ABSTRACT
The HIV-1 reservoir is a major obstacle to complete eradication of the virus. Although many proteins and RNAs have been characterized as regulators in HIV-1/AIDS pathogenesis and latency, only a few long noncoding RNAs (lncRNAs) have been shown to be closely associated with HIV-1 replication and latency. In this study, we demonstrated that lncRNA uc002yug.2 plays a key role in HIV-1 replication and latency. uc002yug.2 potentially enhances HIV-1 replication, long terminal repeat (LTR) activity, and the activation of latent HIV-1 in both cell lines and CD4+ T cells from patients. Further investigation revealed that uc002yug.2 activates latent HIV-1 through downregulating RUNX1b and -1c and upregulating Tat protein expression. The accumulated evidence supports our model that the Tat protein has the key role in the uc002yug.2-mediated regulatory effect on HIV-1 reactivation. Moreover, uc002yug.2 showed an ability to activate HIV-1 similar to that of suberoylanilide hydroxamic acid or phorbol 12-myristate 13-acetate using latently infected cell models. These findings improve our understanding of lncRNA regulation of HIV-1 replication and latency, providing new insights into potential targeted therapeutic interventions.
IMPORTANCE The latent viral reservoir is the primary obstacle to curing HIV-1 disease. To date, only a few lncRNAs, which play major roles in various biological processes, including viral infection, have been identified as regulators in HIV-1 latency. In this study, we demonstrated that lncRNA uc002yug.2 is important for both HIV-1 replication and activation of latent viruses. Moreover, uc002yug.2 was shown to activate latent HIV-1 through regulating alternative splicing of RUNX1 and increasing the expression of Tat protein. These findings highlight the potential merit of targeting lncRNA uc002yug.2 as an activating agent for latent HIV-1.
KEYWORDS: HIV-1 latency, lncRNA uc002yug.2, RUNX1, Tat, activation
INTRODUCTION
Long noncoding RNAs (lncRNAs) are a large class of non-protein-coding transcripts involved in many biological and physiological processes. As of now, only a small number of lncRNAs have been functionally characterized, and most of them have important functions in regulating various aspects of chromatin dynamics, gene expression (1, 2), and cancer-related activities, such as cell proliferation, differentiation, and metabolism (3–7).
Recently emerging evidence has demonstrated the role of lncRNA in viral replication. Viruses utilize some viral and cellular lncRNAs to regulate the expression of host and viral genes (8–10). To fight viruses, hosts also utilize lncRNAs, such as HEX1M1 and NEAT1, which activate the host antiviral immune system through the cGAS-STING pathway; NEAT1 also promotes interferon (IFN) responses by acting as a positive feedback for RIG-I signaling (11, 12). In some cases, cellular lncRNAs behave as sponges that sequester microRNAs (miRNAs) and induce the upregulation of miRNA target genes, such as lncRNA highly upregulated in liver cancer (HULC) (13, 14). Recently, some viral lncRNAs and cellular lncRNAs were identified due to their altered expression postinfection through the transcriptome analysis of cells infected with various viruses. For example, 83 disease-related lncRNAs in HIV-1-infected T cells were profiled. The lncRNA NEAT1 was shown to promote virus production through increased nucleus-to-cytoplasm export of Rev-dependent instability element (INS)-containing HIV-1 mRNAs (15). The lncRNA 7SK is closely associated with HIV Tat protein stimulation of transcriptional elongation of the integrated provirus through negative regulation of cyclin T1/CDK9 (16–18), but it is not involved in establishing latent HIV-1 infection (19–22). The lncRNA NRON, a repressor of NFAT, was found to significantly enhance HIV-1 replication in an NFAT-dependent manner when it was knocked down at an early time after infection (23). NRON was further illustrated to be involved in HIV-1 latency by inducing degradation of the viral transactivator protein Tat through the formation of E3 ligase with CUL4B and PMSD11 proteins (24). By use of a transcriptome assay, some lncRNAs were shown to be aberrantly expressed during HIV-1 replication and interact with the virus through the proteasome, apoptosis, DNA damage, and other pathways (25). However, the number of lncRNAs identified to date is certainly only a fraction of the many that are likely to affect HIV-1 replication.
The lncRNA uc002yug.2, 2,564 bp in length and located in chromosome 21, was initially identified at a higher level of expression in esophageal squamous cell carcinoma (ESCC) than in paired peritumoral tissue. In ESCC, uc002yug.2 was found to promote carcinogenesis by facilitating alternative splicing of RUNX1 to produce the shorter isoform RUNX1a (26). The human RUNX1 gene belongs to one of a family of transcription factors that serve as master regulators of development and has been proven to serve crucial functions in several cancers (27). Core binding factor β (CBF-β), which regulates the folding and DNA-binding activity of RUNX family proteins through forming a heterodimeric transcription complex with RUNX, was identified as a regulator required for HIV-1 replication (28, 29). RUNX1 has also been demonstrated to be involved in HIV-1 latency and was shown to repress HIV-1 replication in T cells by binding with the HIV-1 LTR, while its inhibitor was able to enhance the activation of latent HIV-1 (30).
HIV-1 latency is a state in which no or little viral RNA can be detected, and it represents a major barrier to eradication of AIDS. While host transcriptional silencing is thought to play a key role in HIV-1 latency, insufficient Tat transactivation activity is thought to result in impaired transcription of viral genes and the establishment of latency in cell culture experiments (31). As mentioned above, lncRNA NRON was shown to maintain the latency state of HIV-1 by specifically inducing Tat protein degradation (24). This finding demonstrated lncRNA as a class of promising factors for investigating the mechanism of HIV-1 latency. Since lncRNAs account for a large proportion of the human genome, other lncRNAs that contribute to HIV-1 latency need to be further explored.
In this study, we investigated the potential role of lncRNA uc002yug.2 in HIV-1 replication and reactivation of latent viruses. The results showed that uc002yug.2 could enhance HIV-1 replication and LTR activity, which is closely associated with its ability to reduce RUNX1b and -1c. Furthermore, we next demonstrated that RUNX1b and -1c but not RUNX1a inhibited HIV-1 replication and was required for latent viruses. Interestingly, we also observed that in certain cells, such as Jurkat, uc002yug.2 did not always downregulate RUNX1b and -1c and upregulate RUNX1a as reported previously (26), indicating that another mechanism is employed by uc002yug.2 to regulate HIV-1 replication. Further investigation showed that overexpression of uc002yug.2 increased the expression of Tat protein, further supporting our hypothesis that Tat plays an important role in uc002yug.2-mediated enhancement of replication and activation of latent HIV-1 in addition to RUNX1b and -1c. Importantly, in CD4+ T cells from HIV-1-infected individuals treated with highly active antiretroviral therapy (HAART) upon T-cell activation, uc002yug.2 could activate latent HIV-1. Taken together, our data provide important information and broaden our understanding of the lncRNA regulatory effect on HIV-1 replication and latency. Therefore, research into this field will help to identify new cellular pathways and develop new therapeutic tools for the treatment of HIV-1 infection.
RESULTS
uc002yug.2 enhances HIV-1 replication.
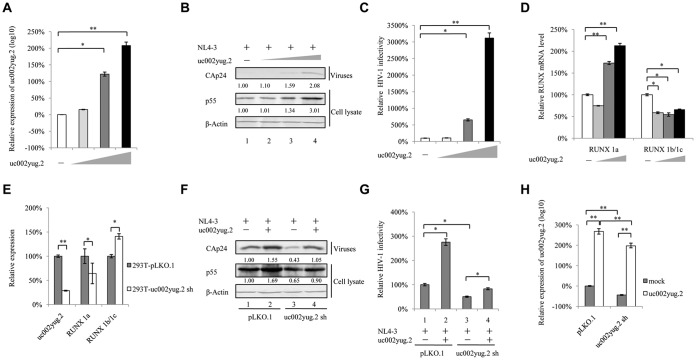
In testing of various cell lines, human embryonic kidney 293T (HEK293T) and TZM-bl cells showed higher uc002yug.2 mRNA levels than those of Jurkat and HeLa cell lines (data not shown). Whether uc002yug.2 affects the replication of HIV-1 was confirmed by its overexpression in HeLa cells or reducing its endogenous level using short hairpin RNA (shRNA) in HEK293T cells. Forty-eight hours after transfection with the pNL4-3 expression vector plus VR1012 or uc002yug.2 expression vector, HeLa cells were harvested for immunoblotting and reverse transcription-quantitative PCR (qRT-PCR) analysis. With increased amounts of uc002yug.2 (Fig. 1A), p55 expression in the cell lysate and capsid p24 (CAp24) expression in the viral supernatant from cells were continually increased (Fig. 1B), and infectivity of HIV-1 was greatly increased (∼30-fold) (Fig. 1C), indicating that uc002yug.2 enhanced HIV-1 replication. The mRNA levels of RUNX1a and RUNX1b and -1c were correspondingly upregulated or downregulated by overexpression of uc002yug.2 (Fig. 1D), which is basically consistent with the regulation of RUNX1 isoforms reported by Wu et al. (26).
FIG 1.
uc002yug.2 enhances HIV-1 replication. (A to D) uc002yug.2 overexpression enhances HIV-1 replication in HeLa cells. (A) The pNL4-3 viral vector with increasing amounts of uc002yug.2 (0, 100, 300, and 900 ng) was cotransfected into HeLa cells as indicated. After 48 h, cells and supernatants were harvested. The RNA level of uc002yug.2 was detected with the qRT-PCR assay. (B) p55 levels in cell lysate and CAp24 levels in supernatants were determined by immunoblotting, and the densities of bands were analyzed with ImageJ software to calculate the values relative to that for β-actin. (C) The infectivity of HIV-1 was measured by detecting the luciferase activity after TZM-bl cells were infected with the supernatant for another 48 h. (D) The mRNA levels of RUNX1a and RUNX1b and -1c were detected by qRT-PCR in the same cells as for panel A. (E to H) Knockdown of uc002yug.2 decreases HIV-1 replication in HEK293T cells. The endogenous uc002yug.2 in HEK293T cells was knocked down by using uc002yug.2-specific shRNA. (E) The knockdown of uc002yug.2 and corresponding RUNX1a and RUNX1b and -1c mRNA levels were detected by qRT-PCR. (F) Both control and uc002yug.2sh HEK293T cells were cotransfected with pNL4-3 viral vector plus uc002yug.2 or control vector as indicated. At 48 h after transfection, the cells and supernatants were harvested. p55 levels in cell lysate and CAp24 levels in supernatants were determined by immunoblotting, and the densities of bands were analyzed with ImageJ software to calculate the values relative to that for β-actin. (G) Virus production was measured by detecting the luciferase activity after TZM-bl cells were infected with the supernatant for another 48 h. The infectivity of 293T-pLKO.1 cells transected with pNL4-3 plus control vector was set as 100%. (H) The RNA level of uc002yug.2 in the same cells as described for panel F was detected by qRT-PCR. Results are representative of those from three independent repeats, and the corresponding value of the mock control was set as 1 or 100%. Data are presented as means ± SDs.
