Abstract
Treatment with allogeneic hematopoietic stem cell transplantation (HSCT) is associated with short and long-term toxicities that can result in alterations in sexual functioning. The aims of this prospective evaluation were to determine: (1) associations between HSCT and increased sexual dysfunction 1 year after treatment; and (2) associations between sexual dysfunction, body image, anxiety and depression. This controlled prospective cohort study was conducted from October 2010 to November 2013. Patients completed assessments 2–3 weeks before HSCT (N = 124) and 1 year after treatment (N = 63). Assessment included descriptive data, Sexual Functioning Questionnaire, Body Image Scale and Hospital Anxiety and Depression Scale. The results showed a significant decline in overall sexual function in both men and women (P = < 0.001, P = 0.010, respectively), although men generally scored higher than women. Forty-seven percent of men and 60% of women reported at least one physical sexual problem 1 year after HSCT. Patients with chronic GVHD trended toward reporting lower levels of sexual function. Finally, women with chronic GVHD scored lower than those without chronic GVHD on the sexual function problem subscale (P = 0.008). Sexual dysfunction remains a major problem for men and women 1 year after HSCT and requires routine evaluation and treatment after HSCT.
INTRODUCTION
In the last decade, there has been advancements in hematopoietic stem cell transplantation (HSCT) for hematologic malignancies, including an increasing number of patients having access to HSCT, reduced toxicity of treatment regimens and improved survival rates. As a result, HSCT is now available to patients with a broader spectrum of diagnoses, more advanced stages of disease, older age and with more comorbidities.1 The existing evidence concerning sexuality in patients following HSCT may therefore not reflect experiences of patients undergoing HSCT today. Sexual dysfunction is a frequently described problem in quality of life studies after HSCT, and is one of the most common long-term issues following HSCT.2–4 Long-term sexual complications include decreased libido, and difficulty reaching an orgasm in men and women, vaginal dryness, atrophy and stenosis in women, and erectile and ejaculatory dysfunctions in men. These can lead to avoidance of sexual activity, dyspareunia, and sometimes vaginal or penile bleeding and irritation.5–8
Treatment with HSCT is associated with short and long-term toxicities affecting sexual function, due to the conditioning regimen, gonadal function changes that result from treatment and chronic GVHD among other factors.9,10 It is not known how myeloablative and non-myeloablative HSCT influence sexual dysfunction, however, these transplant types differ greatly in their conditioning regimens and risk of chronic GVHD.1,11–14 One of the major determinants of sexual dysfunction is chronic GVHD,8,11,15,16 which can manifest anywhere in the body, however, genital chronic GVHD can lead to severe complications.9,12,17,18 Genital chronic GVHD can lead to vaginal stenosis, scar tissue, soreness and adhesions in blood vessels, rash and increased sensitivity in the skin around the penis.9,12,17,18 The incidence of genital chronic GVHD allogeneic HSCT recipients are reported to be 35–52% in women17,19 and 20% in men.20 Furthermore, chronic GVHD and its treatment with high-dose corticosteroids may induce changes in physical appearance to such an extent that sexuality and body image are negatively affected. Body image changes, anxiety, depression, decreased self-confidence, fear of disease recurrence, concurrent life stress and concerns about infertility are some of the psychological factors that may disrupt the sexual response cycle.21–23
The existing evidence suggests there is a decrease in sexual activity and quality of sex life following HSCT. However, owing to new developments in treatment regimens over the past decade these results may not reflect HSCT patient outcomes today. In addition, few studies are longitudinal and most have not examined the nature of this dysfunction in depth.24 In this study, we hypothesized that HSCT, and specifically female gender, non-myeloablative conditioning regimen and chronic GVHD would be associated with increased sexual dysfunction 1 year after HSCT. The primary aim was to examine the association between treatment with HSCT and sexual dysfunction 1 year after HSCT. Secondary aims were to identify the associations between sexual dysfunction, body image, anxiety and depression.
MATERIALS AND METHODS
The study is a clinically controlled, prospective cohort trial with two test time points. Patient-reported outcomes were completed during the pre-examination week, 2 weeks before HSCT and at the 1-year control visit at the bone marrow transplant outpatient unit. Participants with hematological disease undergoing HSCT were eligible at both assessments if ≥ 18 years and were excluded if unable to read or speak Danish, had a cognitive disorder or if it was assessed unethical to enroll the participant in the study because of poor general health or suspicion of relapse. No exclusions were made for sexual orientation or sexual partner. Participants were approached in the outpatient clinic at the Department of Haematology, Copenhagen University Hospital, Rigshospitalet from October 2010 to November 2012. Patients were identified and recruited by the primary investigator (KHN). Patients received oral and written information, guaranteed anonymity and informed that they could withdraw from the study without explanation and without impacting their treatment or access to care. As the topic of this study can be characterized as sensitive, there was emphasis on building trust and explaining the value of the information at the time of enrollment. All patients who agreed to participate signed a written informed consent and received a stamped addressed envelope for their reply. The project is registered by the Danish Data Protection Agency (registration no. 2010-41-4920) and approved by the Joint Ethics Committee of the Capital Region of Denmark (approval no. H-C-FSP-2010-12).
