Abstract
epithelial Na+ channel, ENaC, is the final arbiter of sodium excretion in the kidneys. As such, discretionary control of ENaC by hormones is critical to the fine-tuning of electrolyte and water excretion and, consequently, blood pressure. Casein kinase 2 (CK2) phosphorylates ENaC. Phosphorylation by CK2 is necessary for normal ENaC activity. We tested the physiological importance of CK2 regulation of ENaC as the degree to which ENaC activity is dependent on CK2 phosphorylation in the living organism is unknown. This was addressed using patch-clamp analysis of ENaC in completely split-open collecting ducts and whole animal physiological studies of sodium excretion in mice. We also used ENaC-harboring CK2 phosphorylation site mutations to elaborate the mechanism. We found that ENaC activity in ex vivo preparations of murine collecting duct had a significant decrease in activity in response to selective antagonism of CK2. In whole animal experiments selective antagonism of CK2 caused a natriuresis similar to benzamil, but not additive to benzamil, suggesting an ENaC-dependent mechanism. Regulation of ENaC by CK2 was abolished by mutation of the canonical CK2 phosphorylation sites in beta and gamma ENaC. Together, these results demonstrate that the appropriate regulation of ENaC by CK2 is necessary for the normal physiological role played by this key renal ion channel in the fine-tuning of sodium excretion.
Keywords: aldosterone, collecting duct, hypertension, renal physiology, sodium excretion, transport
INTRODUCTION
Cardiovascular disease (CVD) is responsible for ~20 million deaths worldwide each year (30), and in industrialized countries 90% of people will develop hypertension in their lifetimes (21). Hypertension is a primary risk factor for CVD, and contributes to the risk of myocardial infarction and stroke. However 95% of cases of hypertension are termed “essential hypertension,” hypertension of an unknown etiology (5). Because the causes of essential hypertension are little understood, this hypertension is often refractory to simple treatment, usually requiring therapy involving a combination of drugs (10). This argues that treating the symptom of increased blood pressure without understanding its underlying causation increases the cost and decreases the efficacy of managing this condition.
By regulating excretion the kidneys modulate the volume and electrolyte content of plasma. Because the circulatory system is a closed system, changes in plasma volume affect blood pressure. The epithelial sodium channel (ENaC) is the final step in the reabsorption of sodium in the renal nephron. The activity of ENaC is under the control of the renin-angiotensin-aldosterone system (RAAS): a key feedback cascade that modulates blood pressure. Discretionary control of sodium reabsorption via ENaC fine-tunes renal sodium and water excretion to set urine output and blood volume. The importance of proper ENaC activity is exemplified by Liddle’s syndrome, a monogenic hypertension caused by gain-of-function mutations in the channel, and by the fact that of eight other known monogenic hypertensions, seven act ultimately, in part or fully, on ENaC through the RAAS (8, 25, 29). Conversely, loss-of-function mutations in ENaC cause inappropriate renal salt wasting and hypotension.
Shi et al. (27) reported that at least three distinct kinases phosphorylate ENaC when the recombinant channel is expressed in Chinese Hamster Ovary (CHO) cells. In a phosphomapping study they identified sites in beta and gamma ENaC phosphorylated by casein kinase II (CK 2) (28). Casein Kinase II is a serine/threonine protein kinase comprising two alpha and two beta subunits. It is regulated by Wnt3a (12), is widely expressed, and has been implicated in a diverse set of biological roles, including embryonic morphogenesis (7), circadian rhythms (1), and inflammation. Prior study of CK2 regulation of ENaC showed that inhibition of CK2 decreased short-circuit currents in isolated mouse trachea and colon as well as amiloride-sensitive current in Xenopus oocytes overexpressing exogenous ENaC (2). Amiloride and its analogs like benzamil inhibit ENaC.
Functional ENaC comprises three similar but distinct subunits, α, β, and γ, which share a conserved secondary and tertiary structure. Mutation of the CK2 binding sites in the β and γ ENaC subunits resulted in an attenuated amiloride-sensitive current in oocytes (2). However, the exact molecular and cellular mechanisms by which CK2 regulates ENaC activity and the physiological importance of this remain obscure. The current investigation elaborates CK2 regulation of ENaC by examining the physiological importance of this regulation in a relevant mammalian model.
