Abstract
Focal segmental glomerulosclerosis (FSGS) is an important cause of nondiabetic chronic kidney disease (CKD). Sodium-glucose cotransporter 2 inhibition (SGLT2i) therapy attenuates the progression of diabetic nephropathy, but it remains unclear whether SGLT2i provides renoprotection in nondiabetic CKD such as FSGS. The primary aim of this pilot study was to determine the effect of 8 wk of dapagliflozin on glomerular filtration rate (GFR) in humans and in experimental FSGS. Secondary end points were related to changes in renal hemodynamic function, proteinuria, and blood pressure (BP). GFR (inulin) and renal plasma flow (para-aminohippurate), proteinuria, and BP were measured in patients with FSGS (n = 10), and similar parameters were measured in subtotally nephrectomized (SNx) rats. In response to dapagliflozin, changes in GFR, renal plasma flow, and 24-h urine protein excretion were not statistically significant in humans or rats. Systolic BP (SBP) decreased in SNx rats (196 ± 26 vs. 165 ± 33 mmHg; P < 0.001), whereas changes were not statistically significant in humans (SBP 112.7 ± 8.5 to 112.8 ± 11.2 mmHg, diastolic BP 71.8 ± 6.5 to 69.6 ± 8.4 mmHg; P = not significant), although hematocrit increased (0.40 ± 0.05 to 0.42 ± 0.05%; P = 0.03). In archival kidney tissue from a separate patient cohort, renal parenchymal SGLT2 mRNA expression was decreased in individuals with FSGS compared with controls. Short-term treatment with the SGLT2i dapagliflozin did not modify renal hemodynamic function or attenuate proteinuria in humans or in experimental FSGS. This may be related to downregulation of renal SGLT2 expression. Studies examining the impact of SGLT2i on markers of kidney disease in patients with other causes of nondiabetic CKD are needed.
Keywords: FSGS, nondiabetic CKD, SGLT2 inhibition
INTRODUCTION
Focal segmental glomerulosclerosis (FSGS) is characterized by proteinuria and renal function decline, leading to chronic kidney disease (CKD) and end-stage renal disease (ESRD; Ref. 15). Approximately 55% of patients with idiopathic FSGS with high-grade proteinuria will proceed to ESRD at 10 yr following diagnosis (40). The FSGS lesion can also develop as the end result of a spectrum of sources of glomerular injury, obesity, and reduced nephron mass. Despite current therapies including renin-angiotensin-aldosterone system (RAAS) inhibitors, antihypertensives, and immunosuppressants, clinical outcomes remain suboptimal.
Beyond possible immune-mediated pathophysiological mechanisms responsible for development of primary FSGS, elevated intraglomerular pressure is associated with CKD progression in patients with FSGS (11). Accordingly, agents that block the RAAS exert renal protection in FSGS, in part via hemodynamic effects to reduce intraglomerular hypertension, single-nephron glomerular filtration rate (GFR), and proteinuria via vasodilatation of the efferent renal arteriole (33). Unfortunately, RAAS blockade does not abolish glomerular hypertension or proteinuria in patients with CKD (24).
In addition to modulation of neurohormonal pathways, studies in animals and in humans with diabetes mellitus have implicated tubular factors in the pathogenesis of increased glomerular pressure and hyperfiltration leading to CKD progression. According to the tubular hypothesis of hyperfiltration, reabsorption of sodium (Na+) and glucose by sodium-glucose cotransporter 2 (SGLT2) at the proximal tubule is an important regulator of renal hemodynamic function (21). Augmented SGLT2 activity in the setting of diabetes reduces Na+ delivery at the distal macula densa, which is sensed as a reduction in effective circulating volume via a process known as tubuloglomerular feedback (TGF; Ref. 43). As a result, agents that increase sodium delivery to the macula densa, including proximal tubular diuretics (i.e., acetazolamide) and SGLT2 inhibition (SGLT2i), lead to afferent vasoconstriction, decreased renal blood flow, and reduced intraglomerular hypertension, thereby reducing proteinuria (47). For example, SGLT2i reduces hyperfiltration and intraglomerular pressure in patients with type 1 diabetes (8). Notably, monotherapy with either SGLT2i or RAAS blockade fails to abolish hyperfiltration, suggesting that the combined use of agents that target both afferent and efferent factors is required to normalize hyperfiltration and renal abnormalities in patients at risk for CKD. In patients with type 2 diabetes and cardiovascular disease, SGLT2i with empagliflozin reduces the composite nephropathy end point (5, 45), and substantial renal benefits have also been reported with canagliflozin in the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program trials (29). Although it is not known whether these benefits extend to patients without diabetes, large clinical trials such as Dapa-CKD (NCT03036150) are underway to determine whether SGLT2 inhibition reduces the risk of progressive nondiabetic CKD. These studies are based on the hypothesis that similarly to diabetes, modulation of TGF may reduce kidney injury that may occur in FSGS due to maladaptive renal hyperfiltration, even in the absence of hyperglycemia. Despite interest in this area, especially in patients with diabetes, very little is known about the effects of SGLT2i on proteinuria, renal function, or blood pressure in patients with nondiabetic CKD.
Accordingly, the aim of this proof-of-concept pilot study was to examine the effect of dapagliflozin as an adjunct to RAAS blockade for 8 wk on renal hemodynamic function, proteinuria, blood pressure, and body weight in patients with FSGS. As the primary end point, we hypothesized that SGLT2i would reduce GFR due to vasoconstrictive effects at the afferent arteriole. For secondary end points, we measured the impact of dapagliflozin on GFR and effective renal plasma flow (ERPF), proteinuria, blood pressure, and metabolic parameters. In a related series of experiments, we assessed the renal impact of dapagliflozin on renal hemodynamic function in subtotally nephrectomized (SNx) rats, which is a model of adaptive FSGS. Histologically, kidney injury in this model resembles human FSGS and develops in part due to raised intraglomerular pressure in the remnant kidney (14). Finally, in a post hoc analysis, to gain additional insight into proteinuric and hemodynamic effects in humans, we further measured levels of SGLT2 mRNA expression in kidney tissue from patients with and without background FSGS.
MATERIALS AND METHODS
Human participants.
The flow chart for participants is shown in Fig. 1. In this open-label, pilot clinical trial, 10 participants with FSGS were treated with 10 mg of dapagliflozin once daily for 8 wk as an add-on to RAAS blockade therapy to evaluate effects on proteinuria and renal hemodynamic function. Detailed inclusion criteria were as follows: 1) male or female subjects diagnosed with biopsy-proven FSGS ≥1 mo before informed consent; 2) ≥45 ml·min−1·1.73 m−2 creatinine-based GFR; 3) age > 18 yr; 4) no history of diabetes mellitus; 5) 18.5–45.0 kg/m2 body mass index; 6) blood pressure (BP) ≥ 100/60 mmHg at screening; 7) therapy with a RAAS inhibitor (an angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, or direct renin inhibitor) for >1 mo; and 8) >30 mg/day and <6 g/day proteinuria. Exclusion criteria at screening were 1) leukocyte- and/or nitrate-positive urinalysis that was untreated, 2) history of organ transplantation, 3) bariatric surgery or gastrointestinal surgeries that induced chronic malabsorption within the past 2 yr, 4) current treatment with systemic corticosteroids, calcineurin inhibitors, or other immunosuppressant therapies, 5) blood dyscrasias or any disorders causing hemolysis or unstable red blood cells, 6) premenopausal women who were nursing, pregnant, or of child-bearing potential and not practicing an acceptable method of birth control, 7) participation in another therapeutic trial with an investigational drug within 30 days before informed consent, 8) alcohol or drug abuse within 3 mo before informed consent that would interfere with trial participation or any ongoing clinical condition that would jeopardize subject safety or study compliance based on investigator judgment, 9) liver disease, defined by serum levels of alanine transaminase, aspartate transaminase, or alkaline phosphatase more than three times upper limit of normal as determined during screening, 10) cardiac, lung, or peripheral vascular disease or stroke, 11) pancreas, pancreatic islet cell, or renal transplant recipient, 12) medical history of cancer or treatment of cancer in the last 5 yr before screening, 13) history of allergy or angioedema with RAAS inhibitor exposure, and 14) kidney disease due primarily to another identifiable condition aside from FSGS. The local Research Ethics Board at the University Health Network (Toronto, Canada) approved the protocol, and all subjects gave informed consent before start of study procedures. The study was conducted according to the International Conference on Harmonization on Good Clinical Practice (https://clinicaltrials.gov/ NCT02585804).
Fig. 1.