In contrast, stable HEK293T cell lines with shRNA targeting uc002yug.2 showed strong suppression of the production of uc002yug.2 mRNA, leading to a corresponding reduction of RUNX1a and increase of RUNX1b and -1c (Fig. 1E). Upon transfection of uc002yug.2 shRNA cells with the pNL4-3 expression vector, the amount of p55 was substantially reduced (by ∼35%) compared with the negative control (Fig. 1F, lanes 1 and 3). Overexpression of uc002yug.2 increased the replication of HIV-1 to 169% (Fig. 1F, lanes 1 and 2), but overexpression of uc002yug.2 in knockdown cells could mostly rescue the amount of p55 (Fig. 1F, lanes 1 and 4). The infectivity of the supernatant produced by uc002yug.2 shRNA cells was reduced using TZM-bl as infectious indicator cells (Fig. 1G, lane 3), whereas increased infectivity was observed in the uc002yug.2 overexpression group (Fig. 1G, lane 2). The mRNA levels of uc002yug.2 are shown in Fig. 1H.
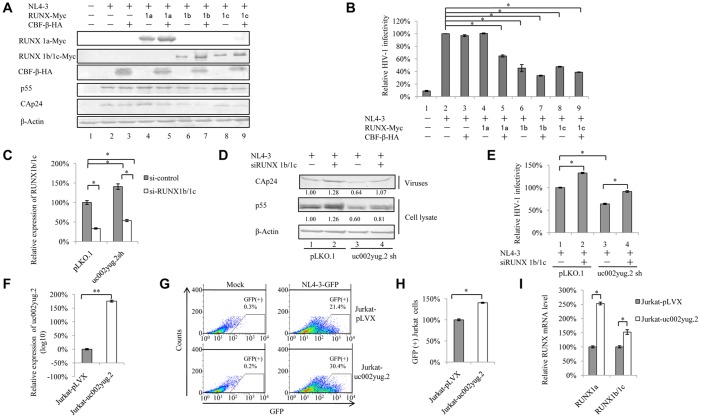
In HEK293T cells, we indeed observed that knockdown of uc002yug.2 induced the reduction of RUNX1a and the increase of RUNX1b and -1c. We then investigated whether the expression of various RUNX1 isoforms resulting from alternative splicing induced by the reduction of uc002yug.2 was responsible for viral replication. The pNL4-3 expression vector plus various RUNX1 isoforms and CBF-β were transfected into HEK293T cells. At 48 h posttransfection, cells were harvested for immunoblotting analysis, and the supernatants were collected and used to infect TZM-bl cells. The results showed that RUNX1a, RUNX1b, and RUNX1c were expressed well. The levels of p55 and CAp24 were reduced in the presence of RUNX1b and -1c, whereas RUNX1a had no effect on HIV-1 replication (Fig. 2A). It has been reported that RUNX1 and CBF-β were capable of repressing HIV-1 transcription (30). We found that in the presence of CBF-β, RUNX1a could impair HIV-1 infectivity, although RUNX1a had no effect on HIV-1 infectivity (Fig. 2B). Moreover, a further reduction of HIV-1 infectivity was also observed when RUNX1b and -1c were cotransfected with CBF-β compared to that with RUNX1b and -1c alone (Fig. 2B), which is consistent with the previous study (30). Thus, alternative splicing of RUNX induced by uc002yug.2 may have contributed to the lower replication capacity of HIV-1 in uc002yug.2-silenced HEK293T cells. In order to confirm whether RUNX1 is closely associated with the effect of un002yug.2 on HIV-1 replication, we investigated the replication of HIV-1 when RUNX1b and -1c were knocked down. Short interfering RNA (siRNA) targeted RUNX1b and -1c was cotransfected with pNL4-3 into uc002yug.2-silenced and control HEK293T cells. Silencing of RUNX1b and -1c increased the replication of HIV-1 to 126% (Fig. 2D, lanes 1 and 2), and silencing of RUNX1b and -1c in uc002yug.2 knockdown cells could mostly rescue the amount of CAp24 (Fig. 2D, lanes 1 and 4). The infectivity of the supernatant produced by uc002yug.2 shRNA cells was reduced when TZM-bl cells were used as infectious indicator cells (Fig. 2E, lane 3), whereas increased infectivity was observed in the uc002yug.2 and RUNX1b and -1c silencing group (Fig. 2E, lane 4). These data further demonstrated that RUNX1 was involved in the ability of uc002yug.2 to regulate HIV-1 replication.
FIG 2.
RUNX1b- and -1c-induced suppression of HIV-1 partially contributes to the effect of uc002yug.2 on HIV-1. (A and B) Overexpression of RUNX1b and RUNX1c inhibits HIV-1 replication in HEK293T cells. (A) The pNL4-3 vector and plasmids expressing RUNX1 isoforms, with or without CBF-β, were cotransfected into HEK293T cells as indicated. At 48 h after transfection, the levels of expression of RUNX1 isoforms, CBF-β, p55, and CAp24 in cells were determined by immunoblotting. (B) HIV-1 NL4-3 infectivity in the supernatant was measured by detecting luciferase activity after TZM-bl cells were infected with the supernatant for another 48 h. The infectivity of 293T cells transfected with pNL4-3 plus control vector was set as 100%. (C to E) RUNX1 is involved in the function of uc002yug.2 to regulate HIV-1 replication. (C) siRNA targeting RUNX1b and -1c was cotransfected with pNL4-3 into uc002yug.2 knockdown and control HEK293T cells. After 48 h, cells and supernatants were harvested. The qRT-PCR assay was used to detect the mRNA level of RUNX1b and -1c. (D) p55 levels in cell lysate and CAp24 levels in supernatants were determined by immunoblotting, and the densities of bands were analyzed with ImageJ software to calculate the values relative to that for β-actin. (E) The infectivity of HIV-1 was measured as described for panel B. (F to I) Overexpression of uc002yug.2 increases HIV-1 replication in Jurkat cells. (F) Jurkat cells were transduced with lentiviruses containing uc002yug.2 or a scramble control, and at 48 h postinfection, puromycin (1 μg/ml) was added to the medium for selection. The RNA level of uc002yug.2 was detected by qRT-PCR. (G) The uc002yug.2-overexpressing or control Jurkat cells were infected with HIV-1 pNL4-3-deltaE-EGFP virus, and GFP-positive cells were measured by flow cytometry at 48 h postinfection. (H) The relative ratio of GFP-positive cells in uc002yug.2-overexpressing or control Jurkat cells was calculated. The ratio of GFP-positive cells in control Jurkat cells infected with pNL4-3-deltaE-EGFP virus was set as 100%. (I) mRNA levels of RUNX1a and RUNX1b and -1c in uc002yug.2-overexpressing or control Jurkat cells was detected with qRT-PCR. Results are representative of those from three independent repeats, and the corresponding value of the mock control was set as 1 or 100%. Data are presented as means ± SDs.
To further confirm that HIV-1 target cells are also affected by uc002yug.2 expression, we overexpressed uc002yug.2 in Jurkat cells using a lentivirus vector containing uc002yug.2 (Fig. 2F). Increased uc002yug.2 expression promoted the infectious HIV-1 yield ∼1.4-fold (Fig. 2G and H). Interestingly, RUNX1a and RUNX1b and -1c both were upregulated by overexpression of uc002yug.2 (Fig. 2I), which may compromise the ability of uc002yug.2 to enhance the replication of HIV-1. Collectively, these data suggested that RUNX1b and -1c are involved in the uc002yug.2 function of enhancing HIV-1 replication, but they are not the only determinant. Other mechanisms may be employed by uc002yug.2 to contribute to the regulation of HIV-1.
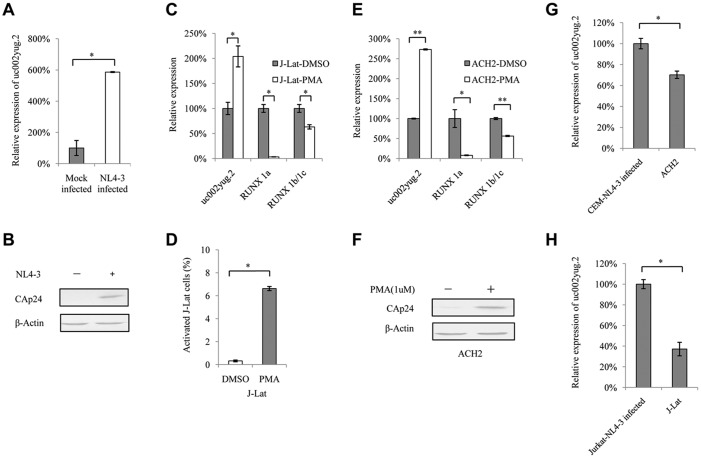
HIV-1 replication upregulates uc002yug.2.
To examine whether uc002yug.2 expression varies along with HIV-1 replication, Jurkat cells were infected with NL4-3 virus produced from transfected HEK293T cells. With HIV-1 replication (Fig. 3B), the mRNA level of uc002yug.2 was increased ∼6-fold (Fig. 3A). Next, we assessed the mRNA level of uc002yug.2 when the latently HIV-1-infected human T cell lines J-Lat 6.3 and ACH-2 were activated by phorbol 12-myristate 13-acetate (PMA). Treatment of J-Lat 6.3 and ACH-2 cells with PMA (1 μM) for 48 h upregulated the mRNA level of uc002yug.2. With increased uc002yug.2, RUNX1b and -1c and RUNX1a both were downregulated (Fig. 3C and E), while PMA stimulated HIV-1 transcription and replication (Fig. 3D and F). These results further support the notion that uc002yug.2 plays a key role during HIV-1 replication but has different regulatory mechanisms for expression of RUNX1 isoforms in different cell lines. In latently infected J-Lat 6.3 and ACH-2 cells, we also observed that the expression levels of uc002yug.2 were lower than those in acutely infected CEM and Jurkat cells with NL4-3 viruses (Fig. 3G and H).
