ABSTRACT
Accurate assembly of viral particles in the potyvirus Plum pox virus (PPV) has been shown to depend on the contribution of the multifunctional viral protein HCPro. In this study, we show that other viral factors, in addition to the capsid protein (CP) and HCPro, are necessary for the formation of stable PPV virions. The CP produced in Nicotiana benthamiana leaves from a subviral RNA termed LONG, which expresses a truncated polyprotein that lacks P1 and HCPro, together with HCPro supplied in trans, was assembled into virus-like particles and remained stable after in vitro incubation. In contrast, deletions in multiple regions of the LONG coding sequence prevented the CP stabilization mediated by HCPro. In particular, we demonstrated that the first 178 amino acids of P3, but not a specific nucleotide sequence coding for them, are required for CP stability and proper assembly of PPV particles. Using a sequential coagroinfiltration assay, we observed that the subviral LONG RNA replicates and locally spreads in N. benthamiana leaves expressing an RNA silencing suppressor. The analysis of the effect of both point and deletion mutations affecting RNA replication in LONG and full-length PPV demonstrated that this process is essential for the assembly of stable viral particles. Interestingly, in spite of this requirement, the CP produced by a nonreplicating viral RNA can be stably assembled into virions as long as it is coexpressed with a replication-proficient RNA. Altogether, these results highlight the importance of coupling encapsidation to other viral processes to secure a successful infection.
IMPORTANCE Viruses of the family Potyviridae are among the most dangerous threats for basically every important crop, and such socioeconomical relevance has made them a subject of many research studies. In spite of this, very little is currently known about proteins and processes controlling viral genome encapsidation by the coat protein. In the case of Plum pox virus (genus Potyvirus), for instance, we have previously shown that the multitasking viral factor HCPro plays a role in the production of stable virions. Here, by using this potyvirus as a model, we move further to show that additional factors are also necessary for the efficient production of potyviral particles. More importantly, a comprehensive screening for such factors led us to the identification of a functional link between virus replication and packaging, unraveling a previously unknown connection of these two key events of the potyviral infection cycle.
KEYWORDS: Potyviridae, capsid protein, sharka, viral replication, virion assembly
INTRODUCTION
With almost 200 assigned representatives sorted in eight different genera, the Potyviridae family is the largest group of plant RNA viruses. Their single-stranded positive-sense RNA genome consists of a single molecule in most cases, with just a few exceptions of viruses having a bipartite genome (1, 2). Members of the genus Potyvirus, by far the largest genus of this family, with 160 assigned species, have a monopartite genome of approximately 10 kb flanked by 5′ and 3′ noncoding regions (NCRs), which is translated in a polyprotein that is further processed by viral proteinases to produce the following mature viral factors: P1, HCPro, P3, 6K1, CI, 6K2, NIa (VPg plus Pro), NIb (viral replicase), and CP (capsid protein) (3). Moreover, RNA polymerase slippage events generate virus-derived RNA variants producing diverse transframe products whose expression varies among potyviruses and include P3N-PIPO, P3N-ALT, and P1N-PISPO (4–8). The genomic RNA is encapsidated by a single core CP that forms characteristic potyviral flexuous rods of 11 to 20 nm in diameter by 680 to 900 nm in length (2, 9). The CP is able to self-assemble in vitro as well as in different heterologous systems (10–13), and once assembled, the particles remain stable after trimming the surface-exposed N- and C-terminal regions of CP by a mild trypsin treatment (14, 15). However, amino acids present at these two regions, in addition to those at the CP central core, are involved in intersubunit interactions required for the initiation of virion assembly (15, 16).
Very little is known about how the potyviral CP specifically packages the cognate genomic RNA. Results reported by Joseph and Savithri (17) suggested that the CP from the potyvirus Pepper vein banding virus produced in Escherichia coli can encapsidate its own mRNA but with very low efficiency. Voloudakis et al. (18), in turn, observed that virus-like particles produced in bacteria by the CP of another potyvirus, Potato virus Y, displayed a stacked-ring structure similar to that of particles formed in vitro by the same CP in the absence of RNA. Some evidence suggests that coating of the potyviral RNA begins near its 5′ terminus (19), but no potyviral origin of assembly or packaging signal has been identified yet.
Recently, it has been shown that a second viral protein, HCPro, is involved in the assembly of potyviral virions (20). HCPro was first recognized as a helper component required for aphid transmission of viral particles (21, 22). Later on, a large number of additional activities were assigned to HCPro (23), although the most important one appears to be the suppression of antiviral RNA silencing (24, 25), as a potyvirus expressing an HCPro without RNA silencing suppression activity is not viable in wild-type plants but can infect RNA silencing-deficient plants (26). Furthermore, in line with this assumption, chimeric viruses in which HCPro was replaced by diverse heterologous RNA silencing suppressors (RSSs) are infectious in RNA silencing-proficient plants (27, 28). Valli et al. (20) recognized a nonessential function of HCPro of the potyvirus Plum pox virus (PPV), which cannot be supplied by other RSSs and contributes to enhance the yield of infectious viral particles with the consequent increase in infectivity of the viral progeny.
Here, we show that HCPro is not the only viral factor required for assembly of the potyviral CP. Our results indicate that PPV CP forms stable particles in plant cells only when it is coexpressed with a replication-competent viral RNA, unraveling a functional link between RNA replication and packaging during potyviral infection.
RESULTS
Enhancement of PPV CP stability by its cognate HCPro depends on additional viral factors.
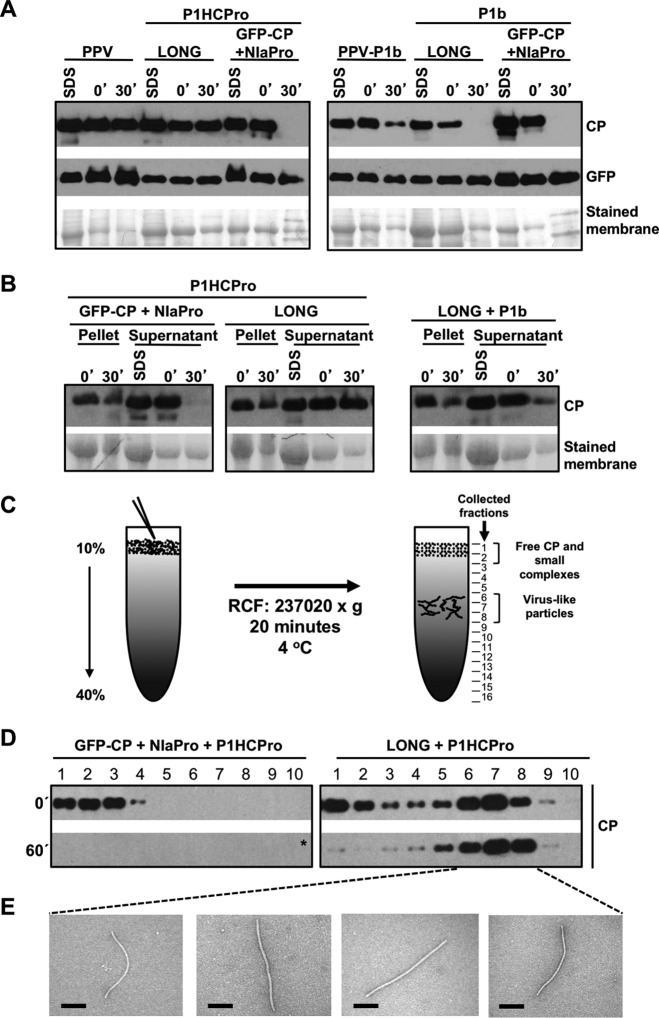
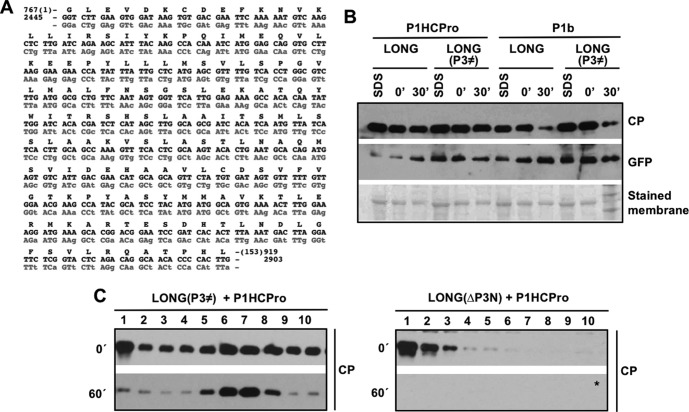
Results reported by Valli et al. (20) showed that PPV CP expressed from pLONG, a plasmid that carries the 5′ and 3′ NCRs flanking the coding sequence of a truncated PPV polyprotein starting in P3, is stable just when it is coexpressed with PPV HCPro. For simplicity, all of the plasmids used in this study, as well as those proteins expressed from them after agroinoculation or agroinfiltration, are shown in Fig. 1. The immunoblot assays shown in Fig. 2 confirmed that CP levels sharply declined when native N. benthamiana extracts derived from leaves expressing LONG and P1b, the RSS of Cucumber vein yellowing virus (CVYV), were incubated for 30 min at 25°C (Fig. 2A, right). In contrast, the levels of CP remained stable when this protein was generated from LONG in the presence of P1HCPro (Fig. 2A, left). As an additional confirmation, equivalent effects over the ex vivo CP stability were obtained when we analyzed native protein extracts deriving from N. benthamiana plants infected with either wild-type PPV or the PPV chimera that expresses CVYV P1b instead of HCPro (Fig. 2A). As expected, the instability of CP in the absence of HCPro was specific in all cases, as the green fluorescent protein (GFP) produced from the same construct, or from the cognate viral genome, remained stable after the indicated incubation period (Fig. 2A).
