Abstract
Chloride is the principal anion of the extracellular fluid and vital for both serum electroneutrality and acid-base homeostasis. The aim of this review is to investigate the relevance of dyschloremia in the critically ill.
An extensive literature research was conducted on www.pubmed.org. In addition, the references of included articles were searched for further possible investigation regarding chloride.
Articles investigating the relevance of dyschloremia in the critically ill were included.
All articles were screened in regard to dyschloremia in the critically ill.
Chloride is essential for blood pressure control, decarboxylation/gas transport, renal function, and gastrointestinal homeostasis. “Dyschloremia,” i.e., serum chloride levels not within the limits of normal, may commonly be observed on ICUs and appear mainly induced by iatrogenic measures (i.e., infusion of chloride-rich fluids). Hypo- and hyperchloremia appear linked to increased mortality in defined ICU populations, but evidence is sparse. Data show that hyperchloremia may not only be linked to hyperchloremic metabolic acidosis, but also to increased hemodynamic instability and vasopressor need (e.g., in patients after major surgery). Nevertheless, it is currently unknown whether such effects would be directly or indirectly mediated. Moreover, recent evidence points to an increased incidence of acute kidney injury and need for renal replacement therapy in patients with advanced hyperchloremia.
Current knowledge on chloride is largely limited by heterogeneous trial design and mostly abundant data on specific fluid replacement strategies. The aim of this review is to summarize key consequences of chloride in critical illness and to discuss implications for daily clinical practice and future research.
Review
The chloride ion (Cl−, molar mass 35.45 g/mol) is the principal extracellular anion in humans [1–3]. Intra- and extracellular chloride concentrations range from 2 to 5 mmol/L (skeletal muscles) to about 90 mmol/L (erythrocytes), and 97–107 mmol/L (plasma). Chloride is vital for maintenance of serum electroneutrality, acid-base balance, fluid homeostasis, osmotic pressure, hydrochloric acid (HCl) production in the gastrointestinal tract, renal function, and for electrical activity in general, e.g., in muscular activity [1, 2].
Hyperchloremia has a high prevalence in critically ill patients with data showing that it may be observed in about 25–45% of ICU patients; however, this seems not acknowledged by previous research or textbooks. Data from a recent prospective observational investigation demonstrate that temporary hyperchloremia may even occur in 75% of ICU patients during the first 24 h of ICU stay [4]. However, despite a rather high prevalence in critically ill patients, few outcome-related data regarding systemic chloride levels exist. The available data indicate that in general, increased disease severity is associated with abnormal chloride levels (reviewed in [1]). This review aims to provide an overview on chloride physiology and to reflect outcome-relevant effects of chloride in critically ill patients.
Physiological functions of chloride—a quick overview
In humans, dietary salt intake is the primary Cl− source (about 6–12 g, respectively 100–200 mmol Cl−) [1, 5]. Cl− is vital for several key physiological functions discussed below (Fig. 1).
Fig. 1.
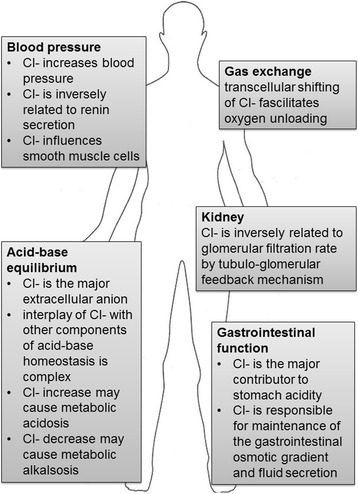
The principal physiological functions of chloride in the human body
Acid-base equilibrium
Cl− is the major extracellular strong ion and is key to maintenance of acid-base homeostasis [1, 6, 7]. Cl− levels are inversely related to bicarbonate [1], which acts as the major acid-base buffer in humans [1]. Cl− was identified as the primary factor influencing the occurrence of metabolic alkalosis and non-anion gap metabolic acidosis in critical illness [6].
The influence of Cl− acid base homeostasis can be explained by the “Stewart approach” (Fig. 2), where the potential proton concentration of a given solute is determined by changes in any of three independent variables: (1) difference in so-called strong ions (SID) where Cl− plays a major role, (2) carbon dioxide partial pressure, and (3) non-volatile weak acid concentration [7, 8]. In addition, Cl− levels are significantly influenced by compensatory factors, urine electrolyte, and bicarbonate concentrations and water homeostasis. The influence of Cl− on acid-base homeostasis is provided in Fig. 3.
Fig. 2.
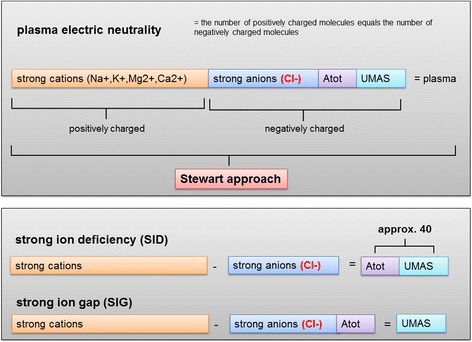
Acid-base physiology according to the Stewart’s approach
Fig. 3.
