Abstract
Inflammation, both acute and chronic, is associated with testosterone deficiency, raising the possibility of a direct causal link. One potential trigger for inflammation in obese men is the passage of intestinal bacteria into the circulation due to a breakdown in mucosal barrier integrity. Recently, we hypothesized that this endotoxin exposure may cause androgen deficiency in obese men. To test this hypothesis, we analyzed the relationship between serum levels of lipopolysaccharide-binding protein (LBP), an indirect measure of endotoxin exposure, against male reproductive hormones, inflammatory markers (C-reactive protein, IL-1β, IL-6, TNF-α), and adiposity in 75 men. Adiposity was positively correlated with endotoxin exposure (LBP) and inflammation (C-reactive protein, IL-6) and negatively correlated with testosterone. Furthermore, endotoxemia (LBP) was negatively correlated with serum testosterone but positively correlated with IL-6. Multivariate analysis revealed a significant, negative correlation between serum IL-6 and free testosterone. In a second interventional study, low-dose endotoxin challenge in lean men produced a transient inflammatory response that was followed by a decline in serum testosterone, without changes in LH or FSH, providing further evidence that endotoxin-driven inflammation may result in impaired Leydig cell function.
Keywords: endotoxin, inflammation, interleukin-6, lipopolysaccharide, obesity, testosterone
INTRODUCTION
Testosterone deficiency (TD), characterized by low testosterone levels and relevant clinical symptoms and signs, is estimated to effect up to 6% of men (11), with projections estimating that 6.5 million American men will develop symptomatic TD by 2025 (2). This is of considerable public health concern, as TD is associated with both impaired physical health (e.g, muscle strength and coordination, osteoporosis, cardiovascular disease) and mental well being (e.g., sexual difficulties, depression, anxiety, sleep disturbance, and impaired cognitive function) plus a decline in reproductive function (11).
TD may result from impaired Leydig cell function, reduced pituitary LH drive, or a combination of the two plus a reduction in testosterone bioavailability because of elevated sex hormone-binding globulin (SHBG) levels. Obesity is considered the single most common cause of TD in the developed world, with more than half of all obese men having TD (9, 23). In terms of mechanisms, inflammation has been suggested as a potential cause for male hypogonadism since several studies have linked various acute and chronic inflammatory conditions (e.g., sepsis, burns, autoimmune diseases) with biochemical TD (3, 8, 21). Large epidemiological studies have linked obesity and systemic inflammation [e.g., raised C-reactive protein (CRP)] with lower testosterone levels (4, 37, 40), whereas administration of proinflammatory cytokines to men (36, 38) or proinflammatory cytokine treatment of Leydig cells in culture (20) has been shown to reduce testosterone production. Taken together, these data provide strong evidence for a link between inflammation and TD.
Recently, we suggested that gut-derived bacterial endotoxin, a pathogen-associated molecular pattern otherwise known as lipopolysaccharide (LPS), may be a key inflammatory trigger responsible for creating TD in obese men (34). Obesity is reported to be associated with low-grade inflammation induced by the passage of intestinal bacteria-derived endotoxin into the systemic circulation, so-called metabolic endotoxemia (6). The human intestine contains on average 1.5 kg (100 trillion) bacteria, with 70% of these being gram negative, containing the potent immune stimulant LPS (6, 34). Normally, these bacteria are prevented from entering the circulation by a combination of tight junctions between epithelial cells, the colonic mucous barrier, and neutralizing secretory IgA antibodies. However, in obesity this barrier is impaired (6), resulting in the passage of bacterial endotoxin into the circulation, where it elicits a systemic inflammatory response through activation of Toll-like receptor 4 (TLR4) expressed on innate immune cells (6).
Although a previous study has reported a negative association between endotoxin exposure and testosterone levels (35), it is presently uncertain whether endotoxin itself is the actual trigger for impaired testosterone production. Therefore, the aim of this study was twofold: first, to examine potential associations between adiposity, endotoxin exposure, inflammation, and testosterone concentration in an observational cohort of reproductive age men; and second, to determine whether experimental administration of low-dose endotoxin to healthy lean men results in reduced testosterone production.
METHODS
Observational Study
Participants.
Participants were 75 men, between the ages of 18 and 50 yr, recruited from a private fertility clinic (Repromed, Adelaide, SA, Australia). Exclusion criteria were documented primary hypogonadism (Klinefelters Syndrome, cryptorchidism, or testicular injury), inflammatory or infectious disease, the consumption of immunosuppressive medication or supplements (e.g., NSAID, corticosteroids, or fish oil), or any hormonal therapy (i.e., aromatase inhibitors, clomiphene citrate, human chorionic gonadotropin, or testosterone). The study was approved by the Institutional Review Board of the University of South Australia Human Ethics Committee (approval no. 0000035369), with informed written consent being obtained from all participants.
Study protocol.
Blood was obtained between 0800 and 1000, with no specific instructions given in relation to exercise or fasting status before venipuncture. On the day of testing, height was measured using a stadiometer, and weight and percentage body fat were measured using bioimpedance digital scales (UM-051; Tanita, Cloverdale, WA, Australia). Waist circumference was measured using a tape measure placed midway between the 12th rib and the iliac crest.
Assays.