We studied the physiological relevance of CK2 regulation of ENaC in ex vivo preparations of native tissue, living animals, and in an exogenous expression system. We identified CK2 phosphorylation of β and γ ENaC as a physiologically important component of normal ENaC activity and a possible mechanism of regulation of ENaC activity and dependent renal sodium excretion. This was evidenced by 4,5,6,7-tetrabromobenzotriazole (TBB) sensitivity of ENaC in native tissue at doses specific to inhibition of CK2, TBB sensitivity in transfected cells being dependent on CK2 phosphorylation sites, and finally by TBB promoting ENaC-dependent sodium excretion in mice. Together these data support CK2 phosphorylation as an important regulator of ENaC activity and sodium handling by the aldosterone-sensitive distal nephron.
MATERIALS AND METHODS
Split-open tubule/single-channel recording.
This technique was performed as described previously (22). In brief, mouse kidney was sectioned transversely and segments of cortical collecting duct were manually microdissected with forceps and adhered to a glass chip coated with polylysine. The chips were transferred to an inverted microscope where the top layer of collecting duct was split open with sharp pipettes. Single-channel patch-clamp in the cell-attached configuration was then performed on the apical membranes of principal cells in the region of the lumen exposed. In some experiments collecting ducts were preincubated for 30 min with 200 nM TBB (24), a potent and highly specific inhibitor of CK2 activity. Channel activity (NPo) was calculated as usual, where N is the number of channels in a patch and Po is the open probability these channels have (17).
Whole cell recordings.
Chinese Hamster Ovary (CHO) cells (from ATCC) were transiently transfected with 2 μg total eGFP, α, β, and γ mENaC cDNA following standard procedures (11). Macroscopic ENaC currents were recorded from CHO cells in the whole cell patch-clamp configuration, with a +60 to −100 mV ramp protocol from a holding potential of +40 mV, using standard protocols (16). Amiloride (10 µM) was applied for 2 min, washed until current was stable, and then TBB was applied for 5 min. Site-directed mutagenesis was performed by Topgene (Toronto, Ontario, Canada).
Animal care and use.
All animal work was performed following protocols approved by the UTHSCSA Department of Laboratory Animal Resources and the Institutional Animal Care and Use Committee.
Metabolic experiments.
Mice were housed in metabolic cages (Techniplast, Buguggiate, Italy), four to a cage. Access to water and chow was allowed ad libitum. Following an acclimation period of 3 days, every 12 h, mouse weight, urine volume, and consumed water were measured, and urine was collected. Urinary [Na+] and [K+] were quantified with a flame photometer (Jenway, Staffordshire, UK). Urine osmolarity was measured with an osmometer (VAPRO 5520, ELITechGroup, Puteaux, France), and urine [creatinine] measured using the QuantiChrom Creatinine Assay (BioAssay Systems, Hayward, CA). Three days before the experiment, mice were switched to and subsequently maintained with a nominally sodium-free diet (<0.01%; TEKLAD, custom diet TD 90229, Envigo, Indianapolis, IN). This maximizes ENaC activity. Four cohorts of mice were subcutaneously injected with 1) 100 µl of 5% ethanol/sterile water (vehicle control), 2) 125 μg/kg TBB in 5% ethanol/sterile saline, 3) 1.4 mg/kg benzamil, or 4) TBB plus benzamil (4). For each trial, each cohort contained four mice. One day after the final injection, mice were euthanized by decapitation under anesthesia, and blood was collected. Hematocrit and plasma creatinine concentration were recorded.
Statistics.
The number of animals used was chosen by power analyses. Summarized data were reported as average ± standard error of the mean (SE). Summarized data were compared with either Student’s t test or a one-way analysis of variance with Dunnett’s posttest as appropriate. Dose-response data were normalized to initial current density, and fit with a four-parameter logistic regression, and accuracy of the fit was reported as R2 value. A P value of 0.05 was selected as the cutoff for statistical significance.
RESULTS
CK2 is necessary for ENaC activity in native collecting duct.
