IL-6 specifically within the male placenta, and sensitizes the HPA stress axis and metabolic dysfunction in male offspring (44). Treatment of pregnant mice with a nonsteroidal anti-inflammatory drug during stress exposure ameliorates the programmatic dysfunction observed in male offspring, suggesting that maternal/fetal immune signaling mediates the effects of maternal stress exposure on placental and/or fetal brain development (44). Activation of the HPA axis is also known to interact with the maternal immune system, with glucocorticoids acting as important immunomodulators (45–46). For example, thymocytes, monocytes, and neutrophils express GR, which mediates the transcriptional effects of glucocorticoids and can alter migration, differentiation, and proliferation (46–47). Clinical studies also report that psychosocial stress during pregnancy decreases lymphocyte activity (48). Other neuropeptide and neuroendocrine factors resulting from activation of the HPA axis can interact with immune cells as well, such as CRF regulation of mast cell degranulation (46; 49). For a more comprehensive review on maternal immune regulation of fetal development, see (40; 50).
Placental epigenetics and neurodevelopmental programming
At the interface of maternal experience and the developing fetus lies the placenta, a tissue that serves as a gatekeeper of maternal signals, admitting critical nutrients and gasses from maternal circulation while blocking pathogenic intruders including viruses and bacteria (51). Here we focus our discussion on the evidence for epigenetic mechanisms in the placenta to promote sex-specific offspring responses to prenatal stress. For additional discussion on the involvement of the placenta in transmitting maternal signals to the developing fetal brain see reviews (51–54).
The fetally-derived trophoblast lineage is the first to differentiate following fertilization, forming the outer blastocyst trophectoderm layer and eventually becoming the dominant cell type of the placenta (55). Male and female placentas express differences in gene expression, largely originating from the X and Y chromosomes (51; 56). Of particular interest are the numerous X- and Y-linked genes that encode epigenetic machinery, including genes that affect the methylation status of the histone transcriptional repressor, H3K27, such as the histone demethylases, UTX and UTY, and the X-linked enzyme, O-linked N-acetylglucosamine transferase (OGT) (57). Differential expression of these and other broad epigenetic mediators are able to establish widespread sex differences in gene expression patterns within male and female trophoblast lineages (58). These sex differences may contribute to sex-specific susceptibility to prenatal perturbations, such as early prenatal stress, where males are more vulnerable (59).
Evidence supporting this hypothesis has been demonstrated in rodents, wherein maternal stress significantly modifies placental gene expression and function in a sex-specific manner (56; 60–62). We previously identified OGT as a placental mediator of the sex-specific effects of prenatal stress on HPA stress axis and metabolic programming (63). OGT is a nutrient-sensing enzyme that biochemically modifies thousands of proteins to promote widespread effects on cellular signaling, cell cycle regulation, proteosomal activity, transcriptional regulation and additional critical cellular functions (for review see (64)). Further, OGT is a known mediator of epigenetic repression via its structural stabilization of the histone H3K27 methyltransferase, EZH2 (65–67), as well as epigenetic activation via its association with the TET proteins at activational histone marks (68–70).
Female placental tissue (mouse and human) has nearly double the level of OGT as male tissue (60). In addition, placental OGT levels are reduced in response to early prenatal stress exposure in both male and female tissue. Might low levels of placental OGT be responsible for producing altered HPA function and endophenotypes of autism and schizophrenia in males exposed to early prenatal stress? To test this hypothesis, we reduced OGT levels in placental trophoblasts in both sexes and found a recapitulation of the stress axis and metabolic dysregulation phenotype found previously only in males (63). Thus, placental OGT activity appears to be a critical mediator of the sex-specific effects of prenatal stress exposure on offspring neurodevelopmental programming. However, as OGT targets myriad proteins and cellular processes, the downstream mechanisms involved in OGT’s sex-specific programming are still unknown.
Maternal preconception stress
Unlike the effects of paternal preconception stress, less is known about the mechanisms by which maternal preconception stress programs offspring outcomes. However, epidemiological evidence suggests that traumatic events prior to conception, such as childhood abuse and development of PTSD, are associated with poor neuropsychiatric outcomes in offspring (71–73). Studies in children whose mothers survived the Holocaust suggest one mechanism whereby maternal preconception stress may affect future generations via alterations in DNA methylation of stress regulatory genes, FKBP5 and GR, associated with enhanced HPA stress axis sensitivity (73–74). Another recent study found that maternal childhood abuse correlates with greater placental CRF production during pregnancy (75), an intriguing finding that suggests integration of stress experience with the female reproductive tract. Further studies are needed to determine how traumatic preconception events alter maternal biology to allow for transmission to future generations.