Metabolic endotoxemia was quantified indirectly by measuring circulating concentrations of LPS-binding protein (LBP), a hepatically derived acute-phase protein, which is upregulated in response to endotoxin (17). LBP in the serum was measured by ELISA (Hycult, Uden, The Netherlands) per the manufacturer’s guidelines, with the minimum detectable concentration of LBP being 4.4 ng/ml. Direct measurement of endotoxin in plasma was not performed because of the well-documented inaccuracies inherent with these measurements (17). CRP was measured in serum using an automated chemiluminesence machine (Integra 800; Roche Diagnostics), with the limit of detection being 1 mg/l. Serum IL-1β, IL-6, and TNF-α were analyzed in duplicate using a multiplex immunoassay (ProcartaPlex Kit, eBioscience, San Diego, CA). The detectable range for each of these cytokines was 0.21–860 IL-1β, 1.06–4,340 IL-6, and 2.17–8,900 pg/ml TNF-α. Serum was analyzed for estradiol, testosterone, SHBG, FSH, and LH using an automated chemiluminescence immunoassay (Cobas 6000 e 601; Roche Diagnostics), with the detectable ranges for each hormone being 18.4–11,010 pmol/l estradiol, 0.087–52.0 nmol/l testosterone, 0.350–200 nmol/l SHBG, 0.1–200 IU/l FSH, and 0.1–200 IU/l LH. Calculated free testosterone (cFT) was determined using the Vermeulen equation (39).
Interventional Study
Participants.
Thirty-three healthy male volunteers between the ages of 18 and 40 yr were recruited by public advertisement, with all assessments and intervention being conducted at the Medical Research Centre of the University Hospital Essen, University of Duisburg-Essen, Essen, Germany. Participants underwent an extensive medical screening conducted by a senior physician, together with blood and clinical chemistry assessments. Only healthy individuals with normal blood and clinical chemistry parameters (i.e., complete blood cell count, CRP, coagulation factors, liver enzymes, and renal function) were recruited. Exclusion criteria were preexisting or current medical or psychiatric conditions, body mass index (BMI) <18 or ≥29 kg/m2, current medications, smoking, and regular high alcohol use. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Review Board of the University of Duisburg-Essen (approval no. 15-6234-BO). Signed, informed consent was obtained, and subjects received financial compensation for their participation in the study.
Study protocol.
Subjects were randomly assigned to receive either endotoxin (n = 17) or placebo (n = 16). An intravenous catheter was inserted into an antecubital forearm vein upon arrival to allow for repeated blood collection and endotoxin/placebo administration. After a rest of 30 min, a baseline blood sample was obtained at 1100, before subjects received an injection of either endotoxin (0.8 ng/kg body wt; reference standard endotoxin from Escherichia coli O113:H10, lot H0K354; United States Pharmacopeia, Rockville, MD) or placebo (sterile, pyrogen-free isotonic NaCl solution; B. Braun Melsungen, Melsungen, Germany). The LPS (2,000 EU/ml) had been subjected to a microbial safety testing routine by the German Federal Agency for Sera and Vaccines (Paul-Ehrlich Institute, Langen, Germany) and was stored in endotoxin-free borosilicate tubes (Pyroquant Diagnostik, Mörfelden-Waldorf, Germany) at −20°C until use. Additional blood samples for cytokine and endocrine analyses were collected at 1, 2, 3, 6, and 24 h after endotoxin or placebo injection. Plasma was separated by centrifugation and was stored at −80°C until analysis.
Assays.
Plasma concentrations of IL-6 were measured by ELISA (Human Quantikine ELISA; R & D Systems, Minneapolis, MN) according to the manufacturer’s protocols. The sensitivity of the assay was 0.70 pg/ml. Concentrations of testosterone, FSH, and LH in plasma were measured by ELISA (IBL International, Hamburg, Germany). The sensitivity of the assays was 0.24 nmol/l testosterone, 1.27 IU/l LH, and 0.86 IU/l FSH. Cross-reactivity of the anti-testosterone antibody with other relevant steroids was 8.67 11β-OH-testosterone, 3.24 11α-OH-testosterone, 1.92 dihydrotestosterone, 0.83 androstendione, and <0.05% DHEA-S, progesterone, and androsterone.
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 7.01 (La Jolla, CA) and IBM SPSS version 23 (SPSS, Chicago, IL). Preliminary analysis was performed to ensure that there was no violation of the assumption of normality and linearity or multicollinearity. Data are expressed as means ± SE when normally distributed or as a median and interquartile range (IQR) when not normally distributed. Correlations were assessed using the Pearson’s method with log transformation of nonnormally distributed data before statistical analysis. Univariate analysis was performed to examine the relationship between testosterone (total and free) and various markers of inflammation and body composition. Multivariate stepwise regression analysis was performed to determine the predictors of total and calculated free testosterone. Endocrine and cytokine data from the interventional study were analyzed by repeated-measures analysis of variance (ANOVA) with “treatment” as between-subject factor and “time” as within-subject factor. Greenhouse-Geisser correction was performed when the assumption of sphericity was violated. If repeated-measures ANOVA revealed a significant treatment × time interaction, Bonferroni-corrected independent samples t-tests were computed to compare endotoxin and placebo groups at the different sampling points.
RESULTS
Observational Study
The demographic, lifestyle, endocrine, and inflammatory status characteristics of participants in the observational study are summarized in Table 1. Most participants were overweight (54.7%) or obese (24%), with only 21.3% being of normal BMI (BMI < 25 kg/m2). Most participants were nonsmokers (93.3%) and without any medical conditions (80%). The median number of standard drinks of alcohol consumed per week was five (IQR 1–10). Two men had non-insulin-dependent diabetes mellitus managed with oral hypoglycemics (metformin), with one man requiring insulin, four subjects were asthmatics, four men were on medications for hypertension or high cholesterol, two men had gastrointestinal reflux disease, and four participants had anxiety/depression.