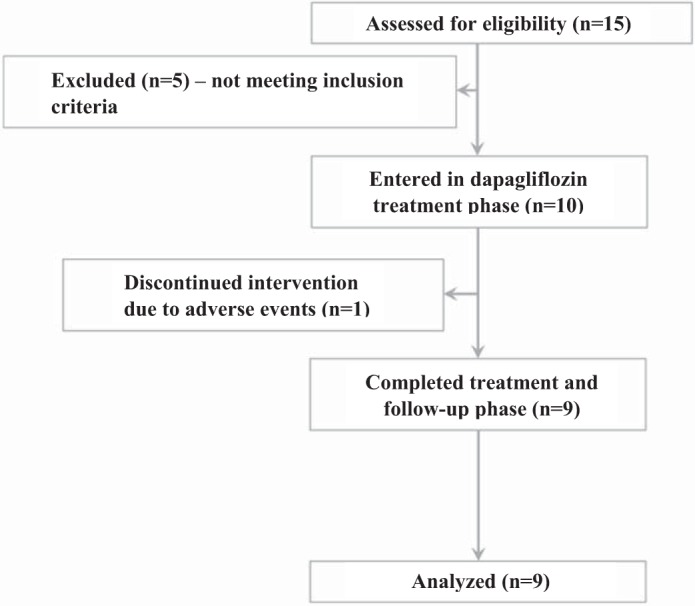
Flow diagram for study participants.
In the human interventional arm of the study, patients with biopsy-proven primary or secondary FSGS were recruited. These were clinical biopsies performed due to signs/symptoms or laboratory values that were compatible with an underlying diagnosis of FSGS. In all cases, secondary glomerular-based diseases were ruled out based on the absence of diabetic nephropathy or immune complex deposition due to other nondiabetic conditions, such as IgA nephropathy. All patients met diagnostic criteria for FSGS with variable foot process effacement on electron microscopy. The diagnosis for FSGS was based on the opinion of the nephropathologist assessing the biopsy and the nephrologist following the patient.
Clinical experimental design.
This clinical trial consisted of five visits: 1) a successful recruitment visit (visit 1, V1) followed by a 1-wk preparation of specified diet, targeting ≥150 mmol/day Na+ and ≤1.5 g·kg−1·day−1 protein, to avoid the effects of circulating volume contraction and/or RAAS activation by low Na+ and hyperfiltration via high protein intake; 2) the first full study visit (visit 2, V2) starting at 0745, when participants underwent baseline measurements of neurohormonal levels, renal hemodynamic function (GFR and ERPF), and blood pressure; 3) the next day, participants started 10 mg po of daily dapagliflozin for 8 wk, during which they would return to the laboratory at 2 wk for a safety assessment (visit 3, V3); 4) the second full study visit (visit 4, V4) starting at 0745 at the end of the 8-wk drug treatment period, when participants had all procedures from V2 repeated; and 5) a 1-wk washout period, when participants returned for a final appointment (visit 5, V5) for safety assessments.
During full study visits, V2 and V4, participants fasted for the duration of their stay. On both days, first blood samples were collected for inulin and para-aminohippurate blank and RAAS mediators using established techniques (28). Following these blood and urine tests, blood pressure was measured using a Critikon automated sphygmomanometer (Tampa, FL) at 30-min intervals. GFR and ERPF (per 1.73 m2) were measured in the supine position using inulin and para-aminohippurate clearances (7). Filtration fraction (GFR/ERPF), renal blood flow [ERPF/(1 − hematocrit)], and renal vascular resistance (mean arterial pressure/renal blood flow) were also calculated using standard methods (3, 26). The Gomez equations were used to calculate efferent resistance (RE), afferent resistance (RA), and glomerular pressure, as described elsewhere (3, 17, 26, 41).
Rodent studies.
Male Sprague-Dawley rats (Charles River, Montréal, Québec, Canada) aged 7 wk were randomized to undergo sham or subtotal nephrectomy surgery, as previously described (1). Briefly, rats were anesthetized with 2.5% isoflurane, the right kidney was removed by subcapsular nephrectomy, and infarction of approximately two-thirds of the left kidney was achieved via ligation of branches of the left renal artery. After surgery, rats were randomly allocated to receive dapagliflozin (1 mg·kg−1·day−1; AstraZeneca, Mölndal, Sweden) in drinking water or drinking water alone (18). Animals were followed for 8 wk with ad libitum access to commercial standard rat chow. After 8 wk of dapagliflozin (or vehicle) treatment, rats were individually housed in metabolic cages for 24 h for determination of urine volume, urine protein (benzethonium chloride method), and urine creatinine (autoanalyzer). Systolic blood pressure (SBP) was determined by tail cuff plethysmography (PowerLab, ADInstruments, Colorado Springs, CO) in conscious rats, as previously described (2). Tail vein venipuncture was used for determination of hematocrit (XN-9000; Sysmex Canada, Mississauga, Ontario, Canada) and fasting blood glucose (OneTouch UltraMini; LifeScan Canada, Burnaby, British Columbia, Canada). The numbers of animals studied at the end of the 8-wk period were as follows: sham + vehicle, n = 18; sham + dapagliflozin, n = 18; SNx + vehicle, n = 17; SNx + dapagliflozin, n = 20. GFR was determined by single-shot FITC-inulin clearance and repeated sampling via the tail vein as previously described (1) in subgroups of rats (sham + vehicle, n = 16; sham + dapagliflozin, n = 10; SNx + vehicle, n = 15; SNx + dapagliflozin, n = 15). Renal plasma flow was determined in conscious unrestrained rats using an adaptation of previously published methods (13, 30) and in subgroups of rats (sham + vehicle, n = 3; sham + dapagliflozin, n = 3; SNx + vehicle, n = 4; SNx + dapagliflozin, n = 5). Briefly, under 2% isoflurane anesthesia, the right femoral artery and right femoral vein were each cannulated with a heparinized (500 IU/ml) PE-50 catheter. Animals were recovered from anesthesia, and para-aminohippurate (PAH; 11.6 mg/ml; Sigma-Aldrich Canada, Oakville, Ontario, Canada) was infused via the right femoral vein with a priming dose of 8 mg/kg and a constant maintenance rate of 0.0267 ml/min. After an equilibration phase of 105 min, three blood samples were obtained from the right femoral artery, one every 15 min, for determination of PAH clearance. All experimental procedures adhered to the guidelines of the Canadian Council on Animal Care and were approved by the St. Michael’s Hospital Animal Care Committee.
Histology.
After euthanasia, kidney tissue was harvested, fixed in 10% neutral buffered formalin, and routinely processed and embedded. Glomerulosclerosis index was determined semiquantitatively in periodic acid-Schiff-stained kidney sections as previously described and in ~60 glomerular profiles for each kidney section (2). Immunohistochemistry was performed as previously described with antibodies in the following concentrations: 1:500 collagen IV (EMD Millipore, Darmstadt, Germany), 1:1,000 JG-12 (Bender MedSystems, Vienna, Austria), and 1:100 ED1 (Bio-Rad, Hercules, CA; Ref. 2). Incubation with phosphate-buffered saline in place of the primary antibody served as the negative control. After incubation with the appropriate horseradish peroxidase-conjugated secondary antibody, sections were labeled with Liquid Diaminobenzidine and Substrate Chromogen (Dako North America, Carpinteria, CA) before counterstaining in Mayer’s hematoxylin. Slides were scanned (Leica Microsystems, Concord, Ontario, Canada) and analyzed using ImageScope 11.1 software (Leica Microsystems). The proportional glomerular area positively immunostaining for collagen IV or with the JG-12 antibody was determined in 30 randomly selected glomerular profiles from each kidney section using ImageScope. Cortical tubulointerstitial ED1 immunostaining was determined in 10 nonoverlapping cortical fields (excluding glomeruli) using the ImageScope ×20 zoom. All histological analyses were performed by an investigator masked to the study groups.
Gene expression in human kidney tissue.
For the determination of SGLT2 mRNA levels, kidney biopsy tissue was examined from 6 individuals with secondary FSGS (biopsy-proven and clinically correlated obesity-related secondary FSGS) and compared with that of kidney tissue obtained at the time of live kidney transplant from 6 healthy donors with normal kidney function (4). The study was approved by the Institutional Research Board of the Health Sciences Centre, University of Manitoba, and was conducted in accordance with the Declaration of Helsinki. RNA was isolated using a Paradise PLUS Reagent System (Arcturus, Mountain View, CA). Real-time PCR was performed using SYBR Green (Wisent Bio Products, St.-Jean-Baptiste, Québec, Canada) on a ViiA 7 PCR system (Thermo Fisher Scientific, Rockford, IL) and with the following primer sequences: SGLT2, forward 5′-GCTGGAGAGAATGGAGCAA-3′, reverse 5′-AGACCACAAGCCAACACCAA-3′; ribosomal protein L32 (RPL32), forward 5′-CAACATTGGTTATGGAAGCAACA-3′, reverse 5′-TGACGTTGTGGACCAGGAACT-3′. Samples were analyzed in duplicate, and data analysis was performed using the Applied Biosystems comparative cycle threshold (CT) method.