FIG 3.
uc002yug.2 expression is upregulated after HIV-1 infection or reactivation. (A and B) HIV-1 infection upregulates uc002yug.2 expression in Jurkat cells. (A) Differences in expression levels of uc002yug.2 between the mock- and HIV-1 NL4-3-infected Jurkat cells were detected by qRT-PCR. (B) Levels of CAp24 from HIV-1 NL4-3 were measured by immunoblotting. (C to F) Reactivation of HIV-1 increases uc002yug.2. HIV-1 latently infected J-Lat 6.3 and ACH-2 cells were utilized for measuring the change of uc002yug.2 during virus reactivation. (C) J-Lat 6.3 cells were treated or not with PMA (1 μM) for 48 h. Differences in expression levels of uc002yug.2, RUNX1a, and RUNX1b and -1c between the mock-infected and reactivated cells were detected by qRT-PCR. (D) HIV-1 reactivation of J-Lat 6.3 cells was measured by detecting GFP-positive cells by flow cytometry. (E) ACH-2 cells were treated or not with PMA (1 μM) for 48 h. Differences in expression levels of uc002yug.2, RUNX1a, and RUNX1b and -1c between the mock-infected and reactivated cells were detected by qRT-PCR. (F) HIV-1 reactivation of ACH-2 cells was measured by immunoblotting with anti-CAp24. (G and H) Low uc002yug.2 level maintains HIV-1 latency. (G) ACH-2 cells are HIV-1 latently infected CEM cells. The uc002yug.2 expression in acutely or latently HIV-1-infected CEM cells was detected using a qRT-PCR assay. (H) J-Lat 6.3 cells are HIV-1 latently infected Jurkat cells. The uc002yug.2 expression in acutely or latently HIV-1-infected Jurkat cells was detected using a qRT-PCR assay. Results are representative of those from three independent repeats. The qRT-PCR results in panels A, C, E, G, and H are represented with corresponding values, with the mock-infected cells set at 100%. Data are presented as means ± SDs.
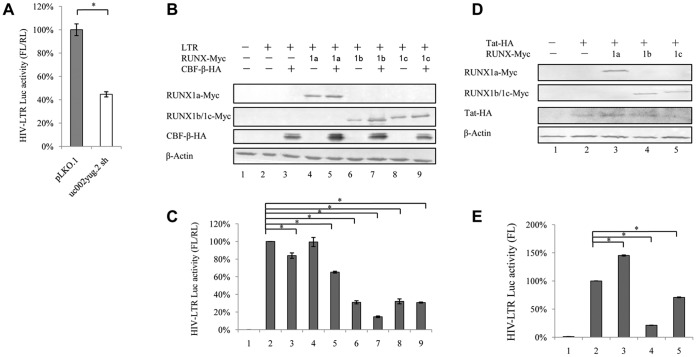
uc002yug.2 activates HIV-1 transcription by the HIV-1 LTR.
In order to examine whether uc002yug.2 affects HIV-1 transcription, we used a reporter luciferase system to detect the effect of uc002yug.2 on HIV-1 promoter activity. HIV-1 promoter activity was significantly reduced when uc002yug.2 was stably depleted in HEK293T cells using shRNA (Fig. 4A), which was concomitant with downregulation of RUNX1a and upregulation of RUNX1b and -1c (Fig. 1E). We then assessed the effects of RUNX1a or RUNX1b and -1c on the basal activity of the HIV-1 LTR in HEK293T cells to confirm whether RUNX1b and -1c are functionally responsible for the suppression of HIV-1 transcription. RUNX1a plus CBF-β slightly reduced HIV-1 LTR activity, but RUNX1b and RUNX1c alone, RUNX1b plus CBF-β, and RUNX1c plus CBF-β all significantly reduced the HIV-1 LTR activity (Fig. 4C). The expression levels of RUNX1a, RUNX1b, RUNX1c, and CBF-β were confirmed in HEK293T cells (Fig. 4B). In TZM-bl cells, which contain an integrated LTR driving expression of firefly luciferase that depends on the expression of Tat to initiate transcription, RUNX1b or -1c but not RUNX1a could suppress the LTR-driven luciferase expression in the presence of Tat protein (Fig. 4D and E).
FIG 4.
uc002yug.2 affects HIV-1 LTR activity in HEK293T cells. (A) Knockdown of uc002yug.2 decreases HIV-1 LTR activity in HEK293T cells. The endogenous uc002yug.2 in HEK293T cells was knocked down as described for Fig. 1E. (A) HIV-1 LTR-luciferase and pRenilla plasmids were cotransfected into control or uc002yug.2 knockdown HEK293T cells. HIV-1 LTR activity was determined by a dual-luciferase reporter assay at 48 h after transfection. (B and C) Overexpression of RUNX1b and RUNX1c inhibits LTR activity in HEK293T cells. (B) The HIV-1 LTR-luciferase, pRenilla, and RUNX1 isoforms, with or without CBF-β plasmids, were cotransfected into HEK293T cells as indicated. At 48 h after transfection, RUNX1a or RUNX1b and -1c and CBF-β expression levels in cells were determined by immunoblotting. (C) HIV-1 LTR activity was determined by a dual-luciferase reporter assay. The luciferase activity of HEK293T cells transfected with HIV-1 LTR-luciferase was determined; pRenilla plus control vector (lane 2) was set as 100%. (D and E) Overexpression of RUNX1b and RUNX1c inhibits HIV-1 LTR activity in TZM-bl cells. (D) In TZM-bl cells, which contain an integrated LTR driving expression of firefly luciferase, Tat and RUNX1 isoforms were cotransfected as indicated. (E) HIV-1 LTR activity was determined at 48 h posttransfection, and the luciferase activity of TZM-bl cells transfected with Tat and control vector (lane 2) was set as 100% (E). Results are representative of those from three independent repeats. Data are presented as means ± SDs.
Expression of uc002yug.2 activates replication of latent HIV-1.
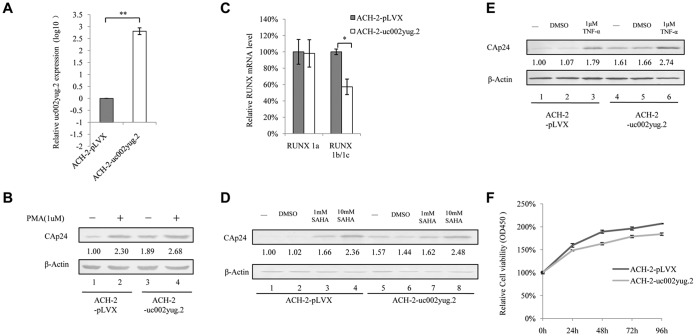
We next investigated whether overexpression of uc002yug.2 would have a function similar to that of PMA in activating latent HIV-1. ACH-2 cells were infected with lentivirus containing uc002yug.2, and stable cell lines were screened for expression of uc002yug.2 (Fig. 5A). ACH-2 cells expressing uc002yug.2 showed significantly increased activation of latent HIV-1, to 189%, as determined by CAp24 amount compared to that in negative-control pLVX cells (Fig. 5B, lanes 1 and 3), and to nearly the same extent as that by PMA (1 μM) treatment alone (Fig. 5B, lanes 2 and 3). Treatment of ACH-2-uc002yug.2 cells with PMA (1 μM) moderately enhanced the expression of HIV-1 CAp24 compared to that in cells stably expressing uc002yug.2 alone (Fig. 5B, lanes 3 and 4), indicating that most of the latent HIV-1 had been activated by uc002yug.2. Interestingly, we indeed found that overexpression of uc002yug.2 downregulated the RUNX1b and -1c mRNA level, which suppressed the transcription of HIV-1 (Fig. 5C). We also tested whether the activation effect of uc002yug.2 on latent HIV-1 could be increased by treatment with another latent reactivation agent, suberoylanilide hydroxamic acid (SAHA). Treatment of ACH-2 cells with 1 mM and 10 mM SAHA greatly increased expression of HIV-1 CAp24 (Fig. 5D, lanes 3 and 4), and stable expression of uc002yug.2 alone in ACH-2 cells could increase CAp24 expression almost to the same level as that induced by 1 mM SAHA (Fig. 5D, lanes 5 and 3). The combination of uc002yug.2 and 1 mM SAHA yielded a slightly stronger activation effect than did uc002yug.2 treatment alone (Fig. 5D, lanes 6 and 7). Similarly, uc002yug.2 alone had the same ability to activate latent ACH-2 cells as 1 μM tumor necrosis factor alpha (TNF-α) (Fig. 5E). These results indicated that the viral activation ability of uc002yug.2 was no less than those of PMA, SAHA, and TNF-α. The proliferation of ACH-2 cells stably expressing uc002yug.2 was measured with Cell Counting Kit-8 (CCK8) to exclude the possibility that rapid proliferation induced by uc002yug.2 could cause the rapid replication of HIV-1. The data showed that ACH-2 stably expressing uc002yug.2 had a lower proliferation rate than ACH-2 cells, but the difference was not statistically significant (Fig. 5F).
FIG 5.
Expression of uc002yug.2 activates latent HIV-1 replication. (A) ACH-2 cells were infected with lentiviruses containing uc002yug.2 or a scramble control, and at 48 h postinfection, puromycin (1 μg/ml) was added to the medium for selection. The expression of uc002yug.2 was determined by qRT-PCR. (B) uc002yug.2-overexpressing or control ACH-2 cells were treated or not with PMA (1 μM), and CAp24 levels were detected by immunoblotting 48 h posttreatment. The densities of bands were analyzed with ImageJ software to calculate the values relative to that for β-actin. (C) Expression levels of RUNX1a and RUNX1b and -1c were determined by qRT-PCR when uc002yug.2 was overexpressed in ACH-2 cells. (D and E) CAp24 levels were detected by immunoblotting 48 h after uc002yug.2-overexpressing or control ACH-2 cells were treated or not with different concentrations of SAHA (0, 1, and 10 mM) (D) or TNF-α (1 μM) (E) for 48 h. The densities of bands were analyzed with ImageJ software to calculate the values relative to that for β-actin. (F) uc002yug.2-overexpressing or control ACH-2 cells at a concentration of 1 × 103 per well were seeded in a 96-well plate. Cell growth was measured using Cell Counting Kit-8 (Transgen, China) according to the manufacturer's instructions at 24 h, 48 h, 72 h, and 96 h. Cell proliferation curves were plotted based on the absorbance at 450 nm using an iMark microplate reader (Bio-Rad) at each time point. The absorbance value at 0 h was set as 100%. Results are representative of those from three independent repeats. Data are presented as means ± SDs.
uc002yug.2 upregulates Tat protein.