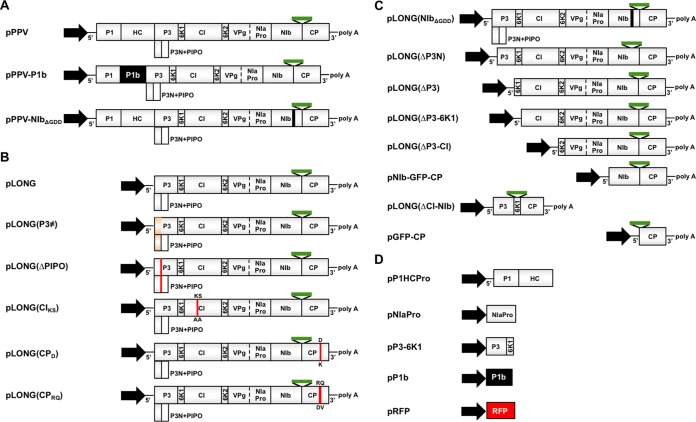
FIG 1.
Schematic representation of constructs used in this study. (A to D) PPV and PPV mutants (A), LONG and LONG mutants (B), LONG deletions (C), and other plasmids (D). The constitutive 35S promoter from Cauliflower mosaic virus is shown as a black arrow. Gray boxes represent cistrons of the indicated viral proteins. A green bar between viral cistrons denotes the presence of the GFP reporter. The presence of both 5′ and 3′ viral noncoding regions and poly(A) are indicated (5′, 3′, and polyA, respectively). P3 coding sequence carries intron I from the potato ST-LS-1 gene to avoid toxicity of P3 in bacteria and increase plasmid stability.
FIG 2.
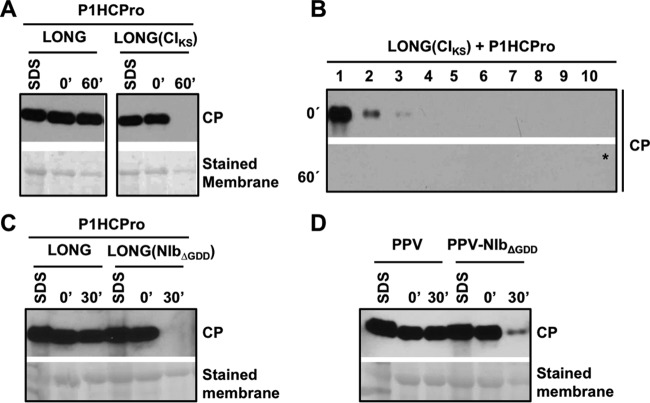
Stabilization of CP and assembly of virion-like particles require other host factors in addition to HCPro. (A) Analysis of the stability of the CP produced by infection or transient expression by agroinfiltration of N. benthamiana plants with the indicated constructs. (B) Analysis of aggregation/precipitation of the CP produced by transient expression by agroinfiltration of N. benthamiana plants with the indicated constructs. For panels A and B, infiltrated patches were collected at 5 days posttreatment. Extracts prepared in SDS buffer (SDS) and the time, in minutes, elapsed after the extraction with phosphate buffer (0′ and 30′) are indicated. Protein accumulation was assessed by immunoblot analysis with polyclonal serum specific for PPV CP and a monoclonal antibody specific for GFP. Membranes stained with Ponceau red showing the RubisCO large subunit were included as loading controls. (C) Schematic representation of viral particle separation by sucrose gradient centrifugation. (D) Analysis of samples after sucrose gradient centrifugation of extracts from N. benthamiana leaves expressing the indicated constructs. Extracts, prepared in phosphate buffer, were loaded on the gradient immediately after being prepared (0′) or after 60 min of incubation at 25°C (60′). Sixteen fractions were collected from the bottom, and the 10 upper ones were subjected to CP-specific immunoblot analysis. The asterisk indicates that the membrane was overexposed. (E) Electron microscopy pictures of virus-like particles trapped with anti-CP serum. Protein extracts before ex vivo incubation (T = 0) were subjected to sucrose gradient centrifugation, and a pool of the indicated fractions was observed under the electron microscope. Black scale bars represent 0.2 μm.
PPV CP was also transiently produced as a GFP-CP fusion, with both proteins connected by a functional NIaPro cleavage site, thus mimicking the C-terminal part of the PPV polyprotein (Fig. 1A and C). Extraction under denaturing conditions showed that similar levels of CP were produced from LONG or GFP-CP plus NIaPro (Fig. 2A). However, the CP produced from GFP-CP drastically differed from that deriving from LONG in being highly unstable in native extracts regardless of whether it was expressed along with PPV HCPro (Fig. 2A). Once again, this instability of CP after 30 min of incubation at room temperature was specific, as GFP, the partner protein in this fusion, remained stable (Fig. 2A).
The disappearance of CP from soluble protein fractions can be explained by either specific degradation or aggregation followed by precipitation. Valli et al. (20) already showed that CP degradation bands appeared after ex vivo incubation only in protein samples where CP disappears. To address whether CP aggregation/precipitation has an influence over its detection in protein-soluble fractions, the presence of this viral factor in the pellets (cell debris) was analyzed by Western blotting. As observed in Fig. 2B, CP was detected in pellets. Importantly, no increase over time in the levels of CP was observed in these pellets independently of whether the expressed viral protein was unstable (Fig. 2B, P1HCPro + GFP-CP + NIaPro and P1b + LONG) or stable (Fig. 2B, P1HCPro + LONG).
The instability of PPV CP in the absence of its cognate HCPro correlates with a defective yield of viral particles (20). To ascertain whether this is the case of CP in the presence of HCPro but in the absence of additional viral factors, native extracts were subjected to sucrose gradient centrifugation either immediately after tissue homogenization or after incubation at 25°C for 60 min. When we normally run this assay with the wild-type PPV, top fractions (1 to 3) contain free CP and CP complexes of small size, whereas middle fractions (6 to 8) form a second peak of CP that corresponds to virion-like particles (Fig. 2C and data not shown). Remarkably, the CP that derived from NIaPro-mediated processing of GFP-CP in the presence of HCPro was only detected in the top fractions and just prior to the incubation (Fig. 2D, left), indicating that CP from GFP-CP is unable to form viral particles and providing an explanation for the instability of this protein in such a context. On the other hand, the CP expressed from LONG behaves as expected for the wild-type virus, with the major part of CP in the middle fractions (Fig. 2D, right). Whereas electron microscopy examination of fractions 6 to 8 showed typical potyviral flexuous rods, these virus-like particles were absent from fractions 1 to 3 (Fig. 2E). Measurements of virions in fractions 6 to 8 indicated that they were slightly shorter than those produced by the wild-type PPV, which is compatible with the encapsidation of a shorter RNA, such as the one produced from pLONG (not shown). This sedimentation profile was not drastically altered by the ex vivo incubation. The only difference was that the amount of CP in fractions 1 to 3 dropped after incubation (Fig. 2D, right, 60′), suggesting that (i) full-length viral particles produced from LONG in the presence of HCPro are highly stable and (ii) a considerable proportion of CP subunits seems to be either free or forming small oligomers, which are degraded after ex vivo incubation. Interestingly, this second observation is in agreement with the idea that CP plays other roles during viral infection beside genome encapsidation (29).
All in all, results displayed in Fig. 2 indicate that formation of PPV virions with the consequent stabilization of the cognate CP requires the contribution of other viral factors in addition to HCPro.
Viral factors contributing to PPV CP stabilization in the presence of HCPro include P3 coding sequence.
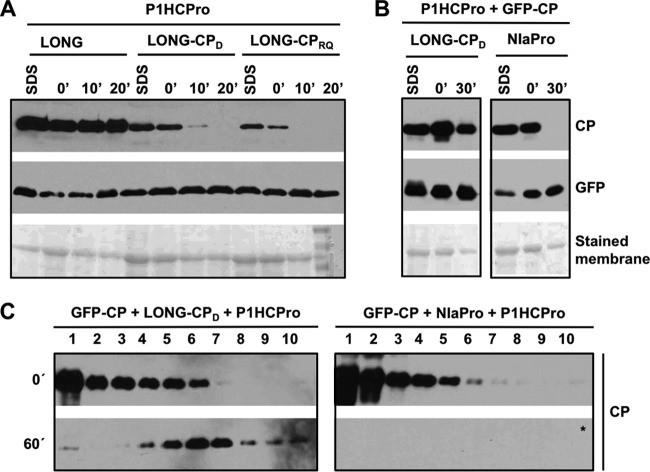
In order to identify viral factors required to stabilize PPV CP other than HCPro, we performed several deletions progressively trimming the N terminus of the LONG polyprotein. Initially, we tested LONG(ΔP3-6K1) (lacking P3-6K1), LONG(ΔP3-CI) (lacking P3-6K1-CI), and NIb-GFP-CP (lacking all PPV proteins except NIb and CP) (Fig. 1C). CP was expressed from all of these plasmids, as well as from pLONG as a positive control, by agroinfiltration in N. benthamiana leaves together with plasmids expressing the proteinase NIaPro, required to separate CP from GFP in NIb-GFP-CP, and HCPro. Accumulation of the CP extracted either under denaturing or native conditions from the agroinfiltrated tissue, as well as CP stability in the native extracts incubated in vitro, were tested by immunoblot analysis (Fig. 3).
FIG 3.