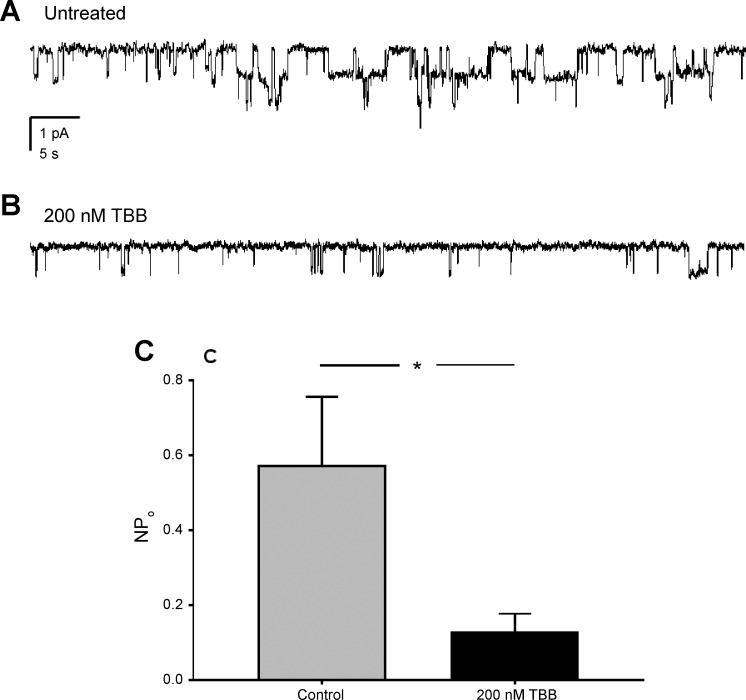
To test the physiological importance of CK2 to the regulation of ENaC, the necessity of this kinase to ENaC activity was quantified in freshly isolated murine collecting ducts. Cortical collecting duct was excised from mouse kidneys, split open, and used in the single-channel patch configuration as described previously (22). Single-channel recordings were made in collecting ducts after incubation for 30 min with vehicle or the specific CK2 inhibitor, TBB (200 nM). TBB significantly reduced ENaC activity in native tissue (Fig. 1, A and B). The activity (NPo) of ENaC in the control group was 0.58 ± 0.18, whereas the NPo of the TBB treated group was 0.13 ± 0.04 (P < 0.05, n = 12–18 from 5 mice). These results demonstrate that CK2 is necessary for normal ENaC activity in the collecting duct of mice, and is consistent with previous reports of short-circuit current in mouse trachea and colon being sensitive to TBB. Absorption of Na+ via ENaC is the primary driver of current across the trachea (20) and colon (14).
Fig. 1.
CK2 is necessary for EnaC activity in the native collecting duct. Current traces of ENaC in cell-attached patches of principal cells in the aldosterone-sensitive distal nephron. The aldosterone-sensitive distal nephron from wild-type C57BL/6 mice were split open and recorded from in the cell-attached mode, either without treatment (control) (A), or after a 30 min pretreatment with 200 nM TBB (B). C: NPo of the untreated group was 0.58 ± 0.18, whereas the NPo of the treated group was 0.13 ± 0.04 (P < 0.05, n = 12–18 from 5 mice). *Significant decrease compared with control, Student’s unpaired t-test.
Physiological relevance of CK2 regulation of ENaC.
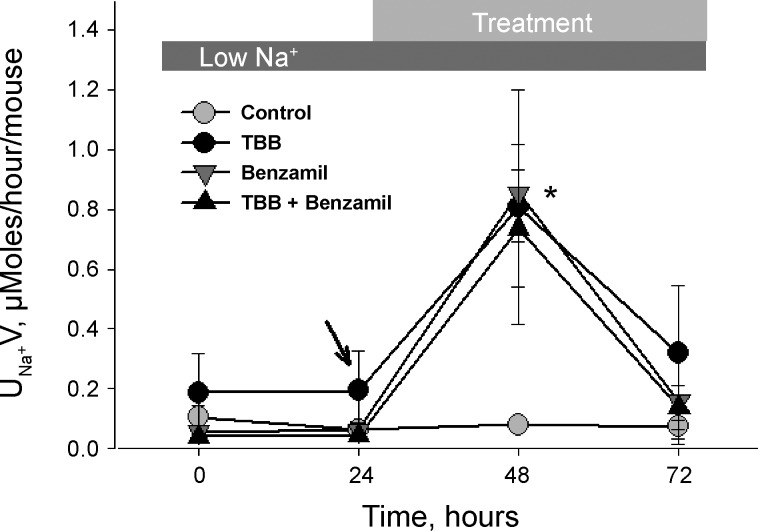
To test the physiological importance of CK2 to regulation of excretion in the living organism and to determine the role played by ENaC in CK2-dependent regulation of excretion, adult C57BL/6 mice were divided into four cohorts and placed in metabolic chambers. Mice were fed a sodium-free diet to enhance ENaC activity. Following a 72-h acclimation period, weight, water intake, and urine output were stable and followed circadian rhythm. During the experimental period, one cohort per trial was treated with vehicle, another with 125 µg/kg TBB in 5% ethanol/sterile saline, another with benzamil, and the final with both TBB and benzamil. These experiments were repeated in triplicate. If TBB has a sodium wasting effect, and it occurs through a mechanism other than ENaC, then an additive effect on sodium excretion is anticipated in the TBB plus benzamil cohort.