Table 1.
Baseline values for participants in the observational study
| Variable | Value (Median, IQR) |
|---|---|
| Age, yr | 35.3 ± 0.75 |
| BMI, kg/m2 | 27.0 (25.5–29.9) |
| Waist circumference, cm | 95.4 ± 1.1 |
| %Body fat | 25.2 ± 0.7 |
| Total testosterone, nmol/l | 15.12 ± 0.54 |
| SHBG, nmol/l | 35.5 ± 1.44 |
| Calculated free testosterone, pmol/l | 301.8 ± 10 |
| Estradiol, pmol/l | 72.8 ± 3.54 |
| LH, IU/l | 4.37 (3.42–5.92) |
| FSH, IU/l | 4.25 (3.05–6.49) |
| CRP, mg/l | 1 (0.9–2) |
| IL-6, pg/ml | 5.67 ± 0.28 |
| TNFα, pg/ml | 0.63 (0.5–0.92) |
| IL-1β, pg/ml | 1.1 (0.79–1.21) |
| LBP, ng/ml | 11.15 (9.21–13.4) |
Values are means ± SE, n = 75 participants. IQR, interquartile range; BMI, body mass index; SHBG, sex hormone-binding globulin; CRP, C-reactive protein; LBP, lipopolysaccharide-binding protein.
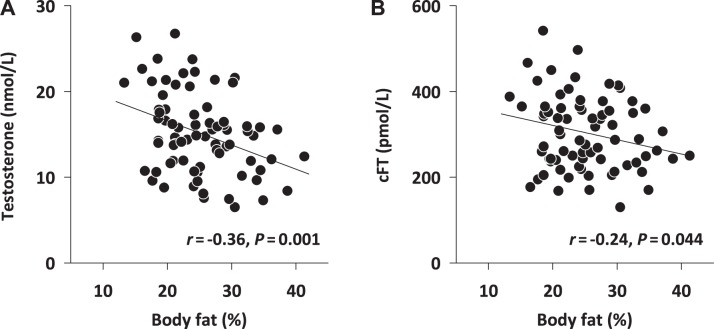
In relation to reproductive hormones, percentage of body fat was most strongly negatively correlated with both total testosterone (r = −0.368, P = 0.001; Fig. 1A) and cFT (r = −0.24, P = 0.044; Fig. 1B) but not with estradiol, LH, or FSH levels. Similar patterns were seen for both waist circumference and BMI, but to a lesser degree (Table 2). In terms of inflammatory status, all three measures of adiposity were significantly positively correlated with serum LBP, IL-6, and CRP (Table 2). However, no significant correlation was observed between serum LBP and either IL-1β or TNF-α.
Fig. 1.
Relationship between individual subjects’ %body fat and total testosterone (A) or calculated free testosterone (cFT; B); n = 75 subjects. Statistics: Pearson product moment correlation.
Table 2.
Correlation matrix between measures of adiposity, inflammation and reproductive hormones
| BMI | Waist circumference | %Body Fat | Testosterone | cFT | LH (log) | FSH (log) | SHBG | E2 | CRP | LBP | IL-6 (log) | IL-1β (log) | TNF-α (log) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | 1 | 0.845** | 0.850** | −0.323** | −0.112 | 0.033 | 0.148 | −0.331** | 0.125 | 0.513** | 0.380** | 0.272* | 0.073 | 0.012 |
| Waist circ. | 1 | 0.880** | −0.350** | −0.159 | −0.049 | 0.044 | −0.352** | 0.162 | 0.412** | 0.332** | 0.334** | 0.050 | 0.080 | |
| % Body fat | 1 | −0.368** | −0.24* | 0.057 | 0.144 | −0.369** | 0.176 | 0.516** | 0.372** | 0.331** | 0.060 | 0.075 | ||
| Testosterone | 1 | 0.485** | 0.190 | 0.036 | 0.584** | 0.270* | −0.477** | −0.25* | −0.365** | −0.131 | −0.042 | |||
| cFT | 1 | 0.123 | 0.096 | 0.055 | 0.149 | −0.210 | −0.114 | −0.287* | −0.037 | −0.065 | ||||
| LH (log) | 1 | 0.444** | 0.203 | 0.059 | 0.085 | 0.308* | 0.080 | −0.097 | −0.016 | |||||
| FSH (log) | 1 | 0.215 | 0.085 | 0.128 | −0.049 | 0.039 | 0.020 | 0.050 | ||||||
| SHBG | 1 | 0.100 | −0.244 | −0.176 | −0.257* | 0.021 | 0.045 | |||||||
| E2 | 1 | −0.076 | 0.073 | −0.094 | −0.165 | −0.172 | ||||||||
| CRP (log) | 1 | 0.348* | 0.458** | 0.273 | 0.028 | |||||||||
| LBP | 1 | 0.408** | −0.006 | −0.159 | ||||||||||
| IL6 (log) | 1 | 0.206 | 0.151 | |||||||||||
| IL-1β (log) | 1 | 0.388** |
BMI, body mass index; cFT, calculated free testosterone; SHBG, sex hormone-binding globulin; E2, estradiol; CRP, C-reactive protein; LBP, lipopolysaccharide-binding protein. Statistical analysis was performed using Pearson’s method, with log transformation of nonnormally distributed data before analysis. All values represent correlation coefficient value (r), with those reaching statistical significance denoted by superscript.