Paternal Mechanisms of Intergenerational Stress Transmission
The number of studies examining the interaction of stress with the paternal germline to impact the programming of offspring development has grown in the last decade, with fascinating results implicating male life experiences in intergenerational transmission. The majority of epigenetic patterning of the male germ cell occurs prenatally, with some de novo DNA methylation and histone modifications occurring postnatally, prior to puberty (for review, see (76)). During spermatogenesis, sperm histones are actively replaced by protamines, highly charged proteins that allow condensation of sperm chromatin to one-tenth that of somatic cells (77). As a result, mature sperm become transcriptionally inert, and are considered resistant to external influences. However, recent studies have now turned the dogma upside down, demonstrating that mature sperm are responsive to homeostatic challenges, including dietary disruption, stress or trauma, and exposure to drugs of abuse, during the sperm maturation stage that occurs in the epididymis (78–82). In the following section we discuss the evidence from human studies and relevant animal models of intergenerational transmission of environmental perturbations through the paternal germline and the potential attributed epigenetic mechanisms. We focus on stress and trauma as these have been the most widely studied exposures in paternal transmission.
Human Studies and Animal Models of Paternal Stress
Retrospective epidemiological studies offer compelling evidence linking stress exposures during the male lifetime with disease risk in subsequent generations. Studies using birth measures and food supply records from the Swedish Famine in 1836 made the first claims for both intergenerational and transgenerational effects occurring through the male lineage. These studies established associations between food supply during early childhood in males with altered health outcomes, including disease risk and longevity, in their sons and grandsons (83–85). Further, studies of a cohort of Holocaust survivors and their adult offspring found an increased prevalence of neuropsychiatric disorders, such as depression and PTSD, and reduced cortisol levels and GR sensitivity in offspring whose fathers were survivors (74; 86). Interestingly, as many of these children were conceived decades following the Holocaust, these outcomes support that traumatic stress promotes lasting effects through the paternal germline (74). Studies of males exposed to chemicals from smoking, high-fat diets, and environmental toxicants also compared germ cell outcomes and reported associated epigenetic changes in sperm (87–89). Human studies investigating molecular signatures of stress in the male germline have not been completed, but would provide valuable insight into novel mechanisms of intergenerational transmission resulting from paternal stress exposures.
Animal models of stress transmission via the paternal lineage provide a unique opportunity to identify germ cell epigenetic mechanisms without the major confounding factor of paternal behavior to consider. In most rodent models, males do not participate in offspring rearing, allowing researchers to isolate the specific contribution of epigenetic changes in paternal germ cells. For example, in our studies, male mice breed with females for a maximum of three days and are removed from the female’s cage immediately following observation of a copulation plug, significantly limiting the impact the stressed male may directly have on maternal behavior or investment (79; 90). Such studies have found that male mice exposed to chronic variable stress, dietary challenges, social defeat stress, and odor-paired fear conditioning have a number of altered epigenetic marks in their germ cells, including increased specific small noncoding RNA and changes in DNA methylation (78; 79; 82; 91–93). Further, these altered epigenetic marks are associated with offspring behavioral, physiological, and metabolic outcomes characteristic of endophenotypes of stress-related neuropsychiatric disorders.
Interestingly, rodent studies have demonstrated germ cell susceptibility to stressful environments across the paternal lifespan. For instance, male mice exposed to maternal separation stress during the perinatal period sired offspring with depressive-like behaviors (94). Our lab has shown that male mice exposed to early prenatal stress present with altered stress coping behaviors and a heightened HPA stress response and transmit this phenotype only to their male, but not female, offspring in the next generation (95). These were two of the first rodent studies demonstrating that male germ cells can be reprogrammed by stress experience during early development. Sperm has distinct periods of differentiation, development, and maturation, and therefore the timing of stress exposure likely impacts distinct mechanisms (7). During the prenatal and perinatal periods, development and epigenetic patterning of germ cell precursors and the surrounding reproductive tissues is dynamic; therefore, stress exposure during these critical windows may disrupt the organization of important processes unique to this period (76).
Other studies examining paternal transmission have demonstrated that stress exposure of adolescent and adult animals alters germ cell programming. For example, male mice exposed to chronic variable stress sire male and female offspring that exhibit a significantly blunted HPA stress response, an endophenotype reflected in subsets of patients with major depressive disorder or PTSD (96–97). Interestingly, this paternal effect occurred whether the sires were exposed to stress over the pubertal window or solely during adulthood, suggesting that stress exposures post-puberty (i.e. following maturation of the male reproductive system) evoke similar mechanisms. In contrast, retrospective studies from Swedish famine cohorts associated nutritional challenge during preadolescence with changes in grandson longevity, while such challenges later in life produced no transgenerational effects (98). This disparity in the timing of germ cell vulnerability between our findings in stress-exposed rodents and the findings from the Swedish cohorts may be dependent on species, timing, or type of perturbation (e.g. psychosocial vs nutritional). Therefore, further studies are needed in order to identify the windows of germ cell vulnerability in humans.