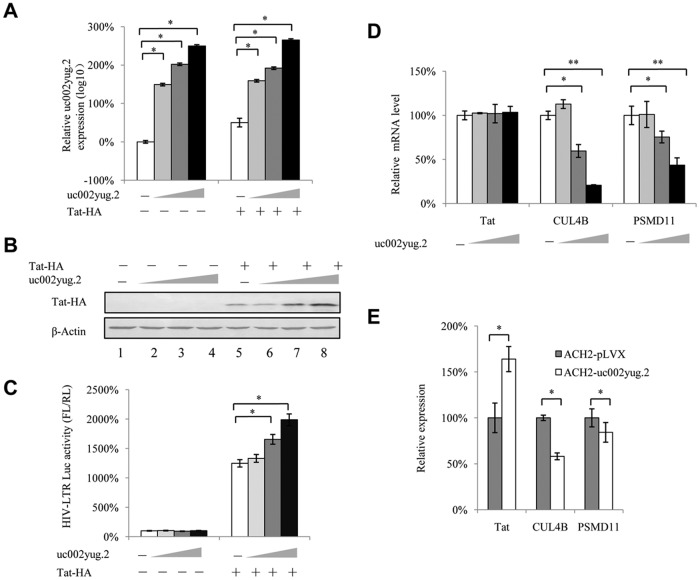
As mentioned above, uc002yug.2 showed different regulatory mechanisms for expression of RUNX1 isoforms in different cell lines, indicating that the RUNX1b and -1c reduction is not the only determinant for activation of latent HIV-1 by uc002yug.2. We speculated that other mechanisms may be utilized by uc002yug.2 to activate latent HIV-1. The Tat protein guarantees the high level of viral transcription through manipulating the 5′ untranslated region (UTR) promoter of HIV-1 and controlling RNA polymerase II (RNAP II). In this study, we examined whether uc002yug.2 could affect the expression of Tat protein. We observed that the expression of Tat was increased in a dose-dependent manner along with increasing amounts of uc002yug.2 expressed in HeLa cells (Fig. 6A and B), and the HIV-1 LTR activity was also significantly increased (Fig. 6C). Moreover, when Tat protein was absent, uc002yug.2 affected HIV-1 LTR activity slightly and without statistical significance, indicating that Tat protein was required in the uc002yug.2-mediated regulation of HIV-1 reactivation.
FIG 6.
uc002yug.2 upregulates Tat protein. (A to C) uc002yug.2 increases HIV-1 LTR activity through upregulating Tat protein. HIV-1 LTR-luciferase reporter, pRenilla, and increasing amounts of uc002yug.2 (0, 100, 300, and 900 ng) with or without Tat plasmids were cotransfected into HeLa cells as indicated. The cells were harvested at 48 h posttransfection. (A) The expression of uc002yug.2 was detected by qRT-PCR. (B) Tat protein levels in cells were determined by immunoblotting, and the densities of bands were analyzed with ImageJ software to calculate the values relative to that for β-actin. (C) The LTR activity was determined by a dual-luciferase reporter assay, and the luciferase activity of HeLa cells transfected with an LTR-luciferase reporter, pRenilla without Tat plus control vector, was set as 100%. (D) mRNA levels of CUL4B and PSMD11 were negatively correlated with increased uc002yug.2 expression. (E) Latent cell line ACH2 stably overexpressing uc002yug.2 also showed downregulated mRNA levels of CUL4B and PSMD11. Results are representative of those from three independent repeats. Data are presented as means ± SDs.
To further understand the mechanism of uc002yug.2 in regulating Tat, we detected the mRNA level of Tat and found that it did not change with increasing amounts of uc002yug.2 (Fig. 6D). As it has been reported that Tat protein could be degraded by the E3 ligase containing CUL4B and PSMD11 proteins (24), we examined the expression of these two proteins; we found that they were both downregulated (Fig. 6D). Moreover, mRNA levels of Tat, CUL4B, and PSMD11 were also detected in ACH-2 cells stably overexpressing uc002yug.2. Consistent with HeLa cells transfected with uc002yug.2, mRNA levels of CUL4B and PSMD11 were decreased, while mRNA levels of Tat were increased, which may be caused by the enhancement of HIV-1 in ACH-2 cells stably overexpressing uc002yug.2 (Fig. 6E). These results indicated that the upregulation of Tat protein by uc002yug.2 may function through the proteasome pathway.
uc002yug.2 affects HIV-1 transcription through the LTR domain.
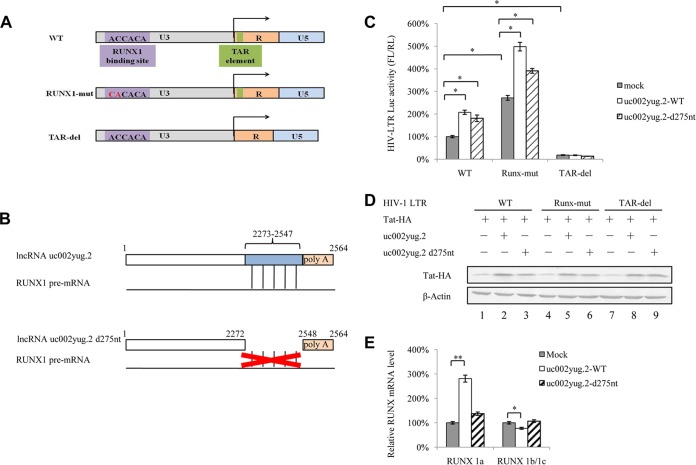
The above-described data support the notion that uc002yug.2 reactivates latent HIV-1 through two mechanisms, which are the reduction of RUNX1b and -1c and the increase of Tat protein level. A schematic diagram of the HIV-1 LTR is shown in Fig. 7A. RUNX1-mut in the LTR was characterized as a mutant affecting RUNX binding. The regulation of Tat in HIV-1 transcription is achieved by binding to the TAR hairpin in the HIV-1 LTR RNA transcript. We next examined whether overexpression of uc002yug.2 would have an effect on LTR mutants in HEK293T cells. The results showed that overexpression of uc002yug.2 increased wild-type LTR activity. The RUNX1-mut LTR released the restriction of RUNX on LTR activity and led to clearly increased LTR activity, especially in the presence of uc002yug.2. The TAR-del LTR lost the Tat influence and showed lower activity, even in the presence of uc002yug.2 (Fig. 7C). The Tat protein also was increased in the presence of uc002yug.2 (Fig. 7D). The above-described data further confirmed that uc002yug.2 affects HIV-1 replication through regulation of RUNX1 and Tat expression. However, uc002yug.2 totally lost the ability to activate the LTR activity when TAR, which is required for Tat binding, was deleted, indicating that RUNX1 might also function through the Tat protein. To further clarify the regulatory mechanism of uc002yug.2 for the HIV-1 LTR, uc002yug.2-d275nt was constructed with a deletion of nucleotides (nt) 2273 to 2547 at its 3′ terminus, a region which is responsible for the alternative splicing of RUNX1 via binding with the RUNX1 pre-mRNA (Fig. 7B). The ability of uc002yug.2-d275nt to increase HIV-1 LTR activity (Fig. 7C) and Tat protein level (Fig. 7D, lane 3) was slightly weaker than that wild-type uc002yug.2 (uc002yug.2-WT), indicating that uc002yug.2-d275nt retained a weaker ability to stimulate the HIV-1 LTR promoter through a functional domain other than the RUNX1 binding domain. The mRNA level of RUNA 1a as well as RUNX1b and -1c in uc002yug.2-WT- and uc002yug.2-d275nt-overexpressing cells was detected by qRT-PCR, and uc002yug.2-d275nt was found to be deficient in causing alternative splicing of RUNX1 pre-mRNA (Fig. 7E). These data further support another mechanism by which uc002yug.2 reactivates latent HIV-1 that is dependent on upregulation of Tat protein, and the alternative splicing of RUNX1 may also affect HIV-1 through the Tat protein.
FIG 7.
uc002yug.2 affects HIV-1 transcription through altering RUNX1 and Tat expression. (A) Schematic of HIV-1 LTR mutants. HIV-1 LTR mutants that had previously been shown to lack RUNX binding ability (middle row) or have a deletion in the TAR element (bottom row) were constructed. (B) Schematic of the uc002yug.2 mutant. Compared with the wild-type uc002yug.2 (uc002yug.2-WT), uc002yug.2-d275nt was constructed to delete 275 nt at its 3′ terminus, which is responsible for the alternative splicing of RUNX1 via binding with RUNX1 pre-mRNA (lower diagram). (C) Effect of uc002yug.2 and its mutants on the HIV-1 LTR as well as its mutants. Wild-type or mutant HIV-1 LTR, pRenilla plasmid plus control vector, or uc002yug.2-WT or uc002yug.2-d275nt was cotransfected into HEK293T cells. After 48 h, cells were harvested. The LTR activity was determined by a dual-luciferase reporter assay, and the luciferase activity of HEK293T cells transfected with wild-type HIV-1 LTR, Tat plus control vector, was set as 100%. (D) Tat protein levels in cells were determined by immunoblotting. (E) The mRNA levels of RUNX1a and RUNX1b and -1c in uc002yug.2-WT-, uc002yug.2-d275nt-, and mock-transfected HEK293T cells were detected by qRT-PCR, and the corresponding value of the mock control was set as 100%. Results are representative of those from three independent repeats. Data are presented as means ± SDs.
uc002yug.2 increases viral replication and reactivation in primary CD4+ T cells.
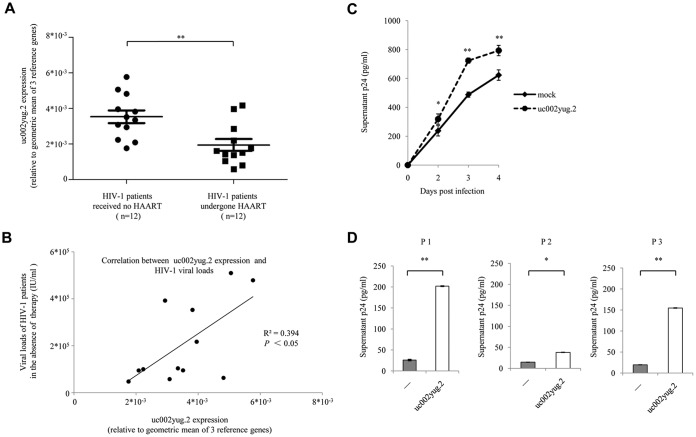
In order to confirm the role of uc002yug.2 in regulating HIV-1, we isolated CD4+ T cells from HIV-1-infected patients. Characteristics of HIV-1-infected patients are listed in Table 1. To minimize the bias that may be caused by heterogeneity of HIV-1 patients, geometric means of three reference genes (the genes for β-actin, glyceraldehyde-3-phosphate dehydrogenase [GAPDH], and hydroxymethyl-bilane synthase [HMBS] were used for normalization according to the MIQE (minimum information for publication of qPCR experiments) guidelines (32–35) when qPCR assay was performed. The expression of uc002yug.2 in HAART-naive HIV-1 patients was significantly higher than that in HIV-1 patients who had undergone HAART for more than 3 years and had undetectable plasma viral loads (Fig. 8A) and correlated with their HIV-1 loads (Fig. 8B). Overexpression of uc002yug.2 in resting CD4+ T cells from three HIV-1-infected individuals who had undergone HAART caused significant reactivation of HIV-1 upon T-cell activation by phytohemagglutinin M (PHA-M), as determined by detecting HIV-1 particles in the supernatants using enzyme-linked immunosorbent assay (ELISA) (Fig. 8D). Moreover, overexpression of uc002yug.2 was capable of increasing HIV-1 replication in CD4+ T cells from healthy donors (Fig. 8C). These data further demonstrated the contribution of uc002yug.2 in regulating HIV-1.