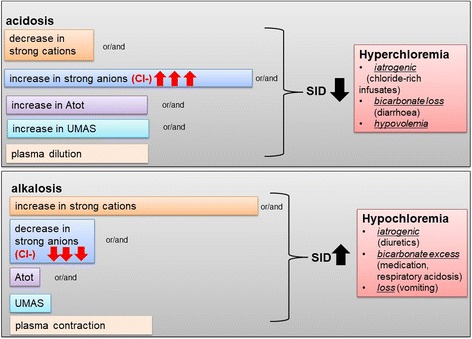
Influence of chloride on acid-base homeostasis
Influence of chloride on renal function and blood pressure
Cl− undergoes free glomerular filtration with 99% being reabsorbed and about 180 mmol of Cl− excreted per day [1, 9]. Cl− reabsorption occurs in the proximal renal tubule (~ 60%) and partly in intercalated cells of the distal nephron. In the ascending part of Henle’s loop, another 25% of Cl− is reabsorbed [1, 9–11]. In euvolemia, Cl− levels regulate active sodium-potassium and Cl− reabsorption [1] by tubular-glomerular feedback with Cl− sensing in the macula densa. This feedback may induce renal afferent vasoconstriction and reduced glomerular filtration [11–14]. Moreover, Cl− levels affect renin secretion and Cl− concentrations at the macula densa and are inversely related to renin-angiotensin-aldosterone system (RAAS) activation [1, 12]. Several preclinical studies showed that Cl− depletion induced stimulation of renin secretion resulting in increased systemic blood pressure [15]. In addition, Cl− concentrations may exert direct effects on smooth muscle cells resulting in vasoconstriction [5].
Gastrointestinal function of chloride
Cl− has two distinct functions in the gastrointestinal (GI) tract: first, it is secreted in form of HCL and is required for protein digestion, microorganism homeostasis, and absorption of nutrients (e.g., calcium, zinc, iron, vitamins, folic acid) [1]. Second, it is responsible for maintenance of the GI osmotic gradient and fluid secretion [1]. The role of Cl− in splanchnic perfusion is discussed controversially with few animal data indicating that increased Cl− levels lead to impaired gastric-pyloric motility, nausea, and vomiting [16].
Effects of chloride on oxygen transport and gas exchange
Intracellular Cl− are lower than extracellular Cl− levels [1]. The extracellular vs. intracellular Cl− distribution mainly depends on cell membrane potentials that are established by transmembrane electrolyte transport [1]. Erythrocytes have low membrane potentials allowing for almost free transmembrane Cl− passage [1] and anion exchange mainly occurs via an Cl−/HCO3− antiport. The underlying physiological mechanism (Cl−/HCO3−exchange) is referred to as “Hamburger shift” and seems key to understanding carbon dioxide (CO2) transport. In fact, when blood passes through the venous system with high CO2 pressures, a chloride efflux and concurrent bicarbonate influx (derived from CO2; CO2 uptake) occurs which is diffused while respective blood is arterialized in the pulmonary system [17, 18]. This Cl− shift plays a role in oxygen (O2) unloading also [17, 18]. Nevertheless, the clinical significance of this effect needs confirmation in subsequent investigations.
Clinical conditions associated with “dyschloremia” on the ICU
Definition of “dyschloremia”
Hypochoremia is usually defined as serum chloride levels below 96–101 mmol/l, while hyperchloremia normally is defined as serum chloride levels higher than 106–111 mmol/l [19–21]. The definition varies depending on the local laboratory. Chloride levels do closely interact with the body’s water contact and are highly susceptible to either plasma contraction or dilution (also see below).
Hypochloremia
Hypochloremia in critically ill patients can be caused by active Cl− loss, e.g., through the GI tract (e.g., vomiting, diarrhea), via inadequate renal Cl− reabsorption or via dilution following infusion of hypotonic fluids [1, 9]. Additionally, Cl− can be lost via the kidneys in cases of increased bicarbonate reabsorption in either chronic respiratory acidosis or hyper-aldosteronism. High-volume bicarbonate infusion may result in Cl− being exchanged for bicarbonate in order to maintain electroneutrality [1]. Key to understanding of hypochloremia thus is assessment of potential iatrogenic effects and/or related use of diuretics [3]. Especially, the use of furosemide is clearly associated with the occurrence of metabolic alkalosis [22]. Plasma contraction further aggravates hypochloremic metabolic alkalosis especially in patients who lose high quantities of chloride-rich fluids (e.g., vomiting) [23].
Hyperchloremia
Hyperchloremia in critically ill patients is mainly due to (1) loss of bicarbonate through the GI or renal tract, (2) as a consequence of “dilution” due to volume loading with fluids with a low bicarbonate concentration, or (3) by excess infusion of Cl−-rich fluids [1]. On the ICU, diarrhea may be the most often reason for bicarbonate loss [1]. Bicarbonate may also be lost through the renal system in renal tubular acidosis (RTA), especially in proximal RTA type II [1, 24].
Further, plasma “dilution” may also decrease bicarbonate levels [25, 26]. This typically results in increased Cl− levels and “dilutional acidosis”—which was also observed after infusion of large quantities of chloride-rich fluids [12, 25, 26].
Hyperchloremia in critical illness most often results from iatrogenic chloride overload (e.g., 0.9% NaCl infusion with 154 mmol/l CL−) [1, 12, 27–30]. Normal saline has a theoretical SID of zero [1] and thus results in development of hyperchloremic metabolic acidosis [1, 12]. Despite growing evidence, 0.9% NaCl is still one of the most widely used crystalloids [12, 30–36]; however, its use is widely debated [37–43]. Importantly, the ICU physician should note that albumin-based replacement fluids may contain rather large quantities of chloride also [44, 45]. Furthermore cases of hyperchloremia on the ICU may result from infusion of HCl, acetazolamide, and/or triamterene therapy, or specific cortisone derivates resulting in NaCl retention [1].
