Abstract
We evaluated the contribution of brown adipose tissue (BAT) sympathetic innervation on central leptin-mediated weight loss. In a short- and long-term study, F344BN rats were submitted to either a denervation of interscapular BAT (Denervated) or a sham operation (Sham). Animals from each group received the Ob (Leptin) or green fluorescent protein (GFP; Control) gene through a single injection of recombinant adeno-associated virus delivered centrally. Changes in body weight were recorded for 14 or 35 days, after which adipose tissues and skeletal muscles were weighed. In both studies, hypothalamic phosphorylated STAT3 (P-STAT3) was significantly higher in Sham-Leptin and Denervated-Leptin groups compared with their respective Control groups (P < 0.01), indicating that leptin signaling was enhanced at the end point. We measured uncoupling protein 1 (UCP1), a marker of BAT thermogenic activity, and found a significant induction in Leptin in Sham animals (P < 0.001) but not in Denervated animals, demonstrating that BAT UCP1 protein was only induced in Sham rats. Both Sham-Leptin and Denervated-Leptin rats lost ~15% of their initial body weight (P < 0.001) by day 14 and reached a maximum of 18% body weight loss that stabilized over week 3 of treatment, indicating that sympathetic outflow to BAT is not required for leptin-mediated weight loss. In summary, interscapular BAT (iBAT) denervation did not prevent body weight loss following central leptin gene delivery. The present data show that sympathetic innervation of iBAT is not essential for leptin-induced body weight loss.
Keywords: iBAT, leptin, P-STAT3, thermogenesis, UCP1, weight loss
INTRODUCTION
The discovery of leptin in 1995 (37) generated a wave of excitement in the field of obesity that rapidly dissipated when researchers established that most overweight individuals display high levels of circulating leptin and are resistant to the effects of leptin (31). Despite the failure of leptin as an antiobesity therapy, investigations on its biology still persist because leptin remains one of the most potent catabolic hormones described in the literature and its weight-reducing effects are not fully understood. Central leptin gene delivery in young and lean rats induces a robust lipolytic activity that results in almost complete depletion of fat stores (30). Leptin functions as an adiposity reporter to its receptors (ObRs) that, upon activation, reduce appetite and trigger strong catabolic signals leading to body weight loss. The main mechanisms through which leptin reduces body mass appear to be centrally mediated and include hypophagia (14), enhanced lipolysis (36), and stimulation of brown adipose tissue (BAT) thermogenesis (17, 29).
Thermogenesis is a physiological process of heat production aimed at preserving body temperature under cold conditions (5). Shivering is the first line of defense; however, prolonged muscle twitches are detrimental to myocyte health. Therefore, recruitment of nonshivering thermogenesis (NST) enables long-term thermoregulation. NST is a process of futile energy expenditure leading to heat production (6). Specifically, NST increases whole body energy expenditure by decreasing biochemical efficiency (18, 34). BAT is specialized in this process due to the abundance and activity of uncoupling protein 1 (UCP1), a protein that dissipates mitochondrial proton motive force as heat (10, 11, 13).
Central administration of leptin in rats induces Ucp1 gene and protein content in interscapular BAT (iBAT) (28, 29). Moreover, Ucp1 was shown to contribute to the fat-reducing effect of leptin (26). Leptin also enhances sympathetic nerve activity and norepinephrine turnover in iBAT (7, 15), thus supporting a role of iBAT thermogenesis in leptin-induced body weight loss. We previously reported that Ucp1 gene induction by leptin is dependent on iBAT sympathetic nerve activity (28). It is well accepted that induction of iBAT UCP1 is involved in leptin-induced weight loss in rodents. However, the importance of iBAT UCP1 to the overall leptin response has never been ascertained. Given that central leptin administration also enhances other catabolic pathways, such as muscle thermogenesis (16, 17), we postulated that induction of iBAT UCP1 through sympathetic outflow may be dispensable for leptin-induced body weight loss. Our hypothesis is further supported by previous findings showing that Ucp1−/− mice are resistant to diet-induced obesity (20) and can adapt to cold environment (32).
To determine whether sympathetic innervation of iBAT is necessary for central leptin-induced body weight loss, we conducted a short- and long-term study employing a recombinant adeno-associated viral (rAAV) construct encoding either Ob (Leptin) or a green fluorescent protein (GFP; Control) gene in iBAT-denervated (Denervated) or sham-operated rats (Sham). Changes in body weight, body composition, and food intake were recorded for 14 days (short-term study) or 35 days (long-term study), after which adipose tissues and skeletal muscles were weighed.
METHODS
Animals.
Six-month-old male Fisher 344 × Brown Norway (F344BN) rats were obtained from the National Institute on Aging Colony at Charles River Laboratories (Wilmington, MA). Adult F344BN rats were selected for their relatively stable body weight under ad libitum access to food compared with other rat strains. Upon arrival, animals were housed individually on a 12:12-h light-dark cycle, and ambient temperature was maintained at 20–23°C. All rats were allowed at least 1 wk to acclimate to their new environment before beginning any experiment. Rats were fed a standard rodent chow (18% kcal from fat, no sucrose, 3.1 kcal/g, Diet 2018; Harlan Teklad, Madison, WI). Health status, body weight, and food intake were monitored daily throughout the study. All experimental protocols were approved by the University of Florida’s Animal Care and Use Committee and in compliance with the Guide for the Care and Use of Laboratory Animals.
Surgeries and groups.
