Abstract
Early life stress (ELS) in humans is associated with elevated proinflammatory markers. We hypothesized that ELS induces activation of the immune response in a rat model of ELS, maternal separation (MatSep), in adulthood. MatSep involves separating pups from the dam from postnatal day 2 to postnatal day 14 for 3 h/day. Control rats are nonseparated littermates. We determined circulating and renal immune cell numbers, renal immune cell activation markers, renal cytokine levels, and the renal inflammatory gene expression response to low-dose lipopolysaccharide (LPS) in male MatSep and control rats. We observed that MatSep did not change the percentage of gated events for circulating CD3+, CD4+, CD8+, and CD4+/Foxp3+ cells or absolute numbers of mononuclear and T cells in the circulation and kidneys; however, MatSep led to an increase in activation of renal neutrophils as well as CD44+ cells. Renal toll-like receptor 4 (TLR4) and interleukin 1 beta (IL-1β) was significantly increased in MatSep rats, specifically in the outer and inner medulla and distal nephron, respectively. Evaluation of renal inflammatory genes showed that in response to a low-dose LPS challenge (2 mg/kg iv) a total of 20 genes were significantly altered in kidneys from MatSep rats (17 genes were upregulated and 3 were downregulated), as opposed to no significant differences in gene expression in control vs. control + LPS groups. Taken together, these findings indicate that MatSep induces priming of the immune response in the kidney.
Keywords: cytokines, early life stress, immune response, kidney, lipopolysaccharide, maternal separation
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death worldwide (27). In the United States alone, it is estimated that ~2,200 people die each day of cardiovascular-related diseases; that is one death every 40 s (49). Obesity, diabetes, and family history are known risk factors for developing CVD (49, 65). Early life stress (ELS) is an additional, relatively newly described risk factor for developing CVD (65). Epidemiological evidence shows that adverse childhood events, such as abuse, low socioeconomic status, childhood maltreatment, and parental separation, lead to increased blood pressure in adult life (2, 4, 66, 67). Not only is blood pressure significantly elevated in adults who experienced adverse childhood events, but they also present with elevated proinflammatory markers and mediators (9, 14, 19). Markers of inflammation have also been described in rat models of ELS (18, 33, 40), although this has not been investigated in detail. The mechanisms involved in the ELS-mediated increase in CVD risk are not fully understood. Prolonged exposure to stress during early development has been suggested to alter the hypothalamic-pituitary-adrenal (HPA) axis leading to impaired developmental pathways and altered regulation of the immune system (18, 33).
Maternal separation (MatSep) is a model of ELS in rodents (40), which involves separating pups from the dam for several hours per day during the first 2 wk of life. Using this animal model and studying the animals in adulthood, our group has previously reported an exaggerated blood pressure response to chronic infusion of angiotensin II (ANG II) as well as exaggerated renal vascular injury in MatSep rats (41). Most importantly, ANG II-infused MatSep male rats display a significant increase in the number of immune cells, specifically T cells, in the renal cortex (41). The ANG II-dependent increase in renal T cell numbers in MatSep rats is of interest because of the important role of T cells in developing CVD, especially hypertension (25, 26). These findings suggest that MatSep may lead to an “ELS-specific priming” of immune cell activation, thereby producing an exaggerated response to secondary stimuli in adulthood.
Proinflammatory cytokines are cell-signaling molecules that modulate immune cell proliferation and differentiation as well as promote hypertension. Increases in circulating proinflammatory cytokines such as interleukin (IL)-1β, IL-4, IL-6, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) have been reported in studies of humans with varying stressors early in the life cycle (14, 18). Miller and Chen (46) demonstrated that ex vivo peripheral blood monocytes from teenagers reporting a harsh family environment had a more robust IL-6 response when exposed to lipopolysaccharide (LPS) compared with teens from a supportive environment. In a separate study, authors found that toll-like receptor (TLR) 3 and TLR5 stimulation led to increased IL-6 production in serum of adults raised in low socioeconomic status compared with adults raised in high socioeconomic status (47). Therefore, the immune response of young or healthy adults with childhood adversity appears to result in a greater level of cytokine production than from adults with less or no childhood adversity.
In light of the kidney’s function as a major regulator of long-term blood pressure, the present study aimed to characterize the renal inflammatory state in adult male MatSep rats. We hypothesized that MatSep induces priming of the immune response in the kidney. To assess the validity of this hypothesis, experiments were designed to first determine the effect of MatSep on circulating and renal immune cell numbers, renal immune cell activation status, and cytokine levels. Specifically, we determined cytokines that had previously been shown to be elevated in humans exposed to childhood adversity (IL-1β, IL-4, IL-6, IFN-γ, TNF-α) as well as IL-17 and IL-10. Second, we assessed the effects of MatSep on chemokine/cytokine gene expression changes in response to an acute challenge with low-dose LPS.
MATERIALS AND METHODS
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at Augusta University and the University of Alabama at Birmingham. Wistar-Kyoto (WKY) rat breeding pairs were purchased from Charles River (Hartford, CT) and acclimatized to the animal housing facility for 2 wk. Rats were fed a standard diet (Teklad 8604, Madison, WI) with free access to water and food. All animals used for the experimental procedures were housed in a controlled 12:12-h light-dark cycle.
Maternal Separation Protocol
The maternal separation (MatSep) protocol was performed using offspring from WKY breeders, as previously described (40). Briefly, from postnatal day 2 to day 14, male pups were removed from the dam and placed in a 30°C incubator with clean bedding for 3 h/day with constant humidity. MatSep male rats were identified by having their tails snipped and cauterized with silver nitrate. Nonseparated male littermates were used as control rats. Weaning occurred on postnatal day 28, and experiments were carried out in adulthood at 12 wk of age. Control and MatSep rats were randomly selected from different litters and assigned to their respective experimental groups.
Blood, Kidney, Kidney Vessels, and Spleen Preparation
Blood.
Blood collected from the abdominal aorta was gently overlaid on 5 ml of the density separating agent Histopaque (Sigma-Aldrich), centrifuged at 400 g for 30 min at room temperature. The buffy coat containing mononuclear cells was resuspended, washed three times in phosphate-buffered saline (PBS), and numbers of mononuclear cells were determined under the microscope using a hemacytometer. Cells were then used in the flow cytometry or magnetic bead separation protocols described below.
Kidney.
Kidneys were harvested, flash-frozen in liquid nitrogen, and maintained at −80°C for ELISA or quantitative real-time PCR (qRT-PCR) analysis. For isolation of mononuclear cells and T lymphocytes, a subset of kidneys was cut in 1- to 2-mm-thick sections and incubated in digestion solution for 1 h at 37°C as previously described (12) and mononuclear cells were isolated by density separation over histopaque. T lymphocytes were then isolated from the mononuclear fraction by following the magnetic bead separation protocol below. The numbers of isolated mononuclear cells and T cells were counted using a hemacytometer to determine the absolute number of cells, and isolated T cells were also processed for qRT-PCR as described below.
Kidney vessel isolation.
