Abstract
Hemolytic uremic syndrome (HUS) is major global health care issue as it is the leading cause of acute kidney injury in children. It is a triad of acute kidney injury, microangiopathic hemolytic anemia, and thrombocytopenia. In recent years, major advances in our understanding of complement-driven inherited rare forms of HUS have been achieved. However, in children 90% of cases of HUS are associated with a Shiga toxin-producing enteric pathogen. The precise pathological mechanisms in this setting are yet to be elucidated. The purpose of this review is to discuss advances in our understanding of the pathophysiology underlying HUS and identify the key questions yet to be answered by the scientific community.
Keywords: complement, hemolytic uremic syndrome, thrombotic microangiopathy
INTRODUCTION
Hemolytic uremic syndrome (HUS) is the leading cause of acute kidney injury in children. It is a triad of acute kidney injury, microangiopathic hemolytic anemia, and thrombocytopenia; first described in 1955 by Gasser et al. (as noted in Ref. 44). Over 90% of childhood HUS cases are associated with infection (19). Shiga toxin-producing Escherichia coli found in contaminated food and water supplies is typically the infective trigger (STEC HUS), although HUS has also been reported following exposure to Shigella, Campylobacter, and Streptococcus pneumoniae (37). In the United States, Shiga toxin associated HUS affects 0.5–2.1 people per 100,000 population per year. Most of these cases are reported in children under 5 yr of age (19).
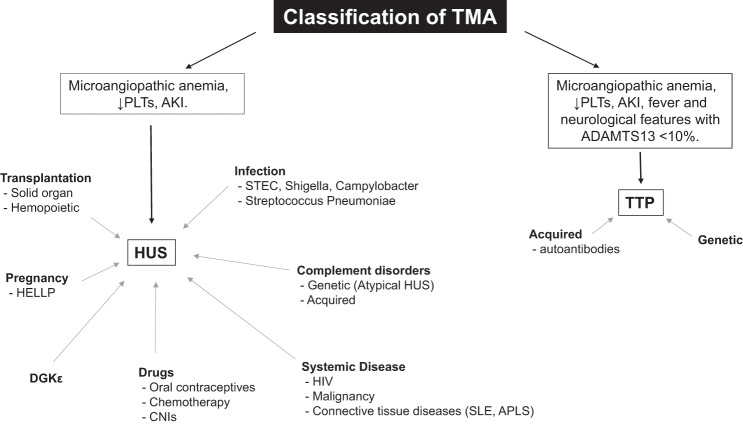
The remaining noninfective HUS cases are termed “atypical.” Atypical HUS is rare, with an estimated incidence in the United States of two cases per million population per year (17). However, unlike Shiga toxin HUS, the majority of adult HUS cases are atypical. These include familial HUS due to genetic abnormalities in complement regulation and other secondary triggers such as pregnancy, drugs, malignancy, connective tissue disorders, and transplantation (Fig. 1) (14). Over the last decade there has been a renewed appreciation and considerable interest in the role of the complement system in renal disease, which has been magnified by the effectiveness of eculizumab (a monoclonal humanized antibody against C5) in the treatment of atypical HUS (17, 24).
Fig. 1.
Classification of thrombotic microangiopathy (TMA). It is now widely accepted that classification of TMA according to etiology (rather than clinical features) is a more useful guide to prognosis and treatment. TMA represents a final common pathway in many disease processes (19). PLTs, platelets; HUS, hemolytic uremic syndrome; AKI, acute kidney injury; CNI, calcineurin inhibitors; SLE, systemic lupus erythematosus; APLS, anti-phospholipid syndrome; STEC, Shiga toxin-producing Escherichia coli; TTP, thrombotic thrombocytopenic purpura; HELLP, hemolysis, elevated liver enzymes, low platelet count syndrome.
This review will focus on the current understanding of the pathogenesis of atypical HUS and STEC HUS and try to identify some of the key questions yet to be answered by the scientific community.
CLASSIFICATION OF HUS
HUS is categorized histopathologically as a thrombotic microangiopathy (TMA). This term was first introduced in 1952 by Symmers (as noted in Ref. 44) to describe capillary wall thickening, swelling, and detachment of the endothelial cell from the basement membrane, accumulation of debris in the subendothelial space, and intraluminal platelet thrombosis, culminating in partial or complete obstruction of the vessel. When this process occurs in the microvasculature of the kidney (as is the case in HUS), renal impairment is seen due to organ ischemia. The microangiopathic hemolytic anemia that ensues is a consequence of erythrocyte shear, resulting in red cell fragmentation. Platelet activation and entrapment in microthrombi as well as trafficking to the reticuloendothelial system lead to thrombocytopenia, local thrombosis, and organ ischemia (19, 35). Thus TMA represents a final common pathway in a number of disease processes that among others include HUS and thrombotic thrombocytopenic purpura (TTP). (Fig. 1). The clinical sequelae observed is dependent on the vascular bed and organ affected (19). Both HUS and TTP are a consequence of endothelial cell injury but are now accepted to be different diseases with distinct underlying mechanisms (21).
ATYPICAL HUS DUE TO COMPLEMENT DYSREGULATION
It is now established that most familial cases of atypical HUS are caused by hyperactivation of the alternative complement pathway as a result of loss-of-function mutations in complement inhibitory regulatory factors or gain of function mutations in C3 or factor B (14). Autoantibodies against regulatory complement factors have also been described in atypical HUS (27). It was the discovery by Warwicker et al. in 1998 (as noted in Ref. 33) that a mutation in factor H led to the development of atypical HUS, which prompted the search and subsequent discovery of over 120 mutations in complement regulatory genes responsible for the disease.
Complement system.
