Abstract
The current study evaluated the role of strain and compulsive trait differences in response to fluvoxamine, a common OCD drug, in two different mouse strains (BIG1 and BIG2) exhibiting a spontaneous compulsive-like phenotype. For compulsive-like nest-building behavior, dose-dependent attenuation of nesting by fluvoxamine was observed for the BIG1 compulsive-like strain during the first hour after administration. No significant differences were found for BIG2 strain during the first hour, although a dose-dependent trend similar to that seen in the BIG1 strain was observed. Fluvoxamine dose-dependently decreased the number of marbles buried in both strains one hour after administration. For anxiety-like behaviors in the open field, no significant drug effects were found for the latency to leave the center and the number of line crossings. Significant strain differences were observed, with the BIG2 strain exhibiting higher anxiety-like behaviors and reduced locomotor activity when compared to the BIG1 strain. Consequently, this study adds predictive validity to our mouse model of OCD, while the anxiety-like differences between the strains add heterogeneity to our mouse model, similar to the heterogeneity seen in OCD.
Keywords: Fluvoxamine, obsessive-compulsive disorder, mouse model, strain, trait, anxiety
Introduction
Obsessive-compulsive disorder (OCD) is an intricate heterogeneous disorder characterized by persistent obsessions (thoughts) and compulsions (repetitive behaviors) (Albert et al., 2013; Karno et al., 1988). In the United States OCD has an estimated lifetime occurrence of about 2.3% (Ruscio et al., 2010). The social functioning, relationships, quality of life and socio-economic status of patients suffering from OCD are significantly impacted (Fontenelle et al., 2010; Hollander et al., 2010). The most common first-line treatments for OCD are selective serotonin reuptake inhibitors (SSRIs) and cognitive behavioral therapy (Bandelow et al., 2012; Kellner, 2010; Pittenger et al., 2005). Fluvoxamine is a first generation SSRI widely used for treatment of OCD (Irons, 2005). It is a potent inhibitor of serotonin reuptake at synapses and has no effect on reuptake of dopamine or norepinephrine (Goodman et al., 1997). Many clinical studies have shown efficacy of fluvoxamine in treating OCD (Goodman et al., 1989a, Mallya et al., 1992; Milanfranchi et al., 1997; Mundo et al., 2001). However, 40–60% of the OCD patients are refractory to the first-line treatments (Nakamae, 2013). Even patients categorized as clinical responders continue to exhibit impairments from their residual obsessions and compulsions (Lack, 2012). Considering such high rates of drug resistance among patients, a better understanding of the efficacy of first-line treatments on specific compulsive traits in OCD subjects of different genetic backgrounds is needed.
OCD has a strong genetic component, which has been established in both human and rodent studies (Hettema et al., 2001; Pato et al., 2002; Taylor, 2013; Ting and Feng, 2011; Wang et al., 2009; Welch et al., 2007). Investigating drug intervention in mouse strains exhibiting spontaneous compulsive-like behaviors can be a valuable starting point for determining if there is any role of genetic background and compulsive traits in influencing drug response. The current mouse model was developed by bidirectionally selecting house mice, Mus musculus, for nest-building behavior (Bult and Lynch, 2000; Lynch, 1980). The stock population for the original selection experiment (Lynch, 1980) was a cross among eight inbred strains yielding the HS/Ibg outbred strain (McClearn et al., 1970; Lynch, 1980). Nest-building behavior, which is homologous to hoarding in humans with OCD (Warneke, 1993), is considered to be a measure of compulsive-like behavior in mice (Greene-Schloesser et al., 2011). Bidirectional selection resulted in three main levels of nest-building behavior (with two replicate strains within each level). The replicates within each level of nesting were maintained separately and subjected to the same selection regime. Hence, all replicates were maintained as separate strains (Bult and Lynch, 2000; Lynch, 1980). Considering replicate strains for comparison is important to guarantee that responses to artificial selection are the result of selection and not due to founder effects or random genetic drift when comparing the different levels of selection, i.e., selection for building big nests or small nests, or random breeding (Bult and Lynch, 2000; Lynch, 1980). The BIG strains (BIG1 and BIG2) consistently display a forty-fold difference in the amount of cotton used when compared to the SMALL strains which display very low levels of nesting. The randomly-bred Control strains serve as a selection control and show intermediate levels of nesting (Bult and Lynch, 2000; Lynch, 1980). We have shown in the past that the replicates within each level of nesting, although subjected to the same selection regime, diverged genetically, probably due to founder effects or random genetic drift (Bult and Lynch, 1996, 2000).
According to Maio et al. (2014), comparing our compulsive-like strains can be useful for understanding the disease mechanism during the OCD condition. Prior studies have shown that our compulsive-like BIG1 and BIG2 strains exhibited varied levels of compulsive-like and anxiety-like behaviors (Mitra et al., 2016a, 2017). The BIG1 and BIG2 strains therefore exhibit face (Mitra et al., 2017) and construct validity (Mitra et al., 2016a) for subgroups of compulsive-like expression as seen in subsets of human patients. In addition, a previous study with inbred mouse strains also found significant variations in expression of drug-induced compulsive-like behaviors (de Hass et al., 2012). The study showed how the A/J mouse strain had higher susceptibility to quinpirole-induced compulsive-like checking behaviors when compared to the C57BL6/J mouse strain (de Hass et al., 2012). Though the predictive validity of the BIG1 strains has been previously established (Greene-Schloesser et al., 2011), this is the first time we have investigated the predictive validity of the BIG2 strains and compared them with the BIG1 strains for better understanding of strain- and trait-based influences on drug effectiveness and behavioral expression. Due to the variations seen in behavioral expression between the compulsive-like BIG1 and BIG2 strains (Mitra et al., 2016a, 2017), we hypothesized that there would be a strain- and trait-specific attenuation of compulsive-like and anxiety-like behaviors due to fluvoxamine administration.