Two surgeries were performed on each animal. For both interventions, rats were anesthetized with isoflurane (2–3%) and administered two analgesics: buprenorphine (0.025 mg/kg sc) and carprofen (5 mg/kg sc) every 24 h for 3 days. Surgical procedures were performed using aseptic techniques. Initially, animals were randomly submitted to either interscapular brown adipose tissue denervation (Denervated) or a sham operation (Sham) as we previously described (19). Two weeks later, after a complete recovery, rats were then submitted to the second surgical intervention. A recombinant adeno-associated virus (rAAV) vector encoding green fluorescent protein (GFP; Control) or Ob gene (Leptin) was delivered through a single injection (3 µl) into the third ventricle using the following coordinates: 1.3 mm anterior to bregma, 0.0 mm from midline to depth of 9.6 mm ventral from the surface of the skull, at an angle of 20° (27). These coordinates were validated using a bromothymol blue dye. The pTR(2)ObW construct encoding the leptin transgene was packaged into rAAV serotype 1 as previously described (38), with the exception that the AAV1 helper plasmid pKRAP1A was used. Dot blot titer of rAAV1-Leptin and GFP contained 5.86 × 1012 and 4.43 × 1012 viral genomes per milliliter, respectively. There were four groups in total: Sham-Control, Denervated-Control, Sham-Leptin, and Denervated-Leptin.
Determination of body composition using time-domain nuclear magnetic resonance.
Body composition was determined weekly starting on day 0 using time-domain nuclear magnetic resonance (TD-NMR; Minispec; Bruker Optics, The Woodlands, TX). The MiniSpec quantifies three components of body composition (fat mass, free body fluid, and lean body mass). The TD-NMR acquires and analyzes signals from all protons in the sample area. Scans were acquired while the rats were restrained into a cylindrical device inserted into the analyzer. The final values comprised the average of two scans for each animal.
Tissue collection, harvesting, and preparation.
Rats were euthanized at week 2 (short-term study) or week 5 (long-term study) under anesthesia (5% isoflurane) 3–6 h after the end of their light cycle by thoracotomy and exsanguination. Several organs and tissues were removed and weighed (Mettler AE 163): hypothalamus, fat depots (mesenteric, perirenal, epididymal, retroperitoneal, and iBAT), and muscles (gastrocnemius, soleus, plantaris, tibialis anterior, and extensor digitorum longus). The hypothalamus was dissected from the brain by a medial incision to the piriform lobes, caudal to the optic chiasm and anterior to the cerebral crus to a depth of 2.5 mm. The hypothalamus and iBAT were sonicated in 270 μl homogenization buffer (10 mM Tris·HCl pH 6.9 and 2% SDS in the presence of phosphatase/protease inhibitors; Thermo Scientific, Rockford, IL). The homogenates, tissues, and plasma samples were stored at −80°C until analyses were performed.
Western analyses.
Protein lysates were separated on a SDS-PAGE gel and transferred to nitrocellulose membranes. Immunoreactivity was detected with ECL prime (GE Healthcare, Piscataway NJ), scanned with a ChemiDoc XRS+ (Bio-Rad, Hercules, CA) and quantified using ImageJ software. All values, including Controls, were normalized to the mean of the Sham-Control group and reported as a percentage. For iBAT thermogenesis capacity, immunoreactivity was assessed with antibodies against UCP1 (Abcam, Cambridge, MA) and normalized to β-tubulin as a loading control. Phospho-STAT3 (P-STAT3; Cell Signaling, Danvers, MA) was determined by comparing the signals obtained using antibodies specific to the phosphorylated protein relative to the signals recorded using GAPDH antibody.
Determination of tissue triglyceride content.
Liver and muscle triacylglycerol concentrations were estimated from glycerol released after ethanolic KOH hydrolysis using a commercial kit (Sigma, St. Louis, MO). Frayn and Maycock (12) have shown that omitting removal of phospholipids leads to only a ±2% error in the determination of tissue triacylglycerol content.
Serum leptin.
Serum leptin levels were determined by enzyme immunoassays (rat leptin ELISA kit, EZRL-83K; Milipore, Waltham, MA). Leptin was measured in the blood that was collected during euthanasia (fed state).
RT-PCR.
Total RNA was isolated from the hypothalamus with TRI reagent (Sigma-Aldrich). Two micrograms of RNA were reverse-transcribed into complementary DNA using high-capacity complementary DNA reverse transcription kits (Applied Biosystems, Waltham, MA). The gene expression of leptin was determined with SYBR Green Supermix using primer sets designed to amplify mutant leptin generated from the vector (forward: 5′-GGCAACGTGCTGGTTATTGT-3′ and reverse: 5′-ATATCCATCACACTGGCGGC-3′) but not from the native sequence. Gapdh (forward: 5′-TCTCTGCTCCTCCCTGTTCT-3′ and reverse: 5′-TACGGCCAAATCCGTTCACA-3′) was used as the housekeeping gene. The IQ (Bio-Rad) was used to detect the amplification level and programmed with an initial step of 3 min at 95°C, followed by 40 cycles for 5 s at 95°C and 15 s at 60°C. All reactions were run in duplicate, and the average of threshold cycle (CT) was used for quantification. The relative quantification of the target genes was determined using the ΔΔCT method. Briefly, the CT values of the target genes were normalized (ΔCT = CTTarget − CTGapdh) and compared with a calibrator (ΔΔCT = ΔCTSample − ΔCTCalibrator). Relative expression (RQ) was calculated using the IQ software (Bio-Rad).
Statistical analyses.