Outcome measures
Descriptive data were obtained from the Danish HSCT database and through medical chart review. Data were entered into the HSCT database by data managers (nurses) through medical chart review. Data included age, education, marital status, occupational status, diagnosis, type of treatment and GVHD status, which included degree, stage, location and length of treatment with corticosteroids. Chronic GVHD was defined according to the NIH criteria14 and graded as limited or extensive and mild, moderate or severe.14 Fertile women were evaluated by a gynecologist at 3 and 6 months, whereas all women were seen systematically 1 year after HSCT for overall genital health including GVHD evaluation. Men were not evaluated at specific time points, however, both men and women were examined for genital GVHD when there were symptoms or other reasons for suspicion of genital GVHD, and then they were referred for further evaluation according to standard clinical practice. Locations of GVHD is registered in the HSCT database for all patients receiving HSCT at specific time points, for example, 3 months, 6 months, 1-year after HSCT. We extracted data on GVHD from the HSCT database and manually examined the medical charts on all patients at follow-up to get a complete picture of GVHD status and symptoms.
Patient-reported outcomes included:
The 37-item Sexual Function Questionnaire (SFQ) has well-defined reliability and validity with HSCT and normative samples,25 assessing nine domains (interest, desire, arousal, orgasm, satisfaction, activity, relationship, masturbation and problems). Male and female versions match in content and scoring with the exception of differing sexual problem content. Frequency of sexual thoughts and behaviors were reported. Sexual responses, satisfaction and problems were rated for intensity or frequency of occurrence during sexual activity. Sexual activity the past month and year are reported and, if not sexually active, respondents check reasons from a list of possibilities. Higher scores indicate better sexual function or less frequent problems. Scores (mean) in overall score and subscales range from 0 to 5. The questionnaire was translated into Danish according to the guidelines from the ‘translation and cultural adaptation group’ in 2010.26 Linguistic validation was done in collaboration with the author of the SFQ Karen Syrjala (KS).
The ‘Body Image Scale’ assesses 10 items: affective (to feel feminine/masculine, to feel attractive), behavioral (avoid people because of appearance) and cognitive (satisfaction with appearance).27 The items are categorized as ‘not at all’, ‘a little’, ‘quite a bit’, ‘very much’ with zero scores representing no symptom/distress, and higher scores representing increasing symptom/distress.
The ‘Hospital Anxiety and Depression Scale’ consists of 14 items, with responses being scored on a scale of 0–3, with 3 indicating higher symptoms of distress. The score for each subscale (anxiety and depression) can range from 0–21 with scores categorized as follows: normal (0–7), mild (8–10), moderate (11–14), severe (15–21).28
Descriptive data were obtained and patient-reported outcomes were completed at baseline and 1 year after HSCT.
Statistical analysis
Statistical analyses were conducted using SPSS for windows version 19 (Copenhagen, Denmark). Longitudinal analyses were conducted on the subset of 63 patients who completed the questionnaire at both time points. Chi-square test was used in measuring difference in demographic characteristics between baseline and 1-year test. In addition, sexual activity, physical problems and reasons for not being sexually active were compared for baseline versus 1 year separately for men and women using paired t-test. The same data were compared for men versus women using independent sample t-test. SFQ scores both overall and subscales were analyzed in relation to gender, treatment regime and chronic GVHD using independent sample t-test. Pearson correlations were used to identify associations between SFQ scores, body image, anxiety and depression. Throughout, two-sided P-values < 0.05 obtained in chi-square tests or t-tests and 95% confidence intervals that included unity were considered indicators of statistical significance.
RESULTS
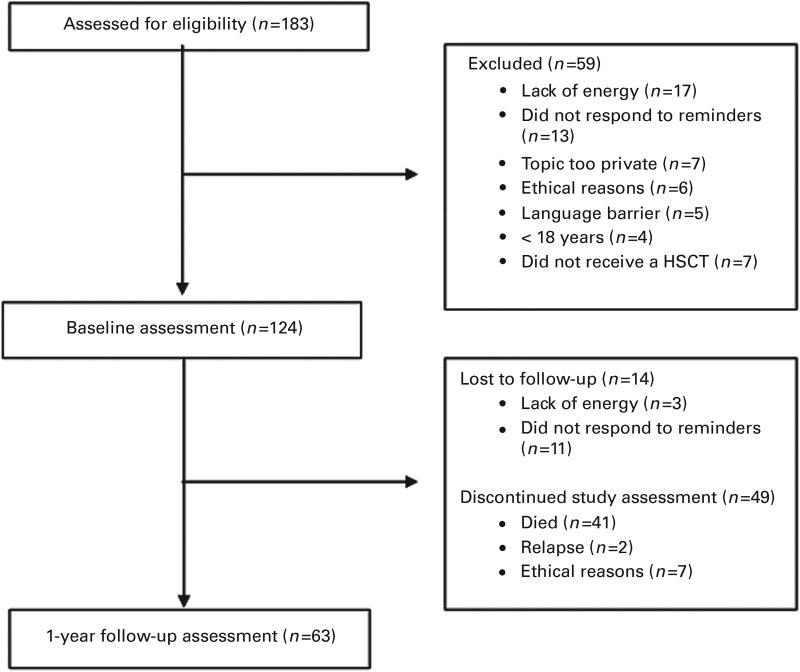
A total of 183 potentially eligible patients were approached for study participation. Of these, 124 patients (77%) provided informed consent and completed questionnaires at baseline and 63 patients (49%) 1 year after HSCT (Figure 1). Reasons for dropout at the 1-year assessment was due to death (n = 41), ethical reasons (n = 7) and relapse (n = 2). Table 1 provides demographic and medical characteristics for participants in the study at both time points. There were no gender differences in any characteristics between baseline and 1 year.
Figure 1.
Consort flowchart.
Table 1.