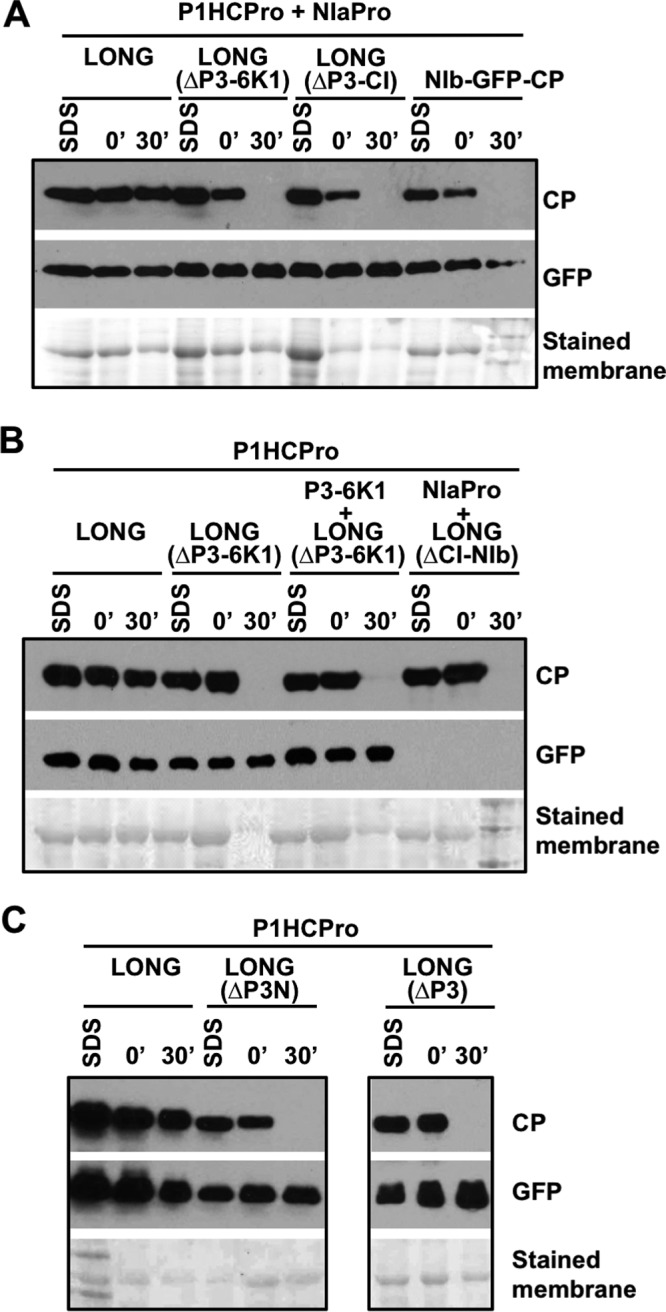
P3-coding sequences contribute to CP stability. All panels (A, B, and C) show analyses of the stability of CP produced by agroinfiltration of N. benthamiana leaves with different deletion mutants of pLONG coexpressed with the indicated constructs. Samples were collected at 5 days postagroinfiltration. Extracts prepared in SDS buffer (SDS) and the time, in minutes, elapsed after extraction with phosphate buffer (0′ and 30′) are indicated. Protein accumulation was assessed by immunoblot analysis with polyclonal serum specific for PPV CP and a monoclonal antibody specific for GFP. Membranes stained with Ponceau red showing the RubisCO large subunit were included as loading controls.
The analysis of the denatured extracts showed good and comparable expression of viral CP from all these constructs. The CP from NIb-GFP-CP was readily separated from GFP by action of the NIaPro proteinase, but it was rapidly degraded during the in vitro incubation of the native extract, as this CP was not even detected after a 30-min incubation (Fig. 3A). Similarly, the CP produced from LONG(ΔP3-6K1) and LONG(ΔP3-CI) showed high degradation rates during the in vitro incubation (Fig. 3A). These results demonstrate that the absence of P3 and 6K1 from the viral polyprotein is enough to promote PPV CP instability, even in the presence of HCPro.
We next analyzed whether the simple fusion of P3-6K1 to GFP-CP [Fig. 1C, pLONG(ΔCI-NIb)] or the expression in trans of P3-6K1 (Fig. 1C, pP3-6K1) together with pLONG(ΔP3-6K1) stabilizes to CP as pLONG does. For the first analysis, LONG(ΔCI-NIb) polyprotein, NIaPro, and P1HCPro were coexpressed, whereas for the second one we coexpressed LONG(ΔP3-6K1), P3-6K1, and P1HCPro. Importantly, neither of these two ways of supplying P3-6K1, either in cis directly linked to GFP-CP or in trans complementing LONG(ΔP3-6K1), was able to provide stability to CP in the in vitro incubation assay, even though the initial levels of CP were similar to those produced from LONG (Fig. 3B). Whereas the CP produced from LONG(ΔCI-NIb) was efficiently separated from GFP by the action of the NIaPro proteinase supplied in trans, we have to remark that NIaPro was unable to cleave efficiently at the C terminus of 6K1, as GFP was not detected in this sample (Fig. 3B). We speculate that the NIaPro-mediated cleavage at the C terminus of 6K1 occurs mainly in cis and that the resulting 6K1-GFP unprocessed factors may be unstable under this condition.
We designed two additional deletions in LONG to define more precisely the viral sequence(s) required to provide stability to CP in the presence of HCPro, LONG(ΔP3) and LONG(ΔP3N), which lack the complete P3 and 178 amino acids at the P3 N terminus, respectively (Fig. 1C). As in the previous experiments, these polyproteins were expressed by agroinfiltration in the presence of HCPro in N. benthamiana leaves (Fig. 3C). The accumulation of CP extracted under denaturing conditions from leaves expressing LONG(ΔP3) and LONG(ΔP3N) appeared to be lower than that produced from LONG (Fig. 3C). More importantly, the CP produced from these two polyproteins and extracted under native conditions was completely degraded after 30 min of in vitro incubation. All these results indicate that the intact P3, as well as other factors present in LONG, is required for PPV CP stabilization in the presence of HCPro.
The amino acid sequence of P3, rather than that of P3N-PIPO or the P3 nucleotide sequence, is required to preserve the stability of PPV CP produced in the presence of HCPro.
Because deletion in both the N terminus and entire P3 amino acid sequences also disrupts P3N-PIPO, results presented in Fig. 3C raised the possibility that instability of PPV CP is due to the lack of this factor. To test this possibility, we introduced two synonymous mutations in the GAA AAA A motif of the P3 cistron from pLONG in order to create pLONG(ΔPIPO) that carries a GAG AAG A motif (underlining indicates the two mutated residues) (Fig. 1B), which abolishes RNA polymerase slippage with the consequent truncation of PIPO (4, 6, 7). As expected from the demonstrated role of P3N-PIPO in cell-to-cell movement (30, 31), the introduced mutations abolished the viral spread from inoculated to neighbor cells (see Fig. 6). Once again, the accumulation of PPV CP extracted either under denaturing or native conditions from tissues expressing wild-type and mutant versions of LONG were tested by immunoblot analysis. Remarkably, the levels of CP extracted with SDS buffer were similar in all cases independently of the LONG version and the presence of either PPV P1HCPro or CVYV P1b (Fig. 4). Extraction following a 30-min incubation showed that, only in the presence of HCPro, the CP produced from LONG(ΔPIPO) was as stable as the one expressed from wild-type LONG (Fig. 4). As expected, CP was unstable independently of its source construct when P1b was used to suppress RNA silencing (Fig. 4). Together, these results indicate that P3N-PIPO and cell-to-cell movement are not required for CP stabilization.
FIG 6.
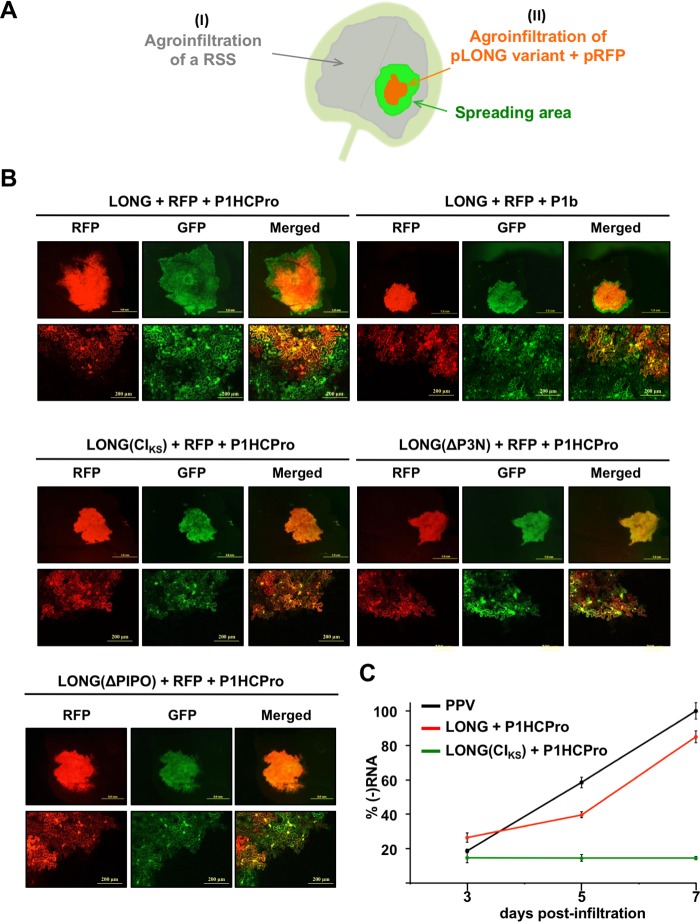
Subviral RNA LONG, which expresses a truncated polyprotein starting at the P3 N terminus, is able to replicate and move cell to cell. (A) Schematic representation of the sequential agroinfiltration assay. An RSS is first expressed in the whole leaf (I), whereas a mix of pLONG and pRFP is later delivered in a patch of the same leaf (II). If the RNA species LONG is able to move locally, then a green fluorescent area is observed under UV around an orange patch (green plus red). (B) Pictures of leaves agroinfiltrated first with pP1HCPro or pP1b and 1 h later with the indicated version of pLONG plus pRFP, taken 5 days after the second infiltration under a stereo (upper) or confocal (bottom) microscope. Merging of the GFP and RFP signals is also shown. (C) Quantification of virus-derived (−)RNA from agroinfiltrated leaves by RT-qPCR. The plot uses average PPV values at 7 days after infiltration equal to 100%. The line shows means ± standard deviations (n = 3 technical replicates). Samples at each time point correspond to a pool of 2 independent plants expressing the indicated viral construct.
FIG 4.
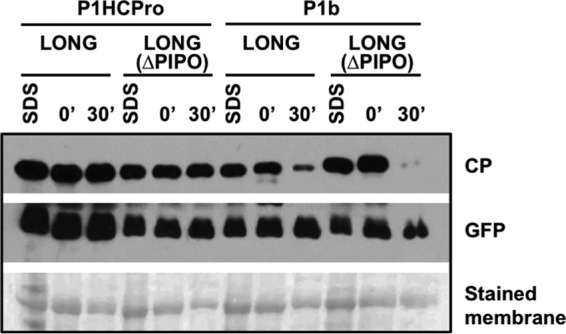
Absence of P3N-PIPO does not affect CP stability. Analysis of the stability of the CP produced by agroinfiltration of N. benthamiana leaves with pLONG or pLONG(ΔPIPO) along with other constructs. Samples were collected at 5 days postagroinfiltration. Extracts prepared in SDS buffer (SDS) and the time, in minutes, elapsed after extraction with phosphate buffer (0′ and 30′) are indicated. Protein accumulation was assessed by immunoblot analysis with polyclonal serum specific for PPV CP and a monoclonal antibody specific for GFP. The membrane stained with Ponceau red showing the RubisCO large subunit was included as a loading control.