Results are expressed as means ± SE. Differences between means were tested for statistical significance (P < 0.05) using a two-way ANOVA with repeated measures for longitudinal analyses using time and groups as main factors. For single-point analyses, two-way ANOVA was performed using iBAT denervation and leptin as main factors. A Bonferroni post hoc test was subsequently employed in the case of significant event (P < 0.05).
RESULTS
Confirmation of leptin gene transduction and denervation.
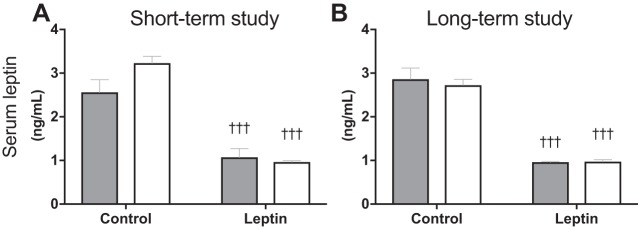
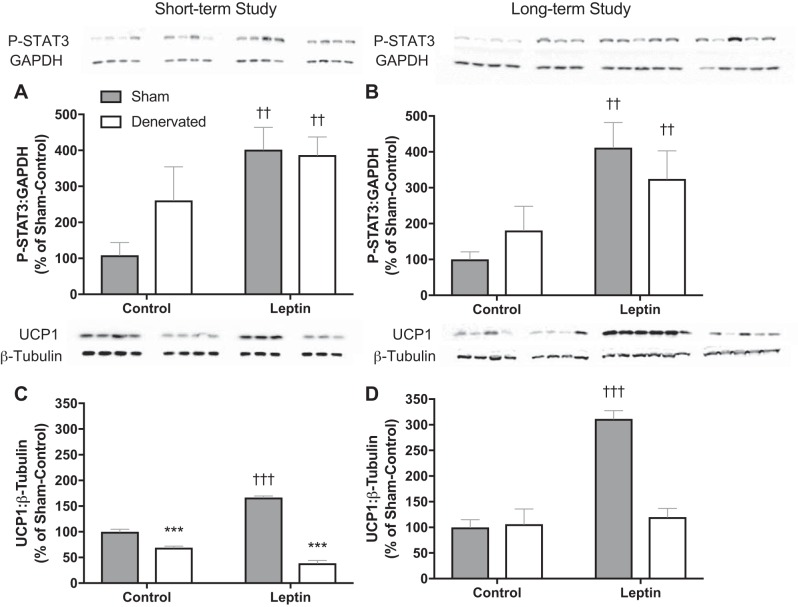
The leptin vector contains a secretory sequence so that leptin is secreted into the third ventricle and can activate leptin receptors throughout the brain. For this reason, we utilized the rAAV serotype, one that readily infects the cells lining the third ventricle but is less specific for neuronal cells and would, therefore, help preserve native neural circuit. Four weeks after vector delivery, leptin transgene expression was 3,000-fold of that amplified in animals treated with the control vector (separate animals, same age and strain; Fig. 1: P < 0.001). We have previously demonstrated that the leptin gene delivered using the same serotype increases cerebrospinal fluid leptin levels by 75% without increasing peripheral leptin levels (30). We here show that leptin-treated animals in the present studies also had significantly lower serum leptin levels compared with their respective control groups (Fig. 2, A and B; P < 0.001). Gene transduction following viral vector delivery in the third ventricle of F344BN was previously confirmed by immunohistochemistry in the brain of rAAV-GFP-injected control rats (9). Six weeks after rAAV injection GFP+ cells were distributed in midline structures along the site of injection in the third ventricle, extending from the anterior commissure to the posterior hypothalamus (9). Central leptin receptor activation was determined by measuring phosphorylation of STAT3 (P-STAT3), a signaling event downstream to leptin receptor b (Ob-Rb) activation. P-STAT3 is essential for leptin regulation of feeding and energy expenditure (35). Both Sham-Leptin and Denervated-Leptin groups exhibited a significantly higher hypothalamic P-STAT3 content (Fig. 3, A and B; P < 0.01) relative to their respective Control group. Indeed, 2 or 5 wk of central leptin gene therapy resulted in a fourfold enrichment in hypothalamic P-STAT3 (Fig. 3, A and B; P < 0.01). We have shown that leptin induces Ucp1 expression through sympathetic outflow (28). Therefore, one method to confirm the absence of sympathetic nerve activity in iBAT is to quantify UCP1 protein content. As expected, UCP1 protein was only induced in Sham-Leptin animals with a nearly twofold increase in the short-term study and a greater than threefold increase in the long-term study (Fig. 3, C and D; P < 0.001). In addition, in the short-term study, there was a small decrease in UCP1 with denervation (Fig. 3C; P < 0.001).
Fig. 1.
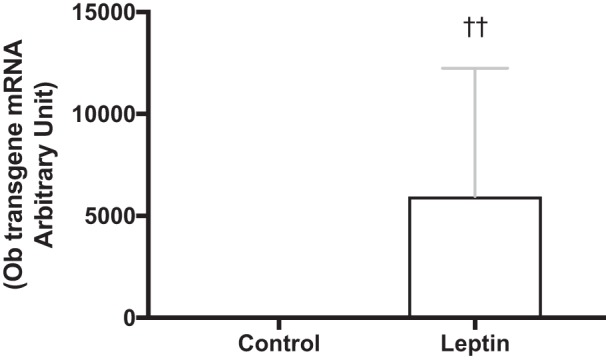
Confirmation of leptin transgene expression in the brain. The hypothalamus from separate rats (same age and strain) that underwent the same treatment as in the long-term study were used to confirm expression of leptin transgene. Values are reported as fold expression levels based on levels recorded in animals injected with the Control vector (n = 10/group). ††P < 0.01, Leptin significantly different from Control group.