Sociodemographic and medical characteristics
| No. (%) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Men | Women | |||||
|
|
|
|||||
| Baseline | Follow-up | P-value | Baseline | Follow-up | P-value | |
| Overall | 83 (66) | 38 (60) | 41 (34) | 25 (40) | ||
| Age, mean (s.d.) | 51.1 (14) | 50.9 (14) | 0.088 | 49.8 (13) | 49.9 (13) | 0.597 |
| Age | ||||||
| 18–29 | 11 (13.3) | 3 (3.6) | 3 (7.3) | 2 (4.9) | ||
| 30–39 | 9 (10.8) | 1 (1.2) | 8 (19.5) | 5 (12.2) | ||
| 40–49 | 20 (24.1) | 11 (13.3) | 9 (22) | 5 (12.2) | ||
| 50–59 | 17 (20.5) | 10 (12) | 7 (17.1) | 5 (12.2) | ||
| 60–69 | 22 (26.5) | 9 (10.8) | 13 (31.7) | 7 (17.1) | ||
| 70–79 | 4 (4.8) | 4 (10.5) | 1 (2.4) | 1 (2.4) | ||
| Marital status | 0.221 | 0.467 | ||||
| Married or cohabiting | 52 (62) | 28 (73.6) | 24 (58) | 13 (52) | ||
| Not married | 32 (38) | 10 (26.4) | 18 (42) | 12 (48) | ||
| Education | 0.949 | 0.877 | ||||
| Short (< 3 years) or no education | 24 (28.9) | 7 (18.4) | 12 (29.3) | 6 (24) | ||
| Middle (3–5 years) | 39 (47.2) | 23 (60.5) | 21 (51.2) | 16 (64) | ||
| Long (> 5 years) | 15 (18.1) | 6 (15.8) | 5 (12.2) | 2 (8) | ||
| During education | 4 (4.8) | 2 (5.3) | 3 (7.3) | 1 (4) | ||
| Occupational status | 0.729 | 0.489 | ||||
| Student | 5 (6) | 4 (10.5) | 2 (4.8) | 0 | ||
| Sick leave | 39 (47) | 6 (15.8) | 25 (61) | 12 (48) | ||
| Normal or early retirement | 26 (31.3) | 15 (39.5) | 12 (29.4) | 8 (32) | ||
| Unemployed | 1 (1.2) | 1 (2.6) | 1 (2.4) | 0 | ||
| Part-time employed | 0 | 7 (18.4) | 0 | 3 (12) | ||
| Full-time employed | 12 (14.5) | 5 (13.2) | 1 (2.4) | 2 (8) | ||
| Diagnosis | 0.230 | 0.737 | ||||
| CML | 3 (3.6) | 2 (5.3) | 2 (4.9) | 4 (16) | ||
| AML | 29 (34.9) | 12 (31.6) | 15 (36.6) | 10 (40) | ||
| CLL | 10 (12) | 8 (21.1) | 0 | 0 | ||
| Acute lymphocytic leukemia | 11 (13.3) | 4 (10.5) | 9 (22) | 5 (20) | ||
| Lymphoma | 0 | 0 | 0 | 0 | ||
| Myelodysplasias | 10 (12) | 5 (13.2) | 6 (14.6) | 3 (12) | ||
| Aplastic anemia | 0 | 0 | 1 (2.4) | 1 (4) | ||
| Other | 20 (24.2) | 7 (18.3) | 8 (19.5) | 2 (8) | ||
| Type of treatment | 0.317 | 0.379 | ||||
| Myeloablative | 34 (41) | 18 (36.8) | 23 (56.1) | 15 (60) | ||
| Non-myeloablative | 49 (59) | 24 (63.2) | 18 (43.9) | 10 (40) | ||
Percentages for the row ‘overall’ reflect the proportion of men compared with women. Percentages for column: baseline and follow-up, reflects proportions with the particular level of age, marital status etc. P-values are measured using chi-square test.
Physical sexual problems
Both genders experienced an increasing number of sexual problems from baseline to 1 year after HSCT (Tables 2 and 3). The largest increase (percent change) among men was in difficulty achieving or maintaining an erection (18.7–47%) and for women it was vaginal dryness (17–60%). At 1 year, 47% men and 60% women experienced at least one physical sexual problem. In men experiencing physical sexual problems between13 and 66% were sexually active within the last month. In women experiencing physical sexual problems between 40 and 80% were sexually active within the last month.
Table 2.
Frequency of male sexual problems > 50% of the time
| Baseline, no. (%) | Follow-up, no. (%) | P-value | Sexually active at follow-up among men with symptom, no. (%) |
|
|---|---|---|---|---|
| Difficulty in getting an erection | 15 (18.7) | 18 (47) | < 0.001 | 4 (22) |
| Lack of sexual interest | 25 (30) | 21 (55) | 0.008 | 11 (52) |
| Loss of erection during sexual intercourse | 22 (27) | 15 (39) | 0.009 | 3 (20) |
| Delayed ejaculation | 12 (14) | 8 (21) | 0.063 | 2 (25) |
| Anxiety about sexual performance | 19 (23) | 17 (45) | 0.037 | 9 (53) |
| Unable to get an orgasm | 8 (10) | 10 (26) | 0.009 | 4 (40) |
| Pain during sexual intercourse | 2 (2) | 6 (16) | 0.017 | 4 (66) |
| Difficulty in pulling back the forskin of the penis | 3 (4) | 8 (21) | 0.007 | 1 (13) |
| Increased sensitivity in the skin around the penis | 5 (6) | 8 (21) | 0.054 | 1 (13) |
| Irritation or bleeding in the skin around the penis | 1 (1) | 4 (11) | 0.023 | 1 (25) |
Percentages for physical problems reflects proportions among all men at baseline and follow-up. P-values are measured using paired t-test.