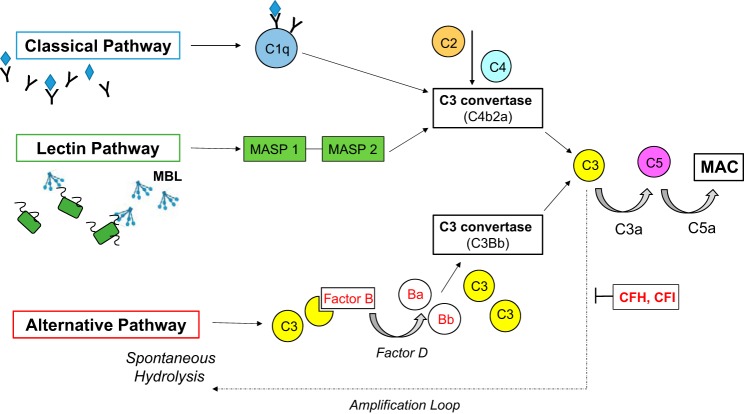
The complement pathway forms part of the innate immune response and consists of 60 proteins that play a vital role in the host defense against pathogens and in the maintenance of tissue homeostasis (1, 33). These proteins can be plasmatic (fluid) or membrane-bound receptors (solid) (43). Three pathways leading to activation of the complement cascade have been described: classic, lectin, and alternative. All culminate in the formation of the membrane attack complex (MAC), leading to the insertion of pores in the target cell membrane, resulting in osmotic lysis and cell death (2) (Fig. 2). The classic pathway is activated by binding of C1q to antibody-antigen complexes. The lectin pathway is triggered by binding of mannose binding lectin (MBL) to mannose-containing carbohydrates on microbial surfaces that activates MBL-associated serine protease 1 and 2 (MASP 1 and MASP 2) (2) (33). Both the classic and lectin pathways lead to the generation of the same C3 convertase C4b2a on target cell surfaces, resulting in proteolytic cleavage of C3 into C3a and C3b (33).
Fig. 2.
The complement cascade. Three pathways have been described leading to activation of the complement pathway; classical, lectin and alternative. All culminate with the formation of the membrane attack complex (MAC) leading to the insertion of pores into the target cell membrane resulting in osmotic lysis and cell death. The alternative pathway differs from the other two in that it is continually active at low levels in the plasma and is activated by spontaneous hydrolysis of a thioester bond in C3. To protect host cells from non-specific destruction complement activation is regulated by a number of plasma (CFH, CFI) and membrane-bound factors (2, 33). MBL, mannose-binding lectin; MASP, MBL-associated serine protease; CFH, complement factor H; CFI, complement factor I.
The alternative pathway differs from the other two in that it is continually active at low levels in the plasma. Spontaneous hydrolysis of the thioester bond in C3 induces a conformational change in the protein, which facilitates binding of complement factor B. Once bound to C3, factor B is cleaved by factor D to Ba and Bb. This generates the alternative pathway C3 convertase C3Bb, resulting in proteolytic cleavage of C3 into C3a and C3b (see Fig. 2) (2) (33, 43). Association of C3b with C3 convertases (generated by any of the 3 pathways) leads to formation of C5 convertases, which cleave factor C5 into C5a and C5b, initiating the assembly of the MAC on target cell surfaces (33). This terminal pathway in the complement cascade also acts to alert the host defenses by the release of chemoattractant and inflammatory mediators; C3a and C5a are potent anaphylatoxins that recruit phagocytes and induce endothelial cell activation (46).
The alternative pathway acts as a positive feedback loop, amplifying complement activity. C3b on target cell surfaces generated by the classic or lectin pathways acts as a site of C3Bb formation, and the activation of alternative pathway itself leads to deposits of C3b on cell surfaces, further potentiating this loop. As such, the alternative pathway may account for up to 80% terminal pathway activity (2). Hence, the complement cascade has great potential for nonspecific destruction, which host cells must be protected from (2).
Complement regulation.
It is essential that complement activation is regulated to prevent excessive cell injury and inflammation (2, 27). Host cells are protected by surface-bound and soluble plasma complement regulatory proteins. Of particular importance are the plasma proteins factor H and factor I, which negatively regulate the C3b amplification loop (Fig. 2). Although found in the plasma both can act in the fluid phase and on cellular surfaces. Their role on cell surfaces and specialized biomembranes (such as the glomerular basement membrane in the kidney), which lack membrane-bound complement regulators, is vital, as they are the only defense against complement attack and thrombus formation (27, 46). This may explain why the kidney is so susceptible to injury in atypical HUS, where impaired regulation of the alternative complement pathway in the glomerulus due to mutation in complement regulators or autoantibodies against them results in loss-of-host protection (12).
Notable membrane-bound complement factors include CD46 [membrane cofactor protein (MCP)], CD55 [decay activating factor (DAF)], CD59, and CR1. By definition, these surface-bound complement regulatory proteins are restricted in activity by their cellular expression and distribution, the exception being CR1, which is expressed by neutrophils, lymphocytes, and erythrocytes, which circulate throughout the body rather than being tissue bound (27, 34).
GENETICS OF ATYPICAL HUS
Atypical HUS is a rare condition associated with significant morbidity and mortality, historically leading to end-stage renal disease in approximately one-half of patients affected (27). Inheritance may be sporadic, autosomal recessive, or autosomal dominant with incomplete penetrance (34). This low-level genetic penetrance highlights the importance of genetic modifiers and environmental triggers in the development of the disease (3). Interestingly, identification of the specific gene polymorphisms resulting in abnormalities of the complement cascade provides powerful prognostic information allowing patients to be counselled about likelihood of posttransplantation recurrence (5, 14). Mutations in Complement factor H are associated with the worst outcomes, whereas those with MCP mutations do far better. This is not surprising given that MCP is a transmembrane protein that is highly expressed in the kidney; hence, kidney transplantation corrects the MCP defect and restores MCP solid phase activity. This is in contrast to mutations in circulating (fluid phase) complement factors H and I, which are predominantly of hepatic origin (33).