Methods
Subjects
Intact compulsive-like BIG male house mouse (Mus musculus) strains (BIG1, BIG2) were used for the study (BIG1 n=36: BIG2 n=38). A total of 12 animals per group per strain were used initially, but 12 mice from BIG1 and 10 mice from BIG2 strain were euthanized due to skin scabbing. Mice were raised in polypropylene cages (27×17×12 cm) with wood shavings, under a 12-12 light-dark cycle at 22±1°C, and with free access to food (Purina Mills, Lab Diet Mouse Diet #5015, St. Louis, MO) and water. Pups were weaned at 19–21 days of age and housed with same-sex littermates. All animals were approximately 60 days old at the start of the experiment.
Drug administration
Fluvoxamine maleate (Sigma Aldrich) was dissolved in physiological saline (pH=7.4) to yield final doses of 10 mg/kg, 20 mg/kg and 40 mg/kg (pH 7–7.4). Saline served as a vehicle (0 mg/kg) control. All male compulsive-like mice were divided into four treatment groups (starting at 12 animals per group) comprising vehicle (sterile saline with pH adjusted to 7.4), 10 mg/kg, 20 mg/kg and 40 mg/kg fluvoxamine. Animals in each group received subcutaneous injections of fluvoxamine/vehicle daily for 17 days.
Experimental design
All animals underwent behavioral testing consecutively, comprising nest building on day 15, marble burying on day 16 and open field test on day 17 of the 17-day fluvoxamine treatment regimen. The sequence of behavioral tests, i.e., doing the anxiety-like tests before the compulsive-like tests or vice versa, does not affect the performance in any of these behaviors in our mouse strains (data not shown).
For nest-building, data were collected during the first, second, third, fourth, fifth hour and 24 h after fluvoxamine administration. Marble burying and open field tests were performed one hour after drug administration. The marble burying test lasted for 15 minutes the and open field test for 3 minutes (Greene-Schloesser et al., 2011; Mitra et al., 2016a,b, 2017). Testing was performed in the light phase of the light-dark cycle and the University of Alaska Fairbanks Institutional Animal Care and Use Committee approved the animal care and experimental procedures (IACUC assurance number 718349).
Compulsive-like nest-building behavior
Nest-building behavior was used as a measure of the compulsive-like phenotype of the mice (Greene-Schloesser et al., 2011). Male mice of both the BIG strains (BIG1 and BIG2) were singly housed and provided with a pre-weighed cotton roll in the cage-top food hopper. The cotton roll was removed and weighed (Bult and Lynch, 1996, 1997, 2000) 1, 2, 3, 4, 5 and 24 h after fluvoxamine administration. The nesting score was defined as the amount of cotton used over a 1-h period (first, second, third, fourth and fifth hour) and over the 24-hour period.
Compulsive-like marble burying behavior
The marble-burying test was also used to determine compulsive-like behavior (Angoa-Perez et al., 2013; Greene-Schloesser et al., 2011; Takeuchi et al., 2002; Thomas et al., 2009). One hour after fluvoxamine administration, mice were individually introduced into a polypropylene cage (37×21×14 cm) containing 20 glass marbles (10 mm in diameter) spaced evenly on wood chip bedding 5 cm deep, without access to food or water, for 15 min (Greene-Schloesser et al., 2011). Testing was carried out in a testing room separate from the housing room. The total number of marbles buried at least 2/3 in the 15-min period was quantified as compulsive-like digging behavior. After the 15-min test, the animals were returned to their home cages.
Anxiety-like open field behavior
Anxiety-like behavior was evaluated using the open field test (Prut and Belzung, 2003; Simon et al., 1994). BIG1 and BIG2 males were transported in their home cages, singly housed, and placed on a rack outside the testing room just prior to the start of testing. The open field apparatus consisted of an open field arena (40 × 40 × 30 cm) with 16 10×10 cm squares on its floor. For testing, animals were placed in the center of the field and allowed to explore the arena for 3 minutes. Latency to leave the central square (20×20 cm) initially and entries into the central zone (20 × 20 cm) after leaving it initially were recorded to quantify anxiety-like behaviors. The total number of line crossings was recorded as a measure of locomotor activity (Frye et al., 2006; Greene-Schloesser et al., 2011; Nosek et al., 2008). The apparatus was cleaned before each test.
Statistical analysis
Values from all behavioral assessments were expressed in mean ± standard error of the mean (SEM). Statistical Analysis System Software (SAS Version 9.4, Cary, NC) was used for statistical analyses. All behavioral measures were tested in a general linear model (GLM) analysis of variance (ANOVA) for effects of strain, drug and strain x drug interaction. If significant effects were found, appropriate post-hoc pair-wise comparisons were conducted using the Tukey's Studentized Range test. The total nesting score was square-root transformed to obtain a more normal distribution (Bult and Lynch, 2000); however, the data are presented as non-transformed nesting scores.