Data analysis.
Variables were checked for the distributional assumption of normality using normal plots. Variables that were positively skewed were natural log-transformed for the analyses. Paired t-tests were used for single measured parameters, and linear mixed models were used for repeated parameters. A two-sided P < 0.05 was considered statically significant. Analyses were stratified by baseline GFR (≥90 or <90 ml·min−1·1.73 m−2) and ≥ and < median 24-h proteinuria. Data are presented as means ± SD for normally distributed variables and medians (range) for positively skewed variables (e.g., renin and aldosterone). Analyses were performed in SAS (version 9.4 for Windows; SAS Institute, Cary, NC). For rodent studies and human gene expression studies, statistical analyses were performed using GraphPad Prism 6 for Mac OS X (GraphPad Software, San Diego, CA). Statistical significance was determined by one-way ANOVA with a Fisher least significant difference test for comparison of multiple groups. Data are presented as means ± SD except skew-distributed rat proteinuria data, which are shown as medians (range) and which were analyzed using a Kruskal-Wallis test with a Dunn post hoc comparison. Gene expression changes in human tissue were compared by Student’s t-test.
RESULTS
Screening clinical characteristics of study cohort.
The study group comprised 10 patients with FSGS within an age range of 27–51 yr (Table 1). All participants had FSGS > 1 mo and were taking concomitant RAAS inhibition for >1 mo (n = 3 taking an angiotensin-converting enzyme inhibitor and n = 7 taking an angiotensin receptor blocker). One participant failed to complete the study and stopped drug intake after 2 wk of treatment due to nonspecific malaise and a feeling of weakness, with no evidence of hypotension, hypoglycemia, or genitourinary tract infection. Although no particular severe adverse event was reported, the participant discontinued study participation but did collect and submit a 24-h urine sample for the primary analysis while on treatment for 2 wk, which was included in the analysis. Renal hemodynamic parameters were not measured in this patient. All study patients returned to the laboratory ~1 wk following last drug intake date for follow-up safety analysis.
Table 1.
Clinical characteristics, hemodynamic function, and biochemistry at baseline and 8 wk
| Baseline (V2) | Posttreatment (V4) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, yr | 37.2 ± 9.2 | ||
| Female sex, N (%) | 4 (40) | ||
| Baseline body mass index, kg/m2 | 30.0 ± 8.2 | ||
| FSGS duration, yr | 5.6 ± 5.3 | ||
| Medications | |||
| Statin, N (%) | 3 (30) | ||
| Xanthine oxidase inhibition, N (%) | 2 (20) | ||
| Calcium channel blocker, N (%) | 1 (10) | ||
| Proton pump inhibitor, N (%) | 1 (10) | ||
| β2-Adrenergic agonist, N (%) | 1 (10) | ||
| Serotonin receptor agonist, N (%) | 1 (10) | ||
| Histamine H2 antagonist | 1 (10) | ||
| Diuretics | 0 | ||
| Renal hemodynamic function | |||
| Glomerular filtration rate | 93.9 ± 18.2 | 85.9 ± 16.9 | 0.22 |
| Effective renal plasma flow | 513.5 ± 161.2 | 496.6 ± 152.0 | 0.19 |
| Filtration fraction | 0.19 ± 0.035 | 0.18 ± 0.039 | 0.85 |
| Renal blood flow | 881.7 ± 287.1 | 853.0 ± 245.6 | 0.42 |
| Renal vascular resistance | 0.11 ± 0.03 | 0.11 ± 0.03 | 0.74 |
| Afferent resistance | 4,117.3 ± 1,588.0 | 3,832.7 ± 1,409.5 | 0.33 |
| Efferent resistance | 935.5 ± 255.1 | 908.7 ± 242.3 | 0.46 |
| Glomerular pressure | 44.6 ± 3.8 | 44.8 ± 4.8 | 0.78 |
| 24-h Urine protein | 2.6 ± 1.9 | 2.4 ± 2.2 | 0.42 |
| Protein-to-creatinine ratio* | 110 (78–352) | 92 (61–446) | 0.65 |
| Systemic hemodynamic parameters, body weight, and urine glucose | |||
| Systolic blood pressure | 112.7 ± 8.5 | 112.8 ± 11.2 | 0.99 |
| Diastolic blood pressure | 71.8 ± 6.5 | 69.6 ± 8.4 | 0.43 |
| Mean arterial pressure | 84.3 ± 6.7 | 84.2 ± 7.8 | 0.97 |
| Body wt | 88.2 ± 25.1 | 87.0 ± 25.4 | 0.11 |
| 24-h Urine glucose, g/day | 0.2 ± 0.2 | 37.5 ± 23.4 | 0.001 |
| Plasma biochemistry | |||
| HbA1c, % | 5.5 ± 0.5 | 5.5 ± 0.6 | 0.85 |
| Hematocrit | 0.40 ± 0.054 | 0.42 ± 0.049 | 0.023 |
| Total protein | 64.7 ± 7.2 | 66.1 ± 6.8 | 0.16 |
| Plasma aldosterone* | 251.0, 149.0–336.0 | 350.0, 224.0–376.0 | 0.17 |
| Plasma renin* | 68.8, 28.0–133.5 | 121.5, 28.4–174.7 | 0.077 |
Values are means ± SD. Twenty-four-hour urine protein is in mg protein per collection period; glomerular filtration rate, effective renal plasma flow, and renal blood flow are in ml·min−1·1.73 m−2; afferent resistance and efferent resistance are in dyn s cm−5; renal vascular resistance is in mmHg·l−1·min−1; systolic blood pressure, diastolic blood pressure, mean arterial pressure, and glomerular pressure are in mmHg; body weight is in kg; hematocrit is in l/l; plasma aldosterone is in pM; plasma renin is in ng/l; and
(median, quartiles 1–3) are in mg/mmol.
Effects of dapagliflozin on proteinuria, renal hemodynamic function, and blood pressure in humans.
Inulin- and PAH-based changes in GFR and ERPF in response to dapagliflozin did not reach statistical significance (Table 1). Similarly, changes in renal vascular resistance, renal blood flow, filtration fraction, RA, RE, and glomerular pressure were not statistically significant (Table 1). In a sensitivity analysis, in which study participants were stratified into two subgroups as a function of GFR (<90 or ≥90 ml·min−1·1.73 m−2 at baseline), there was a significant reduction in GFR following dapagliflozin therapy at 8 wk in those with GFR ≥ 90 ml·min−1·1.73 m−2, and plasma aldosterone increased in this subgroup (Table 2).
Table 2.
Sensitivity analysis by GFRINULIN: change from V2 to V4
| GFR Group | N | Mean Difference ± SD | P Value | |
|---|---|---|---|---|
| Renal hemodynamic function | ||||
| △GFRINULIN | <90 ml·min−1·1.73 m−2 | 4 | +4.13 ± 11.3 | 0.52 |
| ≥90 ml·min−1·1.73 m−2 | 5 | −14.9 ± 11.3 | 0.042 | |
| △ERPF | <90 ml·min−1·1.73 m−2 | 4 | −20.4 ± 33.2 | 0.31 |
| ≥90 ml·min−1·1.73 m−2 | 5 | −31.2 ± 72.7 | 0.39 | |
| Plasma biochemistry | ||||
| △Plasma aldosterone | <90 ml·min−1·1.73 m−2 | 4 | +9.8 ± 334.5 | 0.92 |
| ≥90 ml·min−1·1.73 m−2 | 5 | +106.0 ± 110.2 | 0.017 |
GFR and ERPF are in ml·min−1·1.73 m−2, and plasma aldosterone is in pmol/l.