What is intriguing about complement regulatory proteins (whether soluble or membrane bound) is that they have evolved from a common structural domain characterized by several large genomic repeat regions: the short consensus repeat (8). This facilitates nonhomologous recombination that results in chromosomal rearrangements in these regions that produce diverse outcomes. This has allowed evolution of differential specificities for binding ligands (in this case the various complement proteins) but has also resulted in deletions and duplications of chromosomal fragments that lead to mutated complement regulatory proteins and predisposition to HUS (49).
Factor H is known to be key in discrimination between host cells and invading microbes. Indeed in a recent review by Jokiranta (12), mutations in factor H are responsible for up to 28% of atypical HUS cases with factor I mutations seen in only 8% of patients. Autoantibodies against the C terminus of factor H or factor I can also result in acquired complement dysregulation, accounting for up to 10% of cases (3). In approximately one-third of cases of atypical HUS, the underlying mutation is unknown (12).
When factor H binds to cell surface C3b, it prevents activation and spares the cell from destruction (12). Hence, factor H is central in discriminating self and non-self and regulation of the alternative complement pathway. This is via simultaneous binding of factor H to C3b and cell surface polyanions (sialic acid, glycosaminoglycans, and phospholipids). Until recently, the glycosaminoglycans heparin and heparin sulfate were considered to be the most important polyanions involved in self-protection of host cell surfaces. However, Hy Arinen et al. (7) demonstrated that mutations in factor H specifically disrupt binding to sialic acid. They also showed that removal of sialic acid from glomerular endothelial cells reduced factor H binding and resulted in complement attack (29). Furthermore, sialic acids are under dynamic regulation and as such cell surface composition varies at different ages and conditions, which may contribute to the incomplete genetic penetrance observed in atypical HUS associated with factor H mutations. Fascinatingly, pathogens such as Borrelia burgdorferi, streptococci, and Gram-negative bacteria have evolved to escape complement attack by binding to factor H, thereby mimicking host cell surfaces (29).
The membrane-bound regulators MCP, DAF, and CR1 limit complement activation at the C3 step. CD59 inhibits polymerization of C9 and prevents MAC formation. They are largely species selective and protect host cell surfaces against complement from homologous species (29). Mutations in these cell surface regulators account for 10–15% of atypical HUS cases (12). A lack of DAF and CD59 (due to an acquired defect in anchorage to cell membrane phospholipids) in erythrocytes results in the disease paroxysmal nocturnal hematuria (29). Red blood cells are rendered susceptible to complement attack, and patients develop a tendency to thrombosis. Together, paroxysmal nocturnal hematuria and the glomerular thrombotic microangiopathy seen in atypical HUS suggest that the coagulation system and complement pathways are closely interconnected and that thrombosis itself may act to trigger further complement activation (12, 29).
MCP is expressed by all nucleated cells (i.e., it is absent from erythrocytes). CR1 is present on all human blood cells (except platelets and most T cells) where it acts as a cofactor for the cleavage of C4b and C3b by factor I and deactivates the alternative complement pathway through direct binding to C3b. CR1 also binds to C4b accelerating the decay of classical and MBL convertases (9). In red blood cells, CR1 acts as an immune adherence receptor binding antigens coated in C3b/C4b and transporting them for clearance in the reticuloendothelial system (9). Of particular interest, CR1 is also expressed on selected epithelial cells: the podocyte in the kidney and the retinal pigment cells in the eye. Its role in these cells remains a mystery. Java et al. (9) have recently shown that CR1 is a potent inhibitor of the alternative pathway and binds immune complexes directly that remain on the surface of the epithelial cell. This observation has led to the hypothesis that podocytes are fundamental in modulating the innate immune response through CR1 binding of immune complexes trapped within the glomerulus. However, difficulties with any further study in this area have been that conditionally immortalized podocytes in vitro lack CR1 expression and that mouse erythrocytes lack CR1 (8).
ATYPICAL HUS INDEPENDENT OF COMPLEMENT ACTIVATION
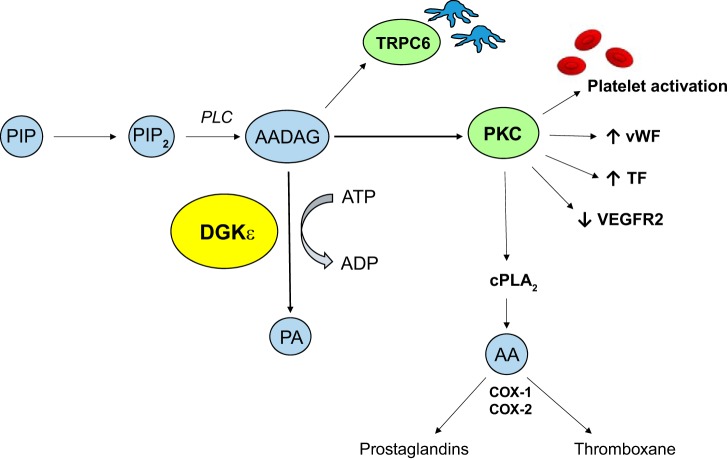
The discovery in 2013 by Lemaire et al. (as noted in Ref. 42) that recessive mutations in DGKε cause atypical HUS in a significant proportion of children in the first year of life has led to an alternative pathophysiological mechanism being proposed for the condition. DGKε is the first gene to be associated with familial HUS that has not been shown to have a direct role in alternative pathway complement activation (4, 25). Diacylglycerol kinases (DGKs) are intracellular lipid kinases that phosphorylate diacylglycerol (DAG) to phosphatidic acid (PA), leading to termination of DAG signaling (4). The DGKε isoform preferentially phosphorylates arachidonic acid containing DAG (AADAG) to PA thereby terminating AADAG signaling (4) (Fig. 3).
Fig. 3.