The TBB-treated mice had a significant increase in urinary Na+ excretion from 0.2 ± 0.1 μmol/h per mouse to 0.8 ± 0.4 μmol/h per mouse during the experimental period, but were indistinguishable from controls during the acclimation period (Fig. 2). This sodium excretion was not different from the sodium excretion observed with injection of benzamil alone at 0.9 ± 0.2 μmol/h per mouse. Moreover, the natriuretic effects of TBB and benzamil were not additive (0.7 ± μmol/h per mouse), suggesting that the change in sodium excretion occurred through the same pathway with both treatments. Analysis of variance with the Dunnett’s posttest showed that each treatment group was significantly different from control (P < 0.05); however, differences between the three experimental groups were not significant. Twenty four hours after the last injection, urinary Na+ excretion returned to near-baseline values. During the experiment neither mouse mass, urinary [K+], or [creatinine] varied significantly. Blood collected after TBB injection showed no significant change to plasma [Na+], [K+], [creatinine], or hematocrit.
Fig. 2.
CK2 regulates sodium excretion in the live animal. Three trials were conducted with groups of four mice housed in metabolic cages and maintained on a sodium-free diet. After a 72-h acclimation period, urinary sodium excretion (UNaV) was measured in 12-h intervals. A: 24 hours after the beginning of the experimental period, mice were injected with 125 μg/kg TBB (black circles), 1.4 μg/kg benzamil (gray triangles), both TBB and benzamil (black triangle), or vehicle (gray circle). Twelve hours after injection, UNaV was significantly greater in TBB-, benzamil-, and TBB + benzamil-treated mice compared with control. *Significant difference compared with control, Dunnett’s posttest.
These results are consistent with TBB treatment causing inhibition of CK2-dependent ENaC activity, resulting in an increase in renal Na+ excretion. This effect is likely to be ENaC-specific because of the overall similarity in profile of treatment with benzamil and TBB, and the fact that treatment with TBB plus benzamil did not produce an additive effect. As such, these observations demonstrate that CK2 regulation of ENaC plays a key role in the control of sodium excretion in the living organism.
TBB dose response and CK2 dependence.
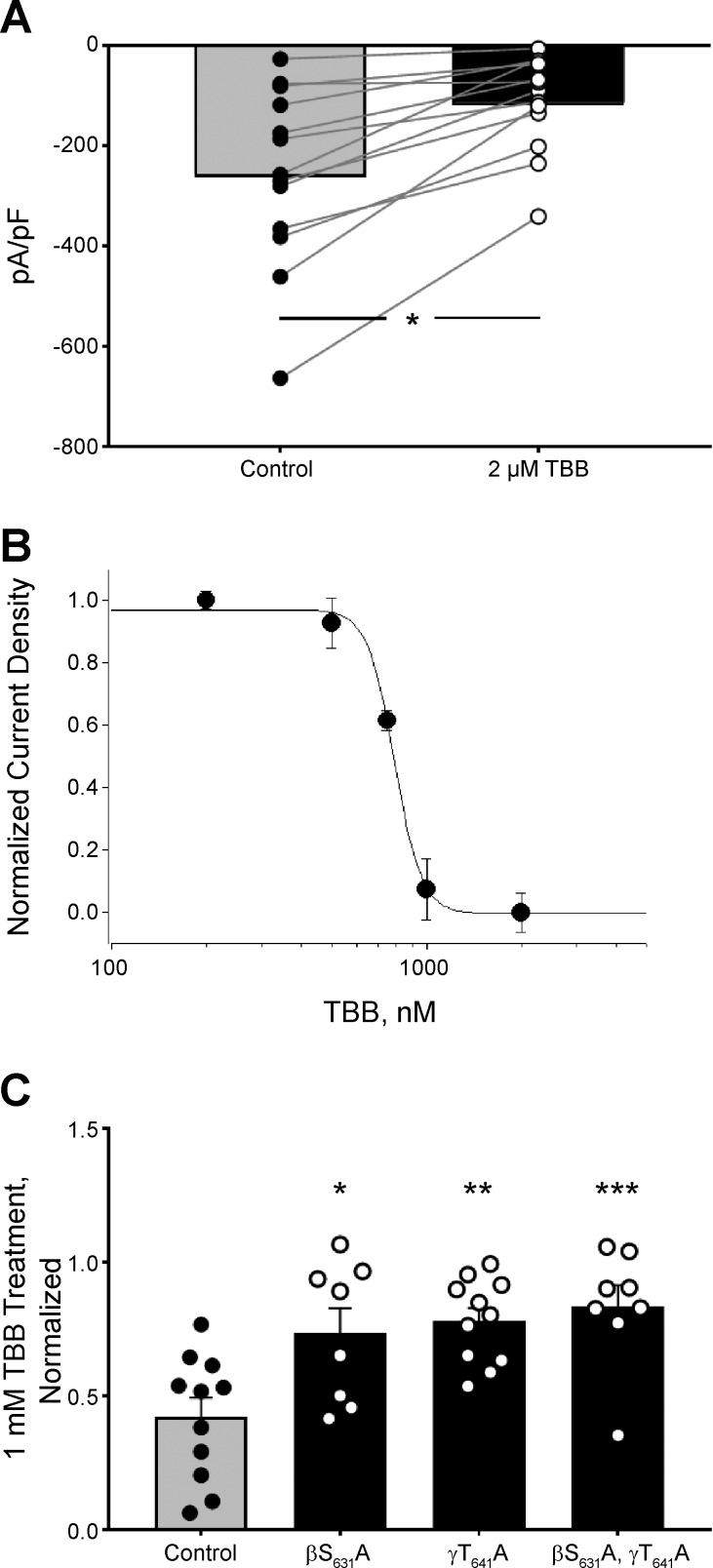