A subset of harvested kidneys were quickly placed in ice-cold Hanks’ balanced salt solution containing protease inhibitors [2 mM phenylmethylsulfonyl fluoride (PMSF), 10 μM leupeptin, 2 μM pepstatin, and 0.0001% aprotinin]. After removal of the renal capsule, a 70-μm sieve mesh (BioDesign, Carmel, NY) was wrapped around the kidney. Renal vessels were isolated by gently grating the tissue with a spatula until only the kidney vessel tree was visualized (62). Kidney vessels were then flash-frozen in liquid nitrogen and kept at −80°C for analysis of gene expression by qRT-PCR as described below.
Spleen and aorta.
Spleens and thoracic aortas were quickly harvested, flash-frozen in liquid nitrogen, and kept at −80°C for analysis of cytokine levels by ELISA as described below.
Flow Cytometry
Circulating mononuclear cells were resuspended in 100 μl of 0.5% BSA PBS and incubated on ice for 15 min in the presence of allophycocyanine-conjugated anti-rat CD3 (clone IF4), fluorescein isothiocyanate-conjugated anti-rat CD4 (clone OX-35) and CD11b (clone OX-42), phycoerythrin-conjugated anti-CD45R (clone HIS24) and CD8 (clone OX-8), peridinin chlorophyll-conjugated anti-rat CD8 (clone OX-8), and Alexa 647-conjugated anti-rat CD11C (clone 8A2) and Foxp3 (clone FJK-16s). All antibodies were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), and Serotec (Bio-Rad, Raleigh, NC). Data acquisition was performed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and analyzed using BD CellQuest software (BD Biosciences).
Isolation of T Cells Using Magnetic Beads
Circulating and kidney mononuclear cells were centrifuged at 300 g for 10 min at room temperature, resuspended in staining buffer, and incubated with MACS pan T cell antibody coupled to magnetic microbeads for 15 min at 4°C (Miltenyi Biotec, San Diego, CA). Cells were then washed, centrifuged, and resuspended in 500 μl of staining buffer and applied to MACS magnetic separation columns (Miltenyi Biotec) to isolate T lymphocytes. Absolute numbers of isolated T lymphocytes were determined using a hemacytometer. Inflammatory gene expression was studied in isolated T cells by qRT-PCR as described below.
Quantitative Real-Time PCR
RNA isolation and cDNA conversion were performed using Qiagen (Valencia, CA) kits, following manufacturer’s instructions. RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The QuantiTect Reverse Transcription Kit was used to convert 1 μg of RNA to cDNA by following the reverse transcription two-step protocol. In step 1, genomic DNA elimination reaction mix was added to the RNA and the reaction was incubated at 42°C for 5 min. The reaction was stopped by immediately placing the samples on ice. In step 2, the reverse transcription components were added to the reaction tube and incubated at 42°C for 15 min to activate the reverse transcriptase, then at 95°C for 3 min to inactivate the reverse transcriptase. Primers for rat IL-1β, TNF-α, IFN-γ, complement component 3 (C3), chemokine (C-X-C motif) ligand 6 (CXCL6), CXCL11, CXCL2, CXCL9, chemokine (C-C motif) ligand 12 (CCL12), CCL19, CXCL1, and CCL3, from Qiagen were used for the amplification step [catalog numbers: QT00187158 (C3), QT02465120 (CXCL6), QT00372302 (CXCL11), QT00184891 (CXCL2), QT00183316 (CXCL9), QT01604624 (CCL12), QT01592724 (CCL19), QT00185528 (CXCL1), and QT00378350 (CCL3)], using 1.25 μl of sample cDNA. Forty cycles of DNA amplification were performed (95°C for 15 s and 55°C for 40 s after the initial DNA denaturing step) (Bio-Rad CFX96 Thermal Cycler. Hercules, CA). mRNA expression of circulating IL-1β, TNF-α, and IFN-γ was normalized to GAPDH, and mRNA expression of C3, CXCL6, CXCL11, CXCL2, CXCL9, CCL12, CCL19, CXCL1, and CCL3 in whole kidneys of untreated and LPS treated MatSep rats was normalized to β-actin. Gene expression was calculated using the 2−ΔΔCt method and normalized to expression levels in control animals, as described in Statistical Analysis.
Cytokine ELISA
Whole kidneys, spleen, and aorta from control and MatSep rats were homogenized in buffer containing 5.8 mM PMSF, 2.3 μM leupeptin, and 7.3 μM pepstatin and 0.5% Triton X-100. The homogenates were sonicated three times on ice and incubated at 4°C for 1 h. After centrifugation at 20,000 g for 20 min at 4°C, protein concentration in the supernatant extract was determined using Quick Start Bradford 1× Dye Reagent (Bio-Rad, Hercules, CA). IL-1β, IL-4, IL-6, IFN-γ, and TNF-α protein levels in the supernatant extract were measured using rat-specific ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Levels of IL-17 and IL-10 were also analyzed in whole kidney homogenates (R&D Systems). All cytokine measurements were normalized to total protein. The minimum detectable level for IL-1β, IL-4, and TNF-α was 5 pg/ml, IFN-γ and IL-10 was 10 pg/ml, IL-17 was 15.6 pg/ml, and IL-6 ranges from 14 to 36 pg/ml.
Immunohistochemical Staining and Analysis
Control and MatSep kidneys were fixed in 10% neutral buffered formalin solution and embedded in paraffin. Kidney sections (0.4 μm) were deparaffinized in a 61°C incubator for 30 min. Tissues were then rehydrated by placing the slides in several changes of ethanol (2 × 100%, 2 × 95%, and 1 × 70%) and finally water. Exogenous peroxidase was blocked by incubating slides in 30% H2O2 for 20 min. Antigen retrieval was performed by placing the slides in a 1:10 dilution of target retrieval solution (DAKO, Carpinteria, CA) and steamed for 35 min. Nonspecific binding was blocked using rodent block R (BioCare Medical, Concord, CA) for 20 min. Slides were incubated for 24 h at 4°C in primary antibodies against IL-1β (1:2,000), Toll-like receptor 4 (TLR4) (1:400; a receptor for LPS), Ki-67 (1:5,000; cell proliferation marker), myeloperoxidase (MPO) (1:2,000; activated neutrophil marker), cluster of differentiation 69 (CD69) (1:10,000; T cell activation marker), or CD44 (1:30,000, hyaluronan receptor, immune cell trafficking, leukocyte adhesion and activation marker) (Abcam, Cambridge, MA). DAB substrate (DAKO) was used to visualize positive stains for IL-1β, TLR4, Ki-67+, MPO+, CD69+, and CD44+.
Numbers of TLR4+, Ki-67+, and MPO+ cells were quantified in renal cortex and outer and inner medulla by counting 10 random microscopic fields (400 μm × 400 μm; 200× magnification) per kidney region in an unbiased manner. Quantification of CD69+ cells was analyzed by counting positive cells in whole kidney sections. For CD44+ cell quantification, 60 random glomeruli/kidney were assessed, and the number of glomeruli presenting CD44+ cells was quantified. Additionally, the number of CD44+ cells/glomerulus was determined at ×400 magnification. Data are expressed as number of positive cells/field. Immunostaining for TLR4 and CD44 was also assessed by analyzing whole kidney scans (×100 magnification) captured with a scanning microscope fitted with a DP73 camera (Olympus America, Melville, NY). Cortical and outer and inner medullary areas of each kidney image were outlined using Metamorph imaging software (Molecular Devices, Sunnyvale, CA), and the amount of positive staining for TLR4 and CD44 within those areas was quantified. Data are expressed as percentage of area of the kidney (cortex, outer or inner medulla) positively staining for TLR4 or CD44.