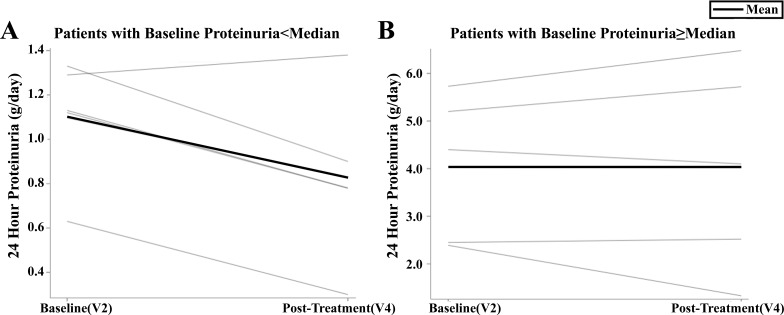
Dapagliflozin therapy for 8 wk did not reduce proteinuria in the overall clinical cohort (Table 1). In a post hoc sensitivity analysis, patients with 24-h protein levels below the median (median 24-h proteinuria: 1.89 g/day) demonstrated a significant reduction in proteinuria (Table 3, Fig. 2). In response to dapagliflozin, numerical reduction in diastolic blood pressure did not reach statistical significance (Table 1).
Table 3.
Sensitivity analysis by 24-h proteinuria: change from V2 to V4
| Proteinuria Group | N | Mean Difference ± SD | P Value | |
|---|---|---|---|---|
| Proteinuria | ||||
| △24-h Proteinuria | 24-h Protein ≥ median | 5 | +0.004 ± 0.72 | 0.99 |
| 24-h Protein < median | 4 | −0.27 ± 0.21 | 0.042 | |
| Renal hemodynamic function | ||||
| △GFR | 24-h Protein ≥ median | 5 | −11.7 ± 10.5 | 0.067 |
| 24-h Protein < median | 4 | +0.13 ± 17.7 | 0.99 | |
| △ERPF | 24-h Protein ≥ median | 5 | −26.8 ± 69.1 | 0.44 |
| 24-h Protein < median | 4 | −25.9 ± 43.2 | 0.32 | |
| Plasma biochemistry | ||||
| △Plasma aldosterone | 24-h Protein ≥ median | 5 | +77.4 ± 19.6 | 0.019 |
| 24-h Protein < median | 4 | +45.5 ± 30.9 | 0.88 |
Twenty-four-hour protein is in g of protein per collection period; GFR and ERPF are in ml·min−1·1.73 m−2; and plasma aldosterone is in pmol/l.
Fig. 2.
Sensitivity analysis by 24-h proteinuria below (A) or above (B) the median for individual participants.
Effect of dapagliflozin on body weight, hematocrit, and RAAS mediators in humans.
Body weight did not change significantly following dapagliflozin therapy (Table 1). On the other hand, hematocrit values and 24-h urine glucose increased significantly from V2 to V4. There were no significant changes in plasma renin or aldosterone levels from V2 to V4 (Table 1).
As part of the sensitivity analysis based on GFR, there was a significant increase in plasma aldosterone levels in those with GFR ≥ 90 ml·min−1·1.73 m−2 at baseline (Table 2). When stratified based on baseline proteinuria, there was a significant increase in plasma aldosterone in those with baseline 24-h proteinuria above the median (Table 3).
Effect of dapagliflozin in subtotally nephrectomized rats.
Table 4 shows the physiological parameters in sham-operated and SNx rats treated with dapagliflozin (or vehicle) for 8 wk. As expected, GFR was reduced in SNx rats in comparison with sham-operated rats. Dapagliflozin treatment had no effect on GFR in either sham or SNx rats. Similarly, ERPF tended to be lower in SNx rats than sham-operated rats, but the difference was not statistically significant, likely because of the relatively small number of rats studied (n = 3–5/group) and the multiple-groups comparison. Dapagliflozin did not modify ERPF in either sham or SNx rats.
Table 4.
Effects of dapagliflozin on renal and metabolic parameters in sham-operated and subtotally nephrectomized (SNx) rats
| Variable | Group | N | Value | Group | N | Value |
|---|---|---|---|---|---|---|
| Body wt, g | Sham + vehicle | 18 | 614 ± 59 | Sham + dapa | 18 | 570 ± 55 |
| SNx + vehicle | 17 | 562 ± 65 | SNx + dapa | 20 | 542 ± 87a | |
| GFR, ml·min−1·kg | Sham + vehicle | 16 | 5.4 ± 2.2 | Sham + dapa | 10 | 4.9 ± 1.7 |
| SNx + vehicle | 15 | 1.8 ± 0.6b,c | SNx + dapa | 15 | 2.3 ± 1.2b,d | |
| ERPF, ml·min−1·kg | Sham + vehicle | 3 | 57.3 ± 15.9 | Sham + dapa | 3 | 58.2 ± 26.8 |
| SNx + vehicle | 4 | 34.2 ± 3.3 | SNx + dapa | 5 | 47.2 ± 43.0 | |
| Systolic blood pressure, mmHg | Sham + vehicle | 18 | 122 ± 14 | Sham + dapa | 18 | 124 ± 13 |
| SNx + vehicle | 17 | 196 ± 26b,c | SNx + dapa | 20 | 165 ± 33b,d,e | |
| Fasting blood glucose, mmol/l | Sham + vehicle | 18 | 6.3 ± 0.7 | Sham + dapa | 18 | 6.1 ± 1.0 |
| SNx + vehicle | 17 | 6.6 ± 0.7 | SNx + dapa | 20 | 6.4 ± 0.6 | |
| 24-h Urine volume, ml | Sham + vehicle | 18 | 25 ± 12 | Sham + dapa | 18 | 48 ± 13b |
| SNx + vehicle | 17 | 38 ± 12a,f | SNx + dapa | 20 | 46 ± 12b | |
| 24-h Urine protein, mg | Sham + vehicle | 18 | 15.1 (11.2–38.0) | Sham + dapa | 18 | 20.1 (10.8–82.2) |
| SNx + vehicle | 17 | 118.4 (18.3–658.4)b,d | SNx + dapa | 20 | 88.9 (9.2–230.9)b,f | |
| Urine protein-to-creatinine ratio, mg/µmol | Sham + vehicle | 17 | 0.11 (0.06–0.22) | Sham + dapa | 18 | 0.11 (0.07–0.64) |
| SNx + vehicle | 17 | 0.74 (0.09–8.39)b,d | SNx + dapa | 20 | 0.66 (0.08–2.16)g,h | |
| Hematocrit, l/l | Sham + vehicle | 18 | 0.434 ± 0.059 | Sham + dapa | 18 | 0.428 ± 0.037 |
| SNx + vehicle | 16 | 0.394 ± 0.041i | SNx + dapa | 18 | 0.379 ± 0.054a,h | |
| Left kidney weight, g | Sham + vehicle | 18 | 1.72 ± 0.17 | Sham + dapa | 18 | 2.27 ± 0.25a |
| SNx + vehicle | 17 | 2.51 ± 0.62b | SNx + dapa | 20 | 2.72 ± 0.76b,h | |
| Left kidney weight to body weight, % | Sham + vehicle | 18 | 0.28 ± 0.04 | Sham + dapa | 18 | 0.40 ± 0.05g |
| SNx + vehicle | 17 | 0.45 ± 0.10b | SNx + dapa | 20 | 0.51 ± 0.14b,d,e |
Values are expressed as means ± SD, except urine protein data, which are expressed as medians (range). dapa, Dapagliflozin.
P < 0.01 vs. sham + vehicle,
P < 0.0001 vs. sham + vehicle,
P < 0.0001 vs. sham + dapagliflozin,
P < 0.001 vs. sham + dapagliflozin,
P < 0.05 vs. SNx + vehicle,
P < 0.05 vs. sham + dapagliflozin,
P < 0.001 vs. sham + vehicle,
P < 0.01 vs. sham + dapagliflozin,
P < 0.05 vs. sham + vehicle.
Urine protein excretion was increased in SNx rats in comparison with sham-operated rats. Similar to effects in humans, dapagliflozin treatment did not impact 24-h urine protein excretion. Twenty-four-hour urine volume was increased in sham-operated rats treated with dapagliflozin and in vehicle-treated SNx rats compared with vehicle-treated sham rats. However, there was no difference in urine volume between dapagliflozin-treated sham and SNx rats, whereas the increase in urine volume in SNx rats treated with dapagliflozin was not statistically significant (P = 0.0504 vs. SNx + vehicle; Table 4). Body weight tended to be lower with dapagliflozin treatment and in SNx rats, but only the comparison of body weight between vehicle-treated sham rats and dapagliflozin-treated SNx rats achieved statistical significance. Fasting blood glucose did not differ between the study groups.
SBP was significantly higher in SNx rats compared with sham-operated rats. Whereas dapagliflozin treatment had no effect on SBP in sham rats, SBP was significantly lower in dapagliflozin-treated SNx rats than in vehicle-treated SNx rats.
Kidney weight was increased both after SNx surgery and with dapagliflozin treatment, with the largest kidney weight seen in dapagliflozin-treated SNx rats. Kidney weight to body weight (in percentage) was significantly larger in dapagliflozin-treated SNx rats than in vehicle-treated SNx rats (P = 0.0491).
