Abstract
Pirfenidone reduces functional decline in patients with Idiopathic Pulmonary Fibrosis (IPF). However, response to treatment is highly heterogeneous. We sought to evaluate whether response to pirfenidone is influenced by the pretreatment rate of forced vital capacity (FVC) decline. Fifty-six IPF patients were categorized as rapid (RP) or slow progressors (SP) based on whether their FVC decline in the year preceding pirfenidone treatment was > or ≤ 10% predicted. Following pirfenidone treatment patients were followed-up every 6 months and up to 24 months. In the entire population, pirfenidone reduced significantly FVC decline from 231 to 49 ml/year at 6 months (T6) (p = 0.003) and this effect was maintained at the 12-, 18- and 24-month time points (p value for trend n.s.). In RP, the reduction of FVC decline was evident at 6 months (36 vs 706 ml/year pretreatment; p = 0.002) and maintained, though to a lesser degree, at 12 (106 ml/year), 18 (176 ml/year) and 24 months (162 ml/year; p value for trend n.s). Among SP, the reduction in FVC decline was not significant at any of the time points analyzed. In conclusion, pirfenidone reduces FVC decline in IPF patients. However, its beneficial effect is more pronounced in patients with rapidly progressive disease.
Introduction
Idiopathic pulmonary fibrosis (IPF), the most common and severe of the idiopathic interstitial pneumonias (IIPs), is a chronic and relentlessly progressive disease of unknown origin with a median survival of 3–5 years from diagnosis1. While the overall prognosis of IPF is poor, its rate of decline and progression to death is highly variable with some patients remaining relatively stable over a prolonged period of time or progressing slowly, and others experiencing a rapid decline2,3. The heterogeneous nature of the disease makes it difficult, if at all possible, to foresee the clinical trajectory in individual patients. Several risk prediction models have been developed in IPF, but their predictive capability is only moderate4–6.
Forced vital capacity (FVC) is a reliable, valid and reproducible measure of disease progression in patients with IPF1,7, and change in FVC percentage predicted (FVC% pred.) over time is a well-established predictor of mortality. Indeed, it has been shown that patients who experience a decrease in FVC% pred. greater than 10% over a 12-month period have a significantly lower 5-year survival compared to patients whose FVC% pred. declines of 10% or less during the same period of time8. While there is no universally agreed upon definition for these two clinical phenotypes, they are commonly referred to as “rapid” and “slow” progressors, respectively. Notably, the observation that rapidly progressive and relatively stable/slowly progressive patients with IPF display distinct gene expression9,10 and inflammatory profiles in the lung parenchyma3 supports the notion that the mechanisms underlying these distinct clinical phenotypes may also be different.
Pirfenidone, a pyridone derivative with anti-inflammatory, anti-fibrotic and anti-oxidant properties, is approved worldwide for the treatment of IPF based on its ability to slow down functional decline and disease progression as shown in three phase III clinical trials11–13. Clinical trials however are usually performed in highly selected patient populations and in clinical settings that reflect only partially real-life clinical practice. Patients enrolled in clinical trials in fact tend to be younger, and have less severe disease and fewer comorbidities, with a shorter follow-up. Yet, reassuringly, outcomes of IPF patients treated with antifibrotic drugs (e.g., pirfenidone and nintedanib) in real-life appear to be comparable to those observed in clinical trials14.
In this longitudinal study, we aimed to assess long-term (24 months) response to pirfenidone treatment in a well-characterized cohort of patients with IPF in a real-life setting. The availability of data from patients followed up before the pirfenidone era gave us the unique opportunity to stratify our patient population in rapid and slow progressors based on their rate of FVC decline in the 12-month pretreatment period.