Impact on clinical outcomes of critically ill patients
Hyperchloremic metabolic acidosis
Hyperchloremic metabolic acidosis results from infusion of considerable quantities of chloride-rich fluids in critically ill patients [27–30, 33, 35, 36, 46–48]. It’s development is dose-dependent and independent of infusion speed [13]. Importantly, hyperchloremic metabolic acidosis may not only affect ICU patients with acute kidney injury (AKI) [28], data also show that a total volume of 2000 ml of chloride-rich infusate may induce hyperchloremic metabolic acidosis in healthy volunteers [13]. Hyperchloremic metabolic acidosis may induce vasodilatation [49–51], altered neurotransmitter function [52, 53], decreased cardiac reactivity [52, 53], and other changes in cellular function [54], as well as decreased endogenous catecholamine release [55].
Current literature clearly indicates that chloride-rich infusates are associated with the temporary occurrence of hyperchloremic metabolic acidosis. However, the significance of the latter and its influence on clinical endpoints such as the occurrence of kidney failure or mortality is not jet clarified.
Renal function
Effects of hyperchloremia on renal function were first investigated over 30 years ago [11, 14]. There is some animal [11, 14, 47] and human data (13) that suggest that renal blood flow and renal cortical perfusion is diminished under chloride infusion. However, a recently published trial [56] does not confirm these findings.
Clinical studies, like animal experiments, showed mixed results regarding patient-centered clinical outcomes (e.g., need for renal replacement therapy (RRT)) in ICU patients [4, 33, 35, 37, 46, 57–62] (see, Table 1). Whereas some clinical trials did not identify changes in serum creatinine or acute kidney injury (AKI) rates in mixed ICU cohorts, cardiac surgery, or sepsis [37, 59, 62], other reports demonstrate increased AKI incidence and need for renal replacement therapy (RRT) [4, 46, 57]. However, the sensitivity analysis of one of these trials showed that the incidence of AKI and need for renal replacement therapy were also influenced by other unidentified confounders [57], so the issue is far from being concluded. This is also confirmed by another recently published retrospective analysis comparing hypertonic (3%) to normal saline in patients undergoing emergent laparotomy that showed no difference in respect to renal outcomes between the groups, even though the chloride levels were significantly higher in the hypertonic saline group [63].
Table 1.
Overview on studies investigating the impact of hyperchloremia on renal function
| Author (year) | Design | Study population | Total study population | Study intervention | Increased incidence of acute kidney injury | Increased need for renal replacement therapy | R |
|---|---|---|---|---|---|---|---|
| Krajewski ML et al. (2015) | Meta-analysis | Perioperative patients | 6253 | 0.9% saline versus other crystalloid infusates | X | Not investigated | [33] |
| Shaw B et al. (2012) | Retrospective cohort study | Patients after open abdominal surgery | 31,920 | 0.9% saline versus other crystalloid infusates | X | Not investigated | [35] |
| Young et al. (2015) | RCT | General ICU population | 2278 | 0.9% saline versus acetate-buffered solute | No difference between the groups | No difference between the groups | [37] |
| Yunos et al. (2012) | Open label, sequential period study (6 month) | General ICU population | 760 | Chloride-rich versus chloride-depleted infusion solutes | X | X | [46] |
| Yunos et al. (2015)a | Open label, sequential period study (1 year) | General ICU population | 2994 | Chloride-rich versus chloride-depleted infusion solutes | X | X | [57] |
| Guirgis FW et al. (2015) | Retrospective cohort study | Patients with sepsis/septic shock | 95 | Chloride-rich versus chloride-depleted infusion solutes | No difference between the groups | No difference between the groups | [59] |
| Shao M et al. (2016) | Retrospective cohort study | General ICU population | 6025 | – | X | Not investigated | [60] |
| Mattinen E et al. (2016) | Subgroup analysis of RCT | General ICU population | 445 | – | X | Not investigated | [4] |
| McCluskey SA (2013) | Retrospective cohort study | Non-cardiac surgery | 22,851 | – | X | Not investigated | [58] |
| Suetrong B et al. (2016) | Retrospective cohort study | Patients with sepsis/septic shock | 240 | – | X | Not investigated | [61] |
| Zhang Z et al. (2013) | Retrospective cohort study | General ICU population | 1221 | – | X | X | [64] |
| McIlroy D et al. (2017 | Open label, sequential period study | Perioperative patients | 1136 | chloride-rich versus chloride-depleted infusion solutes | No difference between the groups | No difference between the groups | [62] |
| Sadan O et al. (2017) | Retrospective cohort study | Patients with subarachnoid hemorrhage | 1267 | – | X | Not investigated | [65] |
| Loftus TJ et al. (2017 | Retrospective cohort study | Patients undergoing emergent laparatomy | 189 | 0.9% saline versus 3% saline | No difference between the groups | No difference between the groups | [63] |
•Sensitivity analysis showed that multiple unknown confounders may have influenced the incidence of AKI and need for RRT in this studySensitivity analysis showed that a multiple unknown confounders may have influenced the incidence of AKI and need for RRT in this study
X = finding was found
In addition, unfortunately, methodology, terminology, amount of total volume applied, and triggers for RRT differed considerably between trials. Overall, it appears that trials with lower total amount of Cl− infusion (i.e., 1–2 L/24 h) found unaffected AKI rates [37, 59], whereas trials with higher infusion rates showed an increased AKI incidence and RRT need [4, 35, 46, 57] suggesting a dose-dependent effect. Despite the enormous heterogeneity in the available literature which makes it almost incomparable, a recently published meta-analysis [33] included randomized and non-randomized trials concluded that use of chloride-rich fluids is associated with a higher AKI risk.