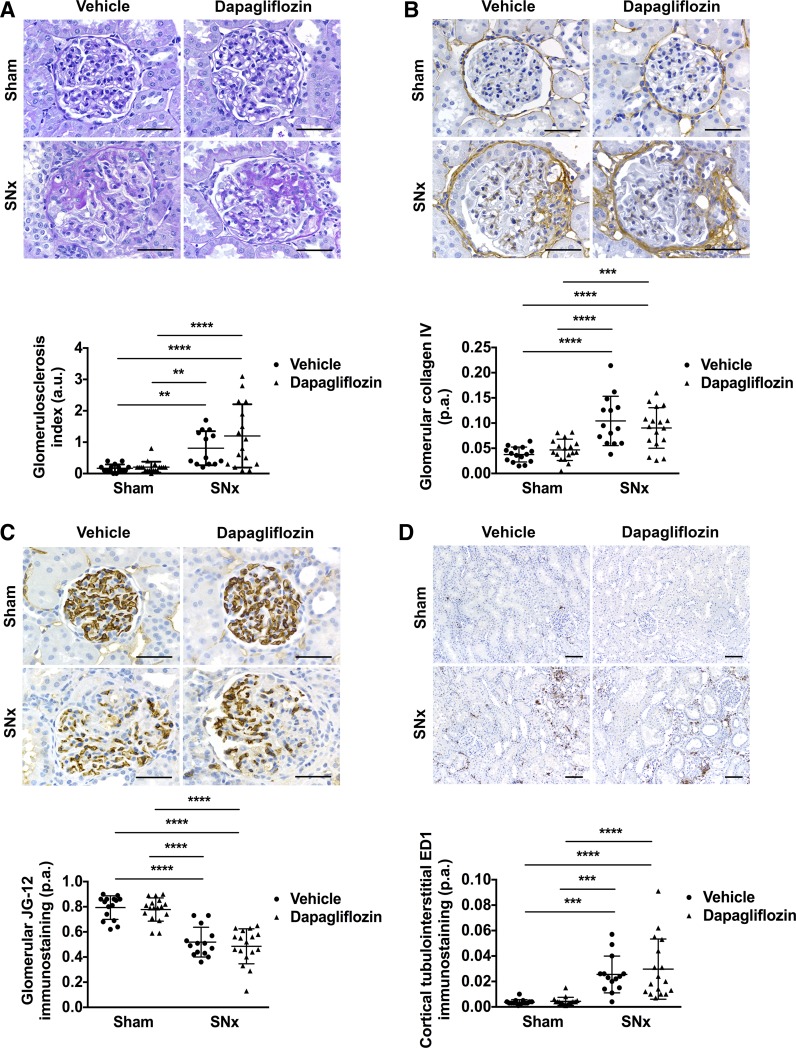
Renal histological parameters in sham and SNx rats treated with vehicle or dapagliflozin were assessed as 1) the degree of glomerulosclerosis on periodic acid-Schiff-stained kidney sections (Fig. 3A), 2) the proportional glomerular area positively immunostaining for type IV collagen (Fig. 3B), 3) glomerular capillary density, determined as the proportional glomerular area immunostained with the monoclonal antibody JG-12, that detects aminopeptidase P expressed on rat renal microvascular endothelial cells (Fig. 3C; Ref. 27), and 4) cortical tubulointerstitial macrophage infiltration, determined as the proportional area of cortical tubulointerstitium immunostained with the monoclonal antibody ED1, that recognizes CD68 expressed on rat macrophages (Fig. 3D). In comparison with sham-operated rats, SNx rats exhibited increased glomerulosclerosis, increased glomerular collagen IV deposition, loss of glomerular capillaries, and increased infiltration of the tubulointerstitium by macrophages (Fig. 3). Dapagliflozin treatment did not affect any of these parameters in either sham or SNx rats (Fig. 3).
Fig. 3.
SGLT2 inhibition with dapagliflozin does not affect histological parameters of renal injury in subtotally nephrectomized (SNx) rats. A: representative photomicrographs of periodic acid-Schiff-stained kidney sections (×400 original magnification) from sham-operated (sham) and SNx rats treated with vehicle or dapagliflozin and quantitation of glomerular injury (glomerulosclerosis index; sham + vehicle, n = 15; sham + dapagliflozin, n = 18; SNx + vehicle, n = 13; SNx + dapagliflozin, n = 16). B: representative photomicrographs of kidney sections (×400 original magnification) from sham and SNx rats treated with vehicle or dapagliflozin stained for collagen IV and quantitation of glomerular collagen IV (sham + vehicle, n = 15; sham + dapagliflozin, n = 17; SNx + vehicle, n = 14, SNx + dapagliflozin, n = 17). C: representative photomicrographs of kidney sections (×400 original magnification) from sham and SNx rats treated with vehicle or dapagliflozin stained with the JG-12 antibody, which detects rat renal microvascular capillaries, and quantitation of glomerular capillary density (sham + vehicle, n = 15; sham + dapagliflozin, n = 17; SNx + vehicle, n = 14; SNx + dapagliflozin, n = 17). D: representative photomicrographs of kidney sections (×100 original magnification) from sham and SNx rats treated with vehicle or dapagliflozin stained with the ED1 antibody that detects rat macrophages and quantitation of cortical tubulointerstitial ED1 immunostaining (sham + vehicle, n = 15; sham + dapagliflozin, n = 17; SNx + vehicle, n = 14; SNx + dapagliflozin, n = 17). A–C: scale bar = 50 µm. D: scale bar = 100 µm. a.u., Arbitrary units; p.a., proportional area. **P < 0.01, ***P < 0.001, ****P < 0.0001.
SGLT2 mRNA levels in the kidneys of humans with obesity-related secondary FSGS.
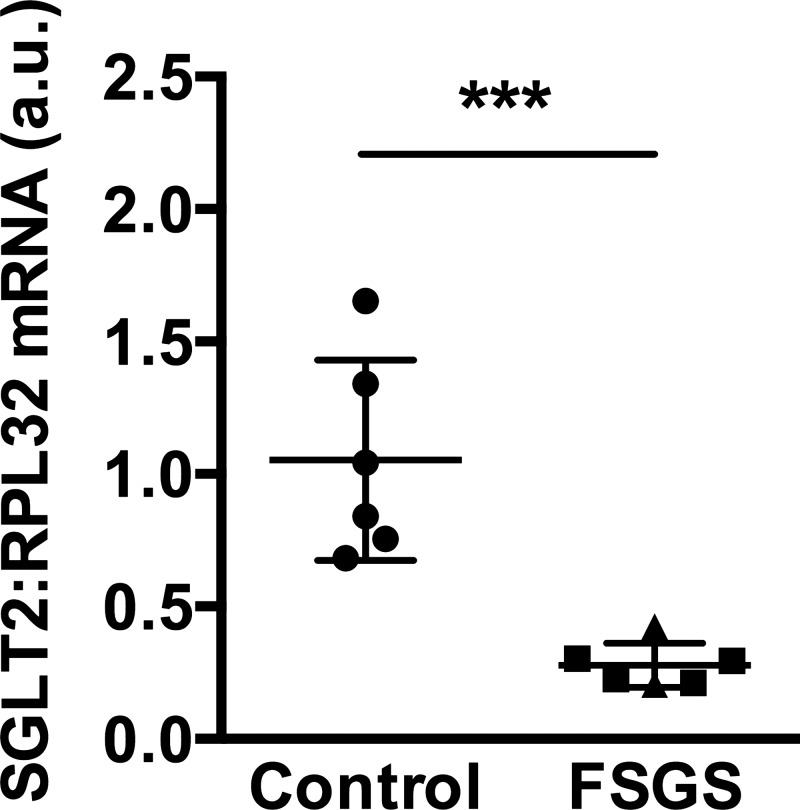
Based on the neutral effect of dapagliflozin on proteinuria in animals and patients with FSGS, we measured SGLT2 mRNA levels in archived biopsy kidney tissue from individuals with FSGS (n = 6) and in healthy controls (n = 6; Ref. 4). All of the patients had obesity-related secondary FSGS (obesity-related kidney disease). In this study, unlike our interventional study with dapagliflozin, we included two samples from patients who had concurrent diabetes mellitus. Five of the six patients with FSGS were male and one was female, the mean age was 32 ± 15 yr, mean serum creatinine was 120 ± 37 µmol/l, and median urine protein-to-creatinine ratio was 338 mg/mmol (range 118–679 mg/mmol, available for 5 patients with 24-h urine protein excretion 1.87 g/24 h in the other patient). Normal control kidney biopsy tissue was obtained from transplanted kidneys before implantation. All of the controls had normal kidney function (estimated GFR > 60 ml·min−1·1.73 m−2), and none had concurrent diabetes. SGLT2 mRNA levels were markedly diminished in kidney tissue from individuals with secondary FSGS (Fig. 4). Among the patients with FSGS, we did not observe a difference in SGLT2 mRNA levels between individuals with and without diabetes (Fig. 4).