P < 0.05;
P < 0.01.
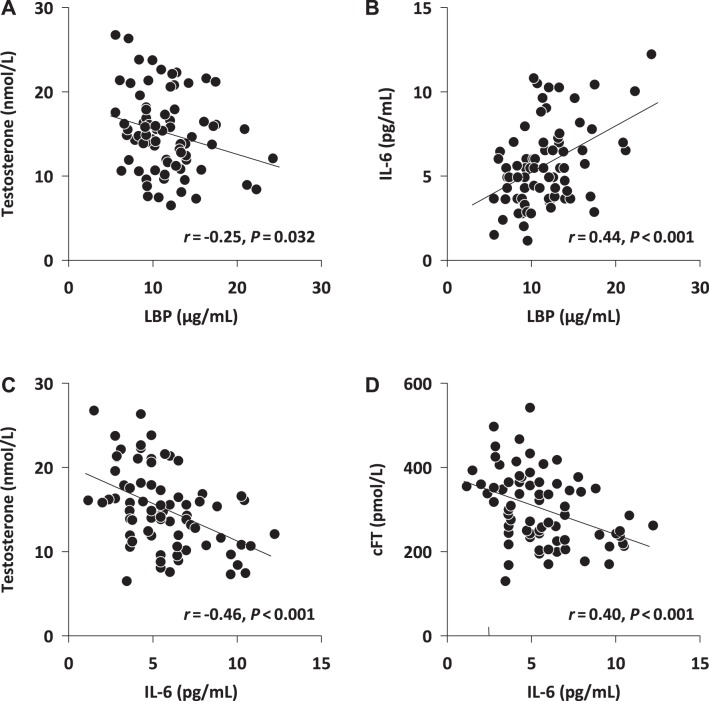
A significant, negative relationship was observed between serum LBP and total testosterone (Fig. 2A), whereas a significant positive relationship was seen between LBP and serum LH (Table 2). However, no significant relationship was observed between LBP and calculated free testosterone (cFT), FSH, or estradiol (Table 2). Serum IL-6 was positively associated with LBP (Fig. 2B) while being negatively correlated with both total testosterone (Fig. 2C) and cFT (Fig. 2D). No significant relationship was observed between either LBP or IL-6 and serum LH, FSH, or estradiol levels.
Fig. 2.
Relationship between individual results for lipopolysaccharide-binding protein (LBP) and total testosterone (A) or calculated free testosterone (cFT; B) and between IL-6 and total testosterone (C) or cFT (D); n = 75 subjects. Statistics: Pearson product moment correlation.
Multivariate regression analysis considering each participant’s age, adiposity (BMI, waist, %adiposity), inflammation (CRP, IL-1β, TNF-α, IL-6, and LBP), and endocrine status [LH, FSH, AMH, and estradiol (E2)] was used to determine the best predictors of total and calculated free testosterone. In the strongest model age, CRP, SHBG, and estrogen explained 55% of the variation in total serum testosterone [F(4,65) = 20.1, β = −0.273 (age), β = −0.203 (CRP), β = 0.543 (SHBG), β = 0.297 (E2), P = 0.003, r2 = 0.549]. A further multivariate analysis using calculated free testosterone (cFT) as the dependent variable resulted in the strongest model consisting of age, serum IL-6, AMH, and estrogen, altogether explaining 29% of the variation in cFT [F(4,66) = 6.845, β = −0.349 (age), β = −0.340 (IL-6), β = 0.224 (E2), β = −0.217 (AMH), P < 0.001, r2 = 0.293]. When considering just IL-6 alone, this cytokine explained 12% of the variation in cFT [F(1,69) = 9.237, β = −0.344, P = 0.003, r2 = 0.118]. Serum total or free testosterone levels were not significantly correlated with IL-1β or TNF-α levels.
Interventional Study
The demographic, endocrine, and inflammatory characteristics of participants in the interventional study are summarized in Table 3. No significant baseline differences in these variables were observed between the endotoxin and placebo groups. CRP levels were below the clinical cutoff (<0.5 mg/l) in all participants.
Table 3.
Baseline values for participants in the interventional study
| Variable | Placebo (n = 16) | Endotoxin (n = 17) | P Value |
|---|---|---|---|
| Age, yr | 25.6 ± 1.6 | 27.1 ± 1.1 | 0.44 |
| BMI, kg/m2 | 24.2 ± 0.6 | 23.6 ± 0.6 | 0.51 |
| IL-6, pg/ml | 1.95 ± 0.35 | 1.31 ± 0.21 | 0.12 |
| TNF-α, pg/ml | 0.83 ± 0.07 | 0.93 ± 0.07 | 0.35 |
| Total testosterone, nmol/l | 14.50 ± 1.07 | 14.71 ± 0.65 | 0.87 |
| LH, IU/l | 3.75 ± 0.49 | 3.48 ± 0.33 | 0.64 |
| FSH, IU/l | 3.66 ± 0.90 | 3.30 ± 0.62 | 0.74 |
Data are means ± SE; n, number of participants.