Fig. 2.
Serum leptin levels. A and B: serum leptin levels measured at the end of the short-term and the long-term study, respectively. †††P < 0.001, significant difference between Leptin and respective Control groups. Short-term study: Sham-Control (n = 7), Sham-Leptin, Denervated-Control, and Denervated-Leptin (n = 8/group). Long-term study: Sham-Control (n = 8), Sham-Leptin (n = 10), Denervated-Control (n = 8), and Denervated-Leptin (n = 9).
Fig. 3.
Validation of the experiment model used. A and B: to assess leptin signaling activity, phosphorylation of STAT3 was assessed in the hypothalamus of rats at the end of the short-term and the long-term study, respectively. C and D: as a measure of interscapular brown adipose tissue (iBAT) thermogenic activity, uncoupling protein 1 (UCP1) content was measured at the end of the short-term and long-term study, respectively. Values are expressed as means ± SE. ††P < 0.01, †††P < 0.001, significant difference between Leptin and respective Control groups. ***P < 0.001, significant difference between Sham and respective Denervated groups. Short-term study: Sham-Control (n = 7), Sham-Leptin, Denervated-Control, and Denervated-Leptin (n = 8/group). Long-term study: Sham-Control (n = 8), Sham-Leptin (n = 10), Denervated-Control (n = 8), and Denervated-Leptin (n = 9).
Leptin-induced comparable body weight and fat mass loss in both Sham and Denervated animals.
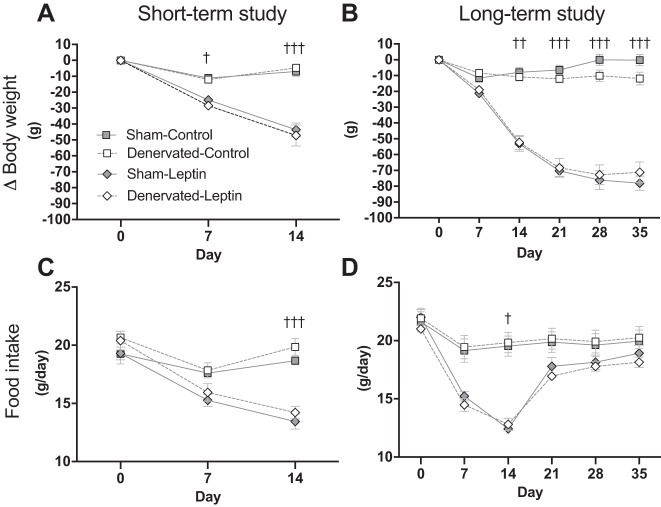
Consistent with our previous studies, central leptin gene delivery resulted in robust and sustained weight loss (Fig. 4, A and B) (9, 30). In the short-term experiment, significant differences between Control and Leptin groups were detected starting at day 7 (Fig. 4A; P < 0.05) and remained until the end of the experiment. In the long-term experiment, statistical differences between Control and Leptin groups could be detected starting day 14 (Fig. 4B; P < 0.01) and persisted until day 35. Contrary to our hypothesis, there was no difference between Sham and Denervated groups (Fig. 4, A and B), indicating that iBAT thermogenesis is not required for leptin-induced weight loss. Similarly, Sham-Leptin and Denervated-Leptin animals in both studies underwent an anorexic phase. In both studies, daily food intake was lower in Leptin than in Control groups at day 14 (Fig. 4C; P < 0.05). However, the long-term study showed that food intake in Leptin-treated rats was normalized to Control level by day 21 (Fig. 4D).
Fig. 4.
Weekly changes in body weight, and food intake. A and B: body weight was recorded daily and weekly changes in [delta (Δ)] body weight was calculated by subtracting body weight recorded at day 0 from body weight recorded at days 0, 7, 14, 21, 28, and 35. In the short-term study, Δbody weight in Sham-Leptin and Denervated-Leptin was significantly different from their respective Control group starting day 7, and differences between those groups remained statistically significant until day 14 (P < 0.05). In the long-term study, Δbody weight in Sham-Leptin and Denervated-Leptin were significantly different from their respective Control group starting day 14, and those differences remained significant until the end of the experiment (P < 0.01). C and D: food intake was recorded on a daily basis. Displayed values are the average of daily food consumption from each week. For both studies, Sham-Leptin and Denervated-Leptin significantly reduced their daily food intake during the 2nd week (day 14; P < 0.05). †P < 0.05, ††P < 0.01, †††P < 0.001, significant difference between Leptin and respective Control groups. Short-term study: Sham-Control (n = 7), Sham-Leptin, Denervated-Control, and Denervated-Leptin (n = 8/group). Long-term study: Sham-Control (n = 8), Sham-Leptin (n = 10), Denervated-Control (n = 8), and Denervated-Leptin (n = 9).