Results
A total of 56 patients were included in the study. Baseline patient demographics and clinical characteristics are summarized in Table 1. Most patients were males (78%) and ex-smokers (71%), with a median age at diagnosis of 67 years (range 37–78). Based on the annual FVC% pred. decline in the pretreatment period, 39 patients were classified as slow (FVC% pred. ≤10%) and 17 as rapid progressors (FVC% pred. >10%). Gender, age at diagnosis, smoking history and functional impairment (as assessed by FVC and DLCO) were similar in the two groups. Notably, a trend towards a higher FVC% pred. at diagnosis was seen in rapid progressors, consistent with previous publications from our group3. Pulmonary function data were available for all patients at the 12-month follow-up, and for most of them at 24 months (38 out of 56, 68%). Eighteen patients did not complete the study due to missed follow-up visits (n = 7), pirfenidone-related adverse effects (n = 2), lung transplantation (n = 3) or death (n = 6). Causes of death were related to chronic respiratory deterioration caused by IPF (n = 5) and left heart failure (n = 1). No acute exacerbations occurred in the entire study population; one possible explanation could be related to the selection bias as patients had to have a long (i.e. at least one year) follow-up without acute exacerbations before initiating pirfenidone treatment.
Table 1.
Demographics and clinical characteristics of the entire population (n = 56), slow progressors (n = 39) and rapid progressors (n = 17).
| Entire population (n=56) | Slow progressors (n=39) | Rapid progressors (n=17) | p value | |
|---|---|---|---|---|
| Male - n (%) | 44 (78%) | 31 (79%) | 13 (76%) | 0.8 |
| Age at diagnosis - years | 67 (37–78) | 67 (37–78) | 67 (54–77) | 0.8 |
| Former smokers - n (%) | 40 (71%) | 28 (72%) | 12 (71%) | 0.9 |
| Smoking history - Pack-Years | 10 (0–60) | 8.5 (0–60) | 15 (0–60) | 0.4 |
| Radiologic diagnosis - n (%) | 37 (66%) | 24 (62%) | 13 (76%) | 0.3 |
| FVC at diagnosis - L | 2.66 (1.19–4.72) | 2.72 (1.19–4.72) | 2.66 (1.69–3.79) | 0.5 |
| FVC at diagnosis - %pred. | 80 (35–116) | 74 (35–116) | 83 (61–105) | 0.1 |
| DLCO at diagnosis - %pred. | 54 (28–114) | 52 (28–114) | 61 (48–75) | 0.1 |
| Transplanted patients - n | 3 | 3 | 0 | 0.5 |
| Deaths - n | 6 | 5 | 1 | 0.4 |
Values are expressed as numbers and (%) or median and (ranges).
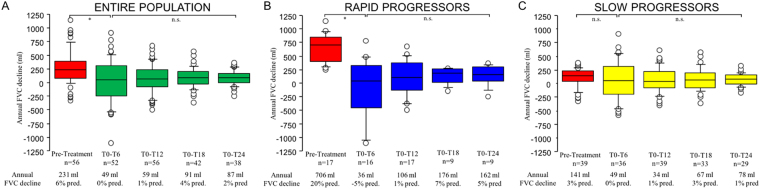
In the pretreatment period, three patients received low-dose steroids, and one N-acetyl cysteine. In the pretreatment period, the median rate of annual FVC decline was 231 ml (range −323 to 1140 ml) in the entire population, 141 ml (range −323 to 375 ml) in slow progressors and 706 ml (range 242 to 1141 ml) in rapid progressors (Fig. 1A–C).
Figure 1.
Panel (A) Annual FVC decline in the entire population (n = 56) before and after pirfenidone treatment at 6 (T0-T6), 12 (T0-T12), 18 (T0-T18) and 24 (T0-T24) months. Overall comparison between all time points was performed using the repeated measurements analysis of variance (ANOVA) (p = 0.03). Pirfenidone reduced significantly the annual decline in FVC already at 6 months (paired t-test, p = 0.003) and this reduction was maintained at 12-, 18- and 24-month follow-up (repeated measures analysis of variance at all time points, p = n.s.). Panel (B) Annual FVC decline in the rapid progressors (n = 17) before and after pirfenidone treatment at 6 (T0-T6), 12 (T0-T12), 18 (T0-T18) and 24 (T0-T24) months. Overall comparison between all time points was performed using the repeated measurements analysis of variance (ANOVA) (p < 0.001). Pirfenidone reduced significantly the annual decline in FVC already at 6 months (paired t-test, p < 0.01) and this reduction was maintained at 12-, 18- and 24-month follow-up (repeated measures analysis of variance at all time points, p = n.s.). Panel (C) Annual FVC decline in the slow progressors (n = 39) before and after pirfenidone treatment at 6 (T0-T6), 12 (T0-T12), 18 (T0-T18) and 24 (T0-T24) months. Overall comparison between all time points was performed using the repeated measurements analysis of variance (ANOVA) (p = 0.1). Pirfenidone did not significantly reduce the annual decline in FVC at any of the time points examined (paired t-test, p = n.s.; repeated measures analysis of variance at all time points, p = n.s.). Negative values mean improvement of FVC. Horizontal bars represent median values, bottom and top of each box plot represents 25th and 75th percentiles, brackets 10th and 90th percentiles, while circles represent outliers. *p value < 0.01, n.s. non significant.