Results
Strain-dependent attenuation of compulsive-like nesting behavior by fluvoxamine
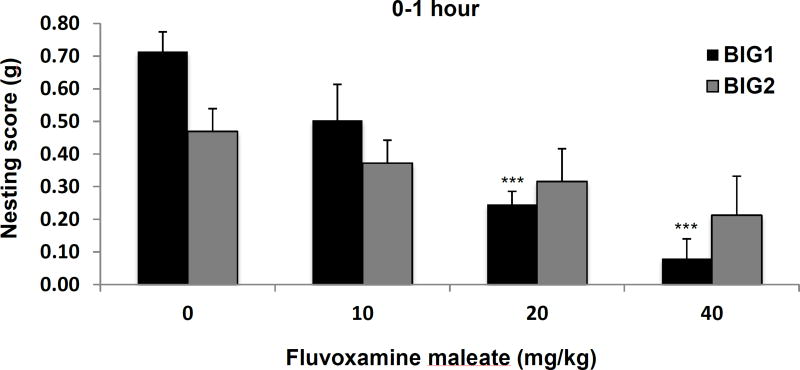
After 1 hour of fluvoxamine administration there was a significant drug effect (F3,74 = 11.73, p<0.001). The 20 and 40 mg/kg doses significantly attenuated the nesting scores for the BIG1 strain when compared to vehicle (Fig. 1). No significant effect of fluvoxamine on nesting in the compulsive-like BIG2 strain was found, although nest-building behavior tended to decrease with increased doses of fluvoxamine (Fig. 1). The strain effect (F1,74 = 1.40, NS) and drug by strain interaction (F3,74 = 2.43, NS) were not significant, which confirmed the trend in the BIG2 strain.
Figure 1.
Compulsive-like nest-building behavior of the BIG1 and BIG2 strains. The data represent the mean (+ SEM) for the nesting score, in grams, for the 0–1 hour period. ***(p<0.001) significant drug effect at 20 and 40 mg/kg relative to vehicle (0 mg/kg) in the BIG1 strain. 0 mg/kg, n=11 and n=11; 10 mg/kg, n=7 and n=12; 20 mg/kg, n=10 and n=8; 40 mg/kg, n=6 and n=9, for BIG1 and BIG2, respectively.
No significant drug effect was found in the second (F3,74 = 1.92, NS), third (F3,74 = 1.45, NS), fourth (F3,74 = 1.93, NS), or fifth (F3,74 = 0.24, NS) hour, or over the whole 24 hours after fluvoxamine administration (F3,74 = 2.01, NS) (data no shown).
Dose-dependent attenuation of compulsive-like marble burying behavior by fluvoxamine in both the BIG strains
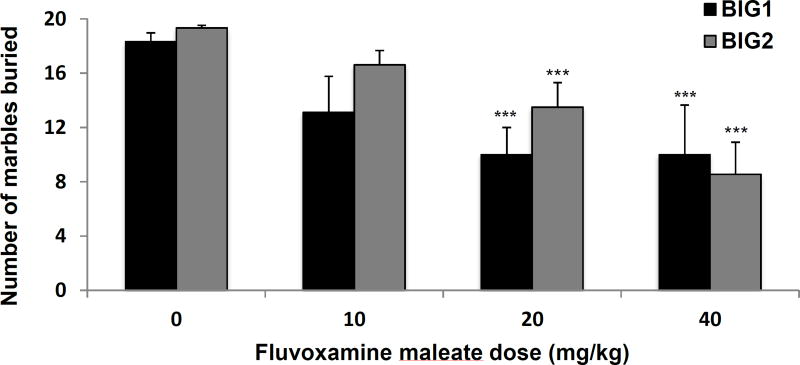
A significant dose-dependent drug effect (F3,74 = 13.08, p<0.001) was observed in the number of marbles buried. The 20 and 40 mg/kg doses showed significant attenuation of marble burying when compared to vehicle in both the BIG1 and BIG2 strains. No significant strain effect (F1,74 = 1.99, NS) and drug by strain interaction (F3,74 = 0.88, NS) were found (Fig. 2).
Figure 2.
Compulsive-like marble burying behavior of the BIG1 and BIG2 strains. The data represent the mean (+ SEM) for total number of marbles buried. ***(p<0.001) indicates significant drug effect at 20 and 40 mg/kg relative to vehicle (0 mg/kg) in both BIG1 and BIG2 strains. 0 mg/kg, n=11 and n=11; 10 mg/kg, n=7 and n=12; 20 mg/kg, n=10 and n=8; 40 mg/kg, n=6 and n=9 for BIG1 and BIG2, respectively.