Although the above findings support a strong response of ENaC to TBB via inhibition of CK2, and TBB is recognized as a specific inhibitor of CK2 (26), it was important to demonstrate that the TBB effect was mediated by CK2, and not a direct effect of TBB on ENaC or through a kinase other than CK2. This was tested by exogenous expression of αβγ mENaC in CHO cells. Cells with good mENaC expression as indicated by eGFP expression were recorded from in the whole cell patch configuration. Treatment with 2 μM TBB caused a significant reduction in the magnitude of amiloride-sensitive macroscopic Na+ current density in these cells from a mean of −257 ± 49 to −114 ± 27 pA/pF (Fig. 3A). This decrease in magnitude was rapid, occurring within 2–5 min after TBB application.
Fig. 3.
CK2 phosphorylation of EnaC is necessary for inhibition by TBB. ENaC-transfected CHO cells were treated with TBB. A: treatment with 2 μM TBB significantly decreased the magnitude of macroscopic ENaC current density in transfected CHO cells (Student’s paired t-test). B: a dose- response curve was created between 200 and 2,000 nM TBB. The IC50 of the TBB-mediated inhibition was 760 nM. R2 = 0.99 for regression to a 4-parameter logistic equation. C: known phosphorylation sites in β and γ ENaC were mutated to alanine and expressed in CHO cells. αβγT649A, αβS631Aγ, or αβS631AγT649A had significantly less sensitivity to inhibition by TBB compared with control (ANOVA, Dunnett’s posttest). *Indicates P < 0.05, **indicates P < 0.01, ***indicates P < 0.005.
A dose-response curve was determined next for the effects of TBB on ENaC in CHO cells (Fig. 3B). The IC50 for the TBB effect on ENaC was 760 nM. This dose is consistent with the IC50 for selective TBB inhibition of CK2 and not other kinases (24).
To determine if inhibition of CK2 phosphorylation of ENaC was necessary for the actions of TBB on the channel, we created and expressed mutant ENaC subunits that had point mutations within canonical CK2 phosphorylation sites at positions shown to be required for CK2 phosphorylation via prior phosphomapping studies (28). If the inhibition of ENaC by TBB occurs through CK2, eliminating phosphorylation at these sites by substitution to amino acids incapable of being phosphorylated will mitigate decreases in ENaC activity in response to treatment with TBB. Mutation of either βS631 to alanine, γT649 to alanine, or both mutations in combination abolished the inhibitory effects of TBB on ENaC (Fig. 3C). This is consistent with TBB sensitivity being dependent on CK2 phosphorylation of ENaC at these sites.