Effect of AADAG signaling. DGKε is an intracellular kinase that preferentially phosphorylates AADAG to PA thereby terminating AADAG signaling. Loss-of-function mutations in DGKε lead to unregulated AADAG signaling and PKC dependent platelet activation and increased production of prothrombotic factors (25, 42). PIP, phosphatidylinositol; PIP2, phosphatidylinositol-4,5-bisphosphate; PLC, phospholipase C; AADAG, arachidonic acid diacylglycerol; ATP, adenosine triphosphate; ADP, adenosine diphosphate; DGKε, diacylglycerol kinase ε; PA, phosphatidic acid; TRCP6, transient receptor potential cation channel 6; PKC, protein kinase C; vWF, von Willebrand factor; TF, tissue factor; VEGFR2, vascular endothelial growth factor receptor 2; cPLA2, cytosolic phospholipase A2; AA, arachidonic acid; COX, cyclooxygenase enzyme.
AADAG is a key intracellular signaling molecule that activates protein kinase C (PKC). AADAG-dependent PKC signaling promotes thrombin-induced platelet activation. In endothelial cells, it has been shown to increase production of prothrombotic factors such as von Willebrand factor and tissue factor; as well as downregulating VEGF receptor VEGFR2 signaling, which has been linked to TMAs previously (25, 42). Bruneau et al. (4) have demonstrated in endothelial cells that knockdown of DGKε results in a proinflammatory and prothrombotic state via over activation of p38 and p44/42 MAP kinases. In podocytes, DAGs have been shown to modify slit diaphragm function and through PKC-dependent pathways downregulate expression of VEGFR2 (25). Hence, the hypothesis is that loss-of-function mutations in DGKε results in sustained AADAG signaling and a subsequent prothrombotic state. This finding has important implications for the management of HUS, which to date have focused on complement blockade.
Vascular endothelial growth factor and HUS
In the kidney, podocytes are the major source of vascular endothelial growth factor (VEGF-A), which is essential for maintaining the nearby glomerular endothelium. The clinical observation that cancer patients treated with the VEGF inhibitor bevacizumab develop a glomerular TMA has led to studies in transgenic mouse models (6). Interestingly, Eremina et al. (6) have shown that disruption of VEGF signaling in mature, fully developed podocyte-specific VEGFA knockout mice results in a glomerular TMA with a phenotype that recapitulates the glomerular injury seen in HUS. Moreover, recent work published by Keir et al. (20) has provided evidence that local VEGF availability modulates complement regulation in the microvasculature of the kidney (and retina) via VEGFR2/PKC-α/CREB signaling.
In further support of the importance of the glomerular microenvironment and local VEGF signaling; Jin et al. (10) have shown that sFLT1 (a soluble VEGF receptor) is produced by podocytes. sFLT1 is known to bind VEGF thereby acting as a decoy receptor to inhibit proangiogenic activity via VEGFR2. High circulating levels of sFLT1 have been linked to preeclampsia, another glomerular disease associated with endotheliosis and complement activation (10, 20). Elimination of podocyte sFLT1 production led to podocyte actin cytoskeleton rearrangement with damage to the glomerular filtration barrier and massive proteinuria. This work illustrates the complexity of glomerular cell cross talk and the autocrine function of sFLT1 in the regulation of podocyte behavior (10).
SHIGA TOXIN-ASSOCIATED HUS
The commonest cause of HUS is infection, which accounts for 90% of cases in childhood (19). The pathophysiology underlying STEC HUS is yet to be determined and as such there are currently no specific treatments for the disease other than supportive care (38). Acute mortality outcomes for STEC HUS have improved from 30 to 5% with the introduction of early dialysis. However, of those patients that survive the initial insult up to 30% develop proteinuria and 18% progress to chronic renal failure. Furthermore, some patients develop extra-renal sequelae such as diabetes mellitus, colonic strictures, and devastating neurological disease (13). A better understanding of the pathophysiology underlying STEC HUS is essential if targeted therapy for this condition is to be developed.
Shiga toxins are exotoxins produced by E. coli, as well as by other bacteria including Shigella dysenteriae and Campylobacter jejuni (11). They consist of a single 30-kDa enzymatic A subunit in noncovalent association with five identical B subunits of 7 kDa each (37, 52). The genes encoding Shiga toxin are found within lambdoid bacteriophages present in all pathogenic STEC. Toxin secretion occurs by phage-mediated bacterial lysis (11). It is the B subunit of Shiga toxin that binds to the glycosphingolipid receptor Gb3. It has been shown that cells lacking Gb3 expression are resistant to the toxic effects of Shiga toxin, meaning the pattern of expression of Gb3 in different cell types is a reliable predictor of Shiga toxin site of action (41).
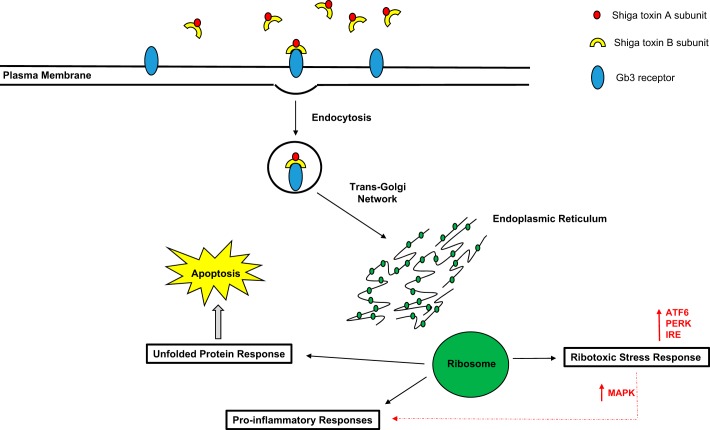
Following binding to Gb3, Shiga toxin is endocytosed and transported in a retrograde manner from the endosome to the trans-Golgi network. From here it is transported to the endoplasmic reticulum (ER), where using the ER-associated degradation machinery it is able to translocate into host cell cytoplasm (53) (Fig. 4). Shiga toxin acts in three main ways: first, it inactivates ribosomes by enzymatically removing an adenine residue from 28S ribosomal RNA. This triggers the ribotoxic stress response leading to MAPK signaling and activation of cytokines and chemokines that result in proinflammatory and proapoptotic pathways (37) (52). Second, the unfolded protein response may be triggered by Shiga toxin unfolding within the ER. Prolonged signaling via the unfolded protein response will induce apoptosis in cells (52). Finally, the binding of the B subunit itself can initiate a cytoplasmic transduction cascade that is distinct from the ribotoxic stress response but that also culminate in a prothrombotic, proinflammatory cellular environment (1a, 37). Attempts made to block Shiga toxin binding with synthetic Shiga toxin binders such as STARFISH or Synsorb-Pk or to inhibit intracellular transport of the toxin with molecules such as Exo1 and Golgicide A have to date been ineffective (11).