Significant strain differences in anxiety-like open field behavior
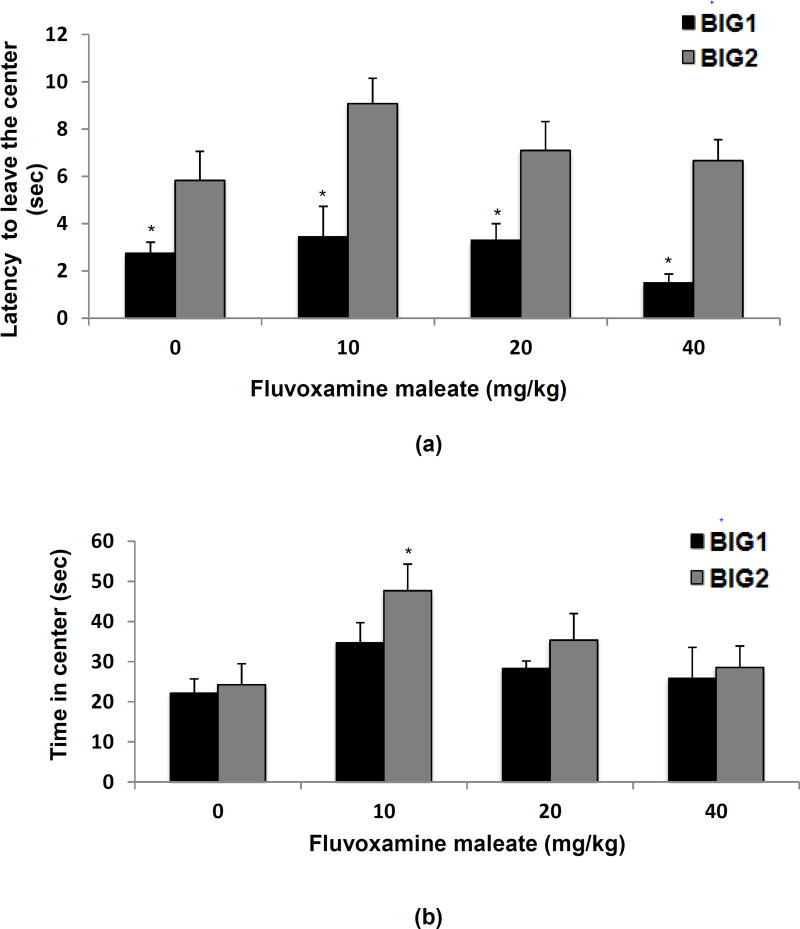
For anxiety-like behavior in the open field, there was a significant strain effect (F1,74 = 39.83 p<0.001) for the latency to leave the central square, with the BIG1 strain taking less time when compared to the BIG2 strain. The drug effect (F3,74 = 2.21, NS) and drug by strain interaction (F3,74 = 0.76, NS) were not significant (Fig. 3a).
Figure 3.
Anxiety-like open field behavior of the BIG1 and BIG2 strains. The data represent the mean (+ SEM) for (a) latency to leave the central square, *(p<0.05) indicates significant strain differences between BIG1 and BIG2 males in each treatment group; (b) the time spent in the central square, *(p<0.05) indicates significant drug effect at 10 mg/kg relative to vehicle (0 mg/kg) in the BIG2 strain; and (c) the total number of line crossings, *(p<0.05) indicates strain differences between BIG1 and BIG2 for the 10 and 20 mg/kg treatment groups. 0 mg/kg, n=11 and n=11; 10 mg/kg, n=7 and n=12; 20 mg/kg, n=10 and n=8; 40 mg/kg, n=6 and n=9 for BIG1 and BIG2, respectively.
For the time spent in the central square the strain effect was marginally significant (F1,74 = 3.73, p>0.05) with the BIG1 mice tending to spend more time in the central square compared to the BIG2 mice. A significant drug effect (F3,74 = 5.12, p<0.005) was primarily attributable to the 10 mg/kg dose, which increased the time spent in the central zone for BIG2 strains compared to the vehicle (Fig. 3b). The drug by strain interaction effect was not significant (F3,74 = 0.47, NS), which supports the view that the response at the 10 mg/kg dose in the BIG2 strain was minor and not replicated in the BIG1 strain.
For the number of line crossings there was a significant strain effect (F1,74 = 19.61, p<0.001), which was due to the BIG1 strains having more line crossings when compared to the BIG2 strains (Fig. 3c). No significant drug effect(F3,74 = 0.77, NS) or drug by strain interaction (F3,74 = 0.19, NS) were found.
Discussion
Our results showed that a fluvoxamine treatment regimen for 15–17 days resulted in significant dose-dependent attenuation of compulsive-like nesting in BIG1 male mice with a similar trend in the BIG2 male mice. This effect was observed following one hour of drug administration on the day of testing. Marble-burying behavior was dose-dependently reduced by fluvoxamine in both BIG1 and BIG2 strains. The dose response in nest-building and marble burying is in congruence with human studies where high doses of fluvoxamine have been found to be more effective for compulsive behaviors when compared to low doses (Fontenelle et al., 2007; Koran et al., 2010; Ordacgi et al., 2009). As fluvoxamine takes several weeks to months to become effective in the treatment of OCD patients (Fontenelle et al., 2007), we focused on the long-term effects of fluvoxamine. We tested the mice 15–17 days after daily injections, because in an earlier study another SSRI, i.e., fluoxetine, had significant effects after two weeks of chronic treatment in the drinking water (Greene-Schloesser et al., 2011). As we did not test the mice after the first injection of fluvoxamine, we cannot rule out acute effects. Fluvoxamine only had a significant effect on nest building in the first hour after administration and not at later time points, and therefore it is possible that the duration of fluvoxamine treatment had too short to see more persistent effects of this drug on compulsive-like nest building. It could also reflect that the response to fluvoxamine persists as long as the drug is active in the system. This is consistent with the refractory concept of OCD where patients have rebound of symptoms as soon as they are taken off the treatment regimen.
The BIG2 strains had a similar non-significant trend as the BIG1 strain in nest-building, which may have been due to the BIG2 males being less sensitive to fluvoxamine, having metabolized fluvoxamine more quickly, or having excreted it more rapidly than BIG1 males. Some OCD patients fail to respond in the typical dosage range used for clinical responders. Success of higher doses of SSRIs for non-responders has been observed (Decloedt and Stein, 2010). However, a higher dose, though beneficial for OCD symptoms, can have severe side effects (Decloedt and Stein, 2010; Bloch et al., 2010) thereby complicating the treatment strategies.