LPS Treatment
Control and MatSep rats were given a single low dose of LPS (2 mg/kg iv; Sigma-Aldrich, St. Louis, MO) or an equal volume of 0.9% NaCl (vehicle). Fourteen hours after LPS or vehicle treatment, animals were euthanized by a single injection of pentobarbital sodium (65 mg/kg). Blood and kidneys were collected for determination of absolute numbers of immune cells, inflammatory gene expression, and inflammatory cytokine levels. Spleens were collected for determinations of inflammatory gene expression and cytokine levels.
mRNA Isolation and RT2 Profiler PCR Array
Renal vessel and whole kidney mRNA isolation and cDNA preparation.
In separate homogenization tubes containing lysis buffer, mRNA was isolated from renal vessels or whole kidneys using an RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions. Total RNA concentration was determined using a NanoDrop 2000 spectrophotometer. For reverse transcription PCR steps, 0.5 μg of RNA was reverse transcribed using an RT2 First Strand Kit (Qiagen) following the manufacturer’s instructions.
PCR arrays.
Qiagen RT2 Profiler PCR Arrays were used to determine differential gene expression of 84 genes in circulating T cells and whole kidney (vehicle and LPS treated) (Rat Inflammatory Cytokines and Receptors; PARN-011A) as well as in renal vessels (Rat TH17 Autoimmunity and Inflammation; PARN-073A) of control and MatSep rats. Gene analyses were performed using a two-step PCR protocol with 40 cycles of 95°C for 15 s and 55°C for 40 s after the initial DNA denaturing step (Bio-Rad CFX96 Thermal Cycler, Hercules, CA). The cycle threshold (Ct) values for individual genes were subtracted from the average of five housekeeping genes included in PCR array plate, reported as change in Ct (ΔCt). Control and MatSep values were normalized to the appropriate control group by subtracting each ΔCt value from the average ΔCt values of the control group to yield ΔΔCt. The reported values were represented as fold change calculated as 2−ΔΔCt.
Statistical Analysis
Data are presented as means ± SE. Unpaired Student’s t-test was used to determine differences between control and MatSep rats for cytokine mRNA and protein expression, immunostaining for Ki-67, CD69, CD44, MPO, and TLR4, and absolute numbers of circulating and kidney mononuclear cells and T-lymphocytes. Differences in circulating immune cell populations detected by flow cytometry were evaluated using one-way ANOVA. Differences in cytokine expression levels assessed by PCR arrays were analyzed using the nonparametric Mann-Whitney test. Significant differences were defined as P < 0.05 using GraphPad Prism Software (San Diego, CA).
RESULTS
MatSep Does Not Change Numbers of Macrophages or T Lymphocytes in Adult Male Rats
The data obtained by flow cytometry revealed that the percentage of gated events of circulating macrophages, total T cells, helper, cytotoxic, and regulatory T cells (CD11b+/CD11c−, CD3+, CD4+, CD8+, and CD4+/FoxP3+ cells, respectively) were not altered by MatSep (Fig. 1A). Paradoxically, circulating percentages of B cells (CD45R+ cells) were significantly decreased in MatSep rats compared with control rats (Fig. 1A). Similarly, absolute numbers of circulating and kidney mononuclear and T cells did not differ between MatSep and control rats (Fig. 1B).
Fig. 1.
A: percent gated events of circulating immune cells. B: absolute numbers of mononuclear and T cells in blood and kidney of control and maternal separation (MatSep) rats. Results are expressed as means ± SE. *P < 0.05 vs. control; n = 4–6/group.
MatSep Induces Increased Renal Levels of IL-1β and TLR4 in Adults
Inflammatory gene PCR array analysis of 84 cytokine and cytokine receptor genes in isolated circulating T cells and renal vessels revealed no differences in gene expression in MatSep rats when compared with control rats. Specific analysis with RT-PCR of mRNA expression of IL-1β, TNF-α, and IFN-γ in isolated circulating T cells revealed significantly increased expression of IL-1β in MatSep rats compared with control rats [fold change vs. control (95% confidence interval): 3.41 (0.58, 4.25), P = 0.0130], whereas mRNA expression of TNF-α [0.807 (−0.38, 0.76), P = 0.476] and IFN-γ [1.05 (−0.92, 0.81), P = 0.896] was similar in MatSep and control rats.
ELISAs were performed to determine cytokine levels in kidneys from control and MatSep rats. Interestingly, MatSep led to increased renal levels of IL-1β when compared with control (Table 1), whereas no differences in renal IL-4 and IL-6 were observed (Table 1). Renal levels of IFN-γ, IL-17, and IL-10 were below the level of detection (<10 pg/ml for IFN-γ and IL-10, <15.6 pg/ml for IL-17) in both control and MatSep groups.
Table 1.
Protein levels of IL-1β, IL-4, IL-6, TNF-α, and IFN-γ in whole kidneys and spleens of control and MatSep rats
| Cytokine | Control | MatSep | P Value |
|---|---|---|---|
| Kidney | |||
| IL-1β | 4.44 ± 0.476 | 7.91 ± 1.02 | 0.0186* |
| IL-4 | 4.52 ± 1.04 | 3.98 ± 1.87 | 0.788 |
| IL-6 | 6.71 ± 0.647 | 5.96 ± 1.10 | 0.578 |
| TNF-α | ND | ND | |
| IFN-γ | BD | BD | |
| Spleen | |||
| IL-1β | 146.1 ± 11.15 | 145.4 ± 9.34 | 0.96 |
| IL-4 | 0.74 ± 0.048 | 0.50 ± 0.080 | 0.024* |
| IL-6 | 3.22 ± 0.15 | 2.90 ± 0.27 | 0.32 |
| TNF-α | 0.94 ± 0.048 | 1.02 ± 0.068 | 0.32 |
| IFN-γ | 9.21 ± 0.48 | 7.61 ± 0.33 | 0.016* |
Values are pg/mg protein ± SE; n = 4–9/group. MatSep, maternal separation; BD, below detection; ND, not detected.
P < 0.05 vs. control.
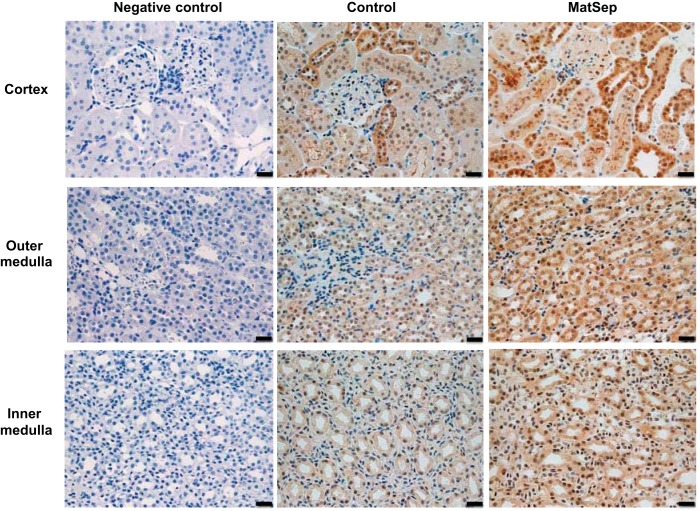
Immunohistochemical staining was used to localize expression of IL-1β in the kidney. The intensity of IL-1β staining was higher in all regions of MatSep compared with control rats (Fig. 2). Particularly, higher intensity of IL-1β was distinct in the distal nephron tubular epithelial cells of MatSep rats compared with control rats (Fig. 2).