TABLE 1.
Characteristics of HIV-1-infected patients in this study
| Characteristic | Datum for patientsa |
|
|---|---|---|
| Not treated with HAARTb | Treated with HAART | |
| No. of patients | 12 | 12 |
| Sex | Male | Male |
| Age (yrs) | 38.6 ± 15.1 | 37.1 ± 8.6 |
| Mode of infection | Sexual transmission | Sexual transmission |
| CD4+ T count (cells/mm3) | 410 ± 76 | 899 ± 172 |
| Viral load (IU/ml) | 2.10 ± 1.8E + 05 | <50 |
| Antiretroviral therapy | No | Yes |
| Duration of antiretroviral therapy (yrs) | 4.4 ± 1.4 | |
| Treatment regimen | Tenofovir disoproxil + lamivudine + efavirenz | |
All values are means ± SDs if not indicated otherwise.
Patients in this group were newly diagnosed HIV-1 patients who had not received HAART.
FIG 8.
uc002yug.2 increases viral replication and reactivation in CD4+ T cells. (A and B) uc002yug.2 was correlated with HIV-1 loads in plasma of HIV-1-infected patients. (A) Expression levels of uc002yug.2 in CD4+ T cells isolated from HIV-1 patients who underwent HAART (n = 12) and HIV-1 patients who had not received HAART (n = 12) were detected by qRT-PCR. The geometric means of the β-actin, GAPDH, and HMBS genes were used for normalization. (B) The HIV-1 loads and uc002yug.2 RNA levels of HIV-1-infected patients who had not received HAART were plotted, and linear regression analysis was performed. The geometric means of the β-actin, GAPDH, and HMBS genes were used for normalization. (C) uc002yug.2 increases viral replication. Primary CD4+ T lymphocytes from healthy donors were nucleofected with uc002yug.2 or control vector and then were infected with HIV-1 NL4-3. HIV-1 production in the supernatant was quantified with p24 ELISA at indicated time points postinfection. (D) uc002yug.2 increases HIV-1 reactivation in primary resting CD4+ T cells from patients. Resting CD4+ T cells were isolated from HAART-treated patients and nucleofected with uc002yug.2 or control vector. P1 to P3 represent three patients. HIV-1 reactivation in CD4+ T cells upon PHA-M (5 ng/ml) stimulation was detected by measuring p24 levels in the supernatants by ELISA.
DISCUSSION
In the current study, we found that lncRNA uc002yug.2 plays an important role in the regulation of HIV-1 transcription and replication as well as reactivation of latent HIV-1. Due to different mRNA levels of uc002yug.2 in various cell lines (data not shown), we overexpressed uc002yug.2 in HeLa cells and stably infected HEK293T cells with a lentivirus encoding shRNA against lncRNA uc002yug.2 to detect its effect on HIV-1 replication. Ectopic expression of uc002yug.2 in HeLa cells potentially enhanced the replication of HIV-1 in a dose-dependent manner (Fig. 1A to D). The depletion of uc002yug.2 in HEK293T cells reduced the replication and infectivity of HIV-1 by ∼35% (Fig. 1F and G), while the mRNA level of RUNX1b and -1c was upregulated (Fig. 1E) as reported by Wu et al. (26). Further investigation confirmed that RUNX1b and -1c but not RUNX1a indeed strongly inhibited HIV-1 replication, in particular when combined with CBF-β (Fig. 2A and B). Upon knockdown of RUNX1b and -1c with siRNA in uc002yug.2-sh cells, the reduced expression and infectivity of HIV-1 were restored compared to those in control uc002yug.2-sh cells (Fig. 2D and E), indicating that upregulation of RUNX1b and -1c induced by uc002yug.2 partially contributed to the suppression of HIV-1 replication. Thus, we deduced that the upregulation of RUNX1b and -1c by knockdown of uc002yug.2 was the main determinant mediating the reduction in HIV-1 infectivity in HEK293T-uc002yug.2sh cells. Our data are consistent with the conclusion that RUNX1 and CBF-β overexpression could reduce expression of viral proteins and viral replication, as reported by Klase et al. (30), and further demonstrate that RUNX1b and -1c but not RUNX1a could inhibit HIV-1 infectivity.
We also observed that lncRNA uc002yug.2 did not always downregulate RUNX1b and -1c. The depletion of uc002yug.2 indeed led to decreased RUNX1a and increased RUNX1b and -1c in HEK293T cells (Fig. 1E), whereas overexpression of uc002yug.2 or upregulated uc002yug.2 by replicating HIV-1 induced the increase in mRNA levels of all RUNX1 isoforms, including RUNX1a, -1b, and -1c, in Jurkat cells (Fig. 2I). These results indicated that uc002yug.2 had different regulatory effects on the expression of RUNX1 isoforms in different cell lines. Upregulation of RUNX1b and -1c in Jurkat cells might compromise the ability of uc002yug.2 to enhance the replication of HIV-1. However, another line of evidence was shown with latently infected cell lines J-Lat 6.3 and ACH-2, in which reactivation of HIV-1 replication using PMA stimulation increased along with increased uc002yug.2, as well as with decreased RUNX1b and -1c, supporting our hypothesis that the regulation of RUNX1b and -1c induced by uc002yug.2 is required for HIV-1 activation (Fig. 3C and E). However, further investigation is needed to elaborate the different regulatory mechanisms of uc002yug.2 for expression of RUNX1 isoforms or the relationship between uc002yug.2 and RUNX1 in various cells. Although the regulation by uc002yug.2 of the expression of RUNX1 isoforms does not always follow the alternative-splicing rule, we speculated that the uc002yug.2-mediated reduction of RUNX1b and -1c in J-Lat 6.3 and ACH-2 cells might partially contribute to the activation of latent HIV-1. In primary CD4+ T cells, expression of uc002yug.2 in HAART-treated HIV-1 patients was significantly lower than that in HIV-1 patients who had not received HAART, and the expression of uc002yug.2 in HAART-naive HIV-1 patients was positively correlated with their HIV-1 loads, further supporting the notion that uc002yug.2 plays a key role in HIV-1 replication and latency.
Increasing the lncRNA uc002yug.2 has been shown to promote proliferation of cells with high endogenous levels of uc002yug.2 (26). In order to distinguish whether the effect of uc002yug.2 on cell proliferation would lead to enhanced HIV-1 replication or reactivation of latent HIV-1, we also examined the growth curve of cells stably expressing uc002yug.2 using the CCK8 assay. As the ACH-2-uc002yug.2 cells showed a growth curve similar to that of ACH-2 cells (Fig. 5F), cell proliferation was determined to not be related to the viral replication and reactivation of HIV-1. It is worth noting that overexpression of uc002yug.2 in ACH-2 cells could activate latent HIV-1 to an extent no less than that with HIV-1-reactivating reagents, including PMA, SAHA, and TNF-α (Fig. 5B, D, and E). These observations indicate the potential merit of targeting lncRNA uc002yug.2 as an activating agent for latent HIV-1. In this study, we performed qRT-PCR following the MIQE guidelines, which make the results more convincing (32–34). To confirm the authenticity of our study, we tested the stability of three reference genes (those for β-actin, GAPDH, and HMBS). The M values, which present the expression stability of reference genes, were calculated with qbase+ software (35). All three reference genes showed high reference target stability (geNorm M < 0.5). Among these reference genes, the β-actin gene, which was used for normalization in our study, showed the highest stability (M value = 0.468), followed by the GAPDH (M value = 0.475) and HMBS (M value = 0.491) genes. Furthermore, several representative experiments in our study were repeated and analyzed by normalization with the geometric means of the 3 reference genes. We found that when normalizing with the geometric means of these genes, the patterns were similar to those of data normalized with β-actin (data not shown), indicating the authenticity of our study.
Among the HIV-1 proteins, Tat has gained more attention for involvement in both the establishment and reactivation of HIV-1 latency based on to its transcriptional regulation function (31, 36). The Tat protein can directly reactivate viral transcription of latently infected cells (37). The compound Ro5-3335 was initially characterized as a Tat antagonist, which was also identified as an inhibitor of CBF leukemia through disrupting the RUNX1–CBF-β interaction (38, 39). Ro5-3335 also activates HIV-1 from latent infection synergistically with SAHA (30). Previous studies hinted that uc002yug.2 might potentially be involved in HIV-1 infection through influencing Tat expression. In this study, we transfected uc002yug.2 and Tat expression vectors into HEK293T cells and found that overexpression of uc002yug.2 greatly enhanced the expression of Tat protein (Fig. 6B). As HIV-1 harbors RUNX1 binding sites and TAR in its LTR promoter region, the enhancement of LTR activity by uc002yug.2 is possibly a function affected by multiple factors. When the binding with RUNX1 was disrupted, the restriction of RUNX1 on LTR activity was released, while the TAR-del LTR almost lost the response to uc002yug.2, indicating that both the ability to cause alternative splicing of RUNX1 pre-mRNA and the ability to increase Tat protein are involved in the function of uc002yug.2. Moreover, uc002yug.2-d275nt, which lost the ability to cause alternative splicing of RUNX1 pre-mRNA compared to the wild type, increased the HIV-1 LTR activity and Tat protein level only to a slightly lower extent, indicating that uc002yug.2-d275nt still retained the ability to stimulate the HIV-1 LTR promoter through a functional domain other than the RUNX1 binding domain. The data further support another mechanism by which uc002yug.2 reactivates latent HIV-1 that is dependent on upregulation of the Tat protein (Fig. 7). Thus, uc002yug.2 could also affect the reactivation of HIV-1 latent virus through regulating Tat protein in CD4+ T cells. However, whether increased Tat expression induced by uc002yug.2 is associated with RUNX1 and the exact mechanism of which uc002yug.2 regulates Tat protein will need to be further investigated.
Taken together, results of this study indicate that uc002yug.2 could not only enhance HIV-1 infectivity but also activate latent HIV-1 through two mechanisms, downregulating RUNX1b and -1c and increasing the Tat expression level. Previous studies and our data further support the notion that Tat protein might play the key role in the regulatory effect of uc002yug.2 on HIV-1 reactivation and that downregulated RUNX1b and -1c also might affect HIV-1 replication and latency through affecting the function of Tat protein (Fig. 9). Therefore, the accumulated data support the view that lncRNAs play regulatory and functional roles in virus-host interplay.