Disease progression in the entire IPF population during treatment
In the IPF population as a whole (n = 56), pirfenidone treatment reduced significantly the rate of annual FVC decline at all the time points examined compared to the pretreatment period. Specifically, the median rate of annual FVC decline changed from 231 ml/year (corresponding to 6% pred./year) in the pretreatment period to 49 ml/year at 6 months (T6) (0% pred., p = 0.003), and this reduction persisted at 12- (59 ml/year, 1% pred.), 18- (91 ml/year, 4% pred.) and 24-month follow-up (87 ml/year, 2% pred.) (p value for trend from T6 to T24 n.s.) (Fig. 1A).
Similar results were observed when the patient populations from Padua, Modena, Foggia and Udine were analyzed separately (data not shown).
Effect of pirfenidone treatment in rapid and slow progressors
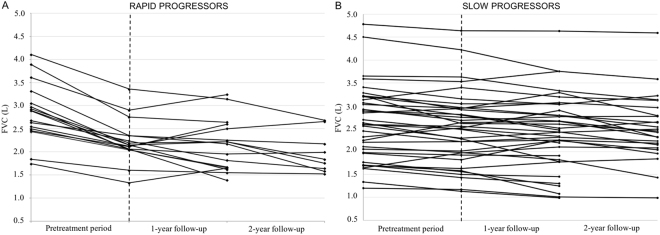
When rapid progressors (n = 17) and slow progressors (n = 39) were analyzed separately, the magnitude of pirfenidone effect on FVC decline varied greatly (Fig. 1B,C and Fig. 2A,B). Indeed, among rapid progressors the beneficial effect of pirfenidone on FVC decline was already evident at 6 months (T6), wherein the annual rate of FVC decline changed from 706 ml/year (20% pred.) in the pretreatment period to 36 ml/year (−5% pred., p = 0.002). The treatment effect was maintained at the 12- (106 ml/year, 1% pred.), 18- (176 ml/year, 7% pred.) and 24-month follow-up (162 ml/year, 5% pred.) (p value for trend from T6 to T24 n.s.) (Fig. 1B).
Figure 2.
Panel (A) Individual FVC trajectories in rapid progressors from pretreatment period through 2-year follow-up. Overall comparison between all time points was performed using the repeated measurements analysis of variance (ANOVA) (p < 0.001). In the pretreatment period, the FVC trajectory of the rapid progressors is depicted by a line with a steep slope. Whereas, after the institution of pirfenidone (vertical dotted line), FVC trajectory is depicted by a line with a significantly flatter slope compared to the pretreatment period (paired t-test, p < 0.01). The FVC values during treatment were stable in the 2-year follow-up (repeated measures analysis of variance at 1-year and 2-year time points, p = n.s.). (B) Individual FVC trajectories in slow progressors from pretreatment period through 2-year follow-up. Overall comparison between all time points was performed using the repeated measurements analysis of variance (ANOVA) (p = n.s.). In the pretreatment period, the FVC trajectory of the slow progressors is depicted by a line with a flat slope. After the institution of pirfenidone (vertical dotted line), FVC trajectory is depicted by a line with a slope as flat as the pretreatment period (paired t-test, n.s.). The FVC values during treatment were stable in the 2-year follow-up (repeated measures analysis of variance at 1-year and 2-year time points, p = n.s.). FVC (on the y axis) is expressed as litres. *p value < 0.01, n.s. non significant.