A previous study with the BIG1 mice has shown that a two-week fluoxetine (SSRI) treatment significantly attenuated nesting and marble burying behavior (Greene-Schloesser et al., 2011) in the BIG1 strain. This is the first time we have used both the BIG strains (BIG1 & BIG2) to compare the compulsive-like behavioral response to a first-line treatment, fluvoxamine. The results supported both nesting and marble-burying for assessment of compulsive-like behavior in our model. This is in contrast to the compulsive-like deer mouse model with spontaneous stereotypy, in which marble burying behavior was not associated with the stereotypy and did not respond to oral escitalopram treatment (Wolamrans et al., 2016b). So for deer mice, marble burying behavior did not appear to be a compulsive-like behavior. Interestingly, stereotypy and nest-building behavior also did not correlate, but 30% of the deer mice, irrespective of these two behaviors, showed unusually large nest-building behavior, which was attenuated by escitalopram (Wolamrans et al., 2016a). So, individual deer mice show different compulsive-like behaviors, i.e., stereotypy or nest building, but these were not correlated, while our BIG nest builders have a predictable compulsive-like phenotype showing both compulsive-like nesting and marble burying (Mitra et al., 2017) that can be attenuated with drugs used in OCD (Greene-Schloesser et al., 2011), as shown in this study. Therefore, it appears that compulsive-like behaviors are species specific and that not all compulsive-like behaviors are genetically correlated or affected by the environment in similar ways.
Locomotor activity was measured in the open field test (Hale et al., 2008) and was not affected by fluvoxamine. This result replicates another study with fluvoxamine in mice in which repeated fluvoxamine administration attenuated marble burying but not locomotion or exploratory behavior (Ichimaru et al., 1995). Our results also conform to a previous study from our lab where fluoxetine treatment did not alter locomotor activity in the wheel running test (Greene-Schloesser et al., 2011). Overall, BIG2 mice showed higher levels of anxiety-like behaviors than BIG1 mice, measured as time to leave the central square and time spent in the center of the open field. The drug effect for time spent in central zone was mainly due to the 10 mg/kg of fluvoxamine increasing central-zone exploration in BIG2 males. However, owing to the lack of a significant strain effect and strain by drug interaction in central zone time, an overall effect of fluvoxamine on anxiety-like behavior cannot be established. Fluvoxamine also did not have any overall effect on locomotor activity. A prior study with mice has shown that fluvoxamine had no anxiolytic effects in different anxiety tests (Ichimaru et al., 1995). Our results show that behavioral responses to fluvoxamine, in the compulsive-like mice, were overall small or absent for anxiety-like measures, while strain differences were observed in anxiety-like behavior and locomotor activity. The strain effect may indicate genetic differences between the two BIG strains due to random genetic drift or founder effects (Bult and Lynch 1996, 2000).
While, fluvoxamine is well tolerated and used widely in the treatment of general anxiety disorder and OCD (Irons, 2005; Freeman et al., 1994; Mundo et al., 2000), not many studies have focused on looking at the effect of fluvoxamine in treating co-morbid anxiety in complex neuropsychiatric disorders (Nadeau et al., 2011). A study on the efficacy of fluvoxamine in treating autism spectrum disorder (ASD) and associated anxiety resulted in a poor response to treatment (Martin et al., 2003). The ego-dystonic and intrusive nature of obsessions differs largely from general anxiety (Langlois et al., 2000, 2000a). In addition to large heterogeneity and variability of anxiety in OCD, this makes it a non-significant indicator for the disorder (Nutt and Malizia, 2006). General anxiety disorders and panic disorders have a late onset when compared to OCD, which has a very early onset (Rosenbaum et al., 1997). In addition, neurocircuitry models proposed for anxiety and OCD differ. While anxiety and panic disorders have been linked to dysregulation of amygdala-ventromedial prefrontal cortex-hippocampus circuitry (Shin and Liberzon, 2010; Rauch et al., 2006), OCD is thought to occur due to abnormal fronto-striatal circuitry (Fitzgerald et al., 2005; Rauch et al., 2007; Greenberg et al., 2010). Other studies with our compulsive-like (BIG) and non-compulsive-like (SMALL) mice indicate that general anxiety appears to be separate from anxiety pertaining to compulsions (Greene-Schloesser et al., 2011; Mitra et al., 2017). This is corroborated by other studies where association of OCD with general anxiety is deemed controversial (Stein et al., 2010).
Currently a lack of consensus exists on the best treatment option for OCD patients (Pittenger et al., 2005). Significant relapse rates have been reported in clinical studies with SSRIs (Abramowitch et al., 2009). Medication differences have also been observed with these first-line treatments. One such example is that of comorbid tics in children with OCD, which reduces the efficacy of SSRI treatment (March et al., 2007). It is however unclear if the same effect is persistent in adults (Lack, 2012). Among various factors, compulsive traits and associated co-morbid symptoms can result in non-responsiveness to treatments in the OCD condition (Koran, 2004). Studies have attributed a strong genetic link to the etiological heterogeneity of OCD (Nestadt et al., 2000; Pauls, 2010). These genes have also been thought to contribute to the disease severity and treatment resistance (Ozaki et al., 2003; Wendland et al., 2008). Quantitative genetic analysis indicates that nesting behavior is a highly polygenic trait (Bult and Lynch, 2000), which is consistent with OCD in humans likely being influenced by many genes (Hettema et al., 2001; Paul et al., 1999; Smoller., 2009; Stein, 2002). Hence, our mouse model selected for varied levels of compulsive-like behavior, i.e., nest-building, provides a critical insight into the role of genetic background and trait in influencing the efficacy of first-line treatments.
Conclusion
This study adds predictive validity to our mouse model of OCD because fluvoxamine, commonly used to treat OCD, dose-dependently decreased nest building behavior and marble burying behavior in the compulsive-like mice. Fluvoxamine had little to no effect on anxiety-like behaviors and locomotor activity, while one compulsive-like strain was more anxious than the other, which adds heterogeneity to our mouse model, similar to the heterogeneity seen in OCD.