DISCUSSION
These studies addressed a gap in our understanding of ENaC regulation. Although it is known that ENaC is phosphorylated by CK2, and that ENaC activity is sensitive to this phosphorylation in oocytes (2), the physiological relevance of this phosphorylation is unknown. The present results are consistent with earlier reports, and expand on them by demonstrating the physiological relevance of CK2 phosphorylation of ENaC in the living organism.
The cytosolic COOH termini of ENaC subunits are rich sites of regulation of the channel by accessory proteins. For instance, Liddle’s syndrome, an inheritable form of hypertension, results from disruption of the proline-rich PY motif (PXXY) in the COOH termini of ENaC subunits. The PY motif mediates interaction with the E3 ubiquitin ligase NEDD4-2, which ubiquitinates the channel to target it for degradation (13). NEDD4-2 mediated degradation is an important point of convergence for several regulatory mechanisms. Phosphorylation of β S633, only two residues downstream of the CK2 site at β S631 studied in this investigation, by the G protein-coupled receptor kinase Grk2 modulates the ability of NEDD4-2 to regulate ENaC, and alters activity (6). Other serine and threonine residues adjacent to the PY motif in β and γENaC are phosphorylated by the MAPK1/2 pathway and influence degradation of the channel (3).
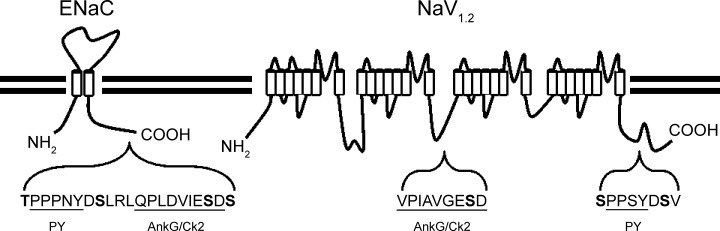
Sequences within the COOH termini of β and γENaC share homology with some stretches in the cytosolic portions of the evolutionarily unrelated sodium channel NaV1.2 (Fig. 4). They each share a common PY motif (PXXY) through which they interact with NEDD4-2. Surrounding the PY motifs in β and γENaC, and NaV1.2, are residues which are phosphorylated by MAPK1/2 (3). Finally both have an intracellular motif with similarity to the canonical Ankyrin G binding motif [(V/A)P(I/L)AXXE(S/D)D] (19), enclosing a residue phosphorylated by CK2. These similarities suggest convergent evolution.
Fig. 4.
NaV1.2 and ENaC have similar regulatory elements suggestive of convergent evolution. β and γ ENaC and NaV1.2 do not share an evolutionary history; however, they share some regulatory motifs, including PY motifs (PXXY) which are required for binding of the E3 ubiqutin ligase NEDD4-2, as well as MAPK1/2-mediated phosphorylation sites (β ENaC T613), and a motif with similarity to the canonical ankyrin G binding motif, (V/A)P(I/L)AXXE(S/D)D. Both ENaC and NaV1.2 are phosphorylated by CK2 at a serine which is the penultimate residue in this putative ankyrin biding sequence.
If this is the case, then the control mechanisms of NaV1.2 may serve as a template for understanding the mechanism by which CK2 regulates ENaC. Phosphorylation by CK2 acts as a molecular “switch” controlling the affinity of NaV1.2 for binding to Ankyrin G (Ank G) (15). AnkG is a cytoskeletal protein responsible for the normal trafficking of NaV1.2 to the axon initial segment (23). Ank G is aldosterone-induced in the collecting duct (9), and an ENaC-Ank G interaction is supported by a recent study showing that Ank G overexpression can influence ENaC activity by affecting membrane trafficking of the channel (18). We speculate that CK2 phosphorylation may act on ENaC, in a manner similar to its action on NaV1.2 by altering the channel’s affinity for binding Ank G.
Independent of molecular mechanism, the current findings demonstrate the physiological importance of CK2 to the normal function of ENaC. Electrophysiological recordings of mENaC in split-open collecting ducts and live animal metabolic experiments demonstrate that phosphorylation by CK2 is necessary for normal ENaC activity. Disruption of normal CK2 activity disrupts normal Na+ excretion, suggesting that regulation of ENaC by CK2 is necessary for normal excretion. Finally, the similarity of the TBB and benzamil actions on Na+ excretion, and their nonadditive effects, demonstrate that ENaC is the primary target of CK2 regulation in the tubule with respect to influencing sodium excretion.