Table 3.
Frequency of female sexual problems > 50% of the time
| Baseline, no. (%) | Follow-up, no. (%) | P-value | Sexually active at follow-up among females with symptoms, no. (%) |
|
|---|---|---|---|---|
| Vaginal dryness | 7 (17) | 15 (60) | 0.042 | 8 (53) |
| Lack of sexual interest | 25 (61) | 12 (48) | 0.197 | 5 (41) |
| Vaginal tightness | 7 (17) | 12 (48) | 0.043 | 5 (41) |
| Pain during sexual intercourse | 6 (15) | 12 (48) | 0.056 | 7 (58) |
| Anxiety about sexual performance | 5 (12) | 7 (28) | 0.451 | 4 (57) |
| Unable to get an orgasm | 8 (20) | 10 (40) | 0.600 | 4 (40) |
| Vaginal bleeding or irritation during sexual intercourse | 5 (12) | 7 (28) | 0.035 | 3 (42) |
| Increased sensitivity of the skin at intimate contact | 8 (20) | 5 (20) | 0.668 | 4 (80) |
| Pain inside or outside of your vagina | 3 (7) | 9 (36) | 0.001 | 4 (44) |
Percentages for physical problems reflect proportions among all men at baseline and follow-up. P-values are measured using paired t-test.
Sexual function and HSCT
Men reported being more frequently sexually active than women in the past year (P = 0.049) and the past month (P = 0.003) at baseline. However, this difference was not significant 1 year after HSCT (Table 4). Reasons for sexual inactivity varied and are shown in Table 4. For men, the most prevalent reason given for absence of sexual activity was physical or sexual problems. For women, lack of libido and physical or sexual problems were equally indicated as reasons for absence of sexual activity.
Table 4.
Reasons for sexual inactivity at baseline and 1-year follow-up after HSCT
| Men | Women | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Baseline | Follow-up | P-value | Baseline | Follow-up | P-value | |
| Sexually active in the past year, no. (%) | 74 (89) | 29 (76) | 0.160 | 31 (76)a | 14 (56) | 0.022 |
| Sexually active in the past month, no. (%) | 57 (69) | 20 (53) | 0.133 | 17 (42)a | 10 (40) | 0.538 |
| Reasons for not being sexually active, no. (%) | ||||||
| I have never been sexually active | 2 (6) | 1 (4) | 1 (2.7) | 1 (15) | ||
| Too tired | 7 (21) | 4 (15) | 10 (27) | 4 (18) | ||
| Not interested, lack of libido | 7 (21) | 6 (22) | 14 (38) | 6 (28) | ||
| Physical or sexual problems | 9 (27) | 13 (48) | 4 (11) | 6 (28) | ||
| Partner not interested | 2 (6) | 1 (4) | 2 (5) | 0 | ||
| Partner has physical problems | 1 (3) | 1 (4) | 1 (3) | 0 | ||
| Partner too tired | 1 (3) | 1 (4) | 0 | 0 | ||
| No partner | 4 (12) | 0 | 5 (14) | 5 (23) | ||
Abbreviation: HSCT=hematopoietic stem cell transplantation.
P < 0.05 baseline values men versus women. Percentages for ‘sexually active in the past year or past month’ reflect proportions among all men and women at baseline and follow-up. P-values are measured using paired t-test.
Men declined significantly in overall SFQ score (P < 0.001) and all SFQ subscales between baseline and 1 year, compared with women who declined in overall score (P = 0.010), orgasm (P = 0.004), relationship (P = 0.043) and problems (P = 0.004) (Table 5). Despite their decline from baseline to 1 year, men had higher overall SFQ scores and subscale scores at both baseline and 1 year compared with women.
Table 5.
Summary of SFQ scores overall and subscales separately for men and women
| Mean (s.d.) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Men | Women | |||||
|
|
|
|||||
| Baseline | Follow-up | P-value | Baseline | Follow-up | P-value | |
| SFQ score, mean (s.d.) | ||||||
| Overall SFQ score | 3.53 (0.7) | 2.96 (0.8) | < 0.001 | 3.11 (0.6)a | 2.73 (0.6) | 0.010 |
| Subscales, mean (s.d.) | ||||||
| Interest | 3.05 (1.5) | 2.18 (1.4) | < 0.001 | 1.77 (1.4)a | 1.73 (1.4) | 0.246 |
| Desire | 3.61 (1.2) | 3.12 (1.4) | 0.005 | 3.49 (1.1) | 4.22 (0.7)b | 0.085 |
| Arousal | 3.36 (1.2) | 2.78 (1.2) | 0.016 | 2.56 (1.3)a | 2.32 (1.0) | 0.989 |
| Orgasm | 3.82 (0.9) | 3.33 (1.2) | 0.021 | 3.22 (12)a | 2.18 (1.2)b | 0.004 |
| Satisfaction | 3.13 (1.7) | 2.29 (1.2) | 0.027 | 1.84 (1.9)a | 1.53 (1.3) | 0.099 |
| Masturbation | 2.94 (1.2) | 2.53 (1.4) | 0.011 | 2.10 (0.7)a | 2.05 (1.0) | 0.889 |
| Relationship | 3.79 (0.8) | 3.47 (0.9) | 0.016 | 3.30 (1.0)a | 2.80 (0.7)b | 0.043 |
| Behavior | 3.20 (0.9) | 2.84 (1.0) | 0.015 | 3.00 (1.2) | 2.73 (0.9) | 0.059 |
| Problems | 4.19 (1.1) | 3.75 (1.2) | < 0.001 | 4.14 (1.2) | 3.47 (1.0) | 0.004 |
Abbreviation: SFQ = Sexual Functioning Questionnaire.