Fig. 4.
SGLT2 mRNA levels are decreased in kidney biopsy tissue from patients with obesity-related secondary focal segmental glomerulosclerosis (FSGS). The triangles indicate mRNA levels in the 2 patients with FSGS who also had diabetes. a.u., Arbitrary units. ***P < 0.001.
DISCUSSION
Animal and human models of diabetes mellitus have shown that SGLT2 inhibition-related natriuresis increases macula densa adenosine generation, thereby activating tubuloglomerular feedback and afferent vasoconstriction leading to attenuation of hyperfiltration (32). Decreased intraglomerular pressure is associated with reduced mechanical stretch and wall tension, suppressing renal inflammation and fibrosis (31). Despite their routine use as antihyperglycemic agents, intrarenal hemodynamic effects of SGLT2i agents are likely related to natriuresis rather than glucosuria. As a consequence of natriuresis rather than glucosuria, the effects of SGLT2i in the kidney may extend to animals and humans without diabetes or ambient hyperglycemia due to ubiquitous effects of proximal natriuresis on TGF (36, 37, 47). In this pilot combined human-rodent study, our aim was to investigate the effect of SGLT2i on renal hemodynamic function as a measure of glomerular hypertension, blood pressure, and proteinuria.
Our first major observation was that although the intervention was generally well-tolerated in this relatively unique SGLT2i study cohort, dapagliflozin did not influence renal hemodynamic function in patients with FSGS or in the animal model used in this series of experiments. Despite the lack of statistical significance overall, GFR did decrease numerically in patients with FSGS at 8 wk in response to SGLT2i to an extent that is similar to that expected in patients with diabetes mellitus (21). The results of our sensitivity analyses further suggest that renal hemodynamic effects are most prominent in individuals with GFR ≥ 90 ml·min−1·1.73 m−2, perhaps due to the greater filtered load of sodium, and in patients with modest proteinuria. Based on these pilot data, patients with FSGS and markers of more mild disease may be more responsive to SGLT2i. Importantly, the impact of changes in proteinuria on CKD progression in secondary FSGS may not be as critically important as they are in primary FSGS. Therefore, significant renal hemodynamic effects in participants with GFR ≥ 90 ml·min−1·1.73 m−2, which may reflect an important physiological reduction in glomerular hypertension, may be physiologically beneficial and preserve renal function over time (23).
Our second major observation was that dapagliflozin did not reduce proteinuria in patients with FSGS, nor were there statistically significant effects observed in the rodent model. Although it is difficult to determine why proteinuria did not decrease in the overall cohort, several plausible explanations are worth considering. First, in a separate cohort of archival human kidney tissue, we found that SGLT2 mRNA expression was decreased in kidney tissue from individuals with FSGS with or without concurrent diabetes mellitus. This is, to our knowledge, the first time that SGLT2 mRNA levels have been reported in patients with nondiabetic CKD. It may in part explain why dapagliflozin had overall neutral renal hemodynamic and antiproteinuric effects and also induced a level of glucosuria that was correspondingly at the lower end of what would be expected based on existing data in nondiabetic individuals (35). The data add to a growing body of studies of SGLT2 expression in human kidney disease that demonstrate heterogeneity in expression of the transporter. For instance, like in our study, Solini et al. (38) also described a reduction in renal SGLT2 expression in kidney tissue from people with type 2 diabetes, whereas Wang et al. (44) reported an increase in renal SGLT2 levels in individuals with diabetic nephropathy. The differences may reflect the clinical characteristics of the patients, level of renal function, sample preparation, or method of analysis. In our study, for example, control tissue was obtained by wedge biopsy, and tissue from patients with FSGS was obtained as core biopsy. It is also worth highlighting that although decreased SGLT2 mRNA levels may be interpreted as being suggestive of decreased SGLT2 protein or transporter activity, this cannot necessarily be assumed. Despite these limitations and potential issues around sampling bias, decreased SGLT2 mRNA expression in kidney tissue from individuals with FSGS may reflect proximal tubular cell injury and/or the absence of a stimulatory hyperglycemic milieu. However, even in two patients with comorbid FSGS and diabetes, SGLT2 mRNA levels were still diminished. Thus, based on renal functional data and SGLT2 mRNA expression, FSGS may not be the ideal pathophysiological setting in which to examine SGLT2 inhibition effects as a model of nondiabetic renal protection.
The lack of an antiproteinuric effect in response to dapagliflozin at 8 wk may also in part reflect the activation of compensatory neurohormonal pathways, such as the RAAS, over time. Although a reduction in proteinuria in glomerular-based disease is a common surrogate marker of renal protection, it is important to note that the mechanisms responsible for renal protection with SGLT2i, including in the setting of diabetes, remain unknown. Therefore, the lack of effect on proteinuria in the setting of FSGS does not necessarily rule out beneficial effects, particularly in light of apparent acute effects on GFR in the preserved renal function range. The importance of elucidating the renal protective effect of SGLT2i in novel settings including nondiabetic CKD was further highlighted by the CANVAS Program results, demonstrating significant reductions in diabetic nephropathy end points (29).
In post hoc sensitivity analyses, when stratified for GFR at baseline, those individuals who had GFR ≥ 90 ml·min−1·1.73 m−2 exhibited a significant increase in plasma aldosterone following dapagliflozin therapy. Since RAAS activation can lead to intraglomerular hypertension, increased plasma aldosterone may have attenuated the maximal antiproteinuric effects of dapagliflozin over time (9).
SGLT2 inhibitors typically lead to sustained reductions in SBP by 4–6 mmHg and diastolic blood pressure by 1–2 mmHg in patients with type 2 diabetes and baseline blood pressure values of approximately 130–140 mmHg systolic and 75–85 mmHg diastolic (21). In patients with type 1 diabetes with baseline blood pressure values (111/64 mmHg) that were similar to those in the present cohort, empagliflozin treatment for 8 wk reduced SBP by ∼2.5 mmHg (8). Therefore, although not statistically significant, the magnitude of the diastolic blood pressure reduction observed in patients in the present study was in the expected range. In contrast, there was a significant decrease in SBP in hypertensive SNx rats. Whether these blood-pressure-lowering effects would have been accentuated in patients with hypertension and FSGS is not known but should be examined in future work. For the mechanism of blood pressure lowering, dapagliflozin was associated with a significant rise in hematocrit in patients with FSGS, and RAAS mediators tended to be higher after dapagliflozin, suggesting a contraction in plasma volume. SGLT2 inhibition-associated increases in hematocrit have been reported in studies involving patients with type 1 and type 2 diabetes and likely represent natriuresis-associated hemoconcentration (8, 21), although direct effects on erythropoiesis have also been hypothesized (12). In conjunction with studies demonstrating long-term plasma volume contraction using I131-labeled albumin in response to SGLT2 inhibitors, the rise in hematocrit likely reflects hemoconcentration on the basis of natriuresis, leading to blood pressure lowering.
In addition to a lowering of BP in SNx rats, dapagliflozin treatment was also associated with a significant increase in kidney weight in sham-operated rats and a significant increase in kidney weight-to-body weight ratio in SNx rats. This phenomenon has been described before in rats and may relate to diuresis-induced enlargement of the tubule lumen or to tubule cell hypertrophy as a consequence of increased SGLT1-mediated glucose reabsorption (34). Whether this effect had any bearing on proteinuria or on histological indices is unclear. Nonetheless, administration of dapagliflozin at the dose employed in the present study (1 mg·kg−1·day−1) for the duration of study (8 wk) in this model of nondiabetic CKD had no significant effect on proteinuria or glomerular or tubulointerstitial injury. We cannot, however, rule out the possibility that a higher dose SGLT2i, longer-term treatment, or the use of less selective SGLT2/SGLT1 inhibitor agents may be associated with greater effects on proteinuria via hemodynamic or anti-inflammatory effects (6, 19, 20).