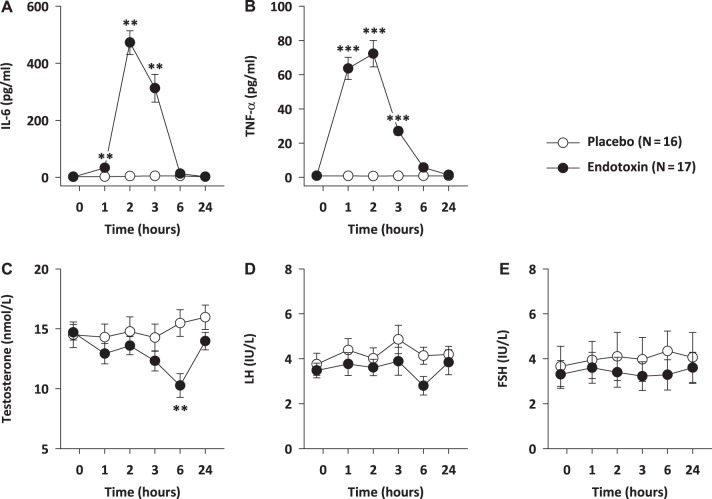
Low-dose endotoxin administration induced an acute systemic inflammatory response, as evident from a significant increase in plasma IL-6 [treatment × time: F(5,155) = 68.39, P < 0.001] and TNF-α concentration [treatment × time: F(5,155) = 63.64, P < 0.001]. This increase was transient for both cytokines, peaking at 2 h post-LPS injection and returning to pretreatment levels by 6 h (Fig. 3, A and B). This inflammatory response was followed by a decline in plasma testosterone levels [treatment × time: F(5,155) = 7.03, P < 0.001], with significant group differences at 6 h, before returning to baseline by 24 h post-LPS administration (Fig. 3C). No significant changes in plasma gonadotrophin levels (LH, FSH) were observed in relation to the LPS infusion (Fig. 3, D and E).
Fig. 3.
Effects of experimental endotoxemia on plasma cytokines, total testosterone, and gonadotropin hormones. Plasma concentrations of IL-6 (A), TNF-α (B), total testosterone (C), LH (D), and FSH (E) before and after intravenous injection of endotoxin (0.8 ng LPS/kg body wt) or placebo. Means ± SE are shown. Repeated-measures ANOVA with Bonferroni-corrected independent t-tests, **P ≤ 0.01; ***P ≤ 0.001.
DISCUSSION
Whereas previous studies have reported an association between male hypogonadism and inflammation (4, 35–38, 40), this paper is the first to conclusively report a rapid decline in testosterone production in healthy reproductive age men following experimental administration of low-dose endotoxin. This result mirrors previous animal studies (28, 32), but importantly, it confirms the interaction between endotoxin exposure, subsequent inflammation, and impaired testicular function in men.
Our observational data have also confirmed significant, positive correlations between chronic low-level exposure to endotoxin (increased serum LBP) and inflammation (raised serum IL-6) and a reduction in testosterone levels. The positive correlation between serum IL-6 and measures of adiposity was strongest for waist circumference and weakest for BMI, reflecting the importance of central (truncal) obesity as a driver of inflammation (25). Similarly, there was a stronger negative correlation between waist circumference and testosterone compared with BMI, as has been previously described (16). These observations suggest that chronic low-grade endotoxin exposure and its associated inflammatory response (IL-6) typically seen in central obesity are likely to play some role in obesity-related TD (4, 6, 16). However, we acknowledge that adipose tissue itself is also a major producer of inflammatory cytokines (25) and as such will be a significant driver of inflammation independent of endotoxin exposure. Importantly, these adipose tissue-derived cytokines are known to impair intestinal tight junction function (7), resulting in an increase in intestinal permeability, which creates the potential for a positive feedback loop generating more endotoxemia and inflammation. In addition, obese individuals often consume a high-fat diet, with dietary fat eliciting the production of chylomicrons that facilitate migration of bacterial endotoxin across the gut wall into the circulation (6). As such, the inflammatory state observed in obese men is most likely a combination of all of these proinflammatory forces.
Although our experimental approach, as a proof-of-concept study, clearly shows that administration of a relatively low dose of endotoxin is capable of impairing testosterone production, we acknowledge that this observation may be only partially relevant to obesity-related metabolic endotoxemia, where serum IL-6 levels are present at significantly lower levels. However, it is still possible that chronic low-grade endotoxin exposure may result in localized activation of testicular macrophages, impairing adjacent Leydig cell function, without producing a detectable systemic (i.e., peripheral blood) cytokine response. This may explain why we did not observe any significant relationships between testosterone and serum TNF-α and IL-1β levels despite both of these cytokines being inhibitors of testosterone production in vitro. Alternatively, because of their high expression of TLR4, Leydig cells may be particularly susceptible to direct LPS inhibition (19, 32) independent of cytokine action.