Among slow progressors, the reduction of the annual FVC decline compared to the pretreatment period did not reach statistical significance at any of the time points examined, i.e. at 6 months (T6) (49 ml/year, 0% pred. vs. 141 ml/year, 3% pred. pretreatment, p = 0.3) nor in any of the other follow-up time points (34 ml/year, 1% pred. at 12 months; 67 ml/year, 3% pred. at 18 months; 78 ml/year, 1% pred. at 24 months) (p value for trend from T6 to T24 n.s.) (Fig. 1C).
Discussion
Pirfenidone is approved worldwide for the treatment of IPF based on its ability to slow down functional decline and disease progression11–13. The heterogeneous nature of IPF makes it difficult to foresee the clinical trajectory in individual patients, and this heterogeneity could conceivably also affect treatment response. In the present study, we report on the long-term (up to 24 months) efficacy of pirfenidone treatment in patients with IPF. These patients had been followed for a prolonged time before starting anti-fibrotic treatment, during which we had the opportunity to categorize them as rapid or slow progressors based on the rate of their FVC decline in the pretreatment period3. In our patient population as a whole, compared to the pretreatment period, pirfenidone reduced significantly the decline in FVC already at 6 months, and this reduction was maintained at the 12-, 18- and 24-month follow-up. However, the effect of pirfenidone differed considerably between slow and rapid progressors, being significantly more pronounced in the latter group at all time points.
Before starting pirfenidone, our patients were followed-up for a median of 15 months, and this gave us the possibility to monitoring changes in FVC during an extended period of time, which, in turn, provided a reliable basis for the definition of rapid and slow disease progression. FVC is a valid and reproducible measure of disease progression in patients with IPF1,7,15. IPF patients who experience a decrease of >10% in FVC% pred. over a 12-month period (rapid progressors) display a significantly lower 5-year survival than patients whose FVC% pred. declines ≤10% (slow progressors)8. Given the differences in the described rates of decay in IPF, it is conceivable that treatment response might also differ in subjects with rapid and slow decline. Accordingly, it is important to prospectively investigate this possibility.
When we considered our whole population together, the annual rate of FVC decline in the pretreatment period was 231 ml/year (6% pred.) similar to what has been reported in the placebo arms of other treatment trials13. With pirfenidone, the rate of FVC decline was variably but significantly reduced already after 6 months of treatment, an improvement that was maintained during the entire 24-month study duration. Overall, after one year of pirfenidone treatment, 7 (12%) patients experienced a FVC decline >10% compared with 17 patients (30%) in the pretreatment period (OR 3.0, p = 0.02), corresponding to a relative reduction of 59%, while the absolute fall of FVC decreased from a pretreatment value of 231 ml/year to 59 ml/year (p < 0.05), consistent with a previous study in Japanese patients16. Our data provide, we believe, a reliable picture of the effect of pirfenidone treatment on the rate of FVC change between the pretreatment and follow-up period, since each patient served as its own control, which clearly strengthens our findings.
The effect of pirfenidone differed significantly between slow and rapid progressors. Among rapid progressors, the median decline of FVC prior to treatment was 706 ml/year, which was reduced to 36 ml/year after 6 months of treatment. Overall, the pirfenidone effect was significantly maintained throughout the two-year study period. Pirfenidone was also beneficial for patients with slow pretreatment decline since, although the rate of FVC decline did not change significantly, it seemed to stabilise the disease.
Our results are in line with those of previous retrospective analyses of lung function changes in patients with mild to moderate IPF treated with pirfenidone17,18. These studies showed that patients with progressive disease (e.g., FVC% pred. decline > 10% per year) may benefit substantially from pirfenidone treatment, which may even result in improvement of FVC, as shown by Loeh et al.18, while patients with slowly progressive disease tend to experience disease stability under treatment. The assessment of disease progression and treatment response in patients with IPF is complicated by its variable clinical course2. In a recent post-hoc analysis of patients from the placebo arms of the CAPACITY and ASCEND trials19, Nathan and colleagues observed a weak negative correlation between changes in FVC% pred. during two consecutives 6-month intervals, that the authors interpreted as a reflection of the variability in both the magnitude and direction of change. An important source of variability in that study could have been the combined analysis of slow and rapid progressors together, particularly since the follow-up period was relatively short (i.e., 6 months). In our study, the median pretreatment observation period was 15 months, which minimizes the possibility that the observed rate of disease progression was confounded by the inherent intra-individual variability in longitudinal change in FVC.