GRANTS
This research was supported by American Heart Association Grants 15GR-NT-22930030 and 17GR-NT-3292002 (to J. D. Stockand) and 17POST-33660468 (to J. M. Berman) and by National Institutes of Health Grants T32-HL-00744536A1 (to J. D. Stockand and J. M. Berman), K12GM111726 (to J. M. Berman), and F32-DK-104572 (to E. Mironova).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.B. and J.D.S. conceived and designed research; J.M.B. and E.M. performed experiments; J.M.B., E.M., and J.D.S. analyzed data; J.M.B., E.M., and J.D.S. interpreted results of experiments; J.M.B. and E.M. prepared figures; J.M.B. drafted manuscript; J.M.B. and J.D.S. edited and revised manuscript; J.M.B., E.M., and J.D.S. approved final version of manuscript.
REFERENCES
- 1.Allada R, Meissner R-A. Casein kinase 2, circadian clocks, and the flight from mutagenic light. Mol Cell Biochem 274: 141–149, 2005. doi: 10.1007/s11010-005-2943-1. [DOI] [PubMed] [Google Scholar]
- 2.Bachhuber T, Almaça J, Aldehni F, Mehta A, Amaral MD, Schreiber R, Kunzelmann K. Regulation of the epithelial Na channel by the protein kinase CK2. J Biol Chem 283: 13225–13232, 2008. doi: 10.1074/jbc.M704532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth RE, Stockand JD. Targeted degradation of ENaC in response to PKC activation of the ERK1/2 cascade. Am J Physiol Renal Physiol 284: F938–F947, 2003. doi: 10.1152/ajprenal.00373.2002. [DOI] [PubMed] [Google Scholar]
- 4.Cantone A, Yang X, Yan Q, Giebisch G, Hebert SC, Wang T. Mouse model of type II Bartter’s syndrome. I. Upregulation of thiazide-sensitive Na-Cl cotransport activity. Am J Physiol Renal Physiol 294: F1366–F1372, 2008. doi: 10.1152/ajprenal.00608.2007. [DOI] [PubMed] [Google Scholar]
- 5.Carretero OA, Oparil S. Essential hypertension. I. Definition and etiology. Circulation 101: 329–335, 2000. doi: 10.1161/01.CIR.101.3.329. [DOI] [PubMed] [Google Scholar]
- 6.Dinudom A, Fotia AB, Lefkowitz RJ, Young JA, Kumar S, Cook DI. The kinase Grk2 regulates Nedd4/Nedd4-2-dependent control of epithelial Na+ channels. Proc Natl Acad Sci USA 101: 11886–11890, 2004. doi: 10.1073/pnas.0402178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez I, Degano IR, Chea K, Cha J, Toselli P, Seldin DC. CK2α is essential for embryonic morphogenesis. Mol Cell Biochem 356: 209–216, 2011. doi: 10.1007/s11010-011-0961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelheit O, Hanukoglu I, Gizewska M, Kandemir N, Tenenbaum-Rakover Y, Yurdakök M, Zajaczek S, Hanukoglu A. Novel mutations in epithelial sodium channel (ENaC) subunit genes and phenotypic expression of multisystem pseudohypoaldosteronism. Clin Endocrinol (Oxf) 62: 547–553, 2005. doi: 10.1111/j.1365-2265.2005.02255.x. [DOI] [PubMed] [Google Scholar]
- 9.Edinger RS, Coronnello C, Bodnar AJ, LaFramboise WA, Benos PV, Ho J, Johnson JP, Butterworth MB. Aldosterone regulates microRNAs in the cortical collecting duct to alter sodium transport. J Am Soc Nephrol 25: 2445–2457, 2014. doi: 10.1681/ASN.2013090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank J. Managing hypertension using combination therapy. Am Fam Physician 77: 1279–1286, 2008. [PubMed] [Google Scholar]
- 11.Gamper N, Stockand JD, Shapiro MS. The use of Chinese hamster ovary (CHO) cells in the study of ion channels. J Pharmacol Toxicol Methods 51: 177–185, 2005. doi: 10.1016/j.vascn.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Wang H-Y. Casein kinase 2 is activated and essential for Wnt/beta-catenin signaling. J Biol Chem 281: 18394–18400, 2006. doi: 10.1074/jbc.M601112200. [DOI] [PubMed] [Google Scholar]
- 13.Goulet CC, Volk KA, Adams CM, Prince LS, Stokes JB, Snyder PM. Inhibition of the epithelial Na+ channel by interaction of Nedd4 with a PY motif deleted in Liddle’s syndrome. J Biol Chem 273: 30012–30017, 1998. doi: 10.1074/jbc.273.45.30012. [DOI] [PubMed] [Google Scholar]