Given that CP stability was not recovered by providing P3-6K1 in trans to LONG(ΔP3-6K1) (Fig. 3B), we then considered the possibility that the instability of the CP produced from LONG(ΔP3N) is due to the absence of a cis-acting RNA sequence present in the first 534 nucleotides (nt) of the P3 coding sequence. To test this possibility, the plasmid pLONG(P3≠) was constructed by recoding the first 153 amino acids of P3 with synonymous mutations (Fig. 1 and 5A). Hence, LONG(P3≠) was transiently expressed along PPV P1HCPro or CVYV P1b, and the stability of CP was assessed by in vitro incubation of native extracts followed by immunoblot detection (Fig. 5B). The analysis of extracts prepared under denaturing conditions showed that the accumulation of CP was similar regardless of whether it was expressed from the wild-type LONG or LONG(P3≠) in the presence of either PPV HCPro or CVYV P1b (Fig. 5B). As expected, in the absence of HCPro, the CP produced from LONG and LONG(P3≠) was unstable after native extraction (Fig. 5B). In contrast, no degradation of CP was observed after 30 min of incubation when this viral protein was generated from LONG and LONG(P3≠) in the presence of PPV HCPro. This result rules out the possibility that a specific nucleotide sequence located at the 5′-terminal region of the P3 cistron confers stability to PPV CP in the presence of HCPro.
FIG 5.
Amino acid sequence of P3 rather than a particular nucleotide sequence is required for CP stability and proper assembly of virion-like particles. (A) Recoding of the first 153 amino acids of PPV P3 with synonymous mutations. A fragment of the P3 wild-type coding sequence (black) is aligned against the recoded P3 version (gray). The introduced synonymous mutations are indicated in lowercase. Amino acids (black, one-letter code) are indicated at the top of the nucleotide sequence. Nucleotide and amino acid positions using PPV-NK-GFP (EF569215.1) as reference are indicated. (B) Analysis of the stability of the CP produced by agroinfiltration of N. benthamiana leaves with the indicated pLONG mutants along with other constructs. Samples were collected at 5 days postagroinfiltration. Extracts prepared in SDS buffer (SDS) and the time, in minutes, elapsed after extraction with phosphate buffer (0′ and 30′) are indicated. Protein accumulation was assessed by immunoblot analysis with polyclonal serum specific for PPV CP and a monoclonal antibody specific for GFP. The membrane stained with Ponceau red showing the RubisCO large subunit was included as a loading control. (C) Sucrose gradient centrifugation of extracts of N. benthamiana leaves agroinfiltrated with the indicated constructs. Extracts, prepared in phosphate buffer, were loaded on the gradient immediately after being prepared (0′) or after 60 min of incubation at 25°C (60′). Sixteen fractions were collected from the bottom, and the 10 upper ones were subjected to CP-specific immunoblot analysis. The asterisk indicates that the membrane was overexposed.
The instability of PPV CP produced in the absence of the N-terminal region of P3 is associated with a defect in the assembly of virion-like particles.
Data shown in Fig. 2D associate the instability of PPV CP coexpressed only with HCPro with a defect in the formation of virion-like particles. To verify whether the susceptibility to degradation of PPV CP produced in the absence of a complete P3 is also linked to a deficiency in virion assembly, native extracts of N. benthamiana expressing P1HCPro along with either LONG(ΔP3N) or LONG(P3≠) were subjected to sucrose gradient centrifugation. In order to get additional information, the centrifugation was carried out immediately after tissue homogenization and also after 60 min of incubation at 25°C. The immunoblot analysis of the sucrose gradient fractions showed that the CP produced from LONG(P3≠) is able to form virion-like particles that migrate mainly in fractions 6 and 7 of the gradient, which remain stable after the in vitro incubation (Fig. 5C, left). In contrast, no CP signal was detected at internal fractions of the gradient when the CP was produced from LONG(ΔP3N), not even when the extract was centrifuged immediately after being prepared (Fig. 5C, right). This result indicates that PPV CP produced in the presence of a truncated P3 is unable to be assembled in structures similar to viral particles.
The viral RNA encoded by pLONG is able to replicate and spread locally in the presence of HCPro.
Assembly of PPV CP in large particles is observed not only in the context of the viral infection but also when it is transiently expressed as part of the polyprotein LONG in the presence of HCPro (Fig. 2D); however, this last combination is unable to establish a systemic infection (data not shown). Since the spreading defect of the particles produced by pLONG could be due to the lack of a continuous supply of RSS activity, we decided to assess the replication and propagation ability of viral products derived from LONG when the RSS HCPro is coexpressed both inside and outside the primary delivery area of pLONG. With this aim, first P1HCPro was transiently expressed in the whole N. benthamiana leaves, whereas LONG was later expressed just in a restricted area of the same leaves. The red fluorescent protein (RFP) was expressed along with LONG to visualize the leaf area in which LONG was originally confined (Fig. 6A). Five days after the experiment started, leaves were examined under an epifluorescence microscope with filters suitable to discriminate the GFP and RFP signals expressed from pLONG and pRFP, respectively. In addition, small leaf fragments were excised and examined with a confocal microscope. Figure 6B clearly shows green fluorescence beyond the red fluorescent area, which indicates that LONG is being expressed outside the region where pLONG was originally delivered. A similar result was obtained when pLONG was delivered in leaf already expressing the heterologous RSS P1b (Fig. 6B). This last result indicates that the RNA silencing suppression activity of HCPro, rather than its ability to facilitate the assembly of viral particles, is needed for the movement of pLONG from the delivery area to the neighbor cells.
To verify that the spread of GFP fluorescence outside the primary expression zone is due to the ability of the subviral RNA species expressed from pLONG to replicate in the presence of an RSS, we used the same experimental approach. In this particular case, we expressed a variant of LONG that carries a mutated version of CI (KS91,92AA) that inhibits PPV replication (32) [Fig. 1B, pLONG(CIKS)]. In contrast to what we observed for the wild-type pLONG, the expression of GFP from pLONG(CIKS) strictly overlapped that of RFP (Fig. 6B). Finally, to formally confirm that pLONG replicates, we carried out a strand-specific reverse transcription-quantitative PCR (RT-qPCR) analysis to measure accumulation of the viral negative-strand RNA [(−)RNA] produced during replication at 3, 5, and 7 days after agroinfiltration. As expected, the accumulation of wild-type PPV (−)RNA strongly increased over time due to replication (Fig. 6C). Importantly, accumulation of pLONG-derived (−)RNA was boosted during the time course at a level similar to that of wild-type PPV, whereas (−)RNA from pLONG(CIKS) stayed at a basal level over time. All these results indicate that a subviral RNA species of PPV that includes the 5′ and 3′ NCRs and the coding sequence of the viral polyprotein starting from P3 is able to replicate and spread from cell to cell in the presence of an RSS.
Finally, we took advantage of the system based on two consecutive agroinfiltrations (Fig. 6A) to test the ability of the above-mentioned pLONG(ΔPIPO) to spread locally. The perfect overlapping of GFP and RFP signals in patches expressing LONG(ΔPIPO) indicates that mutations introduced in the GA6 slippage motif at the P3 cistron abolished cell-to-cell movement of pLONG (Fig. 6B). This observation strongly supports the idea that pLONG(ΔPIPO) is not expressing P3N-PIPO.
RNA replication is an essential requirement for CP stability and formation of PPV viral particles.
The above-described results show (i) the strict requirement of HCPro and an intact P3 for the accumulation of stable CP and virion-like particles (Fig. 3 and 5) and (ii) the ability of subviral RNA expressed from pLONG, which is able to produce particles formed by stable CP (Fig. 2), to replicate in the presence of HCPro (Fig. 6). To assess the possibility that these two observations were related, we analyzed the ability of pLONG(ΔP3N) to move cell to cell in the presence of HCPro by sequential coagroinfiltration following the scheme outlined in Fig. 6A. The result is presented in Fig. 6B and shows that the GFP expressed from LONG(ΔP3N), like that from LONG(CIKS) and in contrast with that from LONG, was unable to spread beyond the primary delivery area delimited by RFP. Taking into account previous reports proposing a key role of P3 in viral replication (33–35), this result suggests that the lack of the P3 N terminus prevents replication and supports the hypothesis that this defect impedes the assembly of CP in stable particles. With these results, however, we cannot rule out the possibility that the role of the P3 N terminus on virion assembly is independent of its participation during viral replication.
To have more direct evidence of the relevance of RNA replication for CP stability and virion assembly, we studied the CP produced from LONG(CIKS), which carries an intact P3 but lacks RNA replication ability. Either wild-type LONG or LONG(CIKS) was transiently expressed along with P1HCPro, and CP accumulation and stability were further assessed by immunoblot detection. The extraction with a denaturing buffer showed that accumulation of CP from LONG(CIKS) was only slightly lower than that from LONG, in spite of the replication incompetence of the subviral LONG(CIKS) RNA (Fig. 7A). However, and contrasting with the high stability of the CP produced from LONG, the CP expressed by the replication-incompetent LONG(CIKS) RNA was completely degraded when it was extracted under native conditions and then incubated for 60 min (Fig. 7A).
FIG 7.