Fig. 2.
Representative images of IL-1β protein expression in the renal cortex, outer medulla, and inner medulla of control and MatSep rats. n = 6–8/group. Scale bar, 20 μm.
Toll-like receptors (TLR), especially TLR4, have been linked to the expression of proinflammatory cytokines such as IL-1β (22, 45). Thus we further probed the expression of TLR4 in the kidney sections. Interestingly, TLR4 expression was prominent in the brush border of proximal tubules in both control and MatSep rats similarly (Fig. 3A). TLR4 expression in the renal medulla was observed in renal interstitial cells (Fig. 3A). Analysis of the number of TLR4-immunopositive cells in the renal medulla showed that MatSep rats had significantly increased numbers of TLR4-positive cells compared with control rats (Fig. 3).
Fig. 3.
A: representative images of TLR4 in the renal cortex, outer medulla, and inner medulla of control and MatSep rats. B: percentage of cortex, outer medulla, and inner medulla stained positive for TLR4. C: numbers of TLR4-positive cells in the outer medulla and inner medulla of control and MatSep rats. *P < 0.05 vs. control. n = 5–7/group. Black scale bar, 50 μm; white scale bar, 20 μm; inset scale bar, 2 mm.
MatSep Is Associated with Increased Cellular Proliferation, Increased Numbers of Activated Neutrophils, and Cd44+ Cells in the Kidneys
Compared with the control group, MatSep rats displayed significantly increased cellular proliferation (as indicated by Ki-67 positive staining) in the outer medulla where the Ki-67 staining appears to be localized to the vasa recta (Fig. 4). Ki-67 positive staining was observed within the cortical tubules and inner medullary interstitium, yet was similar between control and MatSep rats (Fig. 4).
Fig. 4.
Left: representative images of proliferating cells (Ki-67-positive cells) in the renal cortex, outer medulla, and inner medulla of control and MatSep rats. Right: quantification of Ki-67-positive cells in cortex, outer medulla, and inner medulla of control and MatSep rats. *P < 0.05 vs. control; n = 6–8/group. Scale bar, 20 μm. Arrows denote Ki-67-positive cells.
To further examine the activation status of immune cells, we immunostained for myeloperoxidase (MPO)-, CD44-, and CD69-positive cells. The number of activated neutrophils, as evidenced by MPO-positive cells, was significantly higher in the renal cortex and outer medulla of MatSep rats compared with control rats (Fig. 5). Image analysis of whole kidney scans demonstrated no significant difference in the percentage of CD44-positive staining present in cortex or outer medulla between the groups (Fig. 6, A and B); however, there was a trend in MatSep rats to show increased expression of CD44 in the outer medulla (P = 0.146; Fig. 6, A and B). Upon further examination of the renal cortex at higher magnification, MatSep rats presented a greater percentage of glomeruli containing CD44+ cells, as well as a greater number of CD44+ cells/glomerulus than control rats (Fig. 6, C and D). Renal medullary CD44-positive staining was found in tubular localizations with similar staining in both controls and MatSep rats (Fig. 6). CD69-positive cells were observed in both control and MatSep kidneys within distinct interstitial cells. We found no statistically significant difference in the number of CD69-positive cells in the renal cortex (control vs. MatSep, positive cells/renal cortex; 26.0 ± 6.58 vs. 34.22 ± 5.81; P = 0.37) or in the renal medulla (control vs. MatSep, positive cells/renal medulla; 18.83 ± 6.06 vs. 34.67 ± 6.92; P = 0.12) of control and MatSep rats.
Fig. 5.
Left: representative images of activated neutrophils (MPO-positive cells) in the renal cortex, outer medulla, and inner medulla of control and MatSep rats. Right: quantification of MPO-positive cells in cortex, outer medulla, and inner medulla of control and MatSep rats. *P < 0.05 vs. control; n = 6–8/group. Scale bar, 20 μm. Arrows denote MPO positive cells.
Fig. 6.
A: percentage of renal cortex or renal outer medulla stained positive for CD44; n = 5–8/group. B: representative images of CD44+ protein expression in renal outer medulla of control and MatSep rats. Scale bar, 50 μm. C: representative images of CD44 staining in cortex from control and MatSep rats. Yellow circles and arrows indicate glomeruli containing CD44+ cells, and purple circles and arrows indicate glomeruli with no CD44+ cells. Black arrows in the inset photos designate CD44+ cells within the glomerulus. Black scale bar, 100 μm; white scale bar, 20 μm. D: percentage of glomeruli containing CD44+ cells and average number of CD44+ cells/glomerulus in control and MatSep rat renal cortex. n = 5–8/group.
MatSep Sensitizes Rats to an Immune Challenge in Adulthood
To determine if MatSep sensitizes rats to an immune challenge in adulthood, male MatSep and control rats were challenged with a single low dose (2 mg/kg iv injection) of LPS. Absolute numbers of mononuclear and T cells in the blood and kidneys were similar in LPS-treated control and MatSep rats (Fig. 7).
Fig. 7.
Absolute number of mononuclear and T cells in blood and kidney of control and MatSep rats after low-dose LPS treatment. n = 5–7/group.
We analyzed changes in expression of 84 genes encoding inflammatory cytokines and receptors in whole kidneys in the presence and absence of low-dose LPS treatment. Low-dose LPS treatment in control rats resulted in no changes in gene expression when compared with control rats without LPS treatment (P > 0.05 for all 84 genes). On the contrary, the response to low-dose LPS treatment in MatSep rats was more dramatic with 20 genes altered: 17 of the 84 genes were upregulated, and 3 of the 84 genes were downregulated (Table 2). To verify these array results, RT-PCR quantitative measurements were determined for C3, CXCL6, CXCL11, CXCL2, CXCL9, CCL12, CCL19, CXCL1, and CCL3. All the genes except CXCL6 were found to be significantly increased in MatSep rats with LPS treatment compared with MatSep rats treated with vehicle (Table 3). Surprisingly, no differences were observed in the abundance of renal IL-1β, IFN-γ, IL-6, or IL-4 in the kidneys of LPS-treated control vs. LPS-treated MatSep rats (Table 4).
Table 2.