P < 0.05 baseline scores men versus women using independent t-test.
P < 0.05 follow-up scores men versus women using independent t-test. All visible P-values are measured using paired t-test.
Sexual function and conditioning regimen
Both men and women regardless of their conditioning regime (myeloablative or non-myeloablative) declined significantly in overall SFQ scores from baseline to 1 year (P < 0.05). Yet there was no significant difference between the two conditioning regimens in overall SFQ or SFQ subscales. However, women treated with non-myeloablative conditioning regimen scored significantly lower than myeloablative women in overall SFQ score (P = 0.034), arousal (P = 0.008) and interest (P = 0.024) at 1 year.
Sexual function and GVHD
More men than women were diagnosed with chronic GVHD during the first year after HSCT (Table 6). Approximately 80% of them still had chronic GVHD 1 year after HSCT and were still receiving corticosteroid treatment. We found no incidence of genital GVHD recorded in the HSCT database in any respondents. Yet, with a medical chart review we found there were two female patients diagnosed with genital GVHD by 1.5 and 2 years post HSCT. These two women also had high scores on physical sexual problems (vaginal dryness, vaginal tightness and pain during intercourse) at 1 year. Furthermore, we found that men, in contrast to women, even when reporting high scores on physical sexual problems in our assessments, did not report genital symptoms to their nurse or doctor. It also appears from the medical charts that the few men who reported genital problems were referred to a urologist (n = 4), an endocrinologist (n = 3) or a department of growth and reproduction (n = 3). All women were evaluated on the earlier described time points after HSCT.
Table 6.
Chronic GVHD status
| No. (%) | |||
|---|---|---|---|
|
|
|||
| Men | Women | P-value | |
| Diagnosed with chronic GVHD, no. (%) | 16 (41) | 6 (25) | 0.189 |
| Chronic GVHD present at 1-year follow-up for those diagnosed | 13 (81) | 5 (83) | 0.634 |
| Degree of chronic GVHD | 0.238 | ||
| Limited | 8 (50) | 3 (50) | |
| Extensive | 8 (50) | 3 (50) | |
| Degree of chronic GVHD | 0.069 | ||
| Mild | 12 (75) | 6 (100) | |
| Moderate | 4 (25) | 0 | |
| Severe | 0 | 0 | |
| Organs involved | |||
| Skin | 11 (69) | 2 (33) | 0.037 |
| Eye | 5 (31) | 5 (84) | 0.406 |
| Oral cavity | 6 (38) | 4 (67) | 0.585 |
| Lung | 3 (19) | 1 (17) | 0.585 |
| Gastrointestinal tract | 1 (6) | 0 | 0.437 |
| Liver | 2 (13) | 1 (17) | 0.865 |
| Genitals | 0 | 0 | |
| Duration of treatment with corticosteroids | 0.408 | ||
| < 6 months | 9 (56) | 1 (17) | |
| > 6 months | 7 (44) | 5 (83) | |
| Still in treatment at 1-year follow-up | 15 (94) | 6 (100) | 0.267 |
Percentages for ‘diagnosed with chronic GVHD’ reflects proportions compared with all men and women, respectively, at 1-year follow-up. Percentages for ‘degree of chronic GVHD’ reflect the total proportion in men and women diagnosed with chronic GVHD, respectively. The total percentage for ‘organs involved’ is > 100% as some patients have more than one organ involved.
There was no difference in overall SFQ scores and subscales between men or women with chronic GVHD versus without chronic GVHD (Table 6). Although participants with chronic GVHD scored lower descriptively on overall SFQ and subscales, the differences were not significant in both genders. Women with chronic GVHD scored significantly lower on the SFQ problems subscale (P = 0.008), but not on the overall SFQ or other subscales.
Sexual function, anxiety, depression and body image
The correlation between sexual function, anxiety, depression and body image at 1 year was examined in bivariate correlations (complete data are attached as Supplementary material). In men, decreasing depression correlated strongly with better overall sexual functioning (P < 0.001), as well as orgasm (P = 0.014), satisfaction (P = 0.004) and relationship (P = 0.018). Furthermore, there was a negative correlation between anxiety and orgasm (P = 0.048) and better body image in men (P = 0.013). In women, anxiety was not correlated with sexual function or body image. In fact, only arousal correlated with depression in women (P = 0.005). Body image was not correlated with sexual function in either men or women.
DISCUSSION
Although there has been progress in the treatment of hematological malignancy with HSCT during the last decade, this study finds that sexual dysfunction is still a considerable problem for patients undergoing allogeneic HSCT and worsening 1 year after HSCT. The results also emphasize that changes in patient characteristics, in regard to diagnosis, stage of disease, age and general health condition, may have a significant influence on the incidence and prevalence of sexual dysfunction.