Mutations disturbing RNA replication cause instability of CP and prevent its assembly in virion-like particles. (A, C, and D) Analysis of the stability of the CP produced by agroinfiltration (A and C) or agroinoculation (D) of N. benthamiana leaves with the indicated constructs. Samples from infiltrated leaves were collected at 5 days after treatment. Extracts prepared in SDS buffer (SDS) and the time, in minutes, elapsed after extraction with phosphate buffer (0′, 30′, or 60′) are indicated. Protein accumulation was assessed by immunoblot analysis with polyclonal serum specific for PPV CP. Membranes stained with Ponceau red showing the RubisCO large subunit were included as loading controls. (B) Sucrose gradient centrifugation of extracts of N. benthamiana leaves agroinfiltrated with pLONG(CIKS) and pP1HCPro. Extracts, prepared in phosphate buffer, were loaded on the gradient immediately after being prepared (0′) or after 60 min of incubation at 25°C (60′). Sixteen fractions were collected from the bottom, and the 10 upper ones were subjected to CP-specific immunoblot analysis. The asterisk indicates that the membrane was overexposed.
To investigate whether the instability of the CP produced from LONG(CIKS) is associated with a defect in the assembly of virion-like particles, the above-mentioned native extracts were subjected to sucrose gradient centrifugation immediately after protein extraction and after 60 min of incubation at 25°C. In both cases, CP did not migrate in gradient fractions 6 to 8 (Fig. 7B), which are the fractions having LONG-derived virion-like particles (Fig. 2E).
Although the result with LONG(CIKS) suggests a link between RNA replication and assembly of viral particles, it is not possible to discard that the mutation KS91,92AA in CI affects a putative role of this viral protein in the formation of virions independently of its known function during RNA replication. To rule out this possibility, we transiently expressed P1HCPro in N. benthamiana along with a LONG variant that carries a mutation in the RNA replicase NIb [Fig. 1C, pLONG(NIbΔGDD)]. This mutation deleted the GDD triad, a highly conserved motif that is essential for the RNA polymerase activity of NIb and, consequently, for the replication of the viral RNA (36). Total CP accumulation from LONG(NIbΔGDD) as assessed by immunoblot analysis of denatured extracts was similar to that of the wild-type LONG, which was used as a control (Fig. 7C), showing again that the replication competence of the RNA encoded by diverse pLONG variants does not have a noticeable effect over global CP accumulation. However, in contrast to the high stability of the CP expressed from LONG, the CP that was derived from LONG(NIbΔGDD), like that produced from LONG(CIKS) (Fig. 7A and B), was degraded when native extracts were incubated for 30 min at 25°C (Fig. 7C).
To verify whether the instability of CP associated with the RNA replication deficiency also occurred when it was expressed from a complete viral genome, the ΔGDD mutation was introduced in the full-length cDNA clone pPPV to create pPPV-NIbΔGDD (Fig. 1A), and the accumulation and stability of CP in N. benthamiana leaves agroinoculated with these two viruses were assessed by immunoblot assay. The global CP accumulation estimated by extraction with a denaturing buffer was similar in leaves inoculated with either of the two viruses (Fig. 7D). However, in native extracts the strong degradation of the CP produced by PPV-NIbΔGDD after 30 min of incubation at 25°C contrasted with the high stability of the CP expressed by the wild-type PPV (Fig. 7D). All of these results strengthen the hypothesis that the replication of the viral RNA is essential for correct formation of viral particles and CP stability.
PPV CP produced by a nonreplicating RNA is assembled in stable particles as long as it is expressed in the presence of a replicating viral RNA.
The above-described results demonstrate that RNA replication is a requisite for CP assembly in virion-like particles but do not reveal whether the CP necessarily has to be produced by the replicating RNA. To unravel this issue, we designed an experiment in which a wild-type CP was expressed from a nonreplicating RNA together with a replication-competent RNA that expresses a CP mutant unable to self-interact and form viral particles (37, 38). To do that, we first introduced the D263K or RQ220,221DV mutation in the CP of LONG to create pLONG(CPD) and pLONG-(CPRQ) (Fig. 1B). Native and denatured extracts were prepared from N. benthamiana tissues transiently expressing both wild-type and mutant LONG variants together with P1HCPro, and they were further analyzed by immunoblot assay. CPD and CPRQ showed lower accumulation levels than the wild-type CP, and as expected from their presumed inability to self-interact, they were degraded very quickly (Fig. 8A).
FIG 8.
Viral RNA able to replicate promotes assembly in stable virion-like particles of CP expressed from a nonreplicating RNA. (A and B) Analysis of the stability of the CP (wild type or with the D263K or RQ220,221DV mutation) produced by agroinfiltration of N. benthamiana leaves with the indicated constructs. Samples were collected at 5 days postagroinfiltration. Extracts prepared in SDS buffer (SDS) and the time, in minutes, elapsed after extraction with phosphate buffer (0′, 10′, 20′, or 30′) are indicated. The protocol of extraction in the experiment shown in panel A was slightly modified to handle the extreme instability of the mutated CP variants (see Materials and Methods). Protein accumulation was assessed by immunoblot analysis with polyclonal serum specific for PPV CP and a monoclonal antibody specific for GFP. Membranes stained with Ponceau red showing the RubisCO large subunit were included as loading controls. (C) Sucrose gradient centrifugation of extracts of N. benthamiana leaves agroinfiltrated with the indicated constructs. Extracts, prepared in phosphate buffer, were loaded on the gradient immediately after being prepared (0′) or after 60 min of incubation at 25°C (60′). Sixteen fractions were collected from the bottom, and the 10 upper ones were subjected to CP-specific immunoblot analysis. The asterisk indicates that the membrane was overexposed.
The CP produced from the nonreplicating RNA encoded by pGFP-CP accumulates to high levels, but it is highly unstable and unable to form virion-like particles (Fig. 2). To assess whether the replication-competent RNA encoded by pLONG(CPD) can help the pGFP-CP-derived CP to be assembled in stable particles, we transiently expressed these constructs in the presence of P1HCPro, and CP accumulation and stability were determined by immunoblot analysis of denatured and native extracts. The denaturing extraction revealed high levels of CP accumulation in all cases (Fig. 8B). The CP stability test with native extracts showed that, as expected, the CP produced from GFP-CP (in the presence of NIaPro to promote GFP/CP separation) was rapidly degraded and was undetectable after 30 min of incubation at 25°C (Fig. 8B). In contrast, when GFP-CP was expressed along with LONG(CPD), which by itself produces an extremely unstable CP (Fig. 8A), we observed that a large fraction of CP remained unaltered after 30 min of incubation (Fig. 8B).
Finally, to verify that the CP stabilization by the coexpression of two unstable CPs [GFP-CP and LONG(CPD)] was associated with the assembly of virion-like particles, the above-mentioned native extracts of N. benthamiana leaves were subjected to sucrose gradient centrifugation immediately after extraction and after 60 min of incubation at 25°C (Fig. 8C). As expected from the above-described results, in the gradient centrifuged after 1 h of incubation, no CP signal from GFP-CP was detected in the presence of P1HCPro and NIaPro. On the contrary, the CP from GFP-CP and LONG(CPD) stably assembled in structures that migrated in internal fractions of the gradient equivalent to that of the virion-like particles produced by LONG (Fig. 2). Although the employed technique does not discriminate between the CP produced from GFP-CP and that from LONG(CPD), as the mutation D263K interferes with CP oligomerization, these results support the idea that the viral particles detected in Fig. 8C are formed by the CP produced from GFP-CP. Moreover, the observed sedimentation profile suggests that this protein is coating a long RNA, such as that produced by pLONG(CPD).
DISCUSSION
It has long been known that the CP from potyviruses can be assembled in virion-like particles in vitro and in heterologous systems in the absence of other viral proteins (reference 15 and references therein). However, virion assembly in planta appears to be more complex. The CP expressed from a recombinant PPV that carries a heterologous RSS instead of HCPro is unstable, but it can be assembled into particles mimicking wild-type virions in morphology and sedimentation behavior (20, 39). We have observed now that the CP expressed from the PPV subviral species LONG, which produces a truncated polyprotein starting at the N terminus of P3, is assembled in stable virion-like particles when it is trans-complemented with PPV HCPro. On the contrary, expression of CP and HCPro alone fails to produce stable CP and virion-like particles (Fig. 2), which led us to conclude that the LONG species contributes viral factors that collaborate with CP and HCPro in PPV virion assembly.
In general, virion formation depends on specific RNA motifs, known as origin of assembly sequences (OAS) or packaging signals, which serve as nucleation points to start the genomic RNA coating (40, 41). No OAS has been identified in potyviruses so far. Wu and Shaw (19) reported that virion assembly of the potyvirus Tobacco vein mottling virus starts near the 5′ end of the genomic RNA. The efficient virion assembly of the CP from LONG(P3≠), which carries a drastic modification in the RNA sequence coding for the first 153 amino acids of P3 (Fig. 5), clearly indicates that the defect in virion assembly of the CP produced from LONG(ΔP3N), which lacks this sequence, is not due to disturbance of an internal OAS. Thus, if a sequence at the 5′ terminal region of the PPV genome were used as an OAS, it would be entirely included in the 145 nucleotides that constitute its 5′ NCR, which is part of all LONG constructs.
Deletion of the P1 cistron has been shown to render potyviruses viable (27, 42, 43). We now find that the subviral RNA species LONG is able to replicate even in the absence of P1 and HCPro, as long as an RSS is provided in trans (Fig. 6). Taking into account that big deletions in the PPV 5′ NCR also preserve its replication ability (44), it appears that, unlike other viruses of the Picorna-like supergroup (45, 46), RNA motifs required for RNA replication are very scarce at the 5′ NCR of the potyviral genome. This contrasts with the 3′ NCR, where a large cis-acting element that even includes coding sequences of the CP cistron is required for RNA replication (47, 48).
Different mutations preventing replication of the LONG RNA, which include deletion of the N terminus of P3 or of the GDD motif in the NIb replicase, or the KS91,92AA mutation affecting the Walker A motif of the RNA helicase CI, preclude the assembly of PPV CP in large and stable viral particles (Fig. 5, 6, and 7), evidencing that RNA replication is required for virion formation. The previously shown in vivo interaction between NIb and CP from PPV (49) argues in favor of a connection between replication and virion assembly. Although viral RNA incorporation into virions is independent of replication in the coronavirus Transmissible gastroenteritis virus (50), coupling between replication and packaging is not unusual in animal (51–54) and plant (55) spherical viruses, where direct interactions between their corresponding viral capsid proteins and replicases were also detected (56, 57). Hence, our results show that viral RNA replication is also required for the assembly of filamentous virions in a member of the family Potyviridae, the largest group of plant RNA viruses.