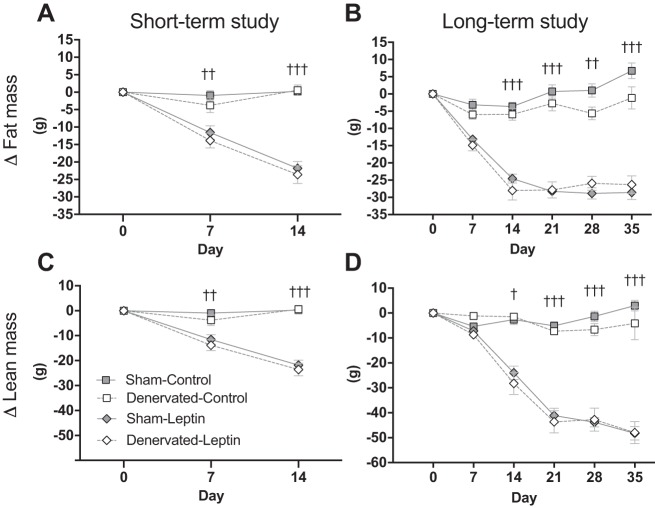
Leptin gene delivery rapidly induced fat mass loss (Fig. 5, A and B; P < 0.01), but again there were no differences between intact and denervated rats. Similar to the decreased body weight, changes in fat mass were statistically different by day 7 in the short-term study (Fig. 5A; P < 0.01) and by day 14 in the long-term study (Fig. 5B; P < 0.01). The long-term study indicates that fat mass radically dropped with a nadir at day 14 (Denervated-Leptin) or day 21 (Sham-Leptin) and remained stable for the remaining of the experiment. On the other hand, Sham-Leptin and Denervated-Leptin groups started to lose lean mass by day 7 (short-term study, Fig. 5C; P < 0.01) or by day 14 (long-term study, Fig. 5D; P < 0.05). Unlike fat mass, lean mass continued to decline until the end of the experiment, which suggests that animals preserved a state of negative energy balance over the course of 35-day central leptin gene therapy.
Fig. 5.
Weekly changes in fat mass and lean mass. A and B: fat mass was assessed on a weekly basis by time-domain (TD)-NMR and weekly changes in Δfat mass were calculated by subtracting absolute fat mass recorded at day 0 from absolute fat mass recorded at days 0, 7, 14, 21, 28, and 35. In the short-term study, Δfat mass in Sham-Leptin (n = 8) and Denervated-Leptin (n = 8) was different than their respective Control group (n = 7 for Sham and n = 8 for Denervated-Control) at days 7 and 14 (P < 0.01). In the long-term study, Δfat mass in Sham-Leptin and Denervated-Leptin was different than their respective Control group by day 14 and differences persisted until day 35 (P < 0.01). C and D: lean mass was assessed on a weekly basis by TD-NMR and weekly changes in Δlean mass were calculated by subtracting absolute lean mass recorded at day 0 from absolute lean mass recorded at days 0, 7, 14, 21, 28, and 35. Similar to Δfat mass, Δlean mass differed between Leptin and respective Control group in both studies. In the short-term study, differences were observed starting day 7 (P < 0.01) whereas in the long-term study, differences were detected starting day 14 (P < 0.05). Differences in Δlean mass remained statistically significant until the end of each study. †P < 0.05, ††P < 0.01, †††P < 0.001, significant difference between Leptin and respective Control groups. Short-term study: Sham-Control (n = 7), Sham-Leptin, Denervated-Control, and Denervated-Leptin (n = 8/group). Long-term study: Sham-Control (n = 8), Sham-Leptin (n = 10), Denervated-Control (n = 8), and Denervated-Leptin (n = 9).
Long-term central leptin gene therapy resulted in fat mass atrophy in both Sham and Denervated animals.
As previously reported, leptin gene administration resulted in adipose tissue atrophy. In fact, the abdominal cavity of Sham-Leptin and Denervated-Leptin rats only contained traces of fat (Table 1). Both leptin and denervation treatments induced loss in iBAT mass (Fig. 5B; P < 0.01). Although changes in lean mass was not significantly affected by Denervation (Fig. 3, C and D), the long-term study shows that skeletal muscle mass was significantly reduced in Denervated-Control and Denervated-Leptin compared with their respective Sham group (Fig. 5C; P < 0.001).
Table 1.