Fig. 4.
Intracellular responses to Shiga toxin binding. The B subunit of Shiga toxin binds to the glycosphingolipid Gb3 in the target cell membrane. Following binding Shiga toxin is endocytosed and transported in a retrograde manner to the trans-Golgi network. From here it is transported to the endoplasmic reticulum (ER) where it triggers 3 responses; the ribotoxic stress response, proinflammatory cytokine release and the unfolded protein response (37) (52). ATF6, activating transcription factor 6; PERK, protein kinase R (PKR)-like endoplasmic reticulum kinase; IRE, iron-response element; MAPK, mitogen-activated protein kinase.
The exact mechanisms underlying the trafficking of Shiga toxin from the gut to the kidney (and other target organs such as the brain) are yet to be elucidated. Interestingly, there is evidence in human intestinal cells that an alternative pathway for toxin translocation exists, which is independent of the Gb3 receptor and results in IL-8 secretion without cytotoxicity (36). Shiga toxin also stimulates the release of proinflammatory cytokines from macrophages, which are also refractory to toxicity due to low-level expression of Gb3 (36). At present, the most widely accepted hypothesis is that neutrophils are responsible for binding and transportation of Shiga toxin to susceptible tissues (37).
Gb3 expression and the effect of Shiga toxin in eukaryotic cells.
As already alluded to, there is an association between the amount of Gb3 expressed on the cell membrane and its sensitivity to Shiga toxin. For example, endothelial cell lines derived from large vessels such as the human umbilical vein (which express low levels of Gb3) are relatively resistant to Shiga toxin in comparison to glomerular endothelial cells (36). Moreover, cells lacking the Gb3 receptor can be rendered sensitive through artificially incorporating Gb3 into their membrane (36). However, the amount of Gb3 extractable from a tissue does not directly correlate to its sensitivity to Shiga toxin. Increasing evidence suggests that the membrane microenvironment consisting of cholesterol, other glycolipids and fatty acids also plays a role in Gb3 receptor binding. It is, therefore, vital to determine how reactive each cell type is when challenged with Shiga toxin not just the amount of Gb3 expressed (37). Furthermore, the Gb3 fatty acid chain length has also been shown to affect how sensitive a cell is to Shiga toxin (36). Therefore, caution is advised when interpreting in vitro studies to acknowledge the differences in the cell membrane fatty acid profiles in culture vs. their in vivo counterparts, which likely affect Gb3 receptor binding to Shiga toxin (39).
All human glomerular cells (mesangial cells, podocytes, and endothelial cells) express Gb3. However, mouse glomerular cells lack Gb3 (19). Human podocytes and glomerular endothelial cells are particularly sensitive to the cytotoxicity of Shiga toxin. Interestingly, human podocytes exposed to the toxin express 60% less VEGFA compared with untreated control cells (19). In light of the finding from Eremina et al. (6) that podocyte-specific VEGFA knockout mice develop a glomerular TMA, it is conceivable that the podocyte may prove to be more important in the pathogenesis of STEC HUS than first thought.
Human and most other animals also express Gb3 on their tubular cells and collecting duct epithelium (37). Consequently, STEC HUS results in primary renal tubular injury and mesangial cell lysis, resulting in the release of proinflammatory chemokines (IL-1 and TNF-α) that potentiate the renal cytotoxicity of Shiga toxin (30). Van Setten et al. (47) have demonstrated that these proinflammatory cytokines increase Gb3 synthesis in glomerular endothelial cells. The expression of such an abundance of biologically reactive Gb3 in the human glomerulus and the upregulation of functional Gb3 expression in response to inflammation in glomerular endothelial cells may provide one explanation as to why the kidney is the predominant target in STEC HUS. Others have suggested that the rich vascular supply of the kidney and filtration rate of blood may increase exposure of the renal microvasculature to Shiga toxin and predispose to renal injury (37).
Animal models of STEC HUS.
The development of rodent models of STEC HUS that recapitulate the glomerular TMA lesions seen in humans is yet to be achieved. Mice challenged with Shiga toxin alone (orally or intraperitoneally) develop lethal tubular disease without histological evidence of glomerular TMA, presumably due to the absence of Gb3 expression in their glomeruli (19). Any vascular changes seen are driven by the combined action of Shiga toxin and lipopolysaccharide (18, 37). In an effort to address this discrepancy several other animal models have been used to study STEC HUS in the rabbit, pig, and baboon.
Rabbits develop acute renal failure following STEC challenge, but this is a variable and inconsistent model. Porcine models have not been described in detail but, similar to murine models, tubular lesions predominate. Primate studies most closely recapitulate the disease process observed in human kidneys but only following intravenous infusion with Shiga toxin (22). Enteric bacterial infection with Shiga toxin-producing E.coli in primates does not lead to HUS, and bacterial translocation from the gut is not seen baboons (23, 51). This is in contrast to humans where STEC induce attaching and effacing lesions and inflammatory cytokine production in intestinal epithelial cells (26). As such, non-human primate models are of use in the validation of observations in mouse studies but are expensive and impractical given the need for intravenous toxin administration. Furthermore, the lack of intestinal injury observed in baboons is a limitation of this model and suggests species-specific host factors are likely to be very important in STEC (23). It is clear that animal models that more closely recapitulate human HUS are needed.