A recent multicentre study20 confirmed the efficacy of pirfenidone in slowing down disease progression in patients with IPF, but suggested that the beneficial effect would be more pronounced in patients with more severe disease. This was not the case in our study, since at diagnosis the severity of the disease in our population was similar in rapid and slow progressors.
The mechanisms through which rapid progressors display a particularly favorable response to pirfenidone treatment is not known but may be related to differences in the described lung pathology in the slow and rapid IPF groups3. Possibly, extracellular matrix deposition and removal may be much more rapid, thus amenable to anti-fibrotic therapy, in the rapid progressive than in relatively stable IPF18,21. Recently, we have shown that explanted lungs from IPF subjects had similar degree of fibrosis deposition and numbers of fibroblast foci in slow and rapid progressors3. This finding might be due to a more rapid fibrous tissue deposition in the rapid decliner perhaps more amenable to lysis and degradation by pirfenidone. Alternatively, pirfenidone might play a prominent immunosuppressive and antioxidant role, with the antioxidant properties contributing to its anti-inflammatory effects, which in turn may account, at least in part, for pirfenidone’s antifibrotic effects22,23. We have reported that rapidly progressive IPF, compared to slow or stable disease, is characterized by a significantly higher innate and adaptive inflammation in the lung parenchyma. This might contribute significantly to the accelerated decline of this group of patients and would also make the disease more responsive to a compound with anti-inflammatory and anti-fibrotic properties3,24.
The presence of an exuberant immune inflammatory infiltrate, found predominantly in the rapid progressors, is consistent with the gene expression profile reported by Boon and colleagues10, who indeed described the activation of important pro-inflammatory pathways that may potentially play a role in the immune activation and disease progression in rapid decliners. In line with these reports it is also the presence of B cell aggregates25 and highly differentiated circulating B cells in patients with IPF26, findings usually observed in autoimmune syndromes. Our findings of an increased number of B lymphocytes and T lymphocytes in the lungs of rapid progressors3 are also in keeping with previous observations showing that lymphocyte density in IPF lung is associated with FVC decline and poor survival27. Although speculative and based on pathological description of the disease, we believe these possibilities deserve some attention since they might explain, at least in part, the different response to pirfenidone treatment.
The rate of discontinuation of pirfenidone due to adverse events observed in our patient population (3.5%) was lower than that observed in the CAPACITY12 (14.8%) and ASCEND13 (14.4%) trials, confirming the safety and tolerability of the drug in clinical practice.
Our long-term prospective study, even if relatively small, provides further evidence of the efficacy of pirfenidone in patients with IPF up to 24 months of treatment and shows that response to therapy is influenced by the rate of decline, slow or rapid, in the pretreatment period. Strengths of our study include the careful patient characterization and the availability of long-term pre- and post-treatment data. Owing to the progressive nature of the disease and the availability worldwide of two efficacious anti-fibrotic drugs (pirfenidone and nintedanib), studies on IPF patients off-treatment will become progressively less common, if at all possible (and ethical). This makes our data regarding the pretreatment period particularly relevant.
Our study has some limitations. Firstly, the size of our study population is relatively small, and it decreased over time; indeed, follow-up data at 24 months were available for 38/56 patients only (68%). Secondly, we limited our analysis to change in FVC. Despite uncertainties about its clinical meaningfulness and handling of missing data, FVC is widely accepted as surrogate of treatment efficacy in IPF both in clinical trials and at regulatory level8,15,28,29. Contrary to clinical trials however, we decided neither to impute missing values for patients who stopped pirfenidone treatment at some point during the observation period nor to set FVC as 0 for patients who died or underwent lung transplantation, as previously done13.
Conclusions
The study confirms that pirfenidone treatment reduces significantly the rate of FVC decline in patients with IPF, an effect that is significantly more pronounced in patients with rapidly progressive disease. Additional studies are required to identify more precisely the underlying differences in disease behavior and treatment response of the slow and rapid decliners in IPF. This will greatly benefit clinical research and daily clinical practice alike.