The use of specific OAS serves to avoid wasting viral resources on coating host RNAs and defective viral genomes, and packaging/replication coupling can help in this task. For instance, it has been observed that encapsidation specificity is reduced in the absence of Brome mosaic virus (BMV) replication, so that viral icosahedral particles can then trap cellular RNAs (55). PPV CP might coat cellular RNAs in the absence of viral replication; however, the absence of CP-containing stable particles from nonreplicating RNAs (Fig. 2, 5, 7, and 8) argues against this possibility and supports the idea that the link between virion assembly and RNA replication is much stronger in PPV than in the case of BMV. An important difference between BMV and PPV deserves to be highlighted here. Efficient packaging of BMV RNA requires the translation of CP from a replication-proficient RNA (55, 58). This seems not to be the case for PPV, as it forms stable virions even when CP is produced from a nonreplicating RNA in the presence of a replicating variant (Fig. 8). Therefore, we hypothesize that viral (and/or host) factors in replication complexes, but not CP, determine which RNA molecule will be packaged.
Potyviral RNA replication has been the subject of multiple cell biology studies that have shed some light on this matter. Based on data from several laboratories working on different potyviral infections, Wei et al. (59) proposed a comprehensive model in which membrane vesicles produced at endoplasmic reticulum (ER) exit sites (60, 61) are targeted to chloroplasts to form factories for viral RNA replication (62). Aggregation of these chloroplasts together with a variety of other elements would form a large perinuclear structure where RNA replication takes place at later times of infection (63). Much less is known about potyviral RNA translation and encapsidation. The fact that most of the discrete membrane vesicles contain viral proteins originated from a single viral genome, as well as host translation factors, led Cotton et al. (64) to suggest that not only RNA replication but also translation occurs in those vesicles. However, the combination of the monophyletic origin of viral proteins in these vesicles, the ability of CP to trans-encapsidate other replicating RNAs (Fig. 8), and the scarce enrichment of CP in potyviral replication vesicles (65) makes it unlikely that such vesicles are the place for virion assembly. We suggest that the large perinuclear structure is the site where the viral RNA synthesized in vesicles is then used for coating with the CP produced, from cellular (GFP-CP) or viral (PPV and LONG) RNAs, in the ER that this large body contains.
However, it is still an open question why the CP synthesized from viral RNAs in replication vesicles forms virus-like particles, whereas the CP produced by RNAs transported from the nucleus does not form stable virions. Current data do not allow discrimination of whether the RNA produced by the cellular RNA polymerase has a peculiarity that prevents its encapsidation or, instead, that the replication-derived viral RNA has certain features promoting its CP coating. An attractive and feasible hypothesis, which is in line with the second option, as well as with the idea that the RNA 5′ end plays a role during encapsidation (19), involves the viral VPg. As VPg is covalently linked to the 5′ end of the RNA synthesized by the viral polymerase but not to that of the RNA produced by host polymerases, it might play an essential role in priming encapsidation. Moreover, it is seductive to suggest that HCPro, which colocalizes with VPg at one end of the viral particle (66) and contributes to proper virion formation (20), participates in the priming.
Genome encapsidation is indeed an important step of the viral infection cycle, and it needs to be thoroughly regulated for multiple reasons. Premature encapsidation can interfere with crucial roles of the viral RNA, namely, translation and replication, whereas unspecific RNA coating wastes viral resources. It is clear then that these processes have to be coordinated in time and space to ensure a productive infection, and it was proposed that CP plays a relevant role in this coordination (67, 68). Indeed, the tight control of the amount of diverse CP forms at each step of the infection process supports this assumption. Such control involves posttranslational modifications like ubiquitination, phosphorylation, and O-GlcNAcylation (69–71), as well as functional interactions with the chaperone HSP70, its cochaperone CPIP, and the E3 ubiquitin ligase CHIP (69, 72). Our data shed light on this intricate scenario by revealing that a link among RNA replication, CP stability, and formation of stable virion-like particles helps to regulate genome encapsidation. It is clear, however, that the data are still very scarce, so that additional works assessing the sites of virion assembly, the kinetics of production and degradation of free and assembled CP, the posttranslational modifications associated with different forms of CP at different times of infection, etc., are required to have a better picture of the processes governing encapsidation/desencapsidation in viruses of the family Potyviridae.
MATERIALS AND METHODS
Plants.
Nicotiana benthamiana plants were cultured in a greenhouse at 18 to 23°C with a 16-h light/8-h dark photoperiod with supplementary illumination.
Plasmids.
A schematic representation of all plasmids used in this study is shown in Fig. 1. The following plasmids have been previously described: pPPV (named pBIN-ICPPVNKGFP-NOSt in reference 73), pLONG (named pLONG-GFP in reference 20), pPPV-P1b (named P1P1b in reference 27), pP1HCPro (named pMDC32-P1HCPro in reference 20), and pNIaPro (pMDC32-NIaPro in reference 28). The plasmid pP3-6K1 uses pMDC32 (74) as a backbone and carries the P3-6K1 coding sequence from PPV (M. Calvo and J. A. García, unpublished results).
pLONG(CIKS) was created by triple ligation of a 4,052-nt DNA fragment from pICPPVGFP-KS91,92AA (32) digested with DraIII/XhoI and two fragments of 16,267 nt and 1,555 nt obtained by digestion of pLONG with ScaI/XhoI and ScaI/DraIII, respectively.
pPPV-NIbΔGDD, pLONG(NIbΔGDD), pLONG(CPD), pLONG(CPRQ), pLONG(ΔP3N), pLONG(ΔP3), pLONG(ΔP3-6K1), pLONG(ΔP3-CI), pLONG(ΔCI-NIb), pNIb-GFP-CP, and pGFP-CP were constructed by an overlapping PCR approach followed by digestion/ligation. The same strategy was followed to build pLONG(P3≠), but in this particular case a synthetic DNA fragment (GeneArt, Invitrogen), which encodes the first 153 amino acids of PPV P3 and carries 144 synonymous mutations (Fig. 5A), was used as one of the PCR templates.
Oligonucleotides used as PCR primers in the different cloning events, the primer combinations used in each PCR, as well as those restriction enzymes used to finally build each plasmid are available upon request. The accuracy of each construct was verified by restriction analysis as well as by Sanger sequencing (Macrogen Spain) of all fragments deriving from PCR amplification.
Transient expression and virus inoculation by infiltration with Agrobacterium tumefaciens.
A. tumefaciens C58C1 cells transformed with the plasmid of interest were cultured at 28°C for 48 h in LB medium with appropriate antibiotics. After transfer to fresh medium, bacteria were further incubated for 16 h. An aliquot of this culture was used to purify plasmid and verify its correctness. Another bacterial aliquot was concentrated by centrifugation at low speed and resuspension of the pellet in a buffer containing 10 mM MgSO4, 10 mM 2-(N-morpholino)ethanesulfonic acid, 150 μM acetosyringone to an optical density at 600 nm of 0.5. The suspended bacteria were incubated at room temperature for 3 h to be further infiltrated in the intercellular spaces of 2 leaves of N. benthamiana plants, grown to the 5/6-leaf stage, by pressing with a 1-ml syringe on the leaf underside.
Analysis of protein stability.
Samples of leaf patches infiltrated with agrobacterium were collected at 5 days after infiltration, ground to fine powder under liquid nitrogen, and stored at −80°C until use. For preparation of protein samples under denaturing conditions, the powder was thawed and homogenized in denaturation/loading buffer (125 mM Tris-HCl, pH 7.5, 2% SDS, 6 M urea, 4% glycerol, 5% β-mercaptoethanol) (2 ml/mg). For extraction under native conditions, 5 mM sodium phosphate, pH 7.5 (2 ml/mg), was used as homogenization buffer. Immediately after extraction or after the indicated times of incubation at 25°C, samples were centrifuged to separate cell debris. After adding concentrated denaturation/loading buffer and boiling, the supernatants were resolved on SDS-PAGE (12% acrylamide). When the presence of CP in cell debris was analyzed, those pellets from the aforementioned centrifugation step were resuspended in denaturation/loading buffer, boiled, and centrifuged, and supernatants were then resolved on SDS-PAGE. When highly unstable CP (CPD263K and CPRQ220,221DV) was expressed in N. benthamiana leaves, incubation was directly stopped by adding concentrated denaturation/loading buffer even before centrifugation.
The electrophoretically resolved proteins were electroblotted to nitrocellulose membranes and subjected to Western blot analysis using anti-CP rabbit serum or a mix of two anti-GFP monoclonal antibodies (Roche Applied Science) as primary detection reagents and horseradish peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch) or sheep anti-mouse IgG (GE Healthcare Life Science) as the secondary antibody, respectively. The immunostained proteins were visualized by enhanced chemiluminescence detection with a LifeABlot kit (Euroclone) in photographic films.
Ultracentrifugation in sucrose gradients.
Native extracts of agroinfiltrated leaves (200 μl), prepared as indicated above, were subjected to centrifugation in continuous sucrose gradients (10 to 40%) of 4.8 ml in a buffer containing 100 mM sodium borate, 10 mM EDTA, pH 8.2. The gradients were centrifuged at 4°C for 20 min at 237,020 × g in an SW55 rotor in a Beckman Coulter Optima L-100 XP ultracentrifuge. Fractions of approximately 300 μl were collected by gravity with a capillary from the bottom of the tube, and aliquots of the fractions were subjected to Western blot analysis as explained above.
Electron microscopy.
The presence of virion-like particles in the indicated fractions from the ultracentrifugation sucrose gradient separation was determined by immunosorbent electron microscopy as previously described (20).
Fluorescence imaging.
GFP and RFP fluorescence of agroinfiltrated tissue was visualized with a Leica MZFLIII epifluorescence microscope and a Leica TCS-SP5 confocal multispectral system. In the epifluorescence microscope, GFP fluorescence was detected with excitation and barrier filters at 470/40 nm and 525/50 nm, whereas RFP visualization was carried out with excitation and barrier filters of 546/10 nm and 570 nm. Images were acquired with an Olympus DP70 digital camera coupled to the microscope.