EVIDENCE OF COMPLEMENT ACTIVATION IN STEC HUS
In the last decade, evidence for the role for complement activation in Shiga toxin-associated HUS has increased. Some patients with STEC HUS have been reported to have low levels of circulating C3 and evidence of increased levels of C3 convertases and factor B (33). However, retrospective analyses of the benefit of complement blockade with eculizumab in patients during STEC HUS outbreaks have been difficult to meaningfully interpret (28).
A recent publication by Ozaki et al. (38) has described a novel mouse model in human MBL-expressing mice (that lack murine MBL). Mice were given intraperitoneal Shiga toxin in combination with or without a monoclonal antibody to human MBL. Fascinatingly, the antibody attenuated Shiga toxin-induced renal injury (38). Conventionally, atypical HUS has been accepted to be a product of dysregulated alternative pathway activation. This paper suggests that STEC HUS may initially trigger the MBL pathway, which then leads to alternative pathway activation and amplification. There is increasing evidence that components of the MBL pathway may be able to activate the alternative pathway directly via a C2 bypass mechanism (32). Indeed, given the high-affinity binding of MBL to the lipopolysaccharide membrane of E. coli, it is feasible that the initial innate immune response to STEC infection in the gut may be predominantly driven by the MBL pathway (48). Furthermore, MASPs activated in the MBL immune response (specifically MASP2) cleave prothrombin to thrombin, resulting in a procoaguable state, which may contribute to the glomerular TMA seen in HUS (16). This interplay between the MBL and alternative complement pathways may even explain the low genetic penetrance of atypical HUS where an environmental trigger is usually required alongside a genetic predisposition (17). However, at odds with the importance of the MBL complement pathway in STEC HUS is the finding that children with MBL deficiency are not predisposed to the condition (40).
Evidently, our understanding of the precise pathophysiological mechanisms underlying STEC HUS is far from complete. Additionally, we do not understand why children and the elderly are more prone to develop STEC HUS. A popular theory had been that children express more renal Gb3 than adults, but studies have shown the reverse (37). It may be the case that the Gb3 expressed in pediatric kidneys is more biologically active due to the membrane microenvironment (37). Another more recent concept in complement biology has been the theory of trained immunity, whereby the innate immune response is capable of immunological memory (31). This may explain why the elderly and immunosuppressed have a higher incidence of STEC HUS.
CONCLUSIONS
Complement pathway hyperactivation is emerging as a potential common mechanism underlying the pathogenesis of glomerular TMA. Identification of the genetic mutations in complement regulatory proteins, C3 convertase components, and anti-CFH antibodies in atypical HUS has led to the advent of a new era of pharmacological complement modulation (33). Indeed, the success of eculizumab in the management of atypical HUS has transformed the management of these patients and resulted in reduced morbidity and mortality (17). However, with the discovery of recessive loss-of-function mutations in DGKε an alternative mechanism for atypical HUS independent of complement activation has been demonstrated, suggesting other non-complement-driven pathways may also be important (42).
In STEC HUS, delineating the role of complement, elucidating which precise signaling pathways are involved, and defining whether there is a cellular target of Shiga toxin in the glomerulus will be important. This will be helped by developing better animal models that more accurately recapitulate human Shiga toxin HUS (28). The challenge for the future lies in identifying the molecular and pathophysiological mechanisms responsible for STEC HUS and subsequent development of specific treatment strategies for this devastating disease.
GRANTS
This review article was supported by Kidney Research UK Clinical Research PhD Fellowship Grant TF_007_20151127 (to E. E. Bowen) and Medical Research Council Senior Research Fellowship Grant MR/K010492/1 (to R. J. Coward).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.E.B. prepared figures; E.E.B. drafted manuscript; E.E.B. and R.J.C. edited and revised manuscript; E.E.B. and R.J.C. approved final version of manuscript.
REFERENCES
- 1.Barnum SR. Complement: a primer for the coming therapeutic revolution. Pharmacol Ther 172: 63–72, 2017. doi: 10.1016/j.pharmthera.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 1a.Betzen C, Plotnicki K, Fathalizadeh F, Pappan K, Fleming T, Bielaszewska M, Karch H, Tönshoff B, Rafat N. Shiga toxin 2a-induced endothelial injury in hemolytic uremic syndrome: a metabolomic analysis. J Infect Dis 213: 1031–1040, 2016. doi: 10.1093/infdis/jiv540. [DOI] [PubMed] [Google Scholar]
- 2.Blatt AZ, Pathan S, Ferreira VP. Properdin: a tightly regulated critical inflammatory modulator. Immunol Rev 274: 172–190, 2016. doi: 10.1111/imr.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brocklebank V, Johnson S, Sheerin TP, Marks SD, Gilbert RD, Tyerman K, Kinoshita M, Awan A, Kaur A, Webb N, Hegde S, Finlay E, Fitzpatrick M, Walsh PR, Wong EKS, Booth C, Kerecuk L, Salama AD. Factor H autoantibody is associated with atypical hemolytic uremic syndrome in children in the United Kingdom and Ireland. Kidney Int 92: 1261–1271, 2017. 10.1016/j.kint.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruneau S, Néel M, Roumenina LT, Frimat M, Laurent L, Frémeaux-Bacchi V, Fakhouri F. Loss of DGKε induces endothelial cell activation and death independently of complement activation. Blood 125: 1038–1046, 2015. doi: 10.1182/blood-2014-06-579953. [DOI] [PubMed] [Google Scholar]