The exact mechanisms by which endotoxin impairs testosterone production are not entirely clear. Experimental administration of LPS to animals has been shown to decrease the frequency and amplitude of LH pulses by suppressing both hypothalamic and anterior pituitary function (10), thereby reducing the pituitary drive for testosterone production. However, we observed no evidence for such an effect in either of our studies. Second, animal studies have confirmed that experimental administration of LPS directly inhibits the production of testosterone by reducing steroidogenic acute regulatory protein (StAR) activity (5, 19), a key protein involved in the initial transfer of cholesterol into mitochondria, where it is converted into testosterone. Rodent studies report that mitochondrial StAR levels fall to only 10% of baseline within 2 h of endotoxin exposure, mediated primarily by mitochondrial oxidative stress, not direct cytokine action (5, 19). Subsequently, several hours later, indirect inhibition of Leydig cell function is mediated by the release of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6, which reduce the activity of other key enzymes involved in steroidogenesis (1, 19, 20). These cytokines are produced by testicular macrophages and Leydig cells themselves in response to LPS (1, 32) plus adipose tissue (18). Therefore, the obesity-related inflammatory inhibition of testicular steroidogenesis is the net effect of a direct and rapid endotoxin-related reduction in StAR activity, followed by a delayed inhibition of steroidogenic enzymes such as cholesterol side chain cleavage (P450) by cytokines released by LPS-activated Leydig cells and macrophages plus adipose tissue independent of endotoxin exposure.
Although most of the work linking inflammatory cytokines to impaired testosterone production has been conducted in animals, there is also some initial evidence of a similar effect in humans. For example, administration of rIL-6 to healthy men, sufficient to produce a peak in plasma IL-6 levels comparable with that observed in our experimental study, has been reported to produce a 40% reduction in serum testosterone in the first 24 h after injection, without significant changes in LH levels (36). These results, plus our own findings, suggest that IL-6 can directly impair Leydig cell steroidogenesis.
It is interesting to speculate from an evolutionary perspective why an inflammatory-testicular function axis exists. Recently, we hypothesized that suppression of testicular function may be an adaptive process, helping protect men from sepsis and preventing them from passing on their genes in times of sickness (34). Testosterone is known to exert a suppressive action on both humoral and cellular immune responses (15), and thus men experience a greater risk of mortality and morbidity when faced with sepsis compared with women (31). As such, a reflex rapid decline in serum testosterone during sepsis similar to that observed in our interventional study will likely improve a man’s ability to fight off infection. Furthermore, in times of infection, sperm DNA damage increases, posing a potential risk to the health of any baby conceived at that time. Therefore, a decline in testosterone and related male libido reduces the chances of him fathering a child during this vulnerable period (34).
These results now provide additional insight into how obesity and gastrointestinal disorders such as IBD, both associated with increased intestinal permeability and endotoxemia (6, 7), may result in TD (3, 9, 11, 16, 24). We recognize that the impact of chronic-low level inflammation on testosterone levels seen in our observational study was relatively minor, thereby suggesting that other mechanisms such the conversion of testosterone to estrogen by adipose tissue aromatase probably play a more dominant role in obesity related hypogonadism (9). However, the recent observation that administration of probiotics, a treatment known to improve intestinal barrier integrity and reduce endotoxemia related inflammation (14), resulted in significant improvements in serum testosterone and spermatogenesis in mice (27) and men (22) underscores the potential importance of this gut-immune system-testicular function axis.
Finally, our observation of a link between obesity, endotoxemia, and TD may help explain why obesity is associated with an increased risk of psychological illness such as depression, anxiety, and social withdrawal. It has been well documented that infections as well as experimental administration of endotoxin can produce “sickness behavior” in healthy men, which is characterized by impaired mood, anxiety, and social disconnection (12, 13). This has previously been explained by inflammatory mediators modulating neurotransmitter release and neuronal function in key areas involved in mood regulation (e.g., the limbic system) (12, 13). However, testosterone is also recognized as playing a key role in regulating mood, with TD being conclusively linked with depression, anxiety, and social withdrawal in men (11). As such, it is possible that metabolic endotoxemia-related TD may play some role in mediating the psychological symptoms of obesity. This mechanism may help explain why reducing endotoxemia-related inflammation through the use of probiotics has been reported to be useful for improving mood in obese patients (30).
The strength of this study is that it provides both observational and interventional data supporting the ability of endotoxin exposure to impair testosterone production. However, we acknowledge that our observational cohort was recruited from an infertility clinic, and although we excluded men with obvious primary testicular failure, it is possible that these men’s gonadal responses may be atypical of the general population, as hypogonadism is certainly more commonly seen in infertile men (26). Future studies investigating the link between endotoxemia and TD in the general population over a broader age range are still needed. Furthermore, although we have presented compelling evidence linking experimental endotoxin exposure with impaired testosterone production, we acknowledge that our observational study results reveal that metabolic endotoxemia and its associated modest increase in IL-6 levels may only partially account for the reduction in serum testosterone in obese men. Although the GELDING theory (34) proposes that a breakdown in the intestinal mucosal barrier is the most likely cause of endotoxemia in obese men, we acknowledge that the data have not provided any direct evidence for an intestinal origin of endotoxin. Future studies that quantify markers of intestinal permeability (e.g., urinary sugar absorption tests, serum zonulin) (7) may help cast light on this issue. Furthermore, since recent ingestion of food has been reported to impact on testosterone levels (33), future studies need to control for fasting status. Finally, the immunoassays and assumptions used to quantify total and calculated free testosterone in our study do have some inaccuracies compared with the gold standards of mass spectroscopy and equilibrium dialysis (29). Although we acknowledge this weakness, these techniques are still widely used, and we do not believe that any related inaccuracies are likely to change the patterns of interaction that we observed between endotoxin exposure and testosterone production.