Our study results may have implications for ongoing and planned clinical trials in the setting of nondiabetic CKD. If effects on GFR and glomerular pressure are most prominent in patients with GFR ≥ 90 ml·min−1·1.73 m−2, then clinical trials focusing on patients with CKD stages 2–4 may miss relevant renal hemodynamic and hence renoprotective effects. On the other hand, patients with lower levels of baseline renal function may exhibit higher baseline BP levels, which may lead to exaggerated antihypertensive responses to SGLT2i. Based on our observations, although current trials will recruit patients with diverse etiologies of nondiabetic CKD, FSGS appears to be a setting where SGLT2i-related effects are less likely to be successful. Moreover, previous studies similarly reported absence of benefit in models of polycystic kidney disease (25, 34) and the remnant kidney model (46), suggesting SGLT2i may be ineffective in these settings. A major caveat is that the impact of SGLT2i on proteinuria in FSGS may be limited to those with lower levels of proteinuria, and effects on GFR, reflecting intraglomerular pressure, may only occur in patients with preserved renal function with GFR ≥ 90 ml·min−1·1.73 m−2. Unfortunately, detecting longer-term benefits in these relatively healthy patients will be difficult in the context of a renal outcome trial due to the prolonged length of time it will likely take to accrue end points.
Our experiments do have important limitations worth mentioning. First, the small sample size in the patient study may have reduced our ability to detect significant differences in physiological parameters such as GFR. We tried to minimize the impact of the small sample size in the patient study by standardizing prestudy conditions, including dietary intake of sodium and protein, and by using gold-standard measures of renal function. Our within-participant study design also helped to minimize physiological variation in parameters such as body mass index, allowing individuals to act as their own control over time. Despite the small sample size, human and rodent model results were consistently neutral for the proteinuria outcome, demonstrating conservation of effects across species as an added value. Second, we acknowledge that, for ethical and practical reasons, SGLT2 expression was not measured prospectively in the kidneys of the participants of the interventional study and neither was histopathology assessed after dapagliflozin treatment. Third, in terms of disease etiology, a further limitation is that the rat experiments used a model of secondary FSGS, whereas our pilot human data included patients with primary FSGS or secondary FSGS. In addition, to understand better the direct impact of SGLT2 inhibition on renal physiological parameters, animals were not treated with background RAAS blockade. In contrast, patients received standard-of-care agents, and RAAS blockade was not discontinued due to ethical considerations. We also recognize that there are potential interspecies differences in responses to SGLT2 inhibitors, which may account for heterogeneous responses and mixed study results to these agents even in animal models of diabetes (16, 39, 42). This underscores the need for larger placebo-controlled trials of SGLT2 inhibition in humans, with and without diabetic kidney disease. Next, we recognize that participants in this trial had preserved renal function. Our results therefore may not be generalizable to patients with significant renal function impairment. We were also not powered to examine the impact of SGLT2 inhibition on renal function in patients with renal hyperfiltration, who may have been included in the GFR ≥ 90 ml·min−1·1.73 m−2 subgroup. Finally, we recognize that other SGLT2 inhibitors might impact renal function differently from dapagliflozin in nondiabetic CKD cohorts and that other members of this drug class require dedicated studies.
In conclusion, SGLT2 inhibition did not influence renal hemodynamic function or lower proteinuria in humans or animals with FSGS. The lack of effect on renal end points in FSGS may be due to decreased renal SGLT2 expression. Future mechanistic studies using SGLT2i should consider including patients with hypertension and those with earlier disease (i.e., higher GFR, less proteinuria) and should also include patients with other etiologies of nondiabetic CKD.
GRANTS
The Treating to Reduce Albuminuria and Normalize Hemodynamic Function in Focal ScLerosis with dApagliflozin Trial Effects (TRANSLATE) trial was funded by AstraZeneca to D. Z. I. Cherney. D. Z. I. Cherney is also supported by funding from the Canadian Institutes of Health Research, the Juvenile Diabetes Research Foundation, the Banting and Best Diabetes Centre, and the Heart & Stroke/Richard Lewar Centre of Excellence and with a University of Toronto Department of Medicine Merit Award. The rodent experiments and gene expression studies were supported by an AstraZeneca Research Grant to A. Advani. H. N. Reich is the recipient of the Gabor Zellerman Chair in Nephrology Research, University Health Network, University of Toronto. J. A. Lovshin is the recipient of a University Health Network Cardiology Renal Endocrine (CaRE) Fellowship Award. P. Bjornstad is supported by National Institute of Diabetes and Digestive and Kidney Diseases Training Grant T32-DK-063687. H. Rajasekeran holds a Banting and Best Diabetes Centre, University of Toronto Training Award. Y. Lytvyn was supported by a Heart & Stroke/Richard Lewar Centre of Excellence Studentship, a Javenthey Soobiah Scholarship, a Queen Elizabeth II/Dr. Arnie Aberman Graduate Scholarship in Science and Technology, a University of Toronto Fellowship in the Department of Pharmacology and Toxicology, and a Diabetes Canada Postdoctoral Fellowship. S. Majumder was supported by a Diabetes Canada Postdoctoral Fellowship. A. Advani is supported by a Diabetes Investigator Award from Diabetes Canada and by a University of Toronto Department of Medicine Merit Award.
DISCLOSURES
D. Z. I. Cherney has acted as a consultant and received honoraria from Boehringer Ingelheim and Lilly, Merck, Janssen, Sanofi, AbbVie, and AstraZeneca and has received research operating funds from Boehringer Ingelheim and Lilly Diabetes Alliance, Merck, AstraZeneca, and Janssen. A. Advani has received research support from AstraZeneca and Boehringer Ingelheim. J. A. Lovshin has received speaker, consulting honoraria, or both from Novo Nordisk, Merck Sharp & Dohme, AstraZeneca, and Eli Lilly.
AUTHOR CONTRIBUTIONS
A.A. and D.Z.I.C. conceived and designed research; H.R., S.M., B.B.B., M.G.K., S.L.A., and D.Z.I.C. performed experiments; H.R., H.N.R., M.A.H., D.C., J.A.L., Y.L., P.B., V.L., J.T., L.C., I.W.G., M.M.S., A.A., and D.Z.I.C. analyzed data; H.R., H.N.R., M.A.H., D.C., J.A.L., Y.L., P.B., V.L., J.T., L.C., A.A., and D.Z.I.C. interpreted results of experiments; H.R., A.A., and D.Z.I.C. prepared figures; H.R., H.N.R., M.A.H., D.C., J.A.L., Y.L., P.B., V.L., J.T., L.C., A.A., and D.Z.I.C. drafted manuscript; H.R., H.N.R., M.A.H., D.C., J.A.L., Y.L., P.B., V.L., J.T., L.C., S.M., B.B.B., M.G.K., S.L.A., I.W.G., M.M.S., A.A., and D.Z.I.C. edited and revised manuscript; H.R., H.N.R., M.A.H., D.C., J.A.L., Y.L., P.B., V.L., J.T., L.C., S.M., B.B.B., M.G.K., S.L.A., I.W.G., M.M.S., A.A., and D.Z.I.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
REFERENCES
- 1.Advani A, Connelly KA, Yuen DA, Zhang Y, Advani SL, Trogadis J, Kabir MG, Shachar E, Kuliszewski MA, Leong-Poi H, Stewart DJ, Gilbert RE. Fluorescent microangiography is a novel and widely applicable technique for delineating the renal microvasculature. PLoS One 6: e24695, 2011. doi: 10.1371/journal.pone.0024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani A, Kelly DJ, Advani SL, Cox AJ, Thai K, Zhang Y, White KE, Gow RM, Marshall SM, Steer BM, Marsden PA, Rakoczy PE, Gilbert RE. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci USA 104: 14448–14453, 2007. doi: 10.1073/pnas.0703577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornstad P, Škrtić M, Lytvyn Y, Maahs DM, Johnson RJ, Cherney DZ. The Gomez equations and renal hemodynamic function in kidney disease research. Am J Physiol Renal Physiol 311: F967–F975, 2016. doi: 10.1152/ajprenal.00415.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LH, Advani SL, Thai K, Kabir MG, Sood MM, Gibson IW, Yuen DA, Connelly KA, Marsden PA, Kelly DJ, Gilbert RE, Advani A. SDF-1/CXCR4 signaling preserves microvascular integrity and renal function in chronic kidney disease. PLoS One 9: e92227, 2014. doi: 10.1371/journal.pone.0092227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherney D, Lund SS, Perkins BA, Groop PH, Cooper ME, Kaspers S, Pfarr E, Woerle HJ, von Eynatten M. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 59: 1860–1870, 2016. doi: 10.1007/s00125-016-4008-2. [DOI] [PubMed] [Google Scholar]