- 5.Durkan AM, Kim S, Craig J, Elliott E. The long-term outcomes of atypical haemolytic uraemic syndrome: a national surveillance study. Arch Dis Child 101: 387–391, 2016. doi: 10.1136/archdischild-2015-309471. [DOI] [PubMed] [Google Scholar]
- 6.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Golam Kabir M, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. New Engl J Med 358: 1129–1136, 2008. 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hy Arinen S, Meri S, Sakari Jokiranta T.. Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome. Blood 27: 2701–2710, 2016. doi: 10.1182/blood-2015-11-680009. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson AC, Weis JH. Comparative functional evolution of human and mouse CR1 and CR2. J Immunol 181: 2953–2959, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Java A, Liszewski MK, Hourcade DE, Zhang F, Atkinson JP. Role of complement receptor 1 (CR1; CD35) on epithelial cells: a model for understanding complement-mediated damage in the kidney. Mol Immunol 67: 584–595, 2015. doi: 10.1016/j.molimm.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin J, Sison K, Li C, Tian R, Wnuk M, Sung HK, Jeansson M, Zhang C, Tucholska M, Jones N, Kerjaschki D, Shibuya M, Fantus IG, Nagy A, Gerber HP, Ferrara N, Pawson T, Quaggin SE. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 151: 384–399, 2012. doi: 10.1016/j.cell.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Johannes L, Römer W. Shiga toxins—from cell biology to biomedical applications. Nat Rev Microbiol 8: 105–116, 2009. doi: 10.1038/nrmicro2279. [DOI] [PubMed] [Google Scholar]
- 12.Jokiranta TS. HUS and atypical HUS. Blood 129: 2847–2856, 2017. doi: 10.1182/blood-2016-11-709865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan BS, Ruebner RL, Spinale JM, Copelovitch L. Current treatment of atypical hemolytic uremic syndrome. Intractable Rare Dis Res 3: 34–45, 2014. doi: 10.5582/irdr.2014.01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karpman D, Loos S, Tati R, Arvidsson I. Haemolytic uraemic syndrome. J Intern Med 281: 123–148, 2017. doi: 10.1111/joim.12546. [DOI] [PubMed] [Google Scholar]
- 16.Karpman D, Tati R. Complement contributes to the pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Kidney Int 90: 726–729, 2016. doi: 10.1016/j.kint.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Kavanagh D, Goodship TH, Richards A. Atypical hemolytic uremic syndrome. Semin Nephrol 33: 508–530, 2013. doi: 10.1016/j.semnephrol.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keepers TR, Psotka MA, Gross LK, Obrig TG. A murine model of HUS: Shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. J Am Soc Nephrol 17: 3404–3414, 2006. doi: 10.1681/ASN.2006050419. [DOI] [PubMed] [Google Scholar]
- 19.Keir L, Coward RJ. Advances in our understanding of the pathogenesis of glomerular thrombotic microangiopathy. Pediatr Nephrol 26: 523–533, 2011. doi: 10.1007/s00467-010-1637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keir LS, Firth R, Aponik L, Feitelberg D, Sakimoto S, Aguilar E, Welsh GI, Richards A, Usui Y, Satchell SC, Kuzmuk V, Coward RJ, Goult J, Bull KR, Sharma R, Bharti K, Westenskow PD, Michael IP, Saleem MA, Friedlander M. VEGF regulates local inhibitory complement proteins in the eye and kidney. J Clin Invest 127: 199–214, 2017. doi: 10.1172/JCI86418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keir LS, Langman CB. Complement and the kidney in the setting of Shiga-toxin hemolytic uremic syndrome, organ transplantation, and C3 glomerulonephritis. Transfus Apher Sci 54: 203–221, 2016. doi: 10.1016/j.transci.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Laszik ZG, Blakey GL. Searching for a valid animal model of hemolytic uremic syndrome. Am J Clin Pathol 118: 323–325, 2002. doi: 10.1309/TC75-EECC-TU3X-FDL9. [DOI] [PubMed] [Google Scholar]
- 23.Lee BC, Mayer CL, Leibowitz CS, Stearns-Kurosawa DJ, Kurosawa S. Quiescent complement in nonhuman primates during E coli Shiga toxin-induced hemolytic uremic syndrome and thrombotic microangiopathy. Blood 122: 803–806, 2013. doi: 10.1182/blood-2013-03-490060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368: 2169–2181, 2013. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 25.Lemaire M, Frémeaux-Bacchi V, Schaefer F, Choi M, Tang WH, Le Quintrec M, Fakhouri F, Taque S, Nobili F, Martinez F, Ji W, Overton JD, Mane SM, Nürnberg G, Altmüller J, Thiele H, Morin D, Deschenes G, Baudouin V, Llanas B, Collard L, Majid MA, Simkova E, Nürnberg P, Rioux-Leclerc N, Moeckel GW, Gubler MC, Hwa J, Loirat C, Lifton RP. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 45: 531–536, 2013. doi: 10.1038/ng.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallick EM, McBee ME, Vanguri VK, Melton-Celsa AR, Schlieper K, Karalius BJ, O’Brien AD, Butterton JR, Leong JM, Schauer DB. A novel murine infection model for Shiga toxin-producing Escherichia coli. J Clin Invest 122: 4012–4024, 2012. doi: 10.1172/JCI62746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medjeral-Thomas NR, Lomax-Browne HJ, Beckwith H, Willicombe M, Mclean AG, Brookes P, Pusey CD, Falchi M, Cook HT, Pickering MC. Circulating complement factor H-related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int 92: 942–952, 2017. doi: 10.1016/j.kint.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menne J, Nitschke M, Stingele R, Abu-Tair M, Beneke J, Bramstedt J, Bremer JP, Brunkhorst R, Busch V, Dengler R, Deuschl G, Fellermann K, Fickenscher H, Gerigk C, Goettsche A, Greeve J, Hafer C, Hagenmüller F, Haller H, Herget-Rosenthal S, Hertenstein B, Hofmann C, Lang M, Kielstein JT, Klostermeier UC, Knobloch J, Kuehbacher M, Kunzendorf U, Lehnert H, Manns MP, Menne TF, Meyer TN, Michael C, Münte T, Neumann-Grutzeck C, Nuernberger J, Pavenstaedt H, Ramazan L, Renders L, Repenthin J, Ries W, Rohr A, Rump LC, Samuelsson O, Sayk F, Schmidt BM, Schnatter S, Schöcklmann H, Schreiber S, von Seydewitz CU, Steinhoff J, Stracke S, Suerbaum S, van de Loo A, Vischedyk M, Weissenborn K, Wellhöner P, Wiesner M, Zeissig S, Büning J, Schiffer M, Kuehbacher T; EHEC-HUS consortium . Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ 345: e4565, 2012. doi: 10.1136/bmj.e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meri S. Self-nonself discrimination by the complement system. FEBS Lett 590: 2418–2434, 2016. doi: 10.1002/1873-3468.12284. [DOI] [PubMed] [Google Scholar]