Stress Programming of Epigenetic Marks in Sperm
The observation that stress exposures across the male lifespan can lead to programming of offspring phenotypes has brought mounting attention to examination of epigenetic marks in sperm (99). Epigenetic marks have been described in mature sperm in both humans and rodents, including DNA methylation, histone PTMs, and small noncoding RNAs, and have been implicated in transmitting environmental information to the next generation (7; 100). In this section, we discuss the evidence supporting the role of sperm epigenetic marks in the transmission and programming of offspring development following paternal stress exposures.
Sperm DNA methylation patterns are well described in normal germ cell development, and specific changes to these patterns have been reported in response to paternal stress exposure, such as maternal separation stress and odor-paired fear conditioning (91; 94). During embryogenesis, the developing germ cell undergoes global erasure of DNA methylation marks. Following this process, de novo DNA methyltransferases specify germ cell methylation patterns that are distinct from those in somatic cells (76). An additional wave of active DNA demethylation of the paternal gamete occurs immediately post-fertilization in the zygote (101). Importantly, some genomic loci are resistant to demethylation, a process of genomic imprinting critical for normal development, as mistakes at imprinted loci can result in neurodevelopmental disorders, including Angelmans and Prader-Willi syndromes (102–103). Changes to sperm DNA methylation have been reported in rodent models of chronic stress experience (91–92; 94). For example, males that experienced odor-paired fear conditioning as adults had decreased DNA methylation at the specific genomic locus of the corresponding odor receptor in their sperm, suggesting a mechanism by which stress experience may produce offspring with specific behavioral changes (91). Intriguingly, in the same study, these sperm DNA methylation changes corresponded to increased offspring behavioral sensitivity to the associated odor. However, DNA methylation changes at this odor receptor were not present in the brains of these offspring, suggesting sperm DNA methylation changes may influence other epigenetic mechanisms, such as histone PTMs, to program the offspring brain. In another study, males exposed to maternal separation stress early in life sired offspring with depressive-like behaviors (94). These altered behaviors were also associated with changes in DNA methylation patterns at loci related to stress regulatory genes and epigenetic pathways in both the paternal germ cell and in the offspring brain. However, how stress induces such site-specific sperm methylation changes and how these changes influence the programming of adult offspring tissues to produce behavioral phenotypes are not known.
Despite the central dogma that mature sperm are transcriptionally inert, populations of small noncoding RNAs (~22–34 bp) have been well described in the mature sperm of humans and animals, including microRNA (miRs), PIWI-associating RNAs, and transfer RNA-derived fragments (tRFs) (104–107). Specifically, sperm miRs are critical for normal embryogenesis, where inhibition of sperm-borne miR-34c in the zygote resulted in zygotic arrest (108). In our studies, male mouse chronic stress experience significantly increased 9 miRs in sire sperm (79). Remarkably, microinjection of these same 9 miRs into fertilized zygotes completely recapitulated the blunted HPA stress axis phenotype reported in our model of paternal stress, providing causal evidence for the role of stress-altered miRs in sperm (109). Other labs have corroborated the crucial role of stress-sensitive sperm RNAs in programming offspring phenotypes via microinjection of total sperm RNA and tRF populations, a class of small non-coding RNAs derived from tRNAs (80; 110–111). The mechanisms by which sperm small noncoding RNAs influence offspring neurodevelopment are not clear. Following zygote miR microinjection, we found a significant reduction of specific stored maternal mRNA populations at the 2-cell stage, supporting sperm miR canonical function in the embryo (109). Intriguingly, in this study, the two most repressed genes were Sirt1 and Ube3a, epigenetic regulators that have been associated with neurodevelopmental and metabolic disorders in humans (112–113). In another study, zygote microinjection of tRFs altered by paternal dietary challenge resulted in changes in zygote gene expression, in particular the repression of endogenous retro-elements (111). Therefore, sperm small noncoding RNAs may alter critical genes during the pre-implantation stages of embryogenesis, resulting in a cascade of cellular events that ultimately reprograms the offspring. Due to the relative instability of RNA molecules compared to more long-lasting epigenetic marks, such as DNA methylation, it is unlikely sperm-derived RNAs are maintained past early stages of embryogenesis, but rather produce a dynamic change in the developmental landscape, possibly additional epigenetic changes, that shapes the embryo trajectory.