Consistent with studies of sexual function in the general population and among other cancer survivors, men reported better sexual function than women.24,29–31 However, at 1 year, HSCT had a persistent negative impact on sexuality in both genders. Yet men declined significantly over time in all aspects of sexual function in contrast to women who began and remained low in function. These findings have not been described in earlier studies that focus primarily on women.24 Our results showed that men experienced a greater deterioration in sexual function than women, which may indicate that men may be more vulnerable to changes induced by treatment with HSCT. On the other hand, women entered HSCT with poorer sexual function and thus experienced greater sexual dysfunction than men at both time points. In contrast, Christensen et al.30 found in a Danish study with healthy adults that both genders had the same prevalence of overall sexual dysfunction (11%). However, it is important to emphasize that the overall SFQ scores in this study are lower, indicating greater sexual dysfunction, than healthy controls and other cancer survivors in studies using the same questionnaire.25
Physical sexual problems either remained consistent or increased and levels of sexual activity decreased for both genders, suggesting that physical difficulties contribute to sexual inactivity. Consistent with similar studies in the general population and among other cancer survivors,9,24,29,30 men had been more frequently sexually active than women before treatment, however, this trend weakened over time. This can be explained by the fact that women’s sexual function was already low at baseline, and a majority of women were post-menopausal before HSCT, which likely contributed to their entry into HSCT with impaired sexual function. In addition, longitudinal studies with HSCT patients (> 2 years of follow-up) show that men to a greater extent reestablish their pre-HSCT level of sexual activity compared with women.4,5 This suggests that men’s sexual dysfunction is more reversible without intervention compared to women. In this study, we found 47% men and 60% women experienced 1 year after HSCT at least one physical sexual problem. Syrjala et al.5 found similar results with 46% males reporting at least one sexual problem versus 80% in women. In both studies, a larger percentage of women reported having at least one problem, indicating that women are at greater risk of experiencing physical problems that can affect level of sexual activity. In this context, it is interesting that both men and women, in this study, who reported physical sexual problems, were not necessarily sexually active 1 year after HSCT.
This study is the first to analyze the association between conditioning regimes and sexual function. We found no evidence linking type of conditioning regime with sexual dysfunction despite great differences in age and risk of chronic GVHD.1,11–14 Similarly, other studies report no difference between allogeneic and autologous HSCT in sexual function.32,33
Chronic GVHD was not significantly related to sexual dysfunction, which may be due to the small number of patients who continued to have chronic GVHD 1 year after HSCT. Women with chronic GVHD did report more sexual problems than those with no chronic GVHD. This suggests that chronic GVHD may be a contributory factor in developing sexual dysfunction particularly in women. This is consistent with a study identifying associations between chronic GVHD and sexual dysfunction.24 In contrast, Schmidt Knutsson et al.20 found genital chronic GVHD in 22 out of 42 (52%) female HSCT recipients, 9 of whom had partial or total vaginal stenosis. In addition, Mueller et al.19 found genital chronic GVHD changes in 31 out of 155 (20%) male HSCT recipients. However, no participants in this study were diagnosed with genital GVHD during the first year after HSCT, although both genders reported a high prevalence of symptoms that could be related to genital GVHD. When reviewing the medical records, we found two women who were diagnosed with genital GVHD after the 1-year follow-up. This indicates that there is a need to focus on the development of genital GVHD after the first year of HSCT. Furthermore by linking the information found in the medical records and the questionnaires, we found that most men, who reported physical sexual problems, did not report these problems to their doctor or nurse. This is of clinical importance, especially in men in clarifying the individual need for further evaluation of genital GVHD and sexual health in general. In all, these results suggest that genital GVHD may be under-reported and consequently under evaluated and treated, particularly for men, and possibly for both genders at later time points of 1 or more years after HSCT.
In addition, there are several reasons that may explain the lack of recognition and diagnosis of (chronic) genital GVHD. First, chronic GVHD treatments may have improved the incidence of genital GVHD in the study cohort. Second, genital symptoms associated with chronic GVHD lack specificity especially in men, and most of them can also be related to the conditions caused by conditioning regimens and previous chemotherapies.12,17 Third, genital involvement in men was absent in the National Institutes of Health consensus publication where organ scoring of genital chronic GVHD only comprises female genital symptoms.14 Moreover, oral steroids, which are often prescribed for other localizations of chronic GVHD, seem to have relative efficacy toward genital GVHD, as suggested by the fact that, in many patients, genital lesions develop soon after or during the tapering of systemic steroids.12 This emphasizes the importance of focusing on risk of increased incidence of genital GVHD following tapering of treatment of systemic steroids for chronic GVHD in other organs and suggests that sexual dysfunction could increase after 1 year for those in whom systemic steroids are tapered.
The evidence concerning male genital GVHD and sexual dysfunction is lacking.19,24 Focus on symptoms and clinical manifestations of genital chronic GVHD is needed in order to improve diagnostic and preventive interventions in men.
Depression correlated with numerous aspects of sexual dysfunction in men, with fewer associations of anxiety with sexual function in men. In contrast, women had a no patterns of correlation between mental health or body image and sexual function other than increasing arousal correlating with increasing depression score. This one finding could be explained by a small sample size in women at 1 year after HSCT. Regardless, the overall results may be explained by the fact that women’s anxiety and depression scores were relatively high and sexual function low already at baseline assessment, perhaps resulting in less room for reactivity to HSCT and ceiling effects for depression and anxiety. For men, this result is consistent with Gruber et al.34 who found significant correlations between anxiety or emotional stress, pain, stress in partnership or family and sexual problems. Therefore, anxiety and depression may be important factors in the development of sexual dysfunction. It is unclear if sexual dysfunction leads to anxiety and depression or vice versa, or both are a result of other factors. Regardless, it is important that health professionals are aware of the correlation between sexual dysfunction and anxiety and depression when implementing preventive and diagnostic interventions in clinical practice.35,36
The strengths of this study include the prospective 1-year follow-up with a relatively large cohort, low dropout rate for those surviving 1 year after HSCT and detailed assessment of sexual function. A limitation of the study is that sexual function before diagnosis was not known. Sample size was insufficient to adjust for age and to examine whether variables (gender, conditioning regime and chronic GVHD) could predict sexual dysfunction. Results are not generalizable to patients undergoing autologous HSCT or other cancer survivors. Further, the Danish version of the SFQ achieved linguistic validation and followed the translation guidelines from the ‘translation and cultural adaptation group’.26 Finally, the SFQ questionnaire does not have cutoff points to indicate sexual dysfunction, which makes it difficult to evaluate whether the changes of SFQ scores are clinically significant.