The confocal microscope was controlled by the program LAS AF v2.7.3 and used a water immersion objective lens (63× objective, 1.20 numeric aperture). Excitation and emission conditions of 488 nm and 515 to 568 nm, and 561 nm and 581 to 649 nm, were used to detect GFP and RFP fluorescence, respectively. Images were processed with the program ImageJ/Fiji v.2.0.0-rc-59/1.51j.
RT-qPCR analysis.
Total RNA extraction from agroinfiltrated patches, cDNA synthesis using a tagged strand-specific primer, and quantitative PCR were carried out by following a previously described method (43).
ACKNOWLEDGMENTS
We thank Beatriz García for technical assistance. We also thank the Electron Microscopy and the Advance Light Microscopy facilities at CNB-CSIC.
This work was supported by the following grants from the Spanish MINECO: BIO2013-49053-R, BIO2016-80572-R (AEI-FEDER), and Plant KBBE PCIN-2013-056 to J.A.G. and BIO2015-73900-JIN (AEI-FEDER) to A.V.
REFERENCES
- 1.Valli A, García JA, López-Moya JJ. 14 May 2015. Potyviridae. In eLS. John Wiley & Sons Ltd, Chichester, United Kingdom. doi: 10.1002/9780470015902.a0000755.pub3. [DOI] [Google Scholar]
- 2.Wylie SJ, Adams M, Chalam C, Kreuze J, Lopez-Moya JJ, Ohshima K, Praveen S, Rabenstein F, Stenger D, Wang A, Zerbini FM, ICTV Report Consortium . 2017. ICTV virus taxonomy profile: Potyviridae. J Gen Virol 98:352–354. doi: 10.1099/jgv.0.000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Revers F, García JA. 2015. Molecular biology of potyviruses. Adv Virus Res 92:101–199. doi: 10.1016/bs.aivir.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Hagiwara-Komoda Y, Choi SH, Sato M, Atsumi G, Abe J, Fukuda J, Honjo MN, Nagano AJ, Komoda K, Nakahara KS, Uyeda I, Naito S. 2016. Truncated yet functional viral protein produced via RNA polymerase slippage implies underestimated coding capacity of RNA viruses. Sci Rep 6:21411. doi: 10.1038/srep21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung BYW, Miller WA, Atkins JF, Firth AE. 2008. An overlapping essential gene in the Potyviridae. Proc Natl Acad Sci U S A 105:5897–5902. doi: 10.1073/pnas.0800468105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodamilans B, Valli A, Mingot A, San León D, Baulcombe D, López-Moya JJ, García JA. 2015. RNA polymerase slippage as a mechanism for the production of frameshift gene products in plant viruses of the Potyviridae family. J Virol 89:6965–6967. doi: 10.1128/JVI.00337-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olspert A, Chung BY, Atkins JF, Carr JP, Firth AE. 2015. Transcriptional slippage in the positive-sense RNA virus family Potyviridae. EMBO Rep 16:995–1004. doi: 10.15252/embr.201540509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mingot A, Valli A, Rodamilans B, San León D, Baulcombe DC, García JA, López-Moya JJ. 2016. The P1N-PISPO trans-frame gene of sweet potato feathery mottle potyvirus is produced during virus infection and functions as an RNA silencing suppressor. J Virol 90:3543–3557. doi: 10.1128/JVI.02360-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamora M, Mendez-Lopez E, Agirrezabala X, Cuesta R, Lavin JL, Sanchez-Pina MA, Aranda MA, Valle M. 2017. Potyvirus virion structure shows conserved protein fold and RNA binding site in ssRNA viruses. Sci Adv 3:eaao2182. doi: 10.1126/sciadv.aao2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald JG, Beveridge TJ, Bancroft JB. 1976. Self-assembly of protein from a flexuous virus. Virology 69:327–331. doi: 10.1016/0042-6822(76)90220-8. [DOI] [PubMed] [Google Scholar]
- 11.Jagadish MN, Ward CW, Gough KH, Tulloch PA, Whittaker LA, Shukla DD. 1991. Expression of potyvirus coat protein in Escherichia coli and yeast and its assembly into virus-like particles. J Gen Virol 72:1543–1550. doi: 10.1099/0022-1317-72-7-1543. [DOI] [PubMed] [Google Scholar]
- 12.Edwards SJ, Hayden MB, Hamilton RC, Haynes JA, Nisbet IT, Jagadish MN. 1994. High level production of potyvirus-like particles in insect cells infected with recombinant baculovirus. Arch Virol 136:375–380. doi: 10.1007/BF01321065. [DOI] [PubMed] [Google Scholar]
- 13.Hammond JM, Sproat KW, Wise TG, Hyatt AD, Jagadish MN, Coupar BE. 1998. Expression of the potyvirus coat protein mediated by recombinant vaccinia virus and assembly of potyvirus-like particles in mammalian cells. Arch Virol 143:1433–1439. doi: 10.1007/s007050050387. [DOI] [PubMed] [Google Scholar]
- 14.Shukla DD, Strike PM, Tracy SL, Gough KH, Ward CW. 1988. The N and C termini of the coat proteins of potyviruses are surface-located and the N-terminus contains the major virus-specific epitopes. J Gen Virol 69:1497–1508. doi: 10.1099/0022-1317-69-7-1497. [DOI] [Google Scholar]
- 15.Anindya R, Savithri HS. 2003. Surface-exposed amino- and carboxy-terminal residues are crucial for the initiation of assembly in Pepper vein banding virus: a flexuous rod-shaped virus. Virology 316:325–336. doi: 10.1016/S0042-6822(03)00593-2. [DOI] [PubMed] [Google Scholar]
- 16.Seo JK, Vo Phan MS, Kang SH, Choi HS, Kim KH. 2013. The charged residues in the surface-exposed C terminus of the Soybean mosaic virus coat protein are critical for cell-to-cell movement. Virology 446:95–101. doi: 10.1016/j.virol.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 17.Joseph J, Savithri HS. 1999. Determination of 3′-terminal nucleotide sequence of pepper vein banding virus RNA and expression of its coat protein in Escherichia coli. Arch Virol 144:1679–1687. doi: 10.1007/s007050050696. [DOI] [PubMed] [Google Scholar]
- 18.Voloudakis AE, Malpica CA, Aleman-Verdaguer ME, Stark DM, Fauquet CM, Beachy RN. 2004. Structural characterization of Tobacco etch virus coat protein mutants. Arch Virol 149:699–712. doi: 10.1007/s00705-003-0247-x. [DOI] [PubMed] [Google Scholar]
- 19.Wu XJ, Shaw JG. 1998. Evidence that assembly of a potyvirus begins near the 5′ terminus of the viral RNA. J Gen Virol 79:1525–1529. doi: 10.1099/0022-1317-79-6-1525. [DOI] [PubMed] [Google Scholar]
- 20.Valli A, Gallo A, Calvo M, Pérez JJ, García JA. 2014. A novel role of the potyviral helper component proteinase contributes to enhance the yield of viral particles. J Virol 88:9808–9818. doi: 10.1128/JVI.01010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atreya CD, Atreya PL, Thornbury DW, Pirone TP. 1992. Site-directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation, and symptom expression in infected tobacco plants. Virology 191:106–111. doi: 10.1016/0042-6822(92)90171-K. [DOI] [PubMed] [Google Scholar]
- 22.Thornbury DW, Hellmann GM, Rhoads RE, Pirone TP. 1985. Purification and characterization of potyvirus helper component. Virology 144:260–267. doi: 10.1016/0042-6822(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 23.Valli AA, Gallo A, Rodamilans B, López-Moya JJ, García JA. 2018. The HCPro from the Potyviridae family: an enviable multitasking helper component that every virus would like to have. Mol Plant Pathol 19:744–763. doi: 10.1111/mpp.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Mallory AC, Smith TH, Vance VB. 1998. A viral suppressor of gene silencing in plants. Proc Natl Acad Sci U S A 95:13079–13084. doi: 10.1073/pnas.95.22.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasschau KD, Carrington JC. 1998. A counterdefensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell 95:461–470. doi: 10.1016/S0092-8674(00)81614-1. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Ruiz H, Takeda A, Chapman EJ, Sullivan CM, Fahlgren N, Brempelis KJ, Carrington JC. 2010. Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defense and small interfering RNA biogenesis during Turnip mosaic virus infection. Plant Cell 22:481–496. doi: 10.1105/tpc.109.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbonell A, Dujovny G, García JA, Valli A. 2012. The Cucumber vein yellowing virus silencing suppressor P1b can functionally replace HCPro in Plum pox virus infection in a host-specific manner. Mol Plant Microbe Interact 25:151–164. doi: 10.1094/MPMI-08-11-0216. [DOI] [PubMed] [Google Scholar]
- 28.Maliogka VI, Calvo M, Carbonell A, García JA, Valli A. 2012. Heterologous RNA-silencing suppressors from both plant- and animal-infecting viruses support plum pox virus infection. J Gen Virol 93:1601–1611. doi: 10.1099/vir.0.042168-0. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov KI, Mäkinen K. 2012. Coat proteins, host factors and plant viral replication. Curr Opin Virol 2:712–718. doi: 10.1016/j.coviro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Wei T, Zhang C, Hong J, Xiong R, Kasschau KD, Zhou X, Carrington JC, Wang A. 2010. Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N-PIPO. PLoS Pathog 6:e1000962. doi: 10.1371/journal.ppat.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayapalani P, Maeshima M, Nagasaki-Takekuchi N, Miller WA. 2012. Interaction of the trans-frame potyvirus protein P3N-PIPO with host protein PCaP1 facilitates potyvirus movement. PLoS Pathog 8:e1002639. doi: 10.1371/journal.ppat.1002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gómez de Cedrón M, Osaba L, López L, García JA. 2006. Genetic analysis of the function of the plum pox virus CI RNA helicase in virus movement. Virus Res 116:136–145. doi: 10.1016/j.virusres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Klein PG, Klein RR, Rodríguez-Cerezo E, Hunt A, Shaw JG. 1994. Mutational analysis of the tobacco vein mottling virus genome. Virology 204:759–769. doi: 10.1006/viro.1994.1591. [DOI] [PubMed] [Google Scholar]