Considering the exciting new evidence that sperm noncoding RNAs are changed by paternal perturbations and reprogram offspring development, mechanistic studies to determine how the male reproductive tract senses changes in the environment and alters sperm content are necessary before interventions can be considered. The source of RNA in mature sperm was previously assumed to be residual from spermatogenic processes (114). However, in the epididymis, the site of important post-testicular sperm maturation, a novel role of epithelial cell extracellular vesicles has recently been proposed in the delivery of small non-coding RNA to maturing sperm (115). For instance, the content of these ‘epididymosomes’ was shown to alter sperm tRFs in response to paternal dietary challenge, supporting that epididymal epithelial cells may be the dynamic mediators between paternal environmental exposures and sperm RNA changes (111).
Lastly, histone PTMs are also potential epigenetic signals in sperm. Roughly 1% of histones in mice and 10% of histones in humans are retained in sperm chromatin following the active exchange of histones with protamines (77; 116). Importantly, retained histones have been mapped to regions of important developmental genes, suggesting they designate those that are critical for post-fertilization function in the zygote (117). As evidence to this point, disruption of the specific histone mark, H3K4me2, in sperm altered gene expression in the two-cell zygote and severely impaired offspring development (118). In addition, sperm from male rats that were administered chronic cocaine showed increased H3 acetylation specifically at the Bdnf promoter in both paternal sperm and in the offspring brain, supporting the hypothesis that retained histone PTMs may denote genes important to offspring development (81; 119). In addition to histone PTMs, protamine biochemical modifications have also been reported, supporting a potential protamine code in sperm that may impart transcriptional effects on embryo development (120). However, as protamines are rapidly replaced with maternal histones post-fertilization (121), how such protamine modifications could influence embryogenesis requires further investigation.
Conclusions
The focus of stress as a risk factor for neuropsychiatric and neurodevelopmental disorders and the mounting evidence for the intergenerational transmission of parental stress exposure brings to light exciting new mechanisms involved in transmission of sex-specific stress signals. Many affected tissues are extra-embryonic and easily accessible (e.g., placenta, semen), and thus the translational potential from animal models to prospective human studies may facilitate development of necessary predictive disease biomarkers. The potential to identify at-risk individuals may then inform clinical decisions, including altering prenatal care and earlier interventions for children. However, the discussion here only begins to appreciate the incredibly complex and multifaceted etiology that contributes to disease risk or resilience. Despite the growing evidence supporting intergenerational inheritance, there remains skepticism as to whether the transmission of parental experience is truly mediated by epigenetic mechanisms in the germ cell. For example, stress exposure may also impact offspring development via changes to parental care (e.g. maternal investment). Further, in paternal inheritance studies, the “sick sperm” hypothesis suggests stress exposure can alter sperm maturation or motility and that offspring development may be affected by the integrity of fertilization or pre-implantation events (122). Therefore, more studies with careful examination of these potentially complex factors are needed to determine the mechanism by which parental stress programs offspring development. Moreover, large prospective cohort studies in which gene x environment influences are considered, such as the NIH-launched initiative Environmental Influences on Child Health Outcomes (ECHO) and the Avon Longitudinal Study of Parents and Children (123–125), will be invaluable to our ability to identify causal mechanisms, who may be at-risk, and in designing prevention and therapeutic measures.
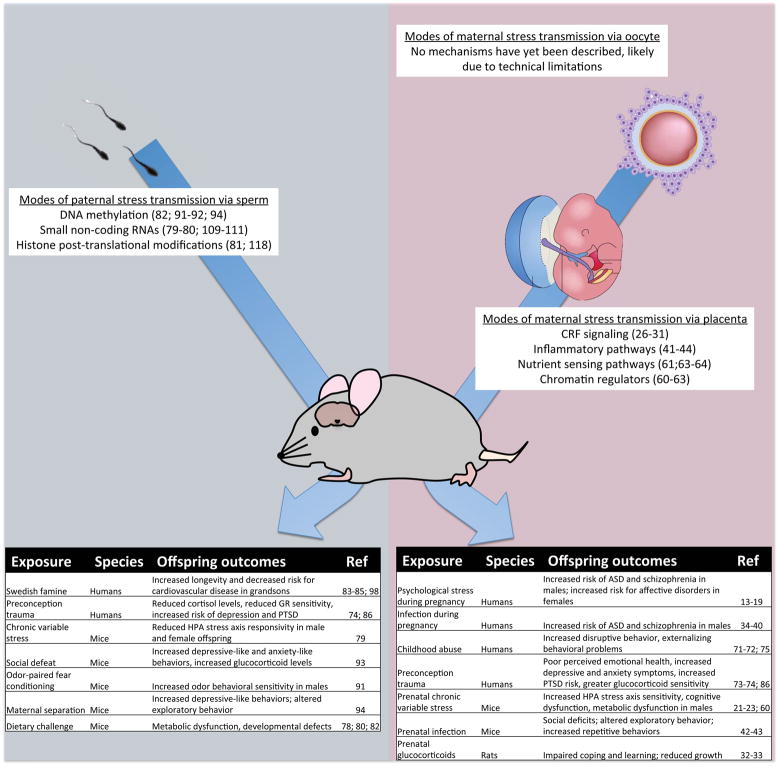
Figure 1. Intergenerational transmission of maternal and paternal stress can impact offspring neurodevelopment.