- 14.Greig ER, Boot-Handford RP, Mani V, Sandle GI. Decreased expression of apical Na+ channels and basolateral Na+, K+-ATPase in ulcerative colitis. J Pathol 204: 84–92, 2004. doi: 10.1002/path.1613. [DOI] [PubMed] [Google Scholar]
- 15.Hien YE, Montersino A, Castets F, Leterrier C, Filhol O, Vacher H, Dargent B. CK2 accumulation at the axon initial segment depends on sodium channel Nav1. FEBS Lett 588: 3403–3408, 2014. doi: 10.1016/j.febslet.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 17.Jospin M, Mariol M-C, Ségalat L, Allard B. Characterization of K+ currents using an in situ patch clamp technique in body wall muscle cells from Caenorhabditis elegans. J Physiol 544: 373–384, 2002. doi: 10.1113/jphysiol.2002.022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klemens CA, Edinger RS, Kightlinger L, Liu X, Butterworth MB. Ankyrin G expression regulates apical delivery of the epithelial sodium channel (ENaC). J Biol Chem 292: 375–385, 2017. doi: 10.1074/jbc.M116.753616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem 278: 27333–27339, 2003. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- 20.Matthews MR. International Handbook of Research in History, Philosophy and Science Teaching. New York: Springer, 2014. [Google Scholar]
- 21.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet 370: 591–603, 2007. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- 22.Mironova E, Bugay V, Pochynyuk O, Staruschenko A, Stockand JD. Recording ion channels in isolated, split-opened tubules. Methods Mol Biol 998: 341–353, 2013. doi: 10.1007/978-1-62703-351-0_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa Y, Rasband MN. The functional organization and assembly of the axon initial segment. Curr Opin Neurobiol 18: 307–313, 2008. doi: 10.1016/j.conb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Pagano MA, Andrzejewska M, Ruzzene M, Sarno S, Cesaro L, Bain J, Elliott M, Meggio F, Kazimierczuk Z, Pinna LA. Optimization of protein kinase CK2 inhibitors derived from 4,5,6,7-tetrabromobenzimidazole. J Med Chem 47: 6239–6247, 2004. doi: 10.1021/jm049854a. [DOI] [PubMed] [Google Scholar]
- 25.Riepe FG, van Bemmelen MXP, Cachat F, Plendl H, Gautschi I, Krone N, Holterhus P-M, Theintz G, Schild L. Revealing a subclinical salt-losing phenotype in heterozygous carriers of the novel S562P mutation in the α subunit of the epithelial sodium channel. Clin Endocrinol (Oxf) 70: 252–258, 2009. doi: 10.1111/j.1365-2265.2008.03314.x. [DOI] [PubMed] [Google Scholar]
- 26.Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (“casein kinase-2”). FEBS Lett 496: 44–48, 2001. doi: 10.1016/S0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- 27.Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H. Interactions of beta and gamma ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. J Biol Chem 277: 13539–13547, 2002. doi: 10.1074/jbc.M111717200. [DOI] [PubMed] [Google Scholar]
- 28.Shi H, Asher C, Yung Y, Kligman L, Reuveny E, Seger R, Garty H. Casein kinase 2 specifically binds to and phosphorylates the carboxy termini of ENaC subunits. Eur J Biochem 269: 4551–4558, 2002. doi: 10.1046/j.1432-1033.2002.03154.x. [DOI] [PubMed] [Google Scholar]
- 29.Welzel M, Akin L, Büscher A, Güran T, Hauffa BP, Högler W, Leonards J, Karges B, Kentrup H, Kirel B, Senses EE, Tekin N, Holterhus PM, Riepe FG. Five novel mutations in the SCNN1A gene causing autosomal recessive pseudohypoaldosteronism type 1. Eur J Endocrinol 168: 707–715, 2013. doi: 10.1530/EJE-12-1000. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization Global Status Report on Noncommunicable Diseases 2014. Geneva, Switzerland: WHO, 2014. [http://www.who.int/nmh/publications/ncd-status-report-2014/en]. [Google Scholar]