Conclusion
The results of our interventional study provide a proof of concept that endotoxin-initiated inflammation is a relevant factor in impairing Leydig cell testosterone production. This short burst of endotoxin exposure can reduce testosterone production by 30% within just 6 h of exposure and may help explain some of the negative “androgen deficiency” type psychological symptoms commonly observed in men during acute infections. Finally, our finding of a link between chronic low-level endotoxin exposure, inflammation (IL-6), and impaired androgen synthesis, together with previous reports linking improvements in testicular function with probiotic use, suggests that interactions between the gut and immune system play a significant role in testicular health. This finding highlights the need for further research to explore enhancement of intestinal barrier integrity as a potential treatment for andrological illness.
GRANTS
This work was supported by internal research grants from the University of Duisburg-Essen and Flinders University.
DISCLOSURES
K. Tremellen holds stock in Monash IVF and has acted as a consultant for MSD and Bayer Consumer Care and also has a financial interest in the male nutraceutical Menevit (Bayer Consumer Care, Australia). The remaining authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
K.T., K.P., M.S., and H.E. conceived and designed research; K.T., N.M., K.P., M.S., and H.E. analyzed data; K.T., N.M., K.P., M.S., and H.E. interpreted results of experiments; K.T. and H.E. prepared figures; K.T. and H.E. drafted manuscript; K.T., K.P., S.B., M.S., and H.E. edited and revised manuscript; K.T., N.M., K.P., S.B., M.S., and H.E. approved final version of manuscript; N.M., S.B., and H.E. performed experiments.
ACKNOWLEDGMENTS
We thank the volunteers involved in this study and the laboratory staff who conducted the hormone and cytokine assays (Dr. Ozlem Tunc, Repromed, Adelaide, SA, Australia; Dr. Tom Chung, Crux Biolab, Melbourne, VIC, Australia; Alexandra Kornowski, University Hospital Essen, Essen, Germany) and Dr. Ingo Spreitzer (Paul-Ehrlich Institute, Langen, Germany) for endotoxin safety testing.
REFERENCES
- 1.Allen JA, Diemer T, Janus P, Hales KH, Hales DB. Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation of mitochondria. Endocrine 25: 265–275, 2004. doi: 10.1385/ENDO:25:3:265. [DOI] [PubMed] [Google Scholar]
- 2.Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 92: 4241–4247, 2007. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 3.Ballinger AB, Savage MO, Sanderson IR. Delayed puberty associated with inflammatory bowel disease. Pediatr Res 53: 205–210, 2003. doi: 10.1203/00006450-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bobjer J, Katrinaki M, Tsatsanis C, Lundberg Giwercman Y, Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One 8: e61466, 2013. doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosmann HB, Hales KH, Li X, Liu Z, Stocco DM, Hales DB. Acute in vivo inhibition of testosterone by endotoxin parallels loss of steroidogenic acute regulatory (StAR) protein in Leydig cells. Endocrinology 137: 4522–4525, 1996. doi: 10.1210/endo.137.10.8828518. [DOI] [PubMed] [Google Scholar]
- 6.Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 124: 11–20, 2016. doi: 10.1016/j.biochi.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24: 503–512, 2012. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christeff N, Benassayag C, Carli-Vielle C, Carli A, Nunez EA. Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. J Steroid Biochem 29: 435–440, 1988. doi: 10.1016/0022-4731(88)90254-3. [DOI] [PubMed] [Google Scholar]
- 9.Corona G, Vignozzi L, Sforza A, Mannucci E, Maggi M. Obesity and late-onset hypogonadism. Mol Cell Endocrinol 418: 120–133, 2015. doi: 10.1016/j.mce.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Daniel JA, Abrams MS, deSouza L, Wagner CG, Whitlock BK, Sartin JL. Endotoxin inhibition of luteinizing hormone in sheep. Domest Anim Endocrinol 25: 13–19, 2003. doi: 10.1016/S0739-7240(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 11.Dean JD, McMahon CG, Guay AT, Morgentaler A, Althof SE, Becher EF, Bivalacqua TJ, Burnett AL, Buvat J, El Meliegy A, Hellstrom WJ, Jannini EA, Maggi M, McCullough A, Torres LO, Zitzmann M. The International Society for Sexual Medicine’s process of care for the assessment and management of testosterone deficiency in adult men. J Sex Med 12: 1660–1686, 2015. doi: 10.1111/jsm.12952. [DOI] [PubMed] [Google Scholar]
- 12.Engler H, Doenlen R, Engler A, Riether C, Prager G, Niemi MB, Pacheco-López G, Krügel U, Schedlowski M. Acute amygdaloid response to systemic inflammation. Brain Behav Immun 25: 1384–1392, 2011. doi: 10.1016/j.bbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Engler H, Brendt P, Wischermann J, Wegner A, Röhling R, Schoemberg T, Meyer U, Gold R, Peters J, Benson S, Schedlowski M. Selective increase of cerebrospinal fluid IL-6 during experimental systemic inflammation in humans: association with depressive symptoms. Mol Psychiatry 22: 1448–1454, 2017. doi: 10.1038/mp.2016.264. [DOI] [PubMed] [Google Scholar]
- 14.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071, 2013. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foo YZ, Nakagawa S, Rhodes G, Simmons LW. The effects of sex hormones on immune function: a meta-analysis. Biol Rev Camb Philos Soc 92: 551–571, 2017. doi: 10.1111/brv.12243. [DOI] [PubMed] [Google Scholar]