Paternal stress exposures influence offspring outcomes (left table), potentially through changes in sperm epigenetic marks. Maternal stress during pregnancy alters placental signaling to reprogram offspring neurodevelopment (right table). Few studies to date have examined maternal preconception stress effects on the oocyte, likely due to current technical barriers.
Acknowledgments
Funding
Studies discussed in this review were funded in part by grants from the National Institutes of Health: MH108286, MH099910, and MH104184.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 2.Campos A, Fogaca M, Aguiar D, Guimaraes F. Animal models of anxiety disorders and stress. Rev Bras Psiquiatr. 2013;35:S101–S111. doi: 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- 3.Bale TL. The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues Clin Neurosci. 2016;18:459–464. doi: 10.31887/DCNS.2016.18.4/tbale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Klengel T, Dias BG, Ressler KJ. Models of Intergenerational and Transgenerational Transmission of Risk for Psychopathology in Mice. Neuropsychopharmacology. 2016;41:219–31. doi: 10.1038/npp.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane M, Robker R, Robertson S. Parenting from before conception. Science. 2014;345:756–760. doi: 10.1126/science.1254400. highwire. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers AB, Bale TL. Germ Cell Origins of Posttraumatic Stress Disorder Risk: The Transgenerational Impact of Parental Stress Experience. Biol Psychiatry. 2015;78:307–14. doi: 10.1016/j.biopsych.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 9.Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity (Edinb) 2010;105:4–13. doi: 10.1038/hdy.2010.54. [DOI] [PubMed] [Google Scholar]
- 10.Bale T. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Guasti A, Fiedler JL, Herrera L, Handa RJ. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm Metab Res. 2012;44:607–18. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–42. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- 13.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–52. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 14.Van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–6. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 15.Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, et al. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35:471–8. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- 16.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA. 2012;109:E1312–9. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronald A, Pennell CE, Whitehouse AJ. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Front Psychol. 2010;1:223. doi: 10.3389/fpsyg.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeWinn KZ, Stroud LR, Molnar BE, Ware JH, Koenen KC, Buka SL. Elevated maternal cortisol levels during pregnancy are associated with reduced childhood IQ. Int J Epidemiol. 2009;38:1700–10. doi: 10.1093/ije/dyp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Robinson M, Malacova E, Jacoby P, Foster J, van Eekelen A. Maternal life stress events in pregnancy link to children’s school achievement at age 10 years. J Pediatr. 2013;162:483–9. doi: 10.1016/j.jpeds.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–56. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 21.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Mueller BR, Bale TL. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav. 2006;88:605–14. doi: 10.1016/j.physbeh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: animal models of an adverse life event. Am J Psychiatry. 2002;159:1265–83. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- 26.Shibasaki T, Odagiri E, Shizume K, Ling N. Corticotropin-releasing factor-like activity in human placental extracts. J Clin Endocrinol Metab. 1982;55:384–6. doi: 10.1210/jcem-55-2-384. [DOI] [PubMed] [Google Scholar]
- 27.Smith R, Mesiano S, Chan EC, Brown S, Jaffe RB. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83:2916–20. doi: 10.1210/jcem.83.8.5020. [DOI] [PubMed] [Google Scholar]
- 28.Wadhwa PD, Sandman CA, Chicz-DeMet A, Porto M. Placental CRH modulates maternal pituitary adrenal function in human pregnancy. Ann N Y Acad Sci. 1997;814:276–81. doi: 10.1111/j.1749-6632.1997.tb46163.x. [DOI] [PubMed] [Google Scholar]
- 29.Ng PC. The fetal and neonatal hypothalamic-pituitary-adrenal axis. Arch Dis Child Fetal Neonatal Ed. 2000;82:F250–4. doi: 10.1136/fn.82.3.F250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fencl MD, Stillman RJ, Cohen J, Tulchinsky D. Direct evidence of sudden rise in fetal corticoids late in human gestation. Nature. 1980;287:225–6. doi: 10.1038/287225a0. [DOI] [PubMed] [Google Scholar]