Effect of acute LPS treatment on the relative mRNA expression of cytokine and receptor genes in whole kidney of MatSep (measured by PCR array)
| Gene | Fold Change | 95% CI | P Value |
|---|---|---|---|
| Complement component 3* | 46.86 | (23.98, 88.44) | 0.0159 |
| Chemokine (C-X-C motif) ligand 6* | 45.54 | (16.46, 89.44) | 0.0159 |
| Chemokine (C-X-C motif) ligand 11* | 21.78 | (15.95, 26.24) | 0.0286 |
| Chemokine (C-X-C motif) ligand 2* | 14.64 | (7.79, 25.05) | 0.0357 |
| Chemokine (C-X-C motif) ligand 9* | 12.51 | (5.09, 16.14) | 0.0159 |
| Chemokine (C-C motif) ligand 12* | 10.91 | (6.46, 19.13) | 0.0357 |
| Secreted phosphoprotein 1* | 7.90 | (2.52, 17.16) | 0.0159 |
| Chemokine (C-C motif) receptor 1* | 7.68 | (3.80, 10.20) | 0.0159 |
| Chemokine (C-C motif) ligand 19* | 4.58 | (3.76, 5.83) | 0.0286 |
| Interleukin 1 beta* | 4.47 | (1.78, 7.20) | 0.0159 |
| Chemokine (C-X-C motif) ligand 1* | 3.50 | (1.92, 5.31) | 0.0159 |
| Chemokine (C-C motif) ligand 3* | 3.29 | (1.04, 5.39) | 0.0286 |
| Integrin alpha M* | 3.16 | (1.34, 4.50) | 0.0159 |
| Chemokine (C-X-C motif) ligand 10* | 2.17 | (0.41, 4.68) | 0.0317 |
| Chemokine (C-C motif) ligand 7* | 1.80 | (1.04, 3.01) | 0.0286 |
| Interleukin 1 receptor, type II* | 1.62 | (0.01, 3.21) | 0.0286 |
| Interleukin 8 receptor, beta* | 0.93 | (0.10, 2.15) | 0.0317 |
| Chemokine (C-C motif) receptor 9† | −0.68 | (−1.40, −0.15) | 0.0357 |
| Macrophage migration inhibitory factor† | −0.65 | (−1.13, −0.27) | 0.0159 |
| Interferon gamma† | −0.45 | (−0.82, −0.06) | 0.0357 |
CI, confidence interval. P < 0.05 vs. MatSep + vehicle. n = 4–5/group.
Upregulated genes in response to LPS.
Downregulated genes in response to LPS.
Table 3.
Effect of acute LPS treatment on the relative mRNA expression of cytokine and receptor genes in whole kidney of MatSep (individual RT-PCR)
| Gene | Fold Change | 95% CI | P Value |
|---|---|---|---|
| C3 | 207 | (27.72, 425.6) | 0.0043* |
| CXCL6 | 76.7 | (15.54, 133.1) | 0.057 |
| CXCL11 | 129 | (14.72, 73.84) | 0.036* |
| CXCL2 | 109 | (4.56, 218.3) | 0.029* |
| CXCL9 | 23.9 | (3.52, 36.32) | 0.0043* |
| CCL12 | 49.1 | (3.03, 65.91) | 0.029* |
| CCL19 | 61.0 | (15.37, 120.1) | 0.0022* |
| CXCL1 | 37.1 | (19.95, 49.4) | <0.0095* |
| CCL3 | 12.3 | (4.16, 21.16) | 0.0095* |
n = 4–6/group.
P < 0.05 vs. MatSep + vehicle.
Table 4.
Protein levels of IL-1β IL-4, IL-6, TNF-α, and IFN-γ in whole kidneys and spleens of control and MatSep rats after treatment with low-dose LPS
| Cytokine | Control + LPS | MatSep + LPS | P Value |
|---|---|---|---|
| Kidney | |||
| IL-1β | 37.64 ± 2.06 | 36.42 ± 4.36 | 0.80 |
| IL-4 | 1.75 ± 0.89 | 0.76 ± 0.27 | 0.33 |
| IL-6 | 13.91 ± 4.45 | 14.17 ± 2.31 | 0.96 |
| TNF-α | BD | BD | |
| IFN-γ | 2.56 ± 0.89 | 1.62 ± 0.48 | 0.39 |
| Spleen | |||
| IL-1β | 334.70 ± 55.85 | 292.2 ± 69.01 | 0.65 |
| IL-4 | 0.36 ± 0.066 | 0.44 ± 0.068 | 0.45 |
| IL-6 | 13.51 ± 5.56 | 8.46 ± 3.0 | 0.45 |
| TNF-α | 2.15 ± 0.25 | 1.35 ± 0.3 | 0.066 |
| IFN-γ | 30.22 ± 6.35 | 29.11 ± 5.57 | 0.90 |
Values are in pg/mg protein ± SE; n = 5–7/group.
MatSep Is Associated with Decreased Levels of IL-4 and IFN-γ in the Spleen
To further determine systemic levels and tissue specificity of cytokine production in control and MatSep rats, we assessed the levels of IL-1β, IL-4, IL-6, TNF-α, and IFN-γ in the spleen, plasma and aorta. IL-4 and IFN-γ were significantly decreased in spleens from MatSep compared with control rats (Table 1), whereas IL-1β, IL-6 and TNF-α were similar between groups. In addition, IL-1β, IL-4, IL-6, IFN-γ, and TNF-α were below detection in the plasma and aorta of control and MatSep rats. LPS treatment did not induce differences in splenic levels of IL-1β, IL-4, IL-6, IFN-γ, and TNF-α (Table 4) in control and MatSep rats.
DISCUSSION
Human studies have reported ELS as an emerging risk factor for CVD (2, 4, 66, 67). An association between ELS and inflammation has also been observed in adults (9). The primary aim of this study was to characterize the renal inflammatory state in adult male MatSep rats. The hypothesis guiding this study was that MatSep induces renal priming of the immune response. We utilized a rat model of ELS, maternal separation (MatSep), to identify ELS-specific inflammatory mediators and/or pathways in adulthood. The main findings of these studies demonstrate that, when compared with control rats, MatSep rats display 1) elevated IL-1β mRNA expression in circulating T cells as well as increased renal levels of IL-1β abundance, particularly localized in distal tubules; 2) increased TLR4-immunopositive interstitial cells in the renal medulla; 3) increased cellular proliferation in the renal medulla; 3) increased renal neutrophil activation; 4) greater numbers of glomerular CD44-immunopositive cells; and 5) heightened renal cytokine and chemokine gene expression in response to LPS treatment. We also found that MatSep and control rats have similar numbers of circulating and renal mononuclear cells, T cells, and T cell subsets, whereas the population of circulating B cell numbers was significantly decreased in MatSep rats. Our results indicate that MatSep leads to “priming” or sensitization of the immune system, resulting in an exaggerated gene expression response to an immune challenge in adulthood. This ELS-mediated sensitization of the immune system may play an important role in promoting cardiovascular disease earlier and more robustly in adulthood.
In humans, ELS is defined as prolonged adverse childhood experiences (ACE) typically occurring during the first decade of life (38). ACEs in humans are described as childhood maltreatment, physical and sexual abuse, parental divorce, war, and low socioeconomic status (2, 15, 67), and increasing numbers of ACEs are associated with greater emotional, immune, and cardiovascular disorders during adult life (8, 9, 15, 67, 69). Some investigators suggest that many adult diseases should be viewed as developmental disorders beginning early in life and could be reduced by the alleviation of or resilience to adversity in childhood (16, 63).