- 6.Cherney DZ, Konvalinka A, Zinman B, Diamandis EP, Soosaipillai A, Reich H, Lorraine J, Lai V, Scholey JW, Miller JA. Effect of protein kinase Cβ inhibition on renal hemodynamic function and urinary biomarkers in humans with type 1 diabetes: a pilot study. Diabetes Care 32: 91–93, 2009. doi: 10.2337/dc08-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hébert RL, Sochett EB. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes 57: 688–695, 2008. doi: 10.2337/db07-1230. [DOI] [PubMed] [Google Scholar]
- 8.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 9.Cherney DZ, Perkins BA, Soleymanlou N, Xiao F, Zimpelmann J, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M, Burns KD. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int 86: 1057–1058, 2014. doi: 10.1038/ki.2014.246. [DOI] [PubMed] [Google Scholar]
- 11.de Mik SM, Hoogduijn MJ, de Bruin RW, Dor FJ. Pathophysiology and treatment of focal segmental glomerulosclerosis: the role of animal models. BMC Nephrol 14: 74, 2013. doi: 10.1186/1471-2369-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 65: 1190–1195, 2016. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 13.Fischer PA, Bogoliuk CB, Ramirez AJ, Sánchez RA, Masnatta LD. A new procedure for evaluation of renal function without urine collection in rat. Kidney Int 58: 1336–1341, 2000. doi: 10.1046/j.1523-1755.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 14.Fogo AB. Animal models of FSGS: lessons for pathogenesis and treatment. Semin Nephrol 23: 161–171, 2003. doi: 10.1053/snep.2003.50015. [DOI] [PubMed] [Google Scholar]
- 15.Fogo AB. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol 11: 76–87, 2015. doi: 10.1038/nrneph.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo LA, Ward MS, Fotheringham AK, Zhuang A, Borg DJ, Flemming NB, Harvie BM, Kinneally TL, Yeh SM, McCarthy DA, Koepsell H, Vallon V, Pollock C, Panchapakesan U, Forbes JM. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep 6: 26428, 2016. doi: 10.1038/srep26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez DM. Evaluation of renal resistances, with special reference to changes in essential hypertension. J Clin Invest 30: 1143–1155, 1951. doi: 10.1172/JCI102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han S, Hagan DL, Taylor JR, Xin L, Meng W, Biller SA, Wetterau JR, Washburn WN, Whaley JM. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes 57: 1723–1729, 2008. doi: 10.2337/db07-1472. [DOI] [PubMed] [Google Scholar]
- 19.Har R, Scholey JW, Daneman D, Mahmud FH, Dekker R, Lai V, Elia Y, Fritzler ML, Sochett EB, Reich HN, Cherney DZ. The effect of renal hyperfiltration on urinary inflammatory cytokines/chemokines in patients with uncomplicated type 1 diabetes mellitus. Diabetologia 56: 1166–1173, 2013. doi: 10.1007/s00125-013-2857-5. [DOI] [PubMed] [Google Scholar]
- 20.Har RL, Reich HN, Scholey JW, Daneman D, Dunger DB, Moineddin R, Dalton RN, Motran L, Elia Y, Deda L, Ostrovsky M, Sochett EB, Mahmud FH, Cherney DZ. The urinary cytokine/chemokine signature of renal hyperfiltration in adolescents with type 1 diabetes. PLoS One 9: e111131, 2014. doi: 10.1371/journal.pone.0111131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 23.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 24.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, de Jong PE, de Zeeuw D, Shahinfar S, Ruggenenti P, Remuzzi G, Levey AS; AIPRD Study Group. Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease . Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 60: 1131–1140, 2001. doi: 10.1046/j.1523-1755.2001.0600031131.x. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor S, Rodriguez D, Riwanto M, Edenhofer I, Segerer S, Mitchell K, Wüthrich RP. Effect of sodium-glucose cotransport inhibition on polycystic kidney disease progression in PCK rats. PLoS One 10: e0125603, 2015. doi: 10.1371/journal.pone.0125603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lytvyn Y, Škrtić M, Yang GK, Lai V, Scholey JW, Yip PM, Perkins BA, Cherney DZ. Plasma uric acid effects on glomerular haemodynamic profile of patients with uncomplicated type 1 diabetes mellitus. Diabet Med 33: 1102–1111, 2016. doi: 10.1111/dme.13051. [DOI] [PubMed] [Google Scholar]
- 27.Matsui K, Nagy-Bojarsky K, Laakkonen P, Krieger S, Mechtler K, Uchida S, Geleff S, Kang DH, Johnson RJ, Kerjaschki D. Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: aminopeptidase P and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels. J Am Soc Nephrol 14: 1981–1989, 2003. doi: 10.1097/01.ASN.0000076078.50889.43. [DOI] [PubMed] [Google Scholar]
- 28.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimpelmann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol 17: 2554–2560, 2006. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 29.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 30.Ossani GP, Fischer PA, Caram SG, Dominguez GN, Monserrat AJ, Masnatta LD. Mild hyperhomocysteinemia promotes renal hemodynamic dysfunction without histopathologic changes in adult rats. Kidney Int 66: 1866–1872, 2004. doi: 10.1111/j.1523-1755.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 31.Petermann AT, Pippin J, Durvasula R, Pichler R, Hiromura K, Monkawa T, Couser WG, Shankland SJ. Mechanical stretch induces podocyte hypertrophy in vitro. Kidney Int 67: 157–166, 2005. doi: 10.1111/j.1523-1755.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 32.Rajasekeran H, Lytvyn Y, Cherney DZ. Sodium-glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: the emerging role of natriuresis. Kidney Int 89: 524–526, 2016. doi: 10.1016/j.kint.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 33.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl 68: S57–S65, 2005. doi: 10.1111/j.1523-1755.2005.09911.x. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez D, Kapoor S, Edenhofer I, Segerer S, Riwanto M, Kipar A, Yang M, Mei C, Wüthrich RP. Inhibition of sodium-glucose cotransporter 2 with dapagliflozin in Han: SPRD rats with polycystic kidney disease. Kidney Blood Press Res 40: 638–647, 2015. doi: 10.1159/000368540. [DOI] [PubMed] [Google Scholar]
- 35.Sha S, Polidori D, Farrell K, Ghosh A, Natarajan J, Vaccaro N, Pinheiro J, Rothenberg P, Plum-Mörschel L. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab 17: 188–197, 2015. doi: 10.1111/dom.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skøtt P, Hommel E, Bruun NE, Arnold-Larsen S, Parving HH. Effects of acetazolamide on kidney function in type 1 (insulin-dependent) diabetic patients with diabetic nephropathy. Diabetologia 31: 806–810, 1988. doi: 10.1007/BF00277481. [DOI] [PubMed] [Google Scholar]
- 37.Skøtt P, Hommel E, Bruun NE, Arnold-Larsen S, Parving HH. The acute effect of acetazolamide on glomerular filtration rate and proximal tubular reabsorption of sodium and water in normal man. Scand J Clin Lab Invest 49: 583–587, 1989. doi: 10.3109/00365518909089139. [DOI] [PubMed] [Google Scholar]
- 38.Solini A, Rossi C, Mazzanti CM, Proietti A, Koepsell H, Ferrannini E. Sodium-glucose co-transporter (SGLT)2 and SGLT1 renal expression in patients with type 2 diabetes. Diabetes Obes Metab 19: 1289–1294, 2017. doi: 10.1111/dom.12970. [DOI] [PubMed] [Google Scholar]
- 39.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group . Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068, 2005. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 41.Tsuda A, Ishimura E, Ohno Y, Ichii M, Nakatani S, Mori K, Fukumoto S, Emoto M, Inaba M. Significant association of poor glycemic control with increased resistance in efferent arterioles–study of inulin and para-aminohippuric acid clearance in humans. Diabetes Res Clin Pract 104: 234–240, 2014. doi: 10.1016/j.diabres.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 42.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Wang XX, Levi J, Luo Y, Myakala K, Herman-Edelstein M, Qiu L, Wang D, Peng Y, Grenz A, Lucia S, Dobrinskikh E, D’Agati VD, Koepsell H, Kopp JB, Rosenberg AZ, Levi M. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J Biol Chem 292: 5335–5348, 2017. doi: 10.1074/jbc.M117.779520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Thai K, Kepecs DM, Gilbert RE. Sodium-glucose linked cotransporter-2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS One 11: e0144640, 2016. doi: 10.1371/journal.pone.0144640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zingerman B, Herman-Edelstein M, Erman A, Bar Sheshet Itach S, Ori Y, Rozen-Zvi B, Gafter U, Chagnac A. Effect of acetazolamide on obesity-induced glomerular hyperfiltration: a randomized controlled trial. PLoS One 10: e0137163, 2015. doi: 10.1371/journal.pone.0137163. [DOI] [PMC free article] [PubMed] [Google Scholar]