- 31.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–49. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 32.Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–9. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 33.Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–8. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- 34.Patterson PH. Maternal infection and autism. Brain Behav Immun. 2012;26:393. doi: 10.1016/j.bbi.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Atladóttir HOO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–30. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 36.Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 2015;45:4015–25. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–90. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–21. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–50. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu WL, Hsiao EY, Yan Z, Mazmanian SK. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain. 2017 doi: 10.1016/j.bbi.2016.11.007. Retrieved from http://www.sciencedirect.com/science/article/pii/S0889159116304974. [DOI] [PMC free article] [PubMed]
- 44.Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 2014;155:2635–46. doi: 10.1210/en.2014-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–35. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 46.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–63. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 47.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrera JA, Alvarado JP, Martínez JE. The psychosocial environment and the cellular immunity in the pregnant patient. Stress and Health. 1988;4:49–56. Wiley Online Library. [Google Scholar]
- 49.Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, et al. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–50. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- 50.Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol. 2012;72:1317–26. doi: 10.1002/dneu.22045. [DOI] [PubMed] [Google Scholar]
- 51.Nugent BM, Bale TL. The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front Neuroendocrinol. 2015;39:28–37. doi: 10.1016/j.yfrne.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Hormones and behavior. 2011 doi: 10.1016/j.yhbeh.2010.06.007. Retrieved from http://www.sciencedirect.com/science/article/pii/S0018506X10001674. [DOI] [PubMed]
- 53.Seckl JR. Prenatal glucocorticoids and long-term programming. European Journal of Endocrinology. 2004 doi: 10.1530/eje.0.151u049. Retrieved from http://www.eje-online.org/content/151/Suppl_3/U49.short. [DOI] [PubMed]
- 54.Matthews SG. Early programming of the hypothalamo–pituitary–adrenal axis. Trends in Endocrinology & Metabolism. 2002 doi: 10.1016/s1043-2760(02)00690-2. Retrieved from http://www.sciencedirect.com/science/article/pii/S1043276002006902. [DOI] [PubMed]
- 55.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–48. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 56.Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci USA. 2010;107:5557–62. doi: 10.1073/pnas.1000440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J Neurosci. 2008;28:4521–7. doi: 10.1523/JNEUROSCI.5382-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nottke A, Colaiácovo MPP, Shi Y. Developmental roles of the histone lysine demethylases. Development. 2009;136:879–89. doi: 10.1242/dev.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–9. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 60.Howerton CL, Morgan CP, Fischer DB, Bale TL. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc Natl Acad Sci USA. 2013;110:5169–74. doi: 10.1073/pnas.1300065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gabory A, Ferry L, Fajardy I, Jouneau L, Gothié J-DD, Vigé A, et al. Maternal diets trigger sex-specific divergent trajectories of gene expression and epigenetic systems in mouse placenta. PLoS ONE. 2012;7:e47986. doi: 10.1371/journal.pone.0047986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bronson SL, Chan JC, Bale TL. Sex-Specific Neurodevelopmental Programming by Placental Insulin Receptors on Stress Reactivity and Sensorimotor Gating. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howerton CL, Bale TL. Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction. Proc Natl Acad Sci USA. 2014;111:9639–44. doi: 10.1073/pnas.1401203111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism and chronic disease. Annu Rev Nutr. 2013;33:205–29. doi: 10.1146/annurev-nutr-071812-161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu C-SS, Lo P-WW, Yeh Y-HH, Hsu P-HH, Peng S-HH, Teng Y-CC, et al. O-GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci USA. 2014;111:1355–60. doi: 10.1073/pnas.1323226111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Myers SA, Panning B, Burlingame AL. Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2011;108:9490–5. doi: 10.1073/pnas.1019289108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gambetta MC, Oktaba K, Müller J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science. 2009;325:93–6. doi: 10.1126/science.1169727. [DOI] [PubMed] [Google Scholar]
- 68.Bauer C, Göbel K, Nagaraj N, Colantuoni C, Wang M, Müller U, et al. Phosphorylation of TET proteins is regulated via O-GlcNAcylation by the O-linked N-acetylglucosamine transferase (OGT) J Biol Chem. 2015;290:4801–12. doi: 10.1074/jbc.M114.605881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dehennaut V, Leprince D, Lefebvre T. O-GlcNAcylation, an Epigenetic Mark. Focus on the Histone Code, TET Family Proteins, and Polycomb Group Proteins. Front Endocrinol (Lausanne) 2014;5:155. doi: 10.3389/fendo.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deplus R, Delatte B, Schwinn MK, Defrance M, Méndez J, Murphy N, et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013;32:645–55. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dubowitz H, Black MM, Kerr MA, Hussey JM, Morrel TM, Everson MD, Starr RH. Type and timing of mothers’ victimization: effects on mothers and children. Pediatrics. 2001;107:728–35. doi: 10.1542/peds.107.4.728. [DOI] [PubMed] [Google Scholar]