Various animal models have been characterized to study the effect of ELS on adult health, including MatSep in rats. The MatSep rat model mimics the behavioral disorders observed in humans with ELS, such as depression and anxiety (35, 44, 68). The association between ELS and CVD risk, such as elevated systolic and diastolic blood pressure (3, 4, 34), and the association with increased proinflammatory mediators (8) are established in humans. The significance of utilizing the MatSep rat model is to understand how ELS alone contributes to the development of cardiovascular disease risks. Our laboratory has used this model to study the effects of ELS on the risk of cardiovascular disease in adulthood (39–41).
The present study showed that MatSep rats specifically express higher levels of IL-1β in the kidney compared with control rats. Macrophages and epithelial cells produce IL-1β, and it plays a critical role in modulating tubular transport and inflammatory responses of both the innate and adaptive immune response through activation of the IL-1 receptor (IL-1R) (6, 50). Zhang et al. (72) recently published that the renal IL-1β/IL-1R pathway plays a role in the development of angiotensin II-induced hypertension. This study demonstrated that activation of the IL-1R prevents maturation of macrophages and blunts the induction of nitric oxide (NO), thereby limiting the NO-dependent natriuresis via the sodium-potassium-two-chloride (NKCC2) transporter (72). Our laboratory previously published that MatSep induces a NO deficient phenotype (39). We propose that the elevated IL-1β levels observed in the distal tubule from MatSep rats may be related to the NO deficient phenotype. Further research is needed to understand whether these pathways are linked in the MatSep model. To begin to understand possible pathways linking ELS to the IL-1β pathway, we examined localization and abundance of TLR4 in the kidneys of control and MatSep rats. We found an approximate twofold increase in the number of TLR4-immunopositive cells specifically in renal medullary interstitial cells in MatSep rats. Activation of TLR4 is known to mediate increased expression of a variety of proinflammatory cytokines including IL-1β (22). Souza et al. (64) recently showed that TLR4 deficiency in renal epithelial cells led to decreased IL-1β expression in vitro. However, further studies are needed to determine whether there is a direct or indirect link between TLR4 signaling and IL-1β in renal tubules of MatSep rats. These observations suggest that MatSep primes the innate immune system within the kidney.
Human studies revealed that chronic psychosocial stress such as depression is associated with increased numbers of circulating neutrophils (42). Blood isolated from depressed and nondepressed patients was analyzed for differences in myeloperoxidase (MPO) content. The results showed increased numbers of circulating neutrophils, elevated circulating monocytes and total number of white blood cell count (42). Since MatSep is a well-studied model for depressive-like behaviors, we assessed activation of neutrophils by MPO-positive cells within the kidney (51). We found a significant increase in MPO-positive cells in MatSep cortex and outer medulla compared with control kidneys, suggesting that ELS leads to a proinflammatory state in adult rat kidneys. Neutrophils are mainly activated by chemokines and cytokines (70). The increased IL-1β in the renal tubular epithelial cells and the upregulation of several cytokines and chemokines may contribute to the MatSep-specific neutrophil activation. Furthermore, we also assessed cellular proliferation by staining for Ki-67, and found a significant elevation specifically in renal outer medulla. Upon close observation, the proliferating cells appear to be localized to the vasa recta although future studies with specific markers are necessary to verify this initial observation. Nevertheless, MatSep is associated with increased cellular proliferation as well as increased activation of neutrophils and, taken together with the elevated abundance of IL-1β and increased TLR4-immunopositive cells, indicates a phenotype with activation of the innate immune system. Taken together, all of these observations suggest that MatSep-induced specific changes may be more specific to the outer medullary region of the kidney, which is a region known to be sensitive to renal injury (13).
CD44 is a glycoprotein expressed on the surface of endothelial cells, leukocytes, epithelial cells, keratinocytes, and fibroblasts and also considered the primary receptor for hyaluronan. CD44 is important in physiological functions including cellular proliferation, lymphocyte activation, hematopoiesis, and cell adhesion and migration (54). Furthermore, various studies have implicated CD44 in renal disease, vascular disease, bacterial infection, liver disease, and in wound healing (28, 52). In rodent models of renal disease, increased expression of CD44 resulted in worsening of disease. For example, in an experimental rat model of crescentic glomerulonephritis, CD44 protein expression was significantly increased in infiltrating macrophages within the glomerular crescents (30). Additionally, migrating leukocytes that adhered to the endothelium also had high expression of CD44 compared with healthy rats (30). This observation correlated with significant increases in proteinuria, serum creatinine, and creatinine clearance in the diseased rats (30). The involvement of CD44 in renal disease has also been reported in humans. Renal biopsy from patients with acute renal allograft rejection showed significant increase in CD44 expression in the tubules and infiltrating cells compared with nonrejecting renal biopsies (57). In a separate study, in biopsies of patients with severe renal histological damage, CD44 expression was significantly higher in interstitial infiltrates and in infiltrating cells in the glomerulus (58). In line with these studies, we report a significant increase in CD44+ cells in glomeruli from MatSep rats compared with control rats, suggesting activation of immune function. Future studies are needed to determine which population of cells expressed CD44 in MatSep glomerulus and whether it affects renal function. Conversely, we stained for the presence of CD69 in control and MatSep kidneys. Unlike CD44, CD69 is an early T cell activation marker. It is a type II C-lectin membrane receptor expressed at low levels in naïve T cells (20). Upon T cell activation, CD69 becomes highly expressed, leading to inflammatory responses (20). In this study, we found no differences in this activation marker in MatSep rats compared with control rats, suggesting that MatSep alone most likely does not activate T cells and the adaptive immune system, although further studies are necessary to fully characterize the T cell activation status in MatSep rats.
We also assessed protein levels of proinflammatory cytokines in the plasma, aorta, and spleen to determine whether the observed cytokine differences between control and MatSep rat kidneys are similar in other tissues or specific to the kidney. Blood vessels participate in inflammatory processes by upregulating expression of cytokines, chemokines, and adhesion molecules to allow extravasation of immune cells into the site of inflammation (71). IL-1β, IL-4, IL-6, IFN-γ, and TNF-α were below the detection limit of the assay in the plasma and aorta of control and MatSep rats, suggesting that MatSep does not elicit a systemic immune response. These findings are interesting because it suggests that the immune programming due to MatSep may be localized to organs. The spleen serves as a reservoir for immune cells and a site for antigen presentation allowing for the release and homing of immune cells to inflammatory sites (7). In the spleen, we observed that IL-4 and IFN-γ were significantly lower in MatSep rats than in control rats, suggesting MatSep induces distinct cytokine profiles in the kidney and spleen.