Acknowledgments
We thank the Biological Research and Diagnostics (BIRD) Facility personnel for excellent routine animal care. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103395 to SM and AB-I and the National Institute of General Medical Sciences of the National Institutes of Health under three linked award numbers RL5GM118990, TL4GM118992 and 1UL1GM118991 to AB-I. This work was also supported by the Alaska Summer Research Academy (ASRA) and the College of Natural Sciences & Mathematics (CNSM). These funding sources did not have a role in the study design, collection, analysis and interpretation of data and submission of this paper for publication. We would also like to thank ASRA students Nailah Bealer, Kira Dickerson, Rachel Evans, Neeshi Hullavarad, Deriene Nickisch, Emily Perkins, and Maia Rothman for collecting the nest building, marble burying, and open field data under the supervision of SM and AB-I.
References
- Abramowitz JS, taylor S, Mckay D. Obsessive compulsive disorder. Lancet. 2009;374:491–499. doi: 10.1016/S0140-6736(09)60240-3. [DOI] [PubMed] [Google Scholar]
- Albert A, Aguglia A, Bramante S, Bogetto F, Maina G. Treatment-resistant obsessive-compulsive disorder (OCD): current knowledge and open questions. Clin Neuropsychiatry. 2013;10(1):19–30. [Google Scholar]
- Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble Burying and Nestlet Shredding as Tests of Repetitive, Compulsive-like Behaviors in Mice. Jove-J Vis Exp. 2013 doi: 10.3791/50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohar J, Moller HJ, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. International journal of psychiatry in clinical practice. 2012;16:77–84. doi: 10.3109/13651501.2012.667114. [DOI] [PubMed] [Google Scholar]
- Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-Analysis of the Dose-Response Relationship of SSRI in Obsessive-Compulsive Disorder. Molecular psychiatry. 2010;15(8):850–855. doi: 10.1038/mp.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult A, Lynch CB. Multiple selection responses in house mice bidirectionally selected for thermoregulatory nest-building behavior: Crosses of replicate lines. Behav Genet. 1996;26:439–446. doi: 10.1007/BF02359488. [DOI] [PubMed] [Google Scholar]
- Bult A, Lynch CB. Nesting and fitness: Lifetime reproductive success in house mice bidirectionally selected for thermoregulatory nest-building behavior. Behav Genet. 1997;27:231–240. doi: 10.1023/a:1025610130282. [DOI] [PubMed] [Google Scholar]
- Bult A, Lynch CB. Breaking through artificial selection limits of an adaptive behavior in mice and the consequences for correlated responses. Behav Genet. 2000;30:193–206. doi: 10.1023/a:1001962124005. [DOI] [PubMed] [Google Scholar]
- Decloedt EH, Stein DJ. Current trends in drug treatment of obsessive–compulsive disorder. Neuropsychiatric Disease and Treatment. 2010;6:233–242. doi: 10.2147/ndt.s3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas R, Seddik A, Oppelaar H, Westenberg HG, Kas MJ. Marked inbred mouse strain difference in the expression of quinpirole induced compulsive like behavior based on behavioral pattern analysis. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2012;22:657–663. doi: 10.1016/j.euroneuro.2012.01.003. 2012. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SE. Error-related hyperactivity of the anterior cingulate cortex in obsessivecompulsive disorder. Biol Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Fontenelle LF, Nascimento AL, Mendlowicz MV. An update on the pharmacological treatment of obsessive-compulsive disorder. Expert opinion on pharmacotherapy. 2007;8:563–583. doi: 10.1517/14656566.8.5.563. [DOI] [PubMed] [Google Scholar]
- Fontenelle IS, Fontenelle LF, Borges MC, Prazeres AM, Range BP, Mendlowicz MV, Versiani M. Quality of life and symptom dimensions of patients with obsessive-compulsive disorder. Psychiatry research. 2010;179:198–203. doi: 10.1016/j.psychres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Freeman CPL, Trimble MR, Deakin JFW, Stokes TM, Ashford JJ. Fluvoxamine Versus Clomipramine in the Treatment of Obsessive-Compulsive Disorder - a Multicenter, Randomized, Double-Blind, Parallel-Group Comparison. J Clin Psychiat. 1994;55:301–305. [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O'Malley B, et al. Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology. 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Delgado PL, Heninger GR, Charney DS. Efficacy of fluvoxamine in obsessive-compulsive disorder. A double-blind comparison with placebo. Archives of general psychiatry. 1989;46:36–44. doi: 10.1001/archpsyc.1989.01810010038006. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Ward H, Kablinger A, Murphy T. Fluvoxamine in the treatment of obsessive-compulsive disorder and related conditions. The Journal of clinical psychiatry. 1997;58:32–49. [PubMed] [Google Scholar]
- Greenberg BD, Suzanne SLR, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35:317–336. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene-Schloesser DM, Van der Zee EA, Sheppard DK, Castillo MR, Gregg KA, Burrow T, Foltz H, et al. Predictive validity of a non-induced mouse model of compulsive-like behavior. Behavioural brain research. 2011;221:55–62. doi: 10.1016/j.bbr.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Polilsen B, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience. 2008;155:659–672. doi: 10.1016/j.neuroscience.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. The American journal of psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hollander E, Stein DJ, Fineberg NA, Marteau F, Legault M. Quality of life outcomes in patients with obsessive-compulsive disorder: relationship to treatment response and symptom relapse. The Journal of clinical psychiatry. 2010;71:784–792. doi: 10.4088/JCP.09m05911blu. [DOI] [PubMed] [Google Scholar]