Interestingly, increased serum Cl− levels alone, independent of i.v. fluid, were associated with a higher AKI risk in several studies [4, 64, 65]. As to chloride levels, studies show that only minimal and/or maximum Cl− levels during ICU stay [61] but not ICU admission levels [64] were associated with an increased AKI incidence. An increase of Cl− serum levels by 10 mmol/l resulted in OR 7.39 for AKI development in one study [65]. However, the number of study focusing on chloride levels independent of i.v. fluids is still a few.
In conclusion, despite the number of studies focusing on the development of AKI and need for RRT in patients receiving chloride-rich infusates, the debate is far from being decided due to the large heterogeneity in the available literature.
Cardiovascular function
Chloride-rich infusions may lead to hemodynamic instability [27, 28, 31, 47]. Hemodynamic effects of chloride-rich fluids were first described by Kellum and coworkers in a rodent sepsis model [47]. In this model, hyperchloremia and associated metabolic acidosis induced decreased arterial pressures [47]. This effect was confirmed in additional studies showing decreased mean arterial blood pressures and cardiac index in rats with abdominal sepsis [31]. In critically ill humans, patients receiving chloride-rich infusions had a volume-dependent increased vasopressor need [27]. A randomized controlled double-blind study by members of our group focusing on normal saline when compared to an acetate-buffered infusion solution in patients undergoing major abdominal surgery even shows, that the effect is not only volume-dependent, but also time-dependent [66]. The mechanisms behind this effect remain somewhat elusive. Further trials comparing other infusion solutes in respect to cardiovascular stability are certainly needed before drawing definitive conclusions.
Hypochloremia seems of particular importance in heart failure patients where low levels of serum chloride indicate advanced disease, and are associated with decreased left ventricular ejection fraction [38, 67–69], increased cardiac function markers (e.g., NT-pro-BNP) [38, 68], and circulating catecholamine levels [70]. In fact, hypochloremia was identified as an independent predictor for adverse outcome in heart failure patients and was recently recognized to predict mortality in affected patients [3].
The influence of chloride on the cardiovascular system may be important for clinicians for several reasons: first, chloride loading may contribute to catecholamine need in critically ill patients. Second, cardiac function may be influenced by chloride levels in a “U-shaped” response curve with both hypo- and hyperchloremia being detrimental for cardiovascular stability (and function). Third, the effect of chloride-“loading” on hemodynamic stability may be dose-dependent. Fourth, in preclinical models, it was shown that simple hyperchloremia may trigger increased blood pressures. However, only concomitant hyperchloremia with metabolic acidosis results in decreased systemic pressures [47]. It thus seems likely that the occurrence of acidosis and not hyperchloremia per se is responsible for observed adverse cardiovascular effects.
Inflammation and coagulation
In several animal models, systemic levels of inflammatory makers were increased following chloride-rich infusions. This was observed in both experimental sepsis [12, 39, 47, 71] and trauma models [40]. In humans, this remains controversially discussed [41, 42] as effects of chloride-rich infusions on inflammatory markers may also be attributed to sodium rather than to Cl−. However, this requires further clarification.
Preclinically, chloride-rich infusions were associated with an increased need for blood products [35, 40, 43]. Moreover, few evidence points to the fact that hyperchloremia may influence plasmatic coagulation cascades [40, 43, 72] and/or platelet function [12]. In humans, several trials and a recent meta-analysis demonstrate increased need for blood product administration in patients receiving chloride-rich infusions [33, 35, 73]. Nevertheless, the effect of acidosis in this context remains uncertain.
Mortality and other patient-centered clinical outcomes
A U-shaped mortality curve was reported in respect to Cl− levels and mortality [12]. This is depicted in Fig. 4. Several large-scale clinical trials in critically ill patients found increased mortality rates in patients treated with chloride-rich infusions [2, 29, 33, 35, 46, 48, 58, 74, 75]. However, this effect was not confirmed in four other large-scale multi-center trials and a recent meta-analysis [4, 33, 37, 46, 76]. Even when a very high Cl− load fluid (hypertonic saline, 3%) was compared to normal Cl− load fluid (0.9% saline), there was no difference in respect to mortality between these groups [76].
Fig. 4.
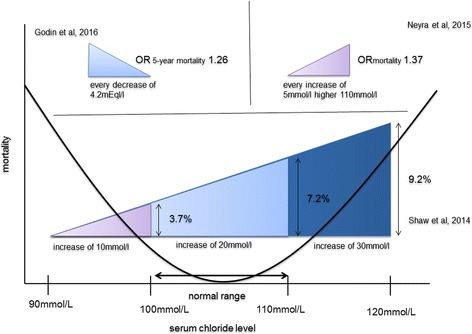
Influence of chloride levels on mortality. *All tables and figures are propriety of the authors and have not been published elsewhere
Hyperchloremia itself (at 72 h after ICU admission) or rise in Cl− levels of > 5 mEq/l was associated with increased in-hospital mortality [2]. Interestingly, two large studies in SIRS patients [29, 48] found that mortality remained lowest in patients with only minimal serum Cl− variation during total hospital stay [29]. In-hospital mortality gradually increased with each 10 mmol/l of serum Cl− level increase [29]. This association was independent of total fluid volume administered, but linked to volume-adjusted chloride-load [29]. Another study in critically ill patients investigating the prognostic potency of acid-base variables to reflect in-hospital mortality identified hyperchloremia and hypoalbuminemia as the only independent factors after adjustment [77].