Methods
Patients and study design
Informed consent was obtained for all study participants. This was a prospective, longitudinal, multicenter study, in which we analyzed a unique and well-characterized cohort of patients with IPF, with a long clinical and functional follow-up before and after the initiation of pirfenidone treatment. Fifty-six patients were selected from four Italian Interstitial Lung Disease (ILD) centers (e.g., University Hospital of Padua, n = 27; University Hospital of Foggia, n = 14; University Hospital of Modena, n = 10; and General Hospital of Udine, n = 5). For all patients, the diagnosis of IPF was made in accordance with current guidelines1.
The peculiarity of this study was to include only patients for whom lung function data were available for at least one year before (pretreatment period) starting pirfenidone treatment. Based on their annual rate of decline in FVC% pred. in the pretreatment period, patients were classified as either “rapid” (decline in FVC% pred. >10%) or “slow” (decline in FVC% pred. ≤10%) progressors. They were then followed-up during pirfenidone treatment every 6 months up to 24 months (follow-up period). At the 12-month follow-up, functional data were available for the entire patient population, whereas at 24 months functional data were available for 38/56 (68%) patients.
Negative values of annual FVC decline during the follow-up indicated amelioration. The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University Hospital of Padua (4280/AO/17).
Additional important clinical and functional parameters, including symptoms, diffusing capacity of the lung for carbon monoxide (DLCO) and 6-minute walking test, were also collected but the amount of missing data did not allow for a meaningful statistical analysis to be performed.
Statistical analysis
Categorical variables were described as absolute (n) and relative values (%) and continuous variable were described as median and range. To compare demographic data and baseline clinical characteristics between rapid and slow progressors Chi-square test for categorical variables and Mann-Whitney test for the continuous variables were used.
In the entire population, as well as in the rapid and slow progressor subgroups, we performed the repeated measurements analysis of variance (ANOVA) at all time points to evaluate the difference in FVC decline between pretreatment and the follow-up period. To evaluate the difference between the pretreatment FVC decline and the first time point available (6 months) in the follow-up period in the entire population, in the rapid progressors and in the slow progressors we performed a paired t-test analysis. Finally, in order to evaluate whether this potential difference was maintained in the follow-up period (6, 12, 18 and 24 months), we performed the repeated measurements analysis of variance (ANOVA) between these time points in the entire population, and in the rapid and slow progressor subgroups.
All data were analysed using SPSS Software version 22.0 (IBM USA). p-values < 0.05 were considered statistically significant.
Data availability
All data generated or analyzed during this study are included in this published article.
Acknowledgements
The authors thank Dr Giulia Patricelli and Dr Ivana Castaniere for their help in data collection. This work was supported in part by the Department of Cardiac, Thoracic and Vascular Sciences, University of Padova, Italy (Grant BIRD 163522) (Prof. Spagnolo).
Author Contributions
D.B. conceived and designed the study, collected and analyzed data, and wrote the manuscript. E.B. conceived and designed the study, supervised data collection and revised the manuscript. D.L. collected data and revised the manuscript. S.C. collected data and revised the manuscript. R.M. collected data and revised the manuscript. F.L. collected data and revised the manuscript. E.C. collected data and revised the manuscript. E.B. collected data, performed the statistical analysis and revised the manuscript. E.C. participated in the study design and manuscript preparation. M.P.F.B. participated in the study design and manuscript preparation. D.G. performed the statistical analysis and revised the manuscript. M.C. conceived and designed the study, supervised data collection, participated in data analysis and revised the manuscript. M.S. conceived and designed the study, supervised data collection, participated in data analysis and revised the manuscript. P.S. conceived and designed the study, supervised data collection, participated in data analysis and revised the manuscript. All authors provided final approval of the submitted work.