Tissue weights
| Sham | Denervated | |||
|---|---|---|---|---|
| Control | Leptin | Control | Leptin | |
| Short-term study | ||||
| Skeletal muscles, g | ||||
| Gastrocnemius | 1.63 ± 0.25 | 1.84 ± 0.04 | 1.90 ± 0.07 | 1.71 ± 0.02 |
| Plantaris | 0.33 ± 0.05 | 0.36 ± 0.01‡ | 0.38 ± 0.02 | 0.34 ± 0.01‡ |
| Soleus | 0.15 ± 0.02 | 0.16 ± 0.00 | 0.17 ± 0.01 | 0.15 ± 0.00 |
| TA | 0.61 ± 0.09 | 0.69 ± 0.01 | 0.73 ± 0.03 | 0.66 ± 0.01 |
| EDL | 0.15 ± 0.02 | 0.16 ± 0.00 | 0.17 ± 0.01 | 0.15 ± 0.01 |
| Adipose tissues, g | ||||
| Mesenteric | 2.16 ± 0.44 | 0.48 ± 0.05‡‡‡ | 2.78 ± 0.13 | 0.28 ± 0.11‡‡‡ |
| Perirenal | 1.27 ± 0.23 | 0.54 ± 0.11‡‡‡ | 1.64 ± 0.14 | 0.27 ± 0.03‡‡‡ |
| Epididymal | 4.36 ± 0.83 | 2.56 ± 0.35‡‡‡ | 5.55 ± 0.17 | 1.27 ± 0.43‡‡‡ |
| Retroperitoneal | 2.63 ± 0.56 | 0.54 ± 0.13‡‡‡ | 2.89 ± 0.15 | 0.14 ± 0.09‡‡‡ |
| iBAT | 0.33 ± 0.05 | 0.22 ± 0.02‡‡‡ | 0.28 ± 0.03*** | 0.14 ± 0.02‡‡‡*** |
| Long-term study | ||||
| Skeletal muscles, g | ||||
| Gastrocnemius | 1.75 ± 0.07 | 1.74 ± 0.08 | 1.33 ± 0.06*** | 1.24 ± 0.10*** |
| Plantaris | 0.35 ± 0.01 | 0.35 ± 0.02 | 0.27 ± 0.01*** | 0.25 ± 0.02*** |
| Soleus | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.12 ± 0.01*** | 0.11 ± 0.01*** |
| Adipose tissues, g | ||||
| Mesenteric | 3.24 ± 0.18 | 0.00 ± 0.00‡‡‡ | 2.8 ± 0.34 | 0.00 ± 0.00‡‡‡ |
| Perirenal | 0.94 ± 0.05 | 0.00 ± 0.00‡‡‡ | 0.80 ± 0.13 | 0.00 ± 0.00‡‡‡ |
| Epididymal | 4.24 ± 0.25 | 0.00 ± 0.00‡‡‡ | 3.81 ± 0.33 | 0.00 ± 0.00‡‡‡ |
| Retroperitoneal | 3.50 ± 0.21 | 0.00 ± 0.00‡‡‡ | 3.28 ± 0.44 | 0.00 ± 0.00‡‡‡ |
| iBAT | 0.37 ± 0.02 | 0.22 ± 0.01‡‡‡ | 0.28 ± 0.03*** | 0.12 ± 0.02‡‡‡*** |
TA, tibialis anterior; EDL, extensor digitorum longus; iBAT, interscapular brown adipose tissue.
P < 0.001, Denervated vs. Sham.
P < 0.05;
P < 0.001 Leptin vs. green fluorescent protein (GFP; Control).
Tissue triglyceride content.
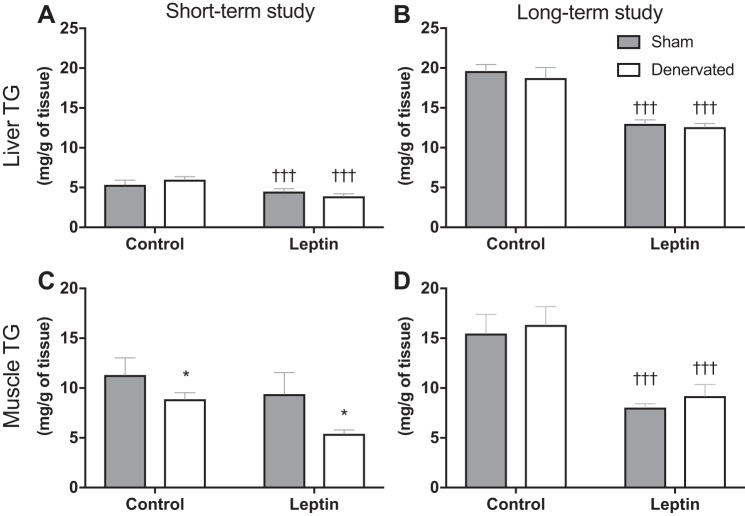
In both studies, Leptin rats displayed significantly lower hepatic triglyceride content than respective Control animals (Fig. 6, A and B; P < 0.001). In skeletal muscle, triglyceride content was not affected by Leptin in the short-term study (Fig. 6C). In contrast, in the long-term study, we found a significant reduction in muscle triglyceride in Leptin compared with Control animals (Fig. 6D; P < 0.001). On the other hand, muscle triglyceride was significantly reduced by Denervation in the short-term study (Fig. 6C; P < 0.05).
Fig. 6.
Tissue triglyceride (TG) content. A and B: liver TG content was measured at the end point of the short-term and the long-term study, respectively. In both studies, liver TG content was significantly lower in Leptin groups relative to their Control groups. C and D: muscle TG content was measured at the end point of the short-term and the long-term study, respectively. A significant effect of Leptin was observed in the long-term study but not in the short-term study. In the short-term study, Denervated animals exhibited significantly lower levels of muscle TG than respective Sham animals. †††P < 0.001, significant difference between Leptin and respective Control groups. *P < 0.05, significant difference between Sham and respective Denervated groups. Short-term study: Sham-Control (n = 7), Sham-Leptin, Denervated-Control, and Denervated-Leptin (n = 8/group). Long-term study: Sham-Control (n = 8), Sham-Leptin (n = 10), Denervated-Control (n = 8), and Denervated-Leptin (n = 9).
DISCUSSION
Understanding the contribution of iBAT sympathetic innervation to leptin-induced weight loss is important to fully comprehend the endogenous mechanisms regulating energy homeostasis. To date, induction of UCP1 is still considered an underlying mechanism for leptin-induced body weight loss (8, 26). Leptin was shown to stimulate energy utilization in ob/ob mice by increasing thermogenic capacity, and it was postulated that decreased Ucp1 expression in BAT of those mice is in part responsible for their enhanced metabolic efficiency and propensity to obesity (8, 21). Chronic leptin treatment increases oxygen consumption and reduces fat mass in wild-type mice but not in Ucp1-KO mice, compared with respective control pair-fed mice (8, 26). However, the significance of iBAT thermogenesis to the overall catabolic effect of leptin has never been ascertained.