Finally, we assessed whether an immune challenge results in differential expression of chemokines/cytokines and their receptors in the kidney of MatSep and control rats. Low-dose LPS treatment showed significant upregulation of 17 genes and downregulation of 3 genes in the MatSep rats (Table 2 and 3), whereas low-dose LPS treatment resulted in no significant changes in gene expression in control rats (P > 0.05). LPS is a known ligand for TLR4, and thus we propose that the MatSep-induced changes in chemokine expression are most likely TLR4-dependent. Chemokines are a group of cytokines with chemotactic properties produced by a variety of cell types and play an important role in recruiting immune cells to the site of an immune response (21). Tissue expression of specific chemokine or chemokine receptors drive distinct immune cell recruitment and response (43). Complement component 3 (C3) showed the highest expression with LPS treatment in MatSep rats. C3 is involved in a host of inflammatory kidney diseases including hereditary renal diseases (32). A number of ligands for C-X-C motif (CXCL) were also highly expressed in response to LPS in MatSep rats. CXCL2 and CXCL6 are neutrophil-recruiting chemoattractants (11, 29), whereas CXCL9 is implicated in crescentric glomerulonephritis (56). CXCL11 and CXCL9 are ligands for CXCR3 (23). The expression of CXCR3 in T cells allows for T cell differentiation and infiltration into injured tissues (23). CXCL1, chemokine motif ligand (CCL) CCL3, CCL19, and CCL12 are involved in recruitment of inflammatory cells in renal diseases as seen in ischemia-reperfusion injury (17, 24, 48) and kidney allograft rejection rodent models (73). Additionally, interleukins are produced by leukocytes and endothelial cells participating in regulating immune cell differentiation (1). The upregulation of chemokines and cytokines that we found in kidneys of MatSep rats in response to LPS supports the hypothesis that ELS primes the immune system.
These data indicate that MatSep induces programming of the innate immune system, especially the TLR4 and IL-1β pathways. Several possible mechanisms exist linking MatSep to the priming of the innate immune system that may be relevant for discussion. Our laboratory previously showed that MatSep induces sensitization of the renal and systemic sympathetic nervous system (SNS) (36). Several recent publications show a link between the SNS and the innate immune response (55, 61). We also previously found that MatSep downregulates endothelin receptor expression (37), and it is well-accepted that the endothelin system regulates immune responses (31, 59, 60) although few details are understood about specific mediators or cell types. Furthermore, MatSep is known to increase activation of the hypothalamic-pituitary-adrenal (HPA) axis during early life (35, 53). The HPA axis is a known modulator of immune function (5). We propose that MatSep-induced modulation of TLR4, IL-1β, SNS sensitivity, endothelin system, and/or the HPA axis during early life reprograms and primes the renal immune system.
Perspectives
Numerous compelling epidemiological studies show that ELS is an independent risk factor in the development of CVD risk. About 35 million children in the United States experience adverse childhood events (10), suggesting that these children are at a higher risk for increased sensitivity to developing CVD in their adult life. Studies to determine the ELS-specific mechanisms of CVD risk are needed. The immune system is a likely culprit mediating ELS-induced CVD risk, yet specific mediator(s) and pathway(s) are unknown. Future studies remain to be done that focus on understanding the interactions and/or cross talk between chemokines/cytokines, innate immune activation, renal function and CVD risk.
GRANTS
Portions of this work were funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant T32-DK-007545 to C. De Miguel, the William Townsend Porter Pre-doctoral Fellowship from the American Physiological Society and National Heart, Lung, and Blood Institute Grants T32-HL-007918–19 to I. E. Obi, F32-HL-116145 to D. H. Ho, K99-R00-HL-111354 to A. S. Loria, and P01-HL-69999 to J. S. Pollock.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.D.M., I.E.O., D.H.H., and J.S.P. conceived and designed research; C.D.M., I.E.O., and D.H.H. performed experiments; C.D.M., I.E.O., D.H.H., and J.S.P. analyzed data; C.D.M., I.E.O., D.H.H., A.S.L., and J.S.P. interpreted results of experiments; C.D.M., I.E.O., and D.H.H. prepared figures; C.D.M., I.E.O., D.H.H., and J.S.P. drafted manuscript; C.D.M., I.E.O., D.H.H., A.S.L., and J.S.P. edited and revised manuscript; C.D.M., I.E.O., D.H.H., A.S.L., and J.S.P. approved final version of manuscript.
REFERENCES
- 1.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol 127: 701–721.e701-770, 2011. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 2.Alastalo H, Räikkönen K, Pesonen AK, Osmond C, Barker DJ, Heinonen K, Kajantie E, Eriksson JG. Cardiovascular morbidity and mortality in Finnish men and women separated temporarily from their parents in childhood–a life course study. Psychosom Med 74: 583–587, 2012. doi: 10.1097/PSY.0b013e31825b3d76. [DOI] [PubMed] [Google Scholar]
- 3.Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Kajantie E, Heinonen K, Forsen TJ, Eriksson JG. Cardiovascular health of Finnish war evacuees 60 years later. Ann Med 41: 66–72, 2009. doi: 10.1080/07853890802301983. [DOI] [PubMed] [Google Scholar]
- 4.Alastalo H, Räikkönen K, Pesonen AK, Osmond C, Barker DJP, Heinonen K, Kajantie E, Eriksson JG. Early life stress and blood pressure levels in late adulthood. J Hum Hypertens 27: 90–94, 2013. doi: 10.1038/jhh.2012.6. [DOI] [PubMed] [Google Scholar]
- 5.Bellavance MA, Rivest S. The HPA-immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol 5: 136, 2014. doi: 10.3389/fimmu.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA 106: 7119–7124, 2009. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity 39: 806–818, 2013. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 163: 1135–1143, 2009. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA 104: 1319–1324, 2007. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Data Resource Center for Childhood and Adolescent Health The Child's Family. 2011. http://www.childhealthdata.org.
- 11.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121: 4930–4937, 2013. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 12.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Sabbahy M, Vaidya VS. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip Rev Syst Biol Med 3: 606–618, 2011. doi: 10.1002/wsbm.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun 27: 8–12, 2013. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14: 245–258, 1998. doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 16.Fortson BL, Klevens J, Merrick MT, Gilbert LK, Alexander SP. Preventing Child Abuse and Neglect: A Technical Package for Policy, Norm, and Programmatic Activities. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, 2016. doi: 10.15620/cdc.38864 [DOI] [Google Scholar]
- 17.Furuichi K, Gao JL, Horuk R, Wada T, Kaneko S, Murphy PM. Chemokine receptor CCR1 regulates inflammatory cell infiltration after renal ischemia-reperfusion injury. J Immunol 181: 8670–8676, 2008. doi: 10.4049/jimmunol.181.12.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galic MA, Spencer SJ, Mouihate A, Pittman QJ. Postnatal programming of the innate immune response. Integr Comp Biol 49: 237–245, 2009. doi: 10.1093/icb/icp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gollwitzer ES, Marsland BJ. Impact of early-life exposures on immune maturation and susceptibility to disease. Trends Immunol 36: 684–696, 2015. doi: 10.1016/j.it.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 20.González-Amaro R, Cortés JR, Sánchez-Madrid F, Martín P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol Med 19: 625–632, 2013. doi: 10.1016/j.molmed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32: 659–702, 2014. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 22.Grishman EK, White PC, Savani RC. Toll-like receptors, the NLRP3 inflammasome, and interleukin-1β in the development and progression of type 1 diabetes. Pediatr Res 71: 626–632, 2012. doi: 10.1038/pr.2012.24. [DOI] [PubMed] [Google Scholar]