This study emphasizes the need for increased focus on sexuality and sexual function in the HSCT clinical setting. Improvements in the diagnosis of genital GVHD and its registration are crucial. Although evidence suggests that genital GVHD is related to GVHD in other organs and the risk increases when tapering systemic steroids, patients are not examined systematically for genital GVHD during treatment for GVHD in other organs. In addition, future studies exploring the degree to which the inevitable decline in endogenous sexual hormones influences sexual function in both men and women are essential in order to understand the mechanisms behind the development of sexual dysfunction. Many treatment options are available, but for patients to be identified to receive these treatments, conversations about sexual dysfunction must be initiated in the medical setting. Dialog about sexual function can help normalize concerns for patients, debunk myths, provide a basis for brief counseling or serve as an entrée for a referral and it is imperative that these conversations become an integral part of cancer care.9
In conclusion, this study confirms that advances made in the treatment of HSCT over the past decade have not reduced the problems related to sexual dysfunction in survivors of HSCT. Patients experience sexual dysfunction 1 year after HSCT, and nearly half of men and a majority of women experienced at least one physical sexual problem at this time. Men experienced a greater decline in all aspects of sexual function, but women had a lower sexual function than men at both time points. These results indicate the need for routine assessment of sexual function, increased attention to genital GVHD symptoms that may be detected with evaluation of sexual symptoms, and increased availability of interventions to improve sexual function for HSCT survivors.
Supplementary Material
Acknowledgments
We thank the dedicated transplant respondents who have participated in this study. This work was supported by grants from The Novo Nordic Foundation.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)
References
- 1.Apperley J, Carreras E, Gluckman E, Masszi T. The 2012 revised edition of the EBMT-ESH Handbook on Haemopoietic Stem Cell Transplantation. Chugai Sanofi Aventis. 2012 [Google Scholar]
- 2.Watson M, Wheatley K, Harrison GA, Zittoun R, Gray RG, Goldstone AH, et al. Severe adverse impact on sexual functioning and fertility of bone marrow transplantation, either allogeneic or autologous, compared with consolidation chemotherapy alone: analysis of the MRC AML 10 trial. Cancer. 1999;86:1231–1239. doi: 10.1002/(sici)1097-0142(19991001)86:7<1231::aid-cncr18>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys CT, Tallman B, Altmaier EM, Barnette V. Sexual functioning in patients undergoing bone marrow transplantation: a longitudinal study. Bone Marrow Transplant. 2007;39:491–496. doi: 10.1038/sj.bmt.1705613. [DOI] [PubMed] [Google Scholar]
- 4.Syrjala KL, Kurland BF, Abrams JR, Sanders JE, Heiman JR. Sexual function changes during the 5 years after high-dose treatment and hematopoietic cell transplantation for malignancy, with case-matched controls at 5 years. Blood. 2008;111:989–996. doi: 10.1182/blood-2007-06-096594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23:6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 6.Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 7.Tierney DK. Sexuality following hematopoietic cell transplantation. Clin J Oncol Nurs. 2004;8:43–47. [PubMed] [Google Scholar]
- 8.Syrjala KL, Roth-Roemer SL, Abrams JR, Scanlan JM, Chapko MK, Visser S, et al. Prevalence and predictors of sexual dysfunction in long-term survivors of marrow transplantation. J Clin Oncol. 1998;16:3148–3157. doi: 10.1200/JCO.1998.16.9.3148. [DOI] [PubMed] [Google Scholar]
- 9.Yi JC, Syrjala KL. Sexuality after hematopoietic stem cell transplantation. Cancer J. 2009;15:57–64. doi: 10.1097/PPO.0b013e318198c758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosher CE, Redd WH, Rini CM, Burkhalter JE, DuHamel KN. Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psychooncology. 2009;18:113–127. doi: 10.1002/pon.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders JE. Chronic graft-versus-host disease and late effects after hematopoietic stem cell transplantation. Int J Hematol. 2002;76:15–28. doi: 10.1007/BF03165081. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch P, Leclerc M, Rybojad M, Petropoulou AD, Robin M, Ribaud P, et al. Female genital chronic graft-versus-host disease: importance of early diagnosis to avoid severe complications. Transplantation. 2012;93:1265–1269. doi: 10.1097/TP.0b013e31824f3dcd. [DOI] [PubMed] [Google Scholar]