In conclusion, even though literature points towards increased mortality rates in patients with hyperchloremia, it remains unclear whether potential effects of Cl− levels on mortality are due to direct or indirect (e.g., acidosis) effects.
Hypochloremia was also linked to mortality in several studies [38, 67, 68, 70, 75, 78] and may be of special importance in patients with heart failure. An inverse relationship of Cl− levels with mortality in patients with compensated and non-compensated heart failure was shown [3, 38, 67]. Some studies showed an independent effect of hypochloremia (< 100 mmol/l) on cardiovascular, non-cardiovascular, and all-cause mortality [38, 67, 79]. All-cause mortality rates were increased even after 5 years of follow-up following initial hypochloremia [67]. Moreover, a recent editorial concludes that reduced serum Cl− levels may be of higher prognostic importance than increased sodium levels in heart failure patients [3].
Unlike with hyperchloremia, hypochloremia is more closely associated with increased mortality and should certainly be considered by intensive care physicians.
Effects of chloride levels on several other patient-centered relevant clinical outcomes were investigated. Hyperchloremia was associated with increased length of mechanical ventilation [33], increased rates of post-operative infectious complications [35, 48], increased readmission rates [48], and increased ICU and hospital length of stay [48, 58, 78]. However, these outcomes need to be further evaluated before drawing any definitive conclusions.
Conclusions
Hypo- or hyperchloremia may often be observed on ICUs, but data on relevant patient-centered clinical outcomes remain sparse. In fact, most studies investigating “dyschloremia” were heterogeneous and hyperchloremia was a result of infusion of normal saline (and thus concomitant sodium infusion). Moreover, different laboratory methods to measure Cl−, definitions of hypo- and/or hyperchloremia, different trial design/methodology, and cohorts under investigation add to a considerable heterogeneity of the available data.
“Dyschloremia,” however, may have a major impact on clinical outcomes in critical illness. Despite growing evidence favoring avoidance of chloride-rich infusions, e.g., 0.9% NaCl is still one of the most widely used crystalloid and some authors argue that despite development of hyperchloremic metabolic acidosis, its clinical significance remains elusive. In respect to renal function, the influence of hyperchloremia on renal function remains somewhat controversial and the available literature very heterogenic and almost incomparable. Therefore, no final conclusions on the topic of AKI incidence and need for RRT in respect to i.v. fluids with elevated chloride-content can be drawn. For cardiovascular aspects, growing evidence indicates that hyperchloremia-associated metabolic acidosis may induce hemodynamic instability. Hyperchloremia also may also have negative effects on coagulation cascades and increased mortality.
Interestingly, hypochloremia was much less studied than hyperchloremia although emerging evidence shows that low chloride levels may largely affect outcome, especially mortality in patients with heart failure.
In conclusion, “dyschloremia” significantly influences several important outcomes in the critically ill. However, much of the discussion is subject to an ongoing debate.
Acknowledgements
Not applicable
Funding
No funding was received for this study.
Availability of data and materials
Not applicable.
Authors’ contributions
CAP designed the strategy, performed the literature review, and drafted the manuscript. DU and SVH revised the manuscript for important intellectual content. JCS designed the strategy, drafted the manuscript, and revised the manuscript for important intellectual. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
All authors consent to publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carmen Andrea Pfortmueller, Phone: +41-31-632-2111, Email: carmen.pfortmueller@insel.ch.
Dominik Uehlinger, Email: Dominik.uehlinger@insel.ch.
Stephan von Haehling, Email: stephan.von.haehling@med-uni-goettingen.de.
Joerg Christian Schefold, Email: joerg.schefold@insel.ch.
References
- 1.Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med. 2012;23(3):203–211. doi: 10.1016/j.ejim.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Neyra JA, Canepa-Escaro F, Li X, Manllo J, Adams-Huet B, Yee J, et al. Association of hyperchloremia with hospital mortality in critically ill septic patients. Crit Care Med. 2015;43(9):1938–1944. doi: 10.1097/CCM.0000000000001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaduganathan M, Pallais JC, Fenves AZ, Butler J, Gheorghiade M. Serum chloride in heart failure: a salty prognosis. Eur J Heart Fail. 2016;18(6):669–671. doi: 10.1002/ejhf.546. [DOI] [PubMed] [Google Scholar]
- 4.Marttinen M, Wilkman E, Petaja L, Suojaranta-Ylinen R, Pettila V, Vaara ST. Association of plasma chloride values with acute kidney injury in the critically ill - a prospective observational study. Acta Anaesthesiol Scand. 2016;60(6):790–799. doi: 10.1111/aas.12694. [DOI] [PubMed] [Google Scholar]
- 5.McCallum L, Lip S, Padmanabhan S. The hidden hand of chloride in hypertension. Pflugers Archiv Eur J Physiol. 2015;467(3):595–603. doi: 10.1007/s00424-015-1690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funk GC, Doberer D, Heinze G, Madl C, Holzinger U, Schneeweiss B. Changes of serum chloride and metabolic acid-base state in critical illness. Anaesthesia. 2004;59(11):1111–1115. doi: 10.1111/j.1365-2044.2004.03901.x. [DOI] [PubMed] [Google Scholar]