- 30.Meyers KE, Kaplan BS. Many cell types are Shiga toxin targets. Kidney Int 57: 2650–2651, 2000. doi: 10.1046/j.1523-1755.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- 31.Netea MG, Joosten LB, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science 352: aaf1098, 2016. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun 68: 688–693, 2000. doi: 10.1128/IAI.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noris GM. Remuzzi G, Noris. M, Mescia F,, Remuzzi G,. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol 8: 622–633, 2012. doi: 10.1038/nrneph.2012.195. . [DOI] [PubMed] [Google Scholar]
- 34.Noris M, Bresin E, Mele C, Remuzzi. G Genetic atypical hemolytic-uremic syndrome. In: Gene Reviews, eited by Adam MP, Ardinger HH, Pagon RA. Seattle, WA: Univ. of Washington, Seattle, 2007. [PubMed] [Google Scholar]
- 35.Noris M, Mele C, Remuzzi G. Podocyte dysfunction in atypical haemolytic uraemic syndrome. Nat Rev Nephrol 11: 245–252, 2015. doi: 10.1038/nrneph.2014.250. [DOI] [PubMed] [Google Scholar]
- 36.O’Loughlin EV, Robins-Browne RM. Effect of Shiga toxin and Shiga-like toxins on eukaryotic cells. Microbes Infect 3: 493–507, 2001. doi: 10.1016/S1286-4579(01)01405-8. [DOI] [PubMed] [Google Scholar]
- 37.Obrig TG. Escherichia coli shiga toxin mechanisms of action in renal disease. Toxins (Basel) 2: 2769–2794, 2010. doi: 10.3390/toxins2122769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozaki M, Kang Y, Tan YS, Pavlov VI, Liu B, Boyle DC, Kushak RI, Skjoedt MO, Grabowski EF, Taira Y, Stahl GL. Human mannose-binding lectin inhibitor prevents Shiga toxin-induced renal injury. Kidney Int 90: 774–782, 2016. doi: 10.1016/j.kint.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poggi P, Mirabella R, Neri S, Assirelli E, Dolzani P, Mariani E, Calder PC, Chatgilialoglu A. Membrane fatty acid heterogeneity of leukocyte classes is altered during in vitro cultivation but can be restored with ad-hoc lipid supplementation. Lipids Health Dis 14: 165, 2015. doi: 10.1186/s12944-015-0166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proulx F, Wagner E, Toledano B, Decaluwe H, Seidman EG, Rivard GE. Mannan-binding lectin in children with Escherichia coli O157:H7 haemmorrhagic colitis and haemolytic uraemic syndrome. Clin Exp Immunol 133: 360–363, 2003. doi: 10.1046/j.1365-2249.2003.02231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Psotka MA, Obata F, Kolling GL, Gross LK, Saleem MA, Satchell SC, Mathieson PW, Obrig TG. Shiga toxin 2 targets the murine renal collecting duct epithelium. Infect Immun 77: 959–969, 2009. doi: 10.1128/IAI.00679-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quaggin SE. DGKE and atypical HUS. Nat Genet 45: 475–476, 2013. doi: 10.1038/ng.2622. [DOI] [PubMed] [Google Scholar]
- 43.Roumenina LT, Rayes J, Frimat M, Fremeaux-Bacchi V. Endothelial cells: source, barrier, and target of defensive mediators. Immunol Rev 274: 307–329, 2016. doi: 10.1111/imr.12479. [DOI] [PubMed] [Google Scholar]
- 44.Ruggenenti P, Noris M, Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int 60: 831–846, 2001. doi: 10.1046/j.1523-1755.2001.060003831.x. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt CQ, Lambris JD, Ricklin D. Protection of host cells by complement regulators. Immunol Rev 274: 152–171, 2016. doi: 10.1111/imr.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Setten PA, van Hinsbergh VW, van der Velden TJ, van de Kar NC, Vermeer M, Mahan JD, Assmann KJ, van den Heuvel LP, Monnens LA. Effects of TNF α on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int 51: 1245–1256, 1997. doi: 10.1038/ki.1997.170. [DOI] [PubMed] [Google Scholar]
- 48.Shang SQ, Chen GX, Shen J, Yu XH, Wang KY. The binding of MBL to common bacteria in infectious diseases of children. J Zhejiang Univ Sci B 6: 53–56, 2005. doi: 10.1631/jzus.2005.B0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skerka C, Chen Q, Fremeaux-Bacchi V, Roumenina LT. Complement factor H related proteins (CFHRs). Mol Immunol 56: 170–180, 2013. doi: 10.1016/j.molimm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Sursal T, Stearns-Kurosawa DJ, Itagaki K, Oh SY, Sun S, Kurosawa S, Hauser CJ. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile SIRS and quantify inflammatory tissue injury in nonhuman primates. Shock 39: 55–62, 2013. doi: 10.1097/SHK.0b013e318276f4ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tesh VL. Activation of cell stress response pathways by Shiga toxins. Cell Microbiol 14: 1–9, 2012. doi: 10.1111/j.1462-5822.2011.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westman K, Storry JR, Olsson ML, Ann-Charlotte Kristoffersson D, Rylander C, Ida Arvidsson JS, Ståhl A, Manea Hedström M, Arvidsson I, Kristoffersson AC, Westman JS, Karpman D. Shiga toxin-induced complement-mediated hemolysis and release of complement-coated red blood cell-derived microvesicles in hemolytic uremic syndrome. J Immunol 194: 2309–2318, 2015. doi: 10.4049/jimmunol.1402470. [DOI] [PubMed] [Google Scholar]