- 13.Passweg JR, Baldomero H, Bregni M, Cesaro S, Dreger P, Duarte RF, et al. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant. 2013;48:1161–1167. doi: 10.1038/bmt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Mattson MR. Graft-versus-host disease: review and nursing implications. Clin J Oncol Nurs. 2007;11:325–328. doi: 10.1188/07.CJON.325-328. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Mourad YR, Lau BC, Barnett MJ, Forrest DL, Hogge DE, Nantel SH, et al. Long-term outcome after allo-SCT: close follow-up on a large cohort treated with myeloablative regimens. Bone Marrow Transplant. 2010;45:295–302. doi: 10.1038/bmt.2009.128. [DOI] [PubMed] [Google Scholar]
- 17.Zantomio D, Grigg AP, MacGregor L, Panek-Hudson Y, Szer J, Ayton R. Female genital tract graft-versus-host disease: incidence, risk factors and recommendations for management. Bone Marrow Transplant. 2006;38:567–572. doi: 10.1038/sj.bmt.1705487. [DOI] [PubMed] [Google Scholar]
- 18.Lara LA, De Andrade JM, Mauad LM, Ferrarese SR, Marana HR, Tiezzi DG, et al. Genital manifestation of graft-vs.-host disease: a series of case reports. J Sex Med. 2010;7:3216–3225. doi: 10.1111/j.1743-6109.2010.01885.x. [DOI] [PubMed] [Google Scholar]
- 19.Mueller SM, Haeusermann P, Rovo A, Halter JP, Passweg J, Itin P, et al. Genital chronic GVHD in men after hematopoietic stem cell transplantation: a single-center cross-sectional analysis of 155 patients. Biol Blood Marrow Transplant. 2013;19:1574–1580. doi: 10.1016/j.bbmt.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Smith Knutsson E, Bjork Y, Broman AK, Helstrom L, Levin Jakobsen AM, Nilsson O, et al. Genital chronic graft-versus-host disease in females: a cross-sectional study. Biol Blood Marrow Transplant. 2014;20:806–811. doi: 10.1016/j.bbmt.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Bruner DW, Calvano T. The sexual impact of cancer and cancer treatments in men. Nurs Clin North Am. 2007;42:555–580. doi: 10.1016/j.cnur.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Barton-Burke M, Gustason CJ. Sexuality in women with cancer. Nurs Clin North Am. 2007;42:531–554. doi: 10.1016/j.cnur.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Weber CS, Fliege H, Arck PC, Kreuzer KA, Rose M, Klapp BF. Patients with haematological malignancies show a restricted body image focusing on function and emotion. Eur J Cancer Care (Engl) 2005;14:155–165. doi: 10.1111/j.1365-2354.2005.00533.x. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen KH, Schjodt I, Jarden M. The impact of hematopoietic stem cell transplantation on sexuality: a systematic review of the literature. Bone Marrow Transplant. 2012;47:716–724. doi: 10.1038/bmt.2011.169. [DOI] [PubMed] [Google Scholar]
- 25.Syrjala KL, Schroeder TC, Abrams JR, Atkins TZ, Brown WS, Sanders JE, et al. Sexual function measurement and outcomes in cancer survivors and matched controls. J Sex Res. 2000;37:213–225. [Google Scholar]
- 26.Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8:94–104. doi: 10.1111/j.1524-4733.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- 27.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer. 2001;37:189–197. doi: 10.1016/s0959-8049(00)00353-1. [DOI] [PubMed] [Google Scholar]
- 28.Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J (Clin Res Ed) 1986;292:344. doi: 10.1136/bmj.292.6516.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derogatis LR, Burnett AL. The epidemiology of sexual dysfunctions. J Sex Med. 2008;5:289–300. doi: 10.1111/j.1743-6109.2007.00668.x. [DOI] [PubMed] [Google Scholar]
- 30.Christensen BS, Gronbaek M, Osler M, Pedersen BV, Graugaard C, Frisch M. Sexual dysfunctions and difficulties in Denmark: prevalence and associated sociodemographic factors. Arch Sex Behav. 2011;40:121–132. doi: 10.1007/s10508-010-9599-y. [DOI] [PubMed] [Google Scholar]
- 31.Christensen BS, Gronbaek M, Osler M, Pedersen BV, Graugaard C, Frisch M. Associations between physical and mental health problems and sexual dysfunctions in sexually active Danes. J Sex Med. 2011;8:1890–1902. doi: 10.1111/j.1743-6109.2010.02145.x. [DOI] [PubMed] [Google Scholar]
- 32.Marks DI, Friedman SH, Delli Carpini L, Nezu CM, Nezu AM. A prospective study of the effects of high-dose chemotherapy and bone marrow transplantation on sexual function in the first year after transplant. Bone Marrow Transplant. 1997;19:819–822. doi: 10.1038/sj.bmt.1700750. [DOI] [PubMed] [Google Scholar]
- 33.Molassiotis A, van den Akker OB, Milligan DW, Goldman JM, Boughton BJ, Holmes JA, et al. Quality of life in long-term survivors of marrow transplantation: comparison with a matched group receiving maintenance chemotherapy. Bone Marrow Transplant. 1996;17:249–258. [PubMed] [Google Scholar]
- 34.Gruber U, Fegg M, Buchmann M, Kolb HJ, Hiddemann W. The long-term psychosocial effects of haematopoetic stem cell transplantation. Eur J Cancer Care (Engl) 2003;12:249–256. [PubMed] [Google Scholar]
- 35.Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J. 2009;15:74–77. doi: 10.1097/PPO.0b013e31819587dc. [DOI] [PubMed] [Google Scholar]
- 36.McKee AL, Jr, Schover LR. Sexuality rehabilitation. Cancer. 2001;92:1008–1012. doi: 10.1002/1097-0142(20010815)92:4+<1008::aid-cncr1413>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.