- 23.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res 317: 620–631, 2011. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo J, Guan Q, Liu X, Wang H, Gleave ME, Nguan CYC, Du C. Relationship of clusterin with renal inflammation and fibrosis after the recovery phase of ischemia-reperfusion injury. BMC Nephrol 17: 133, 2016. doi: 10.1186/s12882-016-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 57: 132–140, 2011. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H, Chen G, Liao D, Zhu Y, Xue X. Effects of berries consumption on cardiovascular risk factors: a meta-analysis with trial sequential analysis of randomized controlled trials. Sci Rep 6: 23625, 2016. doi: 10.1038/srep23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan AR, Racine RR, Hennig MJ, Lokeshwar VB. The role of CD44 in disease pathophysiology and targeted treatment. Front Immunol 6: 182, 2015. doi: 10.3389/fimmu.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jovic S, Linge HM, Shikhagaie MM, Olin AI, Lannefors L, Erjefält JS, Mörgelin M, Egesten A. The neutrophil-recruiting chemokine GCP-2/CXCL6 is expressed in cystic fibrosis airways and retains its functional properties after binding to extracellular DNA. Mucosal Immunol 9: 112–123, 2016. doi: 10.1038/mi.2015.43. [DOI] [PubMed] [Google Scholar]
- 30.Jun Z, Hill PA, Lan HY, Foti R, Mu W, Atkins RC, Nikolic-Paterson DJ. CD44 and hyaluronan expression in the development of experimental crescentic glomerulonephritis. Clin Exp Immunol 108: 69–77, 1997. doi: 10.1046/j.1365-2249.1997.d01-977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 86: 896–904, 2014. doi: 10.1038/ki.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kościelska-Kasprzak K, Bartoszek D, Myszka M, Zabińska M, Klinger M. The complement cascade and renal disease. Arch Immunol Ther Exp (Warsz) 62: 47–57, 2014. doi: 10.1007/s00005-013-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai MC, Huang LT. Effects of early life stress on neuroendocrine and neurobehavior: mechanisms and implications. Pediatr Neonatol 52: 122–129, 2011. doi: 10.1016/j.pedneo.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychol 28: 338–346, 2009. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25: 3091–3098, 2007. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 36.Loria AS, Brands MW, Pollock DM, Pollock JS. Early life stress sensitizes the renal and systemic sympathetic system in rats. Am J Physiol Renal Physiol 305: F390–F395, 2013. doi: 10.1152/ajprenal.00008.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loria AS, D’Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol 299: R185–R191, 2010. doi: 10.1152/ajpregu.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loria AS, Ho DH, Pollock JS. A mechanistic look at the effects of adversity early in life on cardiovascular disease risk during adulthood. Acta Physiol (Oxf) 277–287, 2014. doi: 10.1111/apha.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58: 619–626, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 55: 494–499, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol 304: R121–R129, 2013. doi: 10.1152/ajpregu.00364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maes M, Van der Planken M, Stevens WJ, Peeters D, DeClerck LS, Bridts CH, Schotte C, Cosyns P. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res 26: 125–134, 1992. doi: 10.1016/0022-3956(92)90004-8. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today 20: 254–257, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24: 1161–1192, 2001. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 45.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 1: 135–145, 2001. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 46.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci 21: 848–856, 2010. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA 106: 14716–14721, 2009. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol 159: 2137–2145, 2001. doi: 10.1016/S0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura K, Hayashi H, Kubokawa M. Proinflammatory cytokines and potassium channels in the kidney. Mediators Inflamm 2015: 362768, 2015. doi: 10.1155/2015/362768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191: 677–691, 2010. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patouraux S, Rousseau D, Bonnafous S, Lebeaupin C, Luci C, Canivet CM, Schneck AS, Bertola A, Saint-Paul MC, Iannelli A, Gugenheim J, Anty R, Tran A, Bailly-Maitre B, Gual P. CD44 is a key player in non-alcoholic steatohepatitis. J Hepatol 67: 328–338, 2017. doi: 10.1016/j.jhep.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18: 195–200, 1993. doi: 10.1016/0169-328X(93)90189-V. [DOI] [PubMed] [Google Scholar]
- 54.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4: 33–45, 2003. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 55.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA 110: 16574–16579, 2013. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richard KS, Phoon A, Kitching R, Odobasic D, Jones LK, Semple TJ, Holdsworth SR. T-bet deficiency attenuates renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol 19: 477–485, 2008. doi: 10.1681/ASN.2007030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rouschop KMA, Roelofs JJ, Sylva M, Rowshani AT, Ten Berge IJ, Weening JJ, Florquin S. Renal expression of CD44 correlates with acute renal allograft rejection. Kidney Int 70: 1127–1134, 2006. doi: 10.1038/sj.ki.5001711. [DOI] [PubMed] [Google Scholar]
- 58.Roy-Chaudhury P, Khong TF, Williams JH, Haites NE, Wu B, Simpson JG, Power DA. CD44 in glomerulonephritis: expression in human renal biopsies, the Thy 1.1 model, and by cultured mesangial cells. Kidney Int 50: 272–281, 1996. doi: 10.1038/ki.1996.312. [DOI] [PubMed] [Google Scholar]
- 59.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 56: 942–949, 2010. doi: 10.1161/HYPERTENSIONAHA.110.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saleh MA, Pollock JS, Pollock DM. Distinct actions of endothelin A-selective versus combined endothelin A/B receptor antagonists in early diabetic kidney disease. J Pharmacol Exp Ther 338: 263–270, 2011. doi: 10.1124/jpet.111.178988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: a review. Front Pharmacol 6: 171, 2015. doi: 10.3389/fphar.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider MP, Wach PF, Durley MK, Pollock JS, Pollock DM. Sex differences in acute ANG II-mediated hemodynamic responses in mice. Am J Physiol Regul Integr Comp Physiol 299: R899–R906, 2010. doi: 10.1152/ajpregu.00638.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shonkoff JP, Garner AS; The Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics . The lifelong effects of early childhood adversity and toxic stress. Pediatrics 129: e232–e246, 2012. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 64.Souza AC, Tsuji T, Baranova IN, Bocharov AV, Wilkins KJ, Street JM, Alvarez-Prats A, Hu X, Eggerman T, Yuen PS, Star RA. TLR4 mutant mice are protected from renal fibrosis and chronic kidney disease progression. Physiol Rep 3: e12558, 2015. doi: 10.14814/phy2.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steptoe A, Kivimäki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health 34: 337–354, 2013. doi: 10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- 66.Su S, Wang X, Kapuku GK, Treiber FA, Pollock DM, Harshfield GA, McCall WV, Pollock JS. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension 64: 201–207, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation 131: 1674–1681, 2015. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vetulani J. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol Rep 65: 1451–1461, 2013. doi: 10.1016/S1734-1140(13)71505-6. [DOI] [PubMed] [Google Scholar]
- 69.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry 64: 49–56, 2007. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 70.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 49: 1618–1631, 2010. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 71.Zgraggen S, Ochsenbein AM, Detmar M. An important role of blood and lymphatic vessels in inflammation and allergy. J Allergy (Cairo) 2013: 672381, 2013. doi: 10.1155/2013/672381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab 23: 360–368, 2016. doi: 10.1016/j.cmet.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ziegler E, Gueler F, Rong S, Mengel M, Witzke O, Kribben A, Haller H, Kunzendorf U, Krautwald S. CCL19-IgG prevents allograft rejection by impairment of immune cell trafficking. J Am Soc Nephrol 17: 2521–2532, 2006. doi: 10.1681/ASN.2005070782. [DOI] [PubMed] [Google Scholar]