- 72.Miranda J, de la Osa N, Granero R, Ezpeleta L. Maternal experiences of childhood abuse and intimate partner violence: Psychopathology and functional impairment in clinical children and adolescents. Child Abuse & Neglect. 2011;35:700–711. doi: 10.1016/j.chiabu.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, Binder EB. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol Psychiatry. 2016;80:372–80. doi: 10.1016/j.biopsych.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Yehuda R, Daskalakis N, Lehrner A, Desarnaud F, Bader H, Makotkine I, et al. Influences of Maternal and Paternal PTSD on Epigenetic Regulation of the Glucocorticoid Receptor Gene in Holocaust Survivor Offspring. American Journal of Psychiatry. 2014;171:872–880. doi: 10.1176/appi.ajp.2014.13121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, Wadhwa PD. Maternal Exposure to Childhood Trauma Is Associated During Pregnancy With Placental-Fetal Stress Physiology. Biol Psychiatry. 2016;79:831–9. doi: 10.1016/j.biopsych.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ly L, Chan D, Trasler J. Developmental windows of susceptibility for epigenetic inheritance through the male germline. Seminars Cell Dev Biology. 2015;43:96–105. doi: 10.1016/j.semcdb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction. 2010;139:287–301. doi: 10.1530/REP-09-0281. [DOI] [PubMed] [Google Scholar]
- 78.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–96. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodgers A, Morgan C, Bronson S, Revello S, Bale T. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. The Journal of neuroscience3: the official journal of the Society for Neuroscience. 2013;33:9003–12. doi: 10.1523/JNEUROSCI.0914-13.2013. The Journal of neuroscience3: the official journal of the Society for Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science (New York, NY) 2015 doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 81.Vassoler F, White S, Schmidt H, Sadri-Vakili G, Pierce C. Epigenetic inheritance of a cocaine-resistance phenotype. Nature Neuroscience. 2012;16:42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lambrot Xu, Saint-Phar Chountalos, Cohen Paquet, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nature Communications. 2013;4 doi: 10.1038/ncomms3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjöström M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–66. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 84.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet. 2002;10:682–8. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 85.Kaati G, Bygren LO, Pembrey M, Sjöström M. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet. 2007;15:784–90. doi: 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- 86.Lehrner A, Bierer L, Passarelli V, Pratchett L, Flory J, Bader H, et al. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology. 2014;40:213–220. doi: 10.1016/j.psyneuen.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delbès G, Hales B, Robaire B. Toxicants and human sperm chromatin integrity. Mhr Basic Sci Reproductive Medicine. 2010;16:14–22. doi: 10.1093/molehr/gap087. [DOI] [PubMed] [Google Scholar]
- 88.Marczylo EL, Amoako AA, Konje JC, Gant TW. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012 doi: 10.4161/epi.19794. [DOI] [PubMed] [Google Scholar]
- 89.Donkin I, Versteyhe S, Ingerslev L, Qian K, Mechta M, Nordkap L, et al. Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 2016;23:369–378. doi: 10.1016/j.cmet.2015.11.004. sciencedirect. [DOI] [PubMed] [Google Scholar]
- 90.Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Horm Behav. 2011;59:306–14. doi: 10.1016/j.yhbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dias B, Ressler K. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nature Neuroscience. 2014;17:89–96. doi: 10.1038/nn.3594. Nature Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu L, Lu Y, Jiao Y, Liu B, Li S, Li Y, et al. Paternal Psychological Stress Reprograms Hepatic Gluconeogenesis in Offspring. Cell Metab. 2016;23:735–743. doi: 10.1016/j.cmet.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 93.Dietz D, Laplant Q, Watts E, Hodes G, Russo S, Feng J, et al. Paternal transmission of stress-induced pathologies. Biological psychiatry. 2011;70:408–14. doi: 10.1016/j.biopsych.2011.05.005. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–15. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 95.Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–55. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meewisse M-LL, Reitsma JB, de Vries G-JJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–92. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 97.Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. 2011;13:263–78. doi: 10.31887/DCNS.2011.13.2/jsherin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bygren LO, Kaati G, Edvinsson S. Longevity determined by paternal ancestors’ nutrition during their slow growth period. Acta Biotheor. 2001;49:53–9. doi: 10.1023/a:1010241825519. [DOI] [PubMed] [Google Scholar]