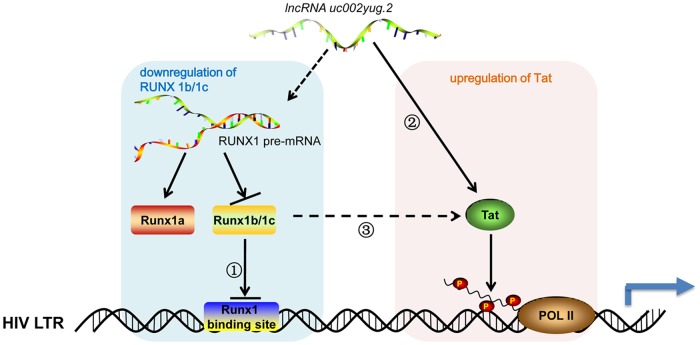
FIG 9.
Model of uc002yug.2 functional reactivation of HIV-1 uc002yug.2 may promote HIV-1 replication and reactivation through two mechanisms, which are the downregulation of RUNX1b and -1c and upregulation of Tat, leading to the activation of HIV-1 LTR. When uc002yug.2 is increased, RUNX1b and -1c would be downregulated due to alternative splicing of RUNX1 pre-mRNA, thereby reducing the suppression of RUNX1b and -1c on HIV-LTR activity (1); meanwhile, Tat protein would be upregulated and exert its transactivator function to promote HIV-1 LTR activity (2); however, whether uc002yug.2 upregulates Tat indirectly through RUNX1 needs to be further explored (3). Solid arrow, pathway with experimental data; dashed arrow, hypothetical potential pathways.
MATERIALS AND METHODS
Ethics statement.
This study was approved by the Ethics Review Committee of the First Hospital of Jilin University. Informed consent was signed by all research participants. Twenty-four HIV-1-infected patients were recruited at Changchun Center for Disease Control and Prevention, Jilin, China, of whom 12 had not received antiretroviral therapy and 12 had undergone HAART for more than 3 years, with undetectable HIV-1 loads in plasma. The viral loads in plasma of HIV-1-infected patients were detected with an HIV-1 load test kit (DA-Z195) from DaAn Gene Company of Sun Yat-Sen University (Guangzhou, China). Characteristics of HIV-1-infected patients are listed in Table 1.
Plasmid construction.
The full-length lncRNA uc002yug.2 was amplified using PCR with total cDNA of human peripheral blood mononuclear cells (PBMCs) as the template and cloned into the VR1012 vector, which was generously provided by Vical (San Diego, CA). uc002yug.2-d275nt was generated in full-length lncRNA uc002yug.2 with deletion of 275 nt at the 3′ terminus, which is responsible for the alternative splicing of Runx1 via binding with RUNX1 pre-mRNA. RUNX1a-myc and RUNX1b-myc were constructed by amplifying the corresponding fragment of human cDNA with a myc epitope tag sequence at the 3′ terminus. RUNX1c-Myc was a gift from A. D. Friedman (The Johns Hopkins Oncology Center, Division of Pediatric Oncology). Hemagglutinin (HA)-tagged CBF-β (CBF-β–HA) has been described previously (29). The HIV-1 infectious clone pNL4-3 and pNL4-3-deltaE-EGFP were obtained from the AIDS Research and Reference Reagents Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). Tat-HA was constructed by amplifying two exons of Tat with a HA epitope tag sequence at its 3′ terminus using pNL4-3 as the template, and then the two fragments were ligated and cloned into the VR1012 vector. The LTR-luciferase plasmid was constructed by amplification of the LTR of pNL4-3 and cloned into the pGL3-basic vector (Promega, Madison, WI). RUNX-mut and TAR-del luciferase plasmids were created in the HIV LTR-luciferase plasmid with PCR-based mutagenesis to construct a mutant affecting RUNX binding with HIV-1 LTR and deleting the TAR hairpin of HIV-1 LTR, respectively. The primer pairs for plasmid construction are listed in Table 2, and all constructs were verified by sequencing. An overview of constructed plasmids is provided in Table 3.
TABLE 2.
Primers used for plasmid construction in this study
| Primer name | Primer direction | Sequence (5′–3′) |
|---|---|---|
| uc002yug.2-F | Forward | GCGTCGACAAGCAGAGCTCCATCAGTC |
| uc002yug.2-R | Reverse | CGGGATCCGAGATGGAGTCTCATTCCATTTCCCAG |
| uc002yug.2-lenti-F | Forward | CCCTCGAGAAGCAGAGCTCCATCAGTC |
| uc002yug.2-lenti-R | Reverse | CGGGATCCGAGATGGAGTCTCATTCCATTTCCCAGGCTGAAGTGCAAC |
| uc002yug.2-d275nt-F | Forward | ATTTCAAGATGGGTCAGGATCCAGATCTGCTGTGCC |
| uc002yug.2-d275nt-R | Reverse | CAGCAGATCTGGATCCTGACCCATCTTGAAATTC |
| uc002yug.2sh-F | Forward | CCGGGCTGGTAGATGCCATTTGTCTCGAGACAAATGGCATCTACCAGCTTTTTG |
| uc002yug.2sh-R | Reverse | AATTCAAAAAGCTGGTAGATGCCATTTGTCTCGAGACAAATGGCATCTACCAGC |
| RUNX1a-Myc-F | Forward | GCGTCGACACCATGCGTATCCCCGTAGATG |
| RUNX1a-Myc-R | Reverse | CGGGATCCTTAAAGATCTTCTTCTGATATGAGTTTTTGTTCTTAACATCTCCAGGGTGCTG |
| RUNX1b-Myc-F | Forward | AAATATGCGGCCGCACCATGCGTATCCCCGTAGATGC |
| RUNX1b-Myc-R | Reverse | GCTCTAGATTAAAGATCTTCTTCTGATATGAGTTTTTGTTCTCAGTAGGGCCTCCACACG |
| CBFβ-HA-F | Forward | GCTAGCAAGATGCCGCGCGTCGTG |
| CBFβ-HA-R | Reverse | AAGCTTACTACGCGTAATCTGGGACGTCGTAAGGGTAGGGTCTTGTTGTCTTCTTGCC |
| HIV-LTR-F | Forward | CGGGGTACCTGGAAGGGCTAATTTGGTCCCA |
| HIV-LTR-R | Reverse | CGCGGATCCTGCTAGAGATTTTCCACACTGA |
| Tat-exon1-F | Forward | GCGTCGACACCATGGAGCCAGTAGATCCTAGACT |
| Tat-exon1-R | Reverse | GGGATTGGGAGGTGGGTTGCTTTGATAGAGAAACTTGATG |
| Tat-exon2-F | Forward | TTCTCTATCAAAGCAACCCACCTCCCAATCCCGAGGGGAC |
| Tat-exon2-R | Reverse | CGGGATCCTTACGCGTAATCTGGGACGTCGTAAGGGTAAATCGAATGGATCTGTCTCTG |
| dTAR-F | Forward | GGGTCTCTCTGGTTAGTCTGGCTAACTAGGGAACCC |
| dTAR-R | Reverse | TCCCTAGTTAGCCAGACTAACCAGAGAGACCCAGTAC |
| RUNXMut-F | Forward | ATCCTTGATCTGTGGATCTCACACACACAAGGCTACTTCC |
| RUNXMut-R | Reverse | GGAAGTAGCCTTGTGTGTGTGAGATCCACAGATCAAGGAT |
TABLE 3.
Overview of plasmids constructed in this study
| Plasmid name | Vector | Restriction enzyme sites | Tag |
|---|---|---|---|
| uc002yug.2 | VR1012 | SalI and BamHI | |
| uc002yug.2-lenti | pLVX-Puro | XhoI and BamHI | |
| uc002yug.2sh | pLKO.1 | AgeI and EcoRI | |
| HIV-LTR | pGL3-basic | KpnI and HindIII | |
| RUNX1a-Myc | VR1012 | SalI and BamHI | C-terminal Myc |
| RUNX1b-Myc | VR1012 | NotI and XbaI | C-terminal Myc |
| CBFβ-HA | pcDNA3.1 | NheI and HindIII | C-terminal HA |
| Tat-HA | VR1012 | SalI and BamHI | C-terminal HA |
RNA isolation and real-time qRT-PCR.
RNA isolation and real-time qRT-PCR were performed by following the MIQE guidelines (32–34). Briefly, RNA was isolated with TRIzol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). RNA samples were treated with RQ-1 DNase (Promega) before reverse transcription. The cDNA generation was performed with a Transcriptor first-strand cDNA synthesis kit (Roche, Basel, Switzerland) according to the supplier's instructions. The real-time qRT-PCR assay was performed on a Roche 480 instrument (Roche) using the FastStart Universal SYBR green Master (Rox) (Roche). The real-time qRT-PCR amplifications were carried out at least in duplicate in a reaction volume of 20 μl consisting of 10 μl of SYBR green solution, 0.5 μl of 10 μmol/liter of each primer, 2 μl of cDNA template, and 7 μl of double-distilled H2O. Amplification of the target fragment was carried out as follows: initial activation at 95°C for 2 min, followed by 45 cycles of 95°C for 15 s, 57°C for 15 s, and 68°C for 20 s. qbase+ software (Biogazelle) was applied to characterize the expression stability of three reference genes (β-actin, GAPDH, and HMBS) (35), and these genes showed high reference target stability (geNorm M < 0.5). To minimize the bias that may be caused by heterogeneity of HIV-1 patients, uc002yug.2 expression in HIV-1 patients was calculated by normalization with the geometric means of the three reference genes (32–35). Primers for real-time qRT-PCR are listed in Table 4.
TABLE 4.
Primers used for qRT-PCR in this study
| Primer name | Primer direction | Sequence (5′–3′) |
|---|---|---|
| uc002yug.2-RT-F | Forward | CCTTCTGCCATGATTGTGAG |
| uc002yug.2-RT-R | Reverse | CTGTGAGACGGTGAAGCA |
| RUNX1a-RT-F | Forward | CCGAGAACCTCGAAGACATC |
| RUNX1a-RT-R | Reverse | GCTGTGTCTTCCTCCTGCAT |
| RUNX1b/1c-RT-Fa | Forward | CGACTCTCAACGGCACCCGA |
| RUNX1b/1c-RT-Ra | Reverse | ATGGCCGACATGCCGATGCC |
| CUL4B-RT-F | Forward | CCCCTCCCCGAGGCTCTTAATTCT |
| CUL4B-RT-R | Reverse | CCACGTACACAGGGAAAAGAGCACA |
| PSMD11-RT-F | Forward | GGGCTTCCAAGCAAGTCCAGACA |
| PSMD11RT-R | Reverse | ACTGGACCATGCTGCCCTGGAA |
| β-Actin-RT-F | Forward | ACCGAGCGCGGCTACAG |
| β-Actin-RT-R | Reverse | CTTAATGTCACGCACGATTTCC |
| GAPDH-RT-F | Forward | TGCACCACCAACTGCTTAGC |
| GAPDH-RT-R | Reverse | GGCATGGACTGTGGTCATGAG |
| HMBS-RT-F | Forward | GGCAATGCGGCTGCAA |
| HMBS-RT-R | Reverse | GGGTACCCACGCGAATCAC |
Since there is no specific sequence for primer design to distinguish RUNX1b and RUNX1c with qRT-PCR, this pair of primers was used to detect the total mRNA of RUNX1b and RUNX1c.