- Ichimaru Y, Egawa T, Sawa A. 5-Ht1a-Receptor Subtype Mediates the Effect of Fluvoxamine, a Selective Serotonin Reuptake Inhibitor, on Marble-Burying Behavior in Mice. Jpn J Pharmacol. 1995;68:65–70. doi: 10.1254/jjp.68.65. [DOI] [PubMed] [Google Scholar]
- Irons J. Fluvoxamine in the treatment of anxiety disorders. Neuropsychiatric disease and treatment. 2005;1:289–299. [PMC free article] [PubMed] [Google Scholar]
- Karno M, Golding JM, Sorenson SB, Burnam MA. The epidemiology of obsessive-compulsive disorder in five US communities. Archives of general psychiatry. 1988;45:1094–1099. doi: 10.1001/archpsyc.1988.01800360042006. [DOI] [PubMed] [Google Scholar]
- Kellner M. Drug treatment of obsessive-compulsive disorder. Dialogues in clinical neuroscience. 2010;12:187–197. doi: 10.31887/DCNS.2010.12.2/mkellner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran LM. Augmentation strategies for treatment resistant obsessive compulsive disorder. Clinical Neuropsychiatry. 2004;1:65–71. [Google Scholar]
- Koran LM, Bromberg D, Hornfeldt CS, Shepski JC, Wang S, Hollander E. Extended-release fluvoxamine and improvements in quality of life in patients with obsessive-compulsive disorder. Comprehensive psychiatry. 2010;51:373–379. doi: 10.1016/j.comppsych.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Lack CW. Obsessive-compulsive disorder: Evidence-based treatments and future directions for research. World journal of psychiatry. 2012;2:86–90. doi: 10.5498/wjp.v2.i6.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois F, Freeston MH, Ladouceur R. Differences and similarities between obsessive intrusive thoughts and worry in a non-clinical population: study 1. Behaviour research and therapy. 2000;38:157–173. doi: 10.1016/s0005-7967(99)00027-3. [DOI] [PubMed] [Google Scholar]
- Lynch CB. Response to divergent selection for nesting behavior in Mus musculus. Genetics. 1980;96:757–765. doi: 10.1093/genetics/96.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio PT, Filgueiras BG, Cunha CD, Estanislau C. Animal models of Obsessive-Compulsive Disorder: Strain differences. World Journal of Neuroscience. 2014;4:240–246. [Google Scholar]
- Mallya GK, White K, Waternaux C, Quay S. Short and long-term treatment of obsessive compulsive disorder. Annals of Clinical Psychiatry. 1992;4:77–80. [Google Scholar]
- March JS, Franklin ME, Leonard H, Garcia A, Moore P, Freeman J, Foa E. Tics moderate treatment outcome with sertraline but not cognitive-behavior therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2007;61:344–347. doi: 10.1016/j.biopsych.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Martin A, Koenig K, Anderson GM, Scahill L. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: A prospective, open-label study. J Autism Dev Disord. 2003;33:77–85. doi: 10.1023/a:1022234605695. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Wilson JR, Meredith W. The use of isogenic and heterogenic mouse stocks in behavioral research. In: Lindzey G, Theissen DD, editors. Contributions to Behavior-Genetic Analysis: The Mouse as a Prototype. Appleton-Century-Crofts; NewYork: 1970. pp. 3–22. 1970. [Google Scholar]
- Milanfranchi A, Ravagli S, Lensi P, Marazziti D, Cassano GB. A double-blind study of fluvoxamine and clomipramine in the treatment of obsessive-compulsive disorder. International clinical psychopharmacology. 1997;12:131–136. doi: 10.1097/00004850-199705000-00002. [DOI] [PubMed] [Google Scholar]
- Mitra S, Bastos CP, Bates K, Pereira GS, Bult-Ito A. Ovarian Sex Hormones Modulate Compulsive, Affective and Cognitive Functions in A Non-Induced Mouse Model of Obsessive-Compulsive Disorder. Frontiers in Behavioral Neuroscience. 2016a;10:215. doi: 10.3389/fnbeh.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Mucha M, Khatri SN, Glenon R, Schulte MK, Bult-Ito A. Attenuation of Compulsive-Like Behavior Through Positive Allosteric Modulation of α4β2 Nicotinic Acetylcholine Receptors in Non-Induced Compulsive-Like Mice. Frontiers in Behavioral Neuroscience. 2016b;10:244. doi: 10.3389/fnbeh.2016.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Bastos CP, Chesworth S, Frye C, Bult-Ito A. Strain and sex based characterization of behavioral expressions in non-induced compulsive-like mice. Physiology & Behavior. 2017;168:103–111. doi: 10.1016/j.physbeh.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundo E, Maina G, Uslenghi C, Grp MS. Multicentre, double-blind, comparison of fluvoxamine and clomipramine in the treatment of obsessive-compulsive disorder. International clinical psychopharmacology. 2000;15:69–76. doi: 10.1097/00004850-200015020-00002. [DOI] [PubMed] [Google Scholar]
- Mundo E, Rouillon F, Figuera ML, Stigler M. Fluvoxamine in obsessive-compulsive disorder: similar efficacy but superior tolerability in comparison with clomipramine. Human psychopharmacology. 2001;16:461–468. doi: 10.1002/hup.317. [DOI] [PubMed] [Google Scholar]