- 99.Jirtle RL, Skinner MK. Nature reviews Genetics. Vol. 8. Nature Publishing Group; 2007. Environmental epigenomics and disease susceptibility; p. 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bohacek J, Mansuy IM. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat Rev Genet. 2015;16:641–52. doi: 10.1038/nrg3964. [DOI] [PubMed] [Google Scholar]
- 101.Wu S, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Bio. 2010;11:607–620. doi: 10.1038/nrm2950. nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lawson HA, Cheverud JM, Wolf JB. Genomic imprinting and parent-of-origin effects on complex traits. Nat Rev Genet. 2013;14:609–17. doi: 10.1038/nrg3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339:448–52. doi: 10.1126/science.1229277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krawetz SA, Kruger A, Lalancette C, Tagett R, Anton E, Draghici S, Diamond MP. A survey of small RNAs in human sperm. Hum Reprod. 2011;26:3401–12. doi: 10.1093/humrep/der329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sendler E, Johnson G, Mao S, Goodrich R, Diamond M, Hauser R, Krawetz S. Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic acids research. n.d;41:4104–17. doi: 10.1093/nar/gkt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kawano M, Kawaji H, Grandjean V, Kiani J, Rassoulzadegan M. Novel small noncoding RNAs in mouse spermatozoa, zygotes and early embryos. PLoS ONE. 2012;7:e44542. doi: 10.1371/journal.pone.0044542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng H, Shi J, Zhang Y, Zhang H, Liao S, Li W, et al. A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Res. 2012;22:1609–12. doi: 10.1038/cr.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu W-MM, Pang RT, Chiu PC, Wong BP, Lao K, Lee K-FF, Yeung WS. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc Natl Acad Sci USA. 2012;109:490–4. doi: 10.1073/pnas.1110368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA. 2015;112:13699–704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–9. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharma U, Conine C, Shea J, Boskovic A, Derr A, Bing X, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2015:aad6780. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herskovits Z, Guarente L. SIRT1 in Neurodevelopment and Brain Senescence. Neuron. 2014;81:471–83. doi: 10.1016/j.neuron.2014.01.028. sciencedirect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greer P, Hanayama R, Bloodgood B, Mardinly A, Lipton D, Flavell S, et al. The Angelman Syndrome Protein Ube3A Regulates Synapse Development by Ubiquitinating Arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. sciencedirect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ostermeier C, Miller D, Huntriss J, Diamond M, Krawetz S. Reproductive biology: Delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154–154. doi: 10.1038/429154a. nature. [DOI] [PubMed] [Google Scholar]
- 115.Belleannée C, Calvo É, Caballero J, Sullivan R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol Reprod. 2013;89:30. doi: 10.1095/biolreprod.113.110486. [DOI] [PubMed] [Google Scholar]
- 116.Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley E, Roloff T, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biology. 2010;17:679–687. doi: 10.1038/nsmb.1821. nature. [DOI] [PubMed] [Google Scholar]
- 117.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–8. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Siklenka K, Erkek S, Godmann M, Lambrot R, McGraw S, Lafleur C, et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science. 2015;350:aab2006. doi: 10.1126/science.aab2006. [DOI] [PubMed] [Google Scholar]
- 119.Wimmer ME, Briand LA, Fant B, Guercio LA, Arreola AC, Schmidt HD, et al. Paternal cocaine taking elicits epigenetic remodeling and memory deficits in male progeny. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.71. [DOI] [PubMed] [Google Scholar]
- 120.Brunner AM, Nanni P, Mansuy IM. Epigenetic marking of sperm by post-translational modification of histones and protamines. Epigenetics Chromatin. 2014;7:2. doi: 10.1186/1756-8935-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McLay DW, Clarke HJ. Remodelling the paternal chromatin at fertilization in mammals. Reproduction. 2003;125:625–33. doi: 10.1530/rep.0.1250625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rando O. Daddy Issues: Paternal Effects on Phenotype. Cell. 2012;151:702–708. doi: 10.1016/j.cell.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Golding J. The Avon Longitudinal Study of Parents and Children (ALSPAC)--study design and collaborative opportunities. European Journal of Endocrinology. 2004 doi: 10.1530/eje.0.151U119. [DOI] [PubMed] [Google Scholar]
- 124.Boyd A, Golding J, Macleod J, Lawlor DA. Cohort profile: the “children of the 90s”—the index offspring of the Avon Longitudinal Study of Parents and Children. International journal …. 2013 doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Golding J. Children of the nineties. A longitudinal study of pregnancy and childhood based on the population of Avon (ALSPAC) West of England medical journal. 1990;105:80–82. Bristol Medico Chirurgical Society. [PMC free article] [PubMed] [Google Scholar]