- 34.Cui XY, Wei TY, Chowda-Reddy RV, Sun GY, Wang AM. 2010. The Tobacco etch virus P3 protein forms mobile inclusions via the early secretory pathway and traffics along actin microfilaments. Virology 397:56–63. doi: 10.1016/j.virol.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Cui X, Yaghmaiean H, Wu G, Wu X, Chen X, Thorn G, Wang A. 2017. The C-terminal region of the Turnip mosaic virus P3 protein is essential for viral infection via targeting P3 to the viral replication complex. Virology 510:147–155. doi: 10.1016/j.virol.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Deng P, Wu Z, Wang A. 2015. The multifunctional protein CI of potyviruses plays interlinked and distinct roles in viral genome replication and intercellular movement. Virol J 12:141. doi: 10.1186/s12985-015-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolja VV, Haldeman R, Robertson NL, Dougherty WG, Carrington JC. 1994. Distinct functions of capsid protein in assembly and movement of tobacco etch potyvirus in plants. EMBO J 13:1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varrelmann M, Maiss E. 2000. Mutations in the coat protein gene of plum pox virus suppress particle assembly, heterologous encapsidation and complementation in transgenic plants of Nicotiana benthamiana. J Gen Virol 81:567–576. doi: 10.1099/0022-1317-81-3-567. [DOI] [PubMed] [Google Scholar]
- 39.Gallo A. 2017. Conexiones entre la replicación del RNA, su empaquetamiento y la proteína HC en la infección de un potyvirus. Ph.D. thesis Universidad Autónoma de Madrid, Madrid, Spain. [Google Scholar]
- 40.Rao AL. 2006. Genome packaging by spherical plant RNA viruses. Annu Rev Phytopathol 44:61–87. doi: 10.1146/annurev.phyto.44.070505.143334. [DOI] [PubMed] [Google Scholar]
- 41.Stockley PG, Twarock R, Bakker SE, Barker AM, Borodavka A, Dykeman E, Ford RJ, Pearson AR, Phillips SE, Ranson NA, Tuma R. 2013. Packaging signals in single-stranded RNA viruses: nature's alternative to a purely electrostatic assembly mechanism. J Biol Phys 39:277–287. doi: 10.1007/s10867-013-9313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verchot J, Carrington JC. 1995. Evidence that the potyvirus P1 proteinase functions in trans as an accessory factor for genome amplification. J Virol 69:3668–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasin F, Simón-Mateo C, García JA. 2014. The hypervariable amino-terminus of P1 protease modulates potyviral replication and host defense responses. PLoS Pathog 10:e1003985. doi: 10.1371/journal.ppat.1003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simón-Buela L, Guo HS, García JA. 1997. Long sequences in the 5′ noncoding region of plum pox virus are not necessary for viral infectivity but contribute to viral competitiveness and pathogenesis. Virology 233:157–162. doi: 10.1006/viro.1997.8574. [DOI] [PubMed] [Google Scholar]
- 45.van Bokhoven H, Le Gall O, Kasteel D, Verver J, Wellink J, van Kammen A. 1993. cis- and trans-acting elements in cowpea mosaic virus RNA replication. Virology 195:377–386. doi: 10.1006/viro.1993.1387. [DOI] [PubMed] [Google Scholar]
- 46.Barton DJ, O'Donnell BJ, Flanegan JB. 2001. 5′ Cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO J 20:1439–1448. doi: 10.1093/emboj/20.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahajan S, Dolja VV, Carrington JC. 1996. Roles of the sequence encoding tobacco etch virus capsid protein in genome amplification: requirements for the translation process and a cis-active element. J Virol 70:4370–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haldeman-Cahill R, Daròs JA, Carrington JC. 1998. Secondary structures in the capsid protein coding sequence and 3′ nontranslated region involved in amplification of the tobacco etch virus genome. J Virol 72:4072–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zilian E, Maiss E. 2011. Detection of plum pox potyviral protein-protein interactions in planta using an optimized mRFP-based bimolecular fluorescence complementation system. J Gen Virol 92:2711–2723. doi: 10.1099/vir.0.033811-0. [DOI] [PubMed] [Google Scholar]
- 50.Morales L, Mateos-Gomez PA, Capiscol C, del Palacio L, Enjuanes L, Sola I. 2013. Transmissible gastroenteritis coronavirus genome packaging signal is located at the 5′ end of the genome and promotes viral RNA incorporation into virions in a replication-independent process. J Virol 87:11579–11590. doi: 10.1128/JVI.01836-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khromykh AA, Varnavski AN, Sedlak PL, Westaway EG. 2001. Coupling between replication and packaging of flavivirus RNA: evidence derived from the use of DNA-based full-length cDNA clones of Kunjin virus. J Virol 75:4633–4640. doi: 10.1128/JVI.75.10.4633-4640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nugent CI, Johnson KL, Sarnow P, Kirkegaard K. 1999. Functional coupling between replication and packaging of poliovirus replicon RNA. J Virol 73:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venter PA, Krishna NK, Schneemann A. 2005. Capsid protein synthesis from replicating RNA directs specific packaging of the genome of a multipartite, positive-strand RNA virus. J Virol 79:6239–6248. doi: 10.1128/JVI.79.10.6239-6248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volkova E, Gorchakov R, Frolov I. 2006. The efficient packaging of Venezuelan equine encephalitis virus-specific RNAs into viral particles is determined by nsP1-3 synthesis. Virology 344:315–327. doi: 10.1016/j.virol.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Annamalai P, Rao AL. 2006. Packaging of brome mosaic virus subgenomic RNA is functionally coupled to replication-dependent transcription and translation of coat protein. J Virol 80:10096–10108. doi: 10.1128/JVI.01186-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seo JK, Kwon SJ, Rao AL. 2012. A physical interaction between viral replicase and capsid protein is required for genome-packaging specificity in an RNA virus. J Virol 86:6210–6221. doi: 10.1128/JVI.07184-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaturvedi S, Rao ALN.. 2014. Live cell imaging of interactions between replicase and capsid protein of Brome mosaic virus using bimolecular fluorescence complementation: implications for replication and genome packaging. Virology 464-465:67–75. [DOI] [PubMed] [Google Scholar]
- 58.Annamalai P, Rofail F, Demason DA, Rao AL. 2008. Replication-coupled packaging mechanism in positive-strand RNA viruses: synchronized coexpression of functional multigenome RNA components of an animal and a plant virus in Nicotiana benthamiana cells by agroinfiltration. J Virol 82:1484–1495. doi: 10.1128/JVI.01540-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei T, Zhang C, Hou X, Sanfacon H, Wang A. 2013. The SNARE protein Syp71 is essential for turnip mosaic virus infection by mediating fusion of virus-induced vesicles with chloroplasts. PLoS Pathog 9:e1003378. doi: 10.1371/journal.ppat.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei T, Wang A. 2008. Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J Virol 82:12252–12264. doi: 10.1128/JVI.01329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lerich A, Langhans M, Sturm S, Robinson DG. 2011. Is the 6 kDa tobacco etch viral protein a bona fide ERES marker? J Exp Bot 62:5013–5023. doi: 10.1093/jxb/err200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei TY, Huang TS, McNeil J, Laliberte JF, Hong J, Nelson RS, Wang AM. 2010. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J Virol 84:799–809. doi: 10.1128/JVI.01824-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grangeon R, Agbeci M, Chen J, Grondin G, Zheng H, Laliberté JF. 2012. Impact on the endoplasmic reticulum and Golgi apparatus of turnip mosaic virus infection. J Virol 86:9255–9265. doi: 10.1128/JVI.01146-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cotton S, Grangeon R, Thivierge K, Mathieu I, Ide C, Wei TY, Wang AM, Laliberté JF. 2009. Turnip mosaic virus RNA replication complex vesicles are mobile, align with microfilaments, and are each derived from a single viral genome. J Virol 83:10460–10471. doi: 10.1128/JVI.00819-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lõhmus A, Varjosalo M, Mäkinen K. 2016. Protein composition of 6K2-induced membrane structures formed during Potato virus A infection. Mol Plant Pathol 17:943–958. doi: 10.1111/mpp.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torrance L, Andreev IA, Gabrenaite-Verhovskaya R, Cowan G, Mäkinen K, Taliansky ME. 2006. An unusual structure at one end of potato potyvirus particles. J Mol Biol 357:1–8. doi: 10.1016/j.jmb.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 67.Mäkinen K, Hafren A. 2014. Intracellular coordination of potyviral RNA functions in infection. Front Plant Sci 5:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Besong-Ndika J, Ivanov KI, Hafrèn A, Michon T, Mäkinen K. 2015. Cotranslational coat protein-mediated inhibition of potyviral RNA translation. J Virol 89:4237–4248. doi: 10.1128/JVI.02915-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lõhmus A, Hafrén A, Mäkinen K. 2017. Coat protein regulation by CK2, CPIP, HSP70 and CHIP is required for potato virus A replication and coat protein accumulation. J Virol 91:e01316-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pérez JJ, Udeshi ND, Shabanowitz J, Ciordia S, Juárez S, Scott CL, Olszewski NE, Hunt DF, García JA. 2013. O-GlcNAc modification of the coat protein of the potyvirus Plum pox virus enhances viral infection. Virology 442:122–131. doi: 10.1016/j.virol.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martínez-Turiño S, Pérez JJ, Hervas M, Navajas R, Ciordia S, Udeshi ND, Shabanowitz J, Hunt DF, García JA. 11 October 2017. Phosphorylation coexists with O-GlcNAcylation in a plant virus protein and influences viral infection. Mol Plant Pathol doi: 10.1111/mpp.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hafrén A, Hofius D, Ronnholm G, Sonnewald U, Mäkinen K. 2010. HSP70 and its cochaperone CPIP promote potyvirus infection in Nicotiana benthamiana by regulating viral coat protein functions. Plant Cell 22:523–535. doi: 10.1105/tpc.109.072413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lucini C. 2004. Expresión de proteínas heterólogas en plantas por medio del virus de la sharka (PPV). Ph.D. thesis. Universidad Politécnica de Madrid, Madrid, Spain. [Google Scholar]
- 74.Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]