Cells and transfection.
Human embryonic kidney 293T (HEK293T) (catalog no. CRL-11268), HeLa (catalog no. CCL-2), and TZM-bl (catalog no. PTA-5659) cells were obtained from American Type Culture Collection (ATCC; Manassas, VA) and cultured as a monolayer in Dulbecco's modified Eagle's medium and minimum essential medium (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; PAN-Biotech, Germany) and maintained at 37°C with 5% CO2 in a humidified atmosphere. J-Lat 6.3 (catalog no. 9846), ACH-2 (catalog no. 349), and CEM (catalog no. 117) cells were obtained from the AIDS Research and Reference Reagents Program, Division of AIDS, NIAID, NIH. Jurkat (catalog no. TIB-152; ATCC), J-Lat 6.3, and CEM cells were cultured in RPMI 1640 medium supplemented with 10% FBS, l-glutamine, and penicillin-streptomycin. ACH-2 cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, HEPES, and l-glutamine. Whole-blood samples were collected from research participants. The PBMCs were isolated through Ficoll gradient centrifugation, and the CD4+ T lymphocytes were then purified from the PBMCs with anti-CD4-specific antibody-coated microbeads (Miltenyi Biotec, Germany) according to the manufacturer's instructions. Adherent cell lines (HEK293T, HeLa, and TZM-bl) were seeded in 12-well plates at a density of 3 × 105 cells/well with a 70% confluent monolayer and were cultured overnight. HEK293T cells were transfected using Lipofectamine 2000 (Invitrogen), while HeLa and TZM-bl cells were transfected using Lipofectamine 3000 (Invitrogen). Human CD4+ T cells were nucleofected using an Amaxa human T-cell Nucleofector kit (Lonza, Switzerland) with the program U-014.
Chemical synthesis of siRNA.
To knock down RUNX1b and -1c, chemically synthesized short interfering RNA (siRNA) and nonspecific control were purchased from RiboBio (Guangzhou, China). The siRNA sequences are as follows: sense, 5′-UGACAACCCUCUCUGCAGAUU-3′, and antisense, 5′-UCUGCAGAGAGGGUUGUCAUU-3′; these sequences target RUN1b and -1c but not RUNX1a.
HIV-1 infection and reactivation.
HeLa cell-derived TZM-bl cells, which contain an integrated HIV-1 LTR promoter, were used to detect the infectivity of HIV-1. TZM-bl cells were seeded in a 24-well plate and infected with supernatant produced by cells transfected with pNL4-3, and luciferase activity was measured with a GloMax 20/20 luminometer (Promega) at 48 h postinfection.
HIV-1 was produced by transfecting pNL4-3 plasmids into HEK293T cells with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Forty-eight hours later, the supernatant was collected and quantified using an HIV-1 p24 ELISA kit. A total of 2 × 106 CD4+ T cells isolated from healthy donors were nucleofected with uc002yug.2 or control vector. Forty-eight hours posttransfection, the cells were infected with the equivalent of 2 ng of HIV-1 p24 antigen. After 3 h of incubation, cells were washed with phosphate-buffered saline (PBS) three times and cultured in RPMI 1640 medium supplemented with interleukin 2 (IL-2; 10 ng/ml). The culture supernatants were collected at various days postinfection and detected using the HIV-1 p24 ELISA kit according to the manufacturer's instructions.
To measure the virus reactivation, HIV-1 latently infected cells, J-Lat 6.3 and ACH-2, were stably overexpressing uc002yug.2 or treated with a latent reactivation agent, PMA (1 μM), TNF-α (1 μM), or suberoylanilide hydroxamic acid (SAHA; 0, 1, or 10 mM) for 48 h. The J-Lat 6.3 cells were analyzed by flow cytometry (FACSCalibur; BD, Franklin Lakes, NJ) to determine the green fluorescent protein (GFP)-positive cells with green fluorescence (FL1, 488 nm), while ACH-2 cells were harvested for immunoblotting.
Luciferase assay.
HEK293T cells were seeded in a 12-well cell culture plate. Transfection was performed using Lipofectamine 2000 according to the manufacturer's instructions. A dual-luciferase reporter assay was performed at 48 h posttransfection using a Promega dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions with a GloMax 20/20 luminometer (Promega).
Immunoblot analysis.
Cells were harvested at 48 h after transfection, infection, or stimulation. Samples were boiled in 1× loading buffer (0.08 M Tris, pH 6.8, with 2.0% SDS, 10% glycerol, 0.1 M dithiothreitol, and 0.2% bromophenol blue), followed by separation by 12% SDS-PAGE. Proteins were transferred onto nitrocellulose membranes, which were then incubated with various primary antibodies against the proteins of interest. Secondary antibodies were alkaline phosphatase (AP)-conjugated anti-human, anti-rabbit, or anti-mouse (Jackson ImmunoResearch, West Grove, PA) antibodies. Proteins were visualized with the substrate 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium (NBT), obtained from Sigma (St. Louis, MO).
The anti-HA antibody (1:1,000 dilution, mouse monoclonal, 901514; Biolegend, San Diego, CA), anti-myc antibody (1:1,000 dilution, mouse monoclonal, 05-724; Millipore, Burlington, MA), anti-β-actin antibody (1:1,000 dilution, mouse monoclonal, A00702-100; Genscript, Piscataway, NJ), and anti-p24 antibody (1:1,000 dilution, AIDS Research and Reference Reagents Program, catalog no. 1513) were used as the primary antibodies. The secondary antibodies were purchased from Jackson ImmunoResearch (115-055-062).
Lentiviral production and transduction.
The full-length human lncRNA-uc002yug.2 sequence was cloned into the lentiviral expression vector pLVX-IRES-neo (Clontech Laboratories Inc., San Francisco, CA). The uc002yug.2 shRNA was subcloned into the pLKO.1-puro shRNA expression vector. Using a four-plasmid transient-cotransfection method, replication-defective vesicular stomatitis virus G protein-pseudotyped viral particles were packaged in HEK293T cells. Lentiviruses were harvested and concentrated. To generate a cell line for stable uc002yug.2 overexpression, Jurkat cells or ACH-2 cells were infected with a control lentivirus or lentivirus overexpressing uc002yug.2. To generate a cell line for stable knockdown of uc002yug.2, a control lentivirus or the lentivirus containing shRNA targeting uc002yug.2 was used to infect HEK293T cells. At 48 h postinfection, puromycin (1 μg/ml) was added to the medium for selection. In order to remove the dead cells, the cell culture medium was replaced every 2 days. Five to 7 days later, expression of uc002yug.2 was detected by qRT-PCR, and subsequent experiments were performed.
Cell proliferation assay.
A total of approximately 1 × 103 ACH-2-plvx or ACH2-uc002yug.2 cells were plated in 96-well plates. Cell proliferation was assessed using Cell Counting Kit-8 (Transgen Biotech, Beijing, China) according to the manufacturer's protocol. Absorbance at 450 nm was recorded using an iMark microplate reader (Bio-Rad, Hercules, CA) at 24 h, 48 h, 72 h, and 96 h.
Reactivation of latent HIV-1 from HAART-treated HIV-1-infected individuals.
Purified resting CD4+ T cells isolated from HIV-1-infected individuals who had undergone HAART were nucleofected with uc002yug.2 or control vector, and then the cells were cultured for another 2 days. Cells were collected for qRT-PCR or stimulated with phytohemagglutinin M (PHA-M) (5 ng/ml; Sigma-Aldrich) for 7 days. HIV-1 activation was detected by measuring the released p24 amount in the supernatants by ELISA.
Statistical analysis.
All data represent three independent experiments and are presented as means ± standard deviations (SDs). Statistical significance was calculated using Student's t test. Significant differences are indicated in figures as follows: *, P < 0.05, and **, P < 0.01.
ACKNOWLEDGMENTS
We thank C. Y. Dai for the critical reagents and P. T. Sarkis for editorial assistance. We thank the AIDS Research and Reference Reagents Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), for critical reagents. We thank A. D. Friedman of The Johns Hopkins Oncology Center, Division of Pediatric Oncology, for the kind gift of plasmid RUNX1c-Myc.
This work was supported in part by funding from the National Natural Science Foundation of China (no. 81672004 and 31270202), the Jilin University Science and Technology Innovative Research Team (JLUSTIRT, 2017TD-05), the Chinese Ministry of Science and Technology (2012CB911102 and 2013ZX10001-005), the Science and Technology Department of Jilin Province (20160101044JC), the Health and Family Planning Commission of Jilin Province (2013Z066), and the Key Laboratory of Molecular Virology, Jilin Province (20102209).
We have no conflict of interest to declare.