- 7.Stewart PA. Independent and dependent variables of acid-base control. Respir Physiol. 1978;33(1):9–26. doi: 10.1016/0034-5687(78)90079-8. [DOI] [PubMed] [Google Scholar]
- 8.Masevicius FD, Dubin A. Has Stewart approach improved our ability to diagnose acid-base disorders in critically ill patients? World J Critical Care Med. 2015;4(1):62–70. doi: 10.5492/wjccm.v4.i1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Critical Care (London, England) 2010;14(4):226. doi: 10.1186/cc9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Planelles G. Chloride transport in the renal proximal tubule. Pflugers Archiv Eur J Physiol. 2004;448(6):561–570. doi: 10.1007/s00424-004-1309-y. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71(3):726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soussi S, Ferry A, Chaussard M, Legrand M. Chloride toxicity in critically ill patients: what’s the evidence? Anaesthesia, Critical Care Pain Med. 2017;36(2):125–130. doi: 10.1016/j.accpm.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256(1):18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox CS, Peart WS. Release of renin and angiotensin II into plasma and lymph during hyperchloremia. Am J Phys. 1987;253(4 Pt 2):F734–F741. doi: 10.1152/ajprenal.1987.253.4.F734. [DOI] [PubMed] [Google Scholar]
- 15.Schmidlin O, Tanaka M, Sebastian A, Morris RC., Jr Selective chloride loading is pressor in the stroke-prone spontaneously hypertensive rat despite hydrochlorothiazide-induced natriuresis. J Hypertens. 2010;28(1):87–94. doi: 10.1097/HJH.0b013e3283316cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tournadre JP, Allaouchiche B, Malbert CH, Chassard D. Metabolic acidosis and respiratory acidosis impair gastro-pyloric motility in anesthetized pigs. Anesth Analg. 2000;90(1):74–79. doi: 10.1097/00000539-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Westen EA, Prange HD. A reexamination of the mechanisms underlying the arteriovenous chloride shift. Physiol Biochem Zool. 2003;76(5):603–614. doi: 10.1086/380208. [DOI] [PubMed] [Google Scholar]
- 18.Brix O, Thomsen B, Nuutinen M, Hakala A, Pudas J, Giardina B. The chloride shift may facilitate oxygen loading and unloading to/from the hemoglobin from the brown bear (Ursus arctos L.) Comparative Biochem Physiol B Comparative Biochem. 1990;95(4):865–868. doi: 10.1016/0305-0491(90)90330-V. [DOI] [PubMed] [Google Scholar]
- 19.Burtis CA AE, Saunders WB MayoClinic Medical Laboratory. https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8460. Accessed 11 Apr 2018
- 20.Utah MUo. User's Guide, ARUP Laboratories 2018 [cited 2018 01.03.2018]. Available from: https://library.med.utah.edu/WebPath/EXAM/LABREF.html
- 21.Kamel G. Chloride Medscape. 2014. [Google Scholar]
- 22.Huang A, Luethi N, Martensson J, Bellomo R, Cioccari L. Pharmacodynamics of intravenous frusemide bolus in critically ill patients. Critical Care Resuscitation. 2017;19(2):142–149. [PubMed] [Google Scholar]
- 23.Luke RG, Galla JH. It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol. 2012;23(2):204–207. doi: 10.1681/ASN.2011070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haque SK, Ariceta G, Batlle D. Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant. 2012;27(12):4273–4287. doi: 10.1093/ndt/gfs493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asano S, Kato E, Yamauchi M, Ozawa Y, Iwasa M. The mechanism of acidosis caused by infusion of saline solution. Lancet. 1966;1(7449):1245–1246. doi: 10.1016/S0140-6736(66)90248-0. [DOI] [PubMed] [Google Scholar]
- 26.Shires GT, Holman J. Dilution acidosis. Ann Intern Med. 1948;28(3):557–559. doi: 10.7326/0003-4819-28-3-557. [DOI] [PubMed] [Google Scholar]
- 27.Pfortmueller CA, Fleischmann E. Acetate-buffered crystalloid fluids: current knowledge, a systematic review. J Crit Care. 2016;35:96–104. doi: 10.1016/j.jcrc.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Potura E, Lindner G, Biesenbach P, Funk GC, Reiterer C, Kabon B, et al. An acetate-buffered balanced crystalloid versus 0.9% saline in patients with end-stage renal disease undergoing cadaveric renal transplantation: a prospective randomized controlled trial. Anesth Analg. 2015;120(1):123–129. doi: 10.1213/ANE.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 29.Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014;40(12):1897–1905. doi: 10.1007/s00134-014-3505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfortmueller CA, Schefold JC. Hypertonic saline in critical illness - a systematic review. J Crit Care. 2017;42:168–177. doi: 10.1016/j.jcrc.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Orbegozo D, Su F, Santacruz C, He X, Hosokawa K, Creteur J, et al. Effects of different crystalloid solutions on hemodynamics, peripheral perfusion, and the microcirculation in experimental abdominal sepsis. Anesthesiology. 2016;125(4):744–754. doi: 10.1097/ALN.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 32.Raghunathan K, Shaw A, Nathanson B, Sturmer T, Brookhart A, Stefan MS, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42(7):1585–1591. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 33.Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102(1):24–36. doi: 10.1002/bjs.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350–1360. doi: 10.1056/NEJMra1412877. [DOI] [PubMed] [Google Scholar]