- 16.Gautier A, Bonnet F, Dubois S, Massart C, Grosheny C, Bachelot A, Aubé C, Balkau B, Ducluzeau PH. Associations between visceral adipose tissue, inflammation and sex steroid concentrations in men. Clin Endocrinol (Oxf) 78: 373–378, 2013. doi: 10.1111/j.1365-2265.2012.04401.x. [DOI] [PubMed] [Google Scholar]
- 17.Gnauck A, Lentle RG, Kruger MC. Chasing a ghost?—Issues with the determination of circulating levels of endotoxin in human blood. Crit Rev Clin Lab Sci 53: 197–215, 2016. doi: 10.3109/10408363.2015.1123215. [DOI] [PubMed] [Google Scholar]
- 18.Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res 113: 1009–1023, 2017. doi: 10.1093/cvr/cvx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hales KH, Diemer T, Ginde S, Shankar BK, Roberts M, Bosmann HB, Hales DB. Diametric effects of bacterial endotoxin lipopolysaccharide on adrenal and Leydig cell steroidogenic acute regulatory protein. Endocrinology 141: 4000–4012, 2000. doi: 10.1210/endo.141.11.7780. [DOI] [PubMed] [Google Scholar]
- 20.Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol 57: 3–18, 2002. doi: 10.1016/S0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 21.Lephart ED, Baxter CR, Parker CR Jr. Effect of burn trauma on adrenal and testicular steroid hormone production. J Clin Endocrinol Metab 64: 842–848, 1987. doi: 10.1210/jcem-64-4-842. [DOI] [PubMed] [Google Scholar]
- 22.Maretti C, Cavallini G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: a pilot study. Andrology 5: 439–444, 2017. doi: 10.1111/andr.12336. [DOI] [PubMed] [Google Scholar]
- 23.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 60: 762–769, 2006. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Toole A, Winter D, Friedman S. Review article: the psychosexual impact of inflammatory bowel disease in male patients. Aliment Pharmacol Ther 39: 1085–1094, 2014. doi: 10.1111/apt.12720. [DOI] [PubMed] [Google Scholar]
- 25.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 69: 29–35, 2005. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Patel DP, Brant WO, Myers JB, Zhang C, Presson AP, Johnstone EB, Dorais JA, Aston KI, Carrell DT, Hotaling JM. Sperm concentration is poorly associated with hypoandrogenism in infertile men. Urology 85: 1062–1067, 2015. doi: 10.1016/j.urology.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Poutahidis T, Springer A, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One 9: e84877, 2014. doi: 10.1371/journal.pone.0084877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy MM, Mahipal SV, Subhashini J, Reddy MC, Roy KR, Reddy GV, Reddy PR, Reddanna P. Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. Reprod Toxicol 22: 493–500, 2006. doi: 10.1016/j.reprotox.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 92: 405–413, 2007. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 30.Schachter J, Martel J, Lin CS, Chang CJ, Wu TR, Lu CC, Ko YF, Lai HC, Ojcius DM, Young JD. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav Immun S0889-1591(17)30415-4, 2017. doi: 10.1016/j.bbi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Schröder J, Kahlke V, Staubach KH, Zabel P, Stüber F. Gender differences in human sepsis. Arch Surg 133: 1200–1205, 1998. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 32.Shang T, Zhang X, Wang T, Sun B, Deng T, Han D. Toll-like receptor-initiated testicular innate immune responses in mouse Leydig cells. Endocrinology 152: 2827–2836, 2011. doi: 10.1210/en.2011-0031. [DOI] [PubMed] [Google Scholar]
- 33.Terrier JE, Isidori AM. How food intakes modify testosterone level. J Sex Med 13: 1292–1296, 2016. doi: 10.1016/j.jsxm.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Tremellen K. Gut endotoxin leading to a decline in gonadal function (GELDING) - a novel theory for the development of late onset hypogonadism in obese men. Basic Clin Androl 26: 7, 2016. doi: 10.1186/s12610-016-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tremellen K, McPhee N, Pearce K. Metabolic endotoxaemia related inflammation is associated with hypogonadism in overweight men. Basic Clin Androl 27: 5, 2017. doi: 10.1186/s12610-017-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsigos C, Papanicolaou DA, Kyrou I, Raptis SA, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on the pituitary-testicular axis. J Interferon Cytokine Res 19: 1271–1276, 1999. doi: 10.1089/107999099312948. [DOI] [PubMed] [Google Scholar]
- 37.Tsilidis KK, Rohrmann S, McGlynn KA, Nyante SJ, Lopez DS, Bradwin G, Feinleib M, Joshu CE, Kanarek N, Nelson WG, Selvin E, Platz EA. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology 1: 919–928, 2013. doi: 10.1111/j.2047-2927.2013.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veldhuis J, Yang R, Roelfsema F, Takahashi P. Proinflammatory cytokine infusion attenuates LH’s feedforward on testosterone secretion: modulation by age. J Clin Endocrinol Metab 101: 539–549, 2016. doi: 10.1210/jc.2015-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84: 3666–3672, 1999. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 40.Yeap BB, Knuiman MW, Divitini ML, Handelsman DJ, Beilby JP, Beilin J, McQuillan B, Hung J. Differential associations of testosterone, dihydrotestosterone and oestradiol with physical, metabolic and health-related factors in community-dwelling men aged 17-97 years from the Busselton Health Survey. Clin Endocrinol (Oxf) 81: 100–108, 2014. doi: 10.1111/cen.12407. [DOI] [PubMed] [Google Scholar]