The thermogenic potential of BAT is attributed to high mitochondria content and uncoupled substrate oxidation from electron transport. Importantly, BAT can oxidize up to 50% of ingested triglycerides and 75% of ingested glucose (24), thus demonstrating the ability of BAT to regulate systemic energy homeostasis. Although UCP1 plays an important role on iBAT thermogenesis, animals that do no express the Ucp1 gene can survive in cold environments (23) and can even be resistant to diet-induced obesity (10), suggesting that UCP1 is not required for the maintenance of energy homeostasis.
This present study employed recombinant adeno-associated viral techniques to enhance central leptin signaling and verify whether iBAT sympathetic innervation is essential to leptin-induced body weight loss. Central leptin overexpression induced UCP1 only in the iBAT of sham and not in the iBAT of denervated rats. Despite the differences in UCP1 levels, similar body weight and fat mass loss between Denervated-Leptin and Sham-Leptin animals were observed in both the short-term and long-term studies. Body composition analyses showed that animals lost a significant amount of fat mass within the first 14 days that remained stable afterwards. It appears that fat stores were almost if not completely depleted after 2 wk of leptin gene delivery in both intact and denervated rats. This rapid loss in fat mass corroborates previously observed catabolic response to leptin (22). Given the very limited amount of white adipose tissue (WAT) that remained at the end our studies, future experiments should focus on analyzing WAT lipolysis and remodeling at earlier time points, before depletion of WAT stores.
The present studies demonstrate that iBAT sympathetic innervation is not required for central leptin signaling-mediated weight loss. This surgical model is superior over using a global Ucp1 knockout because these animals display highly heterogeneous compensatory adaptations to metabolic challenge (33). Additionally, Ucp1 is expressed in WAT; therefore, the knockout model does not distinguish the contribution of BAT thermogenesis from browning of WAT. However, an important limitation of our model is that iBAT was the only denervated BAT depot. Therefore, we cannot exclude the contribution of other intact BAT depots. It is possible that mediastinal, axillary, perirenal, and cervical BAT contributed to the leptin response in iBAT-denervated animals. A new model inhibiting sympathetic nervous system activity in all BAT depots is needed to determine the overall contribution of BAT sympathetic outflow in leptin-treated animals. The importance of the interscapular brown fat pad to the overall BAT thermogenesis has not been clearly described in the literature. Some mouse studies reported that iBAT accounts for 60–70% of total BAT (1, 2) whereas other reports show that iBAT represents only ~25% of all brown fat pads (33). Novel techniques utilizing multiparametric magnetic resonance-based neural network auto-segmentation may help provide more accurate quantification of each BAT depot (3). It was shown that body temperature is under distributed control and has the potential to allow compensation for an event impairing BAT function (25). Consequently, it is possible that iBAT denervation triggered peripheral compensations such as browning of WAT and/or increased muscle-based NST and future studies should aim at identifying those mechanisms. It is also well documented that mechanisms such as increased lipolysis and/or reduced lipogenesis in WAT are important contributors to central leptin-mediated weight loss (4). Moreover, although sympathetic outflow to BAT is not critical, it is possible that in the native state (no denervation) sympathetic outflow to iBAT still may participate in leptin-mediated body weight loss. Nevertheless, given all the attention paid to the role of leptin on brown adipose tissue biology, the determining that iBAT sympathetic innervation is not required for leptin-mediated weight loss highlights the complexities of the mechanisms underlying the role of leptin body weight regulation.
In summary, we found that induction of UCP1 through iBAT sympathetic outflows is not required for leptin-mediated weight loss. Sham-Leptin and Denervated-Leptin animals responded to the same extent in both the short-term study and the long-term study. TD-NMR analyses showed that those animals lost a similar proportion of fat and lean body mass. Future studies should investigate the possible contribution of other thermogenic pathways such as browning of WAT or muscle-based thermogenesis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-091710.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.C., D.M., C.S.C., N.T., and P.J.S. conceived and designed research; I.C., Y.S., and S.M.G. performed experiments; I.C., D.M., and P.J.S. analyzed data; I.C., Y.S., D.M., C.S.C., and P.J.S. interpreted results of experiments; I.C. prepared figures; I.C. drafted manuscript; I.C., Y.S., S.M.G., D.M., C.S.C., N.T., and P.J.S. approved final version of manuscript; D.M., C.S.C., N.T., and P.J.S. edited and revised manuscript.