- 35.Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to plasma-Lyte. Ann Surg. 2012;255(5):821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 36.Lobo DN. Intravenous 0.9% saline and general surgical patients: a problem, not a solution. Ann Surg. 2012;255(5):830–832. doi: 10.1097/SLA.0b013e318250766c. [DOI] [PubMed] [Google Scholar]
- 37.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314(16):1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 38.Grodin JL, Simon J, Hachamovitch R, Wu Y, Jackson G, Halkar M, et al. Prognostic role of serum chloride levels in acute decompensated heart failure. J Am Coll Cardiol. 2015;66(6):659–666. doi: 10.1016/j.jacc.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Zhou F, Peng ZY, Bishop JV, Cove ME, Singbartl K, Kellum JA. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit Care Med. 2014;42(4):e270–e278. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips CR, Vinecore K, Hagg DS, Sawai RS, Differding JA, Watters JM, et al. Resuscitation of haemorrhagic shock with normal saline vs. lactated Ringer's: effects on oxygenation, extravascular lung water and haemodynamics. Critical Care (London, England) 2009;13(2):R30. doi: 10.1186/cc7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan R, Kirschenbaum LA, Larow C, Astiz ME. The effect of resuscitation fluids on neutrophil-endothelial cell interactions in septic shock. Shock (Augusta, Ga) 2011;36(5):440–444. doi: 10.1097/SHK.0b013e3182336bda. [DOI] [PubMed] [Google Scholar]
- 42.Rhee P, Wang D, Ruff P, Austin B, DeBraux S, Wolcott K, et al. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med. 2000;28(1):74–78. doi: 10.1097/00003246-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Kiraly LN, Differding JA, Enomoto TM, Sawai RS, Muller PJ, Diggs B, et al. Resuscitation with normal saline (NS) vs. lactated ringers (LR) modulates hypercoagulability and leads to increased blood loss in an uncontrolled hemorrhagic shock swine model. J Trauma. 2006;61(1):57–64. doi: 10.1097/01.ta.0000220373.29743.69. [DOI] [PubMed] [Google Scholar]
- 44.Gheorghe C, Dadu R, Blot C, Barrantes F, Vazquez R, Berianu F, et al. Hyperchloremic metabolic acidosis following resuscitation of shock. Chest. 2010;138(6):1521–1522. doi: 10.1378/chest.10-1458. [DOI] [PubMed] [Google Scholar]
- 45.Durward A, Skellett S, Mayer A, Taylor D, Tibby SM, Murdoch IA. The value of the chloride: sodium ratio in differentiating the aetiology of metabolic acidosis. Intensive Care Med. 2001;27(5):828–835. doi: 10.1007/s001340100915. [DOI] [PubMed] [Google Scholar]
- 46.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 47.Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125(1):243–248. doi: 10.1378/chest.125.1.243. [DOI] [PubMed] [Google Scholar]
- 48.Shaw AD, Schermer CR, Lobo DN, Munson SH, Khangulov V, Hayashida DK, et al. Impact of intravenous fluid composition on outcomes in patients with systemic inflammatory response syndrome. Critical Care (London, England) 2015;19:334. doi: 10.1186/s13054-015-1045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wray S. Smooth muscle intracellular pH: measurement, regulation, and function. Am J Phys. 1988;254(2 Pt 1):C213–C225. doi: 10.1152/ajpcell.1988.254.2.C213. [DOI] [PubMed] [Google Scholar]
- 50.Daugherty RM, Jr, Scott JB, Dabney JM, Haddy FJ. Local effects of O2 and CO2 on limb, renal, and coronary vascular resistances. Am J Phys. 1967;213(5):1102–1110. doi: 10.1152/ajplegacy.1967.213.5.1102. [DOI] [PubMed] [Google Scholar]
- 51.Haddy FJ, Scott JB. Metabolically linked vasoactive chemicals in local regulation of blood flow. Physiol Rev. 1968;48(4):688–707. doi: 10.1152/physrev.1968.48.4.688. [DOI] [PubMed] [Google Scholar]
- 52.Haunstetter A, Schulze Icking B, Backs J, Kruger C, Haass M. Differential effects of acidosis, high potassium concentrations, and metabolic inhibition on noradrenaline release and its presynaptic muscarinic regulation. Pharmacol Res. 2002;45(3):221–228. doi: 10.1006/phrs.2001.0943. [DOI] [PubMed] [Google Scholar]
- 53.Seyfarth M, Feng Y, Hagl S, Sebening F, Richardt G, Schomig A. Effect of myocardial ischemia on stimulation-evoked noradrenaline release. Modulated neurotransmission in rat, Guinea pig, and human cardiac tissue. Circ Res. 1993;73(3):496–502. doi: 10.1161/01.RES.73.3.496. [DOI] [PubMed] [Google Scholar]
- 54.Pedoto A, Caruso JE, Nandi J, Oler A, Hoffmann SP, Tassiopoulos AK, et al. Acidosis stimulates nitric oxide production and lung damage in rats. Am J Respir Crit Care Med. 1999;159(2):397–402. doi: 10.1164/ajrccm.159.2.9802093. [DOI] [PubMed] [Google Scholar]
- 55.Le Tulzo Y, Shenkar R, Kaneko D, Moine P, Fantuzzi G, Dinarello CA, et al. Hemorrhage increases cytokine expression in lung mononuclear cells in mice: involvement of catecholamines in nuclear factor-kappaB regulation and cytokine expression. J Clin Invest. 1997;99(7):1516–1524. doi: 10.1172/JCI119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olivier PY, Beloncle F, Seegers V, Tabka M, Renou de La Bourdonnaye M, Mercat A, et al. Assessment of renal hemodynamic toxicity of fluid challenge with 0.9% NaCl compared to balanced crystalloid (PlasmaLyte((R))) in a rat model with severe sepsis. Ann Intensive Care. 2017;7(1):66. doi: 10.1186/s13613-017-0286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41(2):257–264. doi: 10.1007/s00134-014-3593-0. [DOI] [PubMed] [Google Scholar]