Competing Interests
PS reports personal fees from Roche/Genentech, personal fees from InterMune, personal fees and non-financial support from Boehringer-Ingelheim, personal fees from Novartis, personal fees from Santhera Pharmaceuticals, outside the submitted work. MS reports grants from Takeda, grants from Guidotti, outside the submitted work. SC reports personal fees from Roche, personal fees from Boehringer Ingelheim, outside the submitted work. FL reports personal fees from Boehringer-Ingelheim, grants and personal fees from Roche, outside the submitted work. The remaining authors report no conflicts of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raghu G, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley B, Collard HR, King TE., Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 3.Balestro E, et al. Immune inflammation and disease progression in idiopathic pulmonary fibrosis. PLoS ONE. 2016;11:e0154516. doi: 10.1371/journal.pone.0154516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.du Bois RM, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011;184:459–466. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 5.Ley B, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Wells AU, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am. J. Respir. Crit. Care Med. 2003;167:962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 7.du Bois RM, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011;184:1382–1389. doi: 10.1164/rccm.201105-0840OC. [DOI] [PubMed] [Google Scholar]
- 8.Collard HR, et al. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 9.Selman M, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS ONE. 2007;2:e482. doi: 10.1371/journal.pone.0000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boon K, et al. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF) PLoS ONE. 2009;4:e5134. doi: 10.1371/journal.pone.0005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi H, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 12.Noble PW, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 13.King TE, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 14.Hughes G, Toellner H, Morris H, Leonard C, Chaudhuri N. Real world experiences: pirfenidone and nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. J. Clin. Med. 2016;5:pii: E78. doi: 10.3390/jcm5090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis - FDA review of pirfenidone and nintedanib. N. Engl. J. Med. 2015;372:1189–1191. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 16.Bando M, et al. Clinical experience of the long-term use of pirfenidone for idiopathic pulmonary fibrosis. Intern. Med. 2016;55:443–448. doi: 10.2169/internalmedicine.55.5272. [DOI] [PubMed] [Google Scholar]
- 17.Okuda R, et al. Safety and efficacy of pirfenidone in idiopathic pulmonary fibrosis in clinical practice. Respir. Med. 2013;107:1431–1437. doi: 10.1016/j.rmed.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Loeh B, et al. Intraindividual response to treatment with pirfenidone in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2015;191:110–113. doi: 10.1164/rccm.201406-1106LE. [DOI] [PubMed] [Google Scholar]
- 19.Nathan SD, et al. Effect of continued treatment with pirfenidone following clinically meaningful declines in forced vital capacity: analysis of data from three phase 3 trials in patients with idiopathic pulmonary fibrosis. Thorax. 2016;71:429–435. doi: 10.1136/thoraxjnl-2015-207011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harari S, et al. Efficacy of pirfenidone for idiopathic pulmonary fibrosis: an Italian real life study. Respir. Med. 2015;109:904–913. doi: 10.1016/j.rmed.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Nkyimbeng T, et al. Pivotal role of matrix metalloproteinase 13 in extracellular matrix turnover in idiopathic pulmonary fibrosis. PLoS ONE. 2013;8:e73279. doi: 10.1371/journal.pone.0073279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasse A, et al. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2009;179:717–723. doi: 10.1164/rccm.200808-1201OC. [DOI] [PubMed] [Google Scholar]
- 23.Gilani SR, et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS ONE. 2010;5:e8959. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim ES, Keating GM. Pirfenidone: a review of its use in idiopathic pulmonary fibrosis. Drugs. 2015;75:219–230. doi: 10.1007/s40265-015-0350-9. [DOI] [PubMed] [Google Scholar]
- 25.Todd N, et al. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J. Inflamm. Res. 2013;6:63–70. doi: 10.2147/JIR.S40673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue J, et al. Plasma B lymphocyte stimulator and B cell differentiation in idiopathic pulmonary fibrosis patients. J. Immunol. 2013;191:2089–2095. doi: 10.4049/jimmunol.1203476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parra ER, Kairalla RA. Ribeiro de Carvalho, C. R., Eher, E. & Capelozzi, V. L. Inflammatory cell phenotyping of the pulmonary interstitium in idiopathic interstitial pneumonia. Respiration. 2007;74:159–169. doi: 10.1159/000097133. [DOI] [PubMed] [Google Scholar]
- 28.Bradford WZ, Cohen AH, Leff JA. Selection of clinically meaningful primary endpoints in phase 3 clinical trials in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013;187:1269–1270. doi: 10.1164/rccm.201204-0770LE. [DOI] [PubMed] [Google Scholar]
- 29.Cottin V, et al. Adherence to guidelines in idiopathic pulmonary fibrosis: a follow-up national survey. Eur. Respir. J. Open Res. 2015;1:pii: 00032–2015. doi: 10.1183/23120541.00032-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.