REFERENCES
- 1.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. 2009. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL. 2009. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A 106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutschner T, Hammerle M, Eissmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, Zornig M, MacLeod AR, Spector DL, Diederichs S. 2013. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res 73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Y, Liu Y, Nie L, Gui Y, Cai Z. 2013. Inducing cell proliferation inhibition, apoptosis, and motility reduction by silencing long noncoding ribonucleic acid metastasis-associated lung adenocarcinoma transcript 1 in urothelial carcinoma of the bladder. Urology 81:209.e1–209.e7. doi: 10.1016/j.urology.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Pandey GK, Mitra S, Subhash S, Hertwig F, Kanduri M, Mishra K, Fransson S, Ganeshram A, Mondal T, Bandaru S, Ostensson M, Akyurek LM, Abrahamsson J, Pfeifer S, Larsson E, Shi L, Peng Z, Fischer M, Martinsson T, Hedborg F, Kogner P, Kanduri C. 2014. The risk-associated long noncoding RNA NBAT-1 controls neuroblastoma progression by regulating cell proliferation and neuronal differentiation. Cancer Cell 26:722–737. doi: 10.1016/j.ccell.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Redis RS, Vela LE, Lu W, Ferreira de Oliveira J, Ivan C, Rodriguez-Aguayo C, Adamoski D, Pasculli B, Taguchi A, Chen Y, Fernandez AF, Valledor L, Van Roosbroeck K, Chang S, Shah M, Kinnebrew G, Han L, Atlasi Y, Cheung LH, Huang GY, Monroig P, Ramirez MS, Catela Ivkovic T, Van L, Ling H, Gafa R, Kapitanovic S, Lanza G, Bankson JA, Huang P, Lai SY, Bast RC, Rosenblum MG, Radovich M, Ivan M, Bartholomeusz G, Liang H, Fraga MF, Widger WR, Hanash S, Berindan-Neagoe I, Lopez-Berestein G, Ambrosio AL, Gomes Dias SM, Calin GA. 2016. Allele-specific reprogramming of cancer metabolism by the long non-coding RNA CCAT2. Mol Cell 61:520–534. doi: 10.1016/j.molcel.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang E, He X, Yin D, Han L, Qiu M, Xu T, Xia R, Xu L, Yin R, De W. 2016. Increased expression of long noncoding RNA TUG1 predicts a poor prognosis of gastric cancer and regulates cell proliferation by epigenetically silencing of p57. Cell Death Dis 7:e2109. doi: 10.1038/cddis.2015.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell M, Kim KY, Chang PC, Huerta S, Shevchenko B, Wang DH, Izumiya C, Kung HJ, Izumiya Y. 2014. A lytic viral long noncoding RNA modulates the function of a latent protein. J Virol 88:1843–1848. doi: 10.1128/JVI.03251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noriega VM, Haye KK, Kraus TA, Kowalsky SR, Ge Y, Moran TM, Tortorella D. 2014. Human cytomegalovirus modulates monocyte-mediated innate immune responses during short-term experimental latency in vitro. J Virol 88:9391–9405. doi: 10.1128/JVI.00934-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossetto CC, Tarrant-Elorza M, Pari GS. 2013. Cis and trans acting factors involved in human cytomegalovirus experimental and natural latent infection of CD14 (+) monocytes and CD34 (+) cells. PLoS Pathog 9:e1003366. doi: 10.1371/journal.ppat.1003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma H, Han P, Ye W, Chen H, Zheng X, Cheng L, Zhang L, Yu L, Wu X, Xu Z, Lei Y, Zhang F. 2017. The long noncoding RNA NEAT1 exerts antihantaviral effects by acting as positive feedback for RIG-I signaling. J Virol 91:e02250-. doi: 10.1128/JVI.02250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morchikh M, Cribier A, Raffel R, Amraoui S, Cau J, Severac D, Dubois E, Schwartz O, Bennasser Y, Benkirane M. 2017. HEXIM1 and NEAT1 long non-coding RNA form a multi-subunit complex that regulates DNA-mediated innate immune response. Mol Cell 67:387–399.e5. doi: 10.1016/j.molcel.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Du Y, Kong G, You X, Zhang S, Zhang T, Gao Y, Ye L, Zhang X. 2012. Elevation of highly up-regulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18. J Biol Chem 287:26302–26311. doi: 10.1074/jbc.M112.342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu G, Wang Y, Lu X, He H, Liu H, Meng X, Xia S, Zheng K, Liu B. 2015. Low mir-372 expression correlates with poor prognosis and tumor metastasis in hepatocellular carcinoma. BMC Cancer 15:182. doi: 10.1186/s12885-015-1214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Chen CY, Yedavalli VS, Jeang KT. 2013. NEAT1 long noncoding RNA and paraspeckle bodies modulate HIV-1 posttranscriptional expression. mBio 4:e00596-. doi: 10.1128/mBio.00596-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras X, Barboric M, Lenasi T, Peterlin BM. 2007. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog 3:1459–1469. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YK, Mbonye U, Hokello J, Karn J. 2011. T-cell receptor signaling enhances transcriptional elongation from latent HIV proviruses by activating P-TEFb through an ERK-dependent pathway. J Mol Biol 410:896–916. doi: 10.1016/j.jmb.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedore SC, Byers SA, Biglione S, Price JP, Maury WJ, Price DH. 2007. Manipulation of P-TEFb control machinery by HIV: recruitment of P-TEFb from the large form by Tat and binding of HEXIM1 to TAR. Nucleic Acids Res 35:4347–4358. doi: 10.1093/nar/gkm443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartholomeeusen K, Fujinaga K, Xiang Y, Peterlin BM. 2013. Histone deacetylase inhibitors (HDACis) that release the positive transcription elongation factor b (P-TEFb) from its inhibitory complex also activate HIV transcription. J Biol Chem 288:14400–14407. doi: 10.1074/jbc.M113.464834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budhiraja S, Famiglietti M, Bosque A, Planelles V, Rice AP. 2013. Cyclin T1 and CDK9 T-loop phosphorylation are downregulated during establishment of HIV-1 latency in primary resting memory CD4+ T cells. J Virol 87:1211–1220. doi: 10.1128/JVI.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haaland RE, Herrmann CH, Rice AP. 2003. Increased association of 7SK snRNA with Tat cofactor P-TEFb following activation of peripheral blood lymphocytes. AIDS 17:2429–2436. doi: 10.1097/00002030-200311210-00004. [DOI] [PubMed] [Google Scholar]
- 22.Sung TL, Rice AP. 2006. Effects of prostratin on cyclin T1/P-TEFb function and the gene expression profile in primary resting CD4+ T cells. Retrovirology 3:66. doi: 10.1186/1742-4690-3-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imam H, Bano AS, Patel P, Holla P, Jameel S. 2015. The lncRNA NRON modulates HIV-1 replication in a NFAT-dependent manner and is differentially regulated by early and late viral proteins. Sci Rep 5:8639. doi: 10.1038/srep08639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Chen C, Ma X, Geng G, Liu B, Zhang Y, Zhang S, Zhong F, Liu C, Yin Y, Cai W, Zhang H. 2016. Long noncoding RNA NRON contributes to HIV-1 latency by specifically inducing tat protein degradation. Nat Commun 7:11730. doi: 10.1038/ncomms11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trypsteen W, Mohammadi P, Van Hecke C, Mestdagh P, Lefever S, Saeys Y, De Bleser P, Vandesompele J, Ciuffi A, Vandekerckhove L, De Spiegelaere W. 2016. Differential expression of lncRNAs during the HIV replication cycle: an underestimated layer in the HIV-host interplay. Sci Rep 6:36111. doi: 10.1038/srep36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Zheng J, Deng J, Zhang L, Li N, Li W, Li F, Lu J, Zhou Y. 2015. LincRNA-uc002yug.2 involves in alternative splicing of RUNX1 and serves as a predictor for esophageal cancer and prognosis. Oncogene 34:4723–4734. doi: 10.1038/onc.2014.400. [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Bae SC, Chuang LS. 2015. The RUNX family: developmental regulators in cancer. Nat Rev Cancer 15:81–95. doi: 10.1038/nrc3877. [DOI] [PubMed] [Google Scholar]
- 28.Jäger S, Kim DY, Hultquist JF, Shindo K, LaRue RS, Kwon E, Li M, Anderson BD, Yen L, Stanley D, Mahon C, Kane J, Franks-Skiba K, Cimermancic P, Burlingame A, Sali A, Craik CS, Harris RS, Gross JD, Krogan NJ. 2012. Vif hijacks CBF-beta to degrade APOBEC3G and promote HIV-1 infection. Nature 481:371–375. doi: 10.1038/nature10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Du J, Evans SL, Yu Y, Yu XF. 2012. T-cell differentiation factor CBF-beta regulates HIV-1 Vif-mediated evasion of host restriction. Nature 481:376–379. doi: 10.1038/nature10718. [DOI] [PubMed] [Google Scholar]
- 30.Klase Z, Yedavalli VS, Houzet L, Perkins M, Maldarelli F, Brenchley J, Strebel K, Liu P, Jeang KT. 2014. Activation of HIV-1 from latent infection via synergy of RUNX1 inhibitor Ro5-3335 and SAHA. PLoS Pathog 10:e1003997. doi: 10.1371/journal.ppat.1003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamori D, Ueno T. 2017. HIV-1 Tat and viral latency: what we can learn from naturally occurring sequence variations. Front Microbiol 8:80. doi: 10.3389/fmicb.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 33.Bustin SA, Benes V, Garson J, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley G, Wittwer CT, Schjerling P, Day PJ, Abreu M, Aguado B, Beaulieu JF, Beckers A, Bogaert S, Browne JA, Carrasco-Ramiro F, Ceelen L, Ciborowski K, Cornillie P, Coulon S, Cuypers A, De Brouwer S, De Ceuninck L, De Craene J, De Naeyer H, De Spiegelaere W, Deckers K, Dheedene A, Durinck K, Ferreira-Teixeira M, Fieuw A, Gallup JM, Gonzalo-Flores S, Goossens K, Heindryckx F, Herring E, Hoenicka H, Icardi L, Jaggi R, Javad F, Karampelias M, Kibenge F, Kibenge M, Kumps C, Lambertz I, Lammens T, Markey A, Messiaen P, Mets E, Morais S, Mudarra-Rubio A, Nakiwala J, Nelis H, Olsvik PA, Pérez-Novo C, Plusquin M, Remans T, Rihani A, Rodrigues-Santos P, Rondou P, Sanders R, Schmidt-Bleek K, Skovgaard K, Smeets K, Tabera L, Toegel S, Van Acker T, Van den Broeck W, Van der Meulen J, Van Gele M, Van Peer G, Van Poucke M, Van Roy N, Vergult S, Wauman J, Tshuikina-Wiklander M, Willems E, Zaccara S, Zeka F, Vandesompele J.. 2013. The need for transparency and good practices in the qPCR literature. Nat Methods 10:1063–1067. doi: 10.1038/nmeth.2697. [DOI] [PubMed] [Google Scholar]
- 34.Bustin SA, Wittwer CT. 2017. MIQE: a step toward more robust and reproducible quantitative PCR. Clin Chem 63:1537–1538. doi: 10.1373/clinchem.2016.268953. [DOI] [PubMed] [Google Scholar]
- 35.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruelas DS, Greene WC. 2013. An integrated overview of HIV-1 latency. Cell 155:519–529. doi: 10.1016/j.cell.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donahue DA, Bastarache SM, Sloan RD, Wainberg MA. 2013. Latent HIV-1 can be reactivated by cellular superinfection in a Tat-dependent manner, which can lead to the emergence of multidrug-resistant recombinant viruses. J Virol 87:9620–9632. doi: 10.1128/JVI.01165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunningham L, Finckbeiner S, Hyde RK, Southall N, Marugan J, Yedavalli VR, Dehdashti SJ, Reinhold WC, Alemu L, Zhao L, Yeh JR, Sood R, Pommier Y, Austin CP, Jeang KT, Zheng W, Liu P. 2012. Identification of benzodiazepine Ro5-3335 as an inhibitor of CBF leukemia through quantitative high throughput screen against RUNX1-CBFbeta interaction. Proc Natl Acad Sci U S A 109:14592–14597. doi: 10.1073/pnas.1200037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu MC, Schutt AD, Holly M, Slice LW, Sherman MI, Richman DD, Potash MJ, Volsky DJ. 1991. Inhibition of HIV replication in acute and chronic infections in vitro by a Tat antagonist. Science 254:1799–1802. [DOI] [PubMed] [Google Scholar]