- 58.McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;117(2):412–421. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- 59.Guirgis FW, Williams DJ, Hale M, Bajwa AA, Shujaat A, Patel N, et al. The relationship of intravenous fluid chloride content to kidney function in patients with severe sepsis or septic shock. Am J Emerg Med. 2015;33(3):439–443. doi: 10.1016/j.ajem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 60.Shao M, Li G, Sarvottam K, Wang S, Thongprayoon C, Dong Y, et al. Dyschloremia is a risk factor for the development of acute kidney injury in critically ill patients. PLoS One. 2016;11(8):e0160322. doi: 10.1371/journal.pone.0160322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suetrong B, Pisitsak C, Boyd JH, Russell JA, Walley KR. Hyperchloremia and moderate increase in serum chloride are associated with acute kidney injury in severe sepsis and septic shock patients. Critical Care (London, England) 2016;20(1):315. doi: 10.1186/s13054-016-1499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McIlroy D, Murphy D, Kasza J, Bhatia D, Wutzlhofer L, Marasco S (2017) Effects of restricting perioperative use of intravenous chloride on kidney injury in patients undergoing cardiac surgery: the LICRA pragmatic controlled clinical trial. Intensive Care Med 43(6):795–806 [DOI] [PubMed]
- 63.Loftus TJ, Efron PA, Bala TM, Rosenthal MD, Croft CA, Smith RS et al (2017) Hypertonic saline resuscitation following emergent laparotomy and temporary abdominal closure. J Trauma Acute Care Surg 84(2):350–357. doi:10.1097/TA.0000000000001730 [DOI] [PMC free article] [PubMed]
- 64.Zhang Z, Xu X, Fan H, Li D, Deng H. Higher serum chloride concentrations are associated with acute kidney injury in unselected critically ill patients. BMC Nephrol. 2013;14:235. doi: 10.1186/1471-2369-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sadan O, Singbartl K, Kandiah PA, Martin KS, Samuels OB (2017) Hyperchloremia is associated with acute kidney injury in patients with subarachnoid hemorrhage. Crit Care Med 45(8):1382–1388. doi:10.1097/CCM.0000000000002497 [DOI] [PubMed]
- 66.Pfortmueller Carmen A (2018) FG-C, Reiterer Christian, Schrott Andrea, Zotti Oliver, Kabon Barbara, Fleischmann Edith, Lindner Gregor. Intraoperative fluid management in patients receiving major abdominal surgery–effects of normal saline versus an acetate-buffered balanced infusion solution on the need for vasopressor support: A prospective double-blind randomised controlled study. Brit J Anaesth 120(2):274–283. doi:10.1016/j.bja.2017.11.088 [DOI] [PubMed]
- 67.Grodin JL, Verbrugge FH, Ellis SG, Mullens W, Testani JM, Tang WH. Importance of abnormal chloride homeostasis in stable chronic heart failure. Circ Heart Fail. 2016;9(1):e002453. doi: 10.1161/CIRCHEARTFAILURE.115.002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radulovic B, Potocnjak I, Dokoza Teresak S, Trbusic M, Vrkic N, Malogorski D, et al. Hypochloraemia as a predictor of developing hyponatraemia and poor outcome in acute heart failure patients. Int J Cardiol. 2016;212:237–241. doi: 10.1016/j.ijcard.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nature reviews. Nephrology. 2016;12(10):610–623. doi: 10.1038/nrneph.2016.113. [DOI] [PubMed] [Google Scholar]
- 70.Testani JM, Hanberg JS, Arroyo JP, Brisco MA, Ter Maaten JM, Wilson FP, et al. Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure. Eur J Heart Fail. 2016;18(6):660–668. doi: 10.1002/ejhf.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130(4):962–967. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]
- 72.Todd SR, Malinoski D, Muller PJ, Schreiber MA. Lactated Ringer's is superior to normal saline in the resuscitation of uncontrolled hemorrhagic shock. J Trauma. 2007;62(3):636–639. doi: 10.1097/TA.0b013e31802ee521. [DOI] [PubMed] [Google Scholar]
- 73.Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93(4):817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Raghunathan K, Murray PT, Beattie WS, Lobo DN, Myburgh J, Sladen R, et al. Choice of fluid in acute illness: what should be given? An international consensus. Br J Anaesth. 2014;113(5):772–783. doi: 10.1093/bja/aeu301. [DOI] [PubMed] [Google Scholar]
- 75.Kimura S, Matsumoto S, Muto N, Yamanoi T, Higashi T, Nakamura K, et al. Association of serum chloride concentration with outcomes in postoperative critically ill patients: a retrospective observational study. J Intensive Care. 2014;2(1):39. doi: 10.1186/2052-0492-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asfar P, Schortgen F, Boisrame-Helms J, Charpentier J, Guerot E, Megarbane B, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017;5(3):180–190. doi: 10.1016/S2213-2600(17)30046-2. [DOI] [PubMed] [Google Scholar]
- 77.Boniatti MM, Cardoso PR, Castilho RK, Vieira SR. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care. 2011;26(2):175–179. doi: 10.1016/j.jcrc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Tani M, Morimatsu H, Takatsu F, Morita K. The incidence and prognostic value of hypochloremia in critically ill patients. TheScientificWorldJOURNAL. 2012;2012:474185. doi: 10.1100/2012/474185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Bacquer D, De Backer G, De Buyzere M, Kornitzer MI. Low serum chloride level a risk factor for cardiovascular mortality? J Cardiovasc Risk. 1998;5(3):177–184. doi: 10.1097/00043798-199806000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