- Nadeau J, Sulkowski ML, Ung D, Wood JJ, Lewin AB, Murphy TK, May JE, et al. Treatment of comorbid anxiety and autism spectrum disorders. Neuropsychiatry-Lond. 2011;1:567–578. doi: 10.2217/npy.11.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamae T. [Novel treatment strategies for refractory patients with obsessive-compulsive disorder] Seishin shinkeigaku zasshi = Psychiatria et neurologia Japonica. 2013;115:997–1003. [PubMed] [Google Scholar]
- Nestadt G, Lan T, Samuels J, Riddle M, Bienvenu OJ, 3rd, Liang KY, Hoehn-Saric R, et al. Complex segregation analysis provides compelling evidence for a major gene underlying obsessive-compulsive disorder and for heterogeneity by sex. American journal of human genetics. 2000;67:1611–1616. doi: 10.1086/316898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek K, Dennis K, Andrus BM, Ahmadiyeh N, Baum AE, Woods LCS, Redei EE. Context and strain-dependent behavioral response to stress. Behav Brain Funct. 2008;4 doi: 10.1186/1744-9081-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Malizia A. Anxiety and OCD - the chicken or the egg? Journal of psychopharmacology. 2006;20:729–731. doi: 10.1177/0269881106068424. [DOI] [PubMed] [Google Scholar]
- Ordacgi L, Mendlowicz MV, Fontenelle LF. Management of obsessive-compulsive disorder with fluvoxamine extended release. Neuropsychiatric disease and treatment. 2009;5:301–308. doi: 10.2147/ndt.s3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Molecular psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Pato MT, Pato CN, Pauls DL. Recent findings in the genetics of OCD. The Journal of clinical psychiatry. 2002;6(63 Suppl):30–33. [PubMed] [Google Scholar]
- Pauls DL. The genetics of obsessive-compulsive disorder: a review. Dialogues in clinical neuroscience. 2010;12:149–163. doi: 10.31887/DCNS.2010.12.2/dpauls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP. The inheritance of obsessive-compulsive disorder. Child and adolescent psychiatric clinics of North America. 1999;8:481–496. viii. [PubMed] [Google Scholar]
- Pittenger C, Kelmendi B, Bloch M, Krystal JH, Coric V. Clinical treatment of obsessive compulsive disorder. Psychiatry. 2005;2:34–43. [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, Cannistraro PA, et al. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biological psychiatry. 2007;61:330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, Kagan J. Behavioral inhibition in childhood: a risk factor for anxiety disorders. Biol Psychiatry. 1997;42:145S. doi: 10.3109/10673229309017052. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatr. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an Index of Anxiety in Mice - Influence of Dopaminergic Transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The Neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Block SR, Young MM. Genetics of anxiety disorders: the complex road from DSM to DNA. Depression and anxiety. 2009;26:965–975. doi: 10.1002/da.20623. [DOI] [PubMed] [Google Scholar]
- Stein DJ. Obsessive-compulsive disorder. Lancet. 2002;360:397–405. doi: 10.1016/S0140-6736(02)09620-4. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Fineberg NA, Bienvenu OJ, Denys D, Lochner C, Nestadt G, Leckman JF, et al. Should OCD be classified as an anxiety disorder in DSM-V? Depress Anxiety. 2010;27(6):495–506. doi: 10.1002/da.20699. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Yatsugi S, Yamaguchi T. Effect of YM992, a novel antidepressant with selective serotonin re-uptake inhibitory and 5-HT2A receptor antagonistic activity, on a marble-burying behavior test as an obsessive-compulsive disorder model. Jpn J Pharmacol. 2002;90:197–200. doi: 10.1254/jjp.90.197. [DOI] [PubMed] [Google Scholar]
- Taylor S. Molecular genetics of obsessive-compulsive disorder: a comprehensive meta-analysis of genetic association studies. Molecular psychiatry. 2013;18:799–805. doi: 10.1038/mp.2012.76. [DOI] [PubMed] [Google Scholar]
- Ting JT, Feng G. Neurobiology of obsessive-compulsive disorder: insights into neural circuitry dysfunction through mouse genetics. Current opinion in neurobiology. 2011;21:842–848. doi: 10.1016/j.conb.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Adamczyk A, Shugart YY, Samuels JF, Grados MA, Greenberg BD, Knowles JA, et al. A screen of SLC1A1 for OCD-related alleles. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the. International Society of Psychiatric Genetics. 2010;153B:675–679. doi: 10.1002/ajmg.b.31001. [DOI] [PubMed] [Google Scholar]
- Warneke L. Anxiety Disorders. Focus on obsessive-compulsive disorder. Canadian Family Physician. 1993;39:1612–1621. [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, DeGuzman TB, McMahon F, Rudnick G, Detera-Wadleigh SD, Murphy DL. SERT Ileu425Val in autism, Asperger syndrome and obsessive-compulsive disorder. Psychiatric genetics. 2008;18:31–39. doi: 10.1097/YPG.0b013e3282f08a06. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolmarans DW, Stein DJ, Harvey BH. Excessive nest building is a unique behavioural phenotype in the deer mouse model of obsessive-compulsive disorder. Journal of Psychopharmacology. 2016a May 6; doi: 10.1177/0269881116645554. pii: 0269881116645554. [DOI] [PubMed] [Google Scholar]
- Wolmarans DW, Stein DJ, Harvey BH. Of mice and marbles: Novel perspectives on burying behavior as a screening test for psychiatric illness. Cognitive, Affective, & Behavioral Neuroscience. 2016b;16:551. doi: 10.3758/s13415-016-0413-8. [DOI] [PubMed] [Google Scholar]