REFERENCES
- 1.Bal NC, Maurya SK, Singh S, Wehrens XH, Periasamy M. Increased reliance on muscle-based thermogenesis upon acute minimization of brown adipose tissue function. J Biol Chem 291: 17247–17257, 2016. doi: 10.1074/jbc.M116.728188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, Tupling AR, Molkentin JD, Periasamy M. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med 18: 1575–1579, 2012. doi: 10.1038/nm.2897 (Erratum. Nat Med 18: 1857, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhanu Prakash KN, Verma SK, Yaligar J, Goggi J, Gopalan V, Lee SS, Tian X, Sugii S, Leow MK, Bhakoo K, Velan SS. Segmentation and characterization of interscapular brown adipose tissue in rats by multi-parametric magnetic resonance imaging. MAGMA 29: 277–286, 2016. doi: 10.1007/s10334-015-0514-3. [DOI] [PubMed] [Google Scholar]
- 4.Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L, Buettner C. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14: 667–675, 2008. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 6.Clarke IJ, Henry BA. Targeting energy expenditure in muscle as a means of combating obesity. Clin Exp Pharmacol Physiol 37: 121–124, 2010. doi: 10.1111/j.1440-1681.2009.05259.x. [DOI] [PubMed] [Google Scholar]
- 7.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature 380: 677, 1996. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 8.Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology 140: 292–300, 1999. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- 9.Dhillon H, Kalra SP, Prima V, Zolotukhin S, Scarpace PJ, Moldawer LL, Muzyczka N, Kalra PS. Central leptin gene therapy suppresses body weight gain, adiposity and serum insulin without affecting food consumption in normal rats: a long-term study. Regul Pept 99: 69–77, 2001. doi: 10.1016/S0167-0115(01)00237-3. [DOI] [PubMed] [Google Scholar]
- 10.Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94, 1997. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 11.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151: 400–413, 2012. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res 21: 139–144, 1980. [PubMed] [Google Scholar]
- 13.Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab 291: E350–E357, 2006. doi: 10.1152/ajpendo.00387.2005. [DOI] [PubMed] [Google Scholar]
- 14.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269: 543–546, 1995. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 15.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100: 270–278, 1997. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry BA, Andrews ZB, Rao A, Clarke IJ. Central leptin activates mitochondrial function and increases heat production in skeletal muscle. Endocrinology 152: 2609–2618, 2011. doi: 10.1210/en.2011-0143. [DOI] [PubMed] [Google Scholar]
- 17.Henry BA, Dunshea FR, Gould M, Clarke IJ. Profiling postprandial thermogenesis in muscle and fat of sheep and the central effect of leptin administration. Endocrinology 149: 2019–2026, 2008. doi: 10.1210/en.2007-1311. [DOI] [PubMed] [Google Scholar]
- 18.Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell 4: 147–155, 2005. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Klein RL, Matheny M, King MA, Meyer EM, Scarpace PJ. Induction of uncoupling protein 1 by central interleukin-6 gene delivery is dependent on sympathetic innervation of brown adipose tissue and underlies one mechanism of body weight reduction in rats. Neuroscience 115: 879–889, 2002. doi: 10.1016/S0306-4522(02)00447-5. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest 111: 399–407, 2003. doi: 10.1172/JCI200315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martins FF, Bargut TCL, Aguila MB, Mandarim-de-Lacerda CA. Thermogenesis, fatty acid synthesis with oxidation, and inflammation in the brown adipose tissue of ob/ob (−/−) mice. Ann Anat 210: 44–51, 2017. doi: 10.1016/j.aanat.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Matheny M, Strehler KY, King M, Tümer N, Scarpace PJ. Targeted leptin receptor blockade: role of ventral tegmental area and nucleus of the solitary tract leptin receptors in body weight homeostasis. J Endocrinol 222: 27–41, 2014. doi: 10.1530/JOE-13-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer CW, Willershäuser M, Jastroch M, Rourke BC, Fromme T, Oelkrug R, Heldmaier G, Klingenspor M. Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. Am J Physiol Regul Integr Comp Physiol 299: R1396–R1406, 2010. doi: 10.1152/ajpregu.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab 13: 238–240, 2011. doi: 10.1016/j.cmet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen NL, Barr CL, Ryu V, Cao Q, Xue B, Bartness TJ. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol 312: R132–R145, 2017. doi: 10.1152/ajpregu.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamatsu-Ogura Y, Uozumi A, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 contributes to fat-reducing effect of leptin. Obes Res Clin Pract 1: 223–290, 2007. doi: 10.1016/j.orcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods 3: 129–149, 1980. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 28.Scarpace PJ, Matheny M. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am J Physiol Endocrinol Metab 275: E259–E264, 1998. doi: 10.1152/ajpendo.1998.275.2.E259. [DOI] [PubMed] [Google Scholar]
- 29.Scarpace PJ, Matheny M, Pollock BH, Tümer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol Endocrinol Metab 273: E226–E230, 1997. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- 30.Scarpace PJ, Matheny M, Zhang Y, Tümer N, Frase CD, Shek EW, Hong B, Prima V, Zolotukhin S. Central leptin gene delivery evokes persistent leptin signal transduction in young and aged-obese rats but physiological responses become attenuated over time in aged-obese rats. Neuropharmacology 42: 548–561, 2002. doi: 10.1016/S0028-3908(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2: 589–593, 1996. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 32.Shabalina IG, Hoeks J, Kramarova TV, Schrauwen P, Cannon B, Nedergaard J. Cold tolerance of UCP1-ablated mice: a skeletal muscle mitochondria switch toward lipid oxidation with marked UCP3 up-regulation not associated with increased basal, fatty acid- or ROS-induced uncoupling or enhanced GDP effects. Biochim Biophys Acta 1797: 968–980, 2010. doi: 10.1016/j.bbabio.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 33.Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev 49: 330–425, 1969. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- 34.Valente A, Jamurtas AZ, Koutedakis Y, Flouris AD. Molecular pathways linking non-shivering thermogenesis and obesity: focusing on brown adipose tissue development. Biol Rev Camb Philos Soc 90: 77–88, 2015. doi: 10.1111/brv.12099. [DOI] [PubMed] [Google Scholar]
- 35.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res 101: 545–559, 2007. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 36.Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Lu YH, Kozlova A, Voss H, Martins GG, Friedman JM, Domingos AI. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163: 84–94, 2015. doi: 10.1016/j.cell.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 38.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ Jr, Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28: 158–167, 2002. doi